Biology-guided engineering of bioelectrical interfaces
Bernadette A.
Miao
 a,
Lingyuan
Meng
a,
Lingyuan
Meng
 *b and
Bozhi
Tian
*b and
Bozhi
Tian
 *acd
*acd
aDepartment of Chemistry, The University of Chicago, Chicago, IL 60637, USA. E-mail: btian@uchicago.edu
bPritzker School of Molecular Engineering, The University of Chicago, Chicago, IL 60637, USA. E-mail: lingyuan@uchicago.edu
cThe James Franck Institute, The University of Chicago, Chicago, IL 60637, USA
dThe Institute for Biophysical Dynamics, The University of Chicago, Chicago, IL 60637, USA
First published on 14th December 2021
Abstract
Bioelectrical interfaces that bridge biotic and abiotic systems have heightened the ability to monitor, understand, and manipulate biological systems and are catalyzing profound progress in neuroscience research, treatments for heart failure, and microbial energy systems. With advances in nanotechnology, bifunctional and high-density devices with tailored structural designs are being developed to enable multiplexed recording or stimulation across multiple spatial and temporal scales with resolution down to millisecond–nanometer interfaces, enabling efficient and effective communication with intracellular electrical activities in a relatively noninvasive and biocompatible manner. This review provides an overview of how biological systems guide the design, engineering, and implementation of bioelectrical interfaces for biomedical applications. We investigate recent advances in bioelectrical interfaces for applications in nervous, cardiac, and microbial systems, and we also discuss the outlook of state-of-the-art biology-guided bioelectrical interfaces with high biocompatibility, extended long-term stability, and integrated system functionality for potential clinical usage.
1. Introduction
Bioelectrical interfaces connect materials and biological systems across various length scales, from subcellular dimensions to tissue and organ levels. The development of interfaces has significantly grown in the last few decades. Since the early 2000s, the field has advanced from patch clamps, microelectrode arrays (MEAs), and field-effect-transistors (FETs) to include minimally invasive, ultrasmall, and biocompatible nanomaterial-based sensing and modulation techniques.1–3 Thus far, research has been utilizing nanoscale conductive materials with rational device structures and efficient fabrication methods to develop new applications in neuroscience, cardiovascular disease research, microbial-related energy systems, and many other expanding areas.4–9 Semiconductors, carbon, metals, and their composites and oxides are materials used in interfaces to catalyze profound progress in the development of deep-brain stimulators, retinal prostheses, implantable artificial pacemakers, and microbial fuel cells as well as in the exploration of personalized medicine.10–14 These developments increase the ability to better understand complex electrophysiological biological processes within and between cells, tissues, and organ systems.Historically, challenges to developing efficient and effective bioelectrical interfaces include improvingsignal-to-noise ratio, optimizing stability, dealing with mechanical mismatch, refining spatiotemporal resolution, and achieving translatability to the clinical setting.15,16 Currently, most researchers are pursuing stable, seamless material-biology interfaces of minimal foreign-body response that operate for chronic time scales and remain functional for large-scale, high-resolution investigation.
Temporally, bioelectrical interfaces can reach sub-microsecond resolution for recording a single-unit action potential for multiple hours, as in monitors of hormones, neurotransmission, and local field potential fluctuation.17 Some biocompatible interfaces can last for months without severe immune responses. Spatially, bioelectrical interfaces enable organelle-level local precision and can also be globally extended to work as whole organ patches.18,19 High-density distributions of thousands of interfacing sites can be achieved by complementary metal-oxide-semiconductor (CMOS) techniques and microelectrode arrays.20
Inorganic nanomaterials are routinely used for bioelectrical interfaces because of their unique features, including but not limited to ultrasmall size, excellent electrical properties (e.g., conductivities), and diverse signal transduction mechanisms for energy conversion.21–23 Additionally, they can be processed via highly scalable fabrication technologies into a broad spectrum of functional devices. There is also an extensive toolbox with various choices and possibilities of material composition. Inorganic nanomaterials are used in bioelectrical interfaces; they serve as essential abiotic–biotic ties, bridging gaps and enabling connections between biological and synthetic systems.
Many organic materials have also been used for bioelectrical interfaces, especially in flexible electronics or electronic skin, stretchable hydrogel electronics, and 3D hydrogel scaffolds.24–26 These organic-based designs also need inorganic nanomaterial components for interconnecting properties because conductive polymers generally have higher operating voltages than conductive inorganic materials, such as highly conductive metals and carbon.27,28 Additionally, organic bioelectrical interfaces can be constructed with inorganic nanostructured surfaces to promote tighter adhesion and thus better signal transduction at interfaces and enable higher spatial resolution.29
This review investigates some of the latest bioelectrical interfaces with rational medical applications in nervous, cardiac, and microbial systems (Fig. 1). While discussing these advances, we highlight the unique properties and behaviors of biological components, materials development, and device fabrication guided by biological features and interfacial integration strategies for biomedical applications and biohybrid systems. We also propose future directions of biology-guided bioelectrical interfaces. We hope that this review will enable better understanding and encourage future research into bioelectrical interfaces.
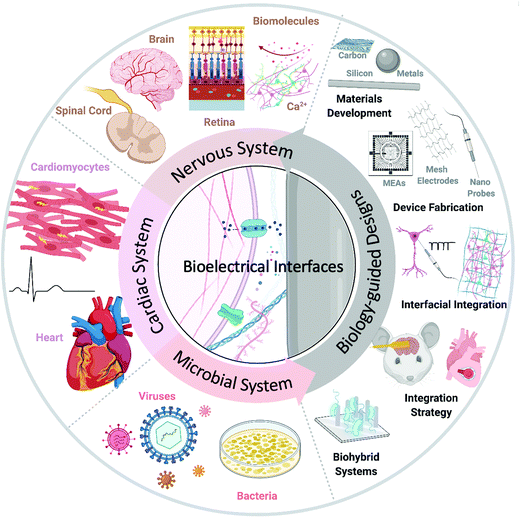 | ||
| Fig. 1 Multisystem biology-guided research. A schematic diagram of biology-guided bioelectrical interfaces, focusing on nervous, cardiac, and microbial systems. Created with BioRender.com. | ||
2. Biology-guided bioelectrical interfaces
Biological systems are complex; each component displays unique properties and is critical for the proper function and behavior of systems. Thus, accurate investigation of the physiological behavior and efficient manipulation of biological activity requires rational development of synthetic materials and devices that fully accommodate special needs.The term “biology guided” broadly applies to aspects involved in interface research where the intended application guides design considerations. For example, to study a network of connected and interacting cells, a device with multiple interface sites needs to be developed. This device should have high-density spatial integration and single-cell resolution with a size similar to the size of the interfaced tissue. For cellular studies, submicron or nanoscale materials are required to provide an interface with minimal footprint; for organ-level studies, we need mechanically soft materials to form tight interfaces that conform with curved surfaces; for implantation in living animals, we need biocompatible, stable designs that are compatible with dynamic movements and withstand natural immune responses.
Currently, some electrical design and implementation strategies for stimulation rely on the plasma membrane's electrical and structural properties to activate and utilize bioelectrical processes.30,31 Specifically, the lipid bilayer is of interest in photothermal interfaces as its electrical capacitance is temperature dependent.32 Illumination induces an increase in the local temperature that increases electrical capacitance, depolarizing the bilayer. Biological systems also inspire research to develop designs that can mimic naturally occurring materials and mechanisms, such as soft-hard composites,33 and refer to applications, such as research interfacing with the nervous and cardiac systems, because of related diseases. Additionally, natural foreign-body and other inflammatory reactions have driven material and device development to improve biocompatibility and stability through matching the modulus of materials, coating surfaces with biomaterials, considering device morphology, and merging integration with biological processes.
Bioelectrical interface research occurs at the intersection of materials development and structural engineering towards various applications. Here, we focus on how biological systems guide designs as well as implementation strategies, specifically those for bioelectrical interface applications. We approach research from these interfaces as they enable us to understand the individual systems involved in the interfacial interaction as well as their effects on each other. Precisely, the biological systems and synthetic materials form a biointerface to enable the investigation of physiological behaviors and rational development of devices. We base our discussion from the “biology-guided” perspective to describe methods that rely on, are inspired by, and interact with biological components. Through this standpoint, we hope to encourage interdisciplinary and fundamental-level research that can advance the understanding of the complexity of biological systems and help elucidate the future of biomedicine.
We arranged our discussion about recent bioelectrical applications using “biology-guided” designs in systems of significant medical importance: nervous system, cardiac system, and microbial system. In the nervous system, we highlight biomolecular (or chemical) and electrical interfaces, extra- and intra-cellular interfaces, and organ-level biomedical applications in the brain, retina, and spinal cord. We also investigate the use of bioelectrical materials and devices in cardiac and microbial systems. We hope to highlight how bioelectrical biological interfaces enable and advance therapeutics that are helping to transform biomedicine.
3. Nervous system
As the most complex part of the human body, the nervous system, comprising the central and peripheral systems, expresses and responds to physiological information through electrical signals, chemical gradients, and biomolecule dynamics. Extending from the brain to the spinal cord, the central nervous system is connected to other vital body components by the peripheral nervous system. The central and peripheral nervous systems enable human life but rely on the proper functionality of related parts, particularly the brain. Current challenges to developments include achieving high spatial integration for multiplexed functionality, intracellular and in vivo understanding, and long-term stability and compatibility.22 Through a more developed understanding of the nervous system, we will be able to create better methods to prevent and mitigate neurological diseases and their effects.Neural electrical interface research advanced with the patch clamp introduced by Neher and Sakmann in the 1970s.34 Recently, the patch clamp has influenced developments, leading to automated, high-throughput pharmaceutical screening platforms and patch-on-a-chip in vitro planar arrays of increased feasibility and reproducibility.35 Since the introduction of the original patch clamp, neural technologies have advanced to include methods and designs that expand spatial integration using microelectrode arrays35–37 and, more recently, nanoelectrode arrays for the investigation of neural networks.20 Along with developing electrode-based multidimensional strategies with increased spatial integration, long-term stability, and multifunctional capabilities, research is required to prompt medical therapies by reducing mechanical mismatch while increasing spatial and temporal functionality.15,22,38,39
Broadly, the scope of bioelectrical studies involves sensing or recording intracellularly and extracellularly in vitro, ex vivo, and in vivo via genetic and nongenetic pathways.40–42 Interest in the nervous system stems from the ability to excite neural tissue and began with the patch clamp for intracellular recording and has expanded to controlling neuronal growth43 and improving biocompatibility and stability with biomimetic and bioinspired designs.44,45 Design materials initially involved platinum, iridium oxide, titanium nitride, and silicon and have expanded to include forms of carbon46–49 with a focus on biocompatibility50 and electrochemical properties, mainly impedance, charge injection capacity, and required voltage, to increase efficiency and functionality.46
Current research centers around neurons, cells through which the brain sends electrical pulses to transmit information but has also involved studying neurotransmitters, non-neuronal cells, and the spinal cord. The sensitivity of neural components to electrical stimuli has achieved therapeutic developments, such as retinal and cochlear implants, which have a significant medical impact that has recently drawn the attention of public and private industries.6,51,52 Another notable example is the use of implantable metal electrodes to determine the spatial origin of seizures and provide deep brain stimulation for neurological diseases.22 Additionally, array-based approaches and other developments harness the advantages of multidimensional designs.36,53,54 For example, Amin et al. used surface chemistry and nanostructured substrates to foster synapse stability and cellular activity in a way that can be expanded to control neuronal growth.55 Through 3D vertical nanopillar arrays, selective guidance of primary hippocampal neurons can be established, showing how biology-guided research can integrate with natural processes, neuronal growth in this case.
Thanks to the launch of several worldwide neurotechnology initiatives, such as the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) in the United States56 and the European Human Brain Project (HBP),57 more creative and scalable approaches, such as those using nanomaterials with projects focused on understanding dynamic actions of the brain, fundamental principles, and origins of disease through ethical scientific research, computing analysis, and worldwide collaboration, are expected to advance bioelectrical interfaces for medical applications.58–60 Exploring synthetic biology and materials science in relation to neural recording and stimulation also offers unique and promising perspectives.52,61 Additionally, various advances in micro- and nano-structured materials highlight the expanding frontiers of this field and the vast potential of leveraging machine intelligence and semiconductor technology.62
In the following subsections, we focus on nanomaterial-based bioelectrical interfaces with applications in the nervous system. Biology has guided research development to focus on these areas for therapeutical treatment and has also influenced designs to interact and integrate with the nervous system. We begin with biomolecular and electrical interfaces that enable measuring neurological activities (Fig. 2) and then investigate organ-level applications in the brain, retina, and spinal cord (Fig. 3).
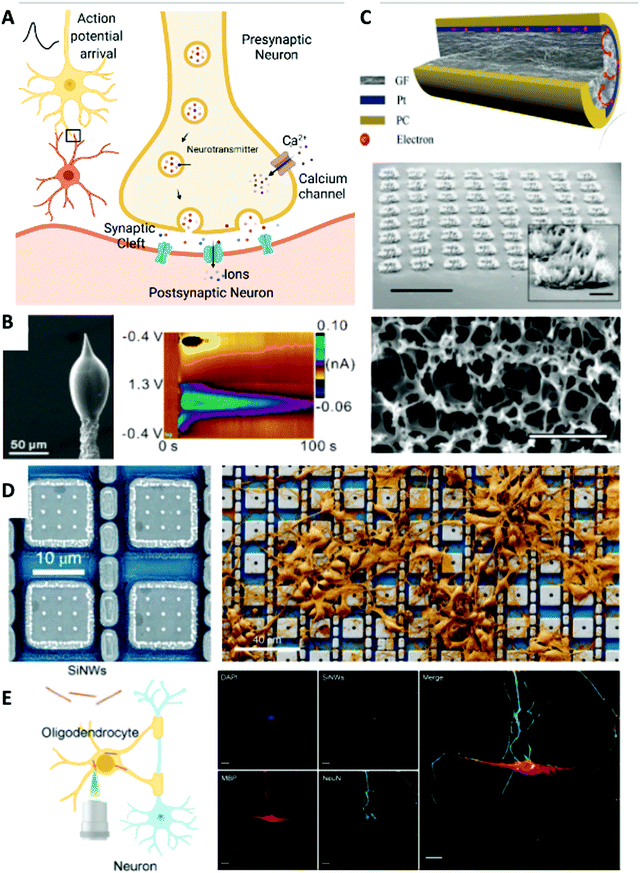 | ||
| Fig. 2 Chemical (biomolecular) and extracellular and intracellular electrical neuronal interfaces. (A) Schematic of neurotransmitter release in a neuronal synaptic cleft. Created with BioRender.com. (B) Left: SEM image of a 3D-printed carbon nanoelectrode. Right: Dopamine detected after acetylcholine (2 pmol) stimulation delivered with the 3D-printed nanoelectrode and a micropipette in an adult fruit fly brain. Reprinted with permission from ref. 76. Copyright 2020, American Chemical Society. (C) Extracellular recording electrode materials. Top: A graphene fiber microelectrode with a platinum coating that shows superior electrical performance. Reprinted with permission from ref. 81. Copyright 2019, Wiley-VCH. Middle: Tilt SEM image of the porous graphene electrode array. Scale bar, 1 mm. Inset: Zoomed-in view of an individual electrode. Scale bar, 100 μm. Bottom: SEM image of the surface morphology of the 3D porous graphene. Scale bar, 2 μm. Reprinted with permission from ref. 82. Copyright 2016, Springer Nature. (D) Intracellular neural recording. Left: Platinum-based electrode that enabled effective intracellular recording. Right: SEM image showing neurons cultured on an electrode array that has thousands of recording sites. Reprinted with permission from ref. 20. Copyright 2020, Springer Nature. (E) Intracellular neural stimulation. Left: Schematic of silicon nanowires (SiNWs) for intracellular optical investigation of subcellular components of neurons. Right: Immunofluorescence confocal image of the nucleus (blue, DAPI), oligodendrocyte (red, MBP), DRG neuron (green, NeuN), internalized SiNWs (white), and merged overlay. Scale bar, 20 μm. Reprinted with permission from ref. 92. Copyright 2020, American Chemical Society. | ||
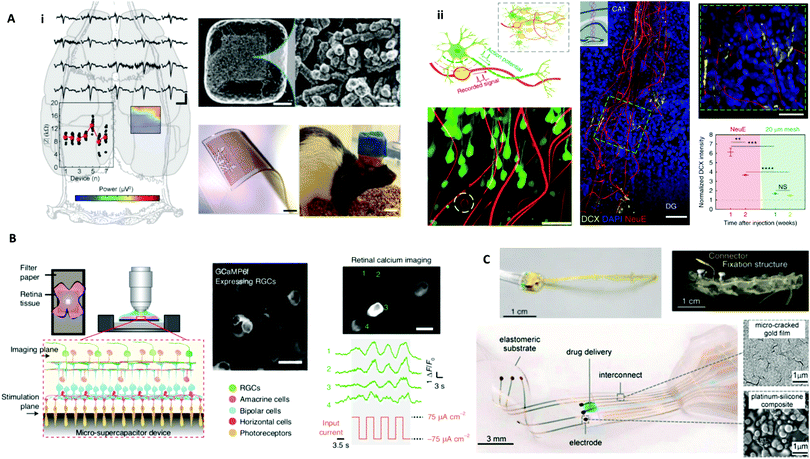 | ||
Fig. 3 Biology-guided interfaces in the nervous system in the brain, retina, and spinal cord. (A) Brain interfaces. i. Left: Recording of somatosensory evoked potentials with an in vivo stretchable electrode grid interfaced with a rat cortex. Upper right, SEM image with an enlarged view on right detailing Pt-coated Au–TiO2 nanowires partially embedded in the PDMS to form the electrode. Scale bars, 20 μm (top middle) and 1 μm (top right). Bottom middle: An image of the electrode grid. Scale bar, 1 mm. Bottom right: Image of a motile rat with in vivo stretchable electrode grid interfaced with the cortex. Scale bar, 10 mm. Reprinted with permission from ref. 39. Copyright 2018, Wiley-VCH. ii. Upper left: Schematic diagram of the neuron-like electronics (NeuE). Bottom left: 3D reconstructed interface of neuron (green) and the NeuE (red) following six weeks of implantation with an electrode in the white dashed circle. Scale bar, 50 μm. Middle: A 3D image of newborn neurons (DCX) grown along NeuE following one week of implantation. Scale bar, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μm. Inset: Schematic of the injection area. Top right: Enlarged view of the green dashed box in the middle image. Scale bar, 50 μm. Inset: Schematic of the injection area. Top right: Enlarged view of the green dashed box in the middle image. Scale bar, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μm. NeuE was indicated by white asterisks. Bottom right: Intensity of DCX near NeuE and 20 μm mesh. Reprinted with permission from ref. 44. Copyright 2019, Springer Nature. (B) Retinal interfaces. Experimental setup using a carbon micro-supercapacitor for retinal stimulation. GCaMP6 expressing RGCs were cultured on a device and calcium imaging shows retinal activation induced by electrical stimulation. Reprinted with permission from ref. 98. Copyright 2020, Springer Nature. (C) Spinal cord interfaces. Top left: Image of a spinal implant. Top right: 3D topography-based reconstruction following five weeks of implantation. Reprinted with permission from ref. 106. Copyright 2016, Springer Nature. Bottom left: Image of e-dura spinal cord implant. Bottom right: SEM images of the gold film and platinum–silicone composite. Reprinted with permission from ref. 45. Copyright 2015, The American Association for the Advancement of Science (AAAS). μm. NeuE was indicated by white asterisks. Bottom right: Intensity of DCX near NeuE and 20 μm mesh. Reprinted with permission from ref. 44. Copyright 2019, Springer Nature. (B) Retinal interfaces. Experimental setup using a carbon micro-supercapacitor for retinal stimulation. GCaMP6 expressing RGCs were cultured on a device and calcium imaging shows retinal activation induced by electrical stimulation. Reprinted with permission from ref. 98. Copyright 2020, Springer Nature. (C) Spinal cord interfaces. Top left: Image of a spinal implant. Top right: 3D topography-based reconstruction following five weeks of implantation. Reprinted with permission from ref. 106. Copyright 2016, Springer Nature. Bottom left: Image of e-dura spinal cord implant. Bottom right: SEM images of the gold film and platinum–silicone composite. Reprinted with permission from ref. 45. Copyright 2015, The American Association for the Advancement of Science (AAAS). | ||
3.1 Extracellular biomolecular and chemical investigation in the nervous system
As a pivotal part of bioelectrical interfaces, chemical and electrical measurements are essential for studying neurological function. Commonly, nervous system processes occur through action potentials, millisecond-long changes in the membrane voltage that serve as electrical signals by which voltage-sensitive ion channels release chemicals. Ion channels can respond to bioelectrical signals as well as physical environmental conditions, such as temperature, mechanical forces, and photons. The nervous system processes and stores electrical and chemical data while also sensing surrounding biological environments to respond to physiological changes.7 This electrochemical complexity has been studied with molecule sensing techniques that have focused on neurotransmitters. Additionally, cell signaling ions, such as calcium ions (Ca2+), have been extensively studied with the help of fluorescent calcium indicators (e.g., Fluo-4 and Cal. 520 AM) and genetically encoded calcium indicators (GECI, specifically GCaMP). Techniques to sense and control the dynamics and release of neurotransmitters and calcium have been developed using bioelectrical interfaces.18In particular, calcium activity has been studied as an indicator in neuron activation and modulation studies because of its role as an intra- and extra-cellular messenger.63,64 Calcium is tied to electric-based biological processes. The interplay between the calcium concentration and neurons relies on electrochemical gradients and voltage-sensitive channels in the plasma membrane. Upon the arrival of an action potential, membrane depolarization opens voltage-gated calcium ion channels, which yields parallel increases in calcium concentration, demonstrating why calcium can be used to track neuronal electrical activity. Following the electrochemical gradient, calcium ions flow into the nerve terminal of lower concentration, triggering the release of neurotransmitters into synaptic clefts. These neurotransmitters sequentially bind to ion channel receptors on the postsynaptic neuron, inducing signal propagation in the neural networks (Fig. 2A). In this way, calcium regulates neuronal activities from neurotransmitter synthesis and release to neuronal excitability. Thus, calcium imaging is widely used to dissect the function and activities of neuronal circuits in cell culture or in living animals. Calcium dynamics at the single-cell level and within neuronal circuits are essential for deciphering the complex nature of neurons.
Currently, several inorganic nanomaterials are reported to induce calcium changes via different mechanisms, for example, photothermal and photocapacitive effects of silicon nanowires and p–i–n membranes,18 magneto-mechanical and magnetothermal transduction using magnetic particles,65 upconversion-enabled optogenetics,66 and in situ generated nitric oxide (NO)-enabled activation of transient receptor potential vanilloid family member 1 (TRPV1).67 Light- and magnetic field-induced calcium activation allows wireless remote control of neuronal activities with spatial and temporal confinement.63 These approaches demonstrate how biology influences research as calcium is a major target because of its relation to electrical activity. Although calcium imaging is generally conducted in vitro, in vivo chronic recording with photostable proteins, such as GCaMP3, has been demonstrated by Ziv and coworkers in freely moving mice over 45 days with cellular resolution in a region of hundreds of hippocampal neurons.68 In zebrafish, whole-brain calcium imaging in vivo in an intact animal is feasible.69
On the other hand, electrical stimulation also induces a burst in calcium to excitable cells, such as neurons and cardiomyocytes. Therefore, nanoelectrodes evoke calcium fluctuation with high spatial and temporal resolution by delivering electrical stimulation, unveiling the connection and physiological complexities of neuron networks.70 Besides providing electrical pulses, nanoelectrodes have also been widely used in sensing local electrochemical potential changes and extracellular and intracellular electrical fields.
Electrochemical potential changes result from local neurotransmitter release and chemical gradient changes can be detected via voltammetry or amperometry.71,72 A nanoelectrode oxidizes and reduces nearby vesicular exocytosis and electroactive biogenic analytes, such as dopamine and norepinephrine. These neurotransmitters or hormones are crucial to neuronal intercellular communication, enabling brain function and neuropathophysiology. Currently, a variety of nanomaterial designs are employed to understand the biological importance of these molecules by developing systems for dopamine monitoring, for example, Pt/3-carboxylate polypyrrole (Pt_CPPy)-based field-effect transistor (FET) sensors,73 Fe3+_CPPy nanoparticles on biodegradable Mg/Si nanomembranes,74 and 3D fuzzy graphene microelectrode arrays for multi-channel subcellular sensing.75 Probing at the nanoscale has been expanded with new synthesis methods. Cao et al. 3D printed carbon nanoelectrodes with Al2O3 insulation for in vivo sensing (Fig. 2B).76 Biology guided this design as the nanoelectrodes were created to be small enough to get close to the synapses that release dopamine to improve the detection limit.
3.2 Extracellular electrical investigation in the nervous system
Electrophysiology in extracellular space is also widely investigated via microelectrode arrays77 and fiber-like electrodes.78,79 Inorganic materials have significantly impacted fundamental scientific studies to improve electrode performance. For example, iridium oxide (IrOx) and porous titanium nitride (TiN) nanocoatings on Pt or Pt/Ir electrode surfaces improve the charge injection limit from 130 μC cm−2 to ∼1 mC cm−2via enlarged surface area from porosity in TiN nanoparticles, and to 5 mC cm−2via reversible faradaic charge transfer between Ir3+ and Ir4+, respectively.80 Structural biology has guided extracellular investigation by forcing the development of electrodes with tips that match the size of neurons (∼100 μm) and mechanical properties that are stiff enough to penetrate tissue yet minimize mechanical mismatch and allow for dynamic movement. This has influenced materials development with nanostructuring, such as with graphene, that provides key electrical characteristics while maintaining structural integrity and flexibility. In a recent work demonstrated by Wang et al., graphene fiber electrodes with platinum coating could synergistically collect current results with low impedance, high surface area, and an unrivaled charge injection capacity (10.34 mC cm−2).81 Detection of neuronal activity in vivo in the rat cerebral cortex with an outstanding signal-to-noise ratio of 9.2 dB was also reported (Fig. 2C, top). A 3D porous graphene-based flexible electrode array has also been fabricated in situ on polyimide films via direct laser engraving and pyrolysis (Fig. 2C, bottom).82 This porous graphene demonstrated superior impedance (2–8 kΩ) and charge injection characteristics (3.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mC cm−2), enabling efficient sensing and stimulation of neural activities. Besides, magnetic resonance imaging (MRI) compatible ultra-small fiber electrodes based on graphene/copper microwires83 and carbon nanotubes (CNTs)84 have also been designed for multifunctional stable recording of single-unit neural signals during which MRI can be simultaneously conducted to map brain activity.
mC cm−2), enabling efficient sensing and stimulation of neural activities. Besides, magnetic resonance imaging (MRI) compatible ultra-small fiber electrodes based on graphene/copper microwires83 and carbon nanotubes (CNTs)84 have also been designed for multifunctional stable recording of single-unit neural signals during which MRI can be simultaneously conducted to map brain activity.
3.3 Intracellular investigation in the nervous system
To investigate neuronal bioelectrical signaling more accurately, intracellular recording of action potentials must be accomplished. Intracellular recording enables substantive understanding of the subcellular and molecular mechanisms that cause neuronal physiological activities, such as membrane depolarization and ion fluctuation. Several MEAs- and silicon nanowire (SiNW)-based FET probes85 have been widely applied to form nano-bioelectrical interfaces at the level of individual axons and dendrites. For example, gold mushroom-shaped microelectrodes86 and a 3D kinked SiNW FET nanoprobe87,88 have enabled intracellular recording and stimulation. Robinson et al. reported a scalable electrode platform composed of vertical nanowires that enables intracellular recording and stimulation of neurons.89 This array-based system is scalable and of high resolution, including at the single-cell level, and allows investigation of signaling processes to better understand neuronal systems. To maximize single-cell level coupling, the authors considered structural biology of neurons, choosing the size of the nanowire array to be a 4 μm square as this would be smaller than the average neuronal cell, designing electrodes with silicon cores and metal tips that could enter the cell interior, and placing a silicon dioxide (glass) shell around the nanowires to ensure tight conformation with the cell membrane to prevent current leakage. Recently, Abbott and colleagues demonstrated how a platinum-based electrode array enabled the recording of over 1700 neurons simultaneously to develop an understanding of synaptic activity and employed biological rationale in designing pixel pads of 20 μm to match the diameter of rat neurons which are about 20 μm (Fig. 2D).20While substrate-bound nanoarrays successfully measure intracellular action potentials via tight interfaces, array-based approaches can be invasive and induce a foreign-body reaction upon implantation.90 Thus, there is a need to reduce the footprint and invasiveness of designs. Freestanding nanomaterials form subcellular interfaces with neurons, and their dimensions are orders of magnitude smaller than that of cells, making them “invisible” to immune cells while increasing sites of contact and offering wireless controllability. Silicon-based nanomaterials have been extensively used for light-induced nongenetic neuron modulation and detailed protocols have been established.48 The ability to photoelectrochemically induce action potentials provides a method to control neural activity.42 Parameswaran et al. used coaxial p-type/intrinsic/n-type (PIN) SiNWs to modulate dorsal root ganglion (DRG) neurons through an atomic gold-enhanced photoelectrochemical process.91 This approach uses the biological principle of membrane depolarization by a photocathodic electrical effect to elicit action potentials and uses SiNWs to tightly conform with neuronal membranes. Rotenberg and collaborators also demonstrated using SiNWs for intracellular optical interrogation of neuron-glia heterocellular interactions with subcellular spatial resolution while not disrupting biological function, such as mitosis, or the cell cytosol since SiNWs can be spontaneously internalized (Fig. 2E).92
3.4 Recording and stimulation in the brain
As one of the most intricate organs, the human brain is comprised of about 100 billion electrically active neurons and 100 billion non-electrically active glial cells, making it difficult to investigate and treat neurological-related medical conditions. Brain cells depend on electrical activity, communication, and the formation of synapse connections. Previously, electric-based approaches have been applied therapeutically to neurological disorders because electrical stimulation can be used in excitable tissue through depolarization and action potentials. Biology-guided designs are critical for interfacing with the brain because of its delicate and complex nature. Creating electrical interfaces is necessary to diagnose and treat neurological disorders. Despite material and technological advances, interfaces can still be improved in terms of their biocompatibility, electrode density, and long-term stability.Curiosity about brain dynamics and therapies has led to the development of brain-machine interfaces which seek to understand, monitor, and treat neurological processes and conditions. Approaches include recording, stimulation, mapping, and directed growth to determine how the brain functions. Interfacing with neural tissue requires biology-guided considerations, mainly biocompatibility and long-term stability, which are related to the soft mechanical nature of the brain. Most material substrates have moduli that lead to mechanical mismatch, which disables performance, stability, and biocompatibility. Thus, research has focused on ways to achieve functional and long-lasting interfaces.
One approach is using materials that closely match the interface's mechanical properties, including stretchability and flexibility. For example, Tybrandt et al. used a stretchable silicon electrode matrix embedded with gold-coated titanium dioxide (TiO2) nanowires to improve spatiotemporal resolution for in vivo surface recording of somatosensory evoked potentials in the cortex of rats (Fig. 3Ai).39 This electrode design that matches the biological mechanics of the brain is formed from platinum-coated gold–TiO2 nanowires that are partially embedded in PDMS. Through this biology-guided approach, the authors achieved high-quality and stable recording for three months after implantation and showed how a reduced size can decrease invasiveness. Flexibility has also been incorporated into designs, such as using a flexible porous graphene electrode array for stimulation and sensing of the cortical surface.82
Yang et al. detailed another way that biology guides development (Fig. 3Aii).44 Despite previous bioinspired and biomimetic efforts, neural probes still yield a mismatch with the brain, leading to inflammation, unstable measurements, and loss of neurons. The authors present neuron-like electronics (NeuE) that merge with neurons by mimicking subcellular structural features and mechanical characteristics. As an example of how biology can guide design, this development was based on the fundamental component of the brain, the neuron, and matches the brain at the network level: electrodes matched the size of the soma and neurite, interconnects had flexibility like axons, and polymer insulation functioned as the myelin sheath to aid in electrical signal transport. The 16-channel probe was fabricated by photolithography with gold interconnects and platinum electrodes, and the top SU-8 layer had distinct layers. The NeuE-brain interface enables 3D mapping post-implantation and facilitates newborn neuronal migration.
3.5 Vision restoration and amplification vision
Closely connected with the brain and vital for vision, the retina senses light and sends electrical pulses to the brain, forming what we see. The retina serves as a model system to study the nervous system under stimulation because of its neural circuitry based on photoreceptors and retinal ganglion cells (RGCs), which can be considered as “input” and “output” neurons, respectively. A standard method to assess retina functionality is the electroretinogram (ERG), which measures electrical potential changes on the corneal surface. Typical designs are in the format of a contact lens that interfaces with the eye; however, these yield irritation and variable recording. Recently, designs guided by the curvature and transparency of the cornea have led to the development of soft and transparent contact lens electrodes for ERG measurements, such as a graphene-based contact lens electrode that enables increased conformity and a tighter interface.93Retinal research often centers around vision degeneration, which has led to restoration and amplification efforts advancing with nanotechnology. Prostheses are an attempt to activate the origin of sight, the retina, and have been applied in animals, such as primates,94 but have only been recently applied to human subjects due to approval processes. Vision restoration research includes developing and applying novel methods for retinal prostheses, such as using indium phosphide and zinc oxide quantum dots to build photoactive photoelectrode-based surfaces to form polarized currents that can induce neuronal activity and electric-based stimulation of RGCs.95 Besides, optogenetic therapies, which have been approved by the US Food and Drug Administration (FDA), have been successfully demonstrated to partially recover vision in a blind human patient.96
RGCs develop first in the retina and are critical for the conversion of light to neural signals. Under some retinal conditions, photoreceptors cannot successfully activate RGCs, which has led to research in alternative activation methods to restore vision. For example, Chen et al. induced RGC stimulation with a TiO2–Au nanowire-based photoelectrical motor.97 The authors demonstrated that this nanomaterial-based motor can be powered by an electrical field generated by ultra-low UV light and considered how to integrate and communicate with the neural system through bioelectrical signaling produced by photoelectric conversion. Most importantly, this system can be used to stimulate calcium ion channels and target single cells and relies on the ability of RGCs to be excited by electrical stimuli. Additionally, Fang et al. recently reported the use of a carbon micro-supercapacitor with hierarchical surface porosity for retinal stimulation (Fig. 3B).98 The electrical potential at the surface of the carbon facilitates the controlled activation of RGCs. Their self-assembly bottom-up fabrication approach using nanoscale micelles and SiO2 templates offers new fabrication routes for device manufacturing. Monolithic carbon minimized biocompatibility issues that result from layer dissociation in binding-based processes and interdigitated electrodes decreased the size to interface at the subcellular length scale.
Wireless approaches, such as the use of nanoparticles, can also be explored as stimulation materials. As demonstrated by Maya-Vetencourt et al., poly[3-hexylthiophene] (P3HT) nanoparticles provide opportunities to recover visual acuity in rats inflicted with retinitis pigmentosa.14 This injection-based approach offers a wireless alternative to retinal prostheses and can potentially be applied to other retinal diseases as well, including age-related macular degeneration. This design was guided by the natural structure of foveal cones, using nanoparticles to achieve broad and effective distribution across the subretinal space.
In addition to restoration, research aims to amplify vision. Commonly, this research targets photoreceptors, showing how developments can be guided by biology by using and integrating with natural components. An example is the extension of the visible spectrum to include NIR light through injections of photoreceptor-binding upconversion nanoparticles (pbUCNPs).13 Following the injection of pbUCNPs and activation by a NIR LED light of low power, the injected mice were able to detect NIR light and shape patterns. Upconversion nanoparticles, which are light-activated particles that convert excited photons in the NIR range to those in the visible and UV light ranges, have also been applied in cancer and energy research.99–102
3.6 Understanding and restoring the spinal cord
Connecting the brain and the rest of the body, the spinal cord serves as a messenger channel critical for the proper function of biological processes. However, once injured, damage to the spinal cord often leads to severe consequences, such as chronic pain and lifelong disability. Upon destruction of the blood-brain spinal cord barrier, an inflammatory response via macrophages and microglia occurs at sites of trauma with an accumulation of inhibitory molecules that form astrological scars and hinder axonal regeneration and spinal cord repair. Given that spinal injury occurs in more than 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people annually, biomedical research is trying to understand the pathology of the damage and therapeutic rehabilitation.103
000 people annually, biomedical research is trying to understand the pathology of the damage and therapeutic rehabilitation.103
Brain-computer or brain-machine interfaces (BCIs/BMIs) have led to improved functional outcomes since the last decade. For example, BrainGate technology, applying the silicon-based monolithic Utah array, directly translated neural activity from two people with tetraplegia into controlled movements of a robotic arm.104 Biological principles guide this approach as it relies on inherent natural signaling, seeking to form an interface to translate neuronal activity into signals understood by external assistive devices. Additionally, a closed-loop demultiplexing BCI, demonstrated by Ganzer et al., has restored the sense of touch and achieved improved sensorimotor functions by decoding sub-perceptual neural signals from the cortex into conscious perception.105 This is based on biological evidence that tactile stimuli can elicit changes in cortical activity in patients with spinal cord injury. A spinal implant delivering electrical stimulation to the spinal cord resulted in locomotion in paralyzed rats (Fig. 3C Top).106 Recently, people with severe spinal cord injury-induced paralysis could regain the ability to walk after epidural electrical stimulation, which is based on remaining brain and neuron spinal cord connections below the spinal cord injury.107
While Utah array-like rigid silicon electrodes are the predominant devices used for clinical research, adverse biological responses and device degradation have driven the development of soft, long-lasting devices with a high signal-to-noise ratio and bifunctional abilities. Currently, spinal cord interfacing research remains limited by the ability to investigate and control spinal cord dynamics, often because the spinal cord presents design challenges as its modulus (0.25–0.3 MPa) requires designs that are durable yet flexible and stretchable, and its movement requires seamless integration to conform to its dynamic movement. An approach to investigate spinal cord circuitry uses flexible and stretchable concentric probes formed by coating polymer fibers with silver nanowire meshes that can combine optical stimulation and electrical recording.108 The need to match mechanical properties to enable lasting interfaces is a case of biology-guided development of materials of specific shape and elasticity. An example reported by Minev et al. mimics the properties of the dura mater to form a soft neural implant that enhances the temporal stability by minimizing mechanical tissue mismatch (Fig. 3C Bottom).45 Their fabrication uses platinum nanoparticles (size 500 nm–1.2 μm) in PDMS silicon paste as electrodes, and stretchable micron-gold as interconnects to transmit electrical excitation and transfer electrophysiological signals. Chemical neuromodulation can be directly delivered to the interfaced intrathecal space with an intimate interface between electrodes and spinal subdural space. The dura mater is the membrane in the spinal cord that helps to protect these parts of the nervous system. The authors demonstrated how a design inspired by the dura mater can be integrated with electronic components and microfluidic channels to form a multifunctional device that enabled paralyzed rats to walk via colocalized electrical and chemical stimulation. In the future, soft neural implants with stable long-term bio-integration and multiple functionalities to sense and stimulate interfaced tissue in high resolution will be designed to assess and treat neurological injuries and disorders.
4. Cardiac system
The heart reliably and rhythmically forms electrical pulses that yield contractions to circulate blood throughout the body. However, misfunction in the heart and circulatory system could lead to adverse cardiovascular disease, a leading cause of death. According to an American Heart Association report, nearly 18 million deaths around the world were related to cardiovascular disease in 2017.109Bioelectrical research is applicable to the cardiac system because processes and characteristics of cardiac diseases that necessitate treatment are often related to electrical functionality. For example, heart contractions originate from depolarization at the sinoatrial node and subsequent electrical conduction signaling activity.110 Currently, treatments are being developed by monitoring and modulating the electrical activity in vitro, in vivo, and ex vivo. We focus on developments for treating conduction abnormalities and regeneration of injured tissue as these are ways to correct and fix damage from cardiac disease.
In the cardiac system, biology has significantly influenced research developments and targets. Translation to the clinical setting is hindered by rigid designs that cannot conform with heart tissue and extremely soft designs that cannot maintain functionality. A conventional approach would design a material for cardiac recording to withstand dynamic movements of the heart and maintain biocompatibility. In contrast, a biology-guided approach might seek to create materials that synergistically interact with cardiac tissue and correct beating abnormalities, yielding a result that supports and amplifies natural processes.
Biology-guided designs include stretchable and flexible materials to withstand the dynamic environment of the heart111 and some that are soft and rubbery to closely match the modulus of heart tissue and deform and stretch with beating.19 Biology has also influenced design integration, such as heart implantation through organogenesis to achieve dimensional and spatial resolution with increased conformability (Fig. 4A),112 formulation of tissue scaffolds to match natural properties,113 and resorbable cardiac pacemakers without leads or batteries.114
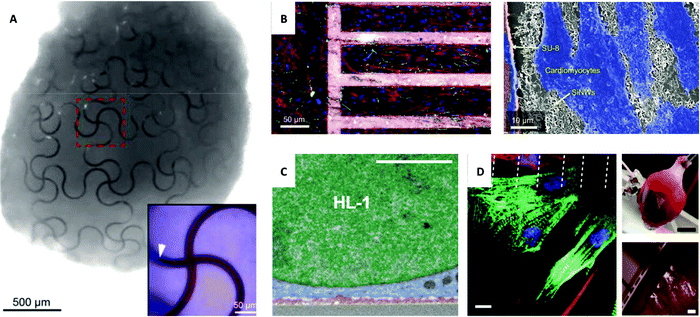 | ||
Fig. 4
In vivo and ex vivo interfaces with the cardiac system. (A) Image of a complete organoid that demonstrates a nano-electrical biointerface. Stretchable SU-8 nanoelectronic mesh interfaced with stem cells through organogenesis to achieve a conformable design. Inset of an enlarged false-colored image with SU-8 ribbons (red) and stem cells (blue) and area of embedded SU-8 ribbon marked with a white arrow. Reprinted with permission from ref. 112. Copyright 2019, American Chemical Society. (B) Left: Confocal image of primary cultured cardiomyocytes grown on fibronectin-coated glass (left) and mesh of polymer and SiNWs (right) with cardiomyocytes stained red, green, and blue and mesh stained pink. Right: SEM image of the interface between cardiomyocytes (blue), SU-8 polymer (orange), and SiNWs (white). Reprinted with permission from ref. 121. Copyright 2019, National Academy of Sciences. (C) Magnified image of SEM cross-section of HL-1 cardiomyocytes cultured on 3D fuzzy graphene. Nucleus (green), cytoplasm (blue), and fuzzy graphene (red). Scale bar, 2 μm. Reprinted with permission from ref. 123. Copyright 2021, AAAS. (D) Left: In vitro interface of a micro-supercapacitor-like device with cardiac cells with dashed lines indicating the edges of carbon electrodes and immunohistochemistry staining of cardiomyocytes (green) and fibroblasts (red) with nuclei (blue) and connexin-43 (magenta). Scale bar, 10 μm. Top right: Ex vivo heart device interface. Scale bar, 5 mm. Bottom right: Magnified view of the cardiac interface. Scale bar, 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μm. Reprinted with permission from ref. 98. Copyright 2021, Springer Nature. μm. Reprinted with permission from ref. 98. Copyright 2021, Springer Nature. | ||
4.1 Addressing conduction abnormalities with recording and stimulation
Cardiovascular disease often disrupts proper conduction activity and leads to beating abnormalities that require correction, which is typically assisted with electronic pacemakers. Although electronic pacemakers effectively correct beating, they are generally invasive with biocompatibility issues and have limited resolution. These challenges are being conquered with developments in extracellular and intracellular recording. For example, vertical plasmonic nanoelectrodes115 and Pt nanopillars that form a heart-on-a-chip can be used for action potential recording.116 The idea of a heart-on-a-chip has also been explored in its use as a biosensor array to determine electrical-based cell signaling communication in the development of cardiac spheroids.117 High-resolution investigation of cardiac systems not only improves understanding of fundamental functionality but can also be used to screen and treat disease. One development that has enabled high-precision recording is complementary metal-oxide-semiconductor (CMOS) circuits, such as the CMOS nanoelectrode array by Abbott et al. that enables single-cell level investigation and stimulation with 1024 pixels.118 Another newly developed recording method follows from attempts to reduce cytotoxicity and inadvertent cell responses by noninvasive monitoring of mirror charges. Barbaglia et al. demonstrate optical-based recording of cardiomyocytes using a device that mirrors ionic currents in a microfluidic chamber.119 This advancement enables future in vivo devices and reliable determination of cardiac cell activities.Intracellular investigation of electrical activity has been performed with structural designs, beginning with silicon nanowires (SiNWs) since they are of a high surface-to-volume ratio and enable electrical readouts with their one-dimensionality. Although SiNWs have enabled intracellular investigation, in vivo studies present challenges. Nevertheless, a nongenetic method was demonstrated to investigate intracellular electrical processes in vivo using SiNWs hybridized with cells. This wireless process relies on laser stimulation and is of high specificity.120 The use of SiNWs has been expanded with other morphological configurations, such as using a mesh composed of polymers and SiNWs to optically modulate cardiac systems, including primary cultured cardiomyocytes (Fig. 4B) and ex vivo rat hearts.121 This composite mesh can also enable modulation in targeted cardiac cells, making it a promising alternative to bulky, invasive, and wire-tethered electrical pacemakers. This approach for regulating heart beating frequency is guided by biological principles behind cardiac conduction disorders that are often characterized by abnormal heart beating.
Other materials have been used for cardiac interfaces. Rastogi et al. discussed the use of a graphene-based electrical platform for investigating intracellular and intercellular signaling and communication of human embryonic stem cell-derived cardiomyocytes.122 The authors synthesized graphene using vapor deposition and gold and graphene microelectrode arrays. This biocompatible and transparent platform is guided by the need to provide an alternative to fluorescent indicators to monitor electrophysiology to better study intracellular and intercellular communication involved in cardiac arrhythmia and neurological disease. Graphene that has been fabricated in other forms has also been used. Dipalo and colleagues demonstrated how a 3D fuzzy graphene microelectrode platform can be used to intracellularly record cardiomyocyte action potentials with high sensitivity (Fig. 4C).123 This approach relies on optoporation of the cell membrane, which guided the authors to design out-of-plane graphene flakes to enable tight interfacial interactions between the electrodes and plasma membrane. Recently, Bruno et al. reported an optical, nongenetic approach to modulate cardiomyocytes and neurons with plasmonic porous metamaterials.124 This method enabled optical and label-free stimulation using pulses from a near-infrared laser that is suitable for tissue penetration in biological systems. Furthermore, Nair et al. developed flexible electrodes for heart modulation using laser ablation to synthesize silicon carbide (SiC) from polydimethylsiloxane (PDMS), where a graphite layer forms between the SiC and PDMS.125 Additionally, carbon-based materials have been used for both in vivo and ex vivo heart modulation, as demonstrated by Fang et al. using monolithic carbon devices (Fig. 4D) with self-assembled hierarchical porosity via a bottom-up approach.98 As these developments show, achieving cardiac interfaces remains challenging but is advancing. The future of pacemakers likely involves the evolution of electronic pacemakers, including smaller and longer-lasting designs, and the introduction of biological pacemakers, such as those based on cell and gene transfer and transplantation. These developments increase the ability to treat individual patient's needs in a way that will advance medicine.
4.2 Regeneration approaches for injured hearts
In addition to efforts to correct beating abnormalities, cardiac therapies involve ways to lessen or reverse damage from cardiac disease. Upon limitation of blood flow to cardiac tissue, cells in the heart do not receive the necessary oxygen and nutrients to survive, leading some cells to die and regions to become injured. While transplants can be used, organ shortages limit this option. Thus, methods have focused on ways to reverse damage or enhance regeneration through tissue engineering. One part of tissue engineering aims to improve conductivity to improve cell proliferation and organization through the incorporation of electrical materials, of which we focus on nanomaterials, that interface with the heart.126 This approach is guided by the biological occurrence that heart failure often results from the loss of cardiomyocytes.Cardiac tissue engineering faces challenges involving arrhythmia due to the implantation of non-electroconductive platforms, which has led researchers to focus on other ways to implant cells since humans cannot produce new cardiomyocytes. Thus, developments must enable electroconductivity that allows for proper conduction following cell growth. For example, Roshanbinfar et al. presented a hydrazide-functionalized nanotube-based electroconductive matrix of biohybrid hydrogels that can be used to grow cardiomyocytes with improved contraction, maturation, and alignment.126 The authors rationalized their approach based on the biological need to form an electroconductive matrix stable under physiological conditions and evaluated its effectivity by measuring biological alignment and organization. Additionally, Kalishwaralal et al. developed a chitosan-based film with selenium nanoparticles to induce electrical conductivity and deliver healthy cells to infarcted tissue.127
5. Microbial system
Although the nervous system and cardiac system have been of significant study in the field of bioelectrical interfaces, research has recently focused on ways to investigate microbial systems with a medical-related focus due to discovered links between microbes and biological systems, such as the influence of the microbiome on the human body, development of viral and bacterial infections, and health connections with clean energy to address climate change. Microbial systems include bacteria and, by some definitions, include viruses that communicate and cooperate in a way that parallels the intercellular signaling of multicellular organisms.128 Better understanding and formation of interfaces with microbial systems will enable the field of bioelectronics to harness favorable genetic characteristics, evolutionary adaptability, and unique biological processes of microbial systems. Similarly, functionalities of microbes can be enhanced with nanostructured materials, such as gold nanoclusters, to introduce photosynthetic properties to non-photosynthetic bacteria.129To investigate microbial systems, methods that are precise and direct are necessary. One method that enables targeted and controlled study is optical stimulation, specifically with Si-based materials. Similar to neural and cardiac interfaces, forming interfaces with bacteria presents challenges due to variation in the length scale and morphology. Approaches that account for microbe morphology and length scale highlight how biology guides microbial system research. Gao et al. reported that multiscale and structured silicon materials can modulate bacterial cells and biofilms.130 This nongenetic method of high spatial and temporal resolution can be used to investigate functions of microbial systems, such as calcium dynamics and biofilm mechanics. Nanowires and microplates were shown to manipulate these bacterial communities and interface with different length scales. For individual planktonic cells of the micrometer length scale, SiNWs were used due to their one-dimensionality and sub-micrometer length that matches tubular bacteria (Fig. 5A), while Si microplates, two-dimensional disc structures, were used to interface with the aggregation of cells in biofilms (Fig. 5B). Improved interfaces can be achieved with mesostructured SiNWs.
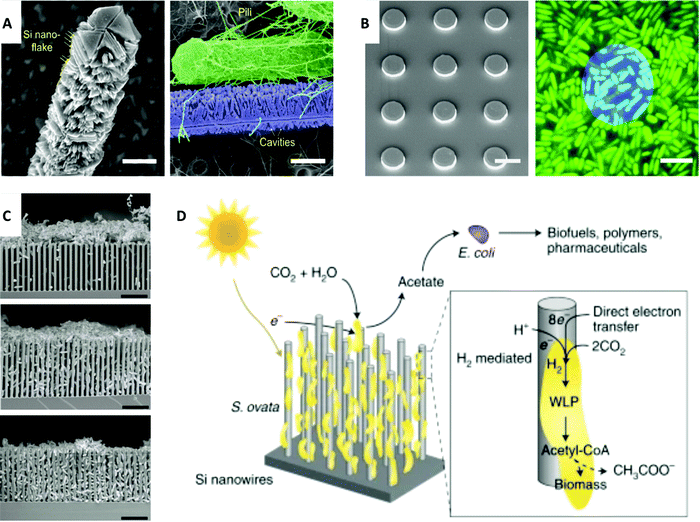 | ||
| Fig. 5 Electrical-related interfaces with microbial systems. (A) Left: SEM image of mesostructured SiNWs with Si nanoflakes. Scale bar, 0.5 μm. Right: SEM image shows a strong interaction between the mesoscale cavities and the bacterial pili. Scale bar, 0.5 μm. (B) Left: SEM image of Si discs. Scale bar, 10 μm. Right: Confocal microscopy image shows Si disc (blue) on biofilms (green). Scale bar, 5 μm. (A) and (B) Reprinted with permission from ref. 130. Copyright 2020, AAAS. (C) SEM images of bacteria interfaced with nanowires. Top: Bacteria aggregation at pH 7.2. Middle: Semi-close packing at pH 6.7. Bottom: Fully packed hybrid structure at pH 6.4. Scale bar, 10 μm. Reprinted with permission from ref. 131. Copyright 2020, Elsevier. (D) Schematic representation of the functionality of photosynthetic S. ovata interfaced with nanowires. Biohybrid system converts CO2 to acetate which can be turned into valuable chemicals with E. coli. Reprinted with permission from ref. 133. Copyright 2020, Springer Nature. | ||
Interfacing with microbial systems can also be addressed with biohybrid systems for energy applications. Photosynthetic biohybrid systems can be used in CO2 reduction and N2 fixation.131,132 Su et al. reported microbe-guided solar-to-chemical conversion supported by electron transfer (Fig. 5C).131 To apply this research to biomedicine, it is possible to use these biosynthetic pathways of bacteria with inorganic light-harvesting processes as alternative activators and energy sources in medical applications in a way that combines living systems with previously nonbiological areas of research. The method to efficiently drive CO2 fixation with solar energy via close-packed nanowire-bacteria hybrids could potentially be adapted to biological modulation, such as in the ability to use microbes to guide chemical synthesis by using genetically engineered E. coli for acetate conversion into biofuels, polymers, and pharmaceuticals (Fig. 5D).133 Such an approach draws from and is guided by the symbiotic principles of biology, such as mutualism and commensalism. In this case, microbes help facilitate critical processes while growing in a directed and viable manner.
Understanding the relationship that microbes have with other cells has also been of interest. For example, Cervera et al. presented theoretical information about how bacteria and non-excitable cells can be synchronized through oscillatory actions.134 They focus on the properties of single cells relevant to the behavior of multiple cells and the properties at the multicellular level that enable the synchronization of bioelectrical oscillations. These oscillatory dynamics are prevalent in various cell types, such as in glioma cells, bacteria, and pancreatic islets and in the developmental stages of chicken embryos. This research was motivated by the biological occurrence of oscillations in cell populations and relied on the biological principles of ion channel proteins that control the transmembrane electrical potential in the cell membrane. This model shows the possibility of controlling the electrical potential and current to manipulate both excitable and non-excitable cells. The applications of work are diverse and quite relevant to current trends, especially those in regenerative medicine and synthetic biology.135
6. Outlook and future directions
The field of bioelectrical interfaces is quickly developing with broad medical applications spanning brain stimulation and controlled neuronal growth, vision restoration and amplification, cardiac modulation and regeneration, and biohybrid microbial systems. New materials development will bring momentum to the direction of bioelectrical interface research. One example is forming nanomaterials from bulk materials, such as using Ti3C2 MXene fabricated microelectrodes to record neural activity with high resolution and reduced impedance compared to traditional microelectrodes (Fig. 6A).136,137 Specifically for clinical translatability, feasible delivery methods and biocompatibility as well as multifunctional designs are of utmost importance as they create efficient ways to monitor, control, and treat medical conditions.138 Zimmerman et al. reported drug-like internalization methods for SiNWs that noninvasively integrate with biological processes to deliver nanoscale components.139 Another delivery method focuses on working with dimensional constraints by using syringe injections to deliver 3D mesh electronics through cavities of much smaller dimensions.140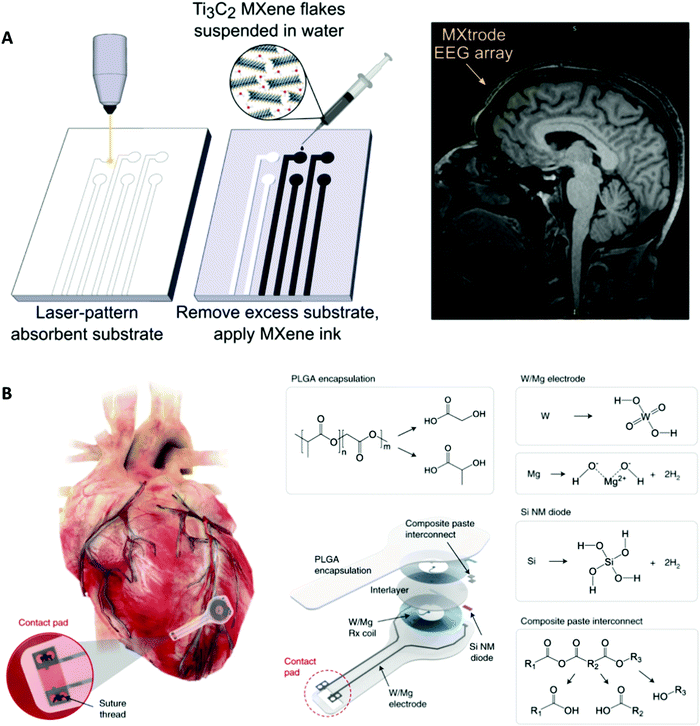 | ||
| Fig. 6 Examples of future biology-guided bioelectrical interfaces. (A) Left: Fabrication of laser-patterned planar and 3D mini-pillar Ti3C2 MXene electrode arrays. Right: MRI compatibility of the MXtrode. MXtrode EEG array was placed on a human forehead. Reprinted with permission from ref. 137. Copyright 2021, AAAS. (B) Schematic and chemical composition of the fully bioresorbable cardiac pacemakers without leads or batteries. Reprinted with permission from ref. 114. Copyright 2021, Springer Nature. | ||
Additionally, research to improve ease of function has included bioresorbable interfaces that rely on biological degradation, such as in a wireless bioresorbable and biocompatible interface for electrical stimulation of injured nervous tissue to improve regeneration and recovery in rodents141 and fully implantable and bioresorbable cardiac pacemakers (Fig. 6B).114 These bioresorbable pacemakers, composed of W/Mg-based inductive electrodes, a radiofrequency PIN Si nanomembrane diode, and a PLGA dielectric layer, enable full resorption via hydrolysis and metabolic activities. This development of a lead-free bioresorbable device is driven by biological necessity to avoid physiological complications, such as those from transcutaneous pacing leads that can cause potential infection and be inadvertently dislodged. These developments also eliminate the need for device removal upon completion of therapy which could lead to laceration and perforation of the myocardium since pacing leads often wrap into fibrotic tissue at the myocardium-device interface.
Specific focus is on bioelectrical signaling that can be integrated with bioengineering and regenerative medicine.135 Bioelectrical signals have also been explored in terms of controlling and targeting cell behavior and gene regulation and embryonic development.8 Clinical challenges, such as accessibility and feasibility of designs as well as approval and markets for these technologies, are present in the development of interface research.52 Recent advances have improved clinical translatability by incorporating biological formation processes and alternatives to device-based approaches, such as optogenetic approaches using radio-luminescent nanoparticles to stimulate genetically engineered light-gated neuron ion channels in vivo.142 Genetic-based devices that interface with neural cells can be used to enable brain imaging, targeted drug delivery, and detection of specific biomolecules and ions. The ability to connect synthetic biology tools, specifically biomolecules and ion detection, shows possible directions of bioelectrical-based research of multifunctional devices. One example demonstrated how flexible bioelectrical technology embedded with nucleic acid-finding CRISPR technology can detect SARS-CoV-2.143 This approach is guided by the ability of synthetic biology to use cell-free reactions in abiotic systems yet enable transcription and translation for nucleic acid detection.
The potential of using bioelectrical interfaces to advance regenerative medicine treatments and integrate with bioengineering for medical applications53 has been explored. Previously, genetic approaches have enabled specific targeting but have been limited by immune responses and ethical concerns; however, the field of genetics-based research is rapidly expanding and enabling new methods. DNA nanotechnology-based devices and scaffolds as materials have been used for cell and tissue engineering.61 These materials are promising because they are biocompatible and highly adaptable in biological systems and do not induce strong negative immune responses.
In addition to new materials, clinical translatability, and genetic-based approaches, future opportunities include investigation and incorporation with cell function, such as reactive oxygen species generation and subcellular dynamics.9,144,145 An example of reactive oxygen species modulation is presented in a study by Zhang et al. in which the authors reported the use of gold nanoclusters to introduce photosynthetic properties to microbial systems.129
We also believe there will be broader applications of bioelectrical interfaces to other health concerns, such as obesity, diabetes, and blindness.5 The use of more specialized materials, robotic systems, and artificial intelligent computer-assisted learning35 will advance rational material design and device integration with durable, functional interfaces to improve clinical translatability.50
7. Conclusion
In this review, we have highlighted what we believe to be some of the latest nano-bioelectrical interface developments in nervous, cardiac, and microbial systems with biology-guided designs in terms of resolution from extra- to intra-cellular investigation, biocompatibility, functional materials for intended applications, and other aspects.Current efforts pursue long-lasting, sensitive, and precise interfaces with minimal damage and multiple functionalities that seamlessly integrate with target biological systems. In applying these developments to biological systems, it is important to consider specific features that are unique and inherent to each system. For example, a stiffness-changeable material can be used for a nervous system interface as stiffness would allow for penetration into brain tissue but reduced rigidity would promote biocompatibility following implantation, a stretchable and flexible substrate could be used for a cardiac interface to enable stable conformation with the dynamic heart, and an interface capable of being formed at various length scales would be necessary to interact with different microbial systems. Many developments in optimizing bioelectrical interfaces stem from our ability to fabricate complex and smart devices with high spatial resolution, large integration, and durable designs from bioinspired and rational designs.
In the future, biology-guided principles, designs, and implementation strategies will emerge with the help of interdisciplinary research involving materials development, clinical translatability, and genetic and cell-based approaches. Through biology-guided considerations, we will be able to properly interface with complex biological systems for in vivo investigation and medical treatments.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the US Air Force Office of Scientific Research (FA9550-20-1-0387), the National Science Foundation (NSF CMMI-1848613, NSF DMR-2011854), and Zhong Ziyi Educational Foundation Award. Bernadette Miao acknowledges support from the Stamps Scholars Program.References
- A. Zhang and C. M. Lieber, Nano-Bioelectronics, Chem. Rev., 2016, 116, 215–257 CrossRef CAS PubMed.
- B. G. Kornreich, The patch clamp technique: Principles and technical considerations, J. Veterinary Cardiology, 2007, 9, 25–37 CrossRef PubMed.
- M. E. Spira and A. Hai, Multi-electrode array technologies for neuroscience and cardiology, Nat. Nanotechnol., 2013, 8, 83–94 CrossRef CAS PubMed.
- K. Hou, C. Yang, J. Shi, B. Kuang and B. Tian, Nano- and Microscale Optical and Electrical Biointerfaces and Their Relevance to Energy Research, Small, 2021, 2100165 CrossRef CAS PubMed.
- V. A. Pavlov and K. J. Tracey, Bioelectronic medicine: updates, challenges and paths forward, Bioelectron. Med., 2019, 5, 1 CrossRef PubMed.
- P. Sanjuan-Alberte and F. J. Rawson, Engineering the spark into bioelectronic medicine, Ther. Delivery, 2019, 10, 139–142 CrossRef CAS PubMed.
- R. Chen, A. Canales and P. Anikeeva, Neural recording and modulation technologies, Nat. Rev. Mater., 2017, 2, 16093 CrossRef CAS PubMed.
- K. A. McLaughlin and M. Levin, Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form, Dev. Biol., 2018, 433, 177–189 CrossRef CAS PubMed.
- P. Sanjuan-Alberte, M. R. Alexander, R. J. M. Hague and F. J. Rawson, Electrochemically stimulating developments in bioelectronic medicine, Bioelectron. Med., 2018, 4, 1 CrossRef PubMed.
- M. Y. Rotenberg and B. Tian, Talking to Cells: Semiconductor Nanomaterials at the Cellular Interface, Adv. Biosyst., 2018, 2, 1700242 CrossRef PubMed.
- S. K. Rastogi, A. Kalmykov, N. Johnson and T. Cohen-Karni, Bioelectronics with nanocarbons, J. Mater. Chem. B, 2018, 6, 7159–7178 RSC.
- S. M. Wellman, et al., A Materials Roadmap to Functional Neural Interface Design, Adv. Funct. Mater., 2018, 28, 1701269 CrossRef PubMed.
- Y. Ma, et al., Mammalian Near-Infrared Image Vision through Injectable and Self-Powered Retinal Nanoantennae, Cell, 2019, 177, 243–255.e15 CrossRef CAS PubMed.
- J. F. Maya-Vetencourt, et al., Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy, Nat. Nanotechnol., 2020, 15, 698–708 CrossRef CAS PubMed.
- G. Hong and C. M. Lieber, Novel electrode technologies for neural recordings, Nat. Rev. Neurosci., 2019, 20, 330–345 CrossRef CAS PubMed.
- C. Sung, et al., Multimaterial and multifunctional neural interfaces: from surface-type and implantable electrodes to fiber-based devices, J. Mater. Chem. B, 2020, 8, 6624–6666 RSC.
- J. A. Frank, M.-J. Antonini and P. Anikeeva, Next-generation interfaces for studying neural function, Nat. Biotechnol., 2019, 37, 1013–1023 CrossRef CAS PubMed.
- Y. Jiang, et al., Rational design of silicon structures for optically controlled multiscale biointerfaces, Nat. Biomed. Eng., 2018, 2, 508–521 CrossRef CAS PubMed.
- K. Sim, et al., An epicardial bioelectronic patch made from soft rubbery materials and capable of spatiotemporal mapping of electrophysiological activity, Nat. Electron., 2020, 3, 775–784 CrossRef CAS.
- J. Abbott, et al., A nanoelectrode array for obtaining intracellular recordings from thousands of connected neurons, Nat. Biomed. Eng., 2020, 4, 232–241 CrossRef CAS PubMed.
- E. N. Schaumann and B. Tian, Biological Interfaces, Modulation, and Sensing with Inorganic Nano-Bioelectronic Materials, Small Methods, 2020, 4, 1900868 CrossRef CAS PubMed.
- H. Acarón Ledesma, et al., An atlas of nano-enabled neural interfaces, Nat. Nanotechnol., 2019, 14, 645–657 CrossRef PubMed.
- P. Fattahi, G. Yang, G. Kim and M. R. Abidian, A Review of Organic and Inorganic Biomaterials for Neural Interfaces, Adv. Mater., 2014, 26, 1846–1885 CrossRef CAS PubMed.
- R. Feiner and T. Dvir, Tissue–electronics interfaces: from implantable devices to engineered tissues, Nat. Rev. Mater., 2017, 3, 17076 CrossRef.
- H. Yuk, B. Lu and X. Zhao, Hydrogel bioelectronics, Chem. Soc. Rev., 2019, 48, 1642–1667 RSC.
- C. J. Bettinger and Z. Bao, Biomaterials-based organic electronic devices, Polym. Int., 2010, 59, 563–567 CAS.
- D. T. Simon, E. O. Gabrielsson, K. Tybrandt and M. Berggren, Organic Bioelectronics: Bridging the Signaling Gap between Biology and Technology, Chem. Rev., 2016, 116, 13009–13041 CrossRef CAS PubMed.
- Y. Fang, et al., Recent advances in bioelectronics chemistry, Chem. Soc. Rev., 2020, 49, 7978–8035 RSC.
- S. G. Higgins, et al., High-Aspect-Ratio Nanostructured Surfaces as Biological Metamaterials, Adv. Mater., 2020, 32, 1903862 CrossRef CAS PubMed.
- Y. Jiang and B. Tian, Inorganic semiconductor biointerfaces, Nat. Rev. Mater., 2018, 3, 473–490 CrossRef PubMed.
- H.-Y. Lou, et al., Membrane curvature underlies actin reorganization in response to nanoscale surface topography, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 23143 CrossRef CAS PubMed.
- A. Prominski, P. Li, B. A. Miao and B. Tian, Nanoenabled Bioelectrical Modulation, Acc. Mater. Res., 2021, 2(10), 895–906 CrossRef CAS PubMed.
- Y. Lin, Y. Fang, J. Yue and B. Tian, Soft–Hard Composites for Bioelectric Interfaces, Trends Chem., 2020, 2, 519–534 CrossRef CAS PubMed.
- A. Verkhratsky, O. A. Krishtal and O. H. Petersen, From Galvani to patch clamp: the development of electrophysiology, Pflügers Archiv, 2006, 453, 233–247 CrossRef CAS PubMed.
- L. A. Annecchino and S. R. Schultz, Progress in automating patch clamp cellular physiology, Brain Neurosci. Adv., 2018, 2, 239821281877656 CrossRef PubMed.
- J. Abbott, T. Ye, D. Ham and H. Park, Optimizing Nanoelectrode Arrays for Scalable Intracellular Electrophysiology, Acc. Chem. Res., 2018, 51, 600–608 CrossRef CAS PubMed.
- Z. Aqrawe, J. Montgomery, J. Travas-Sejdic and D. Svirskis, Conducting polymers for neuronal microelectrode array recording and stimulation, Sens. Actuators, B, 2018, 257, 753–765 CrossRef CAS.
- Y. H. Cho, Y. Park, S. Kim and J. Park, 3D Electrodes for Bioelectronics, Adv. Mater., 2021, 2005805, DOI:10.1002/adma.202005805.
- K. Tybrandt, et al., High-Density Stretchable Electrode Grids for Chronic Neural Recording, Adv. Mater., 2018, 30, 1706520 CrossRef PubMed.
- J. F. Zimmerman and B. Tian, Nongenetic Optical Methods for Measuring and Modulating Neuronal Response, ACS Nano, 2018, 12, 4086–4095 CrossRef CAS PubMed.
- J. Liu, et al., Genetically targeted chemical assembly of functional materials in living cells, tissues, and animals, Science, 2020, 367, 1372 CrossRef CAS PubMed.
- B. Tian, Nongenetic neural control with light, Science, 2019, 365, 457 CrossRef CAS PubMed.
- F. Milos, et al., High Aspect Ratio and Light-Sensitive Micropillars Based on a Semiconducting Polymer Optically Regulate Neuronal Growth, ACS Appl. Mater. Interfaces, 2021, 13, 23438–23451 CrossRef CAS PubMed.
- X. Yang, et al., Bioinspired neuron-like electronics, Nat. Mater., 2019, 18, 510–517 CrossRef CAS PubMed.
- I. R. Minev, et al., Electronic dura mater for long-term multimodal neural interfaces, Science, 2015, 347, 159–163 CrossRef CAS PubMed.
- J. D. Weiland, D. J. Anderson and M. S. Humayun, In vitro electrical properties for iridium oxide versus titanium nitride stimulating electrodes, IEEE Trans. Biomed. Eng., 2002, 49, 1574–1579 CrossRef PubMed.
- E. W. Keefer, B. R. Botterman, M. I. Romero, A. F. Rossi and G. W. Gross, Carbon nanotube coating improves neuronal recordings, Nat. Nanotechnol., 2008, 3, 434–439 CrossRef CAS PubMed.
- Y. Jiang, et al., Nongenetic optical neuromodulation with silicon-based materials, Nat. Protoc., 2019, 14, 1339–1376 CrossRef CAS PubMed.
- F. Vitale, S. R. Summerson, B. Aazhang, C. Kemere and M. Pasquali, Neural Stimulation and Recording with Bidirectional, Soft Carbon Nanotube Fiber Microelectrodes, ACS Nano, 2015, 9, 4465–4474 CrossRef CAS PubMed.
- E. Song, J. Li, S. M. Won, W. Bai and J. A. Rogers, Materials for flexible bioelectronic systems as chronic neural interfaces, Nat. Mater., 2020, 19, 590–603 CrossRef CAS PubMed.
- G. Lee, Y. S. Choi, H.-J. Yoon and J. A. Rogers, Advances in Physicochemically Stimuli-Responsive Materials for On-Demand Transient Electronic Systems, Matter, 2020, 3, 1031–1052 CrossRef.
- N. Obidin, F. Tasnim and C. Dagdeviren, The Future of Neuroimplantable Devices: A Materials Science and Regulatory Perspective, Adv. Mater., 2020, 32, 1901482 CrossRef CAS PubMed.
- Q. Xu, et al., Biomimetic Design for Bio-Matrix Interfaces and Regenerative Organs, Tissue Eng., Part B, 2021, 27(5), 411–429 CrossRef CAS PubMed.
- Y. Park, et al., Three-dimensional, multifunctional neural interfaces for cortical spheroids and engineered assembloids, Sci. Adv., 2021, 7, eabf9153 CrossRef CAS PubMed.
- H. Amin, M. Dipalo, F. De Angelis and L. Berdondini, Biofunctionalized 3D Nanopillar Arrays Fostering Cell Guidance and Promoting Synapse Stability and Neuronal Activity in Networks, ACS Appl. Mater. Interfaces, 2018, 10, 15207–15215 CrossRef CAS PubMed.
- C. I. Bargmann and W. T. Newsome, The Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative and Neurology, JAMA Neurol., 2014, 71, 675 CrossRef PubMed.
- K. Amunts, et al., The Human Brain Project: Creating a European Research Infrastructure to Decode the Human Brain, Neuron, 2016, 92, 574–581 CrossRef CAS PubMed.
- M. C. Mott, J. A. Gordon and W. J. Koroshetz, The NIH BRAIN Initiative: Advancing neurotechnologies, integrating disciplines, PLoS Biol., 2018, 16, e3000066 CrossRef PubMed.
- K. Amunts, et al., The Human Brain Project: Creating a European Research Infrastructure to Decode the Human Brain, Neuron, 2016, 92, 574–581 CrossRef CAS PubMed.
- C. I. Bargmann and W. T. Newsome, The Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative and Neurology, JAMA Neurol., 2014, 71, 675 CrossRef PubMed.
- P. Hivare, C. Panda, S. Gupta and D. Bhatia, Programmable DNA Nanodevices for Applications in Neuroscience, ACS Chem. Neurosci., 2021, 12, 363–377 CrossRef CAS PubMed.
- D. Ham, H. Park, S. Hwang and K. Kim, Neuromorphic electronics based on copying and pasting the brain, Nat. Electron., 2021, 4, 635–644 CrossRef.
- H. Bading, Nuclear calcium signalling in the regulation of brain function, Nat. Rev. Neurosci., 2013, 14, 593–608 CrossRef CAS PubMed.
- L. Leybaert and M. J. Sanderson, Intercellular Ca 2+ Waves: Mechanisms and Function, Physiol. Rev., 2012, 92, 1359–1392 CrossRef CAS PubMed.
- D.-H. Kim, et al., Biofunctionalized magnetic-vortex microdiscs for targeted cancer-cell destruction, Nat. Mater., 2010, 9, 165–171 CrossRef CAS PubMed.
- S. Chen, et al., Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics, Science, 2018, 359, 679–684 CrossRef CAS PubMed.
- J. Park, et al., In situ electrochemical generation of nitric oxide for neuronal modulation, Nat. Nanotechnol., 2020, 15, 690–697 CrossRef CAS PubMed.
- Y. Ziv, et al., Long-term dynamics of CA1 hippocampal place codes, Nat. Neurosci., 2013, 16, 264–266 CrossRef CAS PubMed.
- M. B. Ahrens, M. B. Orger, D. N. Robson, J. M. Li and P. J. Keller, Whole-brain functional imaging at cellular resolution using light-sheet microscopy, Nat. Methods, 2013, 10, 413–420 CrossRef CAS PubMed.
- J. Kwon, et al., Nanoelectrode-mediated single neuron activation, Nanoscale, 2020, 12, 4709–4718 RSC.
- J. A. Ribeiro, P. M. V. Fernandes, C. M. Pereira and F. Silva, Electrochemical sensors and biosensors for determination of catecholamine neurotransmitters: a review, Talanta, 2016, 160, 653–679 CrossRef CAS PubMed.
- X. Liu, Y. Tong and P.-P. Fang, Recent development in amperometric measurements of vesicular exocytosis, TrAC, Trends Anal. Chem., 2019, 113, 13–24 CrossRef CAS.
- J. S. Lee, J. Oh, S. G. Kim and J. Jang, Highly Sensitive and Selective Field-Effect-Transistor NonEnzyme Dopamine Sensors Based on Pt/Conducting Polymer Hybrid Nanoparticles, Small, 2015, 11, 2399–2406 CrossRef CAS PubMed.
- H.-S. Kim, et al., Bioresorbable Silicon Nanomembranes and Iron Catalyst Nanoparticles for Flexible, Transient Electrochemical Dopamine Monitors, Adv. Healthcare Mater., 2018, 7, 1801071 CrossRef PubMed.
- E. Castagnola, R. Garg, S. K. Rastogi, T. Cohen-Karni and X. T. Cui, 3D fuzzy graphene microelectrode array for dopamine sensing at sub-cellular spatial resolution, Biosens. Bioelectron., 2021, 191, 113440 CrossRef CAS PubMed.
- Q. Cao, M. Shin, N. V. Lavrik and B. J. Venton, 3D-Printed Carbon Nanoelectrodes for In Vivo Neurotransmitter Sensing, Nano Lett., 2020, 20, 6831–6836 CrossRef CAS PubMed.
- X. Yuan, et al., Versatile live-cell activity analysis platform for characterization of neuronal dynamics at single-cell and network level, Nat. Commun., 2020, 11, 4854 CrossRef CAS PubMed.
- M. Hejazi, W. Tong, M. R. Ibbotson, S. Prawer and D. J. Garrett, Advances in Carbon-Based Microfiber Electrodes for Neural Interfacing, Front. Neurosci., 2021, 15, 658703 CrossRef PubMed.
- B. Fang, D. Chang, Z. Xu and C. Gao, A Review on Graphene Fibers: Expectations, Advances, and Prospects, Adv. Mater., 2020, 32, 1902664 CrossRef CAS PubMed.
- W. Tong, U. A. Robles and A. Gelmi, Bioelectronics and Neural Interfaces, in Inorganic Materials Series, ed. Spicer, C., Royal Society of Chemistry, Ch. 4, pp. 180–230, 2021 10.1039/9781788019828-00180.
- K. Wang, et al., High-Performance Graphene-Fiber-Based Neural Recording Microelectrodes, Adv. Mater., 2019, 31, 1805867 CrossRef PubMed.
- Y. Lu, H. Lyu, A. G. Richardson, T. H. Lucas and D. Kuzum, Flexible Neural Electrode Array Based-on Porous Graphene for Cortical Microstimulation and Sensing, Sci. Rep., 2016, 6, 33526 CrossRef CAS PubMed.
- S. Zhao, et al., Graphene Encapsulated Copper Microwires as Highly MRI Compatible Neural Electrodes, Nano Lett., 2016, 16, 7731–7738 CrossRef CAS PubMed.
- L. Lu, et al., Soft and MRI Compatible Neural Electrodes from Carbon Nanotube Fibers, Nano Lett., 2019, 19, 1577–1586 CrossRef CAS PubMed.
- B. Tian and C. M. Lieber, Nanowired Bioelectric Interfaces, Chem. Rev., 2019, 119, 9136–9152 CrossRef CAS PubMed.
- S. M. Ojovan, et al., A feasibility study of multi-site,intracellular recordings from mammalian neurons by extracellular gold mushroom-shaped microelectrodes, Sci. Rep., 2015, 5, 14100 CrossRef CAS PubMed.
- B. Tian, et al., Three-Dimensional, Flexible Nanoscale Field-Effect Transistors as Localized Bioprobes, Science, 2010, 329, 830–834 CrossRef CAS PubMed.
- Q. Qing, et al., Free-standing kinked nanowire transistor probes for targeted intracellular recording in three dimensions, Nat. Nanotechnol., 2014, 9, 142–147 CrossRef CAS PubMed.
- J. T. Robinson, et al., Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits, Nat. Nanotechnol., 2012, 7, 180–184 CrossRef CAS PubMed.
- J. W. Salatino, K. A. Ludwig, T. D. Y. Kozai and E. K. Purcell, Glial responses to implanted electrodes in the brain, Nat. Biomed. Eng., 2017, 1, 862–877 CrossRef CAS PubMed.
- R. Parameswaran, et al., Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires, Nat. Nanotechnol., 2018, 13, 260–266 CrossRef CAS PubMed.
- M. Y. Rotenberg, et al., Silicon Nanowires for Intracellular Optical Interrogation with Subcellular Resolution, Nano Lett., 2020, 20, 1226–1232 CrossRef CAS PubMed.
- R. Yin, et al., Soft transparent graphene contact lens electrodes for conformal full-cornea recording of electroretinogram, Nat. Commun., 2018, 9, 2334 CrossRef PubMed.
- P.-H. Prévot, et al., Behavioural responses to a photovoltaic subretinal prosthesis implanted in non-human primates, Nat. Biomed. Eng., 2020, 4, 172–180 CrossRef PubMed.
- H. Bahmani Jalali, et al., Effective Neural Photostimulation Using Indium-Based Type-II Quantum Dots, ACS Nano, 2018, 12, 8104–8114 CrossRef CAS PubMed.
- J.-A. Sahel, et al., Partial recovery of visual function in a blind patient after optogenetic therapy, Nat. Med., 2021, 27, 1223–1229 CrossRef CAS PubMed.
- B. Chen, et al., Photoelectrochemical TiO2–Au-Nanowire-Based Motor for Precise Modulation of Single-Neuron Activities, Adv. Funct. Mater., 2021, 31, 2008667 CrossRef CAS.
- Y. Fang, et al., Micelle-enabled self-assembly of porous and monolithic carbon membranes for bioelectronic interfaces, Nat. Nanotechnol., 2021, 16, 206–213 CrossRef CAS PubMed.
- J. F. Suyver, et al., Novel materials doped with trivalent lanthanides and transition metal ions showing near-infrared to visible photon upconversion, Opt. Mater., 2005, 27, 1111–1130 CrossRef CAS.
- S. Yan, et al., Activating Antitumor Immunity and Antimetastatic Effect Through Polydopamine-Encapsulated Core–Shell Upconversion Nanoparticles, Adv. Mater., 2019, 31, 1905825 CrossRef CAS PubMed.
- S. Wen, et al., Advances in highly doped upconversion nanoparticles, Nat. Commun., 2018, 9, 2415 CrossRef PubMed.
- S. H. Nam, et al., Long-Term Real-Time Tracking of Lanthanide Ion Doped Upconverting Nanoparticles in Living Cells, Angew. Chem., Int. Ed., 2011, 50, 6093–6097 CrossRef CAS PubMed.
- R. Kumar, et al., Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume, World Neurosurgery, 2018, 113, e345–e363 CrossRef PubMed.
- L. R. Hochberg, et al., Reach and grasp by people with tetraplegia using a neurally controlled robotic arm, Nature, 2012, 485, 372–375 CrossRef CAS PubMed.
- P. D. Ganzer, et al., Restoring the Sense of Touch Using a Sensorimotor Demultiplexing Neural Interface, Cell, 2020, 181, 763–773.e12 CrossRef CAS PubMed.
- N. Wenger, et al., Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury, Nat. Med., 2016, 22, 138–145 CrossRef CAS PubMed.
- F. B. Wagner, et al., Targeted neurotechnology restores walking in humans with spinal cord injury, Nature, 2018, 563, 65–71 CrossRef CAS PubMed.
- C. Lu, et al., Flexible and stretchable nanowire-coated fibers for optoelectronic probing of spinal cord circuits, Sci. Adv., 2017, 3, e1600955 CrossRef PubMed.
- S. S. Virani, et al., Heart Disease and Stroke Statistics—2020 Update: A Report From the American Heart Association, Circulation, 2020, 141, e139–e596 Search PubMed.
- E. Cingolani, J. I. Goldhaber and E. Marbán, Next-generation pacemakers: from small devices to biological pacemakers, Nat. Rev. Cardiol., 2018, 15, 139–150 CrossRef PubMed.
- R. Feiner, et al., A Stretchable and Flexible Cardiac Tissue–Electronics Hybrid Enabling Multiple Drug Release, Sensing, and Stimulation, Small, 2019, 15, 1805526 CrossRef PubMed.
- Q. Li, et al., Cyborg Organoids: Implantation of Nanoelectronics via Organogenesis for Tissue-Wide Electrophysiology, Nano Lett., 2019, 19, 5781–5789 CrossRef CAS PubMed.
- X.-P. Li, et al., Electrical stimulation of neonatal rat cardiomyocytes using conductive polydopamine-reduced graphene oxide-hybrid hydrogels for constructing cardiac microtissues, Colloids Surf., B, 2021, 205, 111844 CrossRef CAS PubMed.
- Y. S. Choi, et al., Fully implantable and bioresorbable cardiac pacemakers without leads or batteries, Nat. Biotechnol., 2021, 39, 1228–1238 CrossRef CAS PubMed.
- M. Dipalo, et al., Intracellular and Extracellular Recording of Spontaneous Action Potentials in Mammalian Neurons and Cardiac Cells with 3D Plasmonic Nanoelectrodes, Nano Lett., 2017, 17, 3932–3939 CrossRef CAS PubMed.
- H. Liu, et al., Heart-on-a-Chip Model with Integrated Extra- and Intracellular Bioelectronics for Monitoring Cardiac Electrophysiology under Acute Hypoxia, Nano Lett., 2020, 20, 2585–2593 CrossRef CAS PubMed.
- A. Kalmykov, et al., Organ-on-e-chip: Three-dimensional self-rolled biosensor array for electrical interrogations of human electrogenic spheroids, Sci. Adv., 2019, 5, eaax0729 CrossRef CAS PubMed.
- J. Abbott, et al., CMOS nanoelectrode array for all-electrical intracellular electrophysiological imaging, Nat. Nanotechnol., 2017, 12, 460–466 CrossRef CAS PubMed.
- A. Barbaglia, et al., Mirroring Action Potentials: Label-Free, Accurate, and Noninvasive Electrophysiological Recordings of Human-Derived Cardiomyocytes, Adv. Mater., 2021, 33, 2004234 CrossRef CAS PubMed.
- M. Y. Rotenberg, et al., Living myofibroblast–silicon composites for probing electrical coupling in cardiac systems, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 22531 CrossRef CAS PubMed.
- R. Parameswaran, et al., Optical stimulation of cardiac cells with a polymer-supported silicon nanowire matrix, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 413 CrossRef CAS PubMed.
- S. K. Rastogi, et al., Graphene Microelectrode Arrays for Electrical and Optical Measurements of Human Stem Cell-Derived Cardiomyocytes, Cell. Mol. Bioeng., 2018, 11, 407–418 CrossRef CAS PubMed.
- M. Dipalo, et al., Intracellular action potential recordings from cardiomyocytes by ultrafast pulsed laser irradiation of fuzzy graphene microelectrodes, Sci. Adv., 2021, 7, eabd5175 CrossRef CAS PubMed.
- G. Bruno, et al., All-Optical and Label-Free Stimulation of Action Potentials in Neurons and Cardiomyocytes by Plasmonic Porous Metamaterials, Adv. Sci., 2021, 8, 2100627 CrossRef PubMed.
- V. Nair, et al., Laser writing of nitrogen-doped silicon carbide for biological modulation, Sci. Adv., 2020, 6, eaaz2743 CrossRef CAS PubMed.
- K. Roshanbinfar, et al., Carbon nanotube doped pericardial matrix derived electroconductive biohybrid hydrogel for cardiac tissue engineering, Biomater. Sci., 2019, 7, 3906–3917 RSC.
- K. Kalishwaralal, et al., A novel biocompatible chitosan–Selenium nanoparticles (SeNPs) film with electrical conductivity for cardiac tissue engineering application, Mater. Sci. Eng., C, 2018, 92, 151–160 CrossRef CAS PubMed.
- R. Cavicchioli, et al., Scientists’ warning to humanity: microorganisms and climate change, Nat. Rev. Microbiol., 2019, 17, 569–586 CrossRef CAS PubMed.
- H. Zhang, et al., Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production, Nat. Nanotechnol., 2018, 13, 900–905 CrossRef CAS PubMed.
- X. Gao, et al., Structured silicon for revealing transient and integrated signal transductions in microbial systems, Sci. Adv., 2020, 6, eaay2760 CrossRef CAS PubMed.
- Y. Su, et al., Close-Packed Nanowire-Bacteria Hybrids for Efficient Solar-Driven CO2 Fixation, Joule, 2020, 4, 800–811 CrossRef CAS.
- K. K. Sakimoto, N. Kornienko and P. Yang, Cyborgian Material Design for Solar Fuel Production: The Emerging Photosynthetic Biohybrid Systems, Acc. Chem. Res., 2017, 50, 476–481 CrossRef CAS PubMed.
- S. Cestellos-Blanco, H. Zhang, J. M. Kim, Y. Shen and P. Yang, Photosynthetic semiconductor biohybrids for solar-driven biocatalysis, Nat. Catal., 2020, 3, 245–255 CrossRef CAS.
- J. Cervera, J. A. Manzanares, S. Mafe and M. Levin, Synchronization of Bioelectric Oscillations in Networks of Nonexcitable Cells: From Single-Cell to Multicellular States, J. Phys. Chem. B, 2019, 123, 3924–3934 CrossRef CAS PubMed.
- J. Selberg, M. Gomez and M. Rolandi, The Potential for Convergence between Synthetic Biology and Bioelectronics, Cell Syst., 2018, 7, 231–244 CrossRef CAS PubMed.
- N. Driscoll, et al., Two-Dimensional Ti3C2 MXene for High-Resolution Neural Interfaces, ACS Nano, 2018, 12, 10419–10429 CrossRef CAS PubMed.
- N. Driscoll, et al., MXene-infused bioelectronic interfaces for multiscale electrophysiology and stimulation, Sci. Transl. Med., 2021, 13, eabf8629 CrossRef CAS PubMed.
- N. M. Sakhrani and H. Padh, Organelle targeting: third level of drug targeting, Drug Des. Dev. Ther., 2013, 7, 585–599 CAS.
- J. F. Zimmerman, et al., Cellular uptake and dynamics of unlabeled freestanding silicon nanowires, Sci. Adv., 2016, 2, e1601039 CrossRef PubMed.
- J. Liu, et al., Syringe-injectable electronics, Nat. Nanotechnol., 2015, 10, 629–636 CrossRef CAS PubMed.
- J. Koo, et al., Wireless bioresorbable electronic system enables sustained nonpharmacological neuroregenerative therapy, Nat. Med., 2018, 24, 1830–1836 CrossRef CAS PubMed.
- Z. Chen, et al., Wireless Optogenetic Modulation of Cortical Neurons Enabled by Radioluminescent Nanoparticles, ACS Nano, 2021, 15, 5201–5208 CrossRef CAS PubMed.
- P. Q. Nguyen, et al., Wearable materials with embedded synthetic biology sensors for biomolecule detection, Nat. Biotechnol., 2021, 39, 1366–1374 CrossRef CAS PubMed.
- F. J. Rawson, et al., Fast, Ultrasensitive Detection of Reactive Oxygen Species Using a Carbon Nanotube Based-Electrocatalytic Intracellular Sensor, ACS Appl. Mater. Interfaces, 2015, 7, 23527–23537 CrossRef CAS PubMed.
- T. Tschirhart, et al., Electronic control of gene expression and cell behaviour in Escherichia coli through redox signalling, Nat. Commun., 2017, 8, 14030 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |