A brief overview of classical natural product drug synthesis and bioactivity
Gen
Li†
a,
Mingliang
Lou†
a and
Xiangbing
Qi
 *ab
*ab
aNational Institute of Biological Sciences (NIBS), 7 Science Park Road ZGC Life Science Park, Beijing 102206, China. E-mail: qixiangbing@nibs.ac.cn
bTsinghua Institute of Multidisciplinary Biomedical Research, Tsinghua University, Beijing 100084, China
First published on 20th November 2021
Abstract
Traditional medicines consisting of compounds derived from natural organisms have been used for human health care worldwide since ancient times. Since the last century, huge numbers of bioactive natural entities with diverse chemical scaffolds have been discovered, and some have been explored as clinical medications to treat various diseases. The advent of modern technologies has promoted the discovery of natural product-based pharmaceutical agents. The synthesis of natural products not only paves the way to confirm their molecular structures but also offers the structural modification opportunity to rationally optimize the drug-likeness parameters and evaluate the bioactivity of analogs. By providing a brief overview of a miscellaneous collection of complex natural products synthesis and the efforts of the structure–activity relationship, the present report aims to highlight the impact of chemical synthesis in natural product generation, diversification, bioactivity evaluation, and natural product-based drug development.
1. Introduction
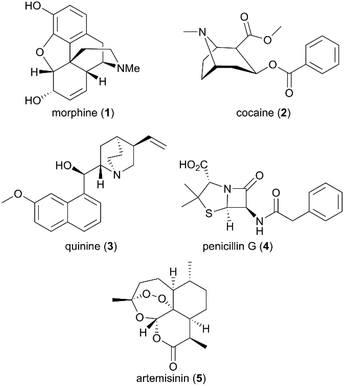
For millennia, nature has provided an invaluable source of traditional medicines from a variety of plants, animals, microorganisms, and minerals for humans to treat a wide spectrum of diseases. Since around 2600 BCE the successful use of natural medicines and remedies, particularly plant-originated substances, has been well documented in a large number of countries.1 The World Health Organization (WHO) estimated in 1985 that more than 4000 million people in the world relied mainly on traditional medicines for their primary health care.2 Since the 19th century, developments in the fields of phytochemistry and analytical chemistry have enhanced humans’ ability to examine natural materials more deeply and to identify pure bioactive constitutes to further leverage the application potential of natural products.Beginning with the isolation of morphine, the first active principle from the opium poppy, by German pharmacist Friedrich Sertürner in 1806, and followed by its commercialization as an analgesic and sedative by Merck in 1827,3 several active natural products were subsequently purified, such as cocaine and quinine from plant coca and cinchona, respectively (Fig. 1).4 However, not until the last century did drug discovery-based natural products come of age due to the scientific breakthroughs in modern chemistry and medicine. The natural products pool was enriched with broader chemical structures and diverse spectrum bioactivities. Compared with conventional synthetic molecules, naturally generated products are characterized by more complex skeletons with a higher molecular mass and a larger number of sp3-hybridized atoms, which can be advantageous for the exploration of small molecule and biomolecule interactions.
Despite the successful discovery of multiple drugs based on original natural products, such as morphine and artemisinin (Fig. 1), they still suffer from source and availability challenges. More importantly, the low success rate of the natural entity in approved drugs emphasizes the importance of structural optimization to improve natural products’ drug-like characteristics during drug development. With the development of new synthetic strategies and methodologies, more complex natural products have been assembled by efficient total synthesis or semisynthesis. The complementation of total synthesis strategies with semisynthetic transformations has been applied widely in the diversity- and bioactivity-oriented synthesis of drug-like entities.
Natural products and their synthetically modified analogs have historically made a remarkable contribution to the development of new drugs. A number of reports and books have reviewed the development of natural product-based drugs,1,5–13 including antibiotic drugs,14–16 antitumor agents,17–20 analgesics,21,22 and antiparasitic drugs.23–25 All the reviews mentioned above emphasize that original natural products play significant roles in the drug discovery and development process. This is also confirmed further in the recent survey provided in serial reviews from Newman and Cragg,5,26–29 which reported that only 463 purely synthetic drugs accounted for 24.6% of 1881 approved new chemical entities between 1981 and 2019. The rest of the new drugs on the market were either derived from the original natural structure or inspired by the rigidly complex skeletons of natural entities; 427 naturally occurring products (N, Newman and Cragg's category) and their structural derivatives (ND) were approved over almost 39 years.
Even though these reviews summarized nicely the natural product-based new drug development, druggability-related synthesis was not intensively tackled. We proposed to provide a brief overview of a miscellaneous collection of complex natural products synthesis and the endeavors of structure–activity relationships (SAR) studies to highlight the impact of chemical synthesis in natural product medicinal chemistry. Given the large number of approved natural product drugs in history, only the representative complex natural products whose chemical synthesis played an important role during the related drugs development are selected here. We admit that some natural products drugs, such as penicillin, vancomycin, ciclosporin, and rapamycin, are also classical and complex, but these are not covered due to limited space. Herein, the total synthesis of eight natural entities that were approved as drugs is presented according to the structural features, and the roles that total synthesis played in drug discovery and development are overviewed. The SAR studies are also discussed extensively.
2. Morphine and morphine derivatives
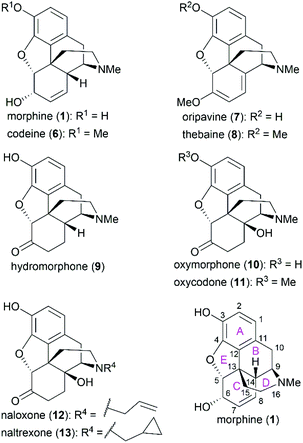
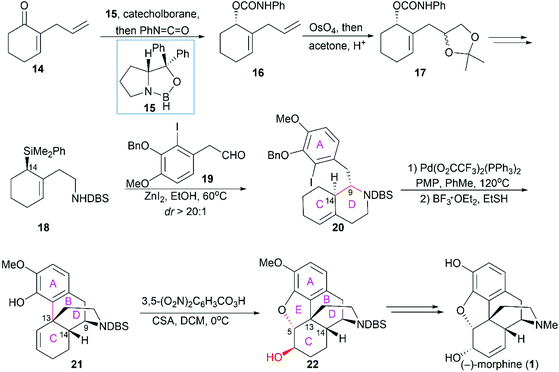
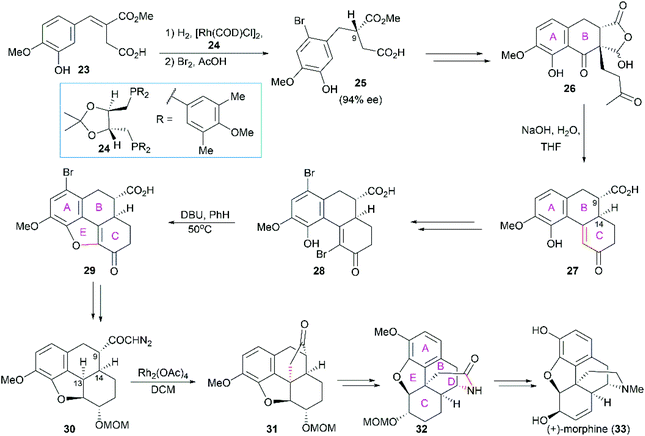
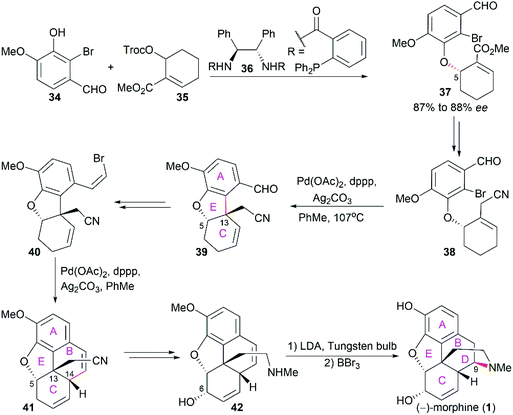
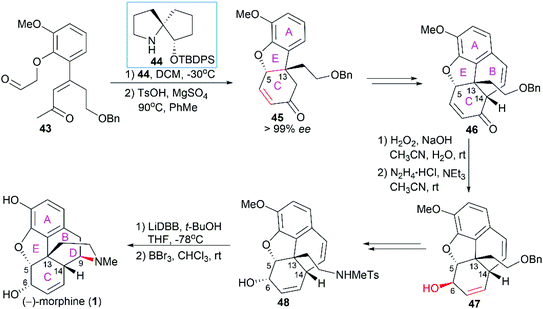
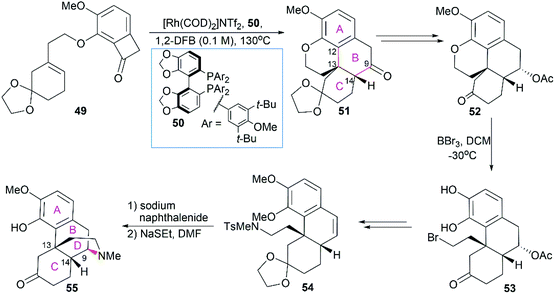
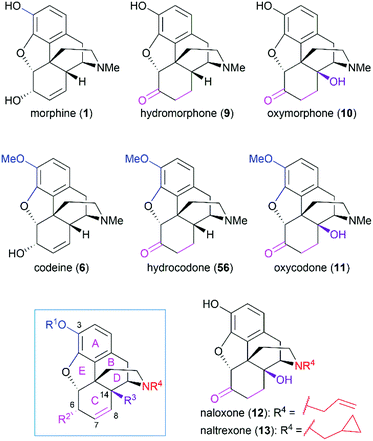
Morphine (1), isolated in 1806 by a German pharmacist, Friedrich Sertürner, is used primarily to treat both acute and chronic moderate-to-severe pain.30–34 It functions through selective binding to the μ-type opioid receptor in the brain and spinal cord.35,36 Many natural analogs and semisynthetic derivatives of morphine have been isolated or synthesized until now, some of which are presented in Fig. 2. The primary source of morphine and its congeners is isolation from the opium poppy, which is greatly affected by climate and political instability.32,37 Although the morphine biosynthesis pathway has been elucidated and the laboratory scale production of morphine in yeast has been realized, major challenges remain to be addressed before the realization of practical strategies for industrial-scale production.38,39 Therefore, total synthesis may be a plausible surrogate for extraction in the production of morphine and its derivatives.The structure of morphine was first proposed by Robinson in 1925, and this structure was supported by Gates’ first total synthesis of morphine in 1952 and later by X-ray analysis in 1954.40–43 As shown in Fig. 2, there are several intriguing structural features of morphine: a rigid pentacyclic bridged/fused ring structure (designated A, B, C, D, and E), five contiguous stereocenters (C5, C6, C9, C13, and C14) including one quaternary stereocenter (C13), an ether linkage between C4 and C5, two hydroxy groups (C3 and C6), a basic tertiary amine moiety, and a double bond between C7 and C8, which make its total synthesis a challenging task. The numerous studies on the synthesis of morphine alkaloids have led to over 40 synthesis routes, which have been summarized in several recent reviews.31,33,34,37,44,45 Selected representative routes for catalytic asymmetric total synthesis of morphine alkaloids will be discussed in the following section.
2.1 Catalytic asymmetric total synthesis of morphine alkaloids
2.2 Structure–activity relationships of morphine derivatives
As the prototypical opioid agonist, morphine (1) has been intensively studied, which led to various types of morphine-related drugs. Representative pentacyclic morphine-based drugs are listed in Fig. 3. There are several structural features that are important for morphine's biological function: the C3 phenolic hydroxy group, C6 allylic hydroxy group and C7–C8 double bond, the B/C cis-fused ring system, the substitution groups at C14, and the amine group. Detailed SAR studies have been summarized previously56 and a brief discussion is provided in the following paragraph.The C3 phenolic hydroxy group is essential for the binding of morphine with the μ receptor. Therefore, codeine (6), the C3 methoxy ether derivative of morphine, shows 10-fold lower potency than morphine.57 However, codeine possesses greatly enhanced oral bioavailability. A similar relationship is observed between codeine-derivatized hydrocodone (56) and morphine-derivatized hydromorphone (9), which are the same as oxycodone (11) and oxymorphone (10).56 These codeine-derivatives function as prodrugs and must undergo CYP2D6-mediated demethylation of their corresponding phenolic methyl ethers before binding to μ receptors.56
The C6 allylic hydroxy group is engaged in a weak hydrogen bond between morphine and its receptor.58 However, removal of the hydroxy group increases the molecular lipophilicity and therefore leads to a 10-fold increase in activity.59 If the alcohol is oxidized to ketone, a 3-fold decrease in activity is observed; further hydrogenation of the C7–C8 double bond led to hydromorphone (9) with more flexibility in ring C, which shows a 6-fold increase in potency compared with morphine.56 Introduction of a β-hydroxy group at C14 transforms hydromorphone (9) into oxymorphone (10), which shows a 2- to 3-fold increase in receptor affinity when compared with hydromorphone (9).
The essential amino nitrogen functions in a protonated form by forming an electrostatic bond with the receptor. Substituents at this nitrogen atom have a great influence on its biological function. A methyl group is commonly observed in morphine-derived agonists; however, naloxone (12) and naltrexone (13), with allyl or cyclopropylmethyl group, respectively, are pure antagonists.
3. Tetracyclines
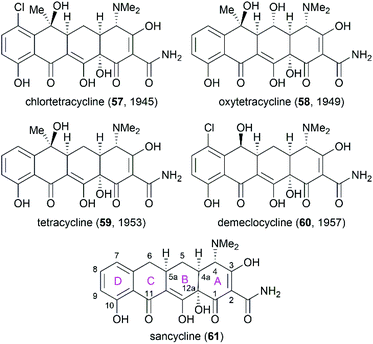
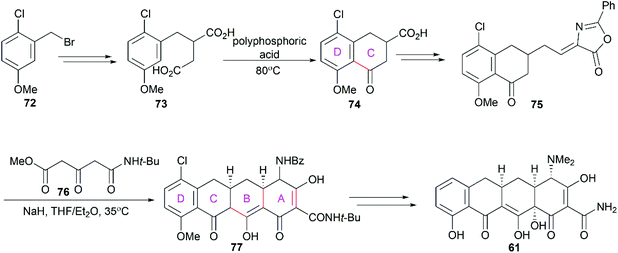
Following the discovery of chlortetracycline (57) by Duggar in 1945,60 several other natural tetracyclines—oxytetracycline (58),61 tetracycline (59),62 and demeclocycline (60),63—were discovered in the following years (Fig. 4). The broad-spectrum activity and low side effects of tetracyclines make them one of the most important classes of antibiotics.64 It is well established that tetracyclines function by binding to the 30S ribosomal subunit, which prevents the association of aminoacyl-tRNA with the bacterial ribosome and therefore inhibits bacterial protein synthesis.65 Over 70 years of widespread use has resulted in the emergence of bacterial resistance to tetracyclines. There are three main mechanisms responsible for tetracycline resistance: (1) efflux pumps reduce the intracellular tetracycline concentration by exporting tetracycline from the cell; (2) ribosomal protection proteins protect ribosomes from tetracycline inhibition via various mechanisms; and (3) protection enzymes inactivate tetracycline through modification and degradation.64,66–68 The growing problem of tetracycline resistance calls for much more investigation on the synthesis of tetracycline derivatives for the development of higher potency antibiotic drugs.In the early 1950s, the structures of chlortetracycline (57) and oxytetracycline (58) were first determined by scientists from Pfizer with the help of Woodward.69–71 Subsequent studies showed that the tetracyclines share one common nucleus, sancycline (61), which is composed of four linearly fused 6-membered rings (designated A, B, C, and D) with four stereocenters. A combination of different substitution groups, for example, chloride, hydroxy, and methyl, with the different substitution positions on the nucleus could lead to various tetracyclines with more structural complexity. The tetracyclines are frangible under an array of conditions: (1) the C6 hydroxy group undergoes dehydration in acidic media; (2) the C4 dimethylamino group epimerizes in mildly acidic conditions; and (3) the B and C rings undergo a retro-Dieckmann reaction in basic media.72,73 The important biological functions and the complicated structural features of tetracyclines have attracted much attention of synthetic chemists.
3.1 Total synthesis of tetracyclines
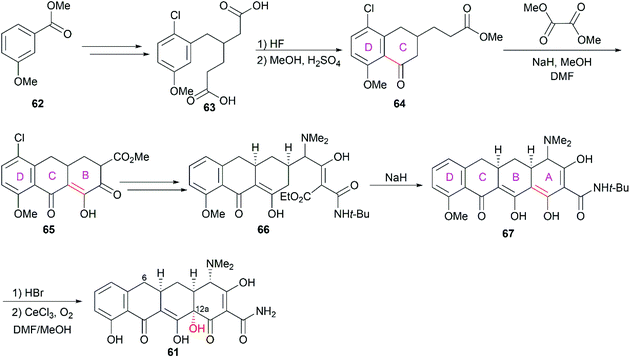
Not long after Woodward's synthesis of sancycline, Shemyakin reported their synthesis of tetracycline.77 Starting from juglone (68), they developed a synthetic strategy similar to that of Woodward (Scheme 7). It should be noted that the C6 hydroxy group was installed using a photooxidation method.78
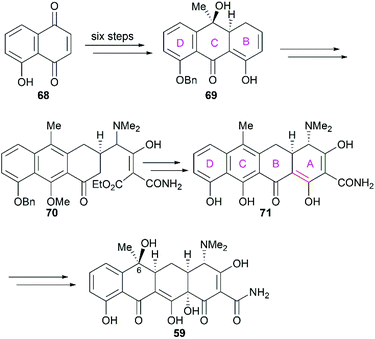
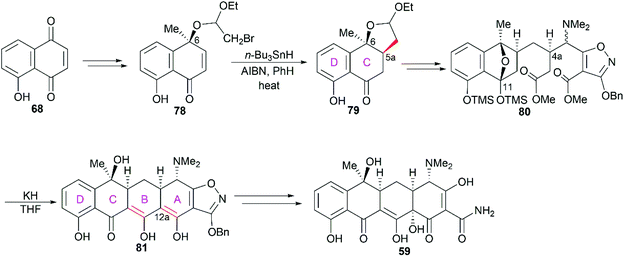
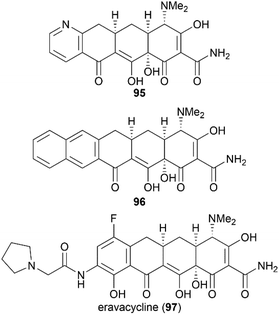
Previous X-ray crystallography studies on the interaction between tetracycline (59) and the 30S ribosomal subunit indicated that the D ring is suitable for modifications to generate more potent antibiotics.65 Therefore, Myers’ late-stage construction of ring C with the concomitant introduction of structurally varied forms of ring D is a promising strategy to synthesize tetracycline derivatives with modified D ring. With continuing optimization, this synthetic strategy is now suitable for industrial-scale application.84–88 What's more, utilizing Myers’ method, countless tetracycline derivatives, which are difficult to synthesize or are inaccessible by semisynthesis, can be constructed. Fig. 5 shows three representative tetracycline derivatives with different substitutions, the 7-azatetracycline 95, the pentacycline 96, and the fluorocycline 97, among which 97, the first fully synthetic tetracycline antibiotic, was approved in 2018.89 To date, more than 3000 tetracycline derivatives have been synthesized based on Myers’ strategy by a company named Tetraphase.90 These tetracycline derivatives with diverse modifications make more detailed SAR studies possible.
3.2 Structure–activity relationships of tetracycline derivatives
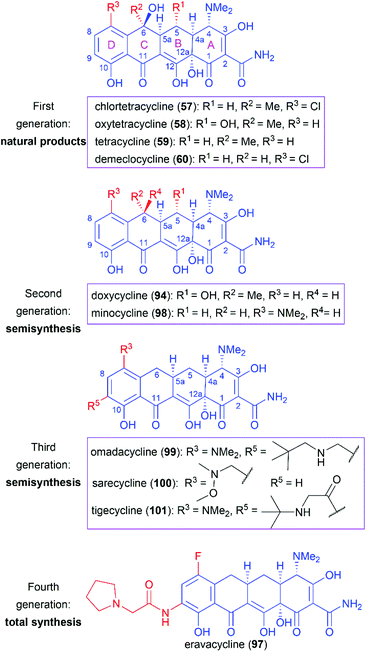
Since the application of chlortetracycline (1) as the first broad-spectrum antibiotic, extensive efforts have been devoted to the SAR studies of tetracyclines, through natural tetracyclines to semisynthetic derivatives and then to fully total synthetic candidates. The tetracycline drugs can be roughly divided into four generations and representative members are presented in Fig. 6.Naturally isolated tetracyclines are the main members of the first-generation drugs. These natural drugs share a common pharmacophore and comprise different substitution groups at C5, C6, and C7. Several structural features are important for their bioactivities. Maintenance of the four linearly fused six-membered carbocyclic skeleton and the α stereochemical configurations of C4, C4a, C5a, and C12a are essential for their high antibacterial activity.64,91,92 Chelation with metal ions has a great influence on tetracyclines’ activity, and the keto–enol systems (C1–C3 and C11–C12) and the carboxamide (C2), which are the sites of interaction with ions, are necessary.64,91 Alkylation of the carboxamide (C2) generally decreases activity, whereas suitable substituents can increase the water solubility, as in the case of the prodrug lymecycline.64,92 Removal of the C4 dimethylamino or its replacement with higher alkylamino groups reduces activity.91 Furthermore, esterification of the C12a hydroxy group diminishes the activity.
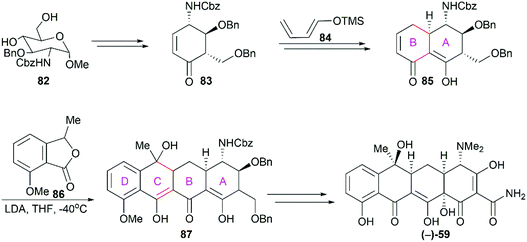
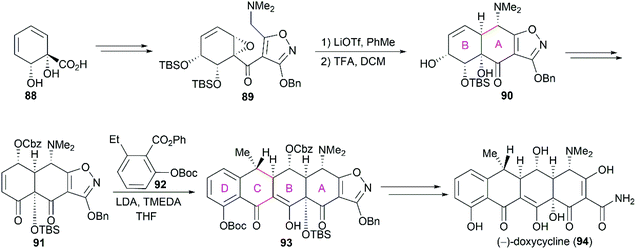
However, as mentioned above, the C6 hydroxy group in these natural products is labile and undergoes dehydration in acidic media to produce anhydrotetracycline, which shows nephrotoxicity.93 Therefore, removal of the C6 hydroxy group and further modifications through semisynthesis led to two second-generation drugs, doxycycline (94) and minocycline (98), which are more stable while maintaining similar or even higher antibacterial activity.94,95
The widespread use of tetracycline antibiotics has brought about serious resistance problems, which has led to the call for more-effective drugs. Investigations on C9-substituted tetracyclines led to the discovery of one glycylcycline named tigecycline (101), which is a derivative of minocycline (98).96 Tigecycline (101) shows high potency on tetracycline-resistant strains that utilize the efflux and ribosomal protection mechanisms of resistance.97 However, tigecycline (101) is only available in an injectable formulation.91 Further modifications led to the discovery of omadacycline (99), which is a C9-aminomethylcycline. Compared with tigecycline (101), omadacycline (99) shows improved oral bioavailability while maintaining activity against tetracycline-resistant bacteria.98 Another C7-aminomethylcycline, sarecycline (100), was approved as a narrow-spectrum tetracycline for the treatment of acne.
The success of the second- and third-generation tetracycline drugs indicates the potential role of the tetracycline pharmacophore in developing new antibiotics. However, the use of semisynthesis, as opposed to total synthesis, for the modification of tetracyclines has inherent limitations. The power of total synthesis is demonstrated by the approval of eravacycline (97),89,99 the first fully synthetic tetracycline drug. We believe that total synthesis will play a much more important role in the campaign against growing antibiotic resistance.
4. Macrolides
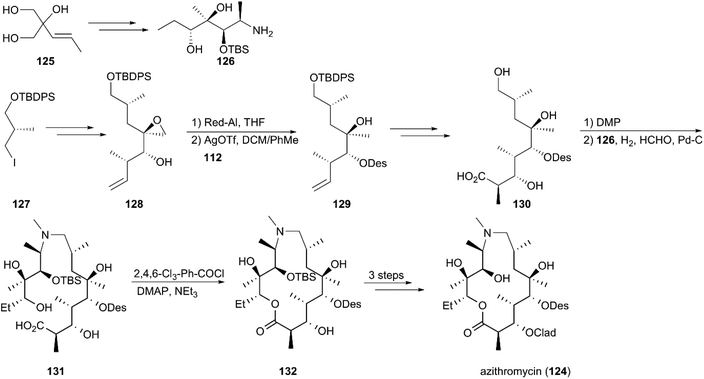
The macrolides, a series of macrocyclic lactones with 14-, 15- and 16-membered rings and decorated with one or two sugar moieties are primarily isolated from different Streptomyces spp. The first macrolide and wide-spread antimicrobial agent, erythromycin (102), was isolated in 1952 from the culture broth of Saccharopolyspora erythera, and its structure features, a highly substituted 14-membered lactone bearing 10 chiral carbon atoms, and configuration were successively defined by chemical and X-ray diffraction studies.100–102 Inspired by its excellent and broad-spectrum activity against Gram-positive pathogens, the mechanism by which erythromycin binds to the 23S RNA of the 50S ribosomal subunit to hamper the exit peptide tunnel and inhibit protein synthesis was elucidated and confirmed further by determining the crystal structure of erythromycin bound to the 50S ribosomal subunit.103,104 Since erythromycin was launched as Ilosone to treat bacterial infections of the respiratory tract, skin, and soft tissues, the problem of varying levels of resistance to macrolides has been uncovered; resistance was characterized as resulting from the expression of efflux proteins in Gram-positive pathogens,105,106 a change in erythromycin binding site in mutated ribosomal proteins,107 and bio-modifications of macrolides by methylase108,109 and esterases.110,111 In addition, erythromycin is unstable, especially in acidic environments, due to ketal formation between the C6 and C12 hydroxy groups and the C9 ketone; this undesired side reaction alters the structural properties and leads to low safety and bioavailability.112 To effectively address these problems and improve the drug-likeness potential of natural macrolides, total synthesis and semisynthesis strategies for the macrolides and their structural analogs have been explored to develop novel lead structures with new molecular features and mechanisms of actions.4.1 Total synthesis of macrolides
4.2 Structure–activity relationships of macrolide derivatives
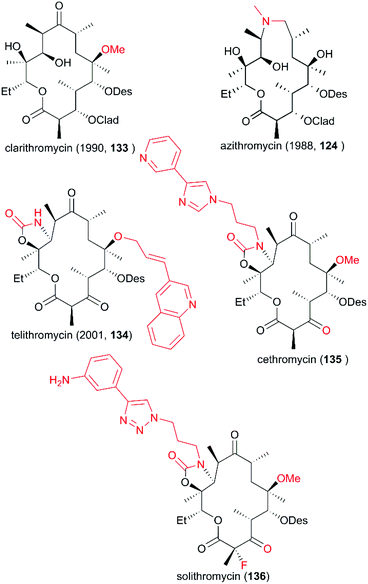
Since the discovery of erythromycin as the first macrolide with broad-spectrum antibacterial activity, the development of more potential novel antibiotic candidates has been spurred, leading to the preparation and testing of hundreds of macrolide derivatives. As mentioned above, spiroketal formation between the ketone at C9 with hydroxy groups at C6 and C11 under acidic conditions contributes to the low stability of erythromycin. To avoid this unpleasant side effect, a more acid-resistant and powerful derivative with a 6-O-methyl group was developed by Ōmura and co-workers in 1984; six years later this derivative, clarithromycin (133), was approved for clinical use (Fig. 7).118 Meanwhile, a 15-membered ring of azamacrolide was furnished through Beckmann rearrangement of erythromycin A oxime at C9. This new strategy from Lazarevski's group led to a novel chemical scaffold with excellent stability and activity; this chemical was approved as a new drug in 1988, named azithromycin (124). In addition, the C3-cladinose moiety was shown to be unnecessary for protein contact.119 The success of the marketed drug telithromycin (134) showed that its ability to bind to the ribosome was improved by the introduction of a C11/C12 carbamate ring with an extended aryl substituted alkyl chain, and binding was also improved by oxidation of the C3 hydroxy group to give C3 ketone without sugar.120–122 However, a more effective antibiotic is urgently needed because of the serious drug resistance problem induced by the widespread use of the antibiotic. The development of more drug-like candidates for clinical applications, such as cethromycin (135) and solithromycin (136), is currently underway.5. Vinca alkaloids
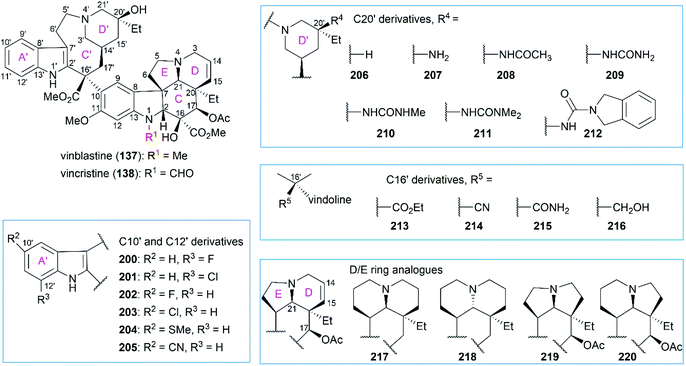
Vinblastine (137) and vincristine (138) are characteristic members of the biologically important vinca alkaloids (Fig. 8). Along with these two alkaloids, derivatives of these alkaloids, including vindesine (139), vinorelbine (140), and vinflunine (141), have been widely applied as well-known antitumor drugs. These molecules function through binding to tubulin, which disrupts microtubule function and results in mitotic arrest and apoptosis.123–127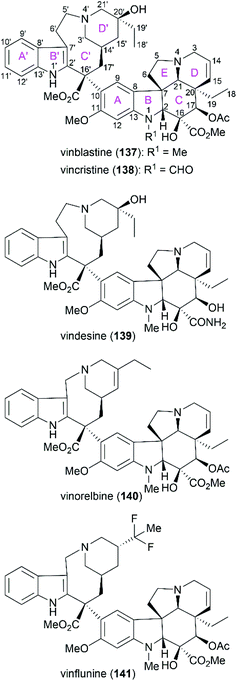 | ||
| Fig. 8 Representative vinca alkaloids and related derivatives used as antitumor drugs and their ring lettering and carbon numbering system. | ||
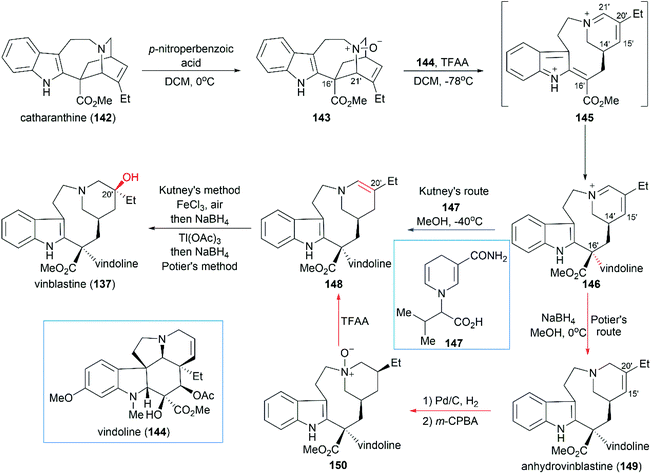
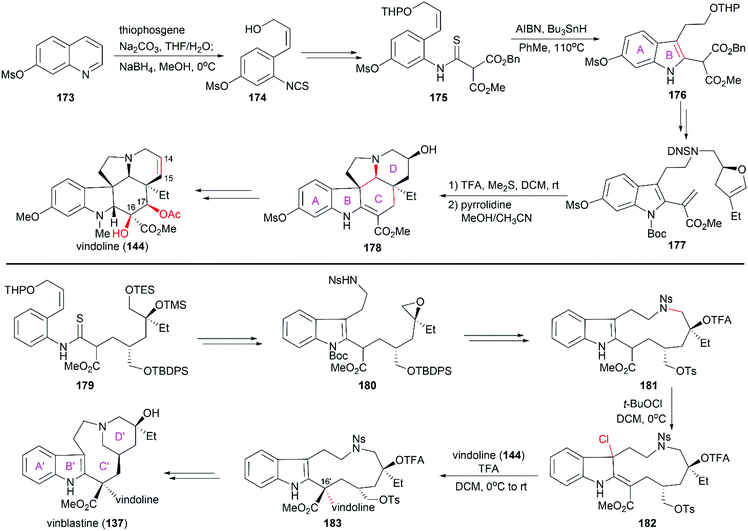
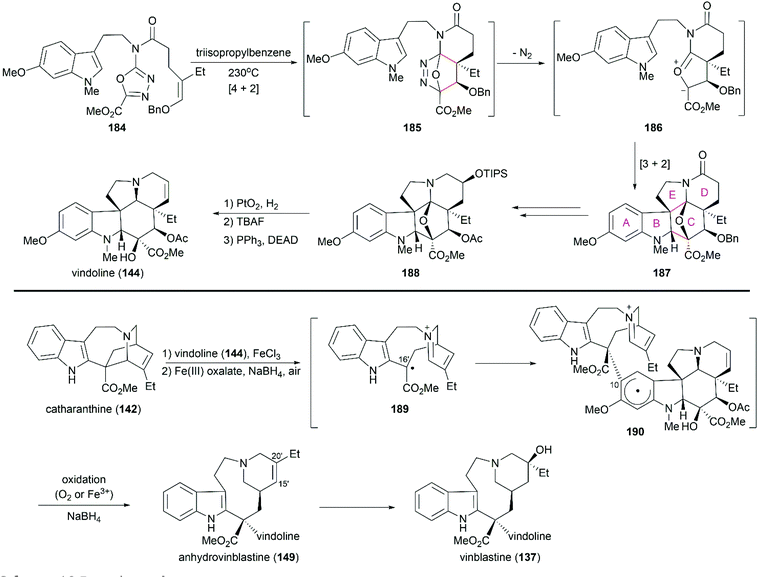
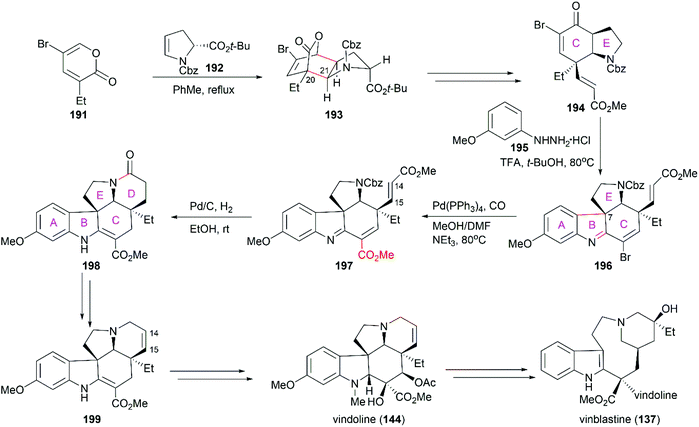
Vinblastine and vincristine were isolated from the leaves of Catharanthus roseus in 1958 and 1961,128–130 respectively, and their absolute configuration was determined by X-ray crystallography in 1965.131 Both vinblastine and vincristine possess an upper velbanamine subunit and a lower vindoline or vindoline-derived subunit. For vinblastine, the lower vindoline subunit contains a pentacyclic skeleton (ABCDE rings), which bears six contiguous stereocenters on ring C with two quaternary chiral centers. The upper velbanamine subunit is composed of four rings, including one 9-membered ring C′. There are three stereocenters in the upper subunit, including the C16′ quaternary chiral center resulted from the linkage between the two subunits. The pharmaceutical importance combined with their intriguing structural features make this kind of alkaloid attractive synthetic targets, and great endeavors from the synthetic community in the past several decades have resulted in several elegant total syntheses of vinblastine and vincristine. These strategies are discussed in the following section.
5.1 Total synthesis of vinca alkaloids
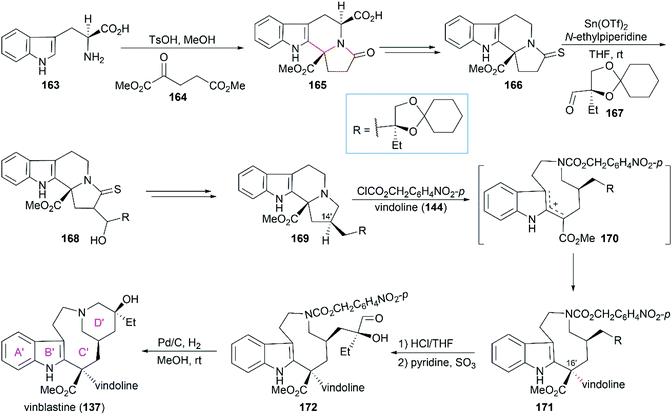
Potier and Kutney applied different strategies for the transformation of iminiumion 146 into vinblastine (137). In Kutney's one-pot operation, a 1,4-reduction of 146 with the carefully designed reductant 147 yielded enamine 148, which was successfully oxidized with O2 in the presence of FeCl3 to establish the C20′ hydroxy group.134 Potier's 1,2-reduction of iminiumion 146 with NaBH4 produced anhydrovinblastine 149, which is a naturally occurring vinca alkaloid. Hydrogenation of the C15′–C20′ double bond and oxidation gave 150. The following Polonovski reaction transformed 150 into enamine 148. Installation of the C20′ hydroxy group was achieved through Tl(OAc)3 mediated oxidation followed by reduction with NaBH4.133
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. It should be noted that the diastereoselectivity of this transformation could be improved to 4
1 dr. It should be noted that the diastereoselectivity of this transformation could be improved to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
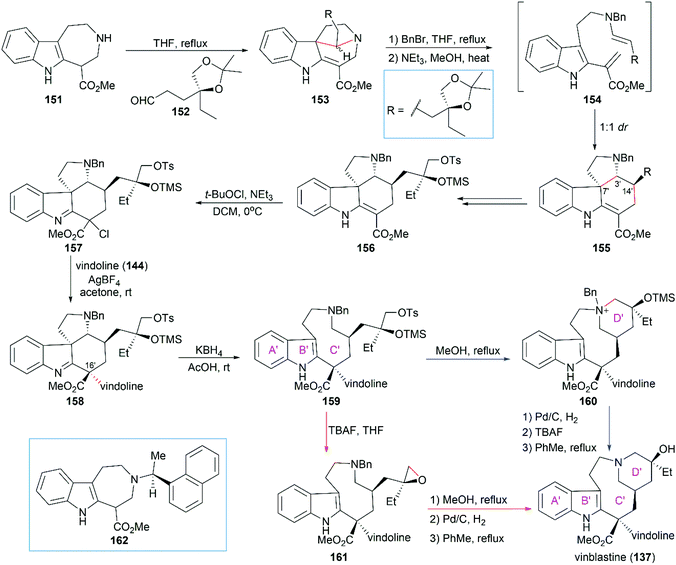
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr when 152 was condensed with 162 which contained a chiral naphthylethyl group. Chlorination of 156 with tert-butyl hypochlorite provided imine compound 157, which coupled smoothly with vindoline (144) in the presence of AgBF4 to give 158 with the desired stereoconfiguration at C16′. KBH4-mediated reductive ring-opening of 158 provided tricyclic compound 159, which possessed the 9-membered ring C′.
1 dr when 152 was condensed with 162 which contained a chiral naphthylethyl group. Chlorination of 156 with tert-butyl hypochlorite provided imine compound 157, which coupled smoothly with vindoline (144) in the presence of AgBF4 to give 158 with the desired stereoconfiguration at C16′. KBH4-mediated reductive ring-opening of 158 provided tricyclic compound 159, which possessed the 9-membered ring C′.
The final D′ ring of the upper unit was constructed by heating the precursor tosylate 159 in MeOH at reflux for 48 h. It is worth noting that no cyclized product was found when 159 was heated in toluene at reflux for two weeks. Hydrogenolytic debenzylation followed by removal of the TMS group led to a higher energy atropisomer of vinblastine, which was transformed into vinblastine (137) by heating at reflux in toluene. An alternative reaction sequence including the epoxide intermediate 161 can also transform 159 into vinblastine (137).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 (C16′ S/R) when the reaction was run in CH3CN/H2O (15
16 (C16′ S/R) when the reaction was run in CH3CN/H2O (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at −15 °C. Hydrolysis of 171 followed by mono-oxidation of the diol and subsequent cyclization through reductive amination provided vinblastine (137).
1) at −15 °C. Hydrolysis of 171 followed by mono-oxidation of the diol and subsequent cyclization through reductive amination provided vinblastine (137).
5.2 Structure–activity relationships of vinblastine derivatives
Extensive SAR studies on vinblastine and related natural or synthesized derivatives led to the approval of several important antitumor drugs, some of which are listed in Fig. 8. However, the emergence of drug resistance caused by overexpression of the drug efflux pump phosphoglycoprotein (Pgp) calls for more potent vinblastine derivatives to be synthesized and evaluated in the future. Since several review papers have given a detailed summary of the SAR results,147–150 we briefly discuss research progress achieved in recent years, especially those in Boger's laboratory (Fig. 9 and Table 1).| Entry | Compound | IC50 (nM) | ||
|---|---|---|---|---|
| L1210b | HCT116b | HCT116/VM46b | ||
| a Values given are IC50 in nM and original activity data can be found in ref. 150–155. b L1210: murine leukemia cell line; HCT116: human colon cancer cell line; HCT116/VM46: resistant human colon cancer cell line, Pgp overexpression. | ||||
| 1 | Vinblastine (137) | 6.0 | 6.8 | 6.0 × 102 |
| 2 | 200 | 50 | 65 | 7.3 × 102 |
| 3 | 201 | 3.3 × 102 | 2.2 × 102 | 5.0 × 103 |
| 4 | 202 | 0.70 | 0.80 | 80 |
| 5 | 203 | 6.2 | 7.6 | 7.2 × 102 |
| 6 | 204 | 50 | 50 | 7.8 × 102 |
| 7 | 205 | 6.3 × 102 | 6.2 × 102 | >1.0 × 104 |
| 8 | 206 | — | 60 | 6.0 × 102 |
| 9 | 207 | — | 6.0 × 102 | >1.0 × 104 |
| 10 | 208 | — | 90 | 7.5 × 103 |
| 11 | 209 | 40 | 7.5 | 4.4 × 103 |
| 12 | 210 | — | 0.82 | 5.3 × 102 |
| 13 | 211 | 5.9 | 2.8 | 80 |
| 14 | 212 | 0.51 | 0.60 | 7.5 |
| 15 | 213 | 60 | 70 | 8.3 × 102 |
| 16 | 214 | 6.3 × 102 | 6.7 × 102 | 7.4 × 103 |
| 17 | 215 | >1.0 × 104 | >1.0 × 104 | >1.0 × 104 |
| 18 | 216 | 6.5 × 103 | 5.8 × 103 | >1.0 × 104 |
| 19 | 217 | 5.7 × 103 | 7.4 × 103 | >1.0 × 104 |
| 20 | 218 | 55 | 80 | 9.0 × 102 |
| 21 | 219 | 5.6 × 102 | 80 | 7.8 × 103 |
| 22 | 220 | 7.2 | 6.5 | 4.5 × 102 |
Although substitution at the C12′ position of ring A′ in the upper velbanamine subunit (200 and 201) often led to reduced activity, substitution at the C10′ gave promising candidates. Applying their one-pot Fe(III)-mediated coupling and oxidation reaction, Boger and co-workers synthesized a series of vinblastine derivatives including the C10′-substituted ones.151 These derivatives exhibited activity that correlated with the size and shape of the substituents: 10′-chlorovinblastine 203 matched the potency of vinblastine; 10′-fluorovinblastine 202 exhibited an 8-fold increase in activity against both a sensitive (HCT116) and a vinblastine-resistant tumor cell line (HCT116/VM46); in contrast, derivatives bearing the larger (SMe, 204) or rigidly extended (CN, 205) substituents were 10–100-fold less potent. According to the X-ray structure of vinblastine bound to tubulin127 and the results of activity assays, the authors proposed that the 10′-fluorine substituent interacts with the protein at a hydrophobic site uniquely sensitive to steric interactions.151
Initial substitution of the C20′ hydroxy group with a hydrogen atom (206) or free amine (207) gave derivatives with less potency.152,153 Acetylation of the free amine (208) resulted in activity improvement, and a derivative with the urea group (209) showed comparable potency with vinblastine. However, these derivatives exhibited a further decrease in activity against a resistant HCT116/VM46 cell line compared with vinblastine. Further substitution of the terminal nitrogen led to improved activity against both sensitive and resistant cell lines (210 and 211). Finally, they found that the isoindoline-substituted compound 212 exhibited a 10-fold increase in activity against the sensitive HCT116 cell line and an 80-fold increase in activity against the resistant HCT116/VM46 cell line.
Replacement of the C16′ methyl ester with an ethyl ester (213), a cyano group (214), a primary carboxamide (215), or a hydroxy group (216) dramatically reduced the activity against both sensitive and resistant cell lines.154 Derivatives (217–220) containing 6,6-, 5,5-, and 5,6-membered DE ring systems, instead of the vindoline 6,5-DE ring, were synthesized.155 Among these analogs, 220 and 218 matched the potency of vinblastine and C17-deacetoxyvinblastine, respectively. Studies also showed that the N1-methyl group, C20 ethyl group, C14–C15 double bond, and C11 methoxy group were all essential to vinblastine's cytotoxic activity.150
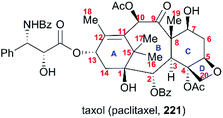
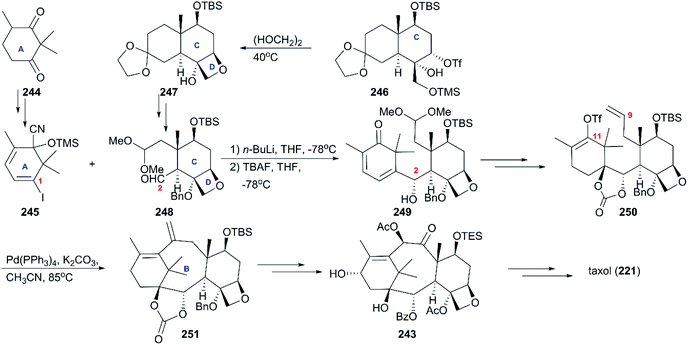
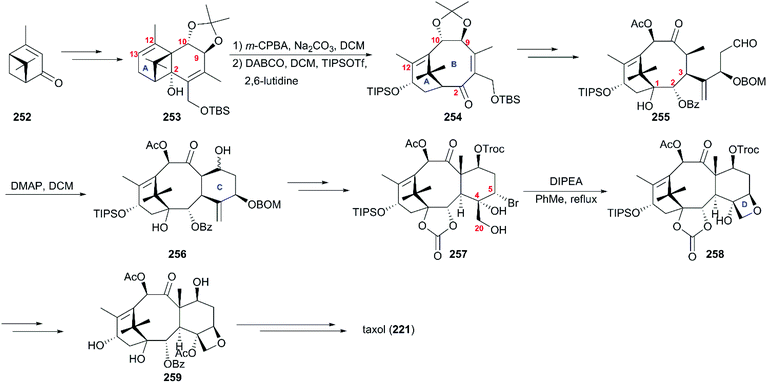
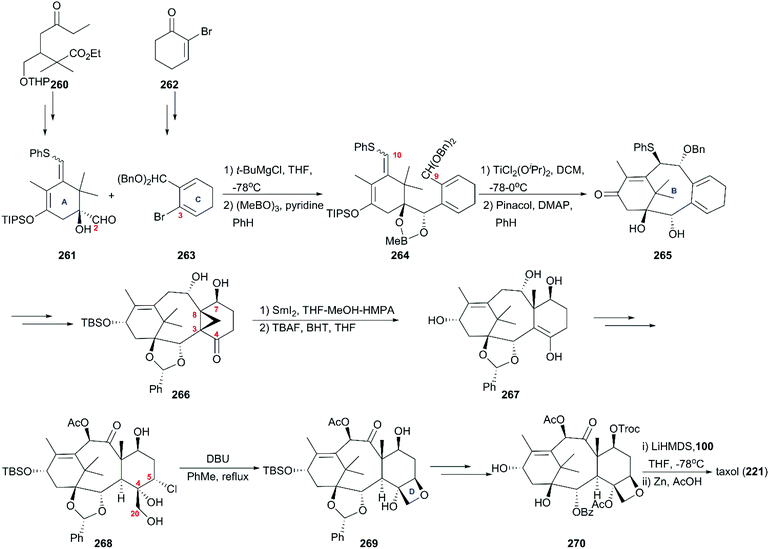
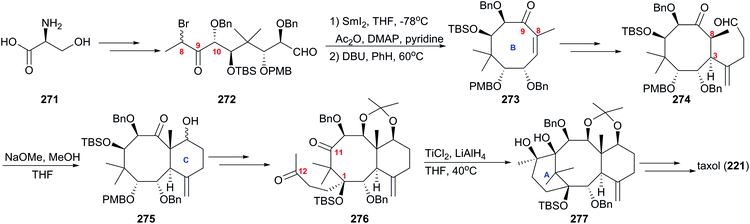
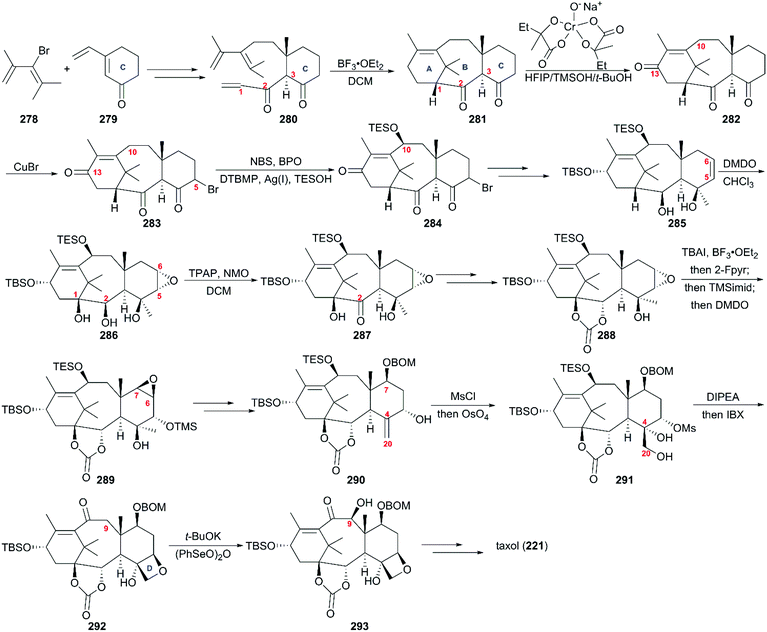
6. Taxol
One of the most famous natural taxane products, taxol (221, paclitaxel) was first isolated from the stem bark of Pacific yew by Wall and Wani in 1966, and in 1971 its complex structure was elucidated by NMR techniques and X-ray crystal structural analysis as a diterpenoid with a unique 6-8-6 tricyclic carbon skeleton bearing 11 stereocenters, an unusual oxetane ring, and a β-phenylisoserine chain ester at C13 (Fig. 10).156 Because of its potent anticancer activity, the worldwide academic and industrial research communities have developed different taxane agents for clinical use in the treatment of various cancers, including the marketed Taxol® and Anzatax®, as well as the synthetic structural derivatives under the tradenames Taxotere®, Jevtana®, and Abraxane®. In addition to its unique structure, taxol also demonstrates modes of action different from those of known anticancer agents. Mechanistic studies showed that during cancer cell mitosis, taxol diffuses through nanopores in the microtubule to bind the β-tubulin subunit on the inner surface of the microtubule, thereby inhibiting the microtubule depolymerization process and arresting the cell in the late G2/M phase, which blocks the cell division and leads to apoptosis. Cancer cells are believed to have a higher cell division rate than “normal” cells; therefore, they are more sensitive to taxol.157–159 As observed for other target-specific anticancer drugs, the mechanism of action discussed above and the long-term use as a first-line treatment have given rise to resistance against taxane anticancer agents. The actual resistance mechanisms are unclear, even though many of these mechanisms have been uncovered in different cell lines. For example, the over-expression of the mdr1 gene gives rise to the drug efflux of the membrane-bound P-glycoprotein. The abnormal expression of microtubule-associated proteins leads to drug resistance.Scarcity of the natural resources of taxane entities hampers the development of taxane-derived anticancer agents, and scientists from academia and industry never stop procuring the taxane products on an industrial scale. Research groups around the world have been working on the synthesis of taxanes and testing accessible synthetic strategies for the construction of the 20-carbon skeleton of the taxane family.
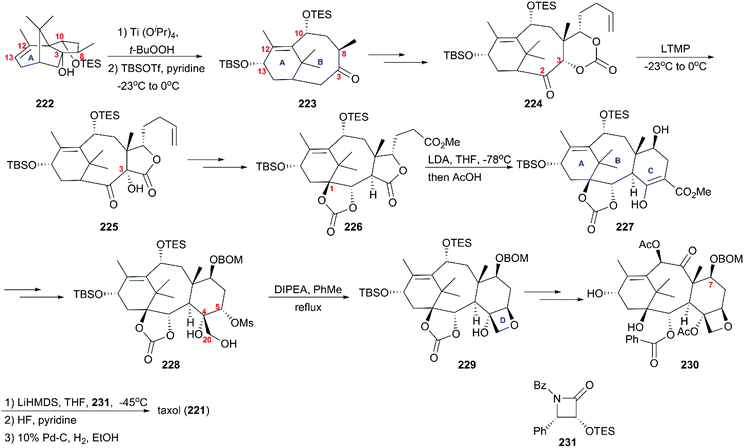
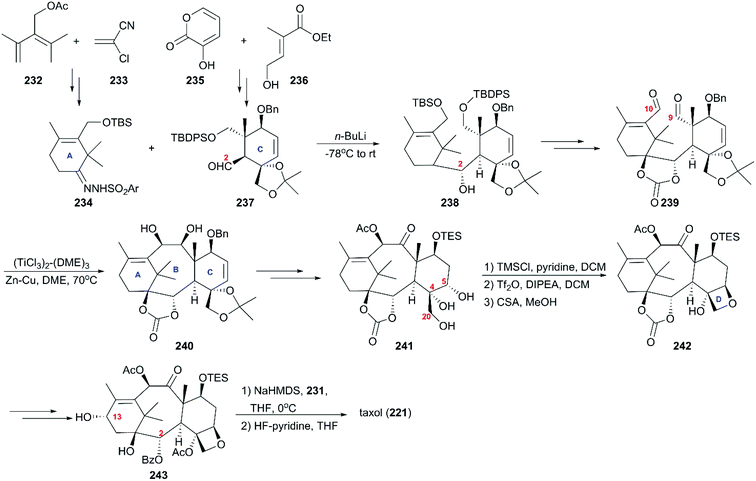
6.1 Total synthesis of taxol
6.2 Structure–activity relationships of taxol derivatives
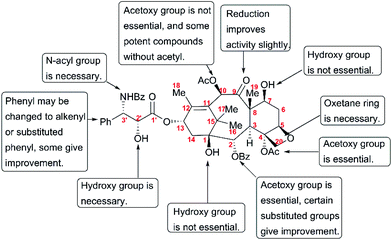
The discovery of taxol as one of the most potent anticancer agents spurred a large number of academic and industrial researchers to investigate the SAR for identifying lead compounds with drug-likeness potential. However, the complex structure of taxol, a heavily oxygenated polycyclic diterpenoid, presents a huge challenge for pharmaceutical studies that call for a substance-rich library. A number of studies have uncovered the still-incomplete SAR information about taxol. This information has been described by several reviews,179–183 and is summarized here in Fig. 11.Studies on the modifications at C1, such as removal and esterification, revealed that the C1-OH is not essential for the activity of taxol.184 In addition, C7-OH, C9-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C10-OAc play insignificant roles in the anticancer activity of taxol.185–188 SAR studies have shown that the benzoyl group at C2 is necessary for activity; 2-deoxy-taxol displayed decreased antitumor activity,189 and 2-benzoate analogs did not lead to any remarkable improvement in biological activity in a microtubule assay or cytotoxicity test.189 The acetoxy group at C4 was also demonstrated to be essential for activity, and the analog 4-deoxytaxol was inactive on several tumor cell lines.190,191 However, oxetane plays a critical role in the bioactivity of taxol, and analogs in which the oxygen atom was replaced by nitrogen, sulfur, or other heteroatoms did not significantly enhance antitumor activity.192,193
O, and C10-OAc play insignificant roles in the anticancer activity of taxol.185–188 SAR studies have shown that the benzoyl group at C2 is necessary for activity; 2-deoxy-taxol displayed decreased antitumor activity,189 and 2-benzoate analogs did not lead to any remarkable improvement in biological activity in a microtubule assay or cytotoxicity test.189 The acetoxy group at C4 was also demonstrated to be essential for activity, and the analog 4-deoxytaxol was inactive on several tumor cell lines.190,191 However, oxetane plays a critical role in the bioactivity of taxol, and analogs in which the oxygen atom was replaced by nitrogen, sulfur, or other heteroatoms did not significantly enhance antitumor activity.192,193
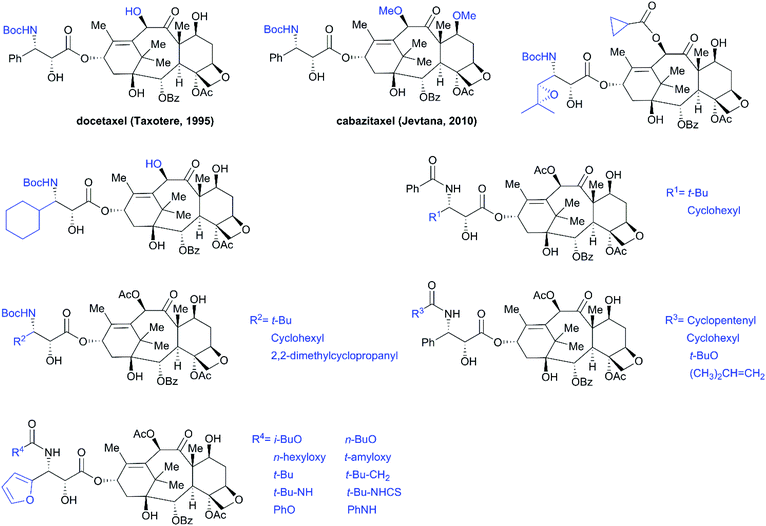
SAR studies revealed that the N-benzoyl-β-phenylisoserine side chain is essential for the antitumor activity of taxol, and this chain has attracted more extensive investigation than any other functional group of taxol.194–198 A number of analogs with a modified side chain exhibit stronger activity than taxol, some of which are listed in Fig. 12, including two marketed drugs, docetaxel (Taxotere®, 1995) and cabazitaxel (Jevtana®, 2010).
7. Artemisinin
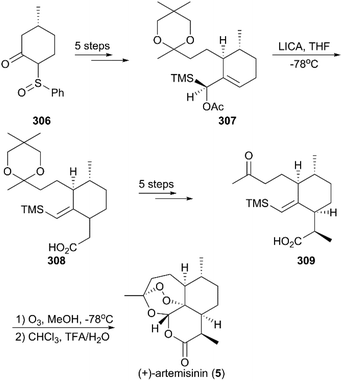
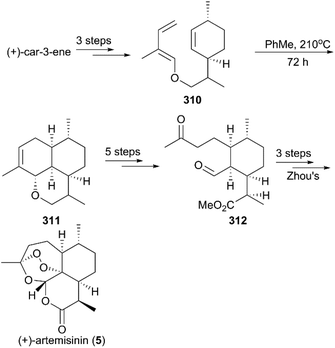
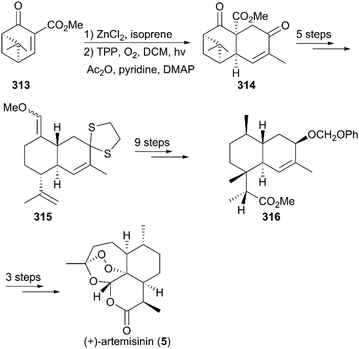
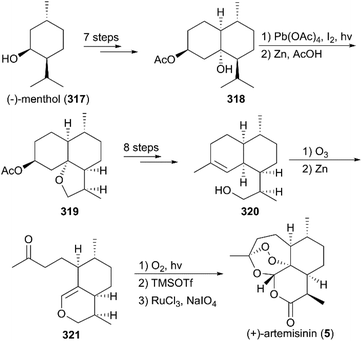
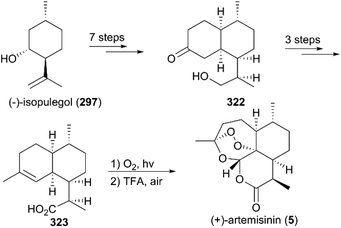
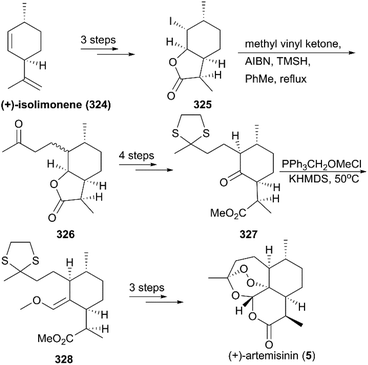
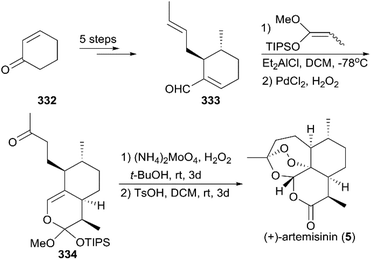
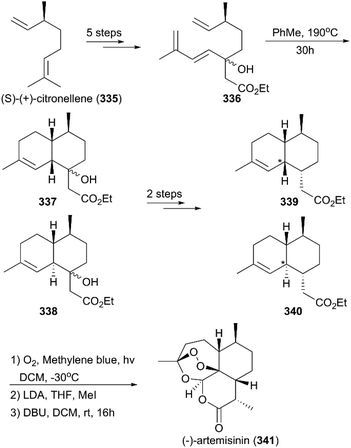
(+)-Artemisinin (QingHaoSu) (Fig. 13, 5), the most used drug against malaria worldwide, was isolated from the traditional Chinese medicine plant Artemisia annua L. in 1972 by Youyou Tu, who shared the 2015 Noble Prize for this discovery,199 and (+)-artemisinin-based combination therapy is advocated by WHO. Artemisinin is a sesquiterpene lactone, and its structure is characterized by a unique 1,2,4-trioxane moiety, a pharmacophore that is a necessary endoperoxide bridge for the development of novel antimalaria drugs through semisynthesis of (+)-artemisinin. So far, pharmacological studies have not fully elucidated the exact mechanism of action of (+)-artemisinin, which is believed to be a pro-drug of carbon-centered free radical that plays a substantial role in antimalarial produced by cleavage of its endoperoxide ring inside erythrocytes. Upon bio-activation, the binding of (+)-artemisinin with low valent ions in hemin leads to reduction of the endoperoxide ring to produce an O radical, which subsequently generates a carbon free radical. This free radical is suggested to alkylate heme in the late stage of the parasite life cycle and react with reduced glutathione (GSH) in the early life-ring stage, after which cellular targets are selectively damaged.200–205 Due to its different mechanism of action, (+)-artemisinin shows higher efficacy against multi-drug-resistant malaria compared with the old antimalarial agent quinine and synthetic drug chloroquine. However, along with the widespread use of (+)-artemisinin-based combination therapy in the world have come reports of resistance from different regions. The mechanism of artemisinin-resistance is unclear enough and mainly attributed to the C580Y mutation in Kelch13 (K13) of parasites, and associated elevation of the lipid product phosphatidylinositol-3-phosphate (PI3P). It may influence host remodeling, functions of the apicoplast and food vacuole, all of which protect the parasites against artemisinin challenge.206,207 Therefore, there is an urgent need to develop novel drugs with new chemical scaffolds and, of course, different mechanisms of action would be excellent. But (+)-artemisinin is still sourced from plants with low yield, which limits the development of more efficient drugs. Therefore, exploring chemical synthesis methods, especially for the total synthesis of (+)-artemisinin, is important for academia and the pharmacy industry.7.1 Total synthesis of artemisinin
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
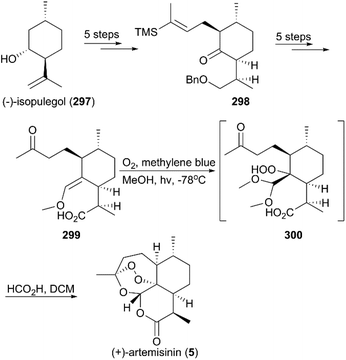
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio in five steps of protection, oxidation, and alkylation reactions. Following the nucleophilic addition of a substituted trimethylsilane reagent, accompanied by deprotection and oxidation, enol ether 299 was generated after desilylation. With the key intermediate 299 in hand, photooxygenation was carried out at −78 °C, and the crude oxidation mixture was treated with acid; the target (+)-artemisinin (5) was achieved in 30% yield after crystallization.
1 diastereomeric ratio in five steps of protection, oxidation, and alkylation reactions. Following the nucleophilic addition of a substituted trimethylsilane reagent, accompanied by deprotection and oxidation, enol ether 299 was generated after desilylation. With the key intermediate 299 in hand, photooxygenation was carried out at −78 °C, and the crude oxidation mixture was treated with acid; the target (+)-artemisinin (5) was achieved in 30% yield after crystallization.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
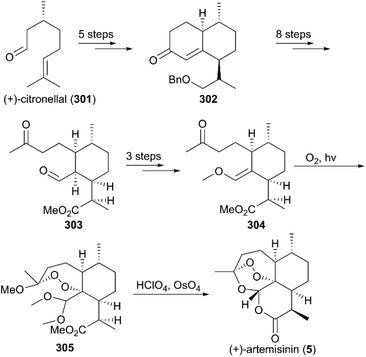
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Scheme 31).214 After sequential oxidations, hydrolysis and methylation, the ketone-aldehyde 312 Zhou reported previously was obtained, and, followed by Zhou's protocol, the synthesis of (+)-artemisinin (5) was completed.
2 (Scheme 31).214 After sequential oxidations, hydrolysis and methylation, the ketone-aldehyde 312 Zhou reported previously was obtained, and, followed by Zhou's protocol, the synthesis of (+)-artemisinin (5) was completed.
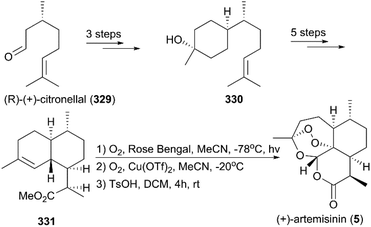
Seven years later, Yadav and co-workers altered the synthetic strategy by using (R)-(+)-citronellal 329 as a starting material instead of (+)-isolimonene 324 (Scheme 36).219 Their new route involved asymmetric 1,4-addition, aldol condensation, ene reaction, and regioselective hydroboration as key steps; synthesis of target molecule 5 was finished in 11 steps.
Although great advances in the total synthesis of artemisinin have been made by academic researchers over the past five decades, because of the complex structure of artemisinin and its derivatives and the drawbacks of the expensive starting material, long reaction routes, and excessive protecting group operations, no synthetic strategy is economically efficient enough for pharmaceutical industry-scale production to date. Therefore, more efforts are needed to design a total synthesis strategy that can be used by industry.
7.2 Structure–activity relationships of artemisinin derivatives
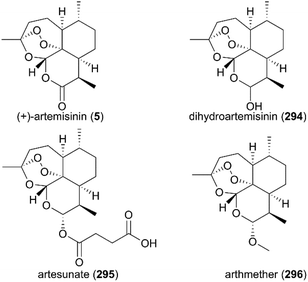
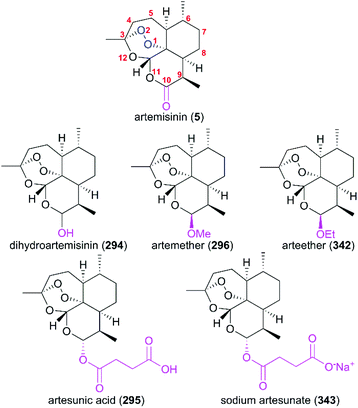
Since the discovery of artemisinin (5) as an excellent antimalaria agent, the structural analogs such as dihydroartemisinin (294), artemether (296), arteether (342), artesunic acid (295), and sodium artesunate (343) have been investigated (Fig. 14); this is because the poor physicochemical properties of artemisinin (6), including its low solubility in both oil and water, and short half-life (t1/2), limits its first-line clinical use. A large number of artemisinin derivatives and analogs have been explored in systematic SAR studies as part of an effort to seek more potential drug candidates.222–229In 2008, the first resistance to artemisinin was reported in Africa. Because of its worldwide use for a long time, WHO recommends artemisinin-based combination therapy (ACT) with different mechanisms and longer half-life instead of monotherapy using artemisinin to avoid the development and spread of resistance to artemisinin. The first-generation artemisinin-derived analogs dihydroartemisinin (294), artemether (296), arteether (342), artesunic acid (295), and sodium artesunate (343) were prepared by semisynthetic modifications at C10 with an acetal linkage; these analogs have improved physicochemical properties compared with their parent molecule, such as higher water-solubility. They are recommended by WHO to be used in combination with therapies such as ASAQ Winthrop (sodium artesunate-amodiaquine), Eurartesim (dihydroartemisinin-piperaquine), and ASMQ (sodium artesunate-mefloquine) for the treatment of malaria.
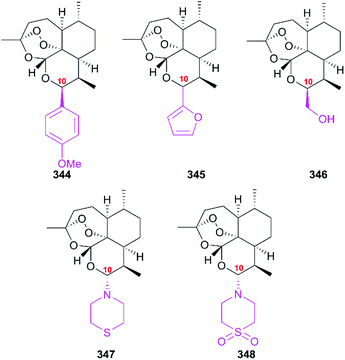
However, the drawbacks of analogs with the acid-sensitive acetal linkage include a short half-life and neurotoxicity. To overcome these drawbacks, the second-generation analogs without acetal groups were designed to improve the drug-like properties; in particular compounds 347 and 348 showed better activity (Fig. 15).230
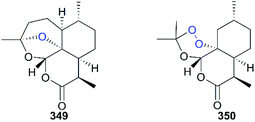
The essential role of the peroxy bridge of artemisinin, which is the pharmacophore, in the development of antimalarials was established early after its discovery, and it was confirmed by the inactive analog deoxoartemisinin 349.231 The orientation of the peroxide bond in the chemical scaffold of analogs is also important. For example, the tricyclic analog 350, which has a flexible peroxide ring displayed weaker antimalarial activity compared with the parent molecule artemisinin (Fig. 16).232
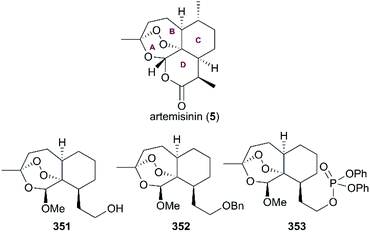
A number of semisynthetic analogs with tricyclic 1,2,4-trixoanes in a rigid scaffold lacking the D ring of artemisinin were developed, and many of them exhibited potential antimalarial activities in lower nanomolar ranges, such as analogs 351, 352, and 353 (Fig. 17).
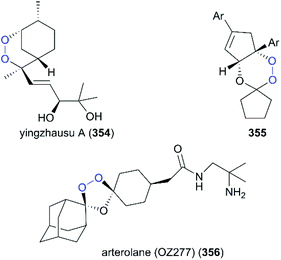
Most semisynthetic drug-like analogs with complex chemical scaffolds are probably not suitable for pharmaceutical industry-scale production. The natural product yingzhausu A (354) has an accessible endoperoxide ring and displays good antimalarial activity. Spurred by this finding, a large number of simple analogs with endoperoxides have been designed and some of them display good antimalarial activity and offer great potential as drug candidates (Fig. 18).233,234
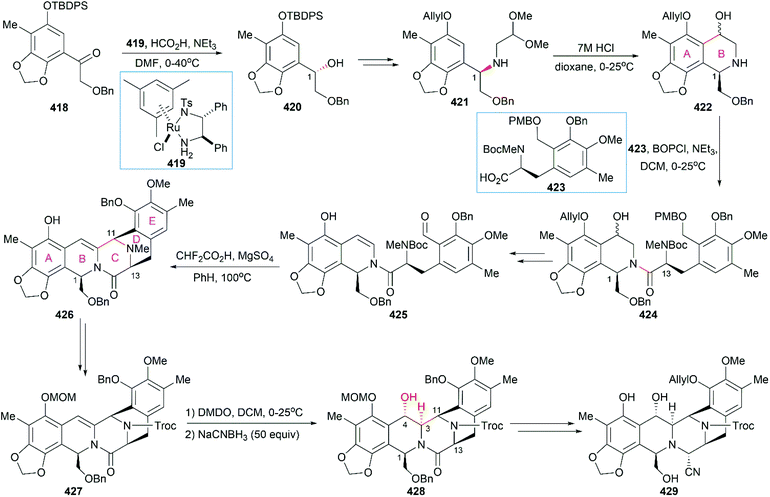
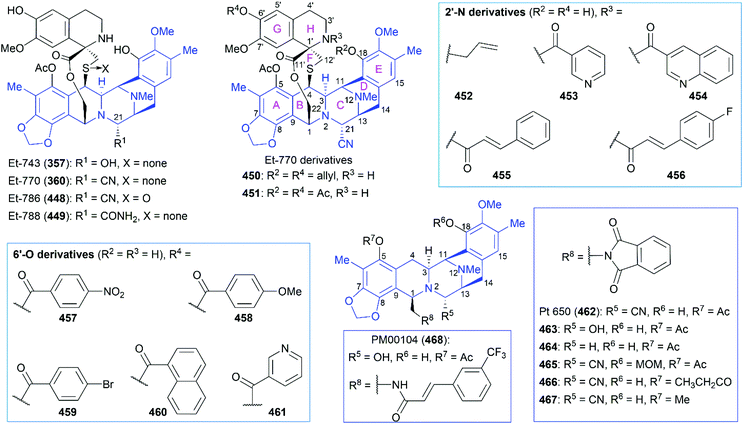
8. Ecteinascidins
Ecteinascidins are a family of marine natural products containing two or three tetrahydroisoquinoline subunits (Fig. 19). Many ecteinascidin members show antiproliferative activity against several tumor cell lines.235,236 Among these bioactive ecteinascidins, ecteinascidin 743 (Et-743, trabectedin, 357) was approved in 2007 for the treatment of advanced soft-tissue sarcoma and ovarian cancer, and lurbinectedin (363), one synthetic ecteinascidin derivative, was approved in 2020 for the treatment of metastatic small cell lung cancer.237,238 Et-743 was first isolated in 1990 from a Caribbean tunicate Ecteinascidia turbinate and its absolute stereochemistry was determined in 1992 with the help of the X-ray crystal structure of its N12-oxide derivative.239,240 Extensive studies of Et-743 show that this molecule functions by forming covalent adducts with DNA after binding to the minor groove of DNA and alkylating DNA at the N2 position of guanine.241–243 The adducts can bend the DNA structure toward the major groove,244,245 disrupt the interaction between DNA and DNA-binding proteins,246 and induce double-strand breaks (DBSs),247 all of which contribute to Et-743's antiproliferative activity on various cancer cell lines.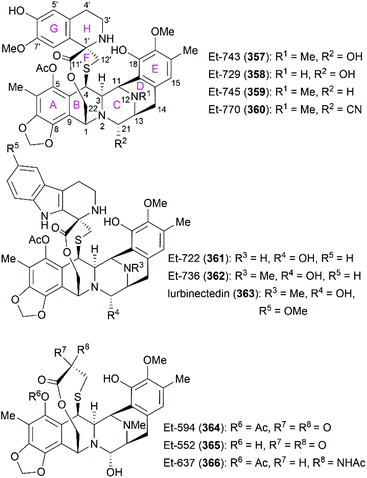 | ||
| Fig. 19 Representative ecteinascidins and ecteinascidin derivatives and their ring lettering and carbon numbering system. | ||
However, the natural source of Et-743 is limited: only 1.0 g of Et-743 can be isolated from about 1.0 ton of tunicate, which hampered its preliminary clinical studies.235,236 In 1996, Corey and co-workers reported the first synthetic route for Et-743, which was initially used for its industrial production.248 A semisynthetic route relying on Corey's synthetic route was developed in 2000 for the commercial manufacture of Et-743.249 Apart from its biological function, Et-743 also has several structural features that make it a quite challenging but attractive synthetic target: (1) three tetrahydroisoquinoline moieties with different substitution patterns; (2) eight rings including one bridged 10-membered lactone ring containing a sulfur atom; (3) seven chiral centers including one spiro center; and (4) a labile carbinolamine functional group. Continuous efforts from the synthetic community have led to outstanding achievements in the total synthesis of Et-743 and we discuss them in the following section.
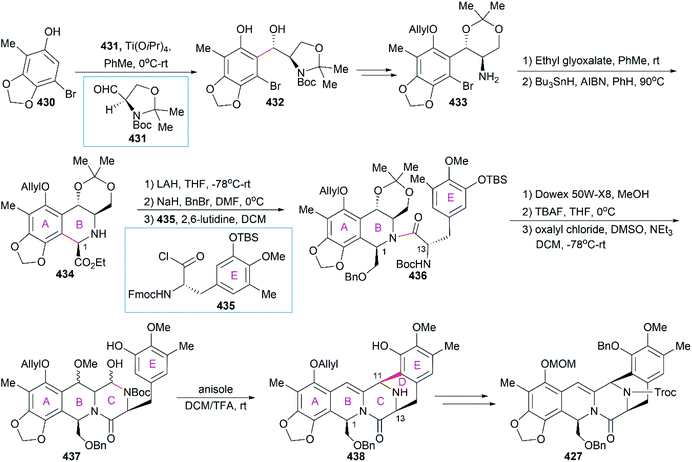
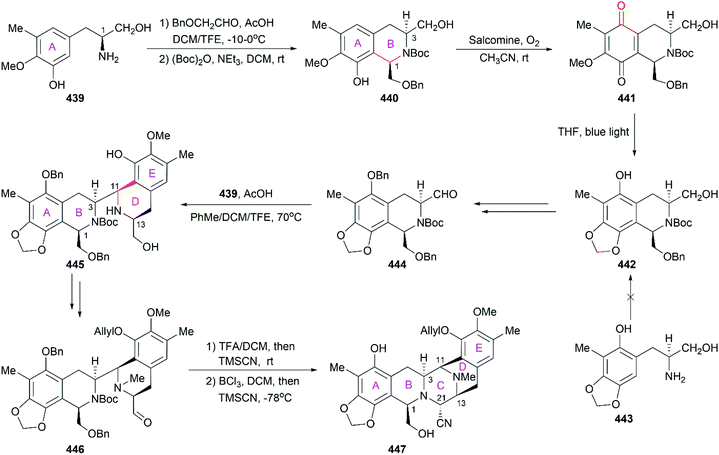
8.1 Total synthesis of Et-743
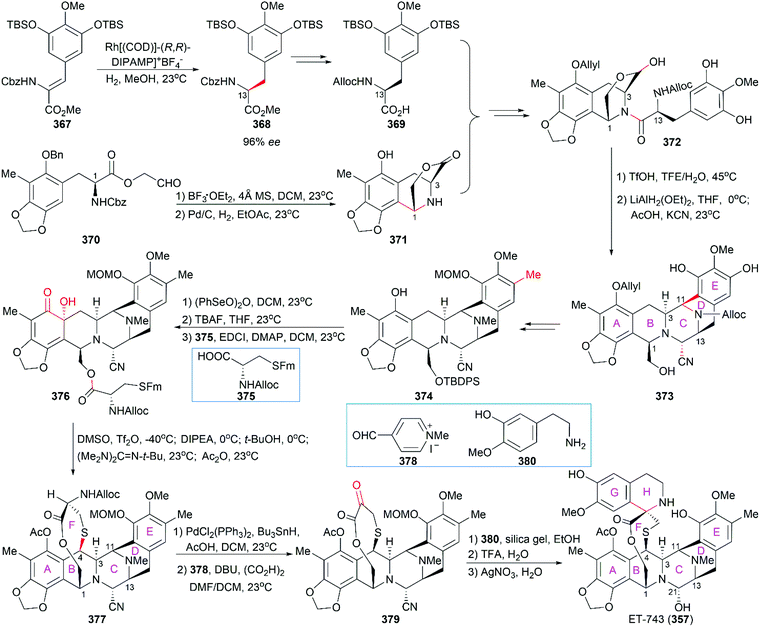
Position-selective hydroxylation with benzeneseleninic anhydride followed by the introduction of the cysteine derivative 375 generated 376, which was ready for the construction of the challenging 10-membered bridged lactone ring F. The construction of 377 from 376 involved a series of transformations: (1) activation of the tertial hydroxy group with the in situ-generated Swern reagent from Tf2O and DMSO, followed by addition of i-Pr2NEt, which led to o-quinone methide; (2) Barton's base-mediated removal of the Fm protecting group, and the subsequent nucleophilic addition of a sulfur ion to the quinone methide generating ring F; and (3) acetylation of the resulting phenoxide group with Ac2O. Removal of the Alloc protecting group and the subsequent transamination with 378 generated the keto lactone 379, which was utilized for the introduction of the third tetrahydroisoquinoline moiety through a Pictet–Spengler reaction with 380. Removal of the MOM protecting group from the C18 phenolic hydroxy group and stereoselective introduction of the C21 hydroxy group completed the total synthesis of Et-743.
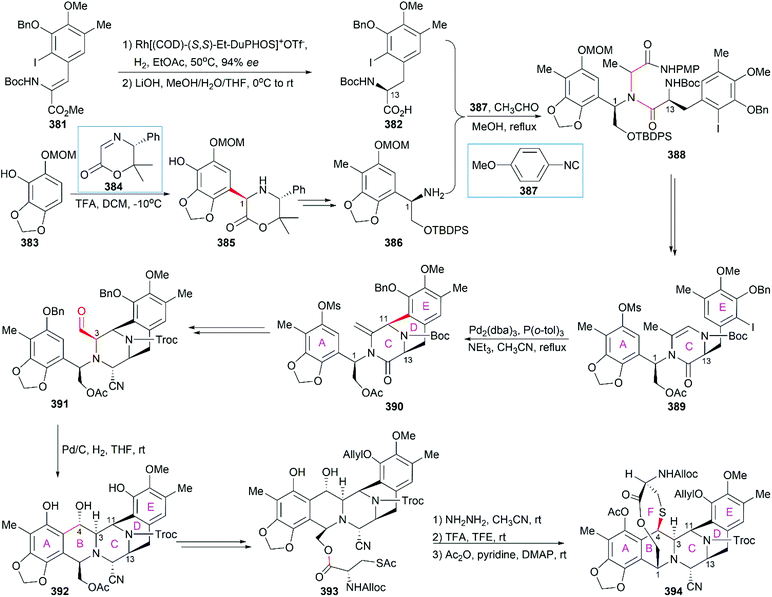
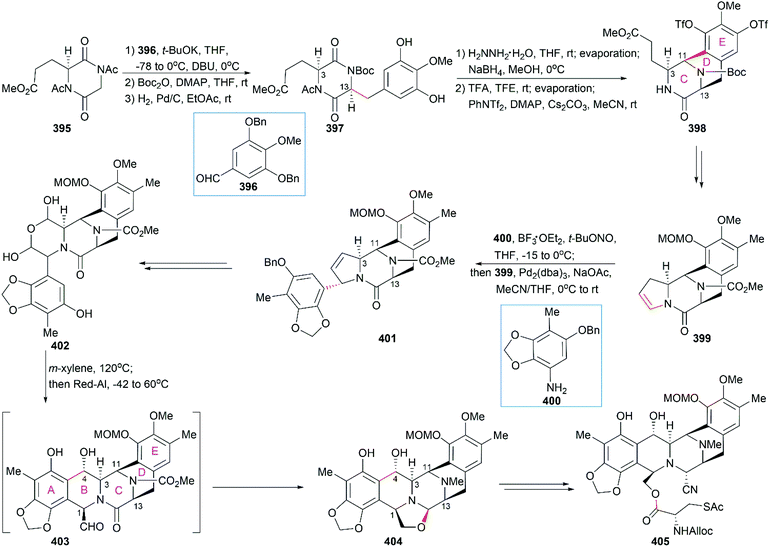
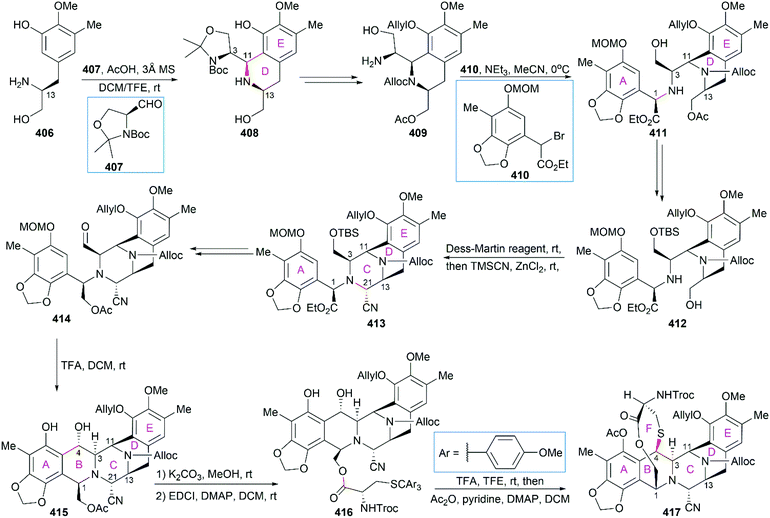
In their optimized second-generation synthetic route, the diacetylated diketopiperazine 395, which was derived from L-glutamic acid, was the only chiral source (Scheme 41).252 Perkin condensation of 395 with aldehyde 396 followed by protection of the amide and hydrogenation stereoselectively constructed the C13 chiral center. Selective reduction of the imide carbonyl group after hydrazinolysis of the acetyl group converted 397 to a suitable intermediate for construction of the bridged C/D ring system through Mannich-type reaction, which was performed in the presence of TFA. Tetracyclic enamide 399, which was derived from 398 with the installation of the methyl group on ring E and several other manipulations, was used as a platform for the introduction of ring A. A regio- and stereo-selective Heck reaction of 399 with a diazonium salt derived from amine 400 successfully led to 401. Manipulation of 401 led to the dialdehyde equivalent 402, which cyclized to form the pentacyclic compound 403 upon heating in m-xylene. Reduction of 403 with Red-Al gave oxazolidine 404, a suitable precursor for the introduction of the cysteine derivative to give 405, an analog of 393, which could be similarly converted to Et-743.
8.2 Structure–activity relationships of ecteinascidin derivatives
Although the reported total synthetic routes are not amenable to scaling-up for manufacturing ET-743 on an industrial scale, transformations developed in these studies provide access to diverse ecteinascidin derivatives, some of which are listed in Fig. 20. These synthesized ecteinascidin derivatives make SAR studies of Et-743 possible and have led to the development of more anti-tumor molecules including the approved drug lurbinectedin (363) and PM00104 (468), which is in phase II clinical trials. We give a short discussion about the SAR studies on ecteinascidin derivatives in the following section (Fig. 20 and Tables 2, 3).| Entry | Compound | IC50 (nM) | ||
|---|---|---|---|---|
| HCT116b | QG56b | DU145b | ||
| a Values given are IC50 in nM and original activity data can be found in ref. 259–262. b HCT116: human colon cancer cell line; QG56: human lung cancer cell line; DU145: human prostate cancer cell line. | ||||
| 1 | Et-770 (360) | 0.71 | 1.6 | 1.6 |
| 2 | Et-788 (449) | 3.5 × 102 | 1.3 × 103 | 9.9 × 102 |
| 3 | 450 | 54 | 1.6 × 102 | 1.0 × 102 |
| 4 | 451 | 1.3 | 9.5 | 4.4 |
| 5 | 452 | 0.50 | 1.8 | 1.2 |
| 6 | 453 | 0.13 | 0.40 | 0.72 |
| 7 | 454 | 0.014 | 0.071 | 0.087 |
| 8 | 455 | 0.053 | 0.33 | 0.24 |
| 9 | 456 | 0.010 | 0.12 | 0.041 |
| 10 | 457 | 0.26 | 0.96 | 0.37 |
| 11 | 458 | 0.31 | 1.7 | 0.62 |
| 12 | 459 | 15 | 36 | 16 |
| 13 | 460 | 13 | 36 | 24 |
| 14 | 461 | 0.20 | 1.0 | 0.51 |
| Entry | Compound | IC50 (nM) | |||
|---|---|---|---|---|---|
| A-549b | HCT116b | A375b | PC-3b | ||
| a Values given are IC50 in nM and original activity data can be found in ref. 257. b A-549: human lung cancer cell line; HCT116: human colon cancer cell line; A375: human melanoma cell line; PC-3: human prostate cancer cell line. | |||||
| 1 | Et-743 (357) | 1.0 | 0.50 | 0.15 | 0.70 |
| 2 | Pt 650 (462) | 0.95 | 0.38 | 0.17 | 0.55 |
| 3 | 463 | 1.0 | 0.61 | 0.20 | 0.53 |
| 4 | 464 | 2.2 × 103 | 1.1 × 103 | 6.1 × 102 | 8.5 × 102 |
| 5 | 465 | 2.3 × 102 | 74 | 66 | 98 |
| 6 | 466 | 2.1 | 1.2 | 0.51 | 2.9 |
| 7 | 467 | 3.1 | 1.4 | 0.55 | 3.1 |
After achieving the first total synthesis of Et-743, Corey and co-workers synthesized a series of Et-743 analogs from a common intermediate, which led to the discovery of one simpler and more stable anti-tumor molecule named Pt 650 (phthalascidin, 462).257 In 2002, Saito and co-workers isolated Et-770 (360), an analog of Et-743, from the pretreated Thai tunicate Ecteinascidia thurstoni with potassium cyanide.258 Both Pt 650 and Et-770 showed antiproliferative activities on various cancer cell lines. Synthesis and evaluation of more synthesized analogs of Pt 650 and Et-770 revealed the structural features for high potent biological activities.257–263
A cyano or a hydroxy group at the C21 position is essential and is responsible for alkylating DNA. Et-788 (449) with an amide group at C21 and 464 with hydrogen at C21 showed a significant decrease in activity compared with their corresponding parent compounds.257,261 Oxidation of the sulfur atom in Et-770 led to Et-786, which resulted in dramatically diminished cytotoxicity.259 Protection of the C18 and C6′ phenolic hydroxy groups with allyl (450) or acetate (451) decreased cytotoxicity.259,260 Protection of the C18 phenolic hydroxy group with MOM (465) also resulted in diminished antitumor activity.257
Modifications at the 2′-N position revealed that the 2′-N-allylated compound 452 showed activity similar to that of Et-770 (360), while several 2′-N amide derivatives including nitrogen-containing heterocycles (453 and 454) and cinnamoyl derivatives (455 and 456) exhibited improved cytotoxicity.260–262 Notably, 454 and 456, respectively, exhibited approximately 50- and 70-fold higher cytotoxicity to the HCT116 cancer cell line than Et-770 (360).261
Evaluation of the cytotoxicity of a series of C6′ aromatic ester derivatives showed that 457 and 458 with a nitro group or a methoxy group on the benzene ring exhibited activities similar to Et-770 (360).262 However, derivatives with a bromo group on the benzene ring (459) or a naphthyl group (460) showed significantly decreased cytotoxicity. The incorporation of a nitrogen-containing heterocyclic ester group (461) resulted in a moderate increase in activity. For Pt 650 analogs, derivatives possessing a propionyl (466) or a methyl (467) protecting group on the C5 phenolic hydroxy group were slightly less effective than the parent compound Pt 650 (462).257 These results indicate the promising role of total synthesis in the future development of ecteinascidin-type and related natural products into drugs.
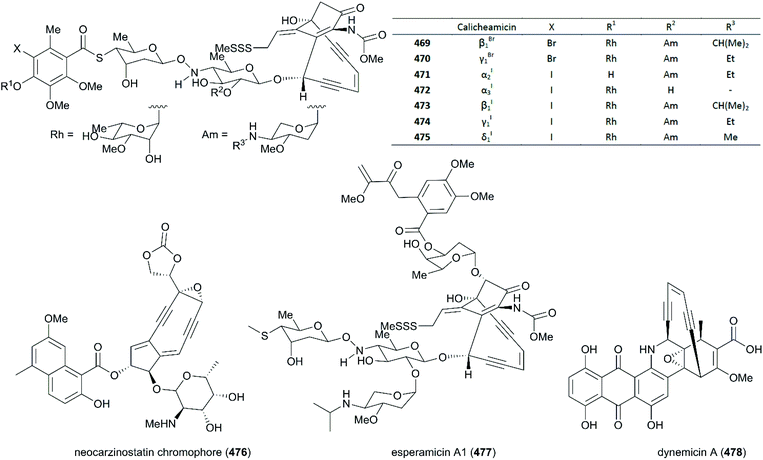
9. Calicheamicin γI1
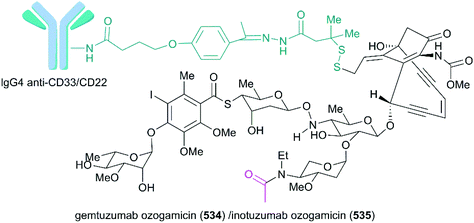
Calicheamicin γI1 (474) is a potent antitumor antibiotic that was isolated from Micromonospora echinospora ssp in 1986. One year later, its structure was defined as an enediyne with a bicyclo[7.3.1]tridec-9-ene-2,6-diyne system, a unique N–O glycosidic linkage, and a methyl trisulfide moiety; other members of the calicheamicin family were also discovered (Fig. 21).264–266 Several natural products containing the enediyne were also found in the 1980s, such as Neocarzinostatin chromophore (476),267 Esperamicin A1 (477),268 and Dynemicin A (478)269 (Fig. 21). Calicheamicin γI1 displays excellent activity against Gram-negative and -positive bacteria at concentrations lower than 1 pg mL−1. It demonstrated extreme toxicity to all cells, indicating that it has extraordinary potency against cancer cells. Investigation of its mechanism showed that calicheamicin γI1 targets and binds to the DNA minor groove, and then undergoes the Bergman cyclization after bioreductive activation to produce a biradical, 1,4-didehydrobenzene, which subsequently abstracts hydrogen atoms from the deoxyribose backbone of DNA to generate sugar radicals. The latter undergo oxygen-dependent reactions and finally induce double-strand DNA breaks.270–272 Because of its excellent activity and site-specific targeting, gemtuzumab ozogamicin (Mylotag®), a calicheamicin γI1 derivative, was approved by the FDA in 2000 as an antibody–drug conjugate (ADC) to treat CD-33-positive myeloid leukemia. Unfortunately, 10 years later it was withdrawn from the market because of its serious side effects. In 2017, inotuzumab ozogamicin (Besponsa®), also derived from calicheamicin γI1, was approved by the FDA as an ADC medication to treat relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia. All in all, the fascinating structure and highly potent drug-likeness characteristics of calicheamicin γI1 have spurred chemists to meet the huge challenge of developing strategies for the total synthesis of calicheamicin γI1.9.1 Total synthesis of calicheamicin γI1
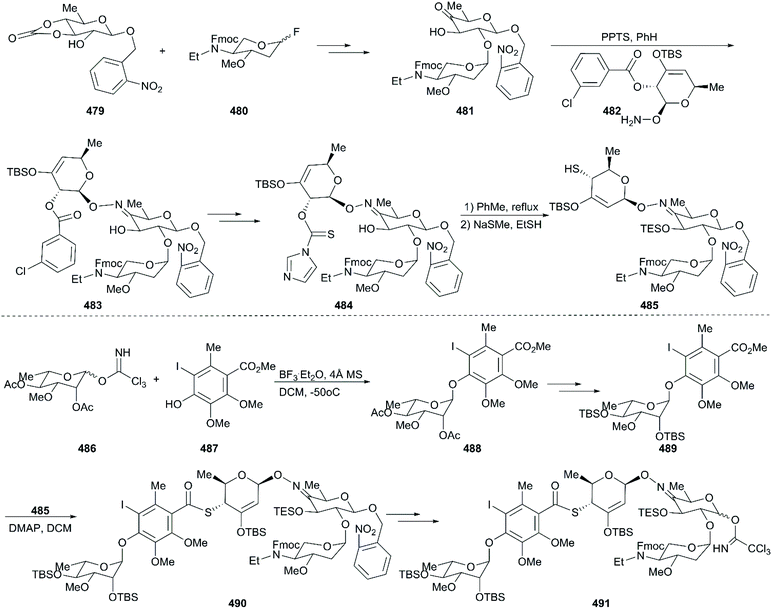
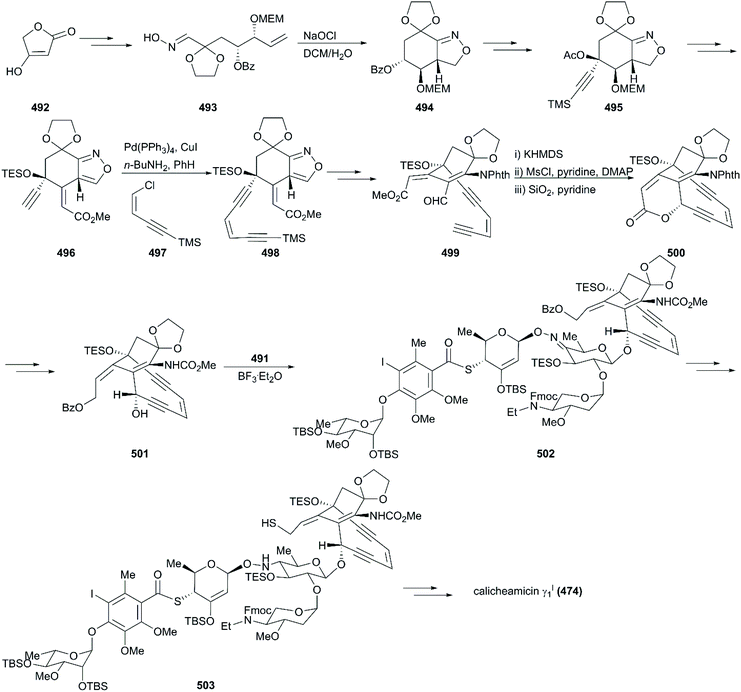
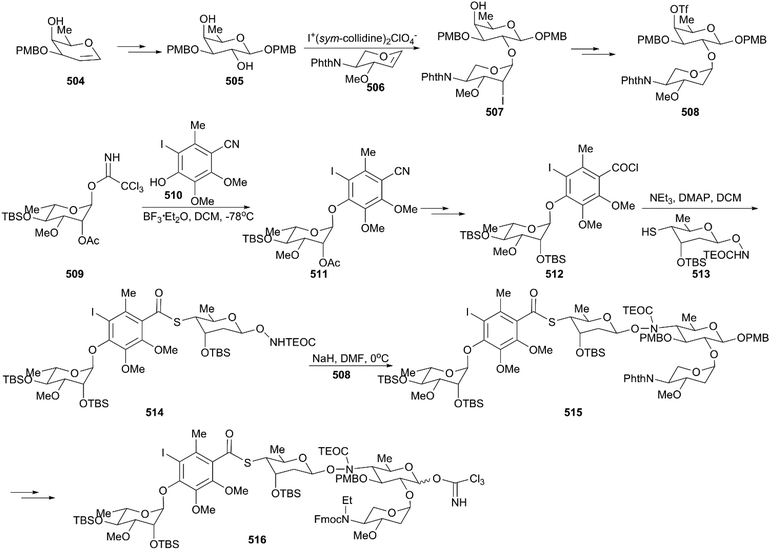
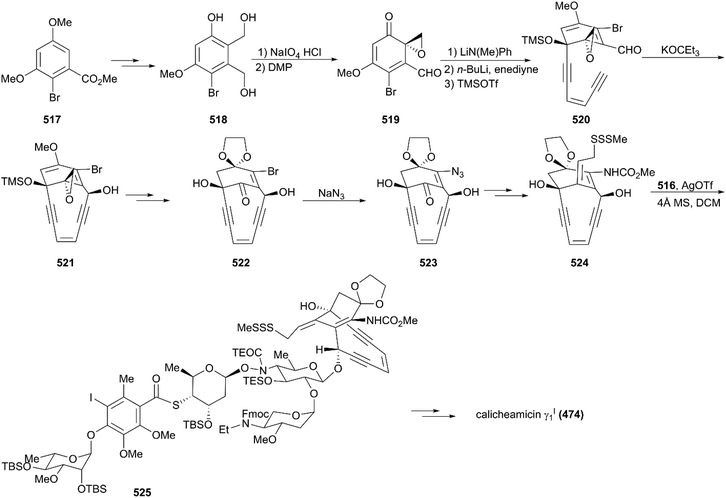
The critical synthesis of aglycon portion is to construct the cyclohexenone precursor of the bicyclic enediyne scaffold using the [3 + 2] nitrile oxide cycloaddition reaction (Scheme 47). The commercially available tetronic acid 492 was used to prepare the aldoxime precursor 493, which underwent an intramolecular 1,3-dipolar cycloaddition reaction using aqueous sodium hypochlorite solution in DCM as an oxidation system to generate isoxazoline 494. After removal of the benzoate group and the Jones oxidation of the resultant secondary hydroxy group, the ketone intermediate was produced. Subsequent addition of lithium(trimethylsilyl)acetylide and acetylation generated intermediate 495 with high stereoselectivity. After careful removal of MEM ether, the resultant secondary alcohol was subjected to Swern oxidation, generating keto isoxazole which was treated with methyl (triphenylphosphoranylidene)acetate followed by protecting group interconversions to generate the olefination product 496 as a single geometrical isomer. This product was subsequently coupled with (Z)-chloroenyne using a Pd(PPh3)4-CuI catalytic system to produce the protected enediyne 498. Cleavage of the isoxazole with molybdenum hexacarbonyl (Mo(CO)6), removal of the TMS group, and phthaloylation of amine gave rise to the desired intermediate 499. The new secondary hydroxy group produced through exposure of 499 to potassium hexamethyldisilazide in toluene was mesylated and subsequently inverted by a neighboring ester group to furnish the lactone 500 in high yield. After exchanging protecting groups and double reduction using DIBAL/NaBH4, the required aglycon intermediate 501 was produced by benzoylation. With the tetrasaccharide donor 491 and aglycon 501 in hand, Schmidt glycosidation was applied to form the coupled product 502 as a single isomer. Then, the target molecule calicheamicin γI1 (474) was generated after multi-step transformations.
The synthesis of the aglycon unit started with the synthesis of unstable triol 518 from the commercially available benzoate 517; 518 was subjected to Becker oxidation to generate an epoxide and, following Dess–Martin oxidation, aldehyde 519 was obtained (Scheme 49). The aldehyde 519 was protected by treating with lithium N-methylanilide, and the keto group was attacked by the lithium acetylide generated from the parent enediyne to give adduct 520 after silylation of the newly formed alcohol by utilizing trimethylsilyl triflate. Then, 520 was subjected to potassium 3-ethyl-3-pentoxide, and the core skeleton 521 was obtained. Protecting group interconversions, acetolysis, and oxidative cleavage were employed to convert 521 to 522. Exposure of 522 to a solution of sodium azide afforded the desired product, azidoenone 523, which underwent subsequent key transformations including reduction of azide, an intramolecular Horner–Wadsworth–Emmons reaction, reduction using NaBH4, and a Mitsunobu reaction, generating trisulfide 524. Remarkably, the Schmidt protocol using silver triflate and molecular sieves was employed to couple trisulfide 524 with the tetrasaccharide donor 516 to forge 525 as a single isomer with moderate yield.
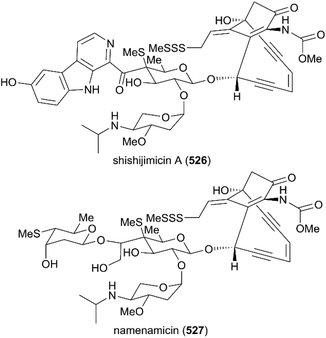
Not long after the discovery of calicheamicin γI1, the structural analogs of calicheamicin, shishijimicin A (526)282 and namenamicin (527),283 were isolated from marine organisms (Fig. 22). Biological assays showed that they both displayed highly potent antitumor activity. Very recently, Nicolaou and co-workers finished the total synthesis of shishijimicin A (526)284,285 and namenamicin (527)286 using a protocol they developed previously, with some modifications. The details of these approaches are not discussed here. The derivatives and analogs of shishijimicin A were also constructed using the total synthesis strategy to explore the ADC payloads for clinical trials against tumors.287
9.2 Structure–activity relationships of calicheamicin γI1 derivatives
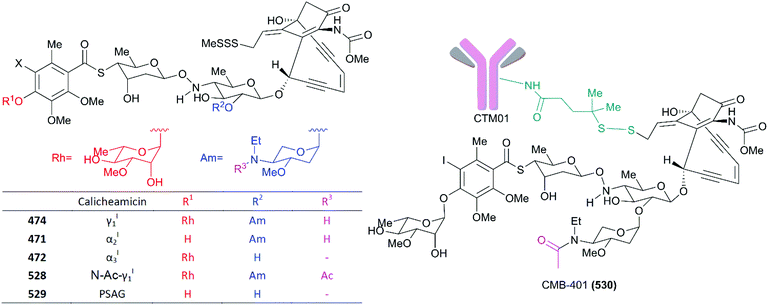
As mentioned above, since calicheamicin γI1 was discovered as a 10-membered enediyne with highly potent cytotoxicity against tumors, a number of synthetic analogs have been designed and used as ADC payloads in preclinical evaluations and clinical tests against tumors. According to the mechanism of action described, the enediyne subunit of calicheamicin γI1 that plays an irreplaceable role in the generation of DNA lesions through the Bergman rearrangement is unchangeable, and the allylic trisulfide serves as a trigger to induce cycloaromatization. Studies of the semisynthetic aglycon analogs revealed that the role of carbohydrate fragments is to facilitate DNA binding and confer target specificity.270,288 To date, SAR research has mainly focused on the modification and simplification of oligosaccharide fragments or trisulfide groups.The four calicheamicin γI1 analogs (Fig. 23), the natural products 471 and 472 and synthetic compounds 528 and 529, were all conjugated to CT-M-01 and the resultant derivatives were tested for therapeutic efficacy against MX-1 xenograft tumors. The results showed that compounds 472 and 528 with the DNA binding unit and the lack or modifications of amino sugar fragments showed stronger therapeutic efficacy than the parent molecule 474 and the derivatives 471 and 529, which missed the rhamnose at the DNA binding region. This result suggested that a rhamnose at the end of the binding region is required for ADC medications.289 CMB-401 (530) was further developed using a more stable amide-disulfide-based linker to conjugate it with hCTMO1 based on the structure of compound 528, and this compound was tested in phase I and phase II studies against epithelial ovarian cancer. However, no patients had objective responses in the phase II investigation. Scientists speculated that the sterically hindered disulfide failed to release the drug from the ADC payload.288
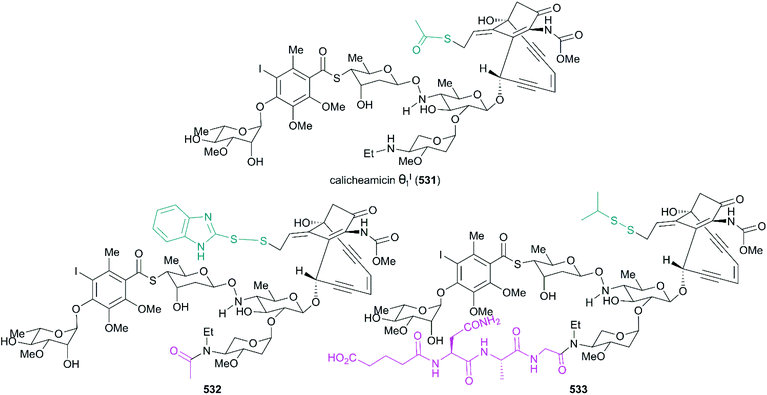
Calicheamicin θI1 (Fig. 24, 531), a novel derivative of calicheamicin γI1, was developed using thioester to replace the methyl trisulfide and prepared through total synthesis by the Nicolaou group. After the test, calicheamicin θI1 was hypothesized to be a powerful biological tool for generating specific DNA lesions because of its highly potent and selective DNA cleaving, apoptosis-inducing, and cytotoxic properties.290 It was also discovered that calicheamicin θI1 (531) effectively suppressed liver metastases in a novel syngeneic model of murine neuroblastoma and CD19-positive malignancies.291,292 In some analogs, the allylic trisulfide was replaced by isopropyl disulfide or functionalized to improve the stability and solubility (Fig. 24, 532 and 533).293,294 Gemtuzumab ozogamicin (Mylotag®, 534) and inotuzumab ozogamicin (Besponsa®, 535) are ADC drugs approved to treat myeloid leukemia and B-cell malignancies by the FDA in 2000 and 2017, respectively. Because of the important role that the linker plays in the effective release of the calicheamicin derivative from the conjugation product, allowing it to access the DNA target, a linker, 4-(4-acetylphenoxy)butanoic acid, was added to condensate drug and antibody. This conjugate showed stability in physiological buffer (pH 7.4) and the calicheamicin derivative was efficiently released inside lysosomes (pH 4.0) (Fig. 25).295
10. Conclusions
In this review, we discussed the total synthesis and SAR studies of eight representative families of natural products applied in the treatment of various human diseases. Although just the tip of the iceberg, these examples demonstrate the essential role of total synthesis in natural product drugs’ discovery: development of efficient transformations for natural products synthesis, especially the deep-seated structural modifications that are otherwise tough to achieve; and providing adequate and sustainable supplies of natural products and their analogs for clinical studies and use. From another standpoint, total syntheses of natural products, which often contain complex structural features, also promote the development of synthetic chemistry by inspiring the discovery of new reactions and reagents for rapid molecular assembly and structural diversifications. In combination with the development of other disciplines, such as synthetic biology and chemical biology, chemical synthesis will continue to play a profound role in functional mechanism elucidation, new biological target identification, and natural product medicinal chemistry.Abbreviations
| AcOH | Acetic acid |
| AIBN | 2,2′-Azobisisobutyronitrile |
| BHT | Butylated hydroxytoluene |
| Boc2O | Di-tert-butyl dicarbonate |
| BOPCl | Bis(2-oxo-3-oxazolidinyl)phosphinic chloride |
| BPO | Benzoyl peroxide |
| CSA | Camphorsulfonic acid |
| DABCO | 1,4-Diazabicyclo[2.2.2]octane |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DCM | Dichloromethane |
| DEAD | Diethyl azodicarboxylate |
| 1,2-DFB | 1,2-Difluorobenzene |
| DIPAMP | (Ethane-1,2-diyl)bis[(2-methoxyphenyl)(phenyl)phosphine] |
| DIPEA | N,N-Diisopropylethylamine |
| DMAP | 4-(N,N-Dimethylamino)pyridine |
| DMDO | Dimethyldioxirane |
| DME | 1,2-Dimethoxyethane |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| Dppp | 1,3-Bis(diphenylphosphino)propane |
| DTBMP | 2,6-Di-tert-butyl-4-methylpyridine |
| EDCI | N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride |
| HFIP | Hexafluoroisopropanol |
| HMPA | Hexamethylphosphoramide |
| IBX | 2-Iodoxybenzoic acid |
| KHMDS | Potassium bis(trimethylsilyl)amide |
| LDA | Lithium diisopropylamide |
| LICA | Lithium N-isopropylcyclohexylamide |
| LiDBB | Lithium di-tert-butylbiphenyl |
| LiHMDS | Lithium bis(trimethylsilyl)amide |
| LTMP | Lithium tetramethylpiperidide |
| m-CPBA | meta-Chloroperoxybenzoic acid |
| MesLi | Mesityllithium |
| MS | Molecular sieve |
| MsCl | Methanesulfonyl chloride |
| NaHMDS | Sodium bis(trimethylsilyl)amide |
| NBS | N-Bromosuccinimide |
| NMO | 4-Methylmorpholine N-oxide |
| NTf2 | Bis(trifluoromethylsulfonyl)imide |
| Pd2(dba)2 | Bis(dibenzylideneacetone)palladium |
| PhNTf2 | Bis(trifluoromethanesulfonyl)aniline |
| PMP | 1,2,2,6,6-Pentamethylpiperidine |
| PPTS | Pyridinium p-toluenesulfonate |
| Red-Al | Sodium bis(2-methoxyethoxy)aluminiumhydride |
| [Rh(COD)Cl]2 | Chloro(1,5-cyclooctadiene)rhodium(I) dimer |
| Rt | Room temperature |
| SARs | Structure–activity relationships |
| TBAF | tert-Butylammonium fluoride |
| TBAI | tert-Butylammonium iodide |
| TBSOTf | tert-Butyldimethylsilyl trifluoromethanesulfonate |
| TESOH | Triethylsilanol |
| Tf2O | Trifluoromethanesulfonic anhydride |
| TFA | Trifluoroacetic acid |
| TFAA | Trifluoroacetic anhydride |
| TFE | 2,2,2-Trifluoroethanol |
| TfOH | Trifluoromethanesulfonic acid |
| THF | Tetrahydrofuran |
| TMEDA | Tetramethylethylenediamine |
| TMSCl | Chlorotrimethylsilane |
| TMSCN | Trimethylsilyl cyanide |
| TMSH | Trimethylsulfonium hydroxide |
| TMSimid | 1-(Trimethylsilyl)imidazole |
| TMSOH | Trimethylsilanol |
| TPAP | Tetrapropylammonium perruthenate |
| TPP | Triphenylphosphine |
| TsOH | p-Toluenesulfonic acid |
Author contributions
X. Q. conceived the study; G. L., M. L. and X. Q. prepared the manuscript; all authors discussed the contents and commented on the manuscript.Conflicts of interest
The authors declare no conflict of interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 21971018). We gratefully acknowledge the Beijing Municipal Government and Tsinghua University for financial support.References
- G. M. Cragg and D. J. Newman, Natural products: a continuing source of novel drug leads, Biochim. Biophys. Acta, Gen. Subj., 2013, 1830, 3670–3695 CrossRef CAS PubMed.
- N. R. Farnsworth, O. Akerele, A. S. Bingel, D. D. Soejarto and Z. Guo, Medicinal plants in therapy, Bull. W. H. O., 1985, 63, 965–981 CAS.
- D. T. Courtwright, Forces of habit: drugs and the making of the modern world, Harvard University Press, 2002, P36 Search PubMed.
- V. Cechinel-Filho, Plant Bioactives and drug discovery John Wiley & Sons, Hoboken, New Jersey, 2011 Search PubMed.
- G. M. Cragg, D. J. Newman and K. M. Snader, Natural products in drug discovery and development, J. Nat. Prod., 1997, 60, 52–60 CrossRef CAS PubMed.
- K. S. Lam, New aspects of natural products in drug discovery, Trends Microbiol., 2007, 15, 279–289 CrossRef CAS PubMed.
- B. L. DeCorte, Underexplored opportunities for natural products in drug discovery, J. Med. Chem., 2016, 59, 9295–9304 CrossRef CAS PubMed.
- Z.-C. Wu and D. L. Boger, The quest for supernatural products: the impact of total synthesis in complex natural products medicinal chemistry, Nat. Prod. Rep., 2020, 37, 1511–1531 RSC.
- A. G. Atanasov, S. B. Zotchev, V. M. Dirsch, the International Natural Product Sciences Taskforce and C. T. Supuran, Natural products in drug discovery: advances and opportunities, Nat. Rev. Drug Discovery, 2021, 20, 200–216 CrossRef CAS PubMed.
- M.-J. U. Ferreira, Natural products in drug discovery and human health, Phytochem. Rev., 2021, 20, 1–4 CrossRef CAS.
- C. A. Dehelean, I. Marcovici, C. Soica, M. Mioc, D. Coricovac, S. Iurciuc, O. M. Cretu and I. Pinzaru, Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy, Molecules, 2021, 26, 1109–1138 CrossRef CAS PubMed.
- A. Nelson and G. Karageorgis, Natural product-informed exploration of chemical space to enable bioactive molecular discovery, RSC Med. Chem., 2021, 12, 353–362 RSC.
- J. Skubnik, V. Pavlickova, T. Ruml and S. Rimpelova, Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy, Plants, 2021, 10, 569–603 CrossRef CAS PubMed.
- C. Hobson, A. N. Chan and G. D. Wright, The antibiotic resistome: a guide for the discovery of natural products as antimicrobial agents, Chem. Rev., 2021, 121, 3464–3494 CrossRef CAS PubMed.
- P. Fernandes and E. Martens, Antibiotics in late clinical development, Biochem. Pharmacol., 2017, 133, 152–163 CrossRef CAS PubMed.
- R. I. Aminov, A brief history of the antibiotic era: lessons learned and challenges for the future, Front. Microbiol., 2010, 1, 1–7 Search PubMed.
- G. M. Cragg, P. G. Grothaus and D. J. Newman, Impact of natural products on developing new anti-cancer agents, Chem. Rev., 2009, 109, 3012–3043 CrossRef CAS PubMed.
- P. Kittakoop, Anticancer drugs and potential anticancer leads inspired by natural products, Elsevier B.V., 2015 Search PubMed.
- A. Mazumder, C. Cerell and M. Diederich, Natural scaffolds in anticancer therapy and precision medicine, Biotechnol. Adv., 2018, 36, 1563–1585 CrossRef CAS PubMed.
- M. Huang, J.-J. Lu and J. Ding, Natural Products in Cancer Therapy: Past, Present and Future, Nat. Prod. Bioprospect., 2021, 11, 5–13 CrossRef PubMed.
- A. J. Goodman, B. L. Bourdonnec and R. E. Dolle, Mu opioid receptor antagonists: recent developments, ChemMedChem, 2007, 2, 1552–1570 CrossRef CAS PubMed.
- H. Buschmann, T. Christoph, E. Friderichs, C. Maul and B. Sundermann, Analgesics-from chemistry and pharmacology to clinical application, Wiley-VCH, Weinheim, Germany, 2002 Search PubMed.
- L. H. Miller and X. Su, Artemisinin: discovery from the Chinese herbal garden, Cell, 2011, 146, 855–858 CrossRef CAS PubMed.
- N. S. Tibon, C. H. Ng and S. L. Cheong, Current progress in antimalarial pharmacotherapy and multi-target drug discovery, Eur. J. Med. Chem., 2020, 188, 111983–112007 CrossRef CAS PubMed.
- H. Madhav and N. Hoda, An insight into the recent development of the clinical candidates for the treatment of malaria and their target proteins, Eur. J. Med. Chem., 2021, 210, 112955–112995 CrossRef CAS PubMed.
- D. J. Newman, G. M. Cragg and K. M. Snader, Natural products as sources of new drugs over the period 1981–2002, J. Nat. Prod., 2003, 66, 1022–1037 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, Natural products as sources of new drugs over the last 25 Years, J. Nat. Prod., 2007, 70, 461–477 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, Natural products as sources of new drugs from 1981 to 2014, J. Nat. Prod., 2016, 79, 629–661 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019, J. Nat. Prod., 2020, 83, 770–803 CrossRef CAS PubMed.
- F. W. Serturner, Darstellung der reinen Mohnsäure (Opiumsaure) nebst einer Chemischen Untersuchung des Opiums mit vorzüglicher Hinsicht auf einen darin neuentdeckten Stoff und die dahin gehörigen Bemerkungen, J. Pharm., 1806, 14, 47–93 Search PubMed.
- B. H. Novak, T. Hudlicky, J. W. Reed, J. Mulzer and D. Trauner, Morphine synthesis and biosynthesis—an update, Curr. Org. Chem., 2000, 4, 343–362 CrossRef CAS.
- P. R. Blakemorea and J. D. White, Morphine, the proteus of organic molecules, Chem. Commun., 2002, 1159–1168 RSC.
- J. Zezula and T. Hudlicky, Recent progress in the synthesis of morphine alkaloids, Synlett, 2005, 388–405 CAS.
- U. Rinner and T. Hudlicky, in Alkaloid synthesis, ed. H.-J. Knolker, Springer Science & Business Media, 2012, vol. 309, pp. 33–66 Search PubMed.
- A. S. Yekkirala, A. E. Kalyuzhny and P. S. Portoghese, Standard opioid agonists activate heteromeric opioid receptors: evidence for morphine and [d-Ala(2)-MePhe(4)-Glyol(5)]enkephalin as selective μ-δ agonists, ACS Chem. Neurosci., 2010, 1, 146–154 CrossRef CAS PubMed.
- A. S. Yekkirala, M. L. Banks, M. M. Lunzer, S. S. Negus, K. C. Rice and P. S. Portoghese, Clinically employed opioid analgesics produce antinociception via μ-δ opioid receptor heteromers in Rhesus monkeys, ACS Chem. Neurosci., 2012, 3, 720–727 CrossRef CAS PubMed.
- J. W. Reed and T. Hudlicky, The quest for a practical synthesis of morphine alkaloids and their derivatives by chemoenzymatic methods, Acc. Chem. Res., 2015, 48, 674–687 CrossRef CAS PubMed.
- T. M. Kutchan, in The alkaloids: chemistry and biology, ed. G. A. Cordell, Academic Press, London, 1998, vol. 50, pp. 257–316 Search PubMed.
- S. Galanie, K. Thodey, I. J. Trenchard, M. F. Interrante and C. D. Smolke, Complete biosynthesis of opioids in yeast, Science, 2015, 349, 6252–6257 CrossRef PubMed.
- J. M. Gulland and R. Robinson, Mem. Proc. – Manchester Lit. Philos. Soc., 1925, 69, 79 CAS.
- M. Gates and G. Tschudi, The synthesis of morphine, J. Am. Chem. Soc., 1952, 74, 1109–1110 CrossRef CAS.
- M. Gates and G. Tschudi, The synthesis of morphine, J. Am. Chem. Soc., 1956, 78, 1380–1393 CrossRef CAS.
- M. F. Mackay and D. C. Hodgki, A crystallographic examination of the structure of morphine, J. Chem. Soc., 1955, 3261–3267 RSC.
- Q. Li and H. Zhang, Research progress on the synthesis of morphine alkaloids, Chin. J. Org. Chem., 2017, 37, 1629–1652 CrossRef CAS.
- S. A. Chambers, J. M. DeSousa, E. D. Huseman and S. D. Townsend, The DARK side of total synthesis: strategies and tactics in psychoactive drug production, ACS Chem. Neurosci., 2018, 9, 2307–2330 CrossRef CAS PubMed.
- C. Y. Hong, N. Kado and L. E. Overman, Asymmetric synthesis of either enantiomer of opium alkaloids and morphinans. Total synthesis of (−)- and (+)-dihydrocodeinone and (−)- and (+)-morphine, J. Am. Chem. Soc., 1993, 115, 11028–11029 CrossRef CAS.
- I. Iijima and K. C. Rice, Studies in the (+)-morphinan series I. An alternative conversion of (+)-dihydrocodeinone into (+)-codeine, Heterocycles, 1977, 6, 1157–1165 CrossRef CAS.
- J. D. White, P. Hrnciar and F. Stappenbeck, Asymmetric synthesis of (+)-morphine. The phenanthrene route revisited, J. Org. Chem., 1997, 62, 5250–5251 CrossRef CAS.
- B. M. Trost and W. Tang, An efficient enantioselective synthesis of (-)-galanthamine, Angew. Chem., Int. Ed., 2002, 41, 2795–2797 CrossRef CAS PubMed.
- B. M. Trost and W. Tang, Enantioselective synthesis of (-)-codeine and (-)-morphine, J. Am. Chem. Soc., 2002, 124, 14542–14543 CrossRef CAS PubMed.
- B. M. Trost, W. Tang and F. D. Toste, Divergent enantioselective synthesis of (-)-galanthamine and (-)-morphine, J. Am. Chem. Soc., 2005, 127, 14785–14803 CrossRef CAS PubMed.
- H. Leisch, A. T. Omori, K. J. Finn, J. Gilmet, T. Bissett, D. Ilceski and T. Hudlicky, Chemoenzymatic enantiodivergent total syntheses of (+)- and (-)-codeine, Tetrahedron, 2009, 65, 9862–9875 CrossRef CAS.
- M. Makarova, M. A. A. Endoma-Arias, H. E. D. Paz, R. Simionescu and T. Hudlicky, Chemoenzymatic total synthesis of ent-oxycodone: second-, third-, and fourth-generation strategies, J. Am. Chem. Soc., 2019, 141, 10883–10904 CrossRef CAS PubMed.
- Q. Zhang, F.-M. Zhang, C.-S. Zhang, S.-Z. Liu, J.-M. Tian, S.-H. Wang, X.-M. Zhang and Y.-Q. Tu, Enantioselective synthesis of cis-hydrobenzofurans bearing all-carbon quaternary stereocenters and application to total synthesis of (-)-morphine, Nat. Commun., 2019, 10, 2507–2513 CrossRef PubMed.
- Y. S.-H. Hou, A. Prichina and G. Dong, Deconstructive asymmetric total synthesis of morphine-family alkaloid (-)-thebainone A, Angew. Chem., Int. Ed., 2021, 60, 13057–13064 CrossRef PubMed.
- D. A. Williams, V. F. Roche and E. B. Roche, in Foye's principles of medicinal chemistry, ed. T. L. Lemke, D. A. Williams, V. F. Roche and S. W. Zito, Lippincott Williams & Wilkins, China, 7 edn, 2012, ch. 20, pp. 675–695 Search PubMed.
- K. Raynor, H. Kong, Y. Chen, K. Yasuda, L. Yu, G. I. Bell and T. Reisine, Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors, Mol. Pharmacol., 1994, 45, 330–334 CAS.
- I. D. Pogozheva, A. L. Lomize and H. I. Mosberg, Opioid receptor three-dimensional structures from distance geometry calculations with hydrogen bonding constraints, Biophys. J., 1998, 75, 612–632 CrossRef CAS PubMed.
- H. E. Hassan, S. L. Mercer, C. W. Cunningham, A. Coop and N. D. Eddington, Evaluation of the P-glycoprotein (Abcb1) affinity status of a series of morphine analogs: Comparative study with meperidine analogs to identify opioids with minimal P-glycoprotein interactions, Int. J. Pharm., 2009, 375, 48–54 CrossRef CAS PubMed.
- T. H. Jukes, Some historical notes on chlortetracycline, Rev. Infect. Dis., 1985, 7(5), 702–707 CrossRef CAS PubMed.
- A. C. Finlay, G. L. Hobby, S. Y. P′an, P. P. Regna, J. B. Routien, D. B. Seeley, G. M. Shull, B. A. Sobin, I. A. Solomons, J. W. Vinson and J. H. Kane, Terramycin, a new antibiotic, Science, 1950, 111, 85–87 CrossRef CAS PubMed.
- M. A. Darken, H. Berenson, R. J. Shirk and N. O. Sjolander, Production of tetracycline by Streptomyces aureofaciens in synthetic media, Appl. Microbiol., 1960, 8, 46–51 CrossRef CAS PubMed.
- J. R. D. McCormick, N. O. Sjolander, U. Hirsch, E. R. Jensen and A. P. Doerschuk, A new family of antibiotics: the demethyltetracyclines, J. Am. Chem. Soc., 1957, 79, 4561–4563 CrossRef CAS.
- I. Chopra and M. Roberts, Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance, Microbiol. Mol. Biol. Rev., 2001, 65, 232–260 CrossRef CAS PubMed.
- D. E. Brodersen, J. William, M. Clemons, A. P. Carter, R. J. Morgan-Warren, B. T. Wimberly and V. Ramakrishnan, The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit, Cell, 2000, 103, 1143–1154 CrossRef CAS PubMed.
- M. C. Roberts, Tetracycline resistance determinants: mechanisms of action, regulation of expression, genetic mobility, and distribution, FEMS Microbiol. Rev., 1996, 19, 1–24 CrossRef CAS PubMed.
- S. R. Connell, D. M. Tracz, K. H. Nierhaus and D. E. Taylor, Ribosomal protection proteins and their mechanism of tetracycline resistance, Antimicrob. Agents Chemother., 2003, 47, 3675–3681 CrossRef CAS PubMed.
- T. H. Grossman, Tetracycline antibiotics and resistance, Cold Spring Harbor Perspect. Med., 2016, 6, a025387–a025387 CrossRef PubMed.
- C. R. Stephens, L. H. Conover, F. A. Hochstein, P. P. Regna, F. J. Pilgrim, K. J. Brunings and R. B. Woodward, Terramycin. VIII. Structure of aureomycin and terramycin, J. Am. Chem. Soc., 1952, 74, 4976–4977 CrossRef CAS.
- F. A. Hochstein, C. R. Stephens, L. H. Conover, P. P. Regna, R. Pasternack, P. N. Gordon, F. J. Pilgrim, K. J. Brunings and R. B. Woodward, The structure of terramycin, J. Am. Chem. Soc., 1953, 75, 5455–5475 CrossRef CAS.
- C. R. Stephens, L. H. Conover, R. Pasternack, F. A. Hochstein, W. T. Moreland, P. P. Regna, F. J. Pilgrim, K. J. Brunings and R. B. Woodward, Structure of aureomycin, J. Am. Chem. Soc., 1954, 76, 3568–3575 CrossRef CAS.
- D. L. J. Clive, Chemistry of the tetracyclines, Q. Rev., Chem. Soc., 1968, 22, 435–456 RSC.
- T. K. Allred, F. Manoni and P. G. Harran, Exploring the boundaries of “practical”: De novo syntheses of complex natural product-based drug candidates, Chem. Rev., 2017, 117, 11994–12051 CrossRef CAS PubMed.
- L. H. Conover, K. Butler, R. B. Johnston, R. B. Korst and R. B. Woodward, The total synthesis of 6-demethyl-6 deoxytetracycline, J. Am. Chem. Soc., 1962, 84, 3222–3224 CrossRef CAS.
- R. B. Woodward, The total synthesis of a tetracycline, Pure Appl. Chem., 1963, 6, 561–573 CAS.
- J. J. Korst, J. D. Johnston, K. Butler, E. J. Bianco, L. H. Conover and R. B. Woodward, The total synthesis of dl-6-demethyl-6-deoxytetracycline, J. Am. Chem. Soc., 1968, 90, 439–457 CrossRef CAS.
- A. I. Gurevich, M. G. Karapetyan, M. N. Kolosov, V. G. Korobko, V. V. Onoprienko, S. A. Popravko and M. M. Shemyakin, Synthesis of 12a-deoxy-5a,6-anhydrotetracycline. The first total synthesis of the naturally occuring tetracycline, Tetrahedron Lett., 1967, 8, 131–134 CrossRef.
- M. S. V. Wittenau, Preparation of tetracyclines by photooxidation of anhydrotetracyclines, J. Org. Chem., 1964, 29, 2746–2748 CrossRef.
- H. Muxfeldt and W. Rogalski, Tetracyclines. V. A total synthesis of (±)-6-deoxy-6-demethyltetracycline, J. Am. Chem. Soc., 1965, 87, 933–934 CrossRef CAS PubMed.
- H. Muxfeldt, G. Haas, G. Hardtmann, F. Kathawala, J. B. Mooberry and E. Vedejs, Tetracyclines. 9. Total synthesis of dl-terramycin, J. Am. Chem. Soc., 1979, 101, 689–701 CrossRef CAS.
- G. Stork, J. J. L. Clair, P. Spargo, R. P. Nargund and N. Totah, Stereocontrolled synthesis of (±)-12a -deoxytetracycline, J. Am. Chem. Soc., 1996, 118, 5304–5305 CrossRef CAS.
- K. Tatsuta, T. Yoshimoto, H. Gunji, Y. Okado and M. Takahashi, The first total synthesis of natural (−)-tetracycline, Chem. Lett., 2000, 29, 646–647 CrossRef.
- M. G. Charest, C. D. Lerner, J. D. Brubaker, D. R. Siegel and A. G. Myers, A convergent enantioselective route to structurally diverse 6-deoxytetracycline antibiotics, Science, 2005, 308, 395–398 CrossRef CAS PubMed.
- M. G. Charest, D. R. Siegel and A. G. Myers, Synthesis of (-)-tetracycline, J. Am. Chem. Soc., 2005, 127, 8292–8293 CrossRef CAS PubMed.
- J. D. Brubaker and A. G. Myers, A practical, enantioselective synthetic route to a key precursor to the tetracycline antibiotics, Org. Lett., 2007, 9, 3523–3525 CrossRef CAS PubMed.
- C. Sun, Q. Wang, J. D. Brubaker, P. M. Wright, C. D. Lerner, K. Noson, M. Charest, D. R. Siegel, Y.-M. Wang and A. G. Myers, A robust platform for the synthesis of new tetracycline antibiotics, J. Am. Chem. Soc., 2008, 130, 17913–17927 CrossRef CAS PubMed.
- D. A. Kummer, D. Li, A. Dion and A. G. Myers, A practical, convergent route to the key precursor to the tetracycline antibiotics, Chem. Sci., 2011, 2, 1710–1718 RSC.
- M. Ronn, Z. Zhu, P. C. Hogan, W.-Y. Zhang, J. Niu, C. E. Katz, N. Dunwoody, O. Gilicky, Y. Deng, D. K. Hunt, M. He, C.-L. Chen, C. Sun, R. B. Clark and X.-Y. Xiao, Process R&D of eravacycline: The first fully synthetic fluorocycline in clinical development, Org. Process Res. Dev., 2013, 17, 838–845 CrossRef CAS.
- A. Mullard, 2018 FDA drug approvals, Nat. Rev. Drug Discovery, 2019, 18, 85–89 CrossRef PubMed.
- W. Y. Zhang, C. Sun, D. Hunt, M. He, Y. Deng, Z. Zhu, C. L. Chen, C. E. Katz, J. Niu, P. C. Hogan, X. Y. Xiao, N. Dunwoody and M. Ronn, Process development and scale-up of fully synthetic tetracycline TP-2758: a potent antibacterial agent with excellent oral bioavailability, Org. Process Res. Dev., 2016, 20, 284–296 CrossRef CAS.
- G. G. Zhanel, K. Homenuik, K. Nichol, A. Noreddin, L. Vercaigne, J. Embil, A. Gin, J. A. Karlowsky and D. J. Hoban, The glycylcyclines, Drugs, 2004, 64, 63–88 CrossRef CAS PubMed.
- A. R. Martin, in Wilson and Gisvold's textbook of organic medicinal and pharmaceutical chemistry, ed. J. N. Delgado and W. A. Remers, Lippincott-Raven Publishers, Philadelphia, 10th edn, 1998, pp. 210–215 Search PubMed.
- K. F. Benitz and H. F. Diermeier, Renal toxicity of tetracycline degradation products, Exp. Biol. Med., 1964, 115, 930–935 CrossRef CAS PubMed.
- M. J. Martell and J. H. Boothe, 6-deoxytetracyclines. VII. Alkylated aminotetracyclines possessing unique antibacterial activity, J. Med. Chem., 1967, 10, 44–46 CrossRef CAS PubMed.
- A. E. Yñigez-Gutierrez and B. O. Bachmann, Fixing the unfixable: the art of optimizing natural products for human medicine, J. Med. Chem., 2019, 62, 8412–8428 CrossRef PubMed.
- P. E. Sum and P. Petersen, Synthesis and structure-activity relationship of novel glycylcycline derivatives leading to the discovery of GAR-936, Bioorg. Med. Chem. Lett., 1999, 9, 1459–1462 CrossRef CAS PubMed.
- P. J. Petersen, N. V. Jacobus, W. J. Weiss, P. E. Sum and R. T. Testa, In vitro and in vivo antibacterial activities of a novel glycylcycline, the 9-t-butylglycylamido derivative of minocycline (GAR-936), Antimicrob. Agents Chemother., 1999, 43, 738–744 CrossRef CAS PubMed.
- L. Honeyman, M. Ismail, M. L. Nelson, B. Bhatia, T. E. Bowser, J. Chen, R. Mechiche, K. Ohemeng, A. K. Verma, E. P. Cannon, A. Macone, S. K. Tanaka and S. Levy, Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline, Antimicrob. Agents Chemother., 2015, 59, 7044–7053 CrossRef CAS PubMed.
- R. B. Clark, D. K. Hunt, M. He, C. Achorn, C.-L. Chen, Y. Deng, C. Fyfe, T. H. Grossman, P. C. Hogan, W. J. O'Brien, L. Plamondon, M. Rönn, J. A. Sutcliffe, Z. Zhu and X.-Y. Xiao, Fluorocyclines. 2. Optimization of the C-9 side-chain for antibacterial activity and oral efficacy, J. Med. Chem., 2012, 55, 606–622 CrossRef CAS PubMed.
- P. F. Wiley, K. Gerzon, E. H. Flynn, M. V. S. Jr., O. Weaver, U. C. Quarck, R. R. Chauvette and R. Monahan, Erythromycin. X.1 Structure of erythromycin, J. Am. Chem. Soc., 1957, 79, 6062–6070 CrossRef CAS.
- R. Place, F. O. R. The, C. Growikg, T. H. Office and H. Office, Erythromycin: another antibiotic, Lancet, 1952, 260, 232–233 Search PubMed.
- D. R. Harris, S. G. McGeachin and H. H. Mills, The structure and stereochemistry of erythromycin A, Tetrahedron Lett., 1965, 6, 679–685 CrossRef.
- A. Contreras and D. Vazquez, Cooperative and antagonistic interactions of peptidyl-tRNA and antibiotics with bacterial ribosomes, Eur. J. Biochem., 1977, 74, 539–547 CrossRef CAS PubMed.
- J. A. Dunkle, L. Xiong, A. S. Mankin and J. H. D. Cate, Structures of the Escherichia coli ribosome with antibiotics bound near the peptidyl transferase center explain spectra of drug action, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 17152–17157 CrossRef CAS PubMed.
- J. Sutcliffe, A. Tait-Kamradt and L. W. Ondarck, Streptococcus pneumoniae and Streptococcus pyogenes resistantto macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system, Antimicrob. Agents Chemother., 1996, 40, 1817–1824 CrossRef CAS PubMed.
- D. Felminghama, R. Cantónb and S. G. Jenkins, Regional trends in β-lactam, macrolide, fluoroquinolone and telithromycin resistance among Streptococcus pneumoniae isolates 2001–2004, J. Infect., 2007, 55, 111–118 CrossRef PubMed.
- A. Canu, B. Malbruny, M. L. Coquemont, T. A. Davies, P. C. Appelbaum and R. Leclercq, Diversity of ribosomal mutations conferring resistance to macrolides, clindamycin, streptogramin, and telithromycin in streptococcus pneumoniae, Antimicrob. Agents Chemother., 2002, 46, 125–131 CrossRef CAS PubMed.
- S. Pestka, R. Vince, R. LeMahieu, F. Weiss, L. Fern and J. Unowsky, Induction of erythromycin resistance in staphylococcus aureus by erythromycin derivatives, Antimicrob. Agents Chemother., 1976, 9, 128–130 CrossRef CAS PubMed.
- N. E. Allen, Macrolide resistance in staphylococcus aureus: induction of macrolide-resistant protein synthesis, Antimicrob. Agents Chemother., 1977, 11, 661–668 CrossRef CAS PubMed.
- H. Ounissi and P. Courvalin, Nucleotide sequence of the gene ereA encoding the erythromycin esterase in Escherichia coli, Gene, 1985, 35, 271–278 CrossRef CAS PubMed.
- M. Arthur, D. Autissier and P. Courvalin, Analysis of the nucleotide sequence of the ereB gene encoding the erythromydn esterase type II, Nucleic Acids Res., 1986, 14, 4987–4999 CrossRef CAS PubMed.
- P. Kurath, P. H. Jones, R. S. Egan and T. J. Perun, Acid degradation of erythromycin A and erythromycin B, Experientia, 1971, 27, 362 CrossRef CAS PubMed.
- R. B. Woodward, E. Logusch, K. P. Nambiar, K. Sakan, D. E. Ward, B. W. Au-Yeung, P. Balaram, L. J. Browne, P. J. Card and C. H. Chen, Asymmetric total synthesis of erythromcin. 1. Synthesis of an erythronolide A secoacid derivative via asymmetric induction, J. Am. Chem. Soc., 1981, 103, 3210–3213 CrossRef.
- R. B. Woodward, B. W. Au-Yeung, P. Balaram, L. J. Browne, D. E. Ward, B. W. Au-Yeung, P. Balaram, L. J. Browne, P. J. Card and C. H. Chen, Asymmetric total synthesis of erythromycin. 2. Synthesis of an erythronolide A lactone system, J. Am. Chem. Soc., 1981, 103, 3213–3215 CrossRef CAS.
- R. B. Woodward, E. Logusch, K. P. Nambiar, K. Sakan, D. E. Ward, B. W. Au-Yeung, P. Balaram, L. J. Browne, P. J. Card and C. H. Chen, Asymmetric total synthesis of erythromycin. 3. Total synthesis of erythromycin, J. Am. Chem. Soc., 1981, 103, 3215–3217 CrossRef CAS.
- P. Breton, P. J. Hergenrother, T. Hida, A. Hodgson, A. S. Judd, E. Kraynack, P. R. Kym, W. C. Lee, M. S. Loft, M. Yamashita and S. F. Martin, Total synthesis of erythromycin B, Tetrahedron, 2007, 63, 5709–5729 CrossRef CAS.
- H. C. Kim and S. H. Kang, Total synthesis of azithromycin, Angew. Chem., Int. Ed., 2009, 48, 1827–1829 CrossRef CAS PubMed.
- S. Morimoto, Y. Takahashi, Y. Watanabe and S. Ōmura, Chemical modification of eryhromycins, J. Antibiot., 1984, 37, 187–189 CrossRef CAS PubMed.
- S. Djokić, G. Kobrehel, G. Lazarevski, N. Lopotar, Z. Tamburašev, B. Kamenar, A. Nagl and I. Vicković, Erythromycin series. Part 11. Ring expansion of erythromycin A oxime by the Beckmann rearrangement, J. Chem. Soc., Perkin Trans. 1, 1986, 1881–1890 RSC.
- J. W. McFarland, C. M. Berger, S. A. Froshauer, S. F. Hayashi, S. J. Hecker, B. H. Jaynes, M. R. Jefson, B. J. Kamicker, C. A. Lipinski, K. M. Lundy, C. P. Reese and C. B. Vu, Quantitative structure−activity relationships among macrolide antibacterial agents: in vitro and in vivo potency against pasteurella multocida, J. Med. Chem., 1997, 40, 1340–1346 CrossRef CAS PubMed.
- W. R. Baker, J. D. Clark, R. L. Stephens and K. H. Kim, Modification of macrolide antibiotics. Synthesis of 11-deoxy-11-(carboxyamino)-6-O-methy¡erythromycin A ll,12-(Cyclic esters) via an intramolecular Michael reaction of O-carbamates with an,/9-unsaturated ketone, J. Org. Chem., 1988, 53, 2340–2345 CrossRef CAS.
- D. Bertrand, S. Bertrand, E. Neveu and P. Fernandes, Molecular characterization of off-target activities of telithromycin: a potential role for nicotinic acetylcholine receptors, Antimicrob. Agents Chemother., 2010, 54, 5399–5402 CrossRef CAS PubMed.
- R. J. Owellen, C. A. Hartke, R. M. Dickerson and F. O. Hams, Inhibition of tubulin-microtubule polymerization by drugs of the Vinca alkaloid class, Cancer Res., 1976, 36, 1499–1502 CAS.
- R. H. Himes, Interactions of the catharanthus (Vinca) alkaloids with tubulin and microtubules, Pharmacol. Ther., 1991, 51, 257–267 CrossRef CAS PubMed.
- M. A. Jordan and L. Wilson, Microtubules as a target for anticancer drugs, Nat. Rev. Cancer, 2004, 4, 253–265 CrossRef CAS PubMed.
- V. F. Roche, in Foye's principles of Medicinal Chemistry, ed. T. L. Lemke, D. A. Williams, V. F. Roche and S. W. Zito, Lippincott Williams & Wilkins, China, 7 edn, 2012, ch. 37, pp. 1244–1250 Search PubMed.
- B. Gigant, C. Wang, R. B. Ravelli, F. Roussi, M. O. Steinmetz, P. A. Curmi, A. Sobel and M. Knossow, Structural basis for the regulation of tubulin by vinblastine, Nature, 2005, 435, 519–522 CrossRef CAS PubMed.
- R. L. Noble, C. T. Beer and J. H. Cutts, Role of chance observations in chemotherapy: vinca rosea, Ann. N. Y. Acad. Sci., 1958, 76, 882–894 CrossRef CAS PubMed.
- G. H. Svoboda, N. Neuss and M. Gorman, Alkaloids of vinca rosea Linn. (Catharanthus roseus G. Don.) V.: Preparation and characterization of alkaloids, J. Am. Pharm. Assoc., Sci. Ed., 1959, 48, 659–666 CrossRef CAS PubMed.
- G. H. Svoboda, Alkaloids of Vinca rosea. IX. Extraction and characterization of leurosidine and leurocristine, Lloydia, 1961, 24, 173–178 CAS.
- J. W. Moncrief and W. N. Lipscomb, Structures of leurocristine (vincristine) and vincaleukoblastine. X-ray analysis of leurocristine methiodide, J. Am. Chem. Soc., 1965, 87, 4963–4964 CrossRef CAS PubMed.
- N. Langlois, F. Gueritte, Y. Langlois and P. Potier, Application of a modification of the Polonovski reaction to the synthesis of vinblastine-type alkaloids, J. Am. Chem. Soc., 1976, 98, 7017–7024 CrossRef CAS PubMed.
- P. Mangeney, R. Z. Andriamialisoa, N. Langlois, Y. Langlois and P. Potier, Preparation of vinblastine, vincristine, and leurosidine, antitumor alkaloids from Catharanthus species (Apocynaceae), J. Am. Chem. Soc., 1979, 101, 2243–2245 CrossRef CAS.
- J. P. Kutney, L. S. L. Choi, J. Nakano, H. Tsukamoto, M. McHugh and C. A. Boulet, A highly efficient and commercially important synthesis of the antimuor Catharanthus alkaloids vinblastine and leurosidine from catharanthine and vindoline, Heterocycles, 1988, 27, 1845–1853 CrossRef CAS.
- M. E. Kuehne, P. A. Matson and W. G. Bornmann, Enantioselective syntheses of vinblastine, leurosidine, vincovaline, and 20′-epi-vincovaline, J. Org. Chem., 1991, 56, 513–528 CrossRef CAS.
- W. G. Bornmann and M. E. Kuehne, A common intermediate providing syntheses of ψ-tabersonine, coronaridine, iboxyphylline, ibophyllidine, vinamidine, and vinblastine, J. Org. Chem., 1992, 57, 1752–1760 CrossRef CAS.
- P. Magnus, J. S. Mendoza, A. Stamford, M. Ladlow and P. Willis, Nonoxidative coupling methodology for the synthesis of the antitumor bisindole alkaloid vinblastine and a lower-half analogue: solvent effect on the stereochemistry of the crucial C-15/C-18′ bond, J. Am. Chem. Soc., 1992, 114, 10232–10245 CrossRef CAS.
- S. Kobayashi, T. Ueda and T. Fukuyama, An efficient total synthesis of (-)-vindoline, Synlett, 2000, 883–886 Search PubMed.
- S. Yokoshima, T. Ueda, S. Kobayashi, A. Sato, T. Kuboyama, H. Tokuyama and T. Fukuyama, Stereocontrolled total synthesis of (+)-vinblastine, J. Am. Chem. Soc., 2002, 124, 2137–2139 CrossRef CAS PubMed.
- T. Kuboyama, S. Yokoshima, H. Tokuyama and T. Fukuyama, Stereocontrolled total synthesis of (+)-vincristine, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11966–11970 CrossRef CAS PubMed.
- T. Miyazaki, S. Yokoshima, S. Simizu, H. Osada, H. Tokuyama and T. Fukuyama, Synthesis of (+)-vinblastine and its analogues, Org. Lett., 2007, 9, 4737–4740 CrossRef CAS PubMed.
- H. Ishikawa, G. I. Elliott, J. Velcicky, Y. Choi and D. L. Boger, Total synthesis of (-)- and ent-(+)-vindoline and related alkaloids, J. Am. Chem. Soc., 2006, 128, 10596–10612 CrossRef CAS PubMed.
- H. Ishikawa, D. A. Colby and D. L. Boger, Direct coupling of catharanthine and vindoline to provide vinblastine: total synthesis of (+)- and ent-(-)-vinblastine, J. Am. Chem. Soc., 2008, 130, 420–421 CrossRef CAS PubMed.
- H. Gotoh, J. E. Sears, A. Eschenmoser and D. L. Boger, New insights into the mechanism and an expanded scope of the Fe(III)-mediated vinblastine coupling reaction, J. Am. Chem. Soc., 2012, 134, 13240–13243 CrossRef CAS PubMed.
- H. Ishikawa, D. A. Colby, S. Seto, P. Va, A. Tam, H. Kakei, T. J. Rayl, I. Hwang and D. L. Boger, Total synthesis of vinblastine, vincristine, related natural products, and key structural analogues, J. Am. Chem. Soc., 2009, 131, 4904–4916 CrossRef CAS PubMed.
- N. Wang, J. Liu, C. Wang, L. Bai and X. Jiang, Asymmetric total syntheses of (-)-jerantinines A, C, and E, (-)-16-methoxytabersonine, (-)-vindoline, and (+)-vinblastine, Org. Lett., 2018, 20, 292–295 CrossRef CAS PubMed.
- L. S. Borman and M. E. Kuehne, in The Alkaloids, ed. A. Brossi and M. Suffness, Academic, San Diego, 1990, vol. 37, pp. 133–144 Search PubMed.
- H. L. Pearce, in The Alkaloids, ed. A. Brossi and M. Suffness, Academic, San Diego, 1990, vol. 37, pp. 145–204 Search PubMed.
- J. Fahy, Modifications in the “upper” or velbenamine part of the vinca alkaloids have major implications for tubulin interacting activities, Curr. Pharm. Des., 2001, 7, 1181–1197 CrossRef CAS PubMed.
- J. E. Sears and D. L. Boger, Total synthesis of vinblastine, related natural products, and key analogues and development of inspired methodology suitable for the systematic study of their structure-function properties, Acc. Chem. Res., 2015, 48, 653–662 CrossRef CAS PubMed.
- H. Gotoh, K. K. Duncan, W. M. Robertson and D. L. Boger, 10′-Fluorovinblastine and 10′-fluorovincristine: synthesis of a key series of modified vinca alkaloids, ACS Med. Chem. Lett., 2011, 2, 948–952 CrossRef CAS PubMed.
- T. J. Barker, K. K. Duncan, K. Otrubova and D. L. Boger, Potent vinblastine C20′ ureas displaying additionally improved activity against a vinblastine-resistant cancer cell line, ACS Med. Chem. Lett., 2013, 4, 985–988 CrossRef CAS PubMed.
- E. K. Leggans, K. K. Duncan, T. J. Barker, K. D. Schleicher and D. L. Boger, A remarkable series of vinblastine analogues displaying enhanced activity and an unprecedented tubulin binding steric tolerance: C20′ urea derivatives, J. Med. Chem., 2013, 56, 628–639 CrossRef CAS PubMed.
- A. Tam, H. Gotoh, W. M. Robertson and D. L. Boger, Catharanthine C16 substituent effects on the biomimetic coupling with vindoline: preparation and evaluation of a key series of vinblastine analogues, Bioorg. Med. Chem. Lett., 2010, 20, 6408–6410 CrossRef CAS PubMed.
- K. D. Schleicher, Y. Sasaki, A. Tam, D. Kato, K. K. Duncan and D. L. Boger, Total synthesis and evaluation of vinblastine analogues containing systematic deep-seated modifications in the vindoline subunit ring system: core redesign, J. Med. Chem., 2013, 56, 483–495 CrossRef CAS PubMed.
- M. C. Wani, H. L. Taylor, M. E. Wall, P. Coggon and A. T. McPhail, Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia, J. Am. Chem. Soc., 1971, 93, 2325–2327 CrossRef CAS PubMed.
- M. A. Jordan, Mechanism of action of antitumor drugs that interact with microtubules and tubulin, Curr. Med. Chem.: Anti-Cancer Agents, 2002, 2, 1–17 CrossRef CAS PubMed.
- M. A. Jordan and L. Wilson, Microtubules as a target for anticancer drugs, Nat. Rev. Cancer, 2004, 4, 253–265 CrossRef CAS PubMed.
- A. Todd, P. W. Groundwater and J. H. Gill, in Anticancer Therapeutics: From Drug Discovery to Clinical Applications, John Wiley & Sons Ltd, 2018, pp. 211–232 Search PubMed.
- R. A. Holton, C. Somoza, H. B. Kim, F. Liang, R. J. Biediger, P. D. Boatman, M. Shindo, C. C. Smith and S. Kim, First total synthesis of taxol. 1. Functionalization of the B ring, J. Am. Chem. Soc., 1994, 116, 1597–1598 CrossRef CAS.
- R. A. Holton, H. B. Kim, C. Somoza, F. Liang, R. J. Biediger, P. D. Boatman, M. Shindo, C. C. Smith and S. Kim, First total synthesis of taxol. 2. Completion of the C and D rings, J. Am. Chem. Soc., 1994, 116, 1599–1600 CrossRef CAS.
- K. C. Nicolaou, Z. Yang, J. J. Liu, H. Ueno, P. G. Nantermet, R. K. Guy, C. F. Claiborne, J. Renaud, E. A. Couladouros, K. Paulvannan and E. J. Sorensen, Total synthesis of taxol, Nature, 1994, 367, 630–634 CrossRef CAS PubMed.
- K. C. Nicolaou, P. G. Nantermet and H. Ueno, Novel chemistry of taxol. Retrosynthetic and synthetic studies, J. Chem. Soc., Chem. Commun., 1994, 295–296 RSC.
- S. J. Danishefsky, J. J. Masters, W. B. Young, J. T. Link, L. B. Snyder, T. V. Magee, D. K. Jung, R. C. A. Isaacs, W. G. Bornmann, C. A. Alaimo, C. A. Coburn and M. J. D. Grandi, Total synthesis of baccatin III and taxol, J. Am. Chem. Soc., 1996, 118, 2843–2859 CrossRef CAS.
- P. A. Wender, N. F. Badham, S. P. Conway, P. E. Floreancig, T. E. Glass, C. Gränicher, J. B. Houze, J. Jänichen, D. Lee, D. G. Marquess, P. L. McGrane, W. Meng, T. P. Mucciaro, M. Mühlebach, M. G. Natchus, H. Paulsen, D. B. Rawlins, J. Satkofsky, A. J. Shuker, J. C. Sutton, R. E. Taylor and K. Tomooka, The pinene path to taxanes. 5. Stereocontrolled synthesis of a versatile taxane precursor, J. Am. Chem. Soc., 1997, 119, 2755–2756 CrossRef CAS.
- P. A. Wender, N. F. Badham, S. P. Conway, P. E. Floreancig, T. E. Glass, J. B. Houze, N. E. Krauss, D. Lee, D. G. Marquess, P. L. McGrane, W. Meng, M. G. Natchus, A. J. Shuker, J. C. Sutton and R. E. Taylor, The pinene path to taxanes. 6. A concise stereocontrolled synthesis of taxol, J. Am. Chem. Soc., 1997, 119, 2757–2758 CrossRef CAS.
- K. Morihira, R. Hara, S. Kawahara, T. Nishimori, N. Nakamura, H. Kusama and I. Kuwajima, Enantioselective total synthesis of taxol, J. Am. Chem. Soc., 1998, 120, 12980–12981 CrossRef CAS.
- H. Kusama, R. Hara, S. Kawahara, T. Nishimori, H. Kashima, N. Nakamura, K. Morihira and I. Kuwajima, Enantioselective total synthesis of (−)-taxol, J. Am. Chem. Soc., 2000, 122, 3811–3820 CrossRef CAS.
- T. Mukaiyama, I. Shiina, H. Iwadare, H. Sakoh, Y. Tani, M. Hasegawa and K. Satoh, Asymmetric total synthesis of Taxol, Chem. – Eur. J., 1999, 5, 121–161 CrossRef CAS.
- T. Doi, S. Fuse, S. Miyamoto, K. Nakai, D. Sasuga and T. Takahashi, A formal total synthesis of taxol aided by an automated synthesizer, Chem. – Asian J., 2006, 1, 370–383 CrossRef CAS PubMed.
- S. Hirai, M. Utsugi, M. Iwamoto and M. Nakada, Formal total synthesis of (−)-taxol through Pd-catalyzed eight-membered carbocyclic ring formation, Chem. – Eur. J., 2015, 21, 355–359 CrossRef CAS PubMed.
- K. Fukaya, Y. Tanaka, A. C. Sato, K. Kodama, H. Yamazaki, T. Ishimoto, Y. Nozaki, Y. M. Iwaki, Y. Yuki, K. Umei, T. Sugai, Y. Yamaguchi, A. Watanabe, T. Oishi, T. Sato and N. Chida, Synthesis of Paclitaxel. 1. Synthesis of the ABC ring of Paclitaxel by SmI2-mediated cyclization, Org. Lett., 2015, 17, 2570–2573 CrossRef CAS PubMed.
- K. Fukaya, K. Kodama, Y. Tanaka, H. Yamazaki, T. Sugai, Y. Yamaguchi, A. Watanabe, T. Oishi, T. Sato and N. Chida, Synthesis of paclitaxel. 2. Construction of the ABCD ring and formal synthesis, Org. Lett., 2015, 17, 2574–2577 CrossRef CAS PubMed.
- S. Jennewein, M. R. Wildung, M. Chau, K. Walker and R. Croteau, Random sequencing of an induced Taxus cell cDNA library for identification of clones involved in Taxol biosynthesis, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 9149–9154 CrossRef CAS PubMed.
- R. Croteau, R. E. Ketchum, R. M. Long, R. Kaspera and M. R. Wildung, Taxol biosynthesis and molecular genetics, Phytochem. Rev., 2006, 5, 75–97 CrossRef CAS PubMed.
- Y. Kanda, H. Nakamura, S. Umemiya, R. K. Puthukanoori, V. R. M. Appala, G. K. Gaddamanugu, B. R. Paraselli and P. S. Baran, Two-phase synthesis of taxol, J. Am. Chem. Soc., 2020, 142, 10526–10533 CrossRef CAS PubMed.
- Y. Kanda, Y. Ishihara, N. C. Wilde and P. S. Baran, Two-phase total synthesis of taxanes: tactics and strategies, J. Org. Chem., 2020, 85, 10293–10320 CrossRef CAS PubMed.
- A. Mendoza, Y. Ishihara and P. S. Baran, Scalable enantioselective total synthesis of taxanes, Nat. Chem., 2012, 4, 21–25 CrossRef CAS PubMed.
- D. G. I. Kingston, Taxol: The chemistry and structure-activity relationships of a novel anticancer agent, Trends Biotechnol., 1994, 12, 222–227 CrossRef CAS PubMed.
- W.-S. Fang and X.-T. Liang, Recent progress in structure activity relationship and mechanistic studies of taxol analogues, Mini-Rev. Med. Chem., 2005, 5, 1–12 CrossRef CAS PubMed.
- D. G. I. Kingston, The shape of things to come: structural and synthetic studies of taxol and related compounds, Phytochemistry, 2007, 68, 1844–1854 CrossRef CAS PubMed.
- Y. Fu, S. Li, Y. Zu, G. Yang, Z. Yang, M. Luo, S. Jiang, M. Wink and T. Efferth, Medicinal chemistry of paclitaxel and its analogues, Curr. Med. Chem., 2009, 16, 3966–3985 CrossRef CAS PubMed.
- J. Żwawiak and L. Zaprutko, A brief history of taxol, J. Med. Sci., 2014, 83, 47–52 CrossRef.
- D. G. I. Kingston, M. D. Chordia, P. G. Jagtap, J. Liang, Y.-C. Shen, B. H. Long, C. R. Fairchild and K. A. Johnston, Synthesis and biological evaluation of 1-deoxypaclitaxel analogues, J. Org. Chem., 1999, 64, 1814–1822 CrossRef CAS PubMed.
- H. Yuan, C. R. Fairchild, X. Liang and D. G. I. Kingston, Synthesis and biological activity of C-6 and C-7 modified paclitaxels, Tetrahedron, 2000, 56, 6407–6414 CrossRef CAS.
- Q. Cheng, T. Oritani, T. Horiguchi, T. Yamada and Y. Mong, Synthesis and biological evaluation of novel 9-functional heterocyclic coupled 7-deoxy-9-Dihydropaclitaxel analogue, Bioorg. Med. Chem. Lett., 2000, 10, 517–521 CrossRef CAS PubMed.
- L. L. Klein, Synthesis of 9-dihydrotaxol: a novel bioactive taxane, Tetrahedron Lett., 1993, 34, 2047–2050 CrossRef CAS.
- H. Ge, V. Vasandani, J. K. Huff, K. L. Audus, R. H. Himes, A. Seelig and G. I. Georg, Synthesis and interactions of 7-deoxy-, 10-deacetoxy, and 10-deacetoxy-7-deoxypaclitaxel with NCI/ADR-RES cancer cells and bovine brain microvessel endothelial cells, Bioorg. Med. Chem. Lett., 2006, 16, 433–436 CrossRef CAS PubMed.
- S. H. Chen, J. M. Wei and V. Farina, Taxol structure-activity relationships: synthesis and biological evaluation of 2-deoxytaxol, Tetrahedron Lett., 1993, 34, 3205–3206 CrossRef CAS.
- A. Datta, L. R. Jayasinghe and G. I. Georg, 4-Deacetyltaxol and 10-acetyl-4-deacetyltaxotere: synthesis and biological evaluation, J. Med. Chem., 1994, 37, 4258–4260 CrossRef CAS PubMed.
- K. A. Neidigh, M. M. Gharpure, J. M. Rimoldi, D. G. I. Kingston, Y. Q. Jiang and E. Hamel, Synthesis and biological evaluation of 4-deacetylpaclitaxel, Tetrahedron Lett., 1994, 35, 6839–6842 CrossRef CAS.
- R. Marder-Karsenti, J. Dubois, L. Bricard, D. Guénard and F. Guéritte-Voegelein, Synthesis and biological evaluation of D-ring-modified taxanes: 15(20)-azadocetaxel analogs, J. Org. Chem., 1997, 62, 6631–6637 CrossRef CAS.
- A. A. L. Gunatilaka, F. D. Ramdayal, M. H. Sarragiotto, D. G. I. Kingston, D. L. Sackett and E. Hamel, Synthesis and biological evaluation of novel paclitaxel (taxol) D-ring modified analogues, J. Org. Chem., 1999, 64, 2694–2703 CrossRef CAS PubMed.
- I. Ojima, J. C. Slater, E. Michaud, S. D. Kuduk, P.-Y. Bounaud, P. Vrignaud, M.-C. Bissery, J. M. Veith, P. Pera and R. J. Bernacki, Syntheses and structure−activity relationships of the second-generation antitumor taxoids: exceptional activity against drug-resistant cancer cells, J. Med. Chem., 1996, 39, 3889–3896 CrossRef CAS PubMed.
- S. M. Ali, M. Z. Hoemann, J. Aube, L. A. Mitscher, G. I. Georg, R. McCall and L. R. Jayasinghe, Novel cytotoxic 3′-(tert-Butyl) 3′-diphenyl analogs of paclitaxel and docetaxel, J. Med. Chem., 1995, 38, 3821–3828 CrossRef CAS PubMed.
- T. C. Boge, R. H. Himes, D. G. V. Velde and G. I. Georg, The effect of the aromatic rings of taxol on biological activity and solution conformation: synthesis and evaluation of saturated taxol and taxotere analogs, J. Med. Chem., 1994, 37, 3337–3343 CrossRef CAS PubMed.
- I. Ojima and S. Lin, Efficient asymmetric syntheses of β-lactams bearing a cyclopropane or an epoxide moiety and their application to the syntheses of novel isoserines and taxoids, J. Org. Chem., 1998, 63, 224–225 CrossRef CAS.
- I. Ojima, T. Inoue and S. Chakravarty, Enantiopure fluorine-containing taxoids: potent anticancer agents and versatile probes for biomedical problems, J. Fluor. Chem., 1999, 97, 3–10 CrossRef CAS.
- J. Wang, C. Xu, Y. K. Wong, Y. Li, F. Liao, T. Jiang and Y. Tu, Artemisinin, the magic drug discovered from traditional Chinese medicine, Engineering, 2019, 5, 32–39 CrossRef CAS.
- A. Robert, F. Benoit-Vical, C. Claparols and B. Meunier, The antimalarial drug artemisinin alkylates heme in infected mice, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 13676–13680 CrossRef CAS PubMed.
- F. T. Aweeka and P. I. German, Clinical pharmacology of artemisinin-based combination therapies, Clin. Pharmacokinet., 2008, 47, 91–102 CrossRef CAS PubMed.
- L. Cui and X. Z. Su, Discovery, mechanisms of action and combination therapy of artemisinin, Expert Rev. Anti-Infect. Ther., 2009, 7, 999–1013 CrossRef CAS PubMed.
- J. Wang, C.-J. Zhang, W. N. Chia, C. C. Y. Loh, Z. Li, Y. M. Lee, Y. He, L.-X. Yuan, T. K. Lim, M. Liu, C. X. Liew, Y. Q. Lee, J. Zhang, N. Lu, C. T. Lim, Z.-C. Hua, B. Liu, H.-M. Shen, K. S. W. Tan and Q. Lin, Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum, Nat. Commun., 2015, 6, 10111–10122 CrossRef CAS PubMed.
- L. Tilley, J. Straimer, N. F. Gnädig, S. A. Ralph and D. A. Fidock, Artemisinin action and resistance in plasmodium falciparum, Trends Parasitol., 2016, 32, 282–296 CrossRef PubMed.
- M. Ouji, J.-M. Augereau, L. Paloque and F. Benoit-Vical, Plasmodium falciparum resistance to artemisinin-based combination therapies: A sword of Damocles in the path toward malaria elimination, Parasite, 2018, 25, 1–12 CrossRef PubMed.
- C. H. Sibley, Understanding artemisinin resistance, Science, 2015, 347, 373–374 CrossRef CAS PubMed.
- A. A. Mbengue, S. S. Bhattacharjee, T. T. Pandharkar, H. Liu, G. Estiu, R. V. Stahelin, S. S. Rizk, D. L. Njimoh, Y. Ryan, K. Chotivanich, C. Nguon, M. Ghorbal, J. J. Lopez-Rubio, M. Pfrender, S. Emrich, N. Mohandas, A. M. Dondorp, O. Wiest and K. Haldar, A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria, Nature, 2015, 520, 683–687 CrossRef CAS PubMed.
- Z. Wang, L. Yang, X. Yang and X. Zhang, Advances in the chemical synthesis of artemisinin, Synth. Commun., 2014, 44, 1987–2003 CrossRef CAS.
- S. K. Sahu, P. K. Behera, P. Choudhury, S. Panda and L. Rout, Strategy and problems for synthesis of antimalaria artemisinin (qinghaosu), ChemistrySelect, 2020, 5, 12333–12344 CrossRef CAS.
- G. Schmid and W. Hofheinz, Total synthesis of qinghaosu, J. Am. Chem. Soc., 1983, 105, 624–625 CrossRef CAS.
- X.-X. Xu, J. Zhu, D.-Z. Huang and W.-S. Zhou, Total synthesis of arteannuin and deoxyarteannuin, Tetrahedron, 1986, 42, 819–828 CrossRef CAS.
- M. A. Avery, C. Jennings-White and W. K. M. Chong, The total synthesis of (+)-artemisinin and (+)-9-desmethyltemesinin, Tetrahedron Lett., 1987, 20, 4629–4632 CrossRef.
- M. A. Avery, W. K. M. Chong and C. Jennings-White, Stereoselective total synthesis of (+)-artemisinin, the antimalarial constituent of Artemisia annua L, J. Am. Chem. Soc., 1992, 114, 974–979 CrossRef CAS.
- T. Ravindranathan, M. A. Kumar, R. B. Menon and S. V. Hiremath, Stereoselective synthesis of artemisinin, Tetrahedron Lett., 1990, 31, 755–758 CrossRef CAS.
- H. J. Liu, W. L. Yeh and S. Yeu Chew, A total synthesis of the antimarial natural product (+)-qinghaosu, Tetrahedron Lett., 1993, 34, 4435–4438 CrossRef CAS.
- J. B. Bhonsle, B. Pandey, V. H. Deshpande and T. Ravindranathan, New synthetic strategies towards (+)-artemisinin, Tetrahedron Lett., 1994, 35, 5489–5492 CrossRef CAS.
- M. G. Constantino, M. B. Jr., G. V. J. Silva and J. Zukerman-Schpector, A novel asymmetric total synthesis of (+)-artemisinin, Synth. Commun., 1196, 26, 321–329 CrossRef.
- J. S. Yadav, R. S. Babu and G. Sabitha, Stereoselective total synthesis of (+)-artemisinin, Tetrahedron Lett., 2003, 44, 387–389 CrossRef CAS.
- J. S. Yadav, B. Thirupathaiah and P. Srihari, A concise stereoselective total synthesis of (+)-artemisinin, Tetrahedron, 2010, 66, 2005–2009 CrossRef CAS.
- C. Zhu and S. P. Cook, A concise synthesis of (+)-artemisinin, J. Am. Chem. Soc., 2012, 134, 13577–13579 CrossRef CAS PubMed.
- J. Krieger, T. Smeilus, M. Kaiser, E.-J. Seo, T. Efferth and A. Giannis, Total synthesis and biological investigation of (−)-artemisinin: the antimalarial activity of artemisinin is not stereospecific, Angew. Chem., Int. Ed., 2018, 57, 8293–8296 CrossRef CAS PubMed.
- J. N. Cumming, D. Wang, S. B. Park, T. A. Shapiro and G. H. Posner, Design, synthesis, derivatization, and structure−activity relationships of simplified, tricyclic, 1,2,4-trioxane alcohol analogues of the antimalarial artemisinin, J. Med. Chem., 1998, 41, 952–964 CrossRef CAS PubMed.
- M. A. Avery, M. Alvim-Gaston, C. R. Rodrigues, E. J. Barreiro, F. E. Cohen, Y. A. Sabnis and J. R. Woolfrey, Structure−activity relationships of the antimalarial agent artemisinin. 6. The development of predictive in vitro potency models using CoMFA and HQSAR methodologies, J. Med. Chem., 2002, 45, 292–303 CrossRef CAS PubMed.
- M. A. Avery, S. Mehrotra, T. L. Johnson, J. D. Bonk, J. A. Vroman and R. Miller, Structure−activity relationships of the antimalarial agent artemisinin. 5. Analogs of 10-deoxoartemisinin substituted at C-3 and C-9, J. Med. Chem., 1996, 39, 4149–4155 CrossRef CAS PubMed.
- M. A. Avery, P. Fan, J. M. Karle, J. D. Bonk, R. Miller and D. K. Goins, Structure−activity relationships of the antimalarial agent artemisinin. 3. Total synthesis of (+)-13-carbaartemisinin and related tetra- and tricyclic structures, J. Med. Chem., 1996, 39, 1885–1897 CrossRef CAS PubMed.
- M. A. Avery, S. Mehrotra, J. D. Bonk, J. A. Vroman, D. K. Goins and R. Miller, Structure−activity relationships of the antimalarial agent artemisinin. 4. Effect of substitution at C-3, J. Med. Chem., 1996, 39, 2900–2906 CrossRef CAS PubMed.
- M. A. Avery, J. D. Bonk, W. K. M. Chong, S. Mehrotra, R. Miller, W. Milhous, D. K. Goins, S. Venkatesan and C. Wyandt, Structure-activity relationships of the antimalarial agent artemisinin. 2. Effect of heteroatom substitution at O-11: synthesis and bioassay of N-alkyl-11-aza-9-desmethylartemisinins, J. Med. Chem., 1995, 38, 5038–5044 CrossRef CAS PubMed.
- M. A. Avery, F. Gao, W. K. M. Chong, S. Mehrotra and W. K. Milhous, Structure-activity relationships of the antimalarial agent artemisinin. 1. Synthesis and comparative molecular field analysis of C-9 analogs of artemisinin and 10-deoxoartemisinin, J. Med. Chem., 1993, 36, 4264–4275 CrossRef CAS PubMed.
- M. K. Tiwari and S. Chaudhary, Artemisinin-derived antimalarial endoperoxides from bench-side to bed-side: Chronological advancements and future challenges, Med. Res. Rev., 2020, 40, 1220–1275 CrossRef CAS PubMed.
- R. K. Haynes, B. Fugmann, J. Stetter, K. Rieckmann, H.-D. Heilmann, H.-W. Chan, M.-K. Cheung, W.-L. Lam, H.-N. Wong, S. L. Croft, L. Vivas, L. Rattray, L. Stewart, W. Peters, B. L. Robinson, M. D. Edstein, B. Kotecka, D. E. Kyle, B. Beckermann, M. Gerisch, M. Radtke, G. Schmuck, W. Steinke, U. Wollborn, K. Schmeer and A. Römer, Artemisone—a highly active antimalarial drug of the artemisinin class, Angew. Chem., Int. Ed., 2006, 45, 2082–2088 CrossRef CAS PubMed.
- Y. Tu, From Artemisia annua L. to artemisinins: the discovery and development of artemisinins and antimalarial agents, Academic Press, 2017 Search PubMed.
- M. A. Avery, F. Gao, W. K. M. Chong, T. F. Hendrickson, W. D. Inman and P. Crews, Synthesis, conformational analysis, and antimalarial activity of tricyclic analogs of artemisinin, Tetrahedron, 1994, 50, 957–972 CrossRef CAS.
- T. N. C. Wells, R. H. V. Huijsduijnen and W. C. V. Voorhis, Malaria medicines: a glass half full?, Nat. Rev. Drug Discovery, 2015, 14, 424–442 CrossRef CAS PubMed.
- S. L. Rawe, in Antimalarial Agents, Elsevier Ltd, 2020, pp. 99–132 Search PubMed.
- C. Cuevas and A. Francesch, Development of Yondelis (trabectedin, ET-743). A semisynthetic process solves the supply problem, Nat. Prod. Rep., 2009, 26, 322–337 RSC.
- V. H. Le, M. Inai, R. M. Williams and T. Kan, Ecteinascidins. A review of the chemistry, biology and clinical utility of potent tetrahydroisoquinoline antitumor antibiotics, Nat. Prod. Rep., 2015, 32, 328–347 RSC.
- S. Yuan, Y.-Q. Luo, J.-H. Zuo, H. Liu, F. Li and B. Yu, New drug approvals for 2020: Synthesis and clinical applications, Eur. J. Med. Chem., 2021, 215, 113284–113317 CrossRef CAS PubMed.
- K. K. C. Liu, S. M. Sakya, C. J. O'Donnell and J. Li, Synthetic approaches to the 2007 new drugs, Mini-Rev. Med. Chem., 2008, 8, 1526–1548 CrossRef CAS PubMed.
- K. L. Rinehart, T. G. Holt, N. L. Fregeau, J. G. Stroh, P. A. Keifer, F. Sun, L. H. Li and D. G. Martin, Ecteinascidins 729, 743, 745, 759A, 759B, and 770: potent antitumor agents from the Caribbean tunicate Ecteinascidia turbinata, J. Org. Chem., 1990, 55, 4512–4515 CrossRef CAS.
- R. Sakai, K. L. Rinehart, Y. Guan and A. H.-J. Wang, Additional antitumor ecteinascidins from a Caribbean tunicate: crystal structures and activities in vivo, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 11456–11460 CrossRef CAS PubMed.
- Y. Pommier, G. Kohlhagen, C. Bailly, M. Waring, A. Mazumder and K. W. Kohn, DNA sequence- and structure-selective alkylation of guanine N2 in the DNA minor groove by ecteinascidin 743, a potent antitumor compound from the caribbean tunicate Ecteinascidia turbinata, Biochemistry, 1996, 35, 13303–13309 CrossRef CAS PubMed.
- B. M. Moore, F. C. Seaman and L. H. Hurley, NMR-based model of an ecteinascidin 743-DNA adduct, J. Am. Chem. Soc., 1997, 119, 5475–5476 CrossRef CAS.
- B. M. Moore, F. C. Seaman, R. T. Wheelhouse and L. H. Hurley, Mechanism for the catalytic activation of ecteinascidin 743 and its subsequent alkylation of guanine N2, J. Am. Chem. Soc., 1998, 120, 2490–2491 CrossRef CAS.
- R. Garcia-Nieto, I. Manzanares, C. Cuevas and F. Gago, Bending of DNA upon binding of ecteinascidin 743 and phthalascidin 650 studied by unrestrained molecular dynamics simulations, J. Am. Chem. Soc., 2000, 122, 7172–7182 CrossRef CAS.
- M. Zewail-Foote and L. H. Hurley, Ecteinascidin 743: a minor groove alkylator that bends DNA toward the major groove, J. Med. Chem., 1999, 42, 2493–2497 CrossRef CAS PubMed.
- A. B. Herrero, C. Martin-Castellanos, E. Marco, F. Gago and S. Moreno, Cross-talk between nucleotide excision and homologous recombination DNA repair pathways in the mechanism of action of antitumor trabectedin, Cancer Res., 2006, 66, 8155–8162 CrossRef CAS PubMed.
- D. G. Soares, A. E. Escargueil, V. Poindessous, A. Sarasin, A. D. Gramont, D. Bonatto, J. A. P. Henriques and A. K. Larsen, Replication and homologous recombination repair regulate DNA double-strand break formation by the antitumor alkylator ecteinascidin 743, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13062–13067 CrossRef PubMed.
- E. J. Corey, D. Y. Gin and R. S. Kania, Enantioselective total synthesis of ecteinascidin 743, J. Am. Chem. Soc., 1996, 118, 9202–9203 CrossRef CAS.
- C. Cuevas, M. Perez, M. J. Martin, J. L. Chicharro, C. Fernandez-Rivas, M. Flores, A. Francesch, P. Gallego, M. Zarzuelo, F. D. L. Calle, J. Garcia, C. Polanco, I. Rodriguez and I. Manzanares, Synthesis of ecteinascidin ET-743 and phthalascidin Pt-650 from cyanosafracin B, Org. Lett., 2000, 2, 2545–2548 CrossRef CAS PubMed.
- E. J. Martinez and E. J. Corey, A new, more efficient, and effective process for the synthesis of a key pentacyclic intermediate for production of ecteinascidin and phthalascidin antitumor agents, Org. Lett., 2000, 2, 993–996 CrossRef CAS PubMed.
- A. Endo, A. Yanagisawa, M. Abe, S. Tohma, T. Kan and T. Fukuyama, Total synthesis of ecteinascidin 743, J. Am. Chem. Soc., 2002, 124, 6552–6554 CrossRef CAS PubMed.
- F. Kawagishi, T. Toma, T. Inui, S. Yokoshima and T. Fukuyama, Total synthesis of ecteinascidin 743, J. Am. Chem. Soc., 2013, 135, 13684–13687 CrossRef CAS PubMed.
- J. Chen, X. Chen, M. Bois-Choussy and J. Zhu, Total synthesis of ecteinascidin 743, J. Am. Chem. Soc., 2006, 128, 87–89 CrossRef CAS PubMed.
- S. Zheng, C. Chan, T. Furuuchi, B. J. D. Wright, B. Zhou, J. Guo and S. J. Danishefsky, Stereospecific formal total synthesis of ecteinascidin 743, Angew. Chem., Int. Ed., 2006, 45, 1754–1759 CrossRef CAS PubMed.
- D. Fishlock and R. M. Williams, Synthetic studies on Et-743. Assembly of the pentacyclic core and a formal total synthesis, J. Org. Chem., 2008, 73, 9594–9600 CrossRef CAS PubMed.
- W. He, Z. Zhang and D. Ma, A scalable total synthesis of the antitumor agents Et-743 and lurbinectedin, Angew. Chem., Int. Ed., 2019, 58, 3972–3975 CrossRef CAS PubMed.
- E. J. Martinez, T. Owa, S. L. Schreiber and E. J. Corey, Phthalascidin, a synthetic antitumor agent with potency and mode of action comparable to ecteinascidin 743, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 3496–3501 CrossRef CAS PubMed.
- K. Suwanborirux, K. Charupant, S. Amnuoypol, S. Pummangura, A. Kubo and N. Saito, Ecteinascidins 770 and 786 from the Thai Tunicate Ecteinascidia thurstoni, J. Nat. Prod., 2002, 65, 935–937 CrossRef CAS PubMed.
- P. Puthongking, C. Patarapanich, S. Amnuoypol, K. Suwanborirux, A. Kubo and N. Saito, Chemistry of ecteinascidins. Part 2. Preparation of 6′-O-acyl derivatives of stable ecteinascidin and evaluation of cytotoxicity, Chem. Pharm. Bull., 2006, 54, 1010–1016 CrossRef CAS PubMed.
- P. Saktrakulkla, S. Toriumi, M. Tsujimoto, C. Patarapanich, K. Suwanborirux and N. Saito, Chemistry of ecteinascidins. Part 3: preparation of 2′-N-acyl derivatives of ecteinascidin 770 and evaluation of cytotoxicity, Bioorg. Med. Chem., 2011, 19, 4421–4436 CrossRef CAS PubMed.
- M. Tsujimoto, W. Lowtangkitcharoen, N. Mori, W. Pangkruang, P. Putongking, K. Suwanborirux and N. Saito, Chemistry of ecteinascidins. Part 4: preparation of 2′-N-acyl ecteinascidin 770 derivatives with improved cytotoxicity profiles, Chem. Pharm. Bull., 2013, 61, 1052–1064 CrossRef CAS PubMed.
- R. Toyoshima, N. Mori, T. Suzuki, W. Lowtangkitcharoen, K. Suwanborirux and N. Saito, Chemistry of ecteinascidins. Part 5: an additional proof of cytotoxicity evaluation of ecteinascidin 770 derivatives, Chem. Pharm. Bull., 2016, 64, 966–969 CrossRef CAS PubMed.
- N. Saito, Chemical research on antitumor isoquinoline marine natural products and related compounds, Chem. Pharm. Bull., 2021, 69, 155–177 CrossRef CAS PubMed.
- M. D. Lee, T. S. Dunne, M. M. Siegel, C. C. Chang, G. O. Morton and D. B. Borders, Calichemicins, a novel family of antitumor antibiotics. 1. Chemistry and partial structure of calichemicin .gamma.1I, J. Am. Chem. Soc., 1987, 109, 3464–3466 CrossRef CAS.
- M. D. Lee, T. S. Dunne, C. C. Chang, G. A. Ellestad, M. M. Siegel, G. O. Morton, W. J. McGahren and D. B. Borders, Calichemicins, a novel family of antitumor antibiotics. 2. Chemistry and structure of calichemicin .gamma.1I, J. Am. Chem. Soc., 1987, 109, 3466–3468 CrossRef CAS.
- M. D. Lee, J. K. Manning, D. R. Williams, N. A. Kuck, R. T. Testa and D. B. Borders, Calicheamicins, a novel family of antitumor antibiotics, J. Antibiot., 1989, 42, 1070–1087 CrossRef CAS PubMed.
- K. Edo, M. Mizugaki, Y. Koide, H. Seto, K. Furihata, N. Ōtake and N. Ishida, The structure of neocarzinostatin chromophore possessing a novel bicyclo-[7,3,0]dodecadiyne system, Tetrahedron Lett., 1985, 26, 331–334 CrossRef CAS.
- J. Golik, G. Dubay, G. Groenewold, H. Kawaguchi, M. Konishi, B. Krishnan, H. Ohkuma, K. Saitoh and T. W. Doyle, Esperamicins, a novel class of potent antitumor antibiotics. 3. Structures of esperamicins A1, A2, and A1b, J. Am. Chem. Soc., 1987, 109, 3462–3464 CrossRef CAS.
- M. Konishi, H. Ohkuma, K. Matsumoto, T. Tsuno, H. Kamei, T. Miyaki, T. Oki, H. Kawaguchi, G. D. Vanduyne and J. Clardy, Dynemicin A, a novel antibiotic with the anthraquinone and 1,5-diyn-3-ene subunit, J. Antibiot., 1989, 42, 1449–1452 CrossRef CAS PubMed.
- S. Walker, R. Landovitz, W. D. Ding, G. A. Ellestad and D. Kahne, Cleavage behavior of calicheamicin gamma 1 and calicheamicin T, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 4608–4612 CrossRef CAS PubMed.
- R. Gébleux and G. Casi, Antibody-drug conjugates: Current status and future perspectives, Pharmacol. Ther., 2016, 167, 48–59 CrossRef PubMed.
- S. Yaghoubi, M. H. Karimi, M. Lotfinia, T. Gharibi, M. Mahi-Birjand, E. Kavi, F. Hosseini, K. S. Sepehr, M. Khatami, N. Bagheri and M. Abdollahpour-Alitappeh, Potential drugs used in the antibody–drug conjugate (ADC) architecture for cancer therapy, J. Cell. Physiol., 2020, 235, 31–64 CrossRef CAS PubMed.
- K. C. Nicolaou, C. W. Hummel, E. N. Pitsinos, M. Nakada, A. L. Smith, K. Shibayama and H. Saimoto, Total synthesis of calicheamicin .gamma.1I, J. Am. Chem. Soc., 1992, 114, 10082–10084 CrossRef CAS.
- R. D. Groneberg, T. Miyazaki, N. A. Stylianides, T. J. Schulze, W. Stahl, E. P. Schreiner, T. Suzuki, Y. Iwabuchi, A. L. Smith and K. C. Nicolaou, Total synthesis of calicheamicin .gamma.1I. 1. Synthesis of the oligosaccharide fragment, J. Am. Chem. Soc., 1993, 115, 7593–7611 CrossRef CAS.
- A. L. Smith, E. N. Pitsinos, C. K. Hwang, Y. Mizuno, H. Saimoto, G. R. Scarlato, T. Suzuki and K. C. Nicolaou, Total synthesis of calicheamicin .gamma.1I. 2. Development of an enantioselective route to (-)-calicheamicinone, J. Am. Chem. Soc., 1993, 115, 7612–7624 CrossRef CAS.
- K. C. Nicolaou, C. W. Hummel, M. Nakada, K. Shibayama, E. N. Pitsinos, H. Saimoto, Y. Mizuno, K. U. Baldenius and A. L. Smith, Total synthesis of calicheamicin .gamma.1I. 3. The final stages, J. Am. Chem. Soc., 1993, 115, 7625–7635 CrossRef CAS.
- M. P. Cabal, R. S. Coleman and S. J. Danishefsky, Total synthesis of calicheamicinone: a solution to the problem of the elusive urethane, J. Am. Chem. Soc., 1990, 112, 3253–3255 CrossRef CAS.
- J. N. Haseltine, M. P. Cabal, N. B. Mantlo, N. Iwasawa, D. S. Yamashita, R. S. Coleman, S. J. Danishefsky and G. K. Schulte, Total synthesis of calicheamicinone: new arrangements for actuation of the reductive cycloaromatization of aglycon congeners, J. Am. Chem. Soc., 1991, 113, 3850–3866 CrossRef CAS.
- S. A. Hitchcock, S. H. Boyer, M. Y. Chu-Moyer, S. H. Olson and S. J. Danishefsky, A convergent total synthesis of calicheamicin γ1′, Angew. Chem., Int. Ed., 1994, 33, 858–862 CrossRef.
- R. L. Halcomb, S. H. Boyer, M. D. Wittman, S. H. Olson, D. J. Denhart, K. K. C. Liu and S. J. Danishefsky, Studies related to the carbohydrate sectors of esperamicin and calicheamicin: definition of the stability limits of the esperamicin domain and fashioning of a glycosyl donor from the calicheamicin domain, J. Am. Chem. Soc., 1995, 117, 5720–5749 CrossRef CAS.
- S. A. Hitchcock, M. Y. Chu-Moyer, S. H. Boyer, S. H. Olson and S. J. Danishefsky, A remarkable glycosidation reaction: the total synthesis of calicheamicin .gamma.1I, J. Am. Chem. Soc., 1995, 117, 5750–5756 CrossRef CAS.
- N. Oku, S. Matsunaga and N. Fusetani, Shishijimicins A−C, novel enediyne antitumor antibiotics from the ascidian didemnum proliferum1, J. Am. Chem. Soc., 2003, 125, 2044–2045 CrossRef CAS PubMed.
- L. A. McDonald, T. L. Capson, G. Krishnamurthy, W.-D. Ding, G. A. Ellestad, V. S. Bernan, W. M. Maiese, P. Lassota, R. A. Kramer and C. M. Ireland, Namenamicin, a new enediyne antitumor antibiotic from the marine ascidian polysyncraton lithostrotum, J. Am. Chem. Soc., 1996, 118, 10898–10899 CrossRef CAS.
- K. C. Nicolaou, Z. Lu, R. Li, J. R. Woods and T.-I. Sohn, Total synthesis of shishijimicin A, J. Am. Chem. Soc., 2015, 137, 8716–8719 CrossRef CAS PubMed.
- K. C. Nicolaou, R. Li, Z. Lu, E. N. Pitsinos, L. B. Alemany, M. Aujay, C. Lee, J. Sandoval and J. Gavrilyuk, Streamlined total synthesis of shishijimicin A and its application to the design, synthesis, and biological evaluation of analogues thereof and practical syntheses of PhthNSSMe and related sulfenylating reagents, J. Am. Chem. Soc., 2018, 140, 12120–12136 CrossRef CAS PubMed.
- K. C. Nicolaou, R. Li, Z. Lu, E. N. Pitsinos and L. B. Alemany, Total synthesis and full structural assignment of namenamicin, J. Am. Chem. Soc., 2018, 140, 8091–8095 CrossRef CAS PubMed.
- K. C. Nicolaou, R. Li, Q. Chen, Z. Lu, E. N. Pitsinos, A. Schammel, B. Lin, C. Gu, H. Sarvaiya, R. Tchelepi, A. Valdiosera, J. Clubb, N. Barbour, V. Sisodiya, J. Sandoval, C. Lee, M. Aujay and J. Gavrilyuk, Synthesis and biological evaluation of shishijimicin A-type linker-drugs and antibody–drug conjugates, J. Am. Chem. Soc., 2020, 142, 12890–12899 CrossRef CAS PubMed.
- A. M. Gillespie, T. J. Broadhead, S. Y. Chan, J. Owen, A. P. Farnsworth, M. Sopwith and R. E. Coleman, Phase I open study of the effects of ascending doses of the cytotoxic immunoconjugate CMB-401 (hCTMO1-calicheamicin) in patients with epithelial ovarian cancer, Ann. Oncol., 2000, 11, 735–741 CrossRef CAS PubMed.
- L. M. Hinman, P. R. Hamann, R. Wallace, A. T. Menendez, F. E. Durr and J. Upeslacis, Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics, Cancer Res., 1993, 53, 3336–3342 CAS.
- K. C. Nicolaou, T. Li, M. Nakada, C. W. Hummel, A. Hiatt and W. Wrasidlo, Calicheamicin θ1′: A rationally designed molecule with extremely potent and selective DNA cleaving properties and apoptosis inducing activity, Angew. Chem., Int. Ed., 1994, 33, 183–186 CrossRef.
- H. N. Lode, R. A. Reisfeld, R. Handgretinger, K. C. Nicolaou, G. Gaedicke and W. Wrasidlo, Targeted therapy with a novel enediyene antibiotic calicheamicin ϑI1 effectively suppresses growth and dissemination of liver metastases in a syngeneic model of murine neuroblastoma, Cancer Res., 1998, 58, 2925–2928 CAS.
- K. M. Bernt, A. Prokop, N. Huebener, G. Gaedicke, W. Wrasidlo and H. N. Lode, Eradication of CD19+ leukemia by targeted calicheamicin θ, Bioconjugate Chem., 2009, 20, 1587–1594 CrossRef CAS PubMed.
- G. Chen, B. K. Alisher, H. Zhang, T. Zhu and Z. Miao, Calicheamicin antibody drug conjugates linking and amidoacetyl group to a sugar moiety on calicheamicin, WO/2017/172907, 2017.
- T. Pillow, Calicheamicin-antibody-drug conjugates and methods of use, WO/2017/068511, 2017.
- A. D. Ricart, Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumab ozogamicin, Clin. Cancer Res., 2011, 17, 6417–6427 CrossRef CAS PubMed.
Footnote |
| † These authors made equal contribution. |
| This journal is © the Partner Organisations 2022 |