Open Access Article
Open Access ArticleElectron count and ligand composition influence the optical and chiroptical signatures of far-red and NIR-emissive DNA-stabilized silver nanoclusters†
Rweetuparna
Guha
 a,
Anna
Gonzàlez-Rosell
a,
Anna
Gonzàlez-Rosell
 a,
Malak
Rafik
a,
Nery
Arevalos
a,
Benjamin B.
Katz
b and
Stacy M.
Copp
a,
Malak
Rafik
a,
Nery
Arevalos
a,
Benjamin B.
Katz
b and
Stacy M.
Copp
 *acd
*acd
aDepartment of Materials Science and Engineering, University of California, Irvine, CA 92697, USA. E-mail: stacy.copp@uci.edu
bDepartment of Chemistry, University of California, Irvine, CA 92697, USA
cDepartment of Physics and Astronomy, University of California, Irvine, CA 92697, USA
dDepartment of Chemical and Biomolecular Engineering, University of California, Irvine, CA 92697, USA
First published on 11th September 2023
Abstract
Near-infrared (NIR) emissive DNA-stabilized silver nanoclusters (AgN-DNAs) are promising fluorophores in the biological tissue transparency windows. Hundreds of NIR-emissive AgN-DNAs have recently been discovered, but their structure–property relationships remain poorly understood. Here, we investigate 19 different far-red and NIR emissive AgN-DNA species stabilized by 10-base DNA templates, including well-studied emitters whose compositions and chiroptical properties have never been reported before. The molecular formula of each purified species is determined by high-resolution mass spectrometry and correlated to its optical absorbance, emission, and circular dichroism (CD) spectra. We find that there are four distinct compositions for AgN-DNAs emissive at the far red/NIR spectral border. These emitters are either 8-electron clusters stabilized by two DNA oligomer copies or 6-electron clusters with one of three different ligand compositions: two oligomer copies, three oligomer copies, or two oligomer copies with additional chlorido ligands. Distinct optical and chiroptical signatures of 6-electron AgN-DNAs correlate with each ligand composition. AgN-DNAs with three oligomer ligands exhibit shorter Stokes shifts than AgN-DNAs with two oligomers, and AgN-DNAs with chlorido ligands have increased Stokes shifts and significantly suppressed visible CD transitions. Nanocluster electron count also significantly influences electronic structure and optical properties, with 6-electron and 8-electron AgN-DNAs exhibiting distinct absorbance and CD spectral features. This study shows that the optical and chiroptical properties of NIR-emissive AgN-DNAs are highly sensitive to nanocluster composition and illustrates the diversity of structure–property relationships for NIR-emissive AgN-DNAs, which could be harnessed to precisely tune these emitters for bioimaging applications.
Introduction
DNA-stabilized silver nanoclusters1 (AgN-DNAs) are emerging as promising emitters for applications in bioimaging and sensing.2,3 AgN-DNAs are known to consist of 10 to 30 Ag atoms protected by one or two single-stranded DNA oligomers, whose sequence selects the size, shape, and photophysical properties of the encapsulated silver nanocluster.4 Researchers have used this sequence-to-structure correlation to synthesize a diverse set of AgN-DNAs with atomically defined sizes and visible to near-infrared (NIR) emission wavelengths.4 NIR-emissive AgN-DNAs have recently gained particular attention for their high fluorescence quantum yields,5 large Stokes shifts,6 and unique photophysical properties for novel bioimaging modalities.7–10 High-throughput studies and machine learning-guided discovery have dramatically expanded the number of known NIR-emissive AgN-DNAs to hundreds of species.11,12 However, the fundamental structure–property relationships of this rapidly growing class of NIR emitters are poorly understood.Detailed studies of compositionally pure AgN-DNAs over the last decade have particularly improved understanding of the compositions of AgN-DNAs with visible fluorescence wavelengths. Atomically precise AgN-DNA species can be isolated by high-performance liquid chromatography (HPLC) and sized by high-resolution electrospray ionization mass spectrometry (ESI-MS) to determine the total number of silver atoms N, the number of DNA strands ns, and the nanocluster charge Qc of the AgN-DNA.4,13–16 With combined knowledge of N and Qc, one can determine the nanocluster's effective valence electron count, N0 = N − Qc, which strongly influences nanocluster electronic structure17 and cannot be provided by crystallography alone. ESI-MS has shown that emissive AgN-DNAs are partially reduced, i.e. N0 < N, and the dominant AgN-DNA excitation peak scales strongly with N0. The correlations of N0 with excitation and emission energies are well-understood for visibly emissive AgN-DNAs: green-emissive AgN-DNAs have N0 = 4 electrons, and red-emissive AgN-DNAs have N0 = 6 electrons.16,18–20
Far less is known about the compositions of NIR-emissive AgN-DNAs with peak emission wavelength λp > 700 nm. Only a few NIR-emissive AgN-DNAs have molecular formulae determined by ESI-MS: four species with λp = 775 to 1000 nm and N0 = 10 to 12 effective valence electrons;11,16,20 two N0 = 8 AgN-DNAs that show evidence for spherical geometries, like other 8-electron superatoms;21 and the recently reported variants of a N0 = 6 Ag16-DNA with λp = 735 nm, an unusually large Stokes shift, two chlorido ligands,22 and solved crystal structures.23 Due to the varying affinities of adenine, cytosine, guanine, and thymine for silver cations,24,25 the combinatorially large space of DNA oligomers may produce a wide array of AgN structures. Moreover, the significant diversities of Stokes shifts, quantum yields, excited state lifetimes, and dark state behaviors of AgN-DNAs in the NIR spectral range6–9,20,21,26,27 further suggest that much about AgN-DNAs has yet to be understood.
To develop an understanding of the structure–property relationships of NIR AgN-DNA emitters, we investigate a large set of 19 different AgN-DNA species at the far red/NIR spectral border, with peak emission λp = 640 nm–820 nm. Several of these NIR AgN-DNAs have previously attracted attention for their notable optical properties.5,6,21–23 12 of the 19 AgN-DNAs in this study do not have molecular formulae assigned by ESI-MS, and only two have previously reported electronic circular dichroism (CD) spectra. We combine HPLC, high-resolution ESI-MS, and CD spectroscopy to correlate AgN core size, electron count, and ligand composition (i.e. the numbers of DNA ligands as well as chlorido ligands) to optical properties. CD spectroscopy is especially sensitive to DNA molecular conformation and to the structural features of chiral metal nanoclusters,28 and CD provides an important bridge with theory.29 AgN-DNAs are known to exhibit UV and visible CD signatures,19,30–33 but no large-scale study has correlated AgN-DNA CD signatures with their compositional or optical properties before. Moreover, the CD spectrum of the Ag16-DNA of known crystal structure was just recently calculated,34 but the experimental CD spectrum of this emitter has not been reported prior to now.
This study shows that unlike the simpler N0-to-color correlation for green- and red-emissive AgN-DNAs, NIR-emissive AgN-DNAs exhibit fluorescence spectra that depend on both valence electron count, N0, and ligand composition. Distinct UV and visible CD signatures are correlated with both N0 and the ligand content of AgN-DNAs, and ligand composition has a particular impact on the Stokes shifts of N0 = 6 AgN-DNAs. Our measured CD spectrum for the chlorido-stabilized Ag16-DNA also agrees well with very recent theoretical calculations.34 This study illustrates the diversity of AgN-DNAs at the far-red/NIR spectral border and shows that ligand chemistry can be used to precisely tune photophysical and chiroptical properties of these nanocluster emitters. Moreover, the compositional and spectral information provided here for a large set of 19 AgN-DNAs provide a rich data set to enable theoretical modeling of AgN-DNA electronic structure and inspire future X-ray crystallographic studies.
Results and discussion
We selected 19 AgN-DNAs with λp = 640 to 820 nm from a library of 10-base DNA oligomers previously designed using machine-learning methods.12,35–37 Emitters were chosen for their λp values in the far-red to NIR spectral region and because they can be isolated by HPLC to obtain a compositionally pure species, which is an essential step that ensures only the emissive AgN-DNA species is probed by ESI-MS and CD spectroscopy. In addition to 15 NIR-emissive AgN-DNAs (λp > 700 nm), we also include four far-red emissive AgN-DNAs (λp < 700 nm) in order to compare to past studies on AgN-DNAs in the visible spectral window.16,20 We consider only 10-base DNA oligomer length here because (1) these are by far the best-studied class of AgN-DNAs, with nearly 4000 DNA sequences sampled to date,5,6,11,12,16,20,35–38 and (2) by focusing on a single oligomer length, we separate the effects of DNA ligand length from DNA ligand conformation on AgN-DNA structural and optical properties.Several NIR AgN-DNAs in this study were previously studied in detail. This includes emitters with molecular formulae determined by ESI-MS: a well-studied λp = 735 nm emissive (DNA)2[Ag16Cl2]8+ and its variants with known structures23,39,40 and N0 = 6;22 and two 8-electron species, (DNA)2[Ag16]8+ and (DNA)2[Ag17]9+.26 Two other well-studied AgN-DNAs investigated here have unknown molecular formulae: a λp = 721 nm emitter with 73% quantum yield5 and a λp = 811 nm emitter with dual ns-lived and μs-lived emission.26 ESI-MS analysis of these latter two species may provide new insights into the origins of their favorable optical properties. About half of the 19 AgN-DNAs have never been studied in detail before.
Mass spectral analysis
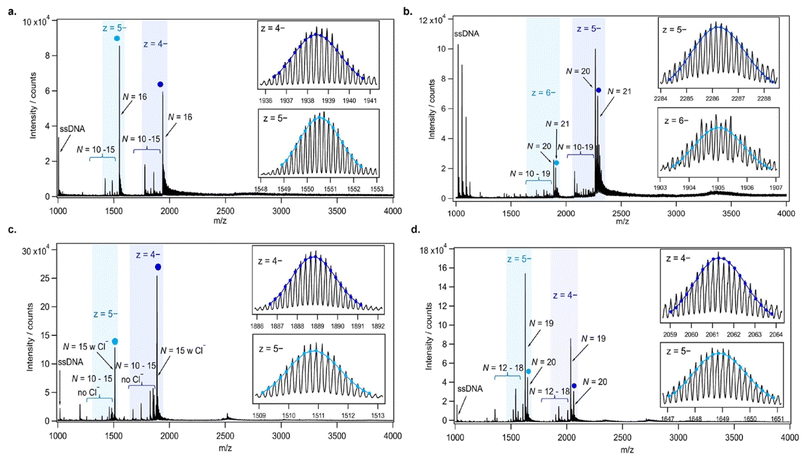
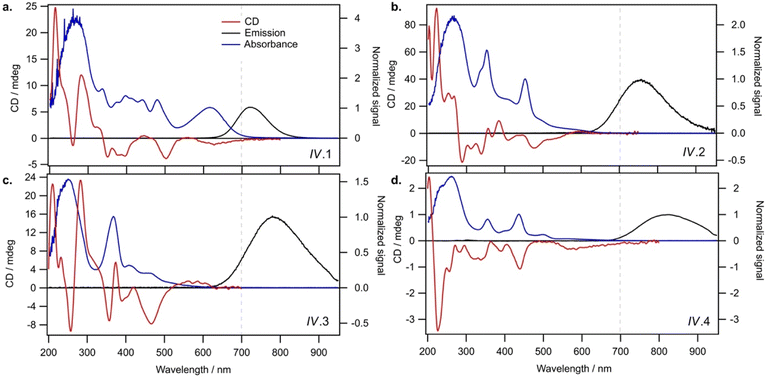
AgN-DNAs were synthesized and then purified by ion-paired reverse-phase HPLC (chromatograms in Fig. S1–S11;† details in Experimental Methods and ESI Table S1†). Following purification, we collected absorbance, emission, and CD spectra for all AgN-DNAs. Composition was determined by negative ion mode ESI-MS, which is well-suited for characterizing noncovalent nucleic acid complexes.41 Experimental mass spectra were fitted to determine each AgN-DNA's total silver content, N, valence electron count, N0, and the number of protecting DNA ligands, ns, using previously established methods4,15,16,31 (see ESI†). Because ESI can remove Ag+ from or fragment AgN-DNA species, here we choose to assign AgN-DNA molecular formula to the largest mass product clearly resolved at multiple charge states, as in prior studies.16,21 For 14 of the species, the largest mass product is also the most abundant product. For I.1, I.3, II.2, III.5, and IV.4, the largest mass product is less abundant than the second-largest mass product, which has one fewer Ag+. Past ESI-MS has shown that III.5 exhibits a largest mass product corresponding to N = 17 Ag atoms,22 but crystallographic studies enable unambiguous assignment of the total silver content as N = 16 in that case.40 Without available crystal structures, it is not possible to discriminate between silvers that are part of the AgN nanocluster core and Ag+ that are more weakly bound adducts, and thus choose to we assign N based on the largest clearly resolved mass spectral product at multiple charge states. We emphasize that isotopic distribution fits to each peak at multiple charge states show that total electron count, N0, for the largest and second-largest mass products are the same for I.1, I.3, II.2, III.5, and IV.4 (Table S3, Fig. S12, S14, S18, and S21†). Thus, it is possible that these AgN-DNAs have Ag+ that are more easily removed by ESI-MS than in other species. X-ray crystallography could be used to unambiguously assign total silver content, which is beyond the scope of this study.Table 1 presents the molecular formulae of all 19 HPLC-purified AgN-DNAs, along with their peak absorbance wavelength(s) and emission wavelength λp. Mass spectral analyses to determine molecular formulae are provided in Tables S2 and S3.† To facilitate comparison in this study, we group AgN-DNAs by ligand composition and N0. ESI-MS shows that the 6-electron AgN-DNAs (N0 = 6) possess three different types of ligand compositions: ns = 2 DNA oligomers per nanocluster (Group I, example in Fig. 1a), ns = 3 DNA oligomers per nanocluster (Group II, example in Fig. 1b), or ns = 2 DNA oligomers and additional chlorido ligand(s) per nanocluster (Group III, example in Fig. 1c). All four 8-electron AgN-DNAs (N0 = 8) are stabilized by ns = 2 DNA oligomers (Group IV, example in Fig. 1d). One mass spectrum for an AgN-DNA from each group in Table 1 is shown in Fig. 1, and all other mass spectra are provided in Fig. S12–S21.†
| AgN-DNA | DNA sequence (5′ to 3′) | N | N 0 | n s | Q c | Abs (nm) | λ p (nm) |
|---|---|---|---|---|---|---|---|
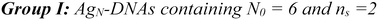
|
|||||||
| I.1 | GTCCGGGCCA | 16 | 6 | 2 | +10 | 530 | 639 |
| I.2 | ACCAATGACC | 15 | 6 | 2 | +9 | 545 | 650 |
| I.3 | CCAGCCCGGA | 15 | 6 | 2 | +9 | 560 | 660 |
| I.4 | GTAGTCCCTA | 16 | 6 | 2 | +10 | 560 | 720 |
| I.5 | ATCCCCTGTC | 17 | 6 | 2 | +11 | 582 | 727 |
| I.6 | AGTCACGACA26 | 16 | 6 | 2 | +10 | 640 | 811 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
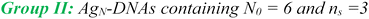
|
|||||||
| II.1 | CCCGGCCGAA | 18 | 6 | 3 | +12 | 630 | 703 |
| II.2 | CCCGGAGAAG5 | 21 | 6 | 3 | +15 | 640 | 721 |
| II.3 | CCTGGGGAAA | 16 | 6 | 3 | +10 | 651 | 726 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
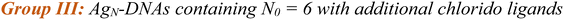
|
|||||||
| III.1 | AACCCCACGT22 | 15 | 6 | 2 | +8 | 496 | 638 |
| III.2 | CACCTAGCGA22,23 | 16 | 6 | 2 | +8 | 525 | 735 |
| III.3 | CACCAAGCGA40 | 16 | 6 | 2 | +8 | 523 | 734 |
| III.4 | CACCCAGCGA40 | 16 | 6 | 2 | +8 | 521 | 734 |
| III.5 | CACCGAGCGA40 | 16 | 6 | 2 | +8 | 521 | 739 |
| III.6 | CACCTAGCG_39 | 16 | 6 | 2 | +8 | 522 | 754 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
|
|||||||
| IV.1 | GCGCAAGATG | 19 | 8 | 2 | +11 | 480, 615 | 720 |
| IV.2 | GACGACGGAT21 | 17 | 8 | 2 | +9 | 350, 410, 465 | 760 |
| IV.3 | ATCTCCACAG21 | 16 | 8 | 2 | +8 | 352, 452 | 800 |
| IV.4 | AGGCGATCAT | 20 | 8 | 2 | +12 | 355, 436, 500 | 820 |
We find that all three of the far-red emissive AgN-DNAs in Table 1 (λp < 700 nm) are 6-electron clusters with ns = 2 DNA ligands (Group I) or with ns = 2 DNA ligands and an additional chlorido ligand (Group III). 11 of the NIR emissive AgN-DNAs (λp > 700 nm) are 6-electron clusters (Group I, Group II, or Group III), while four are 8-electron clusters (Group IV). Notably, there is a significant overlap in λp values for N0 = 6 and N0 = 8 AgN-DNAs. This is unlike the distinct valence electron counts of green-emissive AgN-DNAs (N0 = 4) and red-emissive AgN-DNAs (N0 = 6), which had led to the notion of “magic colors” in this spectral range.16 Unlike the green-to-red spectral region, we find that peak emission wavelength in the far-red to NIR spectral region is not a sole indicator of AgN-DNA valence electron count, as we observe N0 = 6 AgN-DNAs at peak wavelengths up to λp = 811 nm and N0 = 8 AgN-DNAs at peak wavelengths as low as λp = 720 nm.
Table 1 contains the molecular formulae of several notable and previously investigated AgN-DNAs whose compositions we determine here for the first time. We find that I.6, a λp = 811 nm emitter notable for exhibiting dual ns-lived and μs-lived emission,26 has molecular formula (DNA)2[Ag16]10+ (Fig. 1a). The two dominant mass spectral peaks of I.6 at 1938.5 and 1550.5 m/z are well-fitted by calculated isotopic distributions for (DNA)2[Ag16]10+ at charge states of z = 4− and z = 5− (Fig. 1a and Table S2†), confirming that this species has a nanocluster charge of Qc = +10 and an effective valence electron count of N0 = 16 −10 = 6. Mass spectra and isotopic distribution fits for other Group I AgN-DNAs are provided in Fig. S12–S16 and Tables S2, S3.†
We also identify the first known AgN-DNAs stabilized by three copies of the DNA template oligomer (ns = 3). This includes II.2, a previously reported λp = 721 nm NIR AgN-DNA that exhibits an “unusually high” 73% quantum yield.5 Mass spectral analysis shows that II.2 has molecular formula (DNA)3[Ag21]15+, with N0 = 21 −15 = 6 valence electrons and ns = 3 copies of the DNA template (Fig. 1b). Notably, this finding validates the prior observation by Neacşu, et al. that the hydrodynamic volume of II.2, as measured by time-resolved anisotropy, is about twice as large as the volume of another NIR species with ns = 2 DNA strands.5 We also identify two NIR-emitting ns = 3 AgN-DNAs that have never been reported before: II.1, with λp = 703 nm and molecular formula (DNA)3[Ag18]12+, and II.3, with λp = 726 nm and molecular formula (DNA)3[Ag16]10+ (Fig. S17 and S19†).
All three Group II ns = 3 AgN-DNAs are significantly more prone to fragmentation during ESI-MS than ns = 2 AgN-DNAs. (ESI-induced fragmentation is commonly observed for non-covalent DNA complexes,41 including AgN-DNAs.11,16,21,31–33,42) For example, Fig. 1b shows multiple mass spectral peaks corresponding to nanocluster products with N < 21 total silver atoms for II.2, in addition to the largest well-resolved mass spectral peak and its associated Na+ and NH4+ adducts. II.1 and II.3 exhibit similar degrees of fragmentation (Fig. S17 and S19†). We hypothesize that the greater propensity for ESI-induced fragmentation of Group II ns = 3 AgN-DNAs as compared to Group I ns = 2 AgN-DNAs is due to their greater hydrodynamic volume and generally larger values of total silver content N and cluster charge, Qc, which could increase ESI-induced loss of more loosely bound Ag+ and DNA ligands from the AgN-DNAs. Moreover, Neacşu, et al., previously observed that II.2 has limited thermal stability and therefore hypothesized that its high quantum yield results from a AgN core that is weakly bound to its DNA ligands, limiting solvent and/or DNA ligand-mediated nonradiative decay.5 Such a weaker AgN–ligand interaction is consistent with a greater degree of fragmentation by ESI for ns = 3 AgN-DNAs.
Group III includes several recently reported AgN-DNAs with additional adventitious chlorido ligands and N0 = 6 electrons. These chlorido-stabilized AgN-DNAs include III.2 through III.6, which are variants of a well-studied λp = 735 nm (DNA)2[Ag16Cl2]8+ with known crystal structure, and III.1, a λp = 638 nm (DNA)2[Ag15Cl]8+ with one chlorido ligand22 (Fig. 1c). We refer to the additional ligands as “chlorido” in accordance with IUPAC nomenclature.43
Finally, Group IV includes two N0 = 8 AgN-DNAs reported here for the first time, IV.1 and IV.4, and two previously reported N0 = 8 AgN-DNAs21 (IV.2 and IV.3). Fig. 1d shows the mass spectrum and isotopic fits for IV.4. Mass spectra and fit analysis for all N0 = 8 AgN-DNAs are provided in Table S3 and Fig. 1d, S20, S21.† We discuss later that compared to N0 = 6 AgN-DNAs, Group IV AgN-DNAs exhibit highly complex absorbance spectra, without a single distinct peak in the visible spectral region. Thus, Table 1 lists the wavelengths of the two to three well-defined near-UV to visible absorbance peaks for these emitters.
Relationship of molecular formula with Stokes shift
We next analyze the spectral properties and compositions in Table 1 to determine whether general correlations exist between AgN-DNA composition and optical properties. Because three of the four N0 = 8 AgN-DNAs do not have a single distinct longest wavelength absorbance transition, it is not appropriate to assign a single peak absorbance wavelength for these emitters, and we discuss these separately later. Here, we focus the discussion on N0 = 6 AgN-DNAs. Fig. S22† shows no general correlation between peak emission and either N or Qc for N0 = 6 AgN-DNAs and weak correlation between peak absorbance and N or Qc for N0 = 6 AgN-DNAs; in the latter case, chlorido-stabilized AgN-DNAs (Group III) have generally lower peak absorbance wavelength than Group I and II AgN-DNAs without chlorido ligands, and ns = 3 Group II AgN-DNAs have generally higher peak absorbance wavelength than ns = 2 Group I AgN-DNAs. As discussed previously, ESI-induced removal of silvers from less stable AgN-DNAs means that N and Qc as measured by ESI-MS may not always represent the solution-phase total silver content N of an AgN-DNA. X-ray crystallography is needed to confirm N, and ESI-MS is needed to determine N0, which can be unambiguously determined by fitting the isotopic distribution of each peak in the mass spectrum. Crystallographic studies of more AgN-DNA species may improve correlations of peak absorbance/emission and either N or Qc.When λp is plotted against the longest wavelength absorbance peak, we observe that the emitters are roughly grouped by ligand composition, specifically, by the value of ns and the presence or absence of additional chlorido ligands (Fig. 2a). This suggests differences in Stokes shift magnitude among Group I, Group II, and Group III emitters. Fig. 2b confirms these differences, displaying Stokes shift in units of energy (eV) as a function of peak absorbance energy. Group II AgN-DNAs with ns = 3 DNA oligomer ligands possess substantially smaller Stokes shifts than Group I AgN-DNAs, and Group III AgN-DNAs exhibit significantly larger Stokes shifts than other N0 = 6 AgN-DNAs. Given the trends in Fig. S22c and d† these experimental findings support that ligand chemistry has significant effects on the ground state energy levels and the excited-state energy loss (Stokes shift) of AgN-DNAs at the far-red/NIR spectral border. Recent theoretical analysis of the frontier orbitals of III.6, one of the (DNA)2[Ag16Cl2]8+ with known crystal structure, found that most of the frontier orbitals have significant weight on the inorganic Ag16Cl2 core.34 Thus, it is likely that chlorido ligands in this inorganic core will have an effect on ground state and excited state processes of AgN-DNAs.
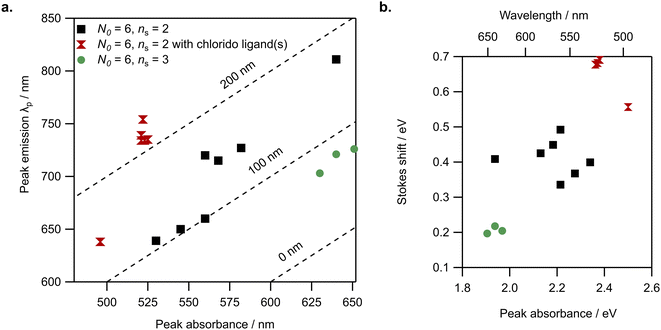 | ||
| Fig. 2 N 0 = 6 AgN-DNA spectral properties are grouped by ligand chemistry. (a) Peak emission (λp) versus peak absorbance wavelength for Group I (black squares), Group II (green circles), and Group III (red double triangles). Dotted lines represent absorbance and emission values corresponding to 0 nm, 100 nm, and 200 nm Stokes shift. (b) Stokes shift versus peak absorbance (units of energy) for N0 = 6 AgN-DNAs. Note: for Group III AgN-DNAs, III.2 through III.6 are essentially the same emitter39,40 and thus have nearly equivalent absorbance and emission values. | ||
Circular dichroism signatures
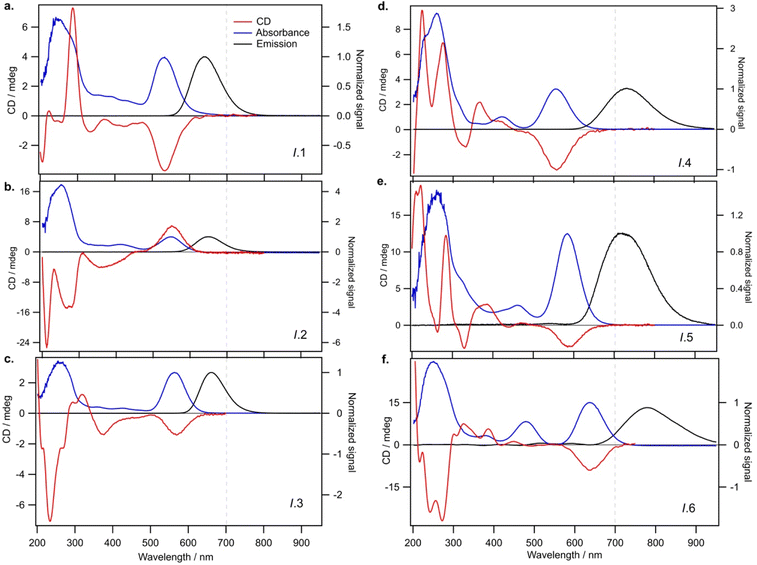
We next investigate how the chiroptical signatures of N0 = 6 AgN-DNAs correlate with ligand composition. CD spectroscopy is a powerful tool for characterizing AgN-DNAs because of its sensitivity to DNA conformation44,45 and its ability to interrogate the origins of electronic transitions in monolayer-protected metal nanoclusters, in combination with theoretical calculations.28 CD signatures in the 200 to 320 nm UV spectral region are assumed to primarily originate from the nucleobases and their interaction with the nanocluster core. Because DNA itself exhibits no chiroptical activity above ca. 320 nm, higher-wavelength CD signatures are assumed to arise due to electronic transitions in the nanocluster. Thus, CD studies of AgN-DNAs could provide information about both DNA ligand conformation and AgN core geometry and electronic structure.All past CD studies of purified AgN-DNAs have reported a distinct monosignate CD transition aligned with the longest wavelength visible or NIR absorbance peak.19,30–33 Four AgN-DNAs with N0 = 4, 6, and 12 were found to exhibit positive Cotton effect for the CD transition aligned with the longest wavelength absorbance peak, as well as six similar UV CD transitions that suggest similar DNA ligand conformations despite widely differing DNA oligomer lengths and AgN-DNA compositions. Quantum chemical calculations qualitatively replicated the seven major CD transitions,30,46 although X-ray crystallography has since shown that thread-like AgN are unrealistic models for AgN-DNAs.23,47 Density functional theory calculations of a N0 = 4 AgN-DNA predicted positive monosignate CD transition aligned with the longest-wavelength absorbance peak.48 In contrast, Petty and coauthors more commonly report negative Cotton effect aligned with the longest-wavelength absorbance peaks of green-emissive AgN-DNAs.19,31–33
Varying chirality in 6-electron AgN-DNAs
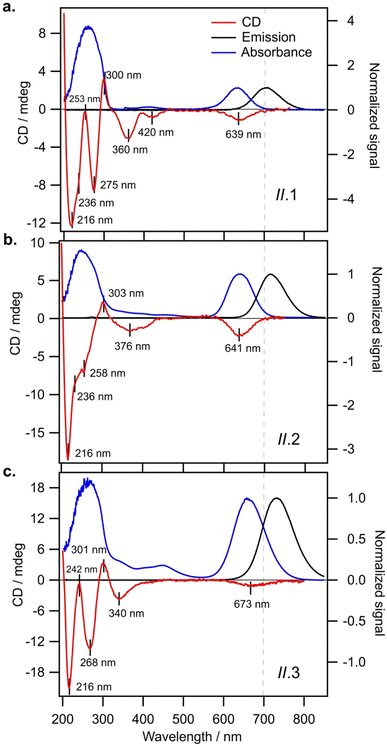
We find that all N0 = 6 AgN-DNAs stabilized by ns = 2 or 3 DNA strands (Groups I and II, respectively) exhibit well-defined monosignate CD transitions aligned with the longest-wavelength absorbance peak (Fig. 3 and 4). These transitions are negative Cotton peaks for all Group I and II emitters except I.2 (λp = 650 nm), which exhibits a strong positive Cotton effect at the longest wavelength absorbance peak (Fig. 3b). The significant prevalence of these negative CD transitions exhibited by Group I and II N0 = 6 AgN-DNAs contrasts with reports by Swasey, et al., of only positive Cotton effect at visible wavelengths for two N0 = 6 AgN-DNAs stabilized by ns = 1 DNA ligands of 28 and 34 nucleobases.36 Our results support that short DNA oligomers can stabilize N0 = 6 AgN-DNAs with either chiral handedness, even for AgN of identical size (I.2 and I.3 both contain N = 15, N0 = 6, and ns = 2). This illustrates the diversity of nanocluster chiralities that can be achieved with DNA oligomer ligands.UV CD signatures of AgN-DNAs
Fig. 3 shows that Group I AgN-DNAs exhibit a greater diversity of UV CD features than the four AgN-DNAs reported by Swasey, et al. This may suggest a variety of DNA conformations around the nanocluster core for Group I AgN-DNAs. We note that II.2, the only Group I AgN-DNA with a positive long-wavelength CD peak, exhibits solely negative CD in the UV region, unlike the other five Group I AgN-DNAs. More emitters should be investigated to determine if UV CD features correlate with the sign of the dominant visible CD feature for AgN-DNAs.Group II AgN-DNAs (ns = 3 DNA strands) share markedly similar UV CD signatures, unlike the more diverse Group I AgN-DNAs. Fig. 4 shows that all Group II AgN-DNAs exhibit negative CD transitions aligned with the longest wavelength absorbance peak and that their UV CD spectra possess distinctly similar transitions, including a strong negative Cotton effect at ca. 216 nm, a positive Cotton effect at ca. 300 nm, and a negative Cotton effect around 340–375 nm (Fig. 4). This high degree of spectral similarity suggests shared conformations of the DNA oligomer ligands around the central AgN for all ns = 3 AgN-DNAs. However, the significant differences between natural DNA secondary structures and the conformation of DNA ligands on the few AgN-DNAs with known crystal structures23,39,40,47 limit the use of well-established CD-to-structure correlations for natural DNA to interpret the structures of AgN-DNA ligands. Given that II.2 is reported to exhibit unusually high 73% quantum yield,5 it is important to understand and learn how to design for the ligand conformation of ns = 3 AgN-DNAs. We encourage experimental work to crystalize and solve the structures of ns = 3 AgN-DNAs, together with theoretical studies to provide better understanding of the origins of their optical properties.
Effects of chlorido ligands on CD signatures
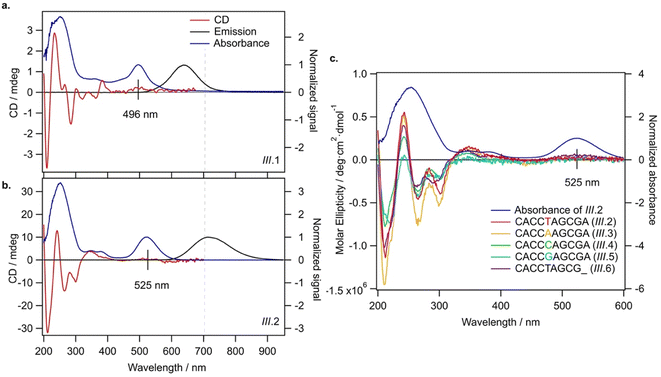
All Group III AgN-DNAs, which are stabilized by both DNA and chlorido ligands, exhibit highly diminished CD signals at visible wavelengths (Fig. 5), in contrast with the well-defined CD signatures of Groups I, II, and IV AgN-DNAs (Fig. 3, 4 and 6). Fig. 5c compares the CD spectra of five variants of the same (DNA)2[Ag16Cl2]8+, all solved by X-ray crystallography23,39,40 (sequences in legend). We observe experimentally that (DNA)2[Ag16Cl2]8+ variants either exhibit no CD signal or very weak positive CD signal at ca. 500 to 600 nm (Fig. 5c). The slight differences in CD spectra of these variants likely result from slight differences in Ag16 structure and ligand conformation as a result of single-base differences in the DNA templates.40Malola, et al., recently used linear response time-dependent density functional theory to calculate ground state absorbance and CD spectra of the “A10” variant, III.6.34 Their study represents the first such theoretical analysis for realistic AgN-DNA systems. The calculated ground state absorbance spectrum agreed well with the experimental absorbance spectrum (Fig. 5b), matching the three dominant absorbance peaks in the 300 to 550 nm spectra region. They also predicted the emitter's CD spectrum to exhibit weak, negative signal in the 500 to 600 nm spectral region and more intense UV CD features. The UV features and suppressed CD signal at visible wavelengths agree with our experimental findings in Fig. 5c, and the suppressed visible CD signal is also in agreement with the weak nanocluster chirality of the X-ray crystal structure of III.6. There is, however, a slight discrepancy in the sign of this weak CD signal in Fig. 5c as compared to predictions by Malola, et al. We hypothesize that this discrepancy may arise from solution-state dynamics of the nanocluster core that were not captured in the calculations. More detailed theoretical studies of nanocluster dynamics may shed light on this discrepancy. We note that the spectra we provide in Fig. 5c would enable a detailed comparison of calculated CD spectra for all five variants of the (DNA)2[Ag16Cl2]8+ emitter.
While further theoretical studies are needed to fully understand the origins of chiroptical activity of AgN-DNAs, the crystal structures of III.2 through III.6 do provide hints. These Ag16 nanoclusters have two chlorido ligands bound to the long faces of the nanocluster with a highly symmetric coordination structure.22,23,39 It may be that chlorido ligands act to “straighten out” the Ag16, reducing its structural chirality and thereby suppressing the CD spectral features that correspond to the lowest-energy excitations of the nanocluster rod,34 which are much more intense in Group I and II AgN-DNAs. Crystallographic studies of AgN-DNAs without chlorido ligands are needed to test this hypothesis. Moreover, crystallographic studies of III.1 are needed to determine the position of its single chlorido ligand and discern how this ligand affects nanocluster chirality and chiroptical activity.
Comparison of 6-electron and 8-electron AgN-DNAs
Lastly, we examine the optical and chiroptical properties of Group IV AgN-DNAs (with N0 = 8 valence electrons). This includes the two N0 = 8 AgN-DNAs that we report for the first time, IV.1 and IV.4, and the previously reported IV.2 and IV.3.21 These emitters exhibit clearly distinct absorbance spectral features compared to all N0 = 6 AgN-DNAs. The four Group IV emitters exhibit multiple distinct absorbance peaks in the near-UV and visible spectral regions (Fig. 6, blue curves), unlike the single dominant peak in the visible spectral region that is exhibited by N0 = 6 AgN-DNAs. IV.1 is the only N0 = 8 emitter with a well-defined high-wavelength absorbance peak, which we hypothesize is due to a slightly different nanocluster geometry than IV.2 through IV.4, which exhibit poorly defined low-intensity absorbance features above ca. 500 nm and significant UV-to-NIR down-conversion, as previously reported for IV.2 and IV.3.21The spectral differences between Groups I and IV clearly illustrate the role of electron count, N0, on AgN-DNA optical properties. Groups I and IV have differing N0, despite both being stabilized by ns = 2 10-base oligonucleotide ligands per AgN. The complex absorbance spectra of Group IV AgN-DNAs (Fig. 6) are in clear contrast with the simpler absorbance spectra of Group I AgN-DNAs, which exhibit a single dominant long wavelength absorbance peak and either less intense peaks or extremely subtle features at shorter near-UV to visible wavelengths (Fig. 3). The CD spectra of Group IV AgN-DNAs (N0 = 8) are also more complex than Groups I, II, III (N0 = 6). This includes the newly reported CD spectra of IV.1 and IV.4 and the previously reported CD spectra of IV.2 and IV.3.21 The distinct differences between the chiroptical and optical properties of superatomic N0 = 8 AgN-DNAs and N0 = 6 AgN-DNAs indicate differences in nanocluster electronic structure and strongly suggest fundamental differences in nanocluster shape. N0 = 6 AgN-DNAs are either known or expected to be rod-shaped.15,16,18,23N0 = 8 AgN-DNAs are hypothesized to possess pseudo-spherical shapes, similar to other ligand-protected 8-electron nanocluster superatoms.21 Thus, N0 plays a clear role in determining the geometry and ground state electronic structure of AgN-DNAs, and differences in N0 produce different classes of NIR AgN-DNA emitters.
Significant current research is focused on the fundamental mechanisms and synthetic control of the chiroptical properties of ligand-protected nanoclusters.28,49–55 CD spectroscopy is highly sensitive to a nanocluster's core, its ligand–core interface, and its ligand shell. Chiroptical signatures of nanoclusters often have complex origins, arising from interactions among the metal cluster core, ligand–metal units, and/or surrounding ligand groups,50 and theoretical studies using structures from X-ray crystallography are often required to elucidate the origins of these CD spectral features. In some cases, chirality transfer from ligand to nanocluster results in strong chiroptical signatures.51 Chirality transfer from metal nanoclusters to adsorbates has also been observed and is of importance for heterogeneous enantioselective catalysis.49 Thus, research into the origins of chiroptical properties of AgN-DNAs will not only advance the fundamental chemistry of nanocluster systems but also has important potential technological applications.
To our knowledge, this is the first detailed study of how the molecular formulae of far-red to NIR-emissive AgN-DNAs dictate their structure and chiroptical properties. Our results show that multidentate DNA ligands are versatile templates for a diverse set of nanocluster structures, with optical properties influenced by both electron count N0 and ligand composition. Variations in electron count and ligand composition produce at least four different classes of NIR-emissive AgN-DNAs with distinct optical properties, and it is possible that an even richer space of possible emitters has yet to be discovered.
While the major experimental challenges of growing single crystals of AgN-DNAs that are suitable for single crystal X-ray diffraction continues to limit progress in understanding their structure–property relationships, this study demonstrates that ESI-MS combined with UV/Vis and CD spectroscopy provides an alternate approach to advance understanding of the solution-phase structures of AgN-DNAs. Moreover, because ground state absorbance and CD spectra can be calculated using ab initio models, the large set of experimental absorbance, emission, and CD spectra of AgN-DNAs presented here will enable theoretical groups to model these emitters while awaiting more X-ray crystal structures to be solved. Importantly, our reports include electron counts for all 19 AgN-DNAs in this study, which are critical for accurate ab initio calculations of their electronic structure.
Conclusions
In summary, we have investigated the compositions and optical properties of 19 atomically precise AgN-DNA species emitting in the far-red to NIR spectral region, each stabilized by a different 10-base DNA oligomer. Molecular formulae determined by ESI-MS show that AgN-DNAs emitting in this spectral region can possess either 6 or 8 total valence electrons (N0) and that unlike visibly emissive AgN-DNAs, emission wavelength in the far red/NIR spectral region is not a sole indicator of electron count. 6-Electron AgN-DNAs have diverse ligand compositions, which appears to strongly influence Stokes shift. AgN-DNAs stabilized by ns = 3 DNA ligands are identified here for the first time, and these exhibit longer wavelength absorbance peaks and shorter Stokes shifts (70 to 80 nm) than AgN-DNAs stabilized by ns = 2 DNA ligands. Additional chlorido ligands are correlated with shorter wavelength absorbance peaks and larger Stokes shifts. UV CD signatures further suggest structural differences in DNA ligand conformation and/or nanocluster chirality amongst the three classes of 6-electron AgN-DNAs. In particular, AgN-DNAs protected solely by DNA ligands have well-defined visible CD transitions, while chlorido-protected AgN-DNAs have significantly suppressed visible CD transitions, agreeing well with emerging theoretical studies and suggesting a lower degree of nanocluster chirality when chlorido ligands are present. Finally, major distinctions exist between both the optical and chiroptical signatures of 8-electron AgN-DNAs versus 6-electron AgN-DNAs, which likely result from significant structural differences in the AgN core geometries of 6-electron and 8-electron nanoclusters and the conformations adopted by their DNA ligands. This work may enable future computational studies to understand the origins of the chiroptical properties of AgN-DNAs. Future efforts to solve the X-ray crystal structures of these AgN-DNAs would significantly expedite the progress of such computational studies, and we hope that researchers will attempt to crystallize the emitters presented here.Experimental section
Synthesis and purification of AgN-DNAs
AgN-DNAs were synthesized by the addition of stoichiometric amounts of AgNO3 (see ESI†) to an aqueous solution of DNA oligomer (Integrated DNA Technologies, standard desalting) in 10 mM ammonium acetate, followed by the partial reduction of the silver content using 0.5 molar ratio of a freshly prepared aqueous solution of NaBH4. For emitter IV.4 only, an elevated storage temperature above 4 °C was used after chemical reduction to form the NIR-emissive species.56 To achieve high enough yields for CD spectroscopy, 5 to 15 mL of solution was typically prepared. The solution was kept at 4 °C (or stated otherwise) in dark until concentration by spin filtering and then purification using reverse-phase HPLC (see ESI†). Following HPLC, the solvent was exchanged into 10 mM ammonium acetate, pH 7.Mass spectrometry
Electrospray ionization mass spectrometry (ESI-MS) was performed using a Waters Xevo G2-XS QTof. Samples were directly injected at 0.1 mL min−1 in negative ion mode with a 2 kV capillary voltage, 30 V cone voltage and no collision energy. Spectra were collected from 1000 to 4000 m/z with an integration time of 1 s. Source and desolvation temperatures were 80 and 150 °C, respectively. Gas flows were 45 L h−1 for the cone, and 450 L h−1 for the desolvation. Samples were injected with 50 mM NH4OAc–MeOH (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) solution at pH 7. These solvent and ESI-MS settings were chosen to minimize product fragmentation from a range of tested conditions, inspired by prior work.13,15,41 Determination of nanocluster size (total number of silvers N, ligand composition i.e., the number of DNA strands ns, and the presence of additional chlorido ligands) and the overall charge, Qc (and hence determine the number of effective neutral silvers N0) was performed by fitting the calculated isotopic distribution of the AgN-DNA to the experimental spectra (details in ESI†). Calculated isotopic distributions were obtained from MassLynx using the chemical formula and corrected for the overall positive charge (oxidation state, Qc) of the nanocluster core.
20) solution at pH 7. These solvent and ESI-MS settings were chosen to minimize product fragmentation from a range of tested conditions, inspired by prior work.13,15,41 Determination of nanocluster size (total number of silvers N, ligand composition i.e., the number of DNA strands ns, and the presence of additional chlorido ligands) and the overall charge, Qc (and hence determine the number of effective neutral silvers N0) was performed by fitting the calculated isotopic distribution of the AgN-DNA to the experimental spectra (details in ESI†). Calculated isotopic distributions were obtained from MassLynx using the chemical formula and corrected for the overall positive charge (oxidation state, Qc) of the nanocluster core.
Optical characterization
Steady-state absorbance and emission spectra were recorded using a thermoelectrically cooled, fiber-coupled spectrometer (Ocean Optics QE65000). Absorbance spectra were collected using a DH-Mini (Ocean Insight) deuterium & tungsten halogen UV-vis-NIR light source. Fluorescence spectra were collected using a UV LED for universal UV excitation.57 Circular dichroism measurements were performed on Chirascan V100 from Applied Photophysics. The concentration of samples was maintained such that the absorbance of the AgN-DNA ranged between 0.8 to 1.0. The CD spectra of AgN-DNA in 10 mM ammonium acetate were recorded from 200 to 800 nm in a quartz cuvette (Starna Cells) of 0.5 mm optical path length at 20 °C with a scanning rate of 1.0 nm interval per 1.0 s. The CD spectrum of each AgN-DNA is the average of three scans with a manual baseline correction to remove contributions from 10 mM NH4OAc.Data availability
UV/Vis absorbance, fluorescence emission, and CD spectra are provided as ESI files.†Author contributions
R. G. and S. M. C. conceived the experiments. R. G., A. G.-R., M. R., and N. A. prepared and characterized atomically precise nanocluster solutions. R. G. and A. G.-R. led data analysis. B. B. K. contributed essential mass spectrometry methods development. R. G. and S. M. C. co-wrote the manuscript with feedback from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by NSF Biophotonics CBET-2025790 and AFOSR FA9550-21-1-0163 and AFOSR DURIP FA9550-22-1-0206. A.G.-R. acknowledges a Balsells Graduate Fellowship. M. R. acknowledges a UC Irvine Engineering Pathway to the PhD Fellowship. The authors thank Hannu Häkkinen and Ara Apkarian for helpful discussions.References
- J. T. Petty, J. Zheng, N. V. Hud and R. M. Dickson, J. Am. Chem. Soc., 2004, 126, 5207–5212 CrossRef CAS PubMed.
- Y. Chen, M. L. Phipps, J. H. Werner, S. Chakraborty and J. S. Martinez, Acc. Chem. Res., 2018, 51, 2756–2763 CrossRef CAS PubMed.
- R. Guha and S. M. Copp, in Modern Avenues in Metal–Nucleic Acid Chemistry, ed. J. Müller and B. Lippert, CRC Press, 25th edn, 2023, pp. 291–342 Search PubMed.
- A. Gonzàlez-Rosell, C. Cerretani, P. Mastracco, T. Vosch and S. M. Copp, Nanoscale Adv., 2021, 3, 1230–1260 RSC.
- V. A. Neacşu, C. Cerretani, M. B. Liisberg, S. M. Swasey, E. G. Gwinn, S. M. Copp and T. Vosch, Chem. Commun., 2020, 56, 6384–6387 RSC.
- S. A. Bogh, M. R. Carro-Temboury, C. Cerretani, S. M. Swasey, S. M. Copp, E. G. Gwinn and T. Vosch, Methods Appl. Fluoresc., 2018, 6, 024004 CrossRef PubMed.
- M. B. Liisberg, Z. S. Kardar, S. M. Copp, C. Cerretani and T. Vosch, J. Phys. Chem. Lett., 2021, 12, 1150–1154 CrossRef CAS PubMed.
- S. Krause, M. R. Carro-Temboury, C. Cerretani and T. Vosch, Phys. Chem. Chem. Phys., 2018, 20, 16316–16319 RSC.
- S. Krause, M. R. Carro-Temboury, C. Cerretani and T. Vosch, Chem. Commun., 2018, 54, 4569–4572 RSC.
- S. Krause, C. Cerretani and T. Vosch, Chem. Sci., 2019, 10, 5326–5331 RSC.
- S. M. Swasey, S. M. Copp, H. C. Nicholson, A. Gorovits, P. Bogdanov and E. G. Gwinn, Nanoscale, 2018, 10, 19701–19705 RSC.
- P. Mastracco, A. Gonzàlez-Rosell, J. Evans, P. Bogdanov and S. M. Copp, ACS Nano, 2022, 16, 16322–16331 CrossRef CAS PubMed.
- D. Schultz and E. G. Gwinn, Chem. Commun., 2012, 48, 5748–5750 RSC.
- K. Koszinowski and K. Ballweg, Chem.–Eur. J., 2010, 16, 3285–3290 CrossRef CAS PubMed.
- D. Schultz, K. Gardner, S. S. R. Oemrawsingh, N. Markešević, K. Olsson, M. Debord, D. Bouwmeester and E. Gwinn, Adv. Mater., 2013, 25, 2797–2803 CrossRef CAS PubMed.
- S. M. Copp, D. Schultz, S. Swasey, J. Pavlovich, M. Debord, A. Chiu, K. Olsson and E. Gwinn, J. Phys. Chem. Lett., 2014, 5, 959–963 CrossRef CAS PubMed.
- H. Häkkinen, Chem. Soc. Rev., 2008, 37, 1847–1859 RSC.
- S. M. Copp, D. Schultz, S. M. Swasey, A. Faris and E. G. Gwinn, Nano Lett., 2016, 16, 3594–3599 CrossRef CAS PubMed.
- J. T. Petty, M. Ganguly, I. J. Rankine, E. J. Baucum, M. J. Gillan, L. E. Eddy, J. C. Léon and J. Müller, J. Phys. Chem. C, 2018, 122, 4670–4680 CrossRef CAS.
- S. M. Copp and A. Gonzàlez-Rosell, Nanoscale, 2021, 13, 4602–4613 RSC.
- A. Gonzàlez-Rosell, R. Guha, C. Cerretani, V. Rück, M. B. Liisberg, B. B. Katz, T. Vosch and S. M. Copp, J. Phys. Chem. Lett., 2022, 13, 8305–8311 CrossRef PubMed.
- A. Gonzàlez-Rosell, S. Malola, R. Guha, N. R. Arevalos, M. F. Matus, M. E. Goulet, E. Haapaniemi, B. B. Katz, T. Vosch, J. Kondo, H. Häkkinen and S. M. Copp, J. Am. Chem. Soc., 2023, 145, 10721–10729 CrossRef PubMed.
- C. Cerretani, H. Kanazawa, T. Vosch and J. Kondo, Angew. Chem., Int. Ed., 2019, 58, 17153–17157 CrossRef CAS PubMed.
- S. M. Swasey, L. E. Leal, O. Lopez-Acevedo, J. Pavlovich and E. G. Gwinn, Sci. Rep., 2015, 5, 10163 CrossRef CAS PubMed.
- S. M. Swasey, F. Rosu, S. M. Copp, V. Gabelica and E. G. Gwinn, J. Phys. Chem. Lett., 2018, 9, 6605–6610 CrossRef CAS PubMed.
- V. Rück, C. Cerretani, V. A. Neacşu, M. B. Liisberg and T. Vosch, Phys. Chem. Chem. Phys., 2021, 23, 13483–13489 RSC.
- J. T. Petty, S. Carnahan, D. Kim and D. Lewis, J. Chem. Phys., 2021, 154, 244302 CrossRef CAS PubMed.
- S. Knoppe and T. Burgi, Acc. Chem. Res., 2014, 47, 1318–1326 CrossRef CAS PubMed.
- K. L. D. M. Weerawardene, H. Häkkinen and C. M. Aikens, Annu. Rev. Phys. Chem., 2018, 69, 205–229 CrossRef CAS PubMed.
- S. M. Swasey, N. Karimova, C. M. Aikens, D. E. Schultz, A. J. Simon and E. G. Gwinn, ACS Nano, 2014, 8, 6883–6892 CrossRef CAS PubMed.
- J. T. Petty, O. O. Sergev, M. Ganguly, I. J. Rankine, D. M. Chevrier and P. Zhang, J. Am. Chem. Soc., 2016, 138, 3469–3477 CrossRef CAS PubMed.
- J. T. Petty, M. Ganguly, I. J. Rankine, D. M. Chevrier and P. Zhang, J. Phys. Chem. C, 2017, 121, 14936–14945 CrossRef CAS.
- J. T. Petty, M. Ganguly, A. I. Yunus, C. He, P. M. Goodwin, Y. H. Lu and R. M. Dickson, J. Phys. Chem. C, 2018, 122, 28382–28392 CrossRef CAS.
- H. Häkkinen, S. Malola and M. F. Matus, J. Phys. Chem. C, 2023, 127(33), 16553–16559 CrossRef.
- S. M. Copp, P. Bogdanov, M. Debord, A. Singh and E. Gwinn, Adv. Mater., 2014, 26, 5839–5845 CrossRef CAS PubMed.
- S. M. Copp, A. Gorovits, S. M. Swasey, S. Gudibandi, P. Bogdanov and E. G. Gwinn, ACS Nano, 2018, 12, 8240–8247 CrossRef CAS PubMed.
- S. M. Copp, S. M. Swasey, A. Gorovits, P. Bogdanov and E. G. Gwinn, Chem. Mater., 2020, 32, 430–437 CrossRef CAS.
- F. Moomtaheen, M. Killeen, J. Oswald, A. Gonzàlez-Rosell, P. Mastracco, A. Gorovits, S. M. Copp and P. Bogdanov, in Proceedings of the 28th ACM SIGKDD International Conference on Knowledge Discovery & Data Mining, 2022 Search PubMed.
- C. Cerretani, J. Kondo and T. Vosch, RSC Adv., 2020, 10, 23854–23860 RSC.
- C. Cerretani, J. Kondo and T. Vosch, CrystEngComm, 2020, 22, 8136–8141 RSC.
- E. Largy, A. König, A. Ghosh, D. Ghosh, S. Benabou, F. Rosu and V. Gabelica, Chem. Rev., 2022, 122, 7720–7839 CrossRef CAS PubMed.
- M. Walter, J. Akola, O. Lopez-Acevedo, P. D. Jadzinsky, G. Calero, C. J. Ackerson, R. L. Whetten, H. Grönbeck and H. Häkkinen, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 9157–9162 CrossRef CAS PubMed.
- R. L. Lundblad, Handb. Biochem. Mol. Biol., 2018, 944–950 Search PubMed.
- J. Kypr, I. Kejnovska, D. Renciuk and M. Vorlickova, Nucleic Acids Res., 2009, 37, 1713–1725 CrossRef CAS PubMed.
- D. M. Gray, R. L. Ratliff and M. R. Vaughan, Methods Enzymol., 1992, 211, 389–406 CAS.
- N. V Karimova and C. M. Aikens, J. Phys. Chem. A, 2015, 119, 8163–8173 CrossRef PubMed.
- D. J. E. Huard, A. Demissie, D. Kim, D. Lewis, R. M. Dickson, J. T. Petty and R. L. Lieberman, J. Am. Chem. Soc., 2019, 141, 11465–11470 CrossRef CAS PubMed.
- X. Chen, M. Boero and O. Lopez-Acevedo, Phys. Rev. Mater., 2020, 4, 065601 CrossRef CAS.
- I. Dolamic, B. Varnholt and T. Bürgi, Nat. Commun., 2015, 6, 1–6 Search PubMed.
- K. R. Krishnadas, L. Sementa, M. Medves, A. Fortunelli, M. Stener, A. Fürstenberg, G. Longhi and T. Bürgi, ACS Nano, 2020, 14, 9687–9700 CrossRef CAS PubMed.
- R. W. Y. Man, H. Yi, S. Malola, S. Takano, T. Tsukuda, H. Häkkinen, M. Nambo and C. M. Crudden, J. Am. Chem. Soc., 2022, 144, 2056–2061 CrossRef CAS PubMed.
- A. Pniakowska, M. Samoć and J. Olesiak-Bańska, Nanoscale, 2023, 15, 8597–8602 RSC.
- M. Monti, G. Brancolini, E. Coccia, D. Toffoli, A. Fortunelli, S. Corni, M. Aschi and M. Stener, J. Phys. Chem. Lett., 2023, 14, 1941–1948 CrossRef CAS PubMed.
- Y. Zhu, J. Guo, X. Qiu, S. Zhao and Z. Tang, Acc. Mater. Res., 2021, 2, 21–35 CrossRef CAS.
- W. D. Si, Y. Z. Li, S. S. Zhang, S. Wang, L. Feng, Z. Y. Gao, C. H. Tung and D. Sun, ACS Nano, 2021, 15, 16019–16029 CrossRef CAS PubMed.
- R. Guha, M. Rafik, A. Gonzàlez-Rosell and S. M. Copp, Chem. Commun., 2023, 59, 10488–10491 RSC.
- P. R. O'Neill, E. G. Gwinn and D. K. Fygenson, J. Phys. Chem. C, 2011, 115, 24061–24066 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and experimental methods; HPLC chromatograms; mass spectra and associated calculated mass distributions. See DOI: https://doi.org/10.1039/d3sc02931j |
| This journal is © The Royal Society of Chemistry 2023 |