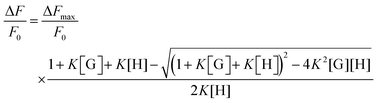A molecularly imprinted nanocavity with transformable domains that fluorescently indicate the presence of antibiotics in meat extract samples†
Azusa
Oshita
a,
Hirobumi
Sunayama
 *a and
Toshifumi
Takeuchi
*a and
Toshifumi
Takeuchi
 *ab
*ab
aGraduate School of Engineering, Kobe University, 1–1, Rokkodai-cho, Nada-ku, Kobe 657–8501, Japan. E-mail: sunayama@penguin.kobe-u.ac.jp; Tel: +81 78 803 6594
bCenter for Advanced Medical Engineering Research & Development (CAMED), Kobe University, 1-5-1 Minatojimaminami-machi, Chuo-ku, Kobe 650-0047, Japan. E-mail: takeuchi@gold.kobe-u.ac.jp; Tel: +81 78 803 6158
First published on 11th May 2022
Abstract
In this study, we aimed to create synthetic polymer receptors with the fluorescence signalling ability, using molecular imprinting, precisely designed template molecules, and site-specific post-imprinting modifications, which can mimic conjugated proteins and are capable of specific molecular recognition, and wherein successful binding can be indicated by a change in fluorescence. A molecularly imprinted APO-type nanocavity with a reconstructable domain was prepared by co-polymerisation of a template molecule containing cephalexin conjugated to polymerisable groups via a Schiff base, a disulphide bond, and a cross-linker, followed by hydrolysis of the Schiff base and a disulphide exchange reaction. Fluorescence-based indication of binding was devised by the Schiff base formation reaction with 4-formylsalicylic acid, and the interacting site was introduced via a disulphide exchange reaction with 4-mercaptobenzoic acid, yielding a multifunctional mature (HOLO)-type molecularly imprinted nanocavity. The ability to indicate binding events using changes in the fluorescence of the HOLO polymer was investigated, and it was revealed that the target antibiotic cephalexin can be selectively detected in aqueous media with high affinity (Ka = 1.1 × 104 M−1). Furthermore, the proposed sensor exhibited the potential to detect spiked cephalexin in chicken extracts with a limit of detection of 18 μM (1.3 ppm). The proposed fluorescence-sensing system based on molecular imprinting and post-imprinting modification is expected to enable the development of advanced materials for the specific detection of trace antibiotics in complex samples.
Introduction
Antibiotics are frequently used as therapeutic agents in humans, livestock, and plants.1 The most famous antibiotic penicillin was discovered in Penicillium notatum by Alexander Fleming in 1928.2 Currently, several antibiotics have been discovered, developed, and are in use; however, the improper or excessive use of antibiotics has resulted in the emergence of certain issues, such as development of drug-resistant bacteria and related diseases. In addition, humans are also exposed to unknown doses of antibiotics upon consumption of contaminated foods, and this has an impact on human health.1,3 Therefore, technologies for sensing antibiotics are becoming increasingly important, especially for food analysis.4 Liquid chromatography is a robust tool used for analysing trace amounts of antibiotics.5–7 However, it requires time-consuming pre-treatments and technical expertise and involves complicated procedures for successful completion of the analysis. Another attractive method for antibiotic analysis, that is, enzyme-linked immunosorbent assay, is highly sensitive and involves the use of antibodies and enzyme-mediated molecular recognition.8 However, antibodies and enzymes have some disadvantages owing to their high cost of production, low stability to chemical/physical stimuli, and arduous quality control processes that are characteristic of naturally occurring materials. To overcome these disadvantages, various synthetic receptors have been developed for antibiotics, including aptamers9 and synthetic polymers. Fluorescence is a highlight attractive method for rapid and sensitive detection of antibiotics. Various fluorescent sensors, including fluorescently labelled proteins10 and aptamers,11 have been reported. Recently fluorescent metal–organic frameworks have also been used for detection of antibiotics.12Molecular imprinting is an attractive technology for the creation of abiotic receptors.13–16 In molecular imprinting, synthetic polymers with binding cavities complementary to the target molecule are created in three simple steps. First, the target molecule (or its analogues) is complexed with functional monomers via covalent or non-covalent interactions. Second, the complex is co-polymerised with co-monomers and cross-linkers, yielding a cross-linked polymer matrix. Finally, the target molecule moiety is removed by washing, resulting in the generation of molecularly imprinted polymers (MIPs). Various MIPs have been reported for antibiotics.17,18
Previously, we reported sophisticated MIPs for antibiotics, called conjugated protein mimics, which have large molecularly imprinted cavities that allow the introduction of various synthetic prosthetic groups for on/off switching of binding activity, photo-responsiveness, and fluorescence signalling as post-imprinting modifications (PIMs).19,20
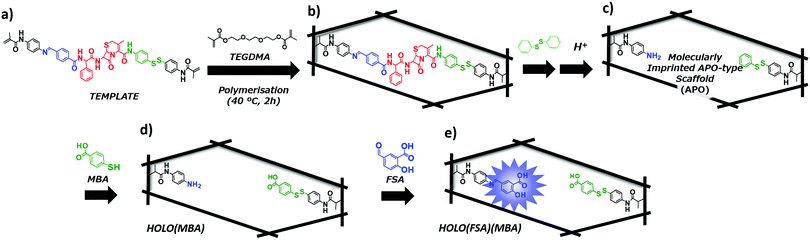
Conjugate proteins are proteins capable of binding non-peptide co-factors or prosthetic groups in their structure; a protein bound to its groups, referred to as ‘Holo-protein’ (or enzyme) exhibits functionality, whereas ‘Apo-protein’ does not have the bound groups. A precisely designed template molecule was synthesised, wherein two functional monomers bearing modifiable parts, a Schiff base moiety and a disulphide bond, were coupled with cephalexin (Fig. 1a). The template was co-polymerised with triethylene glycol dimethacrylate (TEGDMA), as a cross-linker, in dimethyl sulphoxide (DMSO), as the solvent (Fig. 1b). Thereafter, the cross-linked polymer was treated with diphenyl disulphide and acetic acid (AcOH) to remove cephalexin and the interacting parts, via a disulphide exchange reaction and hydrolysis of the Schiff base, yielding a molecularly imprinted immature nanocavity (APO-type scaffold; Fig. 1c). To create a mature (HOLO-type) nanocavity by PIMs, 4-mercaptobenzoic acid (MBA) was introduced using a disulphide exchange reaction (Fig. 1d). MBA was selected as an interacting prosthetic group because its carboxyl group can form hydrogen bonds with the carboxyl group on the target antibiotic. Next, 4-formylsalicylic acid (FSA) was introduced by the re-formation of the Schiff base, resulting in the construction of a HOLO-type nanocavity (Fig. 1e). FSA was selected as a fluorescence signalling prosthetic group because its size was similar to that of the original benzoic acid group. This HOLO polymer was able to detect β-lactam antibiotics with high affinity and selectivity compared with other structurally related compounds. However, the binding of HOLO polymers has only been investigated in organic solvents, like DMSO. For widespread application of the proposed sensing system, its use in aqueous media would be preferable for reducing the environmental load and enhancing sustainability. In addition, ampicillin—having a structure similar to that of the original target, cephalexin—was used in binding experiments as cephalexin is unstable in DMSO, which is the solvent used in the polymerisation step and in the binding experiments. Therefore, in this study, we investigated the fluorescence signalling activity of the HOLO-type molecularly imprinted nanocavity that binds to cephalexin in aqueous media and chicken extract samples.
Experimental
Chemicals and reagents
Dichloromethane, methanol (MeOH), DMSO, N,N-diisopropylethylamine (DIEA), trifluoroacetic acid, acetone, sodium chloride (NaCl), magnesium sulphate, and triethylamine (TEA) were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). AcOH, acetonitrile, boric acid, ethanol, 4-formylbenzoic acid, thioanisole, n-hexane, FSA, ampicillin (Amp), 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile) (V-70), Celite®, sodium hydroxide (NaOH), trisodium citrate dihydrate, disodium hydrogen citrate sesquihydrate, p-aminobenzoic acid (PABA), penicillin G (Pen G), and diphenyl disulphide were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). MBA, cephalexin monohydrate, 4,4′-dithiodianiline, 6-aminopenicillinic acid (6APA), and TEGDMA were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Phosphoric acid was purchased from Katayama Chemical Industries Co., Ltd. (Osaka, Japan). Nafcillin (Naf) was purchased from Toronto Research Chemicals Inc. (Toronto, Canada). Dichloromethane was distilled prior to use. TEGDMA was treated with an inhibitor remover (a packed column) for removing hydroquinones (Sigma-Aldrich, St. Louis, USA) prior to use. Pure water was obtained using a Milli-Q purification system (Millipore). The chicken tender sample was purchased from a local supermarket store (Ikarisuper Co. Ltd., Hyogo, Japan).Synthesis of the template molecule
The template molecule was synthesised as previously described.19,20 The disulphide side functional monomer was synthesised using a coupling reaction between methacryloyl chloride and 4,4-dithiodianiline in the presence of DIEA. The monomer proximal to the Schiff base was synthesised by a Schiff base formation reaction between 4-formylbenzoic acid and N-(4-aminophenyl)methacrylamide, which in turn was synthesised by a coupling reaction between p-phenylenediamine and methacryloyl chloride. Then, the disulphide side monomer was coupled with the tert-butyloxycarbonyl-protected cephalexin via a condensation reaction. After de-protection of the amino group on cephalexin, the monomer proximal to the Schiff base was conjugated to yield the template molecule.Preparation of the APO-type molecularly imprinted cavity and fluorescence signalling HOLO(FSA)(MBA)
The APO-type molecularly imprinted cavity and fluorescence signalling HOLO(FSA)(MBA) were prepared as previously reported. The template molecule (100 mg, 0.1 mmol) and TEGDMA (1403 μL, 5.0 mmol) were dissolved in DMSO (1.71 mL), and N2 gas was bubbled through the solution for 5 min. Polymerisation with V-70 (33 mg, 0.11 mmol) was thermally initiated at 40 °C. After polymerisation for 2 h, the resulting polymer was ground and washed with MeOH using an overnight Soxhlet extraction. After washing, the obtained polymer was dried in an oven at 70 °C to yield cross-linked polymers. The yield was 1571 mg (96.1%).The obtained cross-linked polymer was dispersed in DMSO (156 mL). Thereafter, diphenyl disulphide (262 mg, 1.15 mmol) and TEA (657 μL) were added to it and the resultant mixture was stirred at 60 °C for 24 h. After the reaction, the mixture was filtered, and the obtained polymer was washed with DMSO and MeOH. The polymer was then suspended in AcOH (150 mL) and stirred at room temperature (20–25 °C) for 24 h. After incubation, the mixture was filtered and washed with DMSO and MeOH. The procedures described above were performed twice on the APO-type molecularly imprinted polymer (yield: 1528 mg).
The APO-type polymer (200 mg) was re-suspended in DMSO (30 mL), and then MBA (80 mg, 0.52 mmol) and TEA (100 μL) were added to the suspension. The mixture was stirred at 60 °C for 24 h, followed by filtration and washing with DMSO and MeOH. The obtained polymer was re-suspended in DMSO (20 mL). Thereafter, FSA (80 mg, 0.47 mmol) and TEA (800 μL) were added, and the suspension was stirred at room temperature for 24 h. The mixture was filtered and washed with DMSO and MeOH to yield HOLO(FSA)(MBA).
Binding with cephalexin in aqueous media at different pH values
HOLO(FSA)(MBA) (2 mg) was suspended in 2 mL of Britton–Robinson buffer at various pH values (pH 4.2, 5.1, 6.3, 7.2, and 8.3) and stirred for 10 min at 20 °C. The fluorescence intensity at 468 nm (λex = 365 nm) was measured after 60 min of incubation with various concentrations of cephalexin. The concentrations of cephalexin used were 0, 31.3, 62.5, 125, 250, 500, and 1000 μM. The relative fluorescence intensity (F − F0)/F0 was calculated, where F is the fluorescence intensity at 468 nm after incubation with cephalexin and F0 is the initial fluorescence intensity at 468 nm. The apparent binding constants (Ka) were calculated using graph calculation software (Microsoft Excel 2013, Microsoft Corporation, USA) with the following equation:The equation is generally used with the binding constant for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation, where K is the binding constant, H is determined by fitting raw data to a theoretical curve, G is cephalexin concentration, and ΔFmax is the maximum change in fluorescence.
1 complex formation, where K is the binding constant, H is determined by fitting raw data to a theoretical curve, G is cephalexin concentration, and ΔFmax is the maximum change in fluorescence.
Selectivity test by fluorescence measurements
HOLO(FSA)(MBA) (2 mg) was dispersed in 2 mL of 50 mM Britton–Robinson buffer (pH 7.2) and stirred for 10 min at 20 °C to stabilise the fluorescence intensity. The fluorescence intensity at 468 nm was measured after 60 min incubation of the reference compounds, namely Amp, Pen G, PABA, 6APA, and Naf. The concentration of the reference compounds was 125 μM. Changes in the relative fluorescence were calculated using the formula: (F − F0)/F0.Selectivity test by ultra-performance liquid chromatography (UPLC) measurements
The selectivity of HOLO(FSA)(MBA) was also examined using UPLC. The procedure was similar to that used for the fluorescence measurements. After incubation, polymer particles were removed by filtration using a disposable syringe filter (pore size: 0.3 μm, ADVANTEC). The obtained supernatant was analysed using the following: the UPLC system, LCT-Premier XE (Waters); column, ACQUITY UPLC BEH C18 50 mm × 2.1 mm I.D. 40 °C; flow rate, 0.8 mL min−1; eluent, MeOH/water = 55/45 (v/v); sample size, 10 μL; detection, 254 nm. The experiments were performed in triplicate.Real sample measurements
The chicken tender sample (10 g) was dispersed in a mixture of acetone (10 mL) and Milli-Q water (2 mL), containing NaCl (1 g), trisodium citrate dehydrate (1 g), disodium hydrogen citrate sesquihydrate (0.5 g), and magnesium sulphate (4 g), and homogenised by sonication. The homogenised sample was centrifuged (3000 rpm, 4 °C, 5 min) and cooled to −30 °C. After cooling, the acetone phase was collected. n-Hexane (3 mL) was added to the acetone (0.5 mL) solution and absorbed onto Celite® and an ion exchange resin. The absorbed sample was eluted using MeOH/Milli-Q water (1/1, v/v, 2 mL). The eluate was passed through a C18 column (Cosmosil 75C18-OPN, Nacalai Tesque, Inc., Japan) to yield the sample solution. Cephalexin was diluted with the sample solution to yield a sample solution containing various concentrations of cephalexin (0, 1.9, 3.9, 7.8, 15.5, 31.1, 62.1, 99.1, 124, and 196 μM). The fluorescence intensity at 468 nm of HOLO(FSA)(MBA) was measured after incubation in the prepared sample solution for 60 min at 20 °C. As a control solution, various concentrations of cephalexin (0, 1.9, 3.9, 7.8, 15.5, 31.1, 62.1, 99.1, 124, 196, and 245 μM) were dissolved in MeOH/Milli-Q water (1/1, v/v).Results and discussion
The designed template molecule—wherein cephalexin was connected to two polymerisable groups, via a disulphide bond and a Schiff base—was co-polymerised with TEGDMA as a cross-linker. After a disulphide exchange reaction with diphenyl disulphide and hydrolysis of the Schiff base to remove the cephalexin moiety, a molecularly imprinted nanocavity (APO-type scaffold) was created. The yields of the disulphide exchange reaction and Schiff base hydrolysis were 88.0% and 98.6%, respectively.19To generate a fluorescence signalling HOLO cavity, FSA was reacted with the aniline residue in the APO-type scaffold. In addition, a benzoic acid moiety, MBA, was introduced proximal to the disulphide bond via a disulphide exchange reaction, yielding the HOLO(FSA)(MBA) (Fig. 1). The fluorescence spectra of HOLO(FSA)(MBA) dispersed in aqueous buffer (pH 7.2) were measured at an excitation wavelength of 365 nm, and a fluorescence peak of approximately 480 nm, which was due to the presence of FSA, was observed (Fig. S1, ESI†). The fluorescence intensity of around 480 nm decreased after treatment with AcOH, indicating that the FSA moiety was incorporated into the polymer matrix via the Schiff base.
The fluorescence signalling ability of HOLO(FSA)(MBA) in aqueous media was investigated by fluorescence measurements at an excitation wavelength of 365 nm, and fluorescence intensities at 468 nm were recorded after incubation with various concentrations of cephalexin. Fluorescence intensities at 468 nm increased with the addition of cephalexin (Fig. S2, ESI†). These results implied that fluorescence signalling of cephalexin-imprinted nanocavities was induced in the polymer matrix by cephalexin binding in the cavity prepared via molecular imprinting and the PIM process. The apparent Ka, calculated from the relative fluorescence changes, using curve fitting based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, was 1.1 × 104 M−1 (Fig. 2). This value was comparable to that in the organic solvent, DMSO,20 indicating that the fluorescent HOLO polymer can detect cephalexin in aqueous media as in the organic solvent used in polymer synthesis.
1 binding model, was 1.1 × 104 M−1 (Fig. 2). This value was comparable to that in the organic solvent, DMSO,20 indicating that the fluorescent HOLO polymer can detect cephalexin in aqueous media as in the organic solvent used in polymer synthesis.
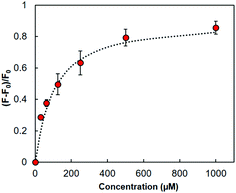 | ||
| Fig. 2 Changes in relative fluorescence intensity at 468 nm in HOLO(FSA)(MBA) upon the addition of cephalexin (λex = 365 nm). The correlation factor of the fitting curve (dashed line) was 0.98. | ||
The fluorescence of FSA depends on the protonation status of the carboxyl group on FSA,21 indicating that the fluorescence signalling properties of HOLO(FSA)(MBA) would be consequently changed. In fact, the initial fluorescence intensity (F0) of HOLO(FSA)(MBA) varied in the aqueous media with different pH values (Fig. S3, ESI†). The fluorescence signalling activity of HOLO(FSA)(MBA) towards cephalexin in aqueous media at various pH values was investigated (Fig. 3). Changes in the relative fluorescence intensity ((F − F0)/F0) in solutions of lower pH were greater than those in solutions of higher pH. These results indicated that a carboxyl group on FSA can interact with the amino group of cephalexin and the benzoic acid moiety proximal to the disulphide bond in the imprinted cavity and can form hydrogen bonds with the carboxyl group on cephalexin. These two complementary interactions synergistically contribute to a greater fluorescence. The apparent Ka in aqueous solution of pH 4 was calculated to be 4.3 × 104 M−1 and was four times higher than that in a solution of pH 7. However, at a higher pH, the ionised benzoic acid moiety in the imprinted cavity and the carboxyl group on cephalexin repel each other, resulting in a lower fluorescence. These results indicate that the designed nanocavity—consisting of a fluorescence signalling FSA moiety and a benzoic acid moiety and connected to the polymer backbone via a Schiff base and a disulphide bond—was successfully created in the polymer matrix.
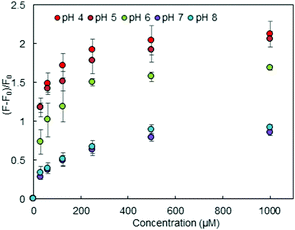 | ||
| Fig. 3 Changes in relative fluorescence intensity in HOLO(FSA)(MBA) on binding cephalexin in aqueous media at different pH values. | ||
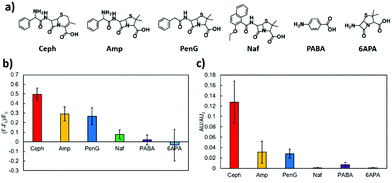
Selectivity tests were performed using structurally related compounds, including Amp, Pen G, Naf, PABA, and 6APA (Fig. 4a). The fluorescence of HOLO(FSA)(MBA) upon binding to cephalexin was greater than that upon binding to the reference compounds (Fig. 4b), revealing the existence of a selective fluorescence signalling cavity. Binding to Amp and PenG resulted in a slightly lower fluorescence intensity compared with that observed upon binding to cephalexin because the chemical structures of these two compounds are similar to that of cephalexin, except for the structure around the carboxy group. These results imply that HOLO(FSA)(MBA) can exhibit a structurally small difference. These sophisticated properties of MIPs have been reported earlier.22,23 However, Naf showed a lower fluorescence intensity because it contains a bulky naphthyl group and hampers binding to the nanocavity. PABA and 6APA showed negligible fluorescence because they are too small to bind to the nanocavity. The binding properties of HOLO(FSA)(MBA) were also found to be similar when examined using UPLC (Fig. 4c). These results indicated that the nanocavity created in HOLO(FSA)(MBA) can selectively bind to the target molecule, cephalexin, and transduce these binding events into changes in fluorescence.
We also examined the fluorescence signalling of HOLO(FSA)(MBA) in a diluted chicken extract sample. A chicken tender was purchased from a local supermarket and treated with acetone, an anion exchange resin, and a C18 column for the removal of fatty acids. The extract was diluted four times with an elution solution (MeOH/water = 1/1, v/v) and spiked with various concentrations of cephalexin (0–196 μM). As shown in Fig. 5, the changes in fluorescence were comparable to those in the control solution (MeOH/water = 1/1, v/v) and the recovery range for cephalexin was 86.3–101%, indicating the feasibility for the use of the nanocavity for specific detection of antibiotics in a complex sample. The fluorescence responses of the HOLO polymer presented a linear calibration range of 0–196 μM with r2 = 0.972 (Fig. S4, ESI†). The limit of detection of cephalexin for the developed fluorescent sensor was calculated as 3SD/m, where m is the slope of the linear range of the calibration curve and SD is the standard deviation for 0 μM cephalexin. The limit of detection was calculated to be 18 μM (1.3 ppm). However, a decrease in the fluorescence signalling ability was observed when an anion exchange resin step was excluded. This indicates that residual fatty acids interfered with the binding of cephalexin to the HOLO polymer (Fig. S5, ESI†). These undesired, non-specific binding events could be reduced by incorporating hydrophilic monomers, such as 2-methacryloyloxyethyl phosphorylcholine24 and oligo ethylene glycol based monomers.25
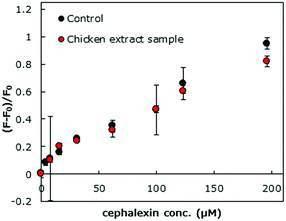 | ||
| Fig. 5 Changes in the fluorescence emitted by HOLO(FSA)(MBA) upon binding with cephalexin in the control medium (MeOH/water = 1/1, v/v) and diluted chicken extract samples. | ||
Conclusions
A fluorescence signalling nanocavity for specific detection of antibiotics in aqueous media was successfully demonstrated, wherein fluorescence-based reporter prosthetic groups and interacting groups were site-selectively introduced together in an APO-type imprinted cavity via Schiff base formation and a disulphide exchange reaction, respectively. The resultant HOLO(FSA)(MBA) could transduce the target antibiotic cephalexin binding events into changes in fluorescence with high selectivity. Furthermore, high recovery rates of cephalexin were observed in cephalexin-spiked chicken extract samples. These results expand the proposed conjugated protein mimic system to complex aqueous media. Therefore, this easy and specific sensing material would be a powerful tool for analysing residual target molecules in complex media, such as food extracts, river water, blood, and urine.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by JSPS KAKENHI (Grant Numbers: 24651261, 15K14943, 16K18300, 19K16342). We also appreciate the financial support provided by System Instruments Co., Ltd. (Tokyo, Japan).References
- M. S. Butler and D. L. Paterson, J. Antibiot., 2020, 73, 329–364 CrossRef CAS PubMed.
- A. Fleming, Br. J. Exp. Pathol., 1929, 10, 226–236 CAS.
- C. A. Michael, D. Dominey-Howes and M. Labbate, Front. Public Health, 2014, 2 Search PubMed.
- S. Ahmed, J. Ning, G. Cheng, I. Ahmad, J. Li, L. Mingyue, W. Qu, M. Iqbal, M. A. B. Shabbir and Z. Yuan, Talanta, 2017, 166, 176–186 CrossRef CAS PubMed.
- S. Joshi, J. Pharm. Biomed. Anal., 2002, 28, 795–809 CrossRef CAS PubMed.
- E. N. Evaggelopoulou and V. F. Samanidou, Food Chem., 2013, 136, 1322–1329 CrossRef CAS PubMed.
- V. F. Samanidou, D. E. Giannakis and A. Papadaki, J. Sep. Sci., 2009, 32, 1302–1311 CrossRef CAS PubMed.
- J. Peng, G. Cheng, L. Huang, Y. Wang, H. Hao, D. Peng, Z. Liu and Z. Yuan, Anal. Bioanal. Chem., 2013, 405, 8925–8933 CrossRef CAS PubMed.
- A. Mehlhorn, P. Rahimi and Y. Joseph, Biosensors, 2018, 8, 54 CrossRef CAS PubMed.
- P.-H. Chan, H.-B. Liu, Y. W. Chen, K.-C. Chan, C.-W. Tsang, Y.-C. Leung and K.-Y. Wong, J. Am. Chem. Soc., 2004, 126, 4074–4075 CrossRef CAS PubMed.
- H. Youn, K. Lee, J. Her, J. Jeon, J. Mok, J.-I. So, S. Shin and C. Ban, Sci. Rep., 2019, 9, 7659 CrossRef PubMed.
- Y. Yang, G. Ren, W. Yang, X. Qin, D. Gu, Z. Liang, D.-Y. Guo and P. Qinhe, Polyhedron, 2021, 194, 114923 CrossRef CAS.
- M. Komiyama, T. Mori and K. Ariga, Bull. Chem. Soc. Jpn., 2018, 91, 1075–1111 CrossRef CAS.
- T. Takeuchi, T. Hayashi, S. Ichikawa, A. Kaji, M. Masui, H. Matsumoto and R. Sasao, Chromatography, 2016, 37, 43–64 CrossRef CAS.
- K. Haupt and K. Mosbach, Chem. Rev., 2000, 100, 2495–2504 CrossRef CAS PubMed.
- T. Takeuchi and H. Sunayama, Chem. Commun., 2018, 54, 6243–6251 RSC.
- N. Tarannum, O. D. Hendrickson, S. Khatoon, A. V. Zherdev and B. B. Dzantiev, Crit. Rev. Anal. Chem., 2020, 50, 291–310 CrossRef CAS PubMed.
- E. Kweinor Tetteh, S. Rathilal, M. Amankwa Opoku, I. D. Amoah and M. N. Chollom, in Advanced Antimicrobial Materials and Applications, ed. Inamuddin, M. I. Ahamed and R. Prasad, Springer Singapore, Singapore, 2021, pp. 393–421 Search PubMed.
- T. Takeuchi, T. Mori, A. Kuwahara, T. Ohta, A. Oshita, H. Sunayama, Y. Kitayama and T. Ooya, Angew. Chem., Int. Ed., 2014, 53, 12765–12770 CrossRef CAS PubMed.
- H. Sunayama, T. Ohta, A. Kuwahara and T. Takeuchi, J. Mater. Chem. B, 2016, 4, 7138–7145 RSC.
- I. P. Pozdnyakov, A. Pigliucci, N. Tkachenko, V. F. Plyusnin, E. Vauthey and H. Lemmetyinen, J. Phys. Org. Chem., 2009, 22, 449–454 CrossRef CAS.
- J. Matsui, Y. Miyoshi, O. Doblhoff-Dier and T. Takeuchi, Anal. Chem., 1995, 67, 4404–4408 CrossRef CAS.
- K. Takeda, A. Kuwahara, K. Ohmori and T. Takeuchi, J. Am. Chem. Soc., 2009, 131, 8833–8838 CrossRef CAS PubMed.
- K. Ishihara, J. Biomed. Mater. Res., Part A, 2019, 107, 933–943 CrossRef CAS PubMed.
- J.-F. Lutz, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3459–3470 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tb00145d |
| This journal is © The Royal Society of Chemistry 2022 |