Engineering surface patterns on nanoparticles: new insights into nano-bio interactions
Boyang
Hu†
ab,
Ruijie
Liu†
c,
Qingyue
Liu†
ac,
Zi'an
Lin
b,
Yiwei
Shi
b,
Jun
Li
b,
Lijun
Wang
ac,
Longjie
Li
*ac,
Xianjin
Xiao
 *c and
Yuzhou
Wu
*c and
Yuzhou
Wu
 *b
*b
aSchool of Life Science and Technology, Wuhan Polytechnic University, Wuhan 430023, China. E-mail: lilongjie@whpu.edu.cn
bHubei Key Laboratory of Bioinorganic Chemistry and Materia Medica, Hubei Engineering Research Center for Biomaterials and Medical Protective Materials, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan 430074, China. E-mail: wuyuzhou@hust.edu.cn
cInstitute of Reproductive Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China. E-mail: xiaoxianjin@hust.edu.cn
First published on 7th February 2022
Abstract
The surface properties of nanoparticles affect their fate in biological systems. Based on nanotechnology and its methodology, pioneering studies have explored the effects of chemical surface patterns on the behavior of nanoparticles and provided many new insights into nano-bio interfaces. In this review, we would like to provide a summary of how the nanoparticle surface pattern modulates its biological effects. The relationship between the surface pattern of nanoparticles and the generated interaction with cell membranes, recognition of viruses and adsorption of proteins was discussed. On this basis, we believe that a reasonable design of the surface microstructure will promote the application of artificial nanoparticles in biomedicine and provide a new strategy for improving the design of nano-drug carriers.
1. Introduction
The surface properties and patterns of nanoparticles significantly affect their interactions with biomolecules, cells and microorganisms.1–4 Nanoparticles with a positively charged or negatively charged surface display distinct cell uptake efficiency and tissue distribution, while the hydrophilicity of nanoparticles also determines their blood circulation, tissue penetration and protein corona adsorption.5 In most studies, the surface chemical properties of synthetic nanoparticles are often presumed to be homogenous. However, in nature, the biorecognition between biomolecules and nanostructures are obviously more complicated compared to synthetic systems. The surface patterns of recognition partners perfectly match with each other with multiple interactions involved, which is essential for the extraordinary specificity.6–9Recently, the advancement of nanofabrication methods allowed creation of nanoparticles with heterogeneous surface patterns, which provide opportunities for fine-tuning and a deeper understanding of nano-bio interactions. The engineered surface patterns starting from “Janus” particles with two phases separated have been further developed to multiple patches with desired geometry and even regular strips.3,10 The recent emerging DNA nanotechnology even enabled precise surface pattern design at pre-encoded positions.7,11–13 Based on these techniques, pioneering studies have explored how chemical surface patterns would influence the nanoparticle behavior in biological systems and provided numerous new insights into the nano-bio interface. These findings are crucial for promoting the biomedical applications of nanoparticles and provided new strategies to improve the design of nanocarriers.3,14–16
In this review, by merging the most recent developments in both nanofabrication strategies to create fine surface patterns and the biological understanding of surface pattern driving biorecognition, we intend to provide more perspectives on how to design surface patterns on nanoparticles to tune their biological effects. The nanoparticles discussed herein are less than 1 μm in diameter considering their potential in nanomedicine, and the surface patterns refer to more than two distinct chemical compositions regularly arranged on the particle surface instead of the statistical mix. The synthetic methods will be firstly summarized, and the relation between surface patterns on nanoparticles and the resulting cell membrane interactions, protein corona adsorption and virus recognition would be further discussed in detail (Scheme 1). We believe that the current knowledge in this field is just a prelude, and the limitations and further potential will be particularly deliberated.
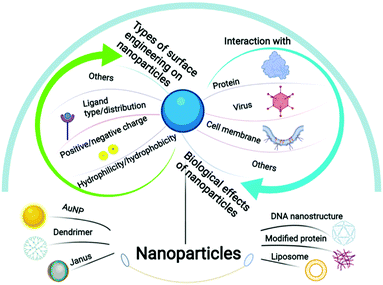 | ||
| Scheme 1 Schematic illustration of the species, types of surface engineering and the biological effects of nanoparticles covered in this review. | ||
2. Biorecognition of natural molecules and artificial nanoparticles
2.1 Mechanism of biorecognition in nature
Accurate recognition between biological molecules is the basis for the development of organisms. Both general and specific interactions occur among many important biological macromolecules, including polysaccharides, proteins, enzymes, and nucleic acids. Intercellular recognition can be achieved by selectively combining surface molecules with the corresponding active substances or signalling molecules, such as protein ion channels, and recognition between antigens and antibodies.17–20 Macrophages recognise aging cells through “eat me” and “don’t eat me” signalling molecules. T cells recognise tumour cells through T cell receptors (TCR), tumour antigens, and MHC molecules. Lipopolysaccharides (LPS) induce inflammatory responses in immune cells by interacting with Toll-like receptor-4 (TLR-4). Therefore, the interactions between biological macromolecules (e.g., proteins, lipids, and polysaccharides) provide a molecular basis for the recognition and cognition of organisms.21–23In nature, the first step of mutual recognition between biomolecules is the structural complementarity of the binding sites of the two molecules, and the second is the generation of sufficient chemical forces between the binding sites. Berger and Milstein et al. found that conformational selection is one of the reasons for the formation of antigen–antibody complexes.24–26 Molecules undergoing molecular recognition exist in the form of conformational complexes. When the ratio of ligands bound by preferentially recognized molecular conformation is reduced, other molecular conformations will change to preferentially bound molecular conformations.24 Chemical forces are mainly van der Waals, hydrogen bond, hydrophobic and electrostatic forces, which arise from the interaction between various conformations.25 Additionally, the charge will affect the recognition and binding of natural biomolecules to a certain extent, such as homogalacturonan (HG) with a low degree of esterification (DE) and chitosan—chitosan with a positive charge in the spatial structure can interact with the carboxyl groups on HG with a low DE.27 Hydrophilicity will also have a certain impact. Common carbohydrates can form a certain hydrophilic or hydrophobic region due to their functional groups and then interact with biomolecules through hydrophilic forces (hydrogen bonds) or hydrophobic forces (hydrophobic bonds). For example, Zeng et al. found that azides, alkynes, ketones or aldehydes can interact with glycoproteins on the cell surface.28,29 Moreover, the interaction between biomolecules and cells also relies on chemical forces such as hydrogen bonds and van der Waals forces. The polysaccharides in the cell wall rely on these weak interactions to arrange tightly, limiting the accessibility of the antibodies to the epitopes. The charged molecules can produce strong polar interactions with the phage, thereby affecting the binding of the antibody to the phage capsid protein in the phage display.25 Cell–cell interactions, such as cell fusion and cell adhesion, are essentially the result of the regulation of cell membranes through chemical forces. Myoblast fusion is a multi-step process involving cell recognition and adhesion, actin cytoskeletal rearrangements, fusogen engagement, lipid mixing and fusion pore formation, ultimately resulting in the integration of two fusion partners.30 This process involves the interaction of many biological molecules such as cell adhesion molecules, adaptor proteins, vesicle trafficking proteins and lipids.31 During the membrane fusion process, hydrophilic lipid heads with the same charge will repel each other due to electrostatic forces.32 Many life activities in nature are closely related to this, such as virus infection, cell migration, vesicle transportation, and fertilization.33
Due to the different chemical properties and physical states of different proteins and lipids on the membrane, based on hydrophobic interactions, electrostatic interactions, van der Waals forces, hydrogen bonds and hydration forces, the membrane surface will form a variety of synergistic lipid raft–protein structure domains (surface pattern). These surface patterns are critically important in mediating life activities.22 For example, cholesterol-rich lipid domains play an important role when Francisella recognizes and infects host cells.34 The lipid–protein pattern formed by the recruitment of the liganded GPI (glycosylphosphatidylinositol)-anchored receptor is very important in specific downstream signal transduction.35 The internalization process mediated by caveolin-1 is also inseparable from the formation of lipid raft-related patterns.36 In conclusion, although the mechanism of lipid domains on membranes to mediate cell functions needs further research, it is undeniable that the formation of these surface patterns occupies an important position in mediating recognition, signal transduction, pathogen invasion and other functions.
2.2 Interaction of nanoparticles with biological systems and the effect of their surface properties
The effect of nanoparticles on biological systems is mainly achieved through the interaction between nanoparticles and cells. The outer interface of human cells is primarily composed of bilayer lipid molecules with saccharides and proteins. Under natural conditions, biological macromolecules can activate many downstream reactions through interactions with cells, which maintain the basic functions of living organisms. Similarly, the interaction between artificial nanoparticles and cell membranes is important for achieving specific functions of nanoparticles. The interaction between nanoparticles and proteins results in the formation of a protein corona, which can cause a downstream effect.37 Nanoparticles can be internalised into cells via receptor/non-receptor-mediated pathways, in which a variety of enzymes and proteins are activated, including guanosine diphosphate (GDP), guanosine triphosphate (GTP), and heat shock proteins (HSP).38 The high endocytosis efficiency of these molecules contributes to their potential application in drug delivery.The surface properties of nanoparticles play a pivotal role in their interaction with cells. Researchers have paid much attention to the effects and related mechanisms of the surface properties of nanoparticles on their interaction with biological systems. Krishnendu et al. found that the formation of a protein corona is closely related to the hydrophilicity and hydrophobicity of nanoparticles, and that the modulation of the surface hydrophobicity of the nanoparticles can effectively reduce the recognition of plasma proteins by macrophages.39 In addition, when interacting with a lipid bilayer membrane, hydrophilic nanoparticles tend to be distributed outside the membrane, while hydrophobic nanoparticles can be embedded inside and can even cause defects on the membrane, leading to bending, budding, or fission.40–43 Surface charge also is a determining factor in the cellular uptake process of nanoparticles. Stronger attraction exists between negatively charged membranes and positively charged nanoparticles, which can enhance the turnover and tilt of phospholipid molecules. Therefore, in many cases, the uptake increases with the augmenting magnitude of charges.4,44 Despite this, negatively charged nanoparticles can accumulate in the serum to a larger extent due to the relative reduction in charge-selective filtration, which results in the drug carrier having a longer retention time.45
Besides, the surface chemistry of nanoparticles (ligand type and ligand distribution) is an important factor affecting their interaction with the cell membrane. Moreover, inspired by the natural spike patterns on virus capsids, researchers synthesize nanoparticles with the corresponding ligand distribution, thus facilitating the interaction with a bio-interface (e.g. higher uptake efficiency achieved by symmetric distribution).46 Since Janus nanoparticles contain heterogeneous regions, they can induce unique biological reactions, which have been widely used in drug delivery47 and biological imaging.48 Therefore, understanding the interaction between nanoparticles and biological interfaces and the effect of the surface patterns of nanoparticles on this interaction is of high significance for the application of nano-drugs.
2.3 Surface engineering on nanoparticles and the biological effect of such modifications
Nanoparticles interact in vivo with cells and biomolecules to produce a “nano-bio interface”. This plays a key role in the reaction of nanoparticles in biological systems. It is widely recognised that the surface properties of nanoparticles are closely related to the “nano-bio interface”. Therefore, surface engineering has emerged to change their surface properties.Surface engineering refers to the use of physical and chemical methods to change the surface chemical properties of particles (functional group structure, electrical, hydrophobic, and hydrophilic). In general, there are two main strategies for surface engineering: “top-down” and “bottom-up”. In the former method, various three-dimensional structures can be easily obtained by using etching technology, but the cost is high, and it is difficult to directly adjust the distance between atoms. The latter emphasises the use of smaller structural units for assembly, such as self-assembly technology with a high yield, and is the current trend in surface engineering. Based on the concept of “bottom-up” surface engineering, many chemical synthesis methods have been applied to the surface engineering of nanoparticles. The ligand-exchange method and ligand-coating method are the two most common methods used to change the surface chemistry. The former involves the substitution of ligands, which can change the nanoparticles from hydrophobic to hydrophilic, which is conducive to their participation in human body transport.49,50 However, the latter focuses on implementing some groups on the nanoparticle surface. For instance, Hauck et al. assembled polyelectrolytes on the nanorod surface in a layer-by-layer manner.51 Furthermore, the stability of nanoparticles can be maintained by surface engineering. To avoid the arbitrary absorbance by proteins, polyethylene glycol (PEG) is one of the best ligands for surface decoration. Moreover, the attachment of PEG significantly decreases the absorbance because of hydration and steric hindrance.52 However, PEG has problems such as low service life caused by oxidation in the biological environment and the blood clearance effect caused by the immune system response. Zwitterionic ligands can bind water molecules effectively via electrostatic induced hydration, and thus the zwitterionic surface can effectively prevent protein adsorption and avoid the dilemma of PEG. Mixed-charge monolayers have been designed on the surface of nanoparticles by various methods, which expand the application of zwitterionic ligands.53,54 To induce specific reactions in organisms, the corresponding ligands can be linked by covalent or non-covalent modifications to a desired location of the already modified nanoparticles.55,56 Therefore, precise control of the pre-modification of these nanoparticles is essential. A monolayer can also be coated on nanoparticles, contributing to stable and multifunctional NPs because of the simultaneous use of stable and functioning ligands.57,58 More importantly, these methods can be used in combination to allow the nanoparticles to respond to specific stimuli in the organism.2
Although many surface engineering methods are proposed, it is still really challenging to create the fine pattern on nanoparticles, which would rely on well-developed characterization techniques and precise control of the activities, positions and density of the functional groups.
3. Fine fabrication of surface patterns on nanoparticles
In this review, we mainly consider AuNPs and dendritic polymers as model particles to analyse how their surface patterns affect their interactions with cell membranes, proteins, and viruses since they are mostly investigated at present. To form accurate surface patterns on these nanoparticles, researchers usually adopt (de)protection methods and precisely control the attributes of the core and the attached ligands. At the same time, as the modifications on emerging DNA nanomaterials are easily regulated, we will also use them as a model to discuss the interaction between ligands and receptors.3.1 Construction of AuNPs with different substructures on their surfaces
AuNPs are particles with a core of gold and a radius of 1–100 nm. For decades, many methods have been proposed for their synthesis, and the shape59 and size60 of the AuNPs can be well controlled. Currently, researchers can obtain nanoparticles of the desired size and shape.61–64 In order to exert biological effects, AuNPs can be modified in many ways to serve as a platform for linking nucleic acids, proteins, and their interactions with biological molecules or membranes, allowing them to be used for detection and drug delivery.65,66 Sulphur-containing groups have been vastly used in the synthesis of gold nanoparticles because of their high affinity to gold and their ease of modification. Since the last century, researchers have developed various methods to synthesize gold nanoparticles,67,68 among which the Brust–Schiffrin method, which can easily control the size and shape of the nanoparticles, is commonly used.69,70Based on this, one of the most representative methods for further surface engineering is to add a mixed self-assembled monolayer (SAM) to a gold nanoparticle surface via (1) co-absorption or (2) thiol-mediated ligand substitution. This pioneering work was conducted by the Stellacci group. In 2004, they first introduced monolayer-protected gold nanoparticles with phase-separated ordered domains—the first striped-pattern nanoparticles. In their experiment, an ordered domain could be easily tailored by adjusting the radius (curvature) of the core and the octane thiol/mercaptopropionic acid ratio. In addition, they concluded that this pattern results from the thermodynamic equilibrium state, resulting in its good stability.71 Subsequently, they made significant progress in synthesizing a series of other gold nanoparticles with various properties, including binary SAM gold nanoparticles, in which two opposite ligand domains are formed (Fig. 1a).72–75 Moreover, the differences between “striped” and “binary” can be explained by the compatibility of the ligands—compatibility leads to a striped pattern, whereas the other leads to “binary”. Based on these observations, they proposed a theory that micro-phase separation is critical to determine the final morphology. For striped gold nanoparticles, the increase in entropy by conformational changes is of high importance. Only when it overcomes the penalty of enthalpy by complete phase separation, a striped pattern occurs, heavily relying on the particle size, i.e. a small radius would give rise to the formation of Janus rather than a striped pattern.75,76
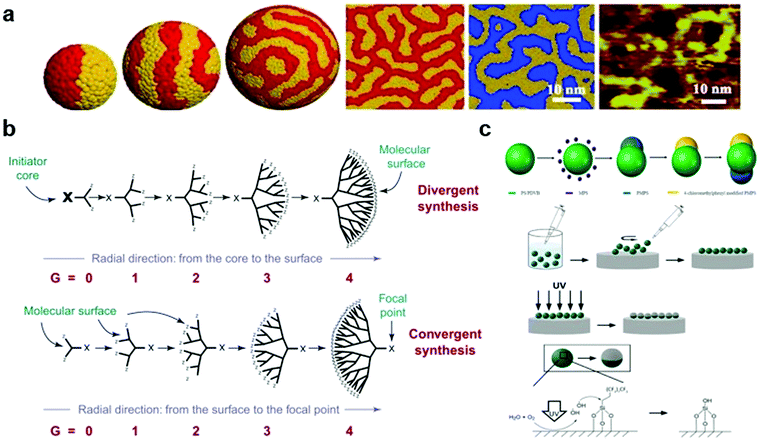 | ||
Fig. 1 (a) Mesoscale simulation results of a fixed length ratio (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) on the surface of gold nanoparticles with different degrees of curvature. (b) Two principles of synthetic methods (divergent synthesis and convergent synthesis) for constructing dendritic macromolecules (dendrons). (c) Illustration of the synthesis of triblock Janus particles by seeded emulsion polymerization and UV irradiation. Reproduced with permission from ref. 75, 16 and 95, copyright (2007) American Physical Society, (2001) Elsevier and (2019) American Chemical Society. 7) on the surface of gold nanoparticles with different degrees of curvature. (b) Two principles of synthetic methods (divergent synthesis and convergent synthesis) for constructing dendritic macromolecules (dendrons). (c) Illustration of the synthesis of triblock Janus particles by seeded emulsion polymerization and UV irradiation. Reproduced with permission from ref. 75, 16 and 95, copyright (2007) American Physical Society, (2001) Elsevier and (2019) American Chemical Society. | ||
To endow gold nanoparticles with a certain function, they can be linked with (bio)polymers. Their preparation strategies can be divided into three types: (1) grafting from—polymerization on the particle surface during their synthesis, (2) grafting to—particles are synthesized and mixed with polymers, and (3) post-synthetic modifications. To stabilize the particles, inorganic polymers, such as PEG and PVA (polyethyleneimine), are conjugated to gold nanoparticles mostly via grafting from/to.77,78 Biomolecules are commonly conjugated to gold nanoparticles through post-synthetic modifications. Gold nanoparticles can be easily prepared using amino acids through the formation of Au–S bonds. Combined with peptides or metal ions, they have been applied in biosensing and molecular computing.79,80 Similarly, DNA undergoing the thiolate process can directly bind to gold nanoparticles aiming for diagnostics and the following construction of a nanodevice.81,82
However, observation of the dynamic process of surface pattern formation can be hardly achieved. Moreover, it is challenging to construct gold nanoparticles with a specific number of ligands, which hinders the preparation of a desirable nanopattern. Moreover, it is relatively simple to exert delicate control on the conjugation of biomolecules to gold nanoparticles. This requires the development of methods to control the thiol activity and improvements in dynamic characterization techniques of nanoparticles.
3.2 Construction of dendrimers with different substructures on their surface
Dendrimers are a family of nanosized three-dimensional polymers characterised by unique dendritic branching structures and compact spherical geometries. Their names come from the Greek word “dendron”, which means “tree”, and refer to the unique organisation of polymer units.83 Dendritic polymers are obtained through an iterative cycle reaction, and one layer of molecules is added in each iterative cycle. There are two main strategies for synthesising dendritic polymers (Fig. 1b).16 The first is the divergence method,84 in which the growth of dendritic units originates from the core site (root). This method assembles monomer modules into radial and branched motifs according to certain dendritic rules and principles.85 The second is the convergence method,86 which starts from the molecules that are about to become the surface of the branch units (that is, from the leaves of the molecular tree) and reaches the root reaction core inward. This requires that a single reaction branch unit is formed first, and then multiple branch units react with the multifunctional core to obtain the dendritic polymer structure.16Polyphenylene dendrimers (PPDs) are unique members of the dendrimer family, whose main chain is mainly composed of substituted benzene rings.87,88 PPDs have a high extent of branching, rigidity and monodispersity. The structure of a PPD contains three levels: core, scaffold, and surface. Compared with other dendrimers, PPDs are more interesting because of their rigidity and non-conformational rearrangement. Dendrimers generally have flexible dendrimers with arms, which makes dendrimers easily undergo conformational rearrangement. Therefore, the surface of dendrimers is obscured by special functionalized sites and cannot function, which is not conducive to practical applications. Because the rigid main chain replaced by the benzene ring endows the PPDs with shape durability, when the core, scaffold, and special sites on the surface are chemically modified and functionalized on the nanoscale, there is no concern about the target properties of the molecule losing effect due to the negative influence brought about by conformational rearrangement.89,90 The shape-persistence feature reveals additional possibilities for PPD applications in areas such as biological research, drug transport, and interface interactions.
The surfaces of PPDs can be finely regulated or modified to have two or more different characteristics, namely, the formation of patched dendrimers with different characteristics on the surface. For example, amphiphilic PPDs have both hydrophobic and hydrophilic functional groups on their surfaces. These amphiphilic PPDs play an important role in the interaction with proteins, cells, or viruses, and they exhibit low cytotoxicity in vitro and in vivo.91 PPDs maintain a unique spherical structure in that solution, and a lipophilic cavity with a specific size is formed inside, which is a protein-like structure and can simulate substances such as human serum protein (HSA) to make “artificial proteins”,91 suggesting that PPDs can be potential carriers of lipophilic drugs. Highly branched, persistent-shaped, monodisperse PPDs consist of a core, a scaffold, and a surface. The core controls and determines the number and geometry of the dendrimers of macromolecules.92,93 Building blocks control the chemical functionalization and properties of the scaffold and surface. The difference in the core or building block type affects the shape and properties of the dendrimer.
PPDs have a rigid, designable surface and a special lipophilic cavity inside, and can interact with cell membranes and viruses. These characteristics provide a broad prospect for the research and application of PPDs. Through the Diels–Alder cycle addition reaction, researchers can prepare various PPDs with different shapes and surface characteristics to explore the interaction between nanoparticles and biological interfaces.
3.3 Construction of “Janus” nanoparticles
Since the concept of Janus nanoparticles (NPs) was first proposed in 1991, research on them has become a hot topic in the field of biochemistry.94 Janus NPs, named after a double-faced Roman God, can be defined as particles with a fine structure consisting of two or more components with different (usually opposite) physical and chemical properties. Due to their unique optical and magnetic properties, Janus NPs have high potential for biopharmaceutical and imaging applications. Similarly, there are two preparation strategies for Janus NPs, “additive” and “subtractive” (Fig. 1c).95 The “additive” strategy is commonly used, including in self-assembly, seed emulsion polymerisation, and phase separation. In the field of self-assembly, one of the pioneering studies was conducted by Muller's research group. In 2001, synthetic “Janus micelles” were first synthesised by Müller using self-assembly technology. His approach of solution-casting–cross-linking–redissolution was relatively simple and laid the foundation for the development of self-assembly technology.96 Thus, a wide variety of Janus particles can be synthesised in a controlled manner via self-assembly.3,97,98 Recently, some “subtractive” methods have been proposed. UV-etching is considered one of the best “subtractive” methods because of its versatility and environmental friendliness. Recently, Chen's research group has made a series of advances in the synthesis of Janus NPs by UV cleavage.99–101 In 2021, they proposed a simpler two-step synthetic method for preparing Janus NPs. They first synthesised a particle film at the gas–solid interface and then placed the film directly under an ultraviolet light source for photodegradation. In a short time, they achieved a coverage rate of 43.49% in the film.102 However, the subtractive method is currently not mature enough, and further studies are encouraged to achieve a high-yield synthesis of Janus particles of interest.3.4 Molecular precision surface engineering by bottom-up synthesis
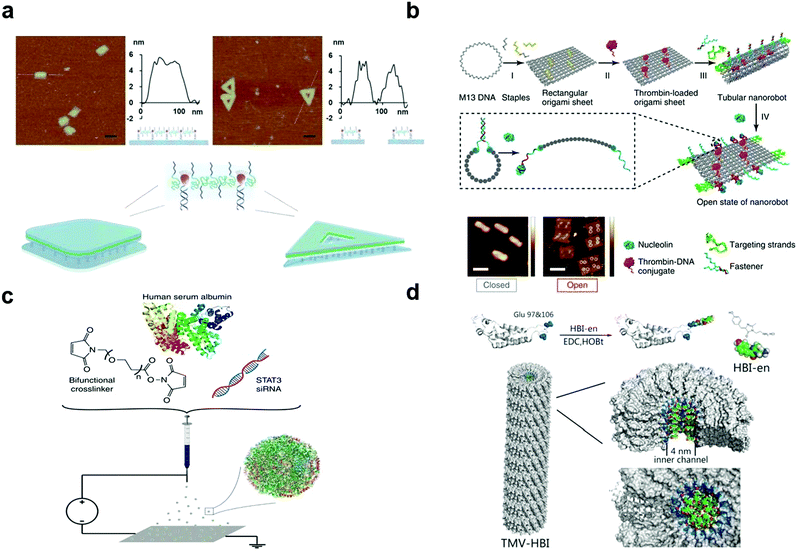 | ||
| Fig. 2 (a) Illustration of the 2D assembly of amphiphilic molecules via a frame-guided process. (b) Scheme of the construction of the DNA origami attached by thrombin. (c) Schematic illustration of the jetting formulation for crosslinked, STAT3i-loaded, iRGD-conjugated, targeted albumin NPs (STAT3iSPNPs). (d) Coupling of HBI-en chromophore and TMV capsid protein with glutamate residues 97 and 106 emphasized (blue). Reproduced with permission from ref. 114, 117, 121 and 123, copyright (2018) Springer Nature, (1996) The Royal Society of Chemistry, (2020) Wiley Online Library and (2020) Springer Nature. | ||
Adenovirus (Ad) is the most frequently used gene transfer vector in clinical trials, and to achieve its clinical applications, various methods have been proposed. There are mainly three biological (genetic) methods to engineer its surface pattern. (1) Insertion of antigenic epitopes in the adenovirus fibre protein is mainly realized by adding targeting ligands to an adenovirus fibre knob, for example, RGD modified adenovirus can bind to integrin.124 In 2015, Hanni succeeded in attaching the epidermal growth factor to the epidermal growth factor receptor for circumventing pre-existing Ad5 immunity in clinical populations.125 (2) The second method is switching the serotype of adenovirus, such as transforming the fibre portion of Ad5 into that of Ad6/Ad35.126,127 (3) The third method is the removal of the liver targeting fibre protein. In this method, Ad can be more biocompatible. For example, Leissner et al. artificially enabled the mutation of an AB loop(S408E) on Ad5, which then lost its ability to bind to cellular receptors.128 Moreover, the mutation of the KKTK motif to GAGA can lead to its inability to bind heparan sulphate proteoglycan.129
Predominantly, there are three methods to chemically engineer the virus surface pattern. (1) Similar to gene modification, attaching polymers with ligands chemically contributes to the specific function of adenovirus (e.g. through interactions with the target tumour tissues).130,131 (2) Conjugation of positive polymers or lipids is a common way to promote adenovirus entry into a membrane. Given the increased electrostatic interactions, the internalization process does not rely on the mediation of a cellular receptor.132,133 (3) As different parts of an organism have unique circumstances, it is also our goal to develop virus-based nanoparticles that can respond to specific surroundings. Considering the relatively acidic microenvironment in tumour tissues, hybridization with pH-responsive polymers enables controllable release only at the tumour site.134,135
3.5 Techniques for the characterization of the nanoparticle surface
To further investigate the surface properties of nanoparticles and to broaden the application prospects of nanoparticles, techniques are needed to characterize the surface patterns on nanoparticles. The attachment of ligands to nanoparticles and the interaction between the whole nanoparticles and their targets are the focus of the nanoparticle field. Some basic methods have been attempted to roughly characterize the surface of nanoparticles. BET (Brunauer–Emmett–Teller) techniques involve a type of gas absorption experiment and have been employed for the characterization of surface area and porosity, indicating the type of biomolecular interaction that follows (e.g. a high surface area and porosity tend to form a protein corona).136 Zeta potential and electrophoretic mobility can provide basic information on the electrical properties of the nanoparticle surface, but they cannot be used to characterize their microscopic charge distribution.137 Nuclear magnetic resonance (NMR) spectroscopy serves as a general tool to characterize ligand density and ligand structures, although it is limited by its sensitivity (Table 1).138| Surface characterization techniques | Usage | Advantages | Disadvantages |
|---|---|---|---|
| BET | Surface area | Rapid and simple | Provides relatively rough information |
| Zeta potential | Electrical properties | Can partially determine the stability of NPs | Cannot provide the detailed charge distribution |
| NMR | Properties of ligands | Relatively comprehensive methods to characterize NPs | Low sensitivity, not suitable for metal NPs which induce changes in local magnetic fields |
| IR methods | Properties of ligands | High sensitivity, merely no restriction on the types of NPs | Qualitative, not quantitative |
| MALDI-TOF | Properties of ligands | Rapid, sensitive, low cost | Mainly used on protein (peptide) related structures |
| AFM/SEM/TEM | Size, shape and morphology | Visualized information, very sensitive | Expensive and laborious |
| HPLC/MS | Linkages between NPs and ligands | Qualitive methods to determine chemical compositions | Involves additional procedures |
| MD | Folded structures of dendrimers | Accurate when combined with other characterization methods | High computational cost |
To provide more detailed information about surface patterns, electron microscopy and atomic force microscopy (AFM) are the main methods proposed thus far. Previous studies have used electron microscopy (transmission electron microscopy) or scanning electron microscopy to elaborately characterize the morphology and growth kinetics of nanoparticles.139,140 AFM, based on the interaction between the probe and the sample, can also directly provide visualized information about nanoparticles.141 These methods have been widely used in studies about origami, dendrimers, etc. to confirm the correct formation of substructures.
In addition, to further characterize surface patterns, infrared techniques with a high sensitivity have been recognized as versatile methods to study the functional groups on nanoparticles. For example, IRRAS (infrared reflection absorption spectroscopy) analyses thin films mainly by a combination of transmission and reflection IR spectroscopy in a mixed mode to achieve high resolution,142 although IR based methods are mainly qualitative not quantitative. Furthermore, elemental analysis can provide information on the elemental composition of surface ligands.143 HPLC/MS (high-performance liquid chromatography/mass spectrometry) can quantitatively characterize the number of nanoparticle surface linkages, which requires some additional procedures before to break the linkages (e.g., Au–S breakage by iodine cleavage).144 As dendrimers are highly branched, MD (molecular dynamics) simulation has been utilized to analyse these complex structures. It can determine the spatial array of branch points and the molecular surface area; what is more, it can provide more convincing results about these complex highly folded conformations when combined with SAXS (small angle X-ray scattering).145 In addition, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectroscopy has been widely applied to assess the ligand labelling efficiency to a virus capsid.146 Moreover, its analysis is based on the comparison between its mass spectrum and the reference in the database.
4. Surface patterns of nanoparticles affect their interaction with the cell membrane
4.1 Structure of the cell membrane: mesoscale domain organization of the plasma membrane
The cell membrane is composed of lipids, proteins, and sugars, which constitute the outermost layer of the cell.21 The cell membrane plays an important role in maintaining cell stability, energy conversion, information transmission, and material transportation. The study of cell membranes has a long history. Among the studies, the membrane fluid mosaic model proposed by Singer and Nicholson in 1972 was supported by ample experimental evidence and has greatly impacted our understanding of cell membranes.147 With the development of experimental technology and in-depth research, researchers have found that not all membrane proteins on the cell membrane are free to move. A large number of facts and experimental results17,148 have proved that the diffusion phenomena of membrane molecules such as transmembrane (TM) proteins, glycosylphosphatidylinositol (GPI)-anchored proteins, and phospholipids are dependent on the regional features, time scale, and actin–base membrane skeleton interaction. This phenomenon is known as hop diffusion.23,149 Based on these features of hop diffusion, researchers have proposed the anchored membrane protein picket model (Fig. 3a and b).18,35 In this model, TM actin-binding proteins were aligned along the membrane skeleton and anchored by underlying cortical actin (CA) filaments, thus forming fences, or pickets, which could effectively block the diffusion of relevant membrane molecules.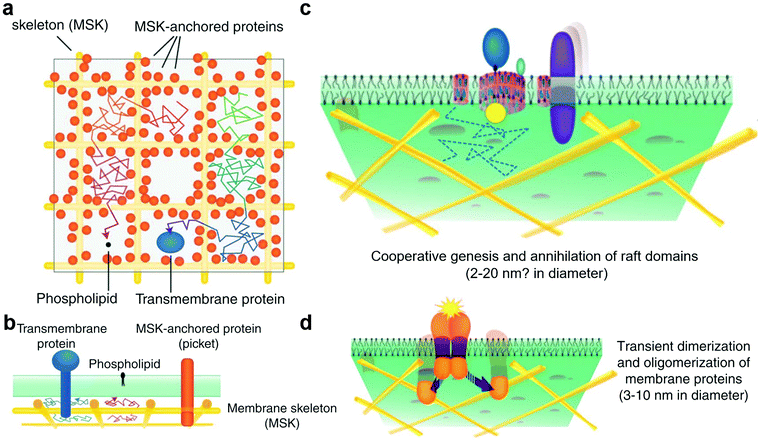 | ||
| Fig. 3 (a) Top view of the anchored membrane protein picket model and membrane compartment. (b) Side view of the anchored membrane protein picket model and membrane compartment. (c) In the raft domain, the size of cholesterol is limited by the membrane compartment. (d) Protein clusters form on the membrane and then disaggregate. Reproduced with permission from ref. 17, copyright (2011) Elsevier. | ||
The cytoplasmic membrane may be divided into three levels on the mesoscale: membrane compartment, raft domain, and protein cluster.150 The mesoscale is larger than the nanoscale and smaller than the microscale. On this scale, the dynamic processes and interactions of domains or molecules at different levels in space and time can be considered, and the important roles played by thermal fluctuations and weak synergy can be included. Based on this, we will introduce the main contents of the mesoscale domains of the plasma membrane in this section.
The formation of membrane compartments151,152 occurred because TM proteins were immobilised on the membrane backbone by an interaction with the filamentous actin fence and then formed fences, or pickets, to partition the cytoplasmic membrane into multiple regions (i.e., membrane compartment) (Fig. 3a and b). The membrane compartment has played an important role in promoting the formation of protein complexes on the membrane surface, the distribution and diffusion of different membrane molecules on the membrane surface, and the size limitation of the raft domain. These effects cannot be separated from the existence of proteins, such as TM proteins, and their molecular interactions.
The raft domain19,22,153 is a nano-scale, heterogeneous, highly dynamic, and relatively ordered membrane domain rich in sterols and phospholipids (Fig. 3c). The formation, size, and existence time of the raft domain are affected by intermolecular affinity and thermal fluctuations. The molecules that affect raft formation include lipids and proteins. Lipid interactions based on cholesterol and saturated acyl chains recruit molecules that gradually form dynamic transient raft complexes. The membrane compartment also plays an important role in the size of the raft domain. The raft domain plays an important role in cell signal transduction, transmembrane transport of substances, and invasion by pathogenic microorganisms. The realisation of these functions may be closely related to the assembly and size regulation of a raft.
Protein clusters are dimers or oligomers of proteins or more complex and large protein complexes (Fig. 3d).154–156 These protein complex domains play an important role in cell signal transduction, transmembrane transport of substances, and other functions. The membrane compartment plays an important role in promoting the formation of protein clusters, such as protein dimers. In the cell signal transduction function, protein complex domains can synergistically assemble cholesterol and saturated lipids to form a raft and form a synergistic complex after binding to related molecules to exert related functions. This synergistic effect is of great significance for the protein to exert its function or for enzyme activity.
The three hierarchical structures on the cell membranes of organisms have dynamic relationships. The cell membrane is a complex system, which is affected not only by the cell surface molecules, but also by the intracellular and extracellular environments. The following sections will introduce how nanoparticles with hydrophobic and hydrophilic surface patterns interact with the cytoplasmic membranes as well as the basic mechanisms and processes involved, which are inseparable from the basic characteristics of the cell membrane and the surface characteristics of nanoparticles.
4.2 Fine-tuning of surface hydrophobicity and charge patterns for membrane interaction
However, in addition to considering the positive effect of hydrophobicity on the cellular uptake of nanoparticles, we should also note the negative effect of surface hydrophobic groups on the adsorption of proteins in organisms and the nonspecific aggregation during circulation.160,161 Therefore, the balance between the hydrophobic and hydrophilic surfaces of nanoparticles plays a very important role in the complete functional path of nanoparticles, such as transportation, ingestion, and drug release in organisms, which deserves further research. This can help researchers design more reasonable and effective nanocarriers, opening up a way to solve practical problems.
From this aspect, nanoparticles with patterned hydrophobic and hydrophilic surfaces should be more preferred to benefit from the hydrophobic interactions with the membrane and also compensate the disadvantages. In recent years, computer simulation has already shown the internalization process and mechanism of nanoparticles with different surface patterns. Li et al. compared striated nanoparticles with hydrophilic and hydrophobic ligands alternately arranged at intervals, fully hydrophilic nanoparticles, fully hydrophobic nanoparticles, and NPs with randomly mixed ligands at the same hydrophilic to hydrophobic ratio.161 They found that striated nanoparticles had the lowest energy barrier and were easy to transport. Through a dissipative particle dynamics (DPD) simulation, they verified the existence of a new type of amphiphilic nanoparticle–ligand complex that spontaneously penetrates the cell membrane.162 Notably, there are also significant differences in the interaction between nanoparticles with different patterns and cell membranes, such as stripes, a random distribution, and other patterns. Zhang et al. simulated four kinds of copolymer-coated nanoparticle complexes (including hydrophilic–hydrophobic (AB), hydrophobic–hydrophilic (BA), hydrophobic–hydrophilic–hydrophobic–hydrophilic (BABA), and random hydrophilic–hydrophobic patterns with different characteristics) by using dissipative particle dynamics to explore the effects of the density, rigidity, and surface pattern on the nanoparticle surfaces during their transport through the cell membrane. They found that BA and BABA patterns play important roles in the transport process.163
Except from hydrophobicity, the surface charge distribution of nanoparticles is also essential in the controlled transport process, and the charge plays an important role in the interaction between nanoparticles and the phospholipid membrane.5,164 For instance, negatively charged AuNPs are not easily fused with cell membranes because they are repelled by electrostatic interactions with the negatively charged cell membranes.165,166 At the same time, positively charged AuNPs usually have high cell membrane permeability. Goodman et al. reported that the cytotoxicity of positively charged AuNPs was much higher than that of negatively charged AuNPs, and this result is likely to be related to their different cellular uptakes.167,168 Ma et al. fabricated different kinds of chitosan-based nanoparticles, which could carry different charges but were identical in other characteristics (the size, shape and hydrophobicity). They incubated these nanoparticles with different cell lines to uncover the biological effects of nanoparticles. The experimental results indicated that positively charged nanoparticles would promote the transmembrane efficiency of nanoparticles. They also observed different intracellular trafficking effects induced by different charged surfaces of nanoparticles.169
These studies strongly supported the importance of constructing hydrophobic patterns and charge structures on nanoparticles for cell interactions. However, the experimental proof of this concept is still challenging. Only dendrimers and AuNPs have been successfully constructed with defined surface hydrophobicity and charge patterns and investigated their influence with cells. Therefore, the following two sections will introduce both systems in detail.
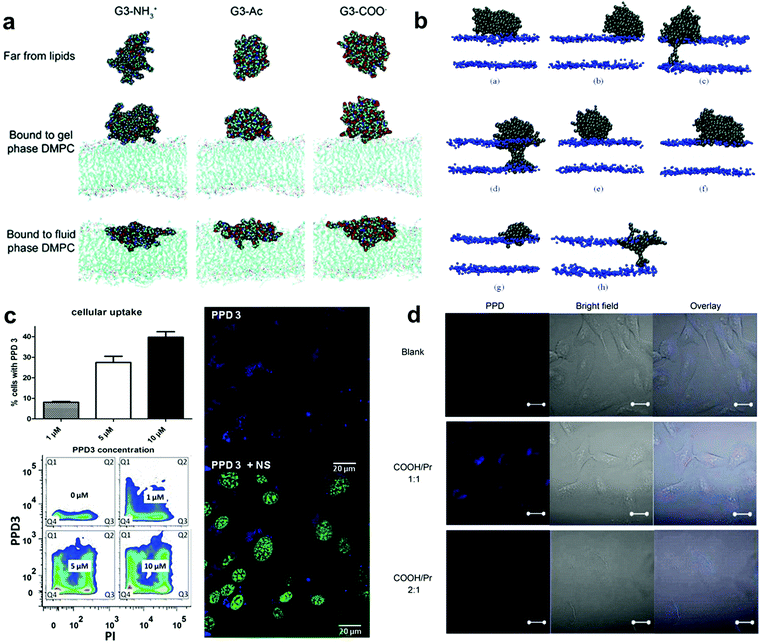 | ||
| Fig. 4 (a) Representative images of G3 with different terminations of the dendrimer in different equilibrated states. (b) Snapshots of different simulations of systems with different dendrimers. (c) PPD3 uptake and influence on brain endothelial cells. (d) Confocal fluorescence images of HLC cells incubated for 24 h with PPD. Reproduced with permission from ref. 173, 176, 91 and 178, copyright (2009) MDPI, (2006) American Chemical Society and (2014), (2017) Wiley Online Library. | ||
By designing proper surface patterns, one could also achieve efficient cell uptake similar to PAMAM but without a high density of positive charges. In the previous section, we introduced the methods to create polyphenylene dendrimers (PPDs) with defined surface patterns. These dendrimers are highly branched, shape durable, and monodisperse. In addition, they can be chemically functionalized at their core, scaffold, and surface, which provides the possibility of regulating the surface characteristics of PPD. Therefore, they are one of the successful models that are used to study the effect of surface patterns on cell interactions.
Müllen and Weil et al. made important contributions to the biological applications of PPDs. In their work in 2014, they demonstrated that PPDs with design alternating hydrophobic and hydrophilic patterns could display similar performance to the natural proteins, demonstrating their biocompatibility and application potential.91 Instead of using positive charges to enhance membrane interactions, the negatively charged hydrophilic groups were adopted in their systems which are normally not preferred for cell uptake. Surprisingly, by optimising the hydrophilic and hydrophobic patterns on the surface, these PPDs could be efficiently taken up by cells and even able to penetrate blood brain barrier models without affecting the membrane integrity.177 PPDs form 3D spheroids in aqueous solution with lipophilic cavities inside (like a protein structure). Researchers assigned the molecule a biological identity similar to that of human serum albumin (HSA), a natural vehicle for transporting lipophilic or poorly water-soluble substances with abundant animal plasma. The characteristics of this new PPD can be summarised as follows: (1) nanometer size, (2) a spherical structure with an amphiphilic surface pattern that promotes the interaction with the cell membrane, and (3) lipophilic internal cavities with different nanoscale dimensions that provide controllable guest uptake. PPDs exhibited good transport in A549 cancer cells and endogenous brain cells and low toxicity in relevant experiments. These results strongly supported that tuning the patterned structures on the nanoparticle surface could be an efficient way to promote cell interactions (Fig. 4c).
In another study, they performed surface tension measurements, X-ray reflex (XR), and sum-frequency generation spectroscopy (SFG) to study the effect of charges of the hydrophilic groups on amphiphilic PPDs (approximately 5 nm in diameter) using the monolayer of a self-assembled cell membrane (1,2-dipalmitoyl-sn-glycero-3-phosphocholine [DPPC]).8 This work showed that the dendrimer interacted with the cell membrane via the electrostatic force of the lipid headgroup, which drove the changes in headgroup alignment, and the negatively charged dendrimer could induce behavioural changes in interface water molecules. The results of this study have deepened the understanding of the interaction between amphiphilic PPD macromolecules and the lipid layer and improved its application in biology.
In their work in 2017, Müllen et al. demonstrated how the type and ratio of hydrophilic groups on amphiphilic PPDs influence the interaction with biological systems and studied their manifestations in cellular uptake and toxicity (Fig. 4d).178 A PPD with a molar ratio of propyl groups to sulfonic acid (i.e. non-polar to polar groups) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the surface feature was used as the parent molecule, and weakly acidic carboxylic or phosphonic groups were used to replace the surface sulfonic acid to explore the effects of acidic changes and the ratio of polarity to non-polarity on the surface of the dendrimer to explore the important effects of the PPD surface-patched architecture on the uptake of substance particles by cells. Different groups had little effect on substance transport and cell uptake. Almost all the dendrimers with a molar ratio of polar to non-polar groups of 1
1 as the surface feature was used as the parent molecule, and weakly acidic carboxylic or phosphonic groups were used to replace the surface sulfonic acid to explore the effects of acidic changes and the ratio of polarity to non-polarity on the surface of the dendrimer to explore the important effects of the PPD surface-patched architecture on the uptake of substance particles by cells. Different groups had little effect on substance transport and cell uptake. Almost all the dendrimers with a molar ratio of polar to non-polar groups of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed high cell uptake, while the dendrimers with a molar ratio of polar to non-polar groups of 2
1 showed high cell uptake, while the dendrimers with a molar ratio of polar to non-polar groups of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 could not cross the cell membrane. Researchers believe that this is because the high density of polar groups enhanced the rejection of PPDs against the cell membrane. This result demonstrates the important role of highly hydrophobic (lipophilic) surface features (i.e., high coverage of hydrophobic groups) in promoting cell uptake. This work is of great significance for the future design of feasible vectors for patched PPD-related drugs and nucleic acids.
1 could not cross the cell membrane. Researchers believe that this is because the high density of polar groups enhanced the rejection of PPDs against the cell membrane. This result demonstrates the important role of highly hydrophobic (lipophilic) surface features (i.e., high coverage of hydrophobic groups) in promoting cell uptake. This work is of great significance for the future design of feasible vectors for patched PPD-related drugs and nucleic acids.
Overall, these studies supported that the hydrophobic and hydrophilic surface patterns could significantly influence the interactions with cell membranes, and particularly the type of hydrophilic groups, hydrophobic groups and their ratio are all essential to tune the interactions. In this regard, the highly branched, shape-persistent, monodisperse PPDs with precisely designable surface patterns and special internal cavity structures offer the possibility and feasibility of their interaction with biological systems and their subsequent applications.
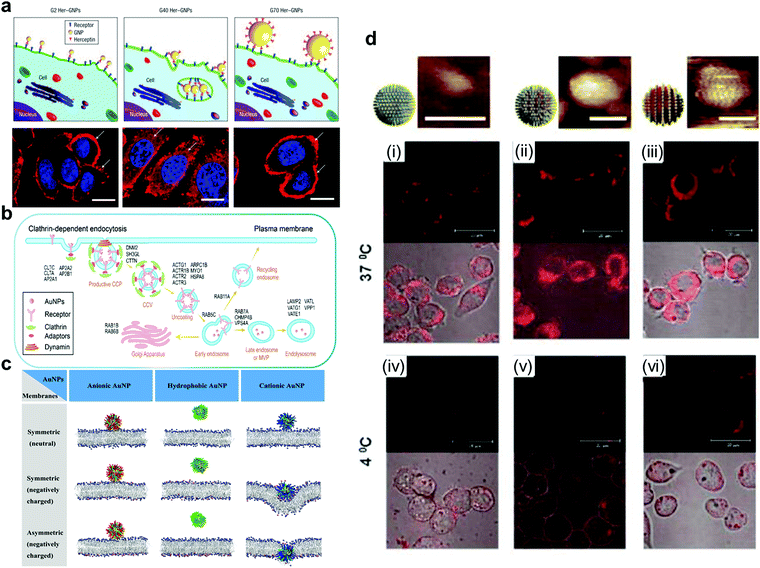 | ||
| Fig. 5 (a) Fluorescence image of ErbB2 receptor localization after treatment with different sizes of Her-GNPs. (b) Illustration of AuNPs internalized by the cell in a receptor-mediated manner. (c) AuNPs with different charges interact with symmetric or asymmetric lipid membranes. (d) AuNPs with different structural organizations of hydrophilic and hydrophobic ligands on the surface display different transmembrane abilities and phenomena when interacting with the cell membrane. Reproduced with permission from ref. 164, 184, 185 and 189, copyright (2008) Springer Nature, (2021) American Chemical Society, (2020) Elsevier and (2010) Wiley Online Library | ||
With the methods discussed in Section 3.1, it is possible to create fine substructures on the surface of AuNPs and thus investigate their effect on membrane interactions in more detail. Recently, Lunnoo et al. theoretically demonstrated that, compared with AuNPs with pure positive/negative charges, zwitterionic particles are less likely to be inside the membrane due to the rise of free energy barrier. In addition, zwitterionic particles prefer to agglomerate, which can further reduce its translocation rate.186 However, as mentioned above, zwitterionic modification can prevent the arbitrary absorbance of these macromolecules, and delicate distribution of these 2 opposite ligands is needed to make a trade-off to overcome the dilemma. Moreover, surface electrical density is also viewed as a co-factor in determining the pathway of the particles into the membrane, which implies that it is essential to control the density of charges for avoiding damage to the membrane.187 This study indicated the promising potential of creating more defined charge separated patterns for fine-tuning of nanoparticle interactions with cell membranes. However, since the techniques to precisely prepare such nanoparticles are still unavailable, this direction remains unexplored.
However, hydrophobic patterns have been created on the AuNP surface which has been shown to strongly influence their interaction with the cell membrane or other biological interfaces. Stellacci et al. have made important contributions to the field. In their early work, they reported related research on AuNPs covered with hydrophobic ligands (octanethiol, OT) and hydrophilic ligands (mercaptopropionic acid, MPA). They found that the self-assembly of AuNPs formed a striped structure, and the transmembrane transport of this particle in fibroblast cells was also observed.188,189 The results from the Stellacci group revealed the potential of nanoparticle surface patterns in regulating the material internalisation mechanism. Gkeka et al. extended the work of Stellacci et al. to explain the mechanism of regular surface patterns promoting transport to some extent by coarse-grained molecular dynamics simulations. Designing nanoparticles with a certain amphiphilic pattern on the surface may be an excellent application scheme.190
The above studies demonstrate that the design of more sophisticated structures (the heterogeneous surfaces) can obtain finer particles. Gao et al. studied the possible effects of hydrophilic and hydrophobic structures of nanoparticles on transmembrane behavior via computer simulations. Homogenous hydrophilic particles can only be absorbed on the membrane surface rather than enter the lipotropy phospholipid bilayer. However, for homogenous lipophilic nanoparticles, it may be easier to be inserted inside the lipotropy phospholipid bilayer, but more difficult to be released. Homogenous lipophilic nanoparticles could even cause substantial disruption to the bilayer while leaving the membrane.191,192 Due to the amphiphilic characteristics of the phospholipid bilayer, AuNPs with amphiphilic patterns are more likely to interact with cell membranes and thus have higher cell uptake efficiency. Stellacci and Alexander-Katz et al. elucidated the mechanisms of spontaneous insertion of amphiphilic AuNPs into the membrane by conducting unbiased atomistic simulations and experimental verification.193 They demonstrated the vesicle fusion-like and non-disruptive behaviour between amphiphilic AuNPs and the membrane.
The structural organization of hydrophilic and hydrophobic ligands on the AuNP surface also play an important role in the interaction between the nanoparticles and cell membrane. Stellacci et al. used hydrophobic and hydrophilic ligands to construct patterns with different structural organization patterns on the particle surface, demonstrating that nanoparticles coated with a ribbon-like alternating arrangement penetrated the cell membrane. However, particles coated with the same ligands but in a random arrangement cannot penetrate the membrane effectively, and can even get trapped in vesicular bodies (Fig. 5d).189 Therefore, the process of AuNP incorporation is a result of many factors.
4.3 Fine-tuning of ligand patterning for receptor mediated cell recognition
However, these studies were all based on the ideal state in which all the ligands on the surface of the nanoparticles bind to the receptor in a one-to-one manner. Therefore, in 2010, Yuan considered ligands that did not bind to the receptor and initially established a functional model of nanoparticle ligand density (ξ) and endocytosis time. His model is similar to a parabola in that, before the turning point, the greater the density, the shorter the endocytosis time; after the turning point, the greater the density, the longer the endocytosis time. At the turning point, the time is the shortest.200 Subsequently, Yuan established a more refined 3-phase model. He summarised the optimal nanoparticle endocytosis parameters, R (radius) ∈ [25, 30] nm and ξ (ligand density) ∈ [0.8, 1], which promoted the design of nanoparticles and their application in organisms.201,202
In addition to these simulations, some cellular experiments have demonstrated the effects of nanoparticle ligand density. The mode of endocytosis of folic acid-modified QDs in HeLa cells was closely related to the density of folic acid. When the density ranged from low to high, the endocytosis mode changed from caveolin-mediated to mixed type and finally to clathrin-mediated.203 Similarly, in 2020, Marine found that the density of the C-type lectin-like molecule-1 binding peptide (cCBP) on the nanoparticle surface determines its way into cells; at a high ligand density, the particle was internalised through the C-type lectin-like molecule-1 (CCL1) receptor, and at a low ligand density it was internalised through the membrane via a non-receptor pathway.204 To better regulate the interaction between nanoparticles and cells, more studies are needed to explore the effect of ligand density on the mechanism of nanoparticle entry into cells.
In 2015, Schubertová used a coarse-grained model to characterise the endocytosis process. In his research, the particles were divided into three categories. For particles with a low ligand density, endocytosis did not occur; for particles with a high ligand density, endocytosis occurred quickly. More importantly, for particles with a medium ligand density, the more symmetrical the ligand distribution, the shorter the endocytosis time. He believed that, for homogeneously distributed nanoparticles, a lower activation energy was required for endocytosis.208 Subsequently, Li et al. considered the mobility of receptors on cell membrane and found the endocytosis rate of nanoparticles was determined by the mutual effect of the distribution of ligands on nanoparticles and the density of receptors on cell membrane. The endocytosis rate of nanoparticles with homogeneously distributed ligands was the fastest only when the density of receptors on the cell membrane was sufficiently high. In most cases, a slightly nonuniform distribution was the best option.209
However, in contrast to these two models, Moradi demonstrated that “clustering distribution” enhanced the efficiency of endocytosis compared to “loose distribution”. He examined the phagocytosis of polystyrene nanoparticles with different distribution patterns of folic acid by bronchial epithelial cells and found that the aggregated distribution of nanoparticles exhibited a lower endocytosis efficiency only in the case of a lower folate density.210 Therefore, cell membrane components (cholesterol, glycoprotein) and intracellular substances (enzymes, reticulin) neglected in previous models might affect receptor-mediated nanoparticle endocytosis.
As mentioned above, DNA origami enables the precise position of ligands, and previously studies have been conducted regarding the influence of the ligand position on receptor-mediated recognition. In 2014, the Teixeira group first demonstrated the feasibility of regulating the receptor function through the nanoscale distribution of ligands (ephrin-A5). They found that the close positioned ligand can promote the recruitment of noun-bound receptors (EphA2) and therefore can activate the pathway to a greater extent.211 Based on this, in 2020, they further found that the located ligands with different interval distances can trigger different downstream transcriptional responses.212 In addition, the distances between aptamers determine the corresponding cellular behaviours.213,214 These results demonstrate the importance of accurately positioning ligands to enable them to perform their desired function.
In summary, under all circumstances, the optimal distribution of ligands needs to be fully considered as all of their factors (size, shape, density, and position) are closely correlated. Therefore, a fully established system to elucidate the effects of these factors on the downstream reactions is needed in this field.
5. Surface patterns affect protein adsorption
5.1 Surface properties of nanoparticles affecting protein corona formation
Because nanoparticles have a high specific surface area, they will interact with the surrounding proteins to produce protein coronas in the biological environment (such as blood and cell lysates).215–217 According to the theory of Derjaguin–Landau–Vervey–Overbeek (DLVO), due to the interaction of van der Waals forces with static electricity, biological macromolecules will be spontaneously deposited on the surface of nanoparticles.218 The nonpolar regions on the surface of the nanoparticles present in the water environment may be more likely to become sites for the binding or adsorption of biological macromolecules.219The formation of a protein corona has a significant impact on the basic characteristics and specific applications of the surface of nanoparticles.220–222 The formation of a protein corona may also have an important impact on the in vivo distribution of nanoparticles, cell uptake mechanisms, the number of nanoparticles ingested,39,161,223–226 and the cytotoxicity of nanoparticles,227–231 and even change the function and biological characteristics of nanoparticle targeting.
The compositions of core materials,232 nano-size,14,233–235 and surface characteristics217,219,220 of nanoparticles are of great significance for the formation of a protein corona. Among the various influencing factors, the surface charge and hydrophilic/hydrophobic characteristics of particles play a key role in various biological phenomena.
Many studies on synthetic nanoparticles have shown the possible influence of surface charges on protein–nanoparticle interactions. As the physiological pH is 7.4, many proteins are negatively charged215 and positively charged nanoparticles may be more likely to interact with them. Boyles et al. investigated the interaction between AuNPs with different surface charges (e.g. chitosan–AuNP conjugates) and proteins in the cell culture. Their results indicated that surface charges are critical for protein–nanoparticle interactions and their metabolic processes.236 With the increase of the quantity of surface charges, the positively charged chitosan–AuNPs adsorbed proteins increasingly. Fleischer et al. found that, after the bovine serum albumin (BSA) was adsorbed on the surface of the nanoparticles, the interaction between the BSA–NP complex and the cell membrane also changed (Fig. 6a).237 A summary of the effects of particle surface charge and hydrophobicity on protein adsorption has been presented by some researchers.217
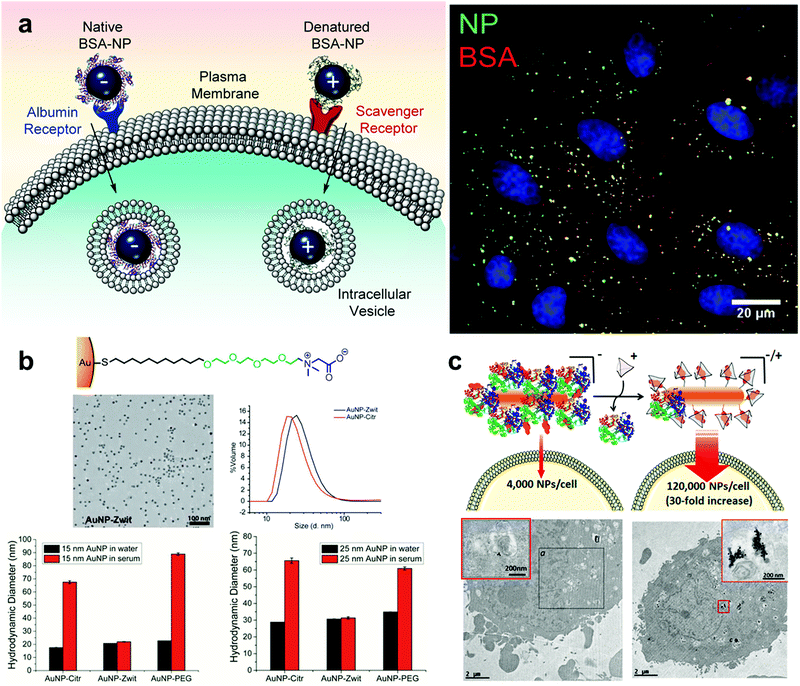 | ||
| Fig. 6 (a) The interaction between the protein corona–AuNP complex and the cell membrane. The positively and negatively charged NPs may adsorb different proteins and bind to different receptors on the cell membrane. (b) Effects of zwitterion and PEG modified nanoparticles on protein corona formation. AuNP-zwit significantly prevented the formation of a protein corona, while AuNP-PEG does the opposite. (c) The internalization of gold nanospheres NS2 in the presence or absence of cage A (in the absence or presence of a protein corona) by HeLa cells. Reproduced with permission from ref. 237, 250 and 251, copyright (2014), (2016), (2020) American Chemical Society. | ||
The surface hydrophilicity and hydrophobicity of nanoparticles also play a key role in the formation of protein coronas. With an increase in the proportion of hydrophobic groups on the surface, the nanoparticles238 show a stronger adsorption capacity for substances such as proteins. At the same time, there was a promoting effect on protein corona formation when the number of surface hydrophilic groups was increased. Due to the different types and surface characteristics of nanoparticles and the different biological fluids used for their incubation, there may have been differences in the relationship between the surface hydrophilicity and hydrophobicity of the particles and the formation of protein coronas.
Based on the knowledge about how surface properties influence protein corona formation, it is anticipated that the surface patterns would also play an important role in fine-tuning protein adsorption on nanoparticles. However, studies on this aspect are still limited. Some studies showed that the use of nanoparticles with amphiphilic surfaces could effectively prevent protein adsorption and protein corona formation in physiological and biological environments.219,239 Many types of complexes and their effects have been explored.219,240 However, the understanding of designable surface patterns and their influence on protein adsorption is more challenging. Most studies in this aspect also used the dendrimer and AuNP systems, which will be discussed in detail in the following sections.
5.2 Fine-tuning of the surface patterns of AuNPs to control protein adsorption
Because of the high free energy on the surface of AuNPs, AuNPs tend to absorb proteins, thus contributing to the formation of protein coronas.241,242 After adsorption of some proteins, AuNPs may lose some functions given by artificial modification and may also have new biological characteristics and recognition sites. AuNPs with opsonin or complement proteins can trigger the immune response of biological organisms, which induces their phagocytosis and clearance by macrophages.39 Therefore, the elucidation of the factors influencing the formation of protein coronas on the surface of AuNPs is essential for their further research and biological applications.The surface patterns (electrical properties, quantity of electricity and ligand pattern) of AuNPs have an important effect on the formation of various protein coronas with different densities and different components.243–246
There exist significant differences in the amount of protein adsorption or composition of a protein corona between particles with different electrical patterns.247 Nanoparticles with homogeneous charge showed a certain application value in the experiment. However, surface modification with a single electrical structure cannot effectively prevent the protein adsorption. The researchers might be able to achieve better results by constructing zwitterionic surface structures that could increase the biological stability of particles. Researchers have developed various types of zwitterionic surfaces that exhibit good adsorption resistance and biostability.53 The construction of zwitterionic patterns on the surface of AuNPs can effectively enhance the biocompatibility of nanoparticles and prevent protein adsorption,248 and this approach has been extensively explored by García et al.249 Gupta et al. demonstrated the ability of AuNPs with zwitterions to effectively prevent protein corona formation in serum, improving the colloidal stability of AuNPs (Fig. 6b).250 Mosquera et al. used host–guest interactions to control the zwitterionic surface characteristics of AuNPs with a large size, and this surface property has an important impact on the formation of protein coronas and cellular uptake. Besides, it is noticed that the protein adsorbed on the surface of nanoparticles can be regulated by adding guest molecules instead of the host macromolecular cage to achieve a reversible process (Fig. 6c).251
Protein coronas bring many limitations to the practical application of AuNPs, such as cytotoxicity,252 biodistribution and cellular uptake of AuNPs. Various substances and methods have been tried to prevent the formation of protein coronas by building surface patterns with different properties. Among them, PEG is one of the most widely used ligands, which can effectively reduce the adsorption of non-specific proteins on the surface of AuNPs and avoid the scavenging phenomenon to some extent, extending the retention time of nanoparticles.253 When the surface density of PEG reaches a high level, the protein corona formation is obviously inhibited, while AuNPs without PEG modification were detected with a higher total amount of proteins.254 Moreover, AuNPs with different PEG surface densities also exhibited different cellular uptake characteristics and internalization phenomena.255
At present, most studies of protein–nanoparticle interactions focus on the homogeneous surfaces, but studies on the heterogeneous surface of nanoparticles are rare. Existing studies have demonstrated the potential of heterogeneous surfaces in regulating protein–nanoparticle interactions. Researchers can design AuNPs with different heterogeneous surfaces to tune nanoparticle–protein interactions. In 2013, Stellacci and Lau et al. studied the interaction between three types of ligand-coated AuNPs ((1) all negatively charged, sulfonated alkanethiols (11-mercapto-1-undecanesulfonate, MUS), (2) a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mixture of MUS and 1-octanethiol (OT), and (3) a 2
1 molar mixture of MUS and 1-octanethiol (OT), and (3) a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mixture of MUS and a branched, apolar version of OT) and tow common serum proteins (ubiquitin and fibrinogen). The results have shown that the adsorption of proteins on AuNPs is influenced by the surface heterogeneity of nanoparticles.256 After that, they designed various types of AuNPs with different surface heterogeneity but the same shape, size, and composition (MUS, MUS/brOT, MUS/OT, MPA/brOT, MPA/OT). The surfaces of these nanoparticles are designed to be randomly distributed or alternating stripe-like. They tested the interaction of these particles with protein mixtures and observed the different characteristics and behaviors of AuNPs with different surface heterogeneity when they interact with proteins.257 As indicated, these studies demonstrated that tunable surface heterogeneity could be a new approach in designing nanoparticles and predicting the fate of nanoparticles.
1 molar mixture of MUS and a branched, apolar version of OT) and tow common serum proteins (ubiquitin and fibrinogen). The results have shown that the adsorption of proteins on AuNPs is influenced by the surface heterogeneity of nanoparticles.256 After that, they designed various types of AuNPs with different surface heterogeneity but the same shape, size, and composition (MUS, MUS/brOT, MUS/OT, MPA/brOT, MPA/OT). The surfaces of these nanoparticles are designed to be randomly distributed or alternating stripe-like. They tested the interaction of these particles with protein mixtures and observed the different characteristics and behaviors of AuNPs with different surface heterogeneity when they interact with proteins.257 As indicated, these studies demonstrated that tunable surface heterogeneity could be a new approach in designing nanoparticles and predicting the fate of nanoparticles.
5.3 Fine-tuning of the surface patterns of dendrimers to control protein adsorption
PAMAM is the first generation of dendrimers and is currently one of the most studied dendrimers. Through in-depth research over the past decades, it has been applied in many fields such as chemistry, biology, and medical treatment.258 PAMAM forms a loose 3D spherical structure composed of repeating units in solution, and its core, branch units, and ends can be independently selected for flexible design.259 Due to its high versatility and flexible modification, PAMAM has broad prospects for applications in drug delivery vehicles,16 nucleic acid delivery vehicles,260,261 and even the simulation of natural proteins.16 However, the entry of PAMAM molecules without reasonable surface modifications faces the same problems as those faced by other nanoparticles (protein adsorption via HSA, Ig, and complement proteins and the formation of a protein corona), significantly impacting the function and fate of nanoparticles.262–264 The combination of PAMAM and HSA, which are used as drug carriers, can increase the lifetime of PAMAM in the circulation.265,266At present, generation and surface chemical modification of dendrimers have been considered to have important effects on their protein affinity and protein corona formation.267,268 Dawson et al. found a high correlation between the generation of cation-modified PAMAM dendrimers and cytotoxicity (Fig. 7a).269 There could also be a correlation between the surface charge and particle size of PAMAM dendrimers and other health hazard events, such as platelet aggregation and thrombosis. Dobrovolskaia and Mcneil et al. demonstrated that large-sized cationic PAMAM dendrimers could induce platelet aggregation by interfering with the integrity of the cell membrane.270 Dawson et al. studied the relationship between the protein corona and the relevant features of the PAMAM dendrimer using electrophotographic mobility techniques and SDS-PAGE and observed a strong interaction between the complement proteins and G6 and G7 dendrimers.271
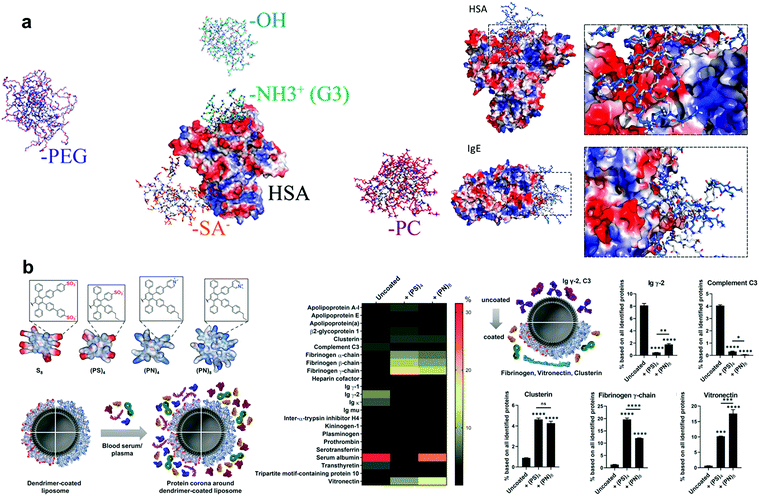 | ||
| Fig. 7 (a) Snapshots of the interaction between dendritic polymers with different surface modifications and human serum proteins (HSA) from DMD simulations. (b) The comparison of protein coronas on PPD-coated liposomes and uncoated liposomes. Reproduced with permission from ref. 269 and 273, copyright (2018) American Chemical Society and (2020) The Royal Society of Chemistry. | ||
Wang et al. systematically investigated the adsorption of human serum albumin (HSA), immunoglobulin (Ig), and other proteins by PAMAM dendrimers with different chemical surfaces using atomistic DMD simulations.272 They used positively charged amine (NH2), negatively charged succinamic acid (SA), neutral hydroxyls (OH), PEG, and photosynthate (PC) to modify the surface characteristics. This study found that these particles could effectively reduce protein adsorption, while the particles on the charged surface were more likely to bind to proteins. This result may have been due to the strong electrostatic interaction between the charged PAMAM and the positively or negatively charged regions of the protein surface. This study emphasises the importance of using amphiphilic surface-modified PAMAM to prevent protein corona formation. Giri et al. explored the mechanism of interaction between HSA and PAMAM dendrimers. In this study, they measured the binding constants (Kb) of a series of PAMAM molecules to HSA in a pH (7.4) aqueous solution using protein-coated silk particles. Furthermore, the researchers studied the PAMAM + HSA complex by combining 1H NMR, saturation transfer difference (STD) NRM, and other methods to explore the specific mechanism of action. The results showed a correlation between the binding constant (Kb) of PAMAM and HSA and the size of the dendrimer, as well as the chemical characteristics of the terminal group. They found that the electrostatic interaction between the charged dendrimer terminal group and the protein residue, the hydrogen bond between the internal group of the dendrimer and the protein residue, the hydrophobic interaction of nonpolar dendrimers with proteins, and the special interaction between the carboxyl group of the dendrimer and the fatty acid binding sites of HSA were the important mechanisms of adsorption of HSA by the PAMAM dendrimer.267 All these results suggest the important influence of the local character difference on the interaction between the particle surface and biological environment.
PPDs are often considered to be highly hydrophobic because of their rigid structure with multiple benzene rings, and they are generally not considered to be strongly associated with biological and other neighbourhoods. However, with in-depth research in recent years, Müllen et al. made important contributions to the further development and biological application of PPDs. PPDs can be subjected to many precisely positioned functional modifications at different structural levels, revealing that, through reasonable preparation, modification, and functional modification, PPDs can play a key role in a series of practical biological applications.234 PPDs have the characteristics of rigidity, shape durability, and accurate modification. These findings provide more possibilities and broader development prospects for in-depth research on the interaction of PPDs with biological interfaces, proteins, nucleic acids, lipids, and related practical applications.
Wagner et al. used a series of PPD-coated liposomal nanocarriers with different surface characteristics to explore the effects of the amphiphilic surface, surface charge, and shape durability on the adsorption of serum proteins and the formation of protein coronas. They also changed and affected the fate and orientation of liposomal nanocarriers in a biological fluid environment by controlling the surface characteristics of PPDs. They proved that the surface charge and hydrophobicity of PPDs played an important role in the formation of the liposome–protein corona, effectively reducing the adsorption of opsonin and complement proteins and inhibiting the cell uptake caused by related immune responses (Fig. 7b).273,274 These results suggest that one can control the biological characteristics of nano-drug carriers by modifying the surface of PPDs.
At present, the widely used PAMAM dendrimers often exhibit strong cytotoxicity and induce adverse immune responses.266,269 Compared with PPDs, they have a flexible structure, which is likely due to the conformational rearrangement of the dendrimers that causes the special sites of surface functionalization of the dendrimers to be “folded” inward and covered, so that they cannot exert their biological or medical effects. PPDs have a rigid three-dimensional structure, a surface that can be effectively and accurately designed, and functional groups can maintain relatively accurate positions therein. The low cytotoxicity of PPDs91 and other characteristics have provided broad prospects for their practical application. At present, in the related application and research field of PPDs, we believe that such macromolecules can play a more important role and have greater potential.
6. Surface patterns affect the interaction of nanoparticles and viruses
6.1 Interaction between AuNPs and viruses
Polyanionic compounds, especially polysulphate, have received increased attention due to their ability to inhibit various enveloped viruses. The polysulphate AuNPs formed by combining polysulphate with AuNPs play an effective role in virus resistance.275 The mutual recognition and binding of polysulphate nanoparticles to viruses are mediated through their binding to the coat protein or capsid protein of viruses. Based on this, researchers often use polysulphate to change and modify AuNPs, making them more conducive to interaction with viruses.276,277 In addition to changing the surface electrical properties of AuNPs, researchers can also modify the surface substructure using specific proteins278 or aptamers279,280 to allow targeted binding to viruses. For example, Chen et al. modified AuNPs with glucose oxidase (GOx) and concanavalin A (ConA) to interact with ConA-glycan to bind to the H3N2 virus.278 Le et al. connected APTA MER against the purified HA protein of J1999V to AuNPs, so that J1999V was wrapped by them to form visual sedimentation and realised rapid visual detection of viruses.280As a result, AuNPs can change their substructures through surface electrical changes and ligand modifications to further interact with the capsid protein of viruses, providing multi-purpose tools for virus detection and removal. If we could design some surface patterns for AuNPs to make them interact with viruses better or more efficiently, we not only can guide the tuning of the characteristics and functions of AuNPs, but may also obtain new compounds with unexpected effects for antiviral therapy in the area of clinical treatment.
Setellacci et al. demonstrated a new anti-viral mechanism through multivalent binding which leads to an irreversible distortion of viruses through the design of ligands on the surface of nanoparticles. Strong particle binding to membranes may result in significant local distortions. They replace the short linkers, 3-mercaptoethylsulfonate (MES), on the surface of AuNPs with long ones, undecanesulfonic acid (MUS), to achieve strong multivalent binding. In vitro experiments showed that MUS-AuNPs had good resistance to the viruses (Fig. 8a).281 At pH 7.4, the aspartic acid and glutamic acid residues on the outer surface of the cowpea mosaic virus (CPMV) capsid protein are deprotonated and negatively charged, respectively. The polycyclic aromatic hydrocarbon (PAH)-modified AuNPs have a positive charge. Under electrostatic interactions, CPMV- and PAH-modified AuNPs can be effectively combined.282 Cowpea chlorotic mottle virus (CCMV) and tobacco mosaic virus can also be combined with AuNPs via electrostatic interactions.283–285 Although this field is in its infancy, the regulation methods are not precise enough, and AuNPs with reasonable surface patterns need to be further developed, we still believe that the concept and approaches introduced here have a chance to guide the production of medically relevant antiviral drugs.
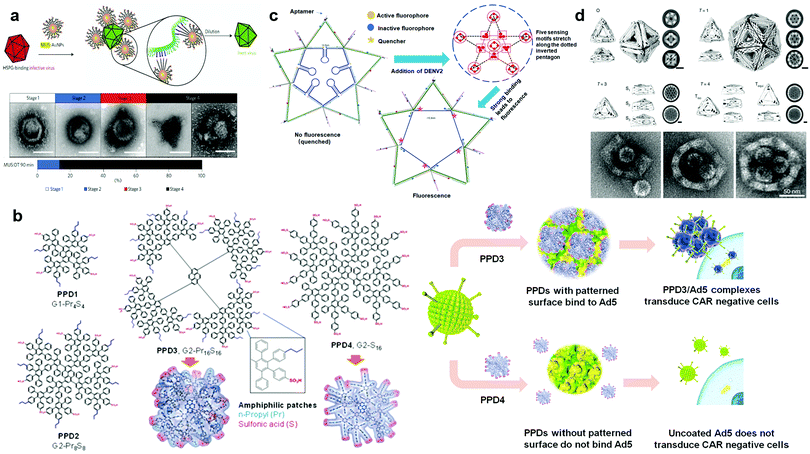 | ||
| Fig. 8 (a) Illustration and TEM images of the interaction between MUS:OT-NPs and the virus. (b) Scheme and TEM images of the course of the assembly and disassembly processes of the virus regulated by dendrons. (c) Illustration of the binding of PPDs to Ad5 and change of the transduction of Ad5. (d) Illustration and results of the detection of DENV by a star-like probe constructed by DNA. (e) Scheme of the assembly of DNA nanocages and TEM images of shells with a missing pentagon vertex engulfing up to three HBV core particles. Reproduced with permission from ref. 281, 258, 7 and 295, copyright (2017), (2020) and (2021) Springer Nature and (2019) American Chemical Society. | ||
6.2 Interaction between dendrimers and viruses
Dendrimers can not only be used as vectors for drugs and nucleic acids to achieve therapeutic purposes, but also for inhibiting virus-related functions. Conventional studies mainly used charges to enhance the interactions between dendrimers and viruses. For instance, dendrimers with cationic terminal functional groups (e.g., polylysine dendrimer, PAMAM) can preferentially bind to HIV envelope proteins (gp41, gp120) and related receptors (CD4 receptors) on host cells.286 HIV infection involves an interaction with gp120. By blocking the formation of the CD4–gp120–chemokine receptor complex, dendrimers can hinder HIV from binding to host cells, which inhibits subsequent viral replication.287 In addition, polyanionic dendrimers were also reported with anti-viral activity. Gastaminza et al. reported the effect of a class of polyanilic carbo silane dendrimers (PCDs) as a nanotool to control the spread of hepatitis C virus (HCV).288 The anti-HCV PCD selected by them was a second-generation carbo silane dendrimer, which was G2-S24P consisting of a poly-core and a surface completely covered by 24 sulfonate groups. G2-S24P may bind to the virus envelope protein at high doses, and the electrostatic repulsion between side-chain molecules that do not participate in the electrostatic interaction with the envelope protein leads to the disruption and irreversible destabilisation of the virus envelope, during which it is likely that a large net negative charge on the surface of G2-S24P plays a key role. The broad-spectrum antiviral activity of PCDs also plays a role in the treatment of HIV and herpes simplex virus (HSV) infection, which has attracted extensive research attention.289–291Except charges, recent studies showed that the design of proper surface patterns could allow binding of dendrimers with viruses independent of electrostatic interactions. Wu et al. adopted an amphiphilic PPD with patterned alternating hydrophobic and hydrophilic groups to bind to the surface of adenovirus type 5 (Ad5) which is a common nucleic acid or drug carrier. They proved that a generation 2 PPD with a high-density amphiphilic pattern combining hydrophilic sulfonic acid groups and hydrophobic n-propyl chains could form a stable PPD/Ad5 complex. Interestingly, the mutation of the positive charges on Ad5 does not affect the binding with the amphiphilic patterned PPD, whereas the PPD with only negatively charged sulfonic acids on the surface also does not exhibit affinity with Ad5. These observations strongly supported that the binding between the PPD and Ad5 does not rely on electrostatic interactions, but most likely depends on the proper hydrophilic and hydrophobic pattern fitting with the surface structures of Ad5. The PPD binds to the Ad5 surface to form a corona structure which could also have an effect on the specific interaction of Ad5 with the cellular coxsackie-adenovirus receptor (CAR) (Fig. 8b). As a consequence, the complex showed a high transduction efficiency of Ad5 in CAR low-expression cells, which was much higher than that of AD5 without PPD3 coating. In particular, this reduced its distribution in the liver and increased its distribution in the heart. This unique structure effectively prevented the binding of endogenous blood coagulation factor X to the virus surface because PPD masked the binding sites on the virus surface. This new idea and method of using dendrimers with amphiphilic patterns to regulate the viral vector brings more possibilities for the biomedical application of PPDs.258,274
6.3 Ligand patterning for virus recognition DNA based architecture interacting with viruses
DNA-based architectures are widely used for the detection and treatment of viruses because of their low toxicity and high programmability. Compared with a single ligand, the whole nanoparticle has a stronger binding capacity for the surface protein of the virus capsid after the ligand is connected to the DNA structure.292 However, the distribution pattern of surface proteins of some viruses is complex, which hinders the interaction of nanoparticles with surface proteins and thus affects the recognition.293,294 Therefore, it is of great significance to finely regulate the distribution of ligands in DNA structures and explore their ability to recognise viruses. One of the pioneering studies was conducted by Rinker, who provided a paradigm for exploring the effects of the spatial distribution of proteins.12 In 2019, Shaw et al. demonstrated that the spatial distribution of antibodies on DNA origami determines the affinity between the antigen and antibody, which reaches a maximum at a distance of 16 nm.6 Furthermore, Kwon et al. proved that, in a two-dimensional structure, the appropriate spatial distribution of ligands of specific DNA structures was necessary for the correct recognition of viruses. In their study, a “star” probe was used to detect dengue virus (DENV). A probe can bind to multiple nucleic acid aptamers. When the linked aptamer binds to the virus surface domain EDIII, its DNA structure is deformed to generate fluorescence signals. Kwon et al. tried three probes at different valencies (5, 6, 7) and found that only the DNA pentagram-like star structure could detect DENV. Although the other two had more aptamers, the detection sensitivity significantly diminished (Fig. 8c).7Additionally, in a three-dimensional structure, the spatial distribution of the ligands of the DNA structure laid the foundation for the mode of binding to the virus. Sigl et al. assembled a series of DNA nanocages with different numbers of subunits to isolate the invading virus from affected cells. DNA nanocages recognise viruses by the binding of antibodies on subunits to virus antigens, and the spatial distribution of antibodies is regulated by subunit topology. The virus is blocked by a semi-octahedral nanocage and a semi-decahedral with a missing pentagon vertex nanocage. Although the former can completely block viruses, the spatial distribution of the latter allows DNA nanocages to block multiple viruses simultaneously, thus having a more efficient blocking efficiency (Fig. 8d).295 However, compared with the extensive research on the interaction between DNA ligand patterns and cells, research on the effect of the ligand pattern on the DNA structure on virus recognition is still rare at present.296,297 Therefore, further research is required in this field.
7. Summary and perspective
Nanomaterials have a wide variety of sizes, morphologies, structures and surface characteristics, and their surface patterns plays a vital role in their biological effects. By adjusting and changing the surface microstructures, nanomaterials with pre-set functions can be designed. On the one hand, we hope that specially designed nanomaterials can interact with biological macromolecules, viruses, cells, etc. to achieve the desired biological effects. For example, the design of amphiphilic surface patterns has attracted increasing attention from researchers. Since the cell membrane has both hydrophilic and hydrophobic regions, patchy nanoparticles with alternative hydrophilic and hydrophobic patches could interact well with them, which provides an important reference for surface modification of nano-drug carriers. Similarly, the surface of the virus capsid also has hydrophilic and hydrophobic regions, and nanoparticles with a patch-like surface have a wide range of applications for the surface modification design of antiviral nano-drugs and gene therapy vectors. Dendrimers can not only provide a model for the study of protein coronas on the surface of nanoparticles, but also contribute to the field of controllable self-assembly of proteins.298 Dendrimers with appropriate size and electrical properties can serve as a “bridge” for the high-ordered assembly of some cricoid proteins. Linking some ligands on the surface of nanoparticles that can interact with the surface receptors of tumor cells can improve the targeting effects of nanoparticles. Introducing groups that are responsive to light, pH and reactive oxygen species could endow nanoparticles with different electrical properties, polarities, and configurations, so as to achieve the regulation of the biological effects of nanoparticles by external conditions. For example, the chemical conformation of polymers with light-responsive groups on the surface can be changed under light, thereby causing pores in the lipid membrane, allowing the release of drug molecules.299On the other hand, an in-depth understanding of the biological effects of the properties of the surface patterns of nanoparticles helps to avoid the rapid removal of nanoparticles with therapeutic functions in organisms. For example, zwitterion modification reduces the formation of a protein corona on the AuNP surface which helps reduce the phagocytosis of AuNPs by opsonin-dependent immune responses. The amphiphilic PPD molecule can shield Ad5 from such molecules as antibody, complement proteins and FX in the blood, so as to prevent Ad5 from being swallowed by macrophages and endocytosed by hepatocytes.
Therefore, research on the surface patterns of nanoparticles will help researchers understand how to construct nanoparticles with different surface properties according to the expected biological effects, so as to improve the biocompatibility and targeting of nanomaterials, reduce their immunogenicity and removal, and provide a model for the biomedical application of nanomaterials. The interaction between artificial nanomaterials and biological macromolecules can provide researchers with new perspectives in the fields of molecular self-assembly and nano-machine construction, and promote the development of novel materials and methods.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was funded by the National Key R&D Program of China (Grant No. 2018YFA0903500), the National Natural Science Foundation of China (Grant No. 51703073) and the Open Fund from Key Laboratory of Cellular Physiology (Shanxi Medical University), Ministry of Education, China (Grant No. CPOF202103).References
- B. Wang, L. Zhang, S. C. Bae and S. Granick, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 18171–18175 CrossRef CAS PubMed.
- K. Kobayashi, J. J. Wei, R. Iida, K. Ijiro and K. Niikura, Polym. J., 2014, 46, 460–468 CrossRef CAS.
- I. M. El-Sherbiny and Y. Abbas, Curr. Pharm. Biotechnol., 2016, 17, 673–682 CAS.
- H. M. Ding and Y. Q. Ma, Nanoscale, 2012, 4, 1116–1122 RSC.
- O. Harush-Frenkel, E. Rozentur, S. Benita and Y. Altschuler, Biomacromolecules, 2008, 9, 435–443 CrossRef CAS PubMed.
- A. Shaw, I. T. Hoffecker, I. Smyrlaki, J. Rosa, A. Grevys, D. Bratlie, I. Sandlie, T. E. Michaelsen, J. T. Andersen and B. Hogberg, Nat. Nanotechnol., 2019, 14, 184–190 CrossRef CAS PubMed.
- P. S. Kwon, S. Ren, S. J. Kwon, M. E. Kizer, L. Kuo, M. Xie, D. Zhu, F. Zhou, F. M. Zhang, D. Kim, K. Fraser, L. D. Kramer, N. C. Seeman, J. S. Dordick, R. J. Linhardt, J. Chao and X. Wang, Nat. Chem., 2020, 12, 26–35 CrossRef CAS PubMed.
- M. Okuno, M. Mezger, R. Stangenberg, M. Baumgarten, K. Mullen, M. Bonn and E. H. Backus, Langmuir, 2015, 31, 1980–1987 CrossRef CAS PubMed.
- S. Pogodin, N. K. H. Slater and V. A. Baulin, ACS Nano, 2011, 5, 1141–1146 CrossRef CAS PubMed.
- Y. Gao and Y. Yu, J. Am. Chem. Soc., 2013, 135, 19091–19094 CrossRef CAS PubMed.
- V. P. Ma and K. Salaita, Small, 2019, 15, e1900961 CrossRef PubMed.
- S. Rinker, Y. Ke, Y. Liu, R. Chhabra and H. Yan, Nat. Nanotechnol., 2008, 3, 418–422 CrossRef CAS PubMed.
- M. Langecker, V. Arnaut, T. G. Martin, J. List, S. Renner, M. Mayer, H. Dietz and F. C. Simmel, Science, 2012, 338, 932–936 CrossRef CAS PubMed.
- S. Schottler, G. Becker, S. Winzen, T. Steinbach, K. Mohr, K. Landfester, V. Mailander and F. R. Wurm, Nat. Nanotechnol., 2016, 11, 372–377 CrossRef PubMed.
- D. C. Luther, R. Huang, T. Jeon, X. Zhang, Y. W. Lee, H. Nagaraj and V. M. Rotello, Adv. Drug Delivery Rev., 2020, 156, 188–213 CrossRef CAS PubMed.
- R. Esfand and D. A. Tomalia, Drug Discovery Today, 2001, 6, 427–436 CrossRef CAS PubMed.
- A. Kusumi, C. Nakada, K. Ritchie, K. Murase, K. Suzuki, H. Murakoshi, R. S. Kasai, J. Kondo and T. Fujiwara, Annu. Rev. Biophys. Biomol. Struct., 2005, 34, 351–378 CrossRef CAS PubMed.
- J. M. Kalappurakkal, P. Sil and S. Mayor, Protein Sci., 2020, 29, 1355–1365 CrossRef CAS PubMed.
- K. Simons and M. J. Gerl, Nat. Rev. Mol. Cell Biol., 2010, 11, 688–699 CrossRef CAS PubMed.
- A. Verma, O. Uzun, Y. Hu, Y. Hu, H. S. Han, N. Watson, S. Chen, D. J. Irvine and F. Stellacci, Nat. Mater., 2008, 7, 588–595 CrossRef CAS PubMed.
- K. Jacobson, P. Liu and B. C. Lagerholm, Cell, 2019, 177, 806–819 CrossRef CAS PubMed.
- M. Cebecauer, M. Amaro, P. Jurkiewicz, M. J. Sarmento, R. Sachl, L. Cwiklik and M. Hof, Chem. Rev., 2018, 118, 11259–11297 CrossRef CAS PubMed.
- T. Fujiwara, K. Ritchie, H. Murakoshi, K. Jacobson and A. Kusumi, J. Cell Biol., 2002, 157, 1071–1081 CrossRef CAS PubMed.
- J. Foote and C. Milstein, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 10370–10374 CrossRef CAS PubMed.
- M. G. Rydahl, A. R. Hansen, S. K. Kracun and J. Mravec, Front. Plant Sci., 2018, 9, 581 CrossRef PubMed.
- D. A. Di Giusto and G. C. King, J. Biol. Chem., 2004, 279, 46483–46489 CrossRef CAS PubMed.
- J. Mravec, S. K. Kracun, M. G. Rydahl, B. Westereng, D. Pontiggia, G. De Lorenzo, D. S. Domozych and W. G. T. Willats, Plant J., 2017, 91, 534–546 CrossRef CAS PubMed.
- K. Tanaka, K. Fukase and S. Katsumura, Synlett, 2011, 2115–2139 CrossRef CAS.
- M. R. Bond and J. J. Kohler, Curr. Opin. Chem. Biol., 2007, 11, 52–58 CrossRef CAS PubMed.
- J. H. Kim and E. H. Chen, J. Cell Sci., 2019, 132, jcs213124 CrossRef CAS PubMed.
- D. M. Lee and E. H. Chen, Annu. Rev. Genet., 2019, 53, 67–91 CrossRef CAS PubMed.
- F. Rodriguez-Perez, A. G. Manford, A. Pogson, A. J. Ingersoll, B. Martinez-Gonzalez and M. Rape, Dev. Cell, 2021, 56, 588–601 CrossRef CAS PubMed.
- L. Li and F. Song, Acta Mech. Sin., 2016, 32, 970–975 CrossRef.
- K. Kubelkova and A. Macela, Front. Cell. Infect. Microbiol., 2019, 9, 241 CrossRef CAS PubMed.
- A. Kusumi, K. G. Suzuki, R. S. Kasai, K. Ritchie and T. K. Fujiwara, Trends Biochem. Sci., 2011, 36, 604–615 CrossRef CAS PubMed.
- M. A. del Pozo, N. Balasubramanian, N. B. Alderson, W. B. Kiosses, A. Grande-Garcia, R. G. Anderson and M. A. Schwartz, Nat. Cell Biol., 2005, 7, 901–908 CrossRef CAS PubMed.
- T. Miclaus, C. Beer, J. Chevallier, C. Scavenius, V. E. Bochenkov, J. J. Enghild and D. S. Sutherland, Nat. Commun., 2016, 7, 11770 CrossRef CAS PubMed.
- T. Tanaka, A. Ogata and M. Narazaki, Clin. Med. Insights: Ther., 2013, 5, CMT.S9282 Search PubMed.
- K. Saha, M. Rahimi, M. Yazdani, S. T. Kim, D. F. Moyano, S. Hou, R. Das, R. Mout, F. Rezaee, M. Mahmoudi and V. M. Rotello, ACS Nano, 2016, 10, 4421–4430 CrossRef CAS PubMed.
- W. H. Binder, R. Sachsenhofer, D. Farnik and D. Blaas, Phys. Chem. Chem. Phys., 2008, 10, 7328 CAS.
- G. Gopalakrishnan, C. Danelon, P. Izewska, M. Prummer, P. Y. Bolinger, I. Geissbuhler, D. Demurtas, J. Dubochet and H. Vogel, Angew. Chem., Int. Ed., 2006, 45, 5478–5483 CrossRef CAS PubMed.
- E. Sackmann, FEBS Lett., 1994, 346, 3–16 CrossRef CAS PubMed.
- H. Noguchi and M. Takasu, Biophys. J., 2002, 83, 299–308 CrossRef CAS PubMed.
- D. Zhang, L. Wei, M. L. Zhong, L. H. Xiao, H. W. Li and J. F. Wang, Chem. Sci., 2018, 9, 5260–5269 RSC.
- Y. L. Dai, C. Xu, X. L. Sun and X. Y. Chen, Chem. Soc. Rev., 2017, 46, 3830–3852 RSC.
- F. Liu, D. Wu and K. Chen, J. Nanopart. Res., 2014, 16, 2556 CrossRef.
- J. L. Tang, K. Schoenwald, D. Potter, D. White and T. Sulchek, Langmuir, 2012, 28, 10033–10039 CrossRef CAS PubMed.
- J. Jiang, H. W. Gu, H. L. Shao, E. Devlin, G. C. Papaefthymiou and J. Y. Ying, Adv. Mater., 2008, 20, 4403–4407 CrossRef CAS.
- K. Davis, B. Cole, M. Ghelardini, B. A. Powell and O. T. Mefford, Langmuir, 2016, 32, 13716–13727 CrossRef CAS PubMed.
- C. I. Mary, M. Senthilkumar and S. M. Babu, Bull. Mater. Sci., 2019, 42, 256 CrossRef.
- T. S. Hauck, A. A. Ghazani and W. C. W. Chan, Small, 2008, 4, 153–159 CrossRef CAS PubMed.
- Y. K. Gong and F. M. Winnik, Nanoscale, 2012, 4, 360–368 RSC.
- X. Liu, H. Li, Q. Jin and J. Ji, Small, 2014, 10, 4230–4242 CAS.
- K. Pombo Garcia, K. Zarschler, L. Barbaro, J. A. Barreto, W. O'Malley, L. Spiccia, H. Stephan and B. Graham, Small, 2014, 10, 2516–2529 CrossRef PubMed.
- D. Li, Z. Y. Miao, C. Y. Bao, X. L. Xu and Q. Zhang, Eur. Polym. J., 2020, 135, 109888 CrossRef CAS.
- S. Q. Qi, J. H. Sun, J. P. Ma, Y. Sun, K. Goossens, H. Li, P. Jia, X. Y. Fan, C. W. Bielawski and J. X. Geng, Nanotechnology, 2019, 30, 024001 CrossRef PubMed.
- S. Rana, A. Bajaj, R. Mout and V. M. Rotello, Adv. Drug Delivery Rev., 2012, 64, 200–216 CrossRef CAS PubMed.
- Y. C. Yeh, B. Creran and V. M. Rotello, Nanoscale, 2012, 4, 1871–1880 RSC.
- R. Sardar and J. S. Shumaker-Parry, J. Am. Chem. Soc., 2011, 133, 8179–8190 CrossRef CAS PubMed.
- M. Grzelczak, J. Perez-Juste, P. Mulvaney and L. M. Liz-Marzan, Chem. Soc. Rev., 2008, 37, 1783–1791 RSC.
- J. Turkevich, P. C. Stevenson and J. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- G. Frens, Nat. Phys. Sci., 1973, 241, 20–22 CrossRef CAS.
- Z. Babaei Afrapoli, R. Faridi Majidi, B. Negahdari and G. Tavoosidana, Nanomed. Res. J., 2018, 3, 190–196 Search PubMed.
- H. Tyagi, A. Kushwaha, A. Kumar and M. Aslam, Nanoscale Res. Lett., 2016, 11, 362 CrossRef PubMed.
- C. Shan, H. Yang, D. Han, Q. Zhang, A. Ivaska and L. Niu, Biosens. Bioelectron., 2010, 25, 1070–1074 CrossRef CAS PubMed.
- E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759–1782 RSC.
- M. Giersig and P. Mulvaney, Langmuir, 1993, 9, 3408–3413 CrossRef CAS.
- C. A. Waters, A. J. Mills, K. A. Johnson and D. J. Schiffrin, Chem. Commun., 2003, 540–541, 10.1039/B211874B,.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802, 10.1039/C39940000801,.
- Z. P. Guven, P. H. J. Silva, Z. Luo, U. B. Cendrowska, M. Gasbarri, S. T. Jones and F. Stellacci, J. Visualized Exp., 2019, 149, e58872 Search PubMed.
- A. M. Jackson, J. W. Myerson and F. Stellacci, Nat. Mater., 2004, 3, 330–336 CrossRef CAS PubMed.
- G. A. DeVries, M. Brunnbauer, Y. Hu, A. M. Jackson, B. Long, B. T. Neltner, O. Uzun, B. H. Wunsch and F. Stellacci, Science, 2007, 315, 358–361 CrossRef CAS PubMed.
- R. J. Barsotti and F. Stellacci, J. Mater. Chem., 2006, 16, 962–965 RSC.
- O. Zeiri, Y. F. Wang, A. Neyman, F. Stellacci and I. A. Weinstock, Angew. Chem., Int. Ed., 2013, 52, 968–972 CrossRef CAS PubMed.
- C. Singh, P. K. Ghorai, M. A. Horsch, A. M. Jackson, R. G. Larson, F. Stellacci and S. C. Glotzer, Phys. Rev. Lett., 2007, 99, 798–799 CrossRef PubMed.
- R. P. Carney, G. A. DeVries, C. Dubois, H. Kim, J. Y. Kim, C. Singh, P. K. Ghorai, J. B. Tracy, R. L. Stiles, R. W. Murray, S. C. Glotzer and F. Stellacci, J. Am. Chem. Soc., 2008, 130, 798–799 CrossRef CAS PubMed.
- T. K. Mandal, M. S. Fleming and D. R. Walt, Nano Lett., 2002, 2, 3–7 CrossRef.
- Z. Zhang, G. Sebe, X. S. Wang and K. C. Tam, Carbohydr. Polym., 2018, 182, 61–68 CrossRef CAS PubMed.
- Y. L. Xianyu, Z. Wang, J. S. Sun, X. F. Wang and X. Y. Jiang, Small, 2014, 10, 4833–4838 CrossRef CAS PubMed.
- R. Raghav and S. Srivastava, Biosens. Bioelectron., 2016, 78, 396–403 CrossRef CAS PubMed.
- M. Larguinho, R. Canto, M. Cordeiro, P. Pedrosa, A. Fortuna, R. Vinhas and P. V. Baptista, Expert Rev. Mol. Diagn., 2015, 15, 1355–1368 CrossRef CAS PubMed.
- R. Schreiber, I. Santiago, A. Ardavan and A. J. Turberfield, ACS Nano, 2016, 10, 7303–7306 CrossRef CAS PubMed.
- S. H. Medina and M. E. H. El-Sayed, Chem. Rev., 2009, 109, 3141–3157 CrossRef CAS PubMed.
- D. Tomalia, H. Baker, J. Dewald, M. Hall, G. Kallos, S. Martin, J. Roeck, J. Ryder and P. Smith, Polym. J., 1985, 17, 117–132 CrossRef CAS.
- D. Tomalia, Macromol. Symp., 1996, 101, 243–255 CrossRef CAS.
- C. Hawker and J. Frechet, J. Am. Chem. Soc., 1990, 112, 7638–7647 CrossRef CAS.
- X. Feng, Y. Liang, L. Zhi, A. Thomas, D. Wu, I. Lieberwirth, U. Kolb and K. Müllen, Adv. Funct. Mater., 2009, 19, 2125–2129 CrossRef CAS.
- B. A. Hammer and K. Mullen, Chem. Rev., 2016, 116, 2103–2140 CrossRef CAS PubMed.
- T. T. Nguyen, M. Baumgarten, A. Rouhanipour, H. J. Rader, I. Lieberwirth and K. Mullen, J. Am. Chem. Soc., 2013, 135, 4183–4186 CrossRef CAS PubMed.
- D. Türp, T.-T.-T. Nguyen, M. Baumgarten and K. Müllen, New J. Chem., 2012, 36, 282–298 RSC.
- R. Stangenberg, Y. Wu, J. Hedrich, D. Kurzbach, D. Wehner, G. Weidinger, S. L. Kuan, M. I. Jansen, F. Jelezko, H. J. Luhmann, D. Hinderberger, T. Weil and K. Müllen, Adv. Healthcare Mater., 2015, 4, 377–384 CrossRef CAS PubMed.
- B. A. G. Hammer, R. Moritz, R. Stangenberg, M. Baumgarten and K. Müllen, Chem. Soc. Rev., 2015, 44, 4072–4090 RSC.
- B. A. G. Hammer and K. Mullen, J. Nanopart. Res., 2018, 20, 262 CrossRef PubMed.
- P. G. d. Gennes, Science, 1992, 256, 495–497 CrossRef PubMed.
- X. T. Yu, Y. J. Sun, F. X. Liang, B. Y. Jiang and Z. Z. Yang, Macromolecules, 2019, 52, 96–102 CrossRef CAS.
- H. Wang, W. M. Hou, Y. Z. Liu, L. Liu and H. Y. Zhao, Macromol. Rapid Commun., 2021, 42, 2000589 CrossRef CAS PubMed.
- G. Liu, J. Tian, X. Zhang and H. Zhao, Chem. – Asian J., 2014, 9, 2597–2603 CrossRef CAS PubMed.
- S. Q. Chen, C. He, H. J. Li, P. Y. Li and W. D. He, Polym. Chem., 2016, 7, 2476–2485 RSC.
- J. S. J. Tan and Z. Chen, J. Colloid Interface Sci., 2019, 546, 285–292 CrossRef CAS PubMed.
- J. S. J. Tan, S. L. Y. Wong and Z. Chen, Adv. Mater. Interfaces, 2020, 7, 1901961 CrossRef CAS.
- J. S. J. Tan, X. Cao, Y. Z. Huang and Z. Chen, Colloids Surf., A, 2020, 589, 124460 CrossRef CAS.
- J. S. J. Tan, C. H. Wong and Z. Chen, Langmuir, 2021, 37, 8167–8176 CrossRef CAS PubMed.
- D. Wu, L. Wang, W. Li, X. Xu and W. Jiang, Int. J. Pharm., 2017, 533, 169–178 CrossRef CAS PubMed.
- K. Halvorsen and W. P. Wong, PLoS One, 2012, 7, e44212 CrossRef CAS PubMed.
- P. W. K. Rothemund, Nature, 2006, 440, 297–302 CrossRef CAS PubMed.
- Y. G. Ke, S. Lindsay, Y. Chang, Y. Liu and H. Yan, Science, 2008, 319, 180–183 CrossRef CAS PubMed.
- J. Hellmeier, R. Platzer, V. Muhlgrabner, M. C. Schneider, E. Kurz, G. J. Schutz, J. B. Huppa and E. Sevcsik, ACS Nano, 2021, 15, 15057–15068 CrossRef CAS PubMed.
- Z. L. Ge, L. J. Guo, G. Q. Wu, J. Li, Y. L. Sun, Y. Q. Hou, J. Y. Shi, S. P. Song, L. H. Wang, C. H. Fan, H. Lu and Q. Li, Small, 2020, 16, e1904857 CrossRef PubMed.
- H. T. Maune, S. P. Han, R. D. Barish, M. Bockrath, W. A. Goddard, P. W. K. Rothemund and E. Winfree, Nat. Nanotechnol., 2010, 5, 61–66 CrossRef CAS PubMed.
- N. V. Voigt, T. Torring, A. Rotaru, M. F. Jacobsen, J. B. Ravnsbaek, R. Subramani, W. Mamdouh, J. Kjems, A. Mokhir, F. Besenbacher and K. V. Gothelf, Nat. Nanotechnol., 2010, 5, 200–203 CrossRef CAS PubMed.
- Z. Liu, Z. Li, H. Zhou, G. Wei, Y. Song and L. Wang, J. Microsc., 2005, 218, 233–239 CrossRef CAS PubMed.
- V. V. Thacker, L. O. Herrmann, D. O. Sigle, T. Zhang, T. Liedl, J. J. Baumberg and U. F. Keyser, Nat. Commun., 2014, 5, 3448 CrossRef PubMed.
- C. Zhou, D. Wang, Y. Dong, L. Xin, Y. Sun, Z. Yang and D. Liu, Small, 2015, 11, 1161–1164 CrossRef CAS PubMed.
- C. Zhou, Y. Zhang, Y. Dong, F. Wu, D. Wang, L. Xin and D. Liu, Adv. Mater., 2016, 28, 9819–9823 CrossRef CAS PubMed.
- J. List, M. Weber and F. C. Simmel, Angew. Chem., Int. Ed., 2014, 53, 4236–4239 CrossRef CAS PubMed.
- Y. Suzuki, M. Endo, Y. Y. Yang and H. Sugiyama, J. Am. Chem. Soc., 2014, 136, 1714–1717 CrossRef CAS PubMed.
- S. Li, Q. Jiang, S. Liu, Y. Zhang, Y. Tian, C. Song, J. Wang, Y. Zou, G. J. Anderson, J. Y. Han, Y. Chang, Y. Liu, C. Zhang, L. Chen, G. Zhou, G. Nie, H. Yan, B. Ding and Y. Zhao, Nat. Biotechnol., 2018, 36, 258–264 CrossRef CAS PubMed.
- K. Lund, A. J. Manzo, N. Dabby, N. Michelotti, A. Johnson-Buck, J. Nangreave, S. Taylor, R. J. Pei, M. N. Stojanovic, N. G. Walter, E. Winfree and H. Yan, Nature, 2010, 465, 206–210 CrossRef CAS PubMed.
- S. F. J. Wickham, J. Bath, Y. Katsuda, M. Endo, K. Hidaka, H. Sugiyama and A. J. Turberfield, Nat. Nanotechnol., 2012, 7, 169–173 CrossRef CAS PubMed.
- A. J. Thubagere, W. Li, R. F. Johnson, Z. B. Chen, S. Doroudi, Y. L. Lee, G. Izatt, S. Wittman, N. Srinivas, D. Woods, E. Winfree and L. L. Qian, Science, 2017, 357, 1095 CrossRef PubMed.
- J. V. Gregory, P. Kadiyala, R. Doherty, M. Cadena, S. Habeel, E. Ruoslahti, P. R. Lowenstein, M. G. Castro and J. Lahann, Nat. Commun., 2020, 11, 1–15 Search PubMed.
- A. E. Czapar and N. F. Steinmetz, Curr. Opin. Chem. Biol., 2017, 38, 108–116 CrossRef CAS PubMed.
- Q. Zhou, F. Wu, M. Wu, Y. Tian and Z. Niu, Chem. Commun., 2015, 51, 15122–15124 RSC.
- J. Fueyo, R. Alemany, C. Gomez-Manzano, G. N. Fuller, A. Khan, C. A. Conrad, T. J. Liu, H. Jiang, M. G. Lemoine, K. Suzuki, R. Sawaya, D. T. Curiel, W. K. A. Yung and F. F. Lang, J. Natl. Cancer Inst., 2003, 95, 652–660 CrossRef CAS PubMed.
- H. Uusi-Kerttula, M. Legut, J. Davies, R. Jones, E. Hudson, L. Hanna, R. J. Stanton, J. D. Chester and A. L. Parker, Hum. Gene Ther., 2015, 26, 320–329 CrossRef CAS PubMed.
- L. Yu, H. Takenobu, O. Shimozato, K. Kawamura, Y. Nimura, N. Seki, K. Uzawa, H. Tanzawa, H. Shimada, T. Ochiai and M. Tagawa, Oncol. Rep., 2005, 14, 831–835 Search PubMed.
- M. Tagawa, K. Kawamura, G. Y. Ma, Q. H. Li, Y. Takei, M. Yamanaka, M. Nakamura, Y. Tada, Y. Takiguchi, K. Tatsumi, N. Suzuki, N. Yamaguchi, K. Hiroshima and H. Shimada, Mol. Ther., 2009, 17, S235–S235 Search PubMed.
- P. Leissner, V. Legrand, Y. Schlesinger, D. A. Hadji, M. van Raaij, S. Cusack, A. Pavirani and M. Mehtali, Gene Ther., 2001, 8, 49–57 CrossRef CAS PubMed.
- S. A. Nicklin, E. Wu, G. R. Nemerow and A. H. Baker, Mol. Ther., 2005, 12, 384–393 CrossRef CAS PubMed.
- J. Kim, H. Y. Nam, T. I. Kim, P. H. Kim, J. Ryu, C. O. Yun and S. W. Kim, Biomaterials, 2011, 32, 5158–5166 CrossRef CAS PubMed.
- P. H. Kim, J. H. Sohn, J. W. Choi, Y. Jung, S. W. Kim, S. Haam and C. O. Yun, Biomaterials, 2011, 32, 2314–2326 CrossRef CAS PubMed.
- Y. Wan, J. F. Han, G. R. Fan, Z. R. Zhang, T. Gong and X. Sun, Biomaterials, 2013, 34, 3020–3030 CrossRef CAS PubMed.
- C. H. Lee, D. Kasala, Y. Na, M. S. Lee, S. W. Kim, J. H. Jeong and C. O. Yun, Biomaterials, 2014, 35, 5505–5516 CrossRef CAS PubMed.
- J. W. Choi, S. J. Jung, D. Kasala, J. K. Hwang, J. Hu, Y. H. Bae and C. O. Yun, J. Controlled Release, 2015, 205, 134–143 CrossRef CAS PubMed.
- J. W. Choi, J. W. Park, Y. Na, S. J. Jung, J. K. Hwang, D. Choi, K. G. Lee and C. O. Yun, Biomaterials, 2015, 65, 163–174 CrossRef CAS PubMed.
- E. C. Arvaniti, M. C. G. Juenger, S. A. Bernal, J. Duchesne, L. Courard, S. Leroy, J. L. Provis, A. Klemm and N. De Belie, Mater. Struct., 2015, 48, 3687–3701 CrossRef CAS.
- J. D. Clogston and A. K. Patri, in Characterization of Nanoparticles Intended for Drug Delivery, ed. S. E. McNeil, Humana Press, Totowa, NJ, 2011 DOI:10.1007/978-1-60327-198-1_6, pp. 63–70.
- C. C. Guo and J. L. Yarger, Magn. Reson. Chem., 2018, 56, 1074–1082 CrossRef CAS PubMed.
- E. A. F. Van Doren, P. J. R. H. De Temmerman, M. A. D. Francisco and J. Mast, J. Nanobiotechnol., 2011, 9, 17 CrossRef CAS PubMed.
- S. Vilayurganapathy, A. Devaraj, R. Colby, A. Pandey, T. Varga, V. Shutthanandan, S. Manandhar, P. Z. El-Khoury, A. Kayani, W. P. Hess and S. Thevuthasan, Nanotechnology, 2013, 24, 095707 CrossRef CAS PubMed.
- M. Quintana, E. Haro-Poniatowski, J. Morales and N. Batina, Appl. Surf. Sci., 2002, 195, 175–186 CrossRef CAS.
- A. I. Lopez-Lorente and B. Mizaikoff, TrAC, Trends Anal. Chem., 2016, 84, 97–106 CrossRef CAS.
- R. Abu Mukh-Qasem and A. Gedanken, J. Colloid Interface Sci., 2005, 284, 489–494 CrossRef CAS PubMed.
- H. Y. Zhou, X. Li, A. Lemoff, B. Zhang and B. Yan, Analyst, 2010, 135, 1210–1213 RSC.
- C. Jana, G. Jayamurugan, R. Ganapathy, P. K. Maiti, N. Jayaraman and A. K. Sood, J. Chem. Phys., 2006, 124, 204719 CrossRef PubMed.
- I. S. Mashhadi, M. R. Safarnejad, M. Shahmirzaie, A. Aliahmadi and A. Ghassempour, Anal. Chem., 2020, 92, 10460–10469 CrossRef PubMed.
- S. J. Singer and G. L. Nicolson, Science, 1972, 175, 720–731 CrossRef CAS PubMed.
- T. Harder, P. Scheiffele, P. Verkade and K. Simons, J. Cell Biol., 1998, 141, 929–942 CrossRef CAS PubMed.
- K. Murase, T. Fujiwara, Y. Umemura, K. Suzuki, R. Iino, H. Yamashita, M. Saito, H. Murakoshi, K. Ritchie and A. Kusumi, Biophys. J., 2004, 86, 4075–4093 CrossRef CAS PubMed.
- S. M. Lu and G. D. Fairn, Crit. Rev. Biochem. Mol. Biol., 2018, 53, 192–207 CrossRef CAS PubMed.
- A. Kusumi, T. K. Fujiwara, R. Chadda, M. Xie, T. A. Tsunoyama, Z. Kalay, R. S. Kasai and K. G. Suzuki, Annu. Rev. Cell Dev. Biol., 2012, 28, 215–250 CrossRef CAS PubMed.
- S. Meinhardt and F. Schmid, Soft Matter, 2019, 15, 1942–1952 RSC.
- D. Lingwood and K. Simons, Science, 2010, 327, 46–50 CrossRef CAS PubMed.
- A. L. Duncan, T. Reddy, H. Koldso, J. Helie, P. W. Fowler, M. Chavent and M. S. P. Sansom, Sci. Rep., 2017, 7, 16647 CrossRef PubMed.
- M. A. Lemmon and J. Schlessinger, Cell, 2010, 141, 1117–1134 CrossRef CAS PubMed.
- I. Levental, D. Lingwood, M. Grzybek, U. Coskun and K. Simons, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 22050–22054 CrossRef CAS PubMed.
- C. E. de Castro, J. J. Bonvent, M. C. da Silva, F. L. Castro and F. C. Giacomelli, Macromol. Biosci., 2016, 16, 1643–1652 CrossRef CAS PubMed.
- S. S. Sun, Y. Y. Huang, C. Zhou, S. S. Chen, M. X. Yu, J. B. Liu and J. Zheng, Bioconjugate Chem., 2018, 29, 1841–1846 CrossRef CAS PubMed.
- Y. C. Guo, E. Terazzi, R. Seemann, J. B. Fleury and V. A. Baulin, Sci. Adv., 2016, 2, e1600261 CrossRef PubMed.
- M. R. Rasch, E. Rossinyol, J. L. Hueso, B. W. Goodfellow, J. Arbiol and B. A. Korgel, Nano Lett., 2010, 10, 3733–3739 CrossRef CAS PubMed.
- Y. Li, X. Chen and N. Gu, J. Phys. Chem. B, 2008, 112, 16647–16653 CrossRef CAS PubMed.
- H. M. Ding, W. D. Tian and Y. Q. Ma, ACS Nano, 2012, 6, 1230–1238 CrossRef CAS PubMed.
- L. Zhang, M. Becton and X. Wang, J. Phys. Chem. B, 2015, 119, 3786–3794 CrossRef CAS PubMed.
- X. B. Quan, C. W. Peng, D. H. Zhao, L. B. Li, J. Fan and J. Zhou, Langmuir, 2017, 33, 361–371 CrossRef CAS PubMed.
- J. Ma, K. Lee, J. Ban, H. Son, J. Koh, G. Hwang, H. Kim, K. Lee, M. Vang, H. Yang, H. Lim, G. Oh, S. Park and K. Yun, J. Nanosci. Nanotechnol., 2013, 13, 3801–3804 CrossRef CAS PubMed.
- C. Freese, M. I. Gibson, H. A. Klok, R. E. Unger and C. J. Kirkpatrick, Biomacromolecules, 2012, 13, 1533–1543 CrossRef CAS PubMed.
- C. A. Goodman, B. L. Frankamp, B. A. Cooper and V. A. Rotello, Colloids Surf., B, 2004, 39, 119–123 CrossRef CAS PubMed.
- E. Frohlich, Int. J. Nanomed., 2012, 7, 5577–5591 CrossRef PubMed.
- Z. G. Yue, W. Wei, P. P. Lv, H. Yue, L. Y. Wang, Z. G. Su and G. H. Ma, Biomacromolecules, 2011, 12, 2440–2446 CrossRef CAS PubMed.
- A. Akesson, K. M. Bendtsen, M. A. Beherens, J. S. Pedersen, V. Alfredsson and M. Cardenas Gomez, Phys. Chem. Chem. Phys., 2010, 12, 12267–12272 RSC.
- A. A. Gurtovenko, J. Anwar and I. Vattulainen, Chem. Rev., 2010, 110, 6077–6103 CrossRef CAS PubMed.
- C. L. Ting and Z. G. Wang, Biophys. J., 2011, 100, 1288–1297 CrossRef CAS PubMed.
- H. Lee and R. G. Larson, Molecules, 2009, 14, 423–438 CrossRef CAS PubMed.
- V. V. Ginzburg and S. Balijepailli, Nano Lett., 2007, 7, 3716–3722 CrossRef CAS PubMed.
- P. R. Leroueil, S. Y. Hong, A. Mecke, J. R. Baker, B. G. Orr and M. M. B. Holl, Acc. Chem. Res., 2007, 40, 335–342 CrossRef CAS PubMed.
- H. Lee and R. G. Larson, J. Phys. Chem. B, 2006, 110, 18204–18211 CrossRef CAS PubMed.
- S. Y. Xiang, J. Wagner, T. Luckerath, K. Mullen, D. Y. W. Ng, J. Hedrich and T. Weil, Adv. Healthcare Mater., 2021, 2101854 Search PubMed.
- B. A. G. Hammer, Y. Wu, S. Fischer, W. Liu, T. Weil and K. Mullen, ChemBioChem, 2017, 18, 960–964 CrossRef CAS PubMed.
- E. C. Cho, L. Au, Q. Zhang and Y. N. Xia, Small, 2010, 6, 517–522 CrossRef CAS PubMed.
- R. C. Van Lehn, P. U. Atukorale, R. P. Carney, Y. S. Yang, F. Stellacci, D. J. Irvine and A. Alexander-Katz, Nano Lett., 2013, 13, 4060–4067 CrossRef CAS PubMed.
- E. Okoampah, Y. S. Mao, S. Y. Yang, S. S. Sun and C. Zhou, Colloids Surf., B, 2020, 196, 111312 CrossRef CAS PubMed.
- A. Akinc and G. Battaglia, Cold Spring Harbor Perspect. Biol., 2013, 5, a016980 CrossRef PubMed.
- G. Sahay, D. Y. Alakhova and A. V. Kabanov, J. Controlled Release, 2010, 145, 182–195 CrossRef CAS PubMed.
- W. Jiang, B. Y. S. Kim, J. T. Rutka and W. C. W. Chan, Nat. Nanotechnol., 2008, 3, 145–150 CrossRef CAS PubMed.
- C. Wang, B. B. Chen, M. He and B. Hu, ACS Nano, 2021, 15, 3108–3122 CrossRef CAS PubMed.
- T. Lunnoo, J. Assawakhajornsak, S. Ruangchai and T. Puangmali, J. Phys. Chem. B, 2020, 124, 1898–1908 CrossRef CAS PubMed.
- X. B. Quan, D. H. Zhao and J. Zhou, Phys. Chem. Chem. Phys., 2021, 23, 23526–23536 RSC.
- A. M. Jackson, J. W. Myerson and F. Stellacci, Nat. Mater., 2004, 3, 330–336 CrossRef CAS PubMed.
- A. Verma and F. Stellacci, Small, 2010, 6, 12–21 CrossRef CAS PubMed.
- P. Gkeka, L. Sarkisov and P. Angelikopoulos, J. Phys. Chem. Lett., 2013, 4, 1907–1912 CrossRef CAS PubMed.
- Y. Li, X. Li, Z. Li and H. Gao, Nanoscale, 2012, 4, 3768–3775 RSC.
- F. L. Tian, T. T. Yue, Y. Li and X. R. Zhang, Sci. China: Chem., 2014, 57, 1662–1671 CrossRef CAS.
- R. C. Van Lehn, M. Ricci, P. H. J. Silva, P. Andreozzi, J. Reguera, K. Voitchovsky, F. Stellacci and A. Alexander-Katz, Nat. Commun., 2014, 5, 4482 CrossRef CAS PubMed.
- N. Adachi, A. Maruyama, T. Ishihara and T. Akaike, J. Biomater. Sci., Polym. Ed., 1994, 6, 463–479 CrossRef CAS PubMed.
- C.-P. Tsai, C.-Y. Chen, Y. Hung, F.-H. Chang and C.-Y. Mou, J. Mater. Chem., 2009, 19, 5737–5743 RSC.
- J. B. Haun and D. A. Hammer, Langmuir, 2008, 24, 8821–8832 CrossRef CAS PubMed.
- S. Tzlil, M. Deserno, W. M. Gelbert and A. Ben-Shaul, Biophys. J., 2004, 86, 2037–2048 CrossRef CAS PubMed.
- G. Bao and X. R. Bao, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9997–9998 CrossRef CAS PubMed.
- H. Gao, W. Shi and L. B. Freund, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 9469–9474 CrossRef CAS PubMed.
- H. Y. Yuan and S. L. Zhang, Appl. Phys. Lett., 2010, 96, 033704 CrossRef.
- H. Yuan, J. Li, G. Bao and S. Zhang, Phys. Rev. Lett., 2010, 105, 138101 CrossRef PubMed.
- Y. G. Srinivasulu, Q. F. Yao, N. Goswami and J. P. Xie, Mater. Horiz., 2020, 7, 2596–2618 RSC.
- C. Dalal, A. Saha and N. R. Jana, J. Phys. Chem. C, 2016, 120, 13324 CrossRef CAS.
- M. A. Ackun-Farmmer, K. L. Alatise, G. Cross and D. S. W. Benoit, Adv. Biosyst., 2020, 4, e2000172 CrossRef PubMed.
- T. K. Hart, A. M. Klinkner, J. Ventre and P. J. Bugelski, J. Histochem. Cytochem., 1993, 41, 265–271 CrossRef CAS PubMed.
- A. M. Wen, P. H. Rambhia, R. H. French and N. F. Steinmetz, J. Biol. Phys., 2013, 39, 301–325 CrossRef CAS PubMed.
- H. M. Ding and Y. Q. Ma, Biomaterials, 2012, 33, 5798–5802 CrossRef CAS PubMed.
- V. Schubertova, F. J. Martinez-Veracoechea and R. Vacha, Soft Matter, 2015, 11, 2726–2730 RSC.
- L. Li, Y. Zhang and J. Wang, R. Soc. Open Sci., 2017, 4, 170063 CrossRef PubMed.
- E. Moradi, D. Vllasaliu, M. Garnett, F. Falcone and S. Stolnik, RSC Adv., 2012, 2, 3025–3033 RSC.
- A. Shaw, V. Lundin, E. Petrova, F. Fordos, E. Benson, A. Al-Amin, A. Herland, A. Blokzijl, B. Hogberg and A. I. Teixeira, Nat. Methods, 2014, 11, 841–846 CrossRef CAS PubMed.
- T. Verheyen, T. Fang, D. Lindenhofer, Y. Wang, K. Akopyan, A. Lindqvist, B. Hogberg and A. I. Teixeira, Nucleic Acids Res., 2020, 48, 5777–5787 CrossRef CAS PubMed.
- K. Liu, C. Xu and J. Y. Liu, J. Mater. Chem. B, 2020, 8, 6802–6809 RSC.
- T. Fang, J. Alvelid, J. Spratt, E. Ambrosetti, I. Testa and A. I. Teixeira, ACS Nano, 2021, 15, 3441–3452 CrossRef CAS PubMed.
- N. Liu, M. Tang and J. Ding, Chemosphere, 2020, 245, 125624 CrossRef CAS PubMed.
- R. K. Mishra, A. Ahmad, A. Vyawahare, P. Alam, T. H. Khan and R. Khan, Int. J. Biol. Macromol., 2021, 175, 1–18 CrossRef CAS PubMed.
- H. Sun, C. Jiang, L. Wu, X. Bai and S. Zhai, Front. Bioeng. Biotechnol., 2019, 7, 414 CrossRef PubMed.
- Y. Li, M. Girard, M. Shen, J. A. Millan and M. Olvera de la Cruz, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 11838–11843 CrossRef CAS PubMed.
- J. L. Kerstetter and W. M. Gramlich, J. Mater. Chem. B, 2014, 2, 8043–8052 RSC.
- Y. Zou, S. Ito, F. Yoshino, Y. Suzuki, L. Zhao and N. Komatsu, ACS Nano, 2020, 14, 7216–7226 CrossRef CAS PubMed.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Nat. Rev. Mater., 2016, 1, 16014 CrossRef CAS.
- R. Tavano, L. Gabrielli, E. Lubian, C. Fedeli, S. Visentin, P. Polverino De Laureto, G. Arrigoni, A. Geffner-Smith, F. Chen, D. Simberg, G. Morgese, E. M. Benetti, L. Wu, S. M. Moghimi, F. Mancin and E. Papini, ACS Nano, 2018, 12, 5834–5847 CrossRef CAS PubMed.
- X. Bai, M. Xu, S. Liu and G. Hu, ACS Appl. Mater. Interfaces, 2018, 10, 20368–20376 CrossRef CAS PubMed.
- M. S. Ehrenberg, A. E. Friedman, J. N. Finkelstein, G. Oberdorster and J. L. McGrath, Biomaterials, 2009, 30, 603–610 CrossRef CAS PubMed.
- L. Digiacomo, F. Cardarelli, D. Pozzi, S. Palchetti, M. A. Digman, E. Gratton, A. L. Capriotti, M. Mahmoudi and G. Caracciolo, Nanoscale, 2017, 9, 17254–17262 RSC.
- N. Feiner-Gracia, M. Beck, S. Pujals, S. Tosi, T. Mandal, C. Buske, M. Linden and L. Albertazzi, Small, 2017, 13, 1701631 CrossRef PubMed.
- M. Das, D. K. Yi and S. S. An, Int. J. Nanomed., 2015, 10, 1521–1545 CAS.
- K. He, X. Liang, T. Wei, N. Liu, Y. Wang, L. Zou, J. Lu, Y. Yao, L. Kong, T. Zhang, Y. Xue, T. Wu and M. Tang, J. Appl. Toxicol., 2019, 39, 525–539 CrossRef CAS PubMed.
- N. Shinohara, G. Zhang, Y. Oshima, T. Kobayashi, N. Imatanaka, M. Nakai, T. Sasaki, K. Kawaguchi and M. Gamo, Part. Fibre Toxicol., 2017, 14, 48 CrossRef PubMed.
- J. C. Y. Kah, C. Grabinski, E. Untener, C. Garrett, J. Chen, D. Zhu, S. M. Hussain and K. Hamad-Schifferli, ACS Nano, 2014, 8, 4608–4620 CrossRef CAS PubMed.
- H. M. Ding and Y. Q. Ma, Biomaterials, 2014, 35, 8703–8710 CrossRef CAS PubMed.
- E. Casals, T. Pfaller, A. Duschl, G. J. Oostingh and V. F. Puntes, Small, 2011, 7, 3479–3486 CrossRef CAS PubMed.
- A. Bekdemir, S. Liao and F. Stellacci, Colloids Surf., B, 2019, 174, 367–373 CrossRef CAS PubMed.
- R. Stangenberg, I. Saeed, S. L. Kuan, M. Baumgarten, T. Weil, M. Klapper and K. Mullen, Macromol. Rapid Commun., 2014, 35, 152–160 CrossRef CAS PubMed.
- N. Duran, C. P. Silveira, M. Duran and D. S. Martinez, J. Nanobiotechnol., 2015, 13, 55 CrossRef PubMed.
- M. S. P. Boyles, T. Kristl, A. Andosch, M. Zimmermann, N. Tran, E. Casals, M. Himly, V. Puntes, C. G. Huber, U. Lutz-Meindl and A. Duschl, J. Nanobiotechnol., 2015, 13, 84 CrossRef PubMed.
- C. C. Fleischer and C. K. Payne, Acc. Chem. Res., 2014, 47, 2651–2659 CrossRef CAS PubMed.
- C. D. Walkey, J. B. Olsen, H. Guo, A. Emili and W. C. Chan, J. Am. Chem. Soc., 2012, 134, 2139–2147 CrossRef CAS PubMed.
- W. J. Yang, K.-G. Neoh, E.-T. Kang, S. L.-M. Teo and D. Rittschof, Prog. Polym. Sci., 2014, 39, 1017–1042 CrossRef CAS.
- L. N. Pilon, S. P. Armes, P. Findlay and S. P. Rannard, Langmuir, 2005, 21, 3808–3813 CrossRef CAS PubMed.
- N. Feliu, D. Docter, M. Heine, P. del Pino, S. Ashraf, J. Kolosnjaj-Tabi, P. Macchiarini, P. Nielsen, D. Alloyeau, F. Gazeau, R. H. Stauber and W. J. Parak, Chem. Soc. Rev., 2016, 45, 2440–2457 RSC.
- L. Lartigue, C. Wilhelm, J. Servais, C. Factor, A. Dencausse, J. C. Bacri, N. Luciani and F. Gazeau, ACS Nano, 2012, 6, 2665–2678 CrossRef CAS PubMed.
- J. Y. Liu and Q. A. Peng, Acta Biomater., 2017, 55, 13–27 CrossRef CAS PubMed.
- F. Charbgoo, M. Nejabat, K. Abnous, F. Soltani, S. M. Taghdisi, M. Alibolandi, W. T. Shier, T. W. J. Steele and M. Ramezani, J. Controlled Release, 2018, 272, 39–53 CrossRef CAS PubMed.
- Y. Ma, J. Hong and Y. Ding, Adv. Healthcare Mater., 2020, 9, 1901448 CrossRef CAS PubMed.
- Z. J. Zhu, T. Posati, D. F. Moyano, R. Tang, B. Yan, R. W. Vachet and V. M. Rotello, Small, 2012, 8, 2659–2663 CrossRef CAS PubMed.
- D. Huhn, K. Kantner, C. Geidel, S. Brandholt, I. De Cock, S. J. H. Soenen, P. R. Gil, J. M. Montenegro, K. Braeckmans, K. Mullen, G. U. Nienhaus, M. Klapper and W. J. Parak, ACS Nano, 2013, 7, 3253–3263 CrossRef PubMed.
- W. Yang, L. Zhang, S. L. Wang, A. D. White and S. Y. Jiang, Biomaterials, 2009, 30, 5617–5621 CrossRef CAS PubMed.
- K. P. Garcia, K. Zarschler, L. Barbaro, J. A. Barreto, W. O'Malley, L. Spiccia, H. Stephan and B. Graham, Small, 2014, 10, 2516–2529 CrossRef CAS PubMed.
- A. Gupta, D. F. Moyano, A. Parnsubsakul, A. Papadopoulos, L. S. Wang, R. F. Landis, R. Das and V. M. Rotello, ACS Appl. Mater. Interfaces, 2016, 8, 14096–14101 CrossRef CAS PubMed.
- J. Mosquera, I. Garcia, M. Henriksen-Lacey, M. Martinez-Calvo, M. Dhanjani, J. L. Mascarenas and L. M. Liz-Marzan, ACS Nano, 2020, 14, 5382–5391 CrossRef CAS PubMed.
- Z. F. Ma, J. Bai and X. E. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 17614–17622 CrossRef CAS PubMed.
- E. Harrison, J. A. Coulter and D. Dixon, Nanomedicine, 2016, 11, 851–865 CrossRef CAS PubMed.
- C. D. Walkey, J. B. Olsen, H. B. Guo, A. Emili and W. C. W. Chan, J. Am. Chem. Soc., 2012, 134, 2139–2147 CrossRef CAS PubMed.
- M. A. Dobrovolskaia, B. W. Neun, S. Man, X. Y. Ye, M. Hansen, A. K. Patri, R. M. Crist and S. E. McNeil, Nanomedicine, 2014, 10, 1453–1463 CrossRef CAS PubMed.
- R. X. Huang, R. P. Carney, F. Stellacci and B. L. T. Lau, Nanoscale, 2013, 5, 6928–6935 RSC.
- R. X. Huang, R. R. Carney, K. Ikuma, F. Stellacci and B. L. T. Lau, ACS Nano, 2014, 8, 5402–5412 CrossRef CAS PubMed.
- Y. Wu, L. Li, L. Frank, J. Wagner, P. Andreozzi, B. Hammer, M. D'Alicarnasso, M. Pelliccia, W. Liu, S. Chakrabortty, S. Krol, J. Simon, K. Landfester, S. L. Kuan, F. Stellacci, K. Mullen, F. Kreppel and T. Weil, ACS Nano, 2019, 13, 8749–8759 CrossRef CAS PubMed.
- E. Blundell, M. J. Healey, E. Holton, M. Sivakumaran, S. Manstana and M. Platt, Anal. Bioanal. Chem., 2016, 408, 5757–5768 CrossRef CAS PubMed.
- X. H. Xu, Y. T. Jian, Y. K. Li, X. Zhang, Z. X. Tu and Z. W. Gu, ACS Nano, 2014, 8, 9255–9264 CrossRef CAS PubMed.
- Y. Dong, T. Yu, L. Ding, E. Laurini, Y. Huang, M. Zhang, Y. Weng, S. Lin, P. Chen, D. Marson, Y. Jiang, S. Giorgio, S. Pricl, X. Liu, P. Rocchi and L. Peng, J. Am. Chem. Soc., 2018, 140, 16264–16274 CrossRef CAS PubMed.
- G. Maiorano, S. Sabella, B. Sorce, V. Brunetti, M. A. Malvindi, R. Cingolani and P. P. Pompa, ACS Nano, 2010, 4, 7481–7491 CrossRef CAS PubMed.
- J. Lv, C. Wang, H. Li, Z. Li, Q. Fan, Y. Zhang, Y. Li, H. Wang and Y. Cheng, Nano Lett., 2020, 20, 8600–8607 CrossRef CAS PubMed.
- F. Yang, Y. Zhang and H. Liang, Int. J. Mol. Sci., 2014, 15, 3580–3595 CrossRef CAS PubMed.
- H. Mandula, J. M. Parepally, R. Feng and Q. R. Smith, J. Pharmacol. Exp. Ther., 2006, 317, 667–675 CrossRef CAS PubMed.
- A. Åkesson, M. Cárdenas, G. Elia, M. P. Monopoli and K. A. Dawson, RSC Adv., 2012, 2, 11245–11248 RSC.
- J. Giri, M. S. Diallo, A. J. Simpson, Y. Liu, W. A. Goddard, R. Kumar and G. C. Woods, ACS Nano, 2011, 5, 3456–3468 CrossRef CAS PubMed.
- E. Gabellieri, G. B. Strambini, D. Shcharbin, B. Klajnert and M. Bryszewska, Biochim. Biophys. Acta, Proteins Proteomics, 2006, 1764, 1750–1756 CrossRef CAS PubMed.
- Z. J. Deng, M. Liang, M. Monteiro, I. Toth and R. F. Minchin, Nat. Nanotechnol., 2010, 6, 39–44 CrossRef PubMed.
- M. A. Dobrovolskaia, A. K. Patri, J. Simak, J. B. Hall, J. Semberova, S. H. De Paoli Lacerda and S. E. McNeil, Mol. Pharmaceutics, 2012, 9, 382–393 CrossRef CAS PubMed.
- A. Akesson, M. Cardenas, G. Elia, M. P. Monopoli and K. A. Dawson, RSC Adv., 2012, 2, 11245–11248 RSC.
- B. Wang, Y. Sun, T. P. Davis, P. C. Ke, Y. Wu and F. Ding, ACS Sustainable Chem. Eng., 2018, 6, 11704–11715 CrossRef CAS PubMed.
- J. Wagner, M. Dillenburger, J. Simon, J. Oberlander, K. Landfester, V. Mailander, D. Y. W. Ng, K. Mullen and T. Weil, Chem. Commun., 2020, 56, 8663–8666 RSC.
- J. Wagner, L. Li, J. Simon, L. Krutzke, K. Landfester, V. Mailander, K. Mullen, D. Y. W. Ng, Y. Wu and T. Weil, Angew. Chem., Int. Ed., 2020, 59, 5712–5720 CrossRef CAS PubMed.
- J. Vonnemann, C. Sieben, C. Wolff, K. Ludwig, C. Bottcher, A. Herrmann and R. Haag, Nanoscale, 2014, 6, 2353–2360 RSC.
- S. A. Staroverov, I. V. Vidyasheva, K. P. Gabalov, O. A. Vasilenko, V. N. Laskavyi and L. A. Dykman, Bull. Exp. Biol. Med., 2011, 151, 436–439 CrossRef CAS PubMed.
- A. S. Blum, C. M. Soto, C. D. Wilson, J. D. Cole, M. Kim, B. Gnade, A. Chatterji, W. F. Ochoa, T. W. Lin, J. E. Johnson and B. R. Ratna, Nano Lett., 2004, 4, 867–870 CrossRef CAS.
- C. Chen, Z. Zou, L. Chen, X. Ji and Z. He, Nanotechnology, 2016, 27, 435102 CrossRef PubMed.
- A. Halder, S. Das, D. Ojha, D. Chattopadhyay and A. Mukherjee, Mater. Sci. Eng., C, 2018, 89, 413–421 CrossRef CAS PubMed.
- T. T. Le, B. Adamiak, D. J. Benton, C. J. Johnson, S. Sharma, R. Fenton, J. W. McCauley, M. Iqbal and A. E. G. Cass, Chem. Commun., 2014, 50, 15533–15536 RSC.
- V. Cagno, P. Andreozzi, M. D'Alicarnasso, P. Jacob Silva, M. Mueller, M. Galloux, R. Le Goffic, S. T. Jones, M. Vallino, J. Hodek, J. Weber, S. Sen, E. R. Janecek, A. Bekdemir, B. Sanavio, C. Martinelli, M. Donalisio, M. A. Rameix Welti, J. F. Eleouet, Y. Han, L. Kaiser, L. Vukovic, C. Tapparel, P. Kral, S. Krol, D. Lembo and F. Stellacci, Nat. Mater., 2018, 17, 195–203 CrossRef CAS PubMed.
- A. A. Aljabali and D. J. Evans, Methods Mol. Biol., 2014, 1108, 97–103 CrossRef CAS PubMed.
- A. A. Khan, E. K. Fox, M. L. Gorzny, E. Nikulina, D. F. Brougham, C. Wege and A. M. Bittner, Langmuir, 2013, 29, 2094–2098 CrossRef CAS PubMed.
- A. Liu, M. V. de Ruiter, S. J. Maassen and J. Cornelissen, Methods Mol. Biol., 2018, 1798, 1–9 CrossRef CAS PubMed.
- O. K. Zahr and A. S. Blum, Methods Mol. Biol., 2014, 1108, 105–112 CrossRef CAS PubMed.
- J. Peng, Z. Wu, X. Qi, Y. Chen and X. Li, Molecules, 2013, 18, 7912–7929 CrossRef CAS PubMed.
- R. Wyatt and J. Sodroski, Science, 1998, 280, 1884–1888 CrossRef CAS PubMed.
- D. Sepulveda-Crespo, J. L. Jimenez, R. Gomez, F. J. De La Mata, P. L. Majano, M. A. Munoz-Fernandez and P. Gastaminza, Nanomedicine, 2017, 13, 49–58 CrossRef CAS PubMed.
- V. Briz, D. Sepulveda-Crespo, A. R. Diniz, P. Borrego, B. Rodes, F. J. de la Mata, R. Gomez, N. Taveira and M. A. Munoz-Fernandez, Nanoscale, 2015, 7, 14669–14683 RSC.
- L. Chonco, M. Pion, E. Vacas, B. Rasines, M. Maly, M. J. Serramia, L. Lopez-Fernandez, J. De la Mata, S. Alvarez, R. Gomez and M. A. Munoz-Fernandez, J. Controlled Release, 2012, 161, 949–958 CrossRef CAS PubMed.
- D. Sepulveda-Crespo, M. J. Serramia, A. M. Tager, V. Vrbanac, R. Gomez, F. J. De La Mata, J. L. Jimenez and M. A. Munoz-Fernandez, Nanomedicine, 2015, 11, 1299–1308 CrossRef CAS PubMed.
- H. Zheng, Y. Lang, J. Yu, Z. Han, B. Chen and Y. Wang, Colloids Surf., B, 2019, 178, 80–86 CrossRef CAS PubMed.
- K. M. Ahmad, Y. Xiao and H. T. Soh, Nucleic Acids Res., 2012, 40, 11777–11783 CrossRef CAS PubMed.
- E. M. Strauch, S. M. Bernard, D. La, A. J. Bohn, P. S. Lee, C. E. Anderson, T. Nieusma, C. A. Holstein, N. K. Garcia, K. A. Hooper, R. Ravichandran, J. W. Nelson, W. Sheffler, J. D. Bloom, K. K. Lee, A. B. Ward, P. Yager, D. H. Fuller, I. A. Wilson and D. Baker, Nat. Biotechnol., 2017, 35, 667–671 CrossRef CAS PubMed.
- C. Sigl, E. M. Willner, W. Engelen, J. A. Kretzmann, K. Sachenbacher, A. Liedl, F. Kolbe, F. Wilsch, S. A. Aghvami, U. Protzer, M. F. Hagan, S. Fraden and H. Dietz, Nat. Mater., 2021, 20, 1281–1289 CrossRef CAS PubMed.
- G. A. O. Cremers, B. Rosier, A. Meijs, N. B. Tito, S. M. J. van Duijnhoven, H. van Eenennaam, L. Albertazzi and T. F. A. de Greef, J. Am. Chem. Soc., 2021, 143, 10131–10142 CrossRef CAS PubMed.
- X. Liu, H. Yan, Y. Liu and Y. Chang, Small, 2011, 7, 1673–1682 CrossRef CAS PubMed.
- H. C. Sun, L. Miao, J. X. Li, S. Fu, G. An, C. Y. Si, Z. Y. Dong, Q. Luo, S. J. Yu, J. Y. Xu and J. Q. Liu, ACS Nano, 2015, 9, 5461–5469 CrossRef CAS PubMed.
- S. C. Sebai, S. Cribier, A. Karimi, D. Massotte and C. Tribet, Langmuir, 2010, 26, 14135–14141 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |
