Adhesive and biodegradable polymer mixture composed of high -biosafety pharmaceutical excipients as non-setting periodontal dressing†
Xiaodan
Zhao‡
 a,
Meiwen
Li‡
a,
Meng
Li
b,
Wenbo
Li
a,
Ang
Li
a,
Yilong
Cheng
a,
Meiwen
Li‡
a,
Meng
Li
b,
Wenbo
Li
a,
Ang
Li
a,
Yilong
Cheng
 *b and
Dandan
Pei
*a
*b and
Dandan
Pei
*a
aKey Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, College of Stomatology, Xi'an Jiaotong University, Xi'an, 710049, China. E-mail: peidandan1986@126.com
bSchool of Chemistry, Xi'an Jiaotong University, Xi'an, 710049, China. E-mail: yilongcheng@mail.xjtu.edu.cn
First published on 7th September 2023
Abstract
Periodontal dressing is a surgical dressing applied to oral wounds after periodontal surgery. Currently, all commercially available setting periodontal dressings are stiff, uncomfortable, with poor aesthetics, and need to be removed at the patient's follow-up visit, which may cause secondary damage. A periodontal dressing with soft texture, biodegradable properties, and that could balance both comfort and aesthetics is urgently desired. Hence, non-setting and degradable dressings were developed using sodium carboxymethyl cellulose, Eudragit S 100 and povidone K30, which were compared with the commercial degradable dressing Reso-pac®. The mucosal adhesion of the dressings was evaluated by lap shear tests, which indicated adequate adhesion. The in vitro swelling rates of the dressings were approximately half that of Reso-pac®, which led to less saliva adsorption and better dimensional stability. The dressings also exhibited satisfactory biocompatibility according to the results of CCK-8, Live/Dead staining, hemolysis, and subcutaneous implantation assays. Moreover, the dressing promoted the healing of full-thickness mucosal wounds in the palatal gingivae of SD rats and contributed to better therapeutic effect than Reso-pac®. Considering the multiple advantages and the pure pharmaceutical excipient formula, we anticipate that this dressing could be a promising product and may enter clinical practice in the near future.
1 Introduction
Periodontal disease, one of the most prevalent oral diseases in the world, causes a significant health burden to society.1 It affects the supporting structures of the teeth (the gingiva, bone and periodontal ligaments) and potentially leads to tooth loss and systemic inflammation.2 Periodontal surgery plays a vital role in the treatment of periodontal disease, but postoperative complications, such as haemorrhage, infection, inflammation, swelling, suppuration and other adverse tissue changes, occur in about 50% of patients.3,4Periodontal dressing is a protective barrier applied to the necks of the teeth and the adjacent tissue after periodontal surgery to alleviate or prevent postoperative complications.5 The invention of and research on periodontal dressing began in 1923. The first periodontal dressing, Wondrpak®, is a representative zinc oxide eugenol dressing.6 However, residual unreacted eugenol in the dressing may induce allergic reactions and burning sensations, and the asbestos components potentially cause lung cancer.7 Therefore, in the 1950s, non-eugenol periodontal dressings, represented by Coe-pak®, were invented, which is currently widely used in clinic.8 The setting of non-eugenol dressing is based on the reaction between metallic oxide and fatty acid. These dressings are free from eugenol and asbestos, thus exhibiting favorable biosafety. The surface smoothness also improves patient comfort. However, the need for mixing, large volume, and the lack of elasticity increase the difficulty of its use.9 Subsequently, Barricaid®, a light-curing dressing mainly composed of urethane dimethacrylate resin, entered the clinic in 1987.10 Under visible light with a spectral output containing 470 nm, it can be cured at the target site after about 10 seconds. Its small size, great aesthetic effect, and easy operation outperform the existing products, but the residual monomers may still cause irritation or possible allergic response after contacting the skin and eyes.6,11
These currently available periodontal dressings are self-setting or light-curing products and are mechanically fixed on the wound area after setting. At the next visit, the extra dressing removal procedure may cause unnecessary mechanical damage and pain for the patient. In 2003, a non-setting periodontal dressing, Reso-pac®, was launched. It can adhere to the surgical site instead of mechanical fixing, remain elastic in the mouth, and dissolve within 1 to 3 days after application. This non-setting dressing improves patient comfort by simplifying the surgical procedure, avoiding secondary mechanical damage, and relieving pain.12 Unfortunately, its high swelling rate and poor dimensional stability greatly restrict the development prospects of the product. In addition, the polyvinyl acetate in the dressing has not been defined as an approved pharmaceutical excipient in many countries.13,14 Recently, several mucoadhesive hydrogels have been developed to promote oral wound healing, such as extracellular matrix-mimicking hydrogels based on chitosan. Even with controllable swelling behaviors and robust mechanical properties, these hydrogels are mostly films, only targeting buccal mucosa wounds or edentulous wounds.15
Sodium carboxymethyl cellulose (SCMC) is an anionic water-soluble polymer with good hydrophilicity, adhesion, and biocompatibility. It is the first cellulose derivative approved by the U.S. Food and Drug Administration.16 Owing to these advantages, SCMC has been extensively used in wound dressing development and acts as an important ingredient of oral mucosal films.17,18 Eudragit S 100, composed of methacrylic acid and methyl methacrylate arranged in a random distribution (the ratio of methyl methacrylate to methacrylic acid is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), is an inactive pharmaceutical excipient widely used as a functional enteric coating agent, which can be dissolved in the ileum and colon. It is highly stable and can be easily combined with other polymers to develop biomedical materials.19 Povidone (PVP), a common inactive pharmaceutical excipient used in many formulations, is a water-soluble and hydrophilic synthetic polymer.20 Due to its non-irritant, nontoxic, and biocompatible characteristics as an excipient, it has also been widely utilized in the development of oral tablets, dressings, and solutions.21–23 For example, polyvinyl alcohol, povidone and poloxamer 407 were used in a mucoadhesive film preparation for buccal drug administration.24 As povidone cannot be absorbed by the mucosa and gastrointestinal tract, it is basically safe to be consumed orally.25 Therefore, we speculate that a non-setting and mucoadhesive periodontal dressing consisting of the above pharmaceutical excipients may be a promising material for oral wound protection. Among the excipients, Eudragit S 100 could bind and wrap SCMC powder to form a uniform lump of material. After the contact of SCMC with saliva and further dissolution, it may further endow the material with favorable mucoadhesive properties. Furthermore, the addition of PVP K30 could increase the solubility of the system and ultimately prevent secondary removal of the dressing through self-degradation.
1), is an inactive pharmaceutical excipient widely used as a functional enteric coating agent, which can be dissolved in the ileum and colon. It is highly stable and can be easily combined with other polymers to develop biomedical materials.19 Povidone (PVP), a common inactive pharmaceutical excipient used in many formulations, is a water-soluble and hydrophilic synthetic polymer.20 Due to its non-irritant, nontoxic, and biocompatible characteristics as an excipient, it has also been widely utilized in the development of oral tablets, dressings, and solutions.21–23 For example, polyvinyl alcohol, povidone and poloxamer 407 were used in a mucoadhesive film preparation for buccal drug administration.24 As povidone cannot be absorbed by the mucosa and gastrointestinal tract, it is basically safe to be consumed orally.25 Therefore, we speculate that a non-setting and mucoadhesive periodontal dressing consisting of the above pharmaceutical excipients may be a promising material for oral wound protection. Among the excipients, Eudragit S 100 could bind and wrap SCMC powder to form a uniform lump of material. After the contact of SCMC with saliva and further dissolution, it may further endow the material with favorable mucoadhesive properties. Furthermore, the addition of PVP K30 could increase the solubility of the system and ultimately prevent secondary removal of the dressing through self-degradation.
In this study, an adhesive, biocompatible, dissolvable, and degradable periodontal dressing was developed using SCMC, PVP K30, and Eudragit S 100 (SPE) (Fig. 1A). SPE with additives such as stearic acid was defined as SPE/S. The samples obtained using different ingredient proportions were characterized in detail by rheology testing. Features such as adhesion strength, swelling ratio, and degradation ratio were further evaluated. The ex vivo and in vivo mucosa residence times on gingiva were tested to determine the adhesion of the developed dressing. Biocompatibility of the dressing was assessed ex vivo and in vivo. Finally, to evaluate the healing effect of this periodontal dressing on the oral mucosal wound, a rat palatal defect model was adopted, and histological analysis was further performed. This study presents a periodontal dressing with non-setting property and favorable wound adaptability. It is worth noting that this dressing also exhibits user-friendliness due to the no-mixing and easy handling features. Considering that the ingredients are all FDA-approved pharmaceutical excipients, we anticipate that this periodontal dressing would be a promising product to enter clinical practice in the near future.
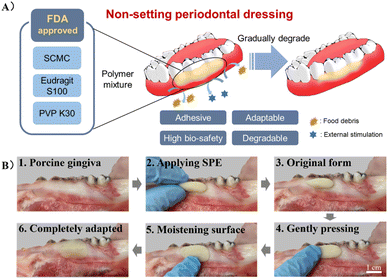 | ||
| Fig. 1 (A) Illustration of the non-setting SPE periodontal dressing. (B) Schematic diagram of the operation procedure for the application of SPE periodontal dressing. | ||
2 Experimental
2.1 Materials
Ethanol was purchased from Guangdong Guanghua Sci-Tech Co., Ltd (China). Glycerol was provided by Shanghai Aladdin Biochemical Co., Ltd (China). Carboxymethyl cellulose (800–1200 mPa s) was obtained from Shanghai Titan Scientific Co., Ltd (China). Stearic acid was bought from Shanghai Shiyi Chemical Reagent Co., Ltd (China). Povidone K30, poly(methacrylic acid, methyl methacrylate) (methacrylic acid/methyl methacrylate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), xylitol and menthol were received from Xi'an Tianzheng Medicinal Materials Co., Ltd (China). HyClone Dulbecco's modified Eagle's medium (DMEM) with high glucose (without phenol red) was supplied by Global Life Sciences Technologies (Shanghai) Co., Ltd (China). Trypsin-EDTA (0.05%) and fetal bovine serum were purchased from Thermo Fisher Scientific Co., Ltd (China). Cell-counting kit-8 (CCK-8) and calcein/PI cell viability assay kit were purchased from Beyotime Institute of Biotechnology (China). Pepsin antigen retrieval solution, phosphate-buffered saline, bovine serum albumin, and spontaneous fluorescence quenching reagent were obtained from Wuhan Servicebio Technology Co., Ltd (China).
2), xylitol and menthol were received from Xi'an Tianzheng Medicinal Materials Co., Ltd (China). HyClone Dulbecco's modified Eagle's medium (DMEM) with high glucose (without phenol red) was supplied by Global Life Sciences Technologies (Shanghai) Co., Ltd (China). Trypsin-EDTA (0.05%) and fetal bovine serum were purchased from Thermo Fisher Scientific Co., Ltd (China). Cell-counting kit-8 (CCK-8) and calcein/PI cell viability assay kit were purchased from Beyotime Institute of Biotechnology (China). Pepsin antigen retrieval solution, phosphate-buffered saline, bovine serum albumin, and spontaneous fluorescence quenching reagent were obtained from Wuhan Servicebio Technology Co., Ltd (China).
2.2 Animals
Male Sprague Dawley (SD) rats aged 8 weeks (250 ± 10 g) and Kunming mice aged 4 weeks (20 ± 2 g) were purchased from the Animal Center of Xi'an Jiaotong University and acclimatized for at least one week. The animals had free access to a laboratory diet and water. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Xi'an Jiaotong University and approved by the Animal Ethics Committee of Health Science Center in Xi'an Jiaotong University (2022-0018).2.3 Periodontal dressing preparation
Based on the initial results, SPE/S-2 was selected to be applied as periodontal dressing (ethanol: 2.00 g, glycerin: 2.00 g, SCMC: 4.00 g, PVP K30: 1.50 g, Eudragit S 100: 0.50 g, stearic acid: 0.20 g, xylitol: 0.15 g, menthol: 0.01 g). For the SPE groups, ethyl alcohol (2.00 g) and glycerin (2.00 g) were mixed as solvents followed by the addition of different amounts of PVP K30 (0.50, 1.50 and 2.50 g). After complete dissolution, different masses of Eudragit S 100 were further added (0.50, 1.00 and 1.50 g) at 40 °C and fully swelled in solution. During the heating process, the mixture was kept sealed to prevent ethanol evaporation. Subsequently, different ratios of SCMC powder (3.00, 4.00 and 5.00 g) were supplemented under stirring. Finally, the optimized SPE groups were determined by the evaluation of the macro properties of the blends. For SPE/S groups, stearic acid (0.20 g) was dissolved in the solvent before adding Eudragit S 100. Xylitol (0.15 g) and menthol (0.01 g) powders were added along with SCMC powder.2.4 Rheological studies
The viscoelastic behavior of the dressings was evaluated through rheological measurement using a Discovery HR-2 Rheometer (TA Instruments). A parallel-plate geometry (25 mm) and 1.0 mm gap were selected, and the test was performed by using an oscillation frequency sweep between 0.1 and 100 rad per s at a constant strain (1%), which was chosen within the linear viscoelastic regime. Meanwhile, an integrated temperature controller was used to maintain the temperature at 25 °C and 37 °C, respectively. The storage modulus (G′), loss modulus (G′′), and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (G′′/G) were recorded, respectively (n = 3).
δ (G′′/G) were recorded, respectively (n = 3).
2.5 Mucoadhesion strength test
The adhesion performance was quantified by lap-shear adhesion tests.26,27 According to previous work, the gingivae were excised from fresh porcine jaw from a local market and prepared into long strips with a width of 10 mm at the end. Dressings were adhered between the overlap of two gingival ends with an adhesion area of 1 cm × 1 cm, and this area was lightly pressed for 2 min under moist condition. After 15 min at 25 °C, the samples were tested with the CMT-1503 electromechanical tester (SUST Inc., China). All tests were conducted with a constant tensile speed of 10 mm min−1. Shear strength was determined by dividing the maximum force by the adhesion area (n = 5).2.6 In vitro residence time
The rotating-disc method based on previous reports was adopted to investigate the in vitro mucosa residence time.28 Briefly, the gingivae were cut to 1.5 cm × 1.0 cm sections and fixed onto a polyethylene terephthalate (PET) mold with cyanoacrylate adhesive and thread. Then, the dressings (0.1 g) were pressed on the gingival tissue for 2 min under moist condition. The PET mold was then immersed in PBS (pH = 6.8) in a beaker under magnetic stirring with the rotating speed of 100 rpm. At specific time intervals, the number of dressings still attached to the gingival tissue was recorded (n = 5).2.7 Swelling and degradation time test
The swelling ratio was assessed to determine the dimensional stability, which affects the adhesion of the dressing to the wound surface and the comfort of patients.29 The swelling test was based on previous reports.30,31 Dressings were immersed in PBS (pH = 6.8) at 37 °C with continuous shaking at 100 rpm. At the predetermined time point, the wet weights (Wt) of the swollen dressings were measured (n = 3). The swelling ratio was calculated using the following equation:| Swelling ratio(%) = (Wt − W0)/W0 × 100%, |
For the degradation test, after the dressings were fully swelled, the weight remaining ratio of the dressings was examined by the same processes and defined by the following equation:
| Degradation(%) = (W0 − Wt)/W0 × 100%, |
2.8 Hemolysis assay and pH value evaluation
Extracts of the present dressings and Reso-pac® were prepared at a ratio of 0.1 g mL−1 based on GBT 1688.12. Red blood cells (RBCs) of rats were washed and diluted to 5% (vol/vol) erythrocyte/PBS suspension and incubated with the extracts of SPE, SPE/S and Reso-pac® at 37 °C for 3 h (n = 3); PBS buffer and Triton X-100 treatments served as negative and positive controls, respectively. After centrifugation for 10 min, the optical absorbance of supernatants was detected at 540 nm,32 and the percentage of hemolysis was calculated by the formula:| Hemolysis ratio(%) = [(ODsample − ODnegative)/(ODpositive − ODnegative)] × 100% |
2.9 Cell viability and cell proliferation evaluation
Human gingival epithelial cells (hGECs) were purchased from Otwo Biotech (Guangzhou) Inc (HTX2651). Cells were cultured to the 4th–6th generation and then seeded in 96-well plates (3 × 103 cells per well) and incubated for 24 h. Extracts were obtained by immersing the swelled dressings into DMEM/high-glucose without phenol red (HyClone, USA) at the concentration of 100 mg mL−1, leaving them at 37 °C for 1 day. Subsequently, the culture medium was replaced with the extracts with 10% (v/v) fetal bovine serum. After incubation for 1 day, cell viability was assessed using a CCK-8 assay kit (Beyotime®, China) (n = 3).33The hGECs were seeded in 96-well plates with a density of 0.5 × 103 cells per well. The cells were incubated with the extracts of SPE, SPE/S and Reso-pac® for 1 and 3 days, respectively. CCK-8 assay was performed at the predetermined timepoint according to the manufacturer's instructions. Furthermore, after being seeded in glass bottom dishes (0.5 × 103 cells per dish) and incubating with extracts for 1 and 3 days, cells were processed with the Live/Dead cytotoxicity tool kit (Beyotime®, China) according to the manufacturer's instructions (n = 3). Green (492 nm) and red (545 nm) fluorescence were observed under a fluorescent microscope (OLYMPUS®, Japan).
2.10 In vivo biological adhesive capacity evaluation
To evaluate the in vivo mucoadhesion of the periodontal dressings, the dressings were applied on the gingivae of SD rats, and the residence time was recorded. Briefly, 0.05 g SPE-2, SPE/S-2 and Reso-pac® were applied on the labial gingivae of the lower teeth of rats (n = 3). A blend of SCMC powder and saline was adopted as the control. After the predetermined time interval, the rats were anesthetized, and the samples remaining on the palate were monitored.342.11 Wound healing studies
The wound healing effect was evaluated using a full-thickness mucosal defect model on the palate of SD rats. The rat was generally anesthetized, and a 3 mm-diameter circular defect was punched on the middle of the palate by a disposable punch biopsy tool (Kai Medical, Kai Industries Co., Ltd, Seki City, Japan), which sharply separated the mucosal tissue. SPE-2 and SPE/S-2 were sterilized by ultraviolet light for 15 minutes and applied to the defect after hemostasis, and the samples were changed every 3 days (n = 10). The rats with wounds and no treatment were set as the blank control group. The healing of the mucosal wound was continuously documented by digital photographs for 3, 6, 9, and 12 days after surgery, and the wound area was measured using Image J software. The degree of wound closure was calculated using the following equation:| Degree of wound closure(%) = (Ax − A0)/A0 × 100%, |
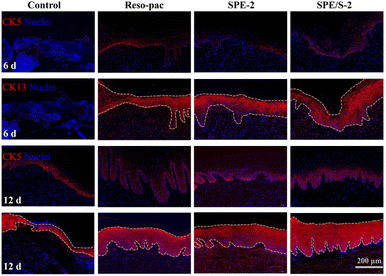
Palate tissues around the defects were collected at 3, 6, and 12 days after surgery for hematoxylin and erosion (H&E) staining. Immunofluorescence staining of CK5 and CK13 was used to evaluate the regeneration of the epithelium at 6 and 12 days after surgery. Anti-CD11b was adopted to evaluate the recruitment of inflammatory cells after 3 days.28,35 The sections were scanned using Pannoramic DESK, P-MIDI, P250 (3DHISTECH Ltd, Hungary). Quantitative analysis was done with the ImageJ software, and the mean fluorescence, normalized to the area of staining, was calculated. At least three different areas were measured in each specimen.
2.12 In vivo biosafety and degradation
The in vivo biosafety and degradation properties of the dressings were evaluated using the subcutaneous implantation mouse model. First, 0.02 g sterile dressings were implanted under the backs of Kunming mice. Reso-pac® and saline were used as control groups. At determined time points, the skin tissues contacting with the sample (1 and 7 days) and major organs (the heart, liver, spleen, lung, and kidney) (14 days) were harvested for histological analysis (n = 3).2.13 Statistical analysis
All data are expressed as mean ± SD. Data from experiments were analyzed using Origin 2022 (OriginLab, Hampton, MA, USA). The statistical differences were calculated using one-way ANOVA followed by Tukey's test with SPSS version 22.0 (IBM Corp, Chicago, IL, USA). For all tests, * indicates P value < 0.05, **P value < 0.01, and ***P value < 0.001.3 Results and discussion
3.1 Fabrication of periodontal dressings
As shown in Fig. S1A,† SPE was fabricated by mixing SCMC, Eudragit S 100 and PVP K30 in ethanol and glycerin, and SPE/S was composed of SPE and additives, in which the mass of the solvent was fixed. Additives include stearic acid, xylitol and menthol, where stearic acid was added for lubrication, emulsification and adjustment of viscosity to develop homogeneous dressing and enhance aesthetics; xylitol and menthol were used to improve the odor and taste of the dressing. The type and ratios of the solute mixtures were modified according to macro observation (Fig. S1B†). It was found that the cohesion of the material gradually weakened and the material was easily broken when the composition ratio of SCMC and Eudragit S 100 was fixed with the increase of PVP. This may be attributed to the fact that PVP reduced the cohesion in Eudragit S 100. When the proportion of Eudragit S 100 was increased and the proportion of the other two components was fixed, adhesion of the material in aqueous solution (pH < 7) decreased. We speculate that Eudragit S 100 formed water-insoluble clumps and prevented the dissolution and adhesion of the inner SCMC. When the SCMC ratio was increased over 4.0 g, the material became hard and lacked elasticity. Four mixtures were chosen to be experimental groups (SPE/S-1, SPE/S-2, SPE-1, and SPE-2) (Table S1†). The macro appearance of the SPE dressing and the schematic diagram of the operation procedure are exhibited in Fig. 1B.Periodontal dressings are widely used for postoperative wounds in periodontal surgery, as they could (1) provide mechanical protection for the surgical wound and therefore facilitate healing; (2) improve patient comfort by isolating the area from external irritations or injuries; (3) prevent post-operative bleeding by maintaining the initial clot in place; (4) support mobile teeth during healing; and (5) provide help in sharping or molding the newly formed tissue.7,8 However, the usage of periodontal dressing has been questioned by some researchers owing to their disadvantages, including accumulating plaque, irritating healthy tissue, and having no effect on healing, clinical parameters, and pain score. Besides, current periodontal dressings could also potentially retain food and increase inflammation.36,37
Compared with conventional setting dressings, the present non-setting degradable dressings have multiple advantages. They are flexible and soft due to their non-setting properties, which could reduce patient discomfort and foreign body sensation.38 Furthermore, they can adhere to the wound surface tightly because of the soft texture (Movie S1†). The adhesion could thus reduce salivary leakage and plaque accumulation, which often occur on setting dressings and may lead to delayed healing.5,29,37 Although the setting dressings may displace the flap, entrap the sutures beneath the dressing and force the dressing material under the flap during placement, these problems can be avoided in degradable dressings.7 It should also be noted that the setting dressings should be removed within 1 week after surgery to prevent alterations in the healing pattern and bacterial growth.38 The removal of dressings requires regular follow-up visits and may cause pain and mechanical damage to the wound, while this process could be exempt by using degradable dressings. Hence, in the present work, a non-setting and degradable periodontal dressing was adopted and evaluated.
3.2 Rheology evaluation of the periodontal dressings
To assess the influence of temperature and additives on the rheological properties of the dressings, an oscillation frequency procedure was performed to monitor the viscoelasticity of SPE and SPE/S. As shown in Fig. S2,† for all dressings, the storage modulus (G′) and loss modulus (G′′) demonstrated a continuous increase with increased frequency (0.1–100 rad s−1). In SPE, the dressings showed a liquid-like behavior (G′′ > G′, tan(δ) > 1). SPE/S presented a shift from a solid-like to liquid-like behavior with the G′′ surpassing G′ as frequency increased; the crossover points between G′ and G′′ (tan(δ) = 1) were observed under the evaluated rheological conditions.39 Given that the addition of additives changed the viscoelasticity of the dressings, it is suggested that stearic acid may change the interaction of Eudragit S 100 or the interface friction between Eudragit S 100 and PVP K30. Subsequently, the G′ and G′′ for frequency sweep at 25 °C and 37 °C were further determined. At different temperatures, similar trends were observed, indicating that the rheological properties of SPE and SPE/S presented a low sensitivity to temperature.40 Moreover, the curves of storage modulus (G′) and loss modulus (G′′) for the present dressings are similar, indicating that the non-setting dressings are not easily deformed. The curves in groups with different SCMC content were also similar, suggesting SCMC content had a negligible effect on the viscoelasticity.3.3 Adhesive property of periodontal dressings
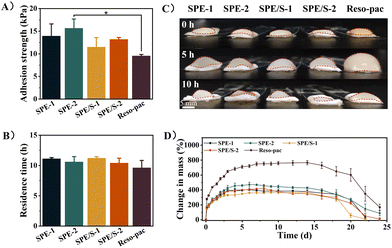
To achieve satisfactory retention in the oral cavity and accomplish favorable wound protection, non-setting periodontal dressings present high requirements for mucoadhesion properties. The lap-shear test method was adopted to examine the mucoadhesion strength of the dressings. To simulate the application scenario, we used porcine keratinized gingiva in the experiment (Fig. S3†). As shown in Fig. 2A, the average adhesion strength of SPE-1, SPE-2, SPE/S-1, and SPE/S-2 were 13.9 ± 2.7, 15.7 ± 2.0, 11.5 ± 2.1 and 13.2 ± 0.4 kPa, respectively, which were higher than the commercially available dressing Reso-pac® (9.5 ± 0.3 kPa). Notably, the adhesion strength of SPE-2 is significantly higher than that of Reso-pac® (P < 0.05). In addition, a higher mucoadhesion strength was observed in the groups with more SCMC, which revealed that the content of SCMC influenced the wet mucoadhesion strength of the dressings. As demonstrated in Movie S2,† SPE-2 can adhere to the porcine mandible, withstanding the water pressure of a full-blast faucet, which suggests its excellent adhesive performance.Then, the rotating-disc method was adopted to investigate the in vitro residence time by recording whether the remaining samples adhered to the porcine gingiva at the predetermined timepoints (Fig. S4†).28 It was found that the present dressings exhibited long-term and strong mucosal adhesion performance in a wet environment, and the in situ retention time reached 11.1 ± 0.2, 10.6 ± 0.9, 11.2 ± 0.2, and 10.4 ± 0.8 h for SPE-1, SPE-2, SPE/S-1, and SPE/S-2, respectively, which were comparable to Reso-pac® (9.6 ± 1.2) (Fig. 2B). Although no statistical difference was observed, the residence times in the experimental group were higher than that of Reso-pac®. Moreover, we also found that the SCMC content and the presence of additives exhibited no significant effect on residence time. The dimensional stability of the dressing has an important influence on the comfort of the patient and is an important indicator of the clinical applicability of dressings. Therefore, we macroscopically tested the swelling property of the dressing in PBS solution. The result revealed that SPE and SPE/S had less dimensional change, while Reso-pac® presented higher swelling behavior (Fig. 2C).
The present dressings demonstrated adequate adhesion performance, higher than 11 kPa, and the ability to adhere to porcine gingiva for over 10 hours in the mucosal adhesion tests using the rotating-disc method. As an anionic polymer, SCMC exhibits excellent skin and mucous membrane compatibility, with high water binding affinity. It can strongly adhere to biological surfaces in transdermal and transmucosal applications.17,18 Typically, the mucosal adhesion of SCMC can be explained by the physical entanglement of polymer chains with mucins, but extensive scientific studies have shown that hydrogen bonds formed between the polymer and mucus are responsible for its adhesive properties.41 It should be noted that the moisture content and relative humidity are key parameters for the adhesion strength of SCMC to ensure its adhesion effect, and thus a period of time with relatively low humidity was necessary.42 Therefore, the wound surface needs to be dried off before application. Compared with previous reports, the adhesion strengths of commercial dressings such as Wondrpak® and Coe-pak® were significantly lower than the present dressings, which may be due to the fact that their retention relies primarily on mechanical locking instead of adhesion.29
3.4 Swelling and degradation behaviours of dressings
The swelling behavior of the dressings was further investigated. In the oral cavity, smaller dimensional changes can lead to greater comfort and better wound protection. As shown in Fig. 2D, SPE and SPE/S were fully swelled after 6 to 9 days of incubation. After this timepoint, the dressings started to degrade, while Reso-pac® reached the equilibrium of full swelling after incubation for up to 14 days. The maximum swelling ratios of SPE-1, SPE-2, SPE/S-1, and SPE/S-2 were 403.9 ± 9.2%, 472.1 ± 32.3%, 365.8 ± 5.9%, and 420.3 ± 44.3%, respectively, and the ratio for Reso-pac® was 729.8 ± 42.0%. This result indicated that SPE and SPE/S showed a smaller swelling ratio and better dimensional stability than Reso-pac®, which is consistent with the observation of the macroscopic properties in the residence time test.For skin wounds, dressings with a high swelling ratio exhibit a good ability to absorb wound exudate and keep the wound moist.43,44 In the oral cavity, however, a high swelling ratio can result in a significant increase of dressing volume and poor dimensional stability due to the absorption of saliva, and this property significantly influences patient comfort. At the same time, uncontrolled swelling often leads to detachment of the adhesion from the wound site due to diminished adhesion and potential pressure from the surrounding tissue. The maximum swelling ratio was higher in dressings with more SCMC, indicating that the dressing hardly maintained its original state in aqueous solution. This may be attributed to the fact that the swelling rate of SCMC is related to its hydrophilic groups.45,46
The weight of the samples was further monitored to evaluate the degradation profile of the dressings. SPE-1 and SPE-2 dressings started degrading after 6 days, while SPE/S-1 and SPE/S-2 exhibited weight loss after 9 and 8 days, respectively. In the longitudinal comparison of each dressing, dressings with less SCMC (SPE-1 and SPE/S-1) totally degraded after 22 days of incubation, while the dressings with more SCMC (SPE-2 and SPE/S-2) completely degraded after 24 days. The incorporation of SCMS increased the resilience of dressings against degradation. Moreover, additives had no obvious effect on the degradation profile of the dressing. The similar degradation time indicated that these dressings should exhibit a similar protective duration as Reso-pac® in clinical practice.
3.5 Hemocompatibility and cell compatibility of dressings
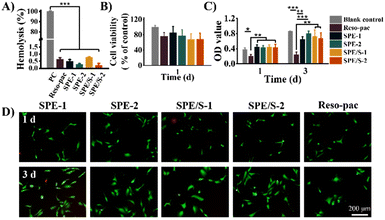
The periodontal dressing covers the wound surface after surgery and may contact with blood directly. Therefore, blood compatibility is a crucial characteristic of periodontal dressing. The hemolysis rate is an important indicator to evaluate the blood compatibility of materials. The international standard for the hemolysis of biological materials is less than 5%.47Fig. 3A illustrates that the hemolysis of all dressings was less than 1%, indicating that the present dressings barely affected the stability of the erythrocyte membrane. In addition, the content of SCMC and additives did not influence the percentage of hemolysis. These results proved that our dressings based on pharmaceutical excipients presented good blood compatibility. The pH values of the dressings were neutral, which also prove the favorable biocompatibility of the present periodontal dressings (Table S2†).The cell compatibility of the dressings was further evaluated. Given that hGECs of the gingival tissue will be in direct contact with the dressing during application, they were selected for cytocompatibility testing. The relative survival rates of hGECs cultured with SPE, SPE/S and Reso-pac® extracts were measured using the CCK-8 assay kit. As shown in Fig. 3B, there was no statistically significant difference in cell viability among all the dressings and blank control groups, indicating that these dressings showed good cell compatibility.
To further determine the effects on cell proliferation, the hGECs were incubated with dressing extracts for 1 and 3 days and then subjected to CCK-8 test and Live/Dead staining. As shown in Fig. 3C, the absorbance at 450 nm increased significantly in SPE and SPE/S, indicating that the present dressings did not interfere with cell proliferation. However, Reso-pac® significantly inhibited cell proliferation, and the OD values after 1 and 3 days were 0.21 ± 0.0 and 0.24 ± 0.1. In addition, this result was also verified by Live/Dead staining (Fig. 3D). The current dressings exhibited negligible influence on the proliferation of hGECs.
Due to the high-biosafety composition and mild preparation process, the cell viability and proliferation mediated by SPE and SPE/S dressings are similar to or even better than the commercially available dressing Reso-pac®. According to Kadkhodazadeh et al., the cytocompatibility of Reso-pac® is better than Coe-pak®.12 Petelin et al. proved that Reso-pac® showed the least inhibitory effects on fibroblast proliferation after 48 h incubation compared with other commercial dressings (e.g., Peripac®, Barricaid®, Fittydent® and Myzotect®-tincture).48 These results indicated that the current dressings feature excellent cytocompatibility compared with other commercially available dressings.
3.6 In vivo residence time
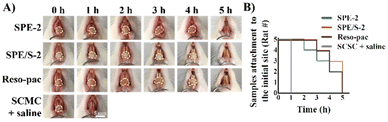
All the above test results proved that the present dressings can be used as a periodontal dressing. Among them, SPE-2 and SPE/S-2 showed favorable adhesion, hemocompatibility and cytocompatibility and thus were chosen as the experimental samples in in vivo tests. To examine the in vivo mucoadhesion of the dressings, the dressings were attached to the labial gingiva of rats’ lower teeth (i.e., keratinized area), and their residence times were measured. As shown in Fig. 4A, the sample of SPE and SPE/S-2 still maintained attachment to the gingival surfaces after 4 h. In contrast, the mixture of SCMC and saline on the gingival surfaces completely fell off within 1 h. The number of rats with residual dressings on their gingivae was then calculated as a function of time (Fig. 4B). Three out of five rats demonstrated residual samples up to 4 h in SPE-2 and SPE/S-2 groups, and two out of five in the Reso-pac® treated group were observed. It was found that the maximum times were similar among the three groups, indicating similar mucoadhesion of SPE-2, SPE/S-2, and Reso-pac®. This result also accorded with the in vitro residence time observations.As the rats were incapable of keeping the dressing in place after anesthesia recovery and attempted to remove and damage the dressings with their tongues and paws during the evaluation process, the in vivo mucoadhesion performance was significantly interrupted.49 However, in clinical practice, patients can follow the doctor's recommendation, which could further prolong the adhesion time of the dressing in the oral cavity. Moreover, the fast detachment in the SCMC + saline group may also be due to poor adhesion of SCMC under the high humidity from saline.
3.7 In vivo wound healing efficacy
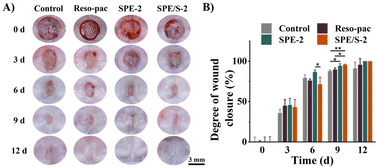
The wound healing efficacy of the dressings was determined using the palate full-thickness excisional wound SD rat model and assessed by macroscopic observation of wound closure as well as histopathological observation. Fig. 5A shows the macroscopic view of the wounds after 0, 3, 6, 9, and 12 days. After 9 days, it was found that satisfactory wound closure was presented in the SPE-2 (94.2 ± 2.8%) and SPE/S-2 (95.5 ± 0.8%) treated groups. Especially, SPE/S-2 significantly accelerated wound closure compared to Reso-pac® (89.6 ± 2.1%) (P < 0.05) and non-treatment (87.8 ± 1.4%) (P < 0.01). Application with SPE and SPE/S-2 resulted in the highest wound closure rate (100.0 ± 0.0%) within 12 days (Fig. 5B).To further investigate the repair process histologically, H&E staining and immunofluorescence staining were performed (Fig. S5†). The defects treated with SPE-2 and SPE/S-2 exhibited a completely regenerated epithelium similar to the normal gingiva after 6 days, whereas the defect treated with Reso-pac® still showed a mucosal break. The epithelium in the Reso-pac® treated group completely regenerated for 9 days, while there was an obvious defect observed in the non-treated group. This may be because these dressings reduced the inflammatory response of the wound and prevented the secondary damage caused by biting and licking.34 At the same time, the dressings prevented the accumulation of food debris near the wound.5 In a previous study, researchers evaluated wound healing on the buccal side of canines using Barricaid® and Reso-pac®, and the results revealed that wounds covered by Reso-pac® exhibited the best epithelization, vascularity, and the least inflammatory reaction in the first 4 days. The parameters observed afterward were similar to wounds with Barricaid® or without packaging.48 Therefore, it can be concluded that the present dressings showed excellent efficiency in promoting the wound healing of oral mucosa.
Moreover, immunofluorescence staining for CK5 (expressed in the basal layer where proliferating cells are located) and CK13 (expression on the prickle cell layer to the keratinized layer) (Fig. 6) exhibited that the wound bed tissue thickness of SPE-2, SPE/S-2, and Reso-pac® was significantly higher compared to the control group after 6 and 12 days. Finally, the inflammatory response was evaluated by CD11b staining (Fig. S6A†). Wounds treated with SPE-2 and SPE/S-2 displayed approximately half the fluorescence intensity of CD11b-positive cells compared to the non-treatment group, which was significantly lower than that in the Reso-pac® treated group (Fig. S6B†). Thus, it was speculated that the present dressings could significantly reduce wound inflammation and provide a beneficial environment for the oral mucosal wound to heal.
3.8 In vivo biosafety and degradation
The in vivo degradation and biosafety of the dressings were characterized through subcutaneous implantation into Kunming mice. The results in Fig. S7A† indicated that there was no significant necrosis or metaplasia in the skin tissue after contact with the dressings for 1 and 7 days. SPE/S-2 showed minimal inflammation among all groups. At the same time, all dressings were completely cleared within 7 days of implantation (Fig. S7B†). Moreover, further evaluation of the major organs (the heart, liver, spleen, lung, and kidney) was performed to detect the histocompatibility of the dressings. Both morphological observation (Fig. S7C†) and H&E staining results (Fig. S7D†) demonstrated no obvious tissue damage or pathological change after the subcutaneous implantation of dressings for 14 days. These results suggested that the current dressings are degradable in vivo and present good biocompatibility.4 Conclusions
A periodontal dressing based on high-biosafety pharmaceutical excipients was designed and developed to reduce postsurgical complications after periodontal surgeries. This novel periodontal dressing improves patient comfort due to its non-setting and degradation characteristics. Compared to the commercially available degradable dressing Reso-pac®, the present dressing also exhibited better dimensional stability, improved mucoadhesion to wet gingival tissue, and satisfactory biocompatibility. Our results illustrate that this dressing provided a stable wound barrier and improved the healing efficiency of oral mucosal wounds in SD rats. With these multiple benefits, we anticipate this periodontal dressing could enter clinical practice and provide satisfactory postoperative care for periodontal patients in the near future.Author contributions
Xiaodan Zhao: methodology, validation, formal analysis, writing – original draft. Meiwen Li: methodology, investigation, formal analysis, visualization, writing – original draft. Meng Li: data curation, validation. Wenbo Li: visualization, validation. Ang Li: supervision, project administration, funding acquisition. Yilong Cheng: conceptualization, supervision, writing – review & editing, supervision, project administration. Dandan Pei: conceptualization, project administration, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 82170927 and 52173139), Outstanding Youth Science Foundation Project of Shaanxi Provincial Department of Science and Technology (No. 2022JC-57), and the Fundamental Research Funds for the Central Universities, Xi'an Jiaotong University (sxtr042021004, xzy012023152 and xzy012023128).References
- H. S. Bawaskar and P. H. Bawaskar, Lancet, 2020, 395, 185–186 CrossRef PubMed.
- D. F. Kinane, P. G. Stathopoulou and P. N. Papapanou, Nat. Rev. Dis. Primers, 2017, 3, 17038 CrossRef PubMed.
- L. J. Heitz-Mayfield and N. P. Lang, Periodontology 2000, 2013, 62, 218–231 CrossRef PubMed.
- R. Pippi, Int. J. Med. Sci., 2017, 14, 721–728 CrossRef PubMed.
- R. Kathariya, H. Jain and T. Jadhav, J. Appl. Biomater. Funct. Mater., 2015, 13, e73–e86 CAS.
- M. B. V. Kumar, V. Narayanan, M. Jalaluddin, S. A. Almalki, S. M. Dey and S. Sathe, J. Contemp. Dent. Pract., 2019, 20, 896–900 CrossRef PubMed.
- S. Soheilifar, M. Bidgoli, J. Faradmal and S. Soheilifar, J. Dent. Tehran, 2015, 12, 151–156 Search PubMed.
- A. Kakar, A. K. Lamba, S. Tandon, F. Faraz and A. Ahad, J. Nat. Sci., Biol. Med., 2018, 9, 65–71 CrossRef PubMed.
- S. Garg, S. A. Arora, S. Chhina, G. Puri, D. Bhardwaj and N. Kaur, Int. J. Pharm. Sci. Res., 2017, 8, 3917–3922 Search PubMed.
- M. V. S. Meghana, J. Deshmukh, M. V. Devarathanamma, K. Asif, L. Jyothi and H. Sindhura, J. Indian. Soc. Periodontol., 2020, 24, 54–59 CrossRef PubMed.
- E. Madan, V. Bharti, K. K. Chaubey, V. K. Arora, R. K. Thakur and A. Nirwal, J. Indian. Soc. Periodontol., 2013, 17, 753–756 CrossRef PubMed.
- M. Kadkhodazadeh, Z. Baghani, M. Torshabi and B. Basirat, J. Oral. Maxillofac. Res., 2017, 8, e3 Search PubMed.
- Ministry of W. Family and C, Indian Pharmacopoeia, Indian Pharmacopoeia Commission, Ghaziabad, 8th edn, 2018.
- Guo jia yao dian wei yuan hui, Pharmacopoeia of the People's Republic of China, China Medical Science Press, Beijing, 2015 edn, 2015 Search PubMed.
- J. Wu, Z. Pan, Z. Y. Zhao, M. H. Wang, L. Dong, H. L. Gao, C. Y. Liu, P. Zhou, L. Chen, C. J. Shi, Z. Y. Zhang, C. Yang, S. H. Yu and D. H. Zou, Adv. Mater., 2022, 34, 2200115 CrossRef CAS PubMed.
- A. Zennifer, P. Senthilvelan, S. Sethuraman and D. Sundaramurthi, Carbohydr. Polym., 2021, 256, 117561 CrossRef CAS PubMed.
- T. W. Wong and N. A. Ramli, Carbohydr. Polym., 2014, 112, 367–375 CrossRef CAS PubMed.
- S. Javanbakht and A. Shaabani, Int. J. Biol. Macromol., 2019, 133, 21–29 CrossRef CAS PubMed.
- S. W. Ko, J. Y. Lee, L. E. Aguilar, Y. M. Oh, C. H. Park and C. S. Kim, J. Nanosci. Nanotechnol., 2019, 19, 2232–2235 CrossRef CAS PubMed.
- A. Sherikar, M. U. M. Siddique, M. More, S. N. Goyal, M. Milivojevic, S. Alkahtani, S. Alarifi, M. S. Hasnain and A. K. Nayak, Oxid. Med. Cell. Longevity, 2021, 2021, 1818538 CrossRef PubMed.
- Y. Cui, Z. Huang, L. Lei, Q. Li, J. Jiang, Q. Zeng, A. Tang, H. Yang and Y. Zhang, Nat. Commun., 2021, 12, 5922 CrossRef CAS PubMed.
- S. Barzegar, M. R. Zare, F. Shojaei, Z. Zareshahrabadi, O. Koohi-Hosseinabadi, M. J. Saharkhiz, A. Iraji, K. Zomorodian and M. Khorram, Int. J. Pharm., 2021, 597, 120288 CrossRef CAS PubMed.
- A. Ibrahim, D. Hassan, N. Kelany, S. Kotb and M. Soliman, Front. Vet. Sci., 2020, 7, 597751 CrossRef PubMed.
- C. F. Vecchi, G. B. Cesar, P. R. Souza, W. Caetano and M. L. Bruschi, Pharm. Dev. Technol., 2021, 26, 138–149 CrossRef CAS PubMed.
- R. C. Rowe, P. J. Sheskey, S. N. C. Owen and American Pharmacists Association, Handbook of pharmaceutical excipients, ed. R. C. Rowe, P. J. Sheskey and M. E. Quinn, APhA/Pharmaceutical Press, London; Chicago, 6th edn, 2009 Search PubMed.
- X. Zhao, Y. Yang, J. Yu, R. Ding, D. Pei, Y. Zhang, G. He, Y. Cheng and A. Li, Biomaterials, 2022, 282, 121387 CrossRef CAS PubMed.
- J. Deng, Y. Tang, Q. Zhang, C. Wang, M. Liao, P. Ji, J. Song, G. Luo, L. Chen, X. Ran, Z. Wei, L. Zheng, R. Dang, X. Liu, H. Zhang, Y. S. Zhang, X. Zhang and H. Tan, Adv. Funct. Mater., 2019, 29, 1809110 CrossRef.
- S. Hu, X. Pei, L. Duan, Z. Zhu, Y. Liu, J. Chen, T. Chen, P. Ji, Q. Wan and J. Wang, Nat. Commun., 2021, 12, 1689 CrossRef CAS PubMed.
- Z. Baghani and M. Kadkhodazadeh, J. Dent. Res. Dent. Clin. Dent. Prospects., 2013, 7, 183–191 Search PubMed.
- P. Chuysinuan, P. Nooeaid, T. Thanyacharoen, S. Techasakul, P. Pavasant and K. Kanjanamekanant, Int. J. Biol. Macromol., 2021, 193, 799–808 CrossRef CAS PubMed.
- T. Zhu, H. Wang, Z. Jing, D. Fan, Z. Liu, X. Wang and Y. Tian, Bioact. Mater., 2021, 8, 12–19 CrossRef PubMed.
- Y. Zhou, T. Ye, C. Ye, C. Wan, S. Yuan, Y. Liu, T. Li, F. Jiang, J. F. Lovell, H. Jin and J. Chen, Bioact. Mater., 2021, 9, 541–553 CrossRef PubMed.
- R. Shi, H. Li, X. Jin, X. Huang, Z. Ou, X. Zhang, G. Luo and J. Deng, Acta Biomater., 2022, 152, 425–439 CrossRef CAS PubMed.
- J. H. Ryu, J. S. Choi, E. Park, M. R. Eom, S. Jo, M. S. Lee, S. K. Kwon and H. Lee, J. Controlled Release, 2020, 317, 57–66 CrossRef CAS PubMed.
- H. M. Hammad, M. M. Hammad, I. N. Abdelhadi and M. S. Khalifeh, Int. J. Dent. Hyg., 2011, 9, 9–16 CrossRef CAS PubMed.
- R. Dahiya, A. Blaggana, V. Panwar, S. Kumar, A. Kathuria and S. Malik, J. Indian. Soc. Periodontol., 2019, 23, 345–350 CrossRef PubMed.
- H. F. Jentsch, G. U. Knofler, R. E. Purschwitz and S. Eick, Oral Health Prev. Dent., 2016, 14, 101–109 Search PubMed.
- R. P. Antoniazzi, A. R. Vieira, J. L. Da Rosa, K. L. Ferrazo, F. B. Zanatta and C. A. Feldens, Acta Odontol. Scand., 2014, 72, 1025–1031 CrossRef PubMed.
- S. M. Hashemnejad, A. Z. M. Badruddoza, B. Zarket, C. R. Castaneda and P. S. Doyle, Nat. Commun., 2019, 10, 2749 CrossRef PubMed.
- Q. Jiang, P. Li, M. Ji, L. Du, S. Li, Y. Liu and Z. Meng, Food Chem., 2022, 372, 131357 CrossRef CAS PubMed.
- P. Schattling, E. Taipaleenmaki, Y. Zhang and B. Stadler, Macromol. Biosci., 2017, 17, 1700060 CrossRef PubMed.
- S. Dong, S. Feng, F. Liu, R. Li, W. Li, F. Liu, G. Shi, L. Chen and Y. Zhang, Int. J. Biol. Macromol., 2021, 179, 398–406 CrossRef CAS PubMed.
- M. F. P. Graca, S. P. Miguel, C. S. D. Cabral and I. J. Correia, Carbohydr. Polym., 2020, 241, 116364 CrossRef CAS PubMed.
- Y. Lu, H. Li, J. Wang, M. Yao, Y. Peng, T. Liu, Z. Li, G. Luo and J. Deng, Adv. Funct. Mater., 2021, 31, 2105749 CrossRef CAS.
- K. M. Salleh, S. Zakaria, M. S. Sajab, S. Gan and H. Kaco, Int. J. Biol. Macromol., 2019, 131, 50–59 CrossRef CAS PubMed.
- Y. Deng, X. Yang, X. Zhang, H. Cao, L. Mao, M. Yuan and W. Liao, Int. J. Biol. Macromol., 2020, 160, 1242–1251 CrossRef CAS PubMed.
- C. Zhang, X. Yang, W. Hu, X. Han, L. Fan and S. Tao, Int. J. Biol. Macromol., 2020, 149, 31–40 CrossRef CAS PubMed.
- M. Petelin, Z. Pavlica, U. Batista, D. Stiblar-Martincic and U. Skaleric, Acta Vet. Hung., 2004, 52, 33–46 CAS.
- S. Nazarnezhada, G. Abbaszadeh-Goudarzi, H. Samadian, M. Khaksari, J. M. Ghatar, H. Khastar, N. Rezaei, S. R. Mousavi, S. Shirian and M. Salehi, Int. J. Biol. Macromol., 2020, 164, 3323–3331 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3bm01314f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |