Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A second life for fruit and vegetable waste: a review on bioplastic films and coatings for potential food protection applications
Danila
Merino
 *a,
Ana Isabel
Quilez-Molina
a,
Giovanni
Perotto
*a,
Ana Isabel
Quilez-Molina
a,
Giovanni
Perotto
 a,
Andrea
Bassani
a,
Andrea
Bassani
 b,
Giorgia
Spigno
b and
Athanassia
Athanassiou
*a
b,
Giorgia
Spigno
b and
Athanassia
Athanassiou
*a
aSmart Materials, Istituto Italiano di Tecnologia, Via Morego, 30, Genoa, 16163, Italy. E-mail: danila.merino@iit.it; danila_m04@hotmail.com; athanassia.athanassiou@iit.it; Tel: +39 010 28961
bDepartment for Sustainable Food Process, Università Cattolica del Sacro Cuore, Via Emilia Parmense 84, Piacenza, 29122, Italy
First published on 28th May 2022
Abstract
In recent decades, significant progress has been made on the development of low environmental impact plastic materials, as alternatives to conventional plastics for food packaging. Research has focused on the engineering of renewable resources of animal or vegetable origin that are rich in polysaccharides and proteins, to produce green bioplastic materials for food packaging, with good mechanical and gas barrier properties. Furthermore, incorporating natural antimicrobials, antioxidants, and pH-sensitive substances in the new eco-friendly materials, smart and active green packaging can be developed. Recently, the preparation of bioplastics and biocomposites directly from the processing of agro-food residues via hydrolysis or digestion was proposed for the production of new added-value products that comply with zero waste and circular economy principles and are expected to impact the future of food packaging significantly. This review aims to revise the various fruit and vegetable agrowaste-based bioplastic and biocomposite systems developed so far, with potential applications in food protection and shelf life extension. The vegetal lignocellulosic and non-lignocellulosic agrowaste composition, processing methods, and properties of the developed biomaterials are addressed. The obtained biocomposites, rich in natural polymers, as cellulose, pectin, starch, zein, etc., can actively protect the packaged food against oxidation or microorganisms, as long as they preserve the raw materials’ phytochemicals in their composition. We focus on simple and easily scalable procedures that either involve green solvents or require low-energy, and lead to films for food packaging or suspensions intended to be applied as coatings directly on fruit or other foodstuff surfaces. All the previously mentioned aspects are extensively reviewed in this manuscript, mainly considering the literature reported during the last five years including the research works of the authors in the field.
1 Introduction
1.1 Current situation in food packaging: towards new biomaterials
Nowadays, food packaging is an indispensable part of the food industry. Food packaging is mainly used to establish a barrier between the food and the environment in order to provide mechanical protection and to reduce food contact with spoilage factors, such as microorganisms, oxygen, and UV-light, as well as to avoid losses of flavors and odors, to extend food shelf life and quality.1 Additionally, food packaging has several other functions, including marketing, information on the product's ingredients and expiry.2From the second half of the 20th century, petroleum-based polymers increased in popularity and ended up dominating the food packaging industry thanks to their excellent properties, versatility, and low price.3 Materials such as polyethylene (PE), polypropylene (PP), and polyethylene terephthalate (PET) are nowadays the most used in the packaging sector, despite their high contribution to the accumulation of plastic waste in the environment. These products originate from non-renewable resources and, most importantly, are non-biodegradable. Their uncontrolled accumulation has raised profound concern about their deleterious effects to the environment. In fact, when they end up in landfills or the oceans, it takes hundreds of years to be biodegraded.1,4 The alternative of their incineration for energy recovery has a severe environmental impact, including the generation of toxic airborne particles and greenhouse gas emissions.2 Although the recycling of such non-biodegradable polymers comes as a better alternative, the gradual chemical deterioration during that process limits the further application of recycled plastics in food packaging. Finally, many of the current plastic packaging items are made by combined polymers, making their recycling technologically challenging and non-economically sustainable. For example, within Europe, one of the best global scenarios regarding the actions against plastic pollution, 24.9% of plastic waste is disposed of in landfills, 42.6% is used for energy production and 32.5% is recycled.5 The immense contribution of plastic packaging products to plastic waste generation is mainly due to their very short lifetime, about 1 year in average, that forces a very quick turnover of materials with lack of economically viable recycling.6,7
In this scenario, more than 60 countries have issued prohibitions on the use of plastic products, such as single-use plastic bags, intending to stop the increasingly severe environmental pollution.8 These prohibitions are in line with the Directive (EU) 2019/904 of the European Parliament and of the European Council of 5 June 2019 on the reduction of the impact of certain plastic products on the environment.9 Furthermore, the European Commission guidelines on single-use plastic products, mainly connected to food protection and consumption, have recently been published in accordance with this Directive (May 31, 2021).10
The above-described situation highlights the immediate need to reduce the use of traditional plastics and a great necessity for new sustainable materials capable of replacing plastics in the food packaging industry and in other short-lived applications. These alternative materials can be considered sustainable only if they have positive implications simultaneously on the environment, economy, and society. Bioplastics that are made of renewable resources, preferably wastes, that can be recyclable and biodegradable at the end of their life-cycle fulfill this requirement,1 since they contribute to the reduction of plastic waste generation, of nonrenewable raw materials dependence, and of water and energy usage.2 The use of vegetable wastes as raw materials for bioplastics preparation has the additional potential to reduce greenhouse gas emissions, since plants uptake carbon dioxide from the atmosphere during their growth and convert it into biomass thanks to the photosynthesis.11 Furthermore, plant biomass offers immeasurable potential for bioplastics preparation through methods that include, but are not limited to, the extraction and isolation of polymers, the possibility to obtain monomers for further synthetic routes and the possibility to disassemble, mix and reassemble the entire biomass to develop blends and composites. Although this review focuses mainly to the latter processing methods, all the above methods have great potentiality and lead to a wide variety of materials and the possibility to tune their properties for specific applications. Thus, they can be the ideal candidates for replacing traditional polymeric packaging in all its forms, i.e. rigid, flexible and coatings. More details about the chemistry of the materials prepared from vegetable waste are introduced in the following section.
The bioplastic packaging needs to maintain its properties unaltered from the moment of the introduction of the food within it, till the moment of its removal for consumption. Successively, it is highly required that its biodegradation starts as soon as it is disposed of, and happens in short time.12 The biodegradation can be triggered by external factors such as humidity, temperature, changes in pH, and UV-light exposure, in the presence of microorganisms or enzymes. Research efforts are being made to improve the end of life compostability of the few already commercially available sustainable biopolymers, such as polylactic acid (PLA), polybutylene succinate (PBS) or polyhydroxyalkanoates (PHAs). These materials are produced from plant biomass through various sequential processes that will be mentioned further down, are rapidly biodegradable in specific conditions (industrial composting sites, with temperatures around 60 °C) and slowly biodegrade over the years when they end up dispersed in the environment, although PHAs are marine-degradable.13 New policies should be implemented to ensure their correct collection and composting, while governments worldwide need to support the increase, expansion and improvement of the industrial compost sites.
On the other hand, biocomposites produced by the direct transformation and reorganization of plant biomass from agrowastes are more easily compostable at environmental conditions, and can possibly be managed together with the organic waste produced at homes. Furthermore, antimicrobial and antioxidant molecules that are present in the biomass of the agrowastes can provide functional properties to such biocomposites, that can help extend food shelf life and quality when used for food packaging.
Finally, it is worth mentioning that sustainable solutions to conventional non-biodegradable plastics could also come from microalgae exploitation. In fact, microalgae can be used to bio-valorize food wastes and food processing waste waters to produce proteins, lipids and carbohydrates, but can also be transformed through reorganization upon solvent processing into composite bioplastic films.14–16
In this manuscript, we review the bioplastics and biocomposites obtained from the processing of biomass from industrial vegetable agro-food by-products, consistent with zero waste and circular economy models, as pictured in Fig. 1, focusing mainly on biocomposites obtained from the biomass deconstruction, separation and reconstruction under a different form.
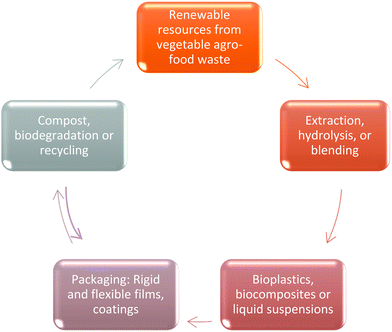 | ||
| Fig. 1 Life cycle of an ideal system for packaging based on the circular economy of agro-food waste. | ||
1.2 Agro-food waste components and their potentiality for new biomaterials in food packaging
The life cycle of a typical food item can be summarized in four phases: (1) production, (2) processing, (3) distribution, and (4) consumption. Food loss can occur at every step of the supply chain and also occurs when food is removed from the chain. According to the Food and Agriculture Organization of the United Nations (FAO), 13.8% of all food produced worldwide is wasted between the harvest on-farm, transport, storage, processing, and wholesale stages.17 For the scope of this manuscript, as food waste we adopt the definition given by the FUSIONS EU project,18 that is “any fraction of food and inedible parts of food removed from the food supply chain to be recovered or disposed of”.19The generation of edible food waste should be reduced by taking precautionary measures at each level from its production to its consumption. Since the growing world population demands more food, minimizing the edible waste could be a viable solution, reducing intensive agriculture. Regarding the inedible discards, there is an extreme need for adequate practices and policies for their management and reuse.20 The inedible parts of agro-food production could be the ideal biomass source for the production of the new sustainable bioplastics. Indeed, agricultural processing by-products such as husks, peels, straw, seeds, and pomace, constitute a reservoir of carbohydrates, proteins, lipids, and phytochemicals,21,22 all invaluable components for new biomaterials. Next, we present analytically all these components of the plants and where they can be found within the plant.
1.3 The plant cell wall
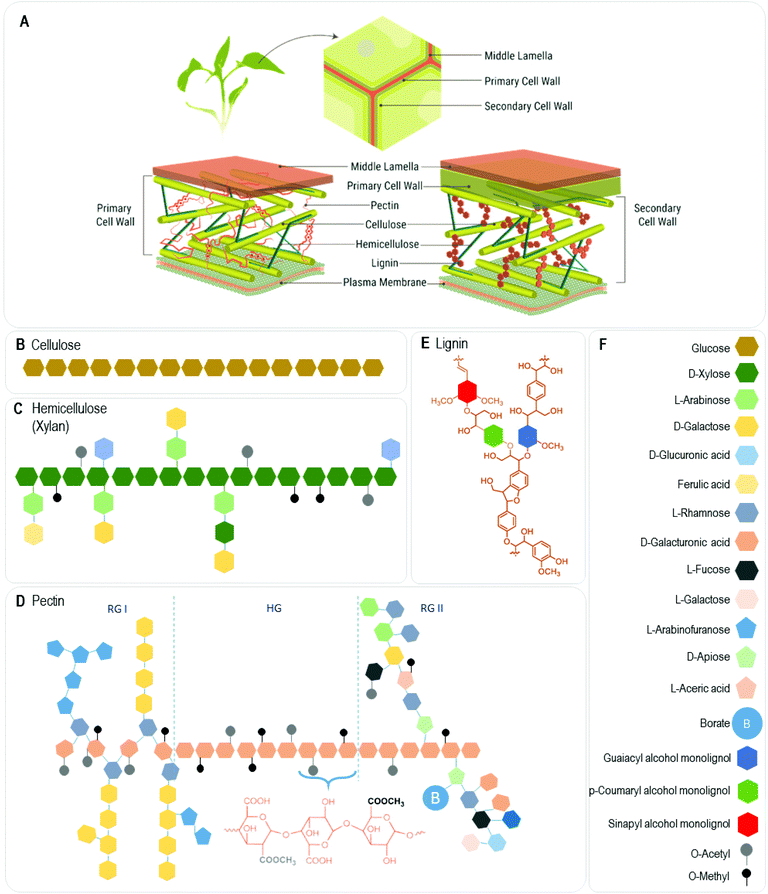
Most of the natural polymers are found in the plant cell walls, playing a fundamental role: they are the key elements that control the wall's resistance, stiffness, and flexibility. Precisely, the cell wall consists of a network of cellulose microfibrils embedded in a highly hydrated amorphous matrix of other complex and heterogeneous non-cellulosic polysaccharides, hemicelluloses, and pectins. As shown in Fig. 2A, there are two types of vegetable cell walls: primary and secondary. The primary walls are typical of growing cells, which require wall flexibility and are generally poorly specialized. Secondary walls form after growth has ceased, they are thick, inflexible, and highly defined in structure and composition.23 Between the walls of neighboring cells, is located the middle lamella, which contains pectin and proteins.24 In addition, vascular plants present a hydrophobic layer called cuticle at their plant-environment interface. It consists of a cutin polymer film with embedded intracuticular waxes, whose main function is to prevent uncontrolled water loss. Wax constituents include long chain hydrocarbons, such as alkanes, primary alcohols, aldehydes, secondary alcohols, ketones, esters, and may also contain triterpenoids, flavonoids, and/or phenolic lipids.25Although both types of walls are based on cellulose microfibrils, the components of the amorphous phase vary widely throughout the plant kingdom. Furthermore, cell walls are dynamic entities, which can change their structure and composition during plant development and in response to abiotic and biotic stress.26 The primary wall is made up of polysaccharides such as cellulose (Fig. 2B), hemicelluloses (Fig. 2C), pectins (Fig. 2D), a small part of structural proteins, and a significant part of water (between 75 and 80%). Hemicelluloses bind cellulose, and together with pectin, they help bind its fibers and prevent the structure from collapsing. The secondary cell wall consists of cellulose, hemicelluloses, and in some cases, lignin (Fig. 2E) with a minimal amount of water (5%).27 Raw materials that include a high content of these three components are called lignocellulosic biomass and include noncommercial material traditionally left on site after harvesting of crops such as trunk, fiber, sugar cane bagasse, plant stalks, vines, hulls, leaves, vegetable matter, sawdust, mill residues, low-quality wood, tops, and limbs.28
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 glucose units linked by β-1,4 bonds in which each glucose residue has a rotation of 180° with respect to the next, resulting in a polymer whose repeating unit is the disaccharide cellobiose.30 These units form long unbranched chains that interact through hydrogen bonds, creating structures of high crystallinity called fibrils, which are then grouped to form microfibrils and that in the presence of hemicellulose and lignin are assembled into the well-known natural fibers. In this way, cellulose fibers have a hierarchical structure with areas of high crystallinity (55–75%) and high chemical and mechanical resistance, connected by amorphous areas, more susceptible to chemical and enzymatic attack.27
000 glucose units linked by β-1,4 bonds in which each glucose residue has a rotation of 180° with respect to the next, resulting in a polymer whose repeating unit is the disaccharide cellobiose.30 These units form long unbranched chains that interact through hydrogen bonds, creating structures of high crystallinity called fibrils, which are then grouped to form microfibrils and that in the presence of hemicellulose and lignin are assembled into the well-known natural fibers. In this way, cellulose fibers have a hierarchical structure with areas of high crystallinity (55–75%) and high chemical and mechanical resistance, connected by amorphous areas, more susceptible to chemical and enzymatic attack.27
Pectins are transported to the wall in highly methyl esterified forms and selectively de-esterify to control matrix stiffness by forming gels in the presence of calcium or boron ions that chelate with charged carboxylic groups forming egg box structures.29,32 Therefore, they show gelling capability, which was explored to prepare hydrogels, coatings, and cross-linked films.33–36 Besides, they were also capable of being processed by melt extrusion in the presence of a plasticizer, showing a thermoplastic characteristic.37
1.4 Other plant intracellular biopolymers
Vegetable derived proteins are often found as a byproduct of food processing, either because they have poor nutritional value or because they are the result of refining of legumes. These proteins, often with the addition of some plasticizers, have been proposed for the production of bioplastics. For example, soy proteins (SP) and wheat gluten (WG) are among the most studied proteins for the production of bioplastics because of their wide availability and low price. SP result from the processing of soy beans after the extraction of the oil. Their main constituents are non-polar aminoacids such as glycine, proline, alanine, valine, acidic amino acids such as aspartic and glutamic acid, and finally basic amino acids such as lysine and arginine.48 Despite their good nutritional value, not all the soy proteins produced are consumed, and a significant portion ends up being discarded. To be processed into bioplastics, soy proteins benefit from the addition of plasticizers such as glycerol or water.48 WG is a relatively cheap protein obtained from wheat processing to obtain starch. Commercially available WG has high protein content (>75%, the rest being starch and lipids) that is due to mostly two proteins: gliadins (that are low-molecular-weight) and glutenins (high-molecular-weight).49,50 Both proteins are prolamine proteins and are storage proteins whose function is to help the germination of the seeds. When combined via S–S bridges, gliadins and glutenins create a strong network with good viscoelastic properties.
Other examples of less known proteins obtained from biomass are zein and kafirin. Zein is a prolamine protein with poor nutritional value and so has limited uses as an ingredient for foodstuff. It is obtained from the processing of corn, in which it has a function as a storage protein providing nitrogen for growing kernels during corn germination and it is composed of glutamine, leucine, proline, and alanine aminoacids.51 Zein proteins are alcohol soluble and form films upon casting, but, similarly to other proteins, their films are usually brittle and fragile, and a plasticizer is needed to provide flexibility.52,53 Similarly, Kafirin is the family of storage proteins in sorghum and are classified as prolamines. Their molecular weight is between 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 961 Da and 27
961 Da and 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da and they are comprised of mostly non-polar amino acids such as proline.54,55 Kafirins are known to be the most hydrophobic prolamines.56 Kafirins are relatively less explored for both food and non-food applications because of the low sorghum production. Despite being the fourth cereal produced worldwide, sorghum represents only 3% of the total cereal production.56 Because of the high hydrophobicity and poor digestibility, kafirins are better suited for non-food applications and are studied for applications ranging from adhesives to bioplastics to biomedical devices.57
000 Da and they are comprised of mostly non-polar amino acids such as proline.54,55 Kafirins are known to be the most hydrophobic prolamines.56 Kafirins are relatively less explored for both food and non-food applications because of the low sorghum production. Despite being the fourth cereal produced worldwide, sorghum represents only 3% of the total cereal production.56 Because of the high hydrophobicity and poor digestibility, kafirins are better suited for non-food applications and are studied for applications ranging from adhesives to bioplastics to biomedical devices.57
1.5 Plant intracellular functional phytochemical compounds
Phytochemicals are plant-derived chemicals. Some of them, called nutraceuticals, can provide health benefits for consumers either in food or in isolation.58 These benefits translate into better nutrition, treatment and prevention of diseases, and delayed aging. They could also reach consumers through packaging, edible or not, based on fruit and vegetable residues.In addition, when phytochemicals are incorporated into the food packaging, they can actively protect food, extending its shelf life and giving information about its quality.59–61 For instance, phenolic compounds provide antioxidant activity, which in some cases can be used to extend the shelf life of food. Besides, some of them also have antimicrobial action and, together with essential oils (EOs), constitute the most commonly used type of phytochemicals for the development of active and sustainable food packaging.62 EOs are mixtures of 20 to 60 different secondary metabolites. Among those, 2 or 3 components predominate, generally terpenes and terpenoids, while other aromatic and aliphatic compounds such as aldehydes and phenols are found in smaller quantities. They are characterized by an intense odor and flavor that vary depending on their constituents and they play a vital role in the plant defense due to their antibacterial, antifungal, and antiviral action.63 These properties make them good candidates to replace chemical preservatives and can be included as active components in food packaging materials.62 In particular, compounds such as linalool, thymol, carvone, carvacrol, citral, and limonene are regulated by the European Commission as flavorings for food products and have been categorized as “generally recognized as safe (GRAS)” ingredients by the Food and Drug Administration.64
It is worth mentioning that the processing of the vegetable wastes can influence the chemical and functional stability of their phytochemicals, so it is a parameter that must be considered.63 Detailed information on these aspects can be found in the literature.65
2 Brief review of the methods of transformation of agrowastes into bioplastics
The most widespread and traditional way to obtain bioplastics from agro-food waste is to extract and purify natural polymers, monomers, or other compounds through quite complex processes briefly mentioned below.Bioplastic films, intended for various applications among which also food packaging, are then obtained mainly through techniques such as blending, solution casting,66–68 chemical synthesis starting from the monomers,69,70 to obtain biopolymers that can be processed by thermomechanical processes like extrusion.71–73 Concerning bioplastic coatings, liquid solutions containing biopolymers and active phytochemicals can be applied onto substrates, i.e. food, either by dipping or casting or spraying techniques. Dipping is suitable when the substrate has an irregular surface, casting when the coating solution is more viscous, while spraying is more appropriate when only one side of the product is supposed to be coated.74,75
Many of the extraction processes of the polymers present in the vegetable cell wall are done with acidic or alkaline solutions or both. For instance, cellulose nanocrystals (CNC) or microcrystalline cellulose (MCC) can be recovered from agro-food residues by a combination of acidic (H2SO4) and alkaline (NaOH) hydrolysis treatments.76 In this process, usually carried out on lignocellulosic biomass, hemicelluloses and lignin can be obtained as byproducts.77,78 Furthermore, pectin polymer found in non-lignocellulosic biomass, is usually obtained by extraction from citrus and apple peels in hot water or hot diluted acid solutions, followed by an isolation step through alcohol precipitation.79,80
Even though these extraction methods are well established, they present some challenges, like the disposal of the acid compounds produced after the acid treatment step. Therefore, different innovative extraction processes are under study and investigation. One of these is the autohydrolysis process, which allows obtaining, for instance, MCC with similar results to acid hydrolysis, taking advantage of high pressure and temperature, avoiding the use of sulfuric or other strong acids.81,82
Intracellular polymers, such as proteins and starch, can also be obtained through acid-alkaline treatments but also through solvent-assisted extractions. For instance, proteins can be extracted by dissolution in an alkaline environment and subsequent precipitation at their isoelectric point with the addition of concentrated solutions of mineral acids like hydrochloric acid and sulfuric acid.83 Alternatively, some proteins are obtained by solvent-assisted extractions. For example, α-zein proteins and a small amount of β-zein proteins can be extracted with 80–85% (v/v) ethanol or 2-propanol, while α, β, γ, and δ-zein proteins are extracted by combining an alcohol with a reducing agent that contributes to the disulfide bonds break and release of zein proteins.45 Similarly, kafirins are usually extracted with ethanol and a reducing agent such as sodium metabisulfite, although this method is usually preceded by an enzymatic pretreatment.84
Starch, another important intracellular polymer, is mainly recovered using wet extraction processes, where different extraction liquids can be utilized to dilute starch in the liquid phase. Sometimes sodium sulfate solution is used to promote the separation.85 Besides, other innovative methods like ultrasonically-enhanced wet extraction or microwave radiation have been reported for starch recovery.86
The isolation through extraction from agro-food wastes of specific compounds, like fatty, organic, or amino acids, or the fermentation of the biomass for obtaining specific monomers is also interesting for the production of new biopolymers. Indeed, these compounds can be the base for biopolymer synthesis, like polylactic acic (PLA) and polyesteramides (PEAs). PEAs are innovative synthetic bio-based and biodegradable polymers, not as well-known as PLA or PHAs. PEAs constitute a promising family of biodegradable materials since they combine a degradable character, afforded by hydrolysable ester groups in the backbone, with relatively good thermal and mechanical properties due to the strong intermolecular hydrogen bonding interactions between their amide groups.87
As mentioned before, plant wastes often contain residual amounts of different bioactive compounds (e.g., glucosinolates, phenolic acids, and flavonoids) characterized by remarkable health-promoting properties with strong immunomodulatory, antimicrobial, antioxidant, and anti-inflammatory actions.88 These bioactive compounds need to be recovered from the waste source through feasible and efficient extraction processes. The most common and easily implementable extraction process is conventional solid–liquid extraction. The choice of the solvents is crucial to maximize the extraction of the target compounds while minimizing co-extraction of undesired components, together with the proper set of the operating conditions of the process (e.g., temperature, time, solid–liquid ratio, etc.) depending on the target bioactive and the specific matrix.89 The main disadvantage of this kind of process is the requirement of expensive and potentially hazardous organic solvents. Therefore, different, potentially greener extraction techniques (e.g., microwave-and ultrasounds-assisted extraction, deep eutectic solvents, etc.) are under investigation.90,91 The release of bioactive compounds from vegetables can be enhanced using electric fields such as pulsed electric and moderate electric fields92 or using enzyme preparations that are environmentally friendly technologies.93 However, the main limitation for the application of enzymes in industrial extraction processes is their relatively high cost.93,94
Apart from the extraction of isolated compounds from plant biomass and their transformation in bioplastics, the latter can also be obtained by the direct processing of the entire agrowaste biomass either by biotechnological or by chemical routes. Regarding, biotechnological transformation, biopolymers can be directly produced through microbial fermentation starting from plants and plant wastes, with most known examples the polyhydroxyalkanoates (PHAs) and the bacterial cellulose (BC). For example, Vega-Castro et al. obtained PHA from pineapple peel waste using Ralsthonia eutropha.95 Before the fermentation process, pineapple peels were hydrolyzed using sulfuric acid (2% v/v). Then, fermentation was conducted inoculating the specific culture. Other authors have also used waste streams as carbon sources for bacterial PHA production, including whey,96 spent coffee grounds,97 grass biomass,98 and fruit waste.99 Similarly, BC is produced by some bacteria, being the ones from the genus Komagataeibacter the most used for research and food applications, since they produce BC with high purity and yield.100 The main difference among these two biopolymers is that BC is synthetized extracellularly, while PHAs are produced intracellularly in the form of granules. Details on the methods of synthesis and properties of bio-based polyesters, polyesteramides and BC are not part of the scope of this manuscript and for more information about them the reader can refer to other recent publications.87,100–102
Regarding chemical transformation, the entire plant biomass from agrowastes, when treated with different acids, such as hydrochloric,12 formic103 and acetic,104 but also with alkali solutions such as ammonium hydroxide,105 results in deconstruction of its plant cell structure and hydrolysis of its components, and eventually, in solutions or dispersions of partially hydrolyzed natural polymers and phytochemicals. The resulting solutions and dispersions, after casting or spraying and solvent evaporation, can reassemble into stand-alone, compact, self-assembled composites, but sometimes, the hydrolyzed vegetable biomass needs to be combined with other additives or natural polymers or biopolymers to obtain consistent materials suitable for food packaging. For instance, when the amount of starch present in the vegetable waste is high enough, the materials obtained are fragile and need a plasticizer.106 Besides, when the fiber content is high, the biocomposites can be blended with other amorphous polymers acting as binding agents.66
The sections 3 and 4 of this review that follow, present protective films and coatings respectively, which contain partially or totally biomass from agrowastes, and are developed for food protection through combinations of the above described processes.
3 Films for food packaging from agro-food waste
In this section, we present examples of agrowaste biomasses transformed into biocomposite films, either as additives or through chemical processing, intended for food packaging. The mechanical, barrier, antioxidant, and antimicrobial properties of the obtained films are discussed with respect to their potential in food protection. A summary of the main results discussed below, is shown in Table 1.| Agro waste | Other components | Solvent/method | Mechanical properties | Moisture content (%) | Antioxidant activity | Barrier properties | Antimicrobial activity | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TS (MPa) | Eb (%) | YM (MPa) | TPC | DPPH | ABTS | FRAP | WVP (×10−10 g per P.a.s.m) | O2P (×10−9 cm3 per m.d.Pa) | Inhibition area (mm2) | |||||
| Abbreviations: TS, Tensile strength; Eb, Elongation at break; YM, Young's modulus; TPC, total polyphenol content; WVP, water vapor permeability; O2P, oxygen permeability; GAE, gallic acid equivalents; RSA, radical scavenging activity and AAE, ascorbic acid equivalents. | ||||||||||||||
| Blueberry pomace fiber | Gelatin waste and glycerol | Distilled water/casting | 1.51 ± 0.06 | 86.33 ± 5.13 | 735.83 ± 3.43 | 21.82 ± 1.19 | Not assessed | Not assessed | Not assessed | Not assessed | 2.4 ± 0.11 | Not assessed | Not assessed | de Moraes Crizel et al., 2016112 |
| Grape skin pomace | Chitosan and glycerol | Acetic acid aqueous solution/casting | Not assessed | Not assessed | Not assessed | 17.05 ± 0.46 | ∼2 mg GAE per g film | Not assessed | Not assessed | 5 mg AAE per g film | 9.3 ± 0.23 | ∼1.2 | Not assessed | Kurek et al., 2019132 |
| Olive pomace flour | Chitosan and glycerol | Acetic acid aqueous solution/casting | 2.89 ± 0.06 | 13.31 ± 0.77 | 12.39 ± 0.39 | 16.47 ± 0.87 | Not assessed | 2.48 ± 0.2 μM Trolox per g film | Not assessed | 7.84 ± 0.2 μmol FeSO4·7H2O | 6.19 ± 0.38 | Not assessed | Not assessed | de Moraes Crizel et al., 2018133 |
| Olive pomace microparticles | Chitosan and glycerol | Acetic acid aqueous solution/casting | 6.51 ± 0.02 | 20.93 ± 0.69 | 16.28 ± 0.83 | 15.04 ± 0.28 | Not assessed | 2.73 ± 0.18 μM Trolox per g film | Not assessed | 11.28 ± 0.23 μmol FeSO4·7H2O | 3.02 ± 0.55 | Not assessed | Not assessed | de Moraes Crizel et al., 2018133 |
| Avocado peel and seed | Pectin and polyglycerol-3 | Acetic acid aqueous solution/casting | 17.9 ± 2.7 | 16.8 ± 6.1 | 341.7 ± 46.3 | Not assessed | Not assessed | 3.4 ± 0.2 μmof Trolox per g dried sample | Not assessed | Not assessed | 18.0 ± 0.6 | 22.5 ± 1.5 | Not assessed | Merino, Bertolacci, et al., 202166 |
| Potato peel | Polyglycerol-3 | Acetic acid aqueous solution/casting | 12.7 ± 1.5 | 18.4 ± 4.3 | 432.1 ± 86.4 | 16.4 ± 3.2 | Not assessed | Not assessed | Not assessed | Not assessed | 3.1 ± 0.3 | 5.5 × 103 | Not assessed | Merino, Paul, et al., 2021106 |
| Coffee extract and husk fibers | Glycerol and corn starch | Not applicable/melt blending and compression molding | 11.2 ± 1.2 | 12.9 ± 2.6 | 386 ± 25 | Not assessed | Not assessed | 79.1 ± 6.6 mg film per g DPPH | Not assessed | Not assessed | 38.8 ± 4.72 | 2.07 ± 0.17 | Not assessed | Collazo-Bigliardi et al., 2019110 |
| Rice extract and husk fibers | Glycerol and corn starch | Not applicable/melt blending and compression molding | 12.1 ± 0.8 | 16.4 ± 3.6 | 541 ± 16 | Not assessed | Not assessed | 114.0 ± 0.7 mg film per g DPPH | Not assessed | Not assessed | 33.8 ± 1.11 | 1.81 ± 0.77 | Not assessed | Collazo-Bigliardi et al., 2019110 |
| Wheat starch and Oat CNCs | Glycerol | Distilled water/casting | 0.7 ± 0.33 | 32.8 ± 0.81 | 56.58 ± 9.06 | 16.08 ± 1.5 | Not assessed | Not assessed | Not assessed | Not assessed | 0.28 ± 0.01 | Not assessed | Not assessed | Bruni et al., 2020116 |
| Phosphorylated wheat starch and Oat CNCs | Glycerol | Distilled water/casting | 3.52 ± 0.14 | 50.20 ± 2.44 | 24.3 ± 1.55 | 21.26 ± 0.71 | Not assessed | Not assessed | Not assessed | Not assessed | 0.13 ± 0.01 | Not assessed | Not assessed | Bruni et al., 2020116 |
| Sugar palm starch and NCC | Glycerol and sorbitol | Distilled water/casting | 11.47 ± 0.1 | 24.42 ± 0.2 | 178.88 ± 0.2 | 12.70 ± 0.38 | Not assessed | Not assessed | Not assessed | Not assessed | 8.17 ± 0.05 | Not assessed | Not assessed | Ilyas et al., 2018117 |
| Banana flour pomace | Glycerol, and KMS | Distilled water/casting | 1.65 ± 0.03 | 21.26 ± 3.28 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | 0.16 ± 0.06 | Not assessed | Not assessed | Jirukkakul, 2016140 |
| Banana puree | Glycerol and KMS | Distilled water/casting | 1.45 ± 0.05 | 17.73 ± 1.97 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | 0.65 ± 0.035 | Not assessed | Not assessed | Jirukkakul, 2016140 |
| Carrot puree | — | Hydrochloric acid in distilled water/casting | 38 ± 5 | ∼6 ± 1 | 1300 ± 200 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | ∼90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 5000 000 ± 5000 |
Not assessed | Perotto et al., 201812 |
| Papaya puree | De-fated soy protein, soy protein starch, glycerol and gelatin | Distilled water/casting | 68.0 ± 0.06 | 22.38 ± 0.06 | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | 15.4 ± 1.36 | 7.84 ± 0.07 | Not assessed | Tulamandi et al., 2016123 |
| Lime peel pectin | Glycerol | Ethanol and distilled water/casting | 14 ± 1.82 | 4.6 ± 1.17 | 441.08 ± 43.17 | 21.92 ± 3.25 | 75.15 ± 2.67 (mg GAE per g film) | 40.22 ± 0.44 μM Trolox per g film | 532.04 ± 472 μM Trolox per g film | Not assessed | 2.12 ± 0.04 | 7.84 ± 0.07 | Not assessed | Rodsamran and Sothornvit, 2019131 |
| Pomelo peel | Glycerol, sodium alginate and tea polyphenols | Distilled water/casting | 61.43 ± 0.15 | 10.74 ± 0.15 | Not assessed | Not assessed | 26.63 ± 0.25 μM Trolox per g film | 51.72 ± 0.02% RSA | Not assessed | Not assessed | 2.02 ± 0.02 | Not assessed | E. coli: 13.65 ± 0.07 mm, S. aureus: 14.71 ± 0.17 mm | Wu et al., 2019127 |
| Mango peel | Glycerol | Citrate buffer solution (pH = 4.5)/casting | Not assessed | Not assessed | Not assessed | 3.11 ± 0.84 | 14.67 ± 3.57 GAE per g film | 51.90 ± 3.57% RSA | Not assessed | Not assessed | 0.88 ± 0.02 | Not assessed | Not assessed | Torres-León et al., 2018125 |
| Mango peel and mango seed extract | Glycerol | Citrate buffer solution (pH = 4.5)/casting | Not assessed | Not assessed | Not assessed | 4.14 ± 1.3 | 44.42 ± 4.82 mg GAE per g film | 63.55 ± 3.62% RSA | Not assessed | Not assessed | 1 ± 0 | Not assessed | Not assessed | Torres-León et al., 2018125 |
Agrowastes discussed in this section include both lignocellulosic biomass such as husks, shells, stems and straw, and non lignocellulosic biomass like pomace, seeds, fruit puree and peels. There are also considered formulations prepared with the addition of extracted phytochemicals such as EOs, waxes, and polyphenols.
3.1 Films with suitable mechanical and barrier properties
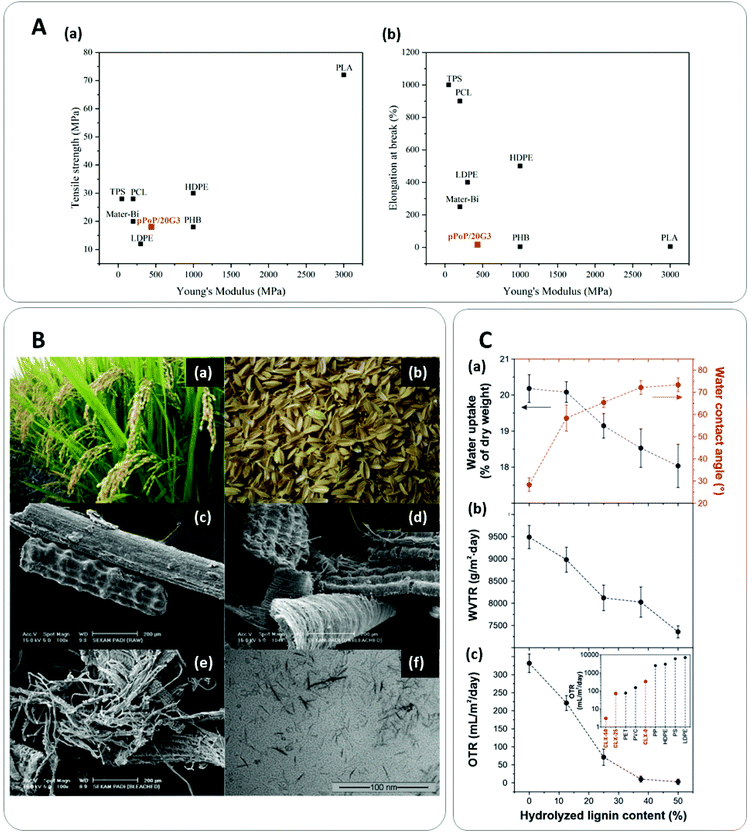
Since the biopolymers present in plant biomass are commonly hydrophilic, with shorter chain lengths and have more complex structures than their petroleum-based counterparts, the mechanical (Fig. 3A) and water vapor barrier properties of the obtained films are usually inferior.106 Therefore, many approaches have been developed so far to overcome these drawbacks and offer efficient, apart from sustainable, films suitable for food transportation and protection.A first approach, is the addition of lignocellulosic biomass to natural polymers. Lignocellulosic biomass in addition to being low-cost, and to show high strength, low density, biodegradability, availability, and renewability, can also effectively improve mechanical and barrier properties of natural polymers when added at relatively small percentages.107,108 For example, the incorporation of lignocellulosic biomass has been demonstrated to improve the mechanical behavior of some starch-based composites.109 Rice and coffee husks were added separately at 5 wt% in a corn starch matrix plasticized with 30 wt% of glycerol. The composite materials were prepared by melt blending followed by compression molding, and mechanical characterization demonstrated a 100% improvement on the tensile strength (TS) with respect to the control starch substrate (5.2 ± 1.6 MPa).110 Similarly, lignocellulosic fibers from corn stalks, husks, and cobs were added to a starch water solution at concentrations ranging from 5 to 20 wt%. The films obtained by casting showed increases in TS and Young's modulus (YM) in the case of 5 and 10 wt% fiber content.109
However, lignocellulosic fiber-reinforced composites often present different manufacturing defects associated with the poor dispersion and big size of the fibers, as well as the lack of compatibility between them and the matrix, which usually leads to fibers’ agglomeration and negatively affects the overall mechanical properties.111 For example, de Moraes Crizel et al., has prepared gelatin-blueberry fiber composite films by casting. The incorporation of 15 wt% of blueberry fibers into gelatin, reduced the TS of the matrix by 40% due to the fibers’ agglomeration, observed by scanning electron microscopy at the cross-section surface.112 Nevertheless, the chemical modification either of the matrix or of the additive biomass, as well as the use of compatibilizers, were able to improve the cohesion between the components. In particular, the butyl methacrylate acid has been employed to enhance the compatibility between coconut shells and regenerated cellulose biocomposites prepared by casting. The regenerated cellulose with 3 wt% of butyl methacrylate acid-treated and untreated coconut shells exhibited a TS of 62 and 55 MPa, respectively. Above this concentration, the strength was reduced due to low-dispersion and the agglomeration of coconut shell fibers.113
Another strategy to avoid fillers agglomeration and composites’ defects is to use purified cellulose nanocrystals (CNCs) at even lower concentrations. CNCs have received growing interest as polymers’ reinforcing agents in recent years, since their uniform distribution into a polymeric matrix improves its mechanical performance and barrier properties.114 In the work of Kargarzadeh et al.,115 the authors prepared composites of cassava starch with untreated rice husk fibers, bleached rice husk fibers, and rice husk-derived CNCs (Fig. 3B) by casting. Results showed that untreated fibers weakened the TS, bleached fibers improved it by 12%, while 6 wt% of CNCs produced an increase of 52% in the TS.115 The authors attributed these results to the different dimensions of the fibers and the lack of cohesion between the untreated fibers and the matrix. Bruni et al. have reported the preparation of biocomposites, by adding CNCs derived from different plant sources (rice, oat, and eucalyptus) into a phosphorylated wheat starch matrix, by the method of solution casting.116 Results indicated that the incorporation of the CNCs resulted in an improvement of the water-resistance and mechanical properties of the materials.116 In the same way, Ilyas et al. have prepared and analyzed the mechanical properties of plasticized sugar palm starch-sugar palm CNCs composites obtained by casting. They found an enhancement in the thermoplastic starch films’ mechanical resistance showing the highest TS at 0.5 wt% of CNCs, the double of the sugar palm starch films.117
Besides their application as reinforcement agents, CNCs have demonstrated to improve the barrier properties of biocomposites when they are well-dispersed in the polymer matrix.114 This happens because the incorporation of CNCs provides reduced gas diffusion by increasing the tortuosity path through the materials.114 For example, in over-ripe papaya puree films obtained by casting, the water vapor diffusion was reduced by 50% with the incorporation of 0.2 wt% of cellulose nanofibers (CNFs), while an improvement in the TS of the neat puree film was also observed.118 Similarly, the water vapor permeability (WVP) of sugar palm starch films obtained by casting decreased up to 18% when 1 wt% of CNCs was loaded.117
Alternatively, the addition of lignin as filler in composites is also being considered as strategy for mechanical and barrier properties improvement. For instance, soy protein isolate films with 30–40% of sulfonated lignin prepared by melt blending followed by compression molding, demonstrated the simultaneous enhancement of the TS and YM of the pure soy protein films together with a decrease in the material's water absorption.119 Interestingly, Tedeschi et al. combined different proportions of cellulose, commercial Kraft lignin, and xylan to fabricate multifunctional lignin-based bioplastics by casting method after treating materials in different concentrations under acidic media. Results showed that lignin increased the stiffness and the oxygen and water transmission barrier of the wood-like biocomposites (Fig. 3C), exhibiting barrier and mechanical properties comparable to petroleum-based and commercial polymers.67
Another strategy used for improving plant-based films’ barrier properties includes the addition of small functional phytochemical substances, such as lipophilic EOs, into a biopolymer matrix. It has been demonstrated that they enhance water resistance and provide films with antimicrobial activity.120 For example, sunflower seed oil substantially affected the water-resistance properties when incorporated into mung bean starch-guar gum films prepared by casting.121 The WVP of this film with 2 wt% of sunflower oil showed a decrease of 30% on the WVP with respect to the film without oil. Similarly, improved water resistance and moisture barrier properties were reported when 50 wt% of apricot kernel EO was incorporated into chitosan films obtained by casting.122 The mixture between the hydrophilic carbohydrates with the hydrophobic EOs creates an emulsion system. The emulsion stability and dispersion of the oil droplets have an essential effect on the material's properties. The average size of oil droplets tends to grow due to the coalescence effect limiting the material's efficiency against water vapor and pathogens growth prevention.120,123 In the work of Li et al., the octenylsuccination of sweet potato starch enhanced the dispersion of the oregano EO (1.5 wt%), reducing in 60% the water vapor permeability of the casted biocomposites films respect to the non-grafted sample with the same content of oregano EO.120 Interestingly, citrus peels, which are rich in cuticular waxes, EOs, and terpenes, provide peel waste-based films with high moisture barrier properties.124 For example, films obtained by casting method based on mango peel presented a WVP of 0.88 × 10−10 g m−1 s−1 Pa−1, which is inferior to the permeability observed for materials prepared from other vegetable wastes,125 but still superior to the one of PE (5.5 × 10−13 g m−1 s−1 Pa−1),126 for example.
Another strategy used to improve polysaccharide-based biocomposite films’ barrier properties is to combine them with proteins to form blends or bilayers.75 Proteins show significate advantages over the polysaccharides discussed in the previous paragraphs, associated with their structure, and in particular with the wide variety of amino acids (the monomers of the proteins) that comprises them. Proteins rich in non-polar and hydrophobic amino acids (e.g. glycine, alanine, proline, valine) such as zein and kerfirin are significantly more water resistant than the polysaccharide biopolymers, while proteins that can inherently crosslink, like wheat gluten proteins, could create strong and elastomeric materials. However, these vegetable proteins are produced in significantly smaller volumes compared to the lignocellulosic components or to the proteins from other sectors such as collagen or keratin. This difference in available volumes of raw materials, makes it difficult to realistically substitute a significant percentage of plastic with protein-derived materials from vegetables. Nevertheless, their unique properties could allow them to be used for high added value-applications, like water resistant coatings of more readily available substrates or as barrier films in multilayer bio-based packaging. For instance, soy protein has been used to improve the WVP of many polysaccharide biopolymers composites. Tulamandi et al., reported that gelatin and defatted soy protein in different proportions, were incorporated into composites made mainly by papaya puree to improve their barrier properties and water resistance. Films produced by casting with 3 wt% of gelatin and 4 wt% of defatted soy protein decreased the water vapor permeability by 34% with respect to the control film.123
3.2 Films with antimicrobial and antioxidant properties
The large amount of bioactive compounds present in the agrowaste biomass may provide the bioplastics derived from it with antioxidant and antimicrobial activities.127,128The most widely explored strategy to prevent the risk of pathogen growth is to incorporate safe antimicrobial additives into the films. For that, organic acids, chitosan biopolymer, nisin peptide, plant extracts, and EOs are usually the options of choice.61,129 For example, the volatile terpenoids and phenolic compounds of different EOs demonstrated to inhibit the microbial activity of E. coli, S. enterica, and L. monocytogenes by both direct and indirect contact (vapor diffusion) when incorporated into an apple peel film, developed upon drop casting from water-based solutions. Remarkable differences were observed between the two methods, indicating that such EOs’ vapor phases led to more significant bacterial inhibitory effects.130
On the other hand, non-lignocellulosic biomass or its by-products such as fruit peels and pomace extracts have been widely selected as ingredients in active food packaging. For instance, in the work of Nur Hanani et al. examples of the excellent antimicrobial and antioxidant properties of plant peels are delivered. These authors have incorporated the peel powders of pomegranate, papaya, and jackfruit into a gelatin matrix, in order to avoid the growth of the pathogens in a gelatin-polyethylene bilayer material.128 The fruit peel powders were incorporated into fish gelatin film-forming solutions and were directly casted on a polyethylene layer. Among them, pomegranate peel powder provided excellent antimicrobial properties against different Gram-positive and Gram-negative bacteria. Simultaneously, papaya peel powder embedded in the bilayer exhibited excellent antioxidant properties, reaching 78% of 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH) inhibition, comparable to a maximum of 76% of inhibition obtained by the same bilayer loaded with chitosan powder.128 Another example of the great antioxidant capacity of fruit peel was reported by Rodsamran and Sothornvit, who incorporated a 10 wt% ethanolic extract of lime peel into lime peel pectin films obtained by casting and enhanced its antioxidant capacity more than 11 times against 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid radical (ABTS) dissolved in water.131 Similarly, bioactive films prepared by solution casting method made of pomelo peel flour and tea polyphenols in different concentrations (5, 10, 15, and 20 wt%) prevented the development of both Gram-positive and Gram-negative bacteria, as well as reduced the lipid oxidation of soybean oil samples placed in a glass vial sealed on the top with the film, limiting the migration of oxygen gas molecules.127
Similarly, fruit pomace was also reported to provide active properties to bioplastics. For example, the fibers and the ethanolic extract of blueberry pomace obtained from juice blueberry processing were isolated and added separately to a gelatin-based matrix to develop active and environmentally-friendly packaging films by the casting technique.112 As expected, the addition of pomace by-products demonstrated excellent antioxidant properties avoiding the lipid oxidation of the packed sunflower oil. For these tests, gelatin films with a fiber concentration of 0.15 g mL−1 were sealed to form packages containing 7 g of sunflower oil, and its oxidation, represented as peroxide value (PV) (mEq O2 per kg oil), was monitored during 13 days using a transparent plastic bag as control. Interestingly, while the oxidation of sunflower oil increased up to 70 mEq O2 per kg after 13 days of storage in the control, the gelatin/pomace based package inhibited by 100% the oil oxidation.112 Kurek et al. have also reported antioxidant properties in chitosan and carboxymethyl cellulose films prepared by casting by adding blueberry and red grape skin pomace extracts in 1, 2, and 4 wt%. In this case, antioxidant activity was measured by Ferric Reducing Antioxidant Power (FRAP) assay, which evaluates the scavenging capacity of polyphenols present in extracts against a radical reagent. Results showed that the antioxidant response was proportional to the concentration of extract and that it was higher when grape skin pomace extract was used. Chitosan films with 4 wt% of the grape skin pomace extract exhibited seven times higher antioxidant activity (mg ascorbic equivalents per g film) than chitosan films with blueberry pomace extract.132 Similarly, de Moraes Crizel et al. have demonstrated that olive pomace incorporated into chitosan films prepared by casting protects walnuts from oxidation when packed in this material. Protected samples showed inferior PV, dienes, and trienes in walnuts, after 31 days of storage, significantly differing (p < 0.05) from the ones non-packaged or packed in a chitosan film or a commercial plastic packaging.133
Furthermore, lignocellulosic biomass was also reported to provide antioxidant properties to bioplastics. For instance, antioxidant bioplastics were reported for thermoplastic corn starch composites prepared upon melt blending and compression molding with rice and coffee husks,110 and for pectin composites films obtained by casting with avocado peels,66 measured this property in all cases against the DPPH radical.
3.3 Films as indicators or sensors of food spoilage
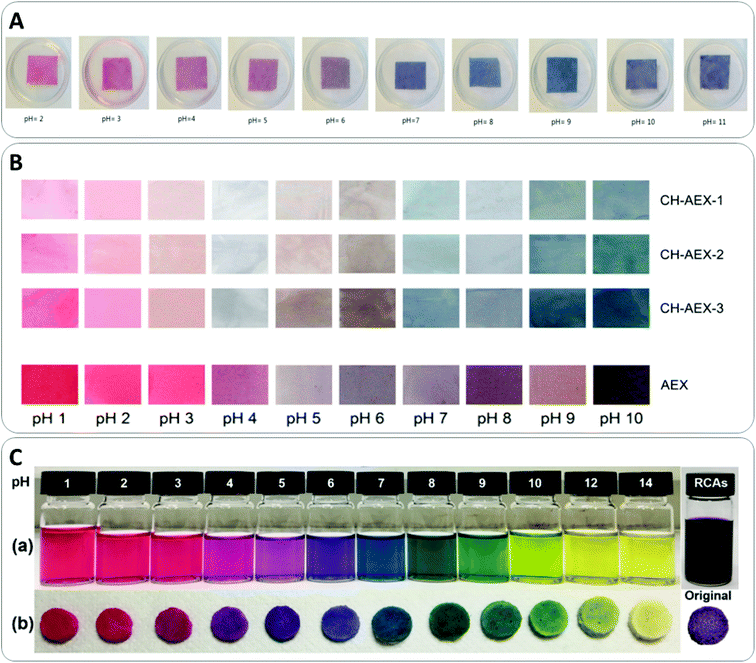
A new trend followed by researchers in the area of intelligent food packaging development uses natural dyes to inform consumers about the quality of food during storage. Phytochemical molecules such as curcumin, alizarin and anthocyanins are sensitive to changes in pH, and respond by a color change upon pH change, that allows consumers to identify the state of freshness of food by naked eye and in real time.134The integration of the bioactive compounds as high-added value compounds in the formulation of bioplastic films can also be exploited to develop additional functionalities, such as radical scavenging or as protection against oxidant agents.135 These compounds are often encapsulated to avoid their degradation by oxidation or by the high temperatures commonly used during industrial processing of polymeric materials such as extrusion and blow-molding.136 Several authors have reported encapsulation methods to incorporate anthocyanin into a biopolymer matrix to obtain active colorimetric pH-sensitive films.136,137 For example, Moazami Goodarzi et al. have reported the development of a starch-filter paper-based film with immobilized anthocyanin of black carrot by immersion of the filter paper into a starch solution to monitor the spoilage of milk in a range of 24 h. During the storage of milk, microbial activity leads to pH changes that can be easily detectable using these innovative bio-based films (Fig. 4A).138 A different work reported black chokeberry pomace extract encapsulation into a chitosan matrix and the realization of films by casting, demonstrating pH-responsive color change: red color at acidic media (pH 1–3) and blue above pH 7 (Fig. 4B).137 In another work, the flower extract of Jamaica (Hibiscus sadbariffa) was incorporated into a nanoclay and added as filler into a thermoplastic corn-starch matrix by extrusion. Results showed that in that case, the incorporation of the extract in the nanoclay reduced the pH-sensibility of the anthocyanin due to the lack of permeability of samples limiting its use as an indicator.136,137
In most cases, the response to changes in pH is evaluated in aqueous media, not entirely appropriate to simulate realistic applications and generally obtaining a slow and non-perceptible optical response at all pH values. The response time is associated to the compact structure of the films or, as mentioned above, to the fact that these substances are usually encapsulated in other matrices to improve their chemical stability. Zia et al.139 have recently managed to overcome this limitation through the development of porous indicators loaded with anthocyanins, which allowed to significantly improve the response time in environments of different pHs (Fig. 4C). To do this, they used anthocyanins extracted from red cabbage and loaded them on freeze-dried porous networks of polyvinylpyrrolidone (PVP) and MCC. Although the PVP polymer is not derived from biomass, this work demonstrates that anthocyanin-based porous systems were capable of rapidly detecting the presence of both acidic and basic vapors in a few seconds and that they can be reused for at least 15 times. These sensors open up the possibility of developing smart packaging for food, although work still needs to be done on the development of completely bio-based systems, in accordance to the principles of green chemistry.
4 Edible active food coatings from agrofood waste
An edible coating is a semi-permeable layer applied directly on food that becomes an integral part of the food product.141,142 The final aim of food coatings is to extend the food shelf-life by reducing the exchange of moisture, O2, CO2, lipids, and volatile molecules between the food and the surrounding environment. As mentioned above for food packaging films, coatings derived from vegetable and fruit by-products also contain bioactive molecules with antioxidant and antimicrobial properties, which can improve the preservation of perishable functional foods.142,143The requirements for a coating material should be linked to the coated food physicochemical properties, which change enormously between the various food types (vegetables, fruits, fish and meat). For example, the high content of lipids and proteins in meats and seafood makes these foods more susceptible to microorganisms’ growth. Therefore, their coatings are expected to present a vital microorganism inhibition capacity. On the other hand, coatings for food such as fruits and vegetables should be focused on slowing down the respiration rate and thus, retarding their ripening.141 Additionally, edible coatings should be tasteless and should not modify the sensory characteristics of the food.1,75 Advances made in the valorization of vegetable and fruit wastes to fulfill these requirements and a review of the latest publications in this field are summarized in Table 2 and included in the following subsections.
| Agro waste | Other components | Solvent | Food coated | Respiration | Weight loss (%) | ΔFirmness (N) | ΔTexture | ΔLightness (L*) | ΔWhiteness index (WI) | ΔLipid oxidation (mg MDA per kg meat) | Antibacterial activity | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ[O2] (%) | Δ[CO2] (%) | ||||||||||||
| Carrot puree | Citric acid | Distilled water | Fresh cut carrot | Not assessed | Not assessed | Coated: 0.8% after 12 days, Uncoated: 1.5% after 12 days | Coated: ↓40% after 12 days, Uncoated: ↓65% after 12 days | Not assessed | Not assessed | Coated: ↑7% after 12 days, Uncoated: ↑40% after 12 days | Not assessed | Not assessed | Wang et al., 2015157 |
| Margo kernel starch | Sorbitol and starch | Distilled water | Tomato fruit | Not assessed | Not assessed | Coated: 8% after 20 days, Uncoated: 12% after 20 days | Coated: ↓50% after 20 days, Uncoated: ↓90% after 20 days | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Nawab, Alam, and Hasnain, 2017171 |
| Pomegranate peel extract | Calcium sulfate | Distilled water | Cherry fruit | Not assessed | Coated: ↑133% after 45 days, Uncoated: ↑186% after 45 days | Coated: 28 after 45 days, Uncoated: 48 after 45 days | Coated: ↓28% after 45 days, Uncoated: ↓42% after 45 days | Not assessed | Coated: ↓1.6% after 45 days, Uncoated: ↓7.1% after 45 days | Not assessed | Not assessed | Not assessed | Parsa et al., 2021148 |
| Rice bran wax | Polysorbate-80 | Distilled water | Tomato fruit | Coated: ↓25% after 122 h, Uncoated: ↓75% after 122 h | Not assessed | Coated: 10% after 27 days, Uncoated: 10% after 18 days | Coated: ↓62.5% after 27 days, Uncoated: ↓62.5% after 18 days | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Abhirami, Modupalli, and Natarajan, 2020144 |
| Sesame protein and mango puree | Maleic acid, glycerol, guar gum and CaCl2 | Distilled water | Fresh cut mango | Not assessed | Not assessed | Not assessed | Coated: ↓33% after 15 days, Uncoated: ↑10% after 15 days | Not assessed | Coated: ↓5.17% after 15 days, Uncoated: ↓21,12% after 15 days | Not assessed | Not assessed | Yes. Analyzed by total plate count. | Sharma et al., 2019152 |
| Sesame protein and mango puree | Succinic acid, glycerol, guar gum and CaCl2 | Distilled water | Fresh cut mango | Not assessed | Not assessed | Not assessed | Coated: ↓30% after 15 days, Uncoated: ↑10% after 15 days | Not assessed | Coated: ↓2.65% in 15 days, Uncoated: ↓21.12% after 15 days | Not assessed | Not assessed | Yes. Analyzed by total plate count. | Sharma et al., 2019152 |
| Sesame protein and mango puree | Citric acid, glycerol, guar gum and CaCl2 | Distilled water | Fresh cut mango | Not assessed | Not assessed | Not assessed | Coated: ↓30% after 15 days, Uncoated: ↑10% after 15 days | Not assessed | Coated: ↓3.50% after 15 days, Uncoated: ↓21.12% after 15 days | Not assessed | Not assessed | Yes. Analyzed by total plate count. | Sharma et al., 2019152 |
| Sesame protein and pineapple juice extract | Glycerol, guar gum and CaCl2 | Distilled water | Fresh cut pineapple | Not assessed | Not assessed | Not assessed | Coated: ↓13% after 15 days, Uncoated: ↓25% after 15 days | Not assessed | Coated: ↓0.13% after 15 days, Uncoated: ↑3.78% after 15 days | Not assessed | Not assessed | Yes. Analyzed by total plate count. | Sharma et al., 2018153 |
| Sesame protein and pineapple juice extract | Maleic acid, glycerol, guar gum and CaCl2 | Distilled water | Fresh cut pineapple | Not assessed | Not assessed | Not assessed | Coated: ↓14% after 15 days, Uncoated: ↓25% after 15 days | Not assessed | Coated: ↓0.65% after 15 days, Uncoated: ↑3.78% after 15 days | Not assessed | Not assessed | Yes. Analyzed by total plate count. | Sharma et al., 2018153 |
| Sesame protein and pineapple juice extract | Succinic acid, glycerol, guar gum and CaCl2 | Distilled water | Fresh cut pineapple | Not assessed | Not assessed | Not assessed | Coated: ↓8% after 15 days, Uncoated: ↓25% after 15 days | Not assessed | Coated: ↑0.76% after 15 days, Uncoated: ↑3.78% after 15 days | Not assessed | Not assessed | Yes. Analyzed by total plate count. | Sharma et al., 2018153 |
| Sesame protein and pineapple juice extract | Citric acid, glycerol, guar gum and CaCl2 | Distilled water | Fresh cut pineapple | Not assessed | Not assessed | Not assessed | Coated: ↓6% after 15 days, Uncoated: ↓25% after 15 days | Not assessed | Coated: ↓0.76% after 15 days, Uncoated: ↑3.78% after 15 days | Not assessed | Not assessed | Yes. Analyzed by total plate count. | Sharma et al., 2018153 |
| Grapefruit seed extract | Carnauba wax, oregano essential oil and Tween 80. | Distilled water | Mandarins | Not assessed | Coated: ↓33% after 28 days, Uncoated: ↓10% after 28 days | Coated: 1% after 28 days, Uncoated: 2% after 28 days | Coated: ↓45% after28 days, Uncoated: ↓50% in 28 days | Not assessed | Coated: ↑9.22% after 21 days, Uncoated: ↑1.57% after 21 days | Not assessed | Not assessed | Yes. Against P. Italicum | Won and Min, 2018154 |
| Sweet potato starch | Glycerol, Tween 80 and thyme essential oil | Distilled water | Shrimp | Not assessed | Not assessed | Not assessed | Not assessed | Coated: ↓6.08% after 8 days, Uncoated: ↓16.54% after 8 days | Not assessed | Not assessed | Coated: ↑<10% after 8 days, Uncoated: ↑100% after 8 days | Yes. Against aerobic bacteria | Alotaibi and Tahergorabi, 2018159 |
| Plantago major seed mucilage | Citrus lemon essential oil | Distilled water | Buffalo meat | Not assessed | Not assessed | Not assessed | Not assessed | Coated: ↓14% after 10 days, Uncoated: ↓22% after 10 days | Coated: ↓7.5% after 10 days, Uncoated: ↓12.5% after 10 days | Not assessed | Not assessed | Yes. Measured against psychrotrophic bacteria, E. coli, S. aureus and Funghi | Noshad et al., 2021167 |
| Grape pomace | Sodium nitrite | Not applicable | Beef sausage | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Not assessed | Coated: ↑3% after 30 days, Uncoated: ↑65% after 30 days | Yes. Measured by total viable count | Riazi et al., 2016172 |
4.1 Coatings reducing fruits and vegetable respiration rate
Fruits and vegetables are living organisms, which means that many biochemical processes are still ongoing after harvesting, with important consequences on their physical appearance and nutritional quality. Respiration is a metabolic process linked to the consumption of oxygen (O2) and the release of carbon dioxide (CO2) and differs from the process of ripening. However, high respiration rates correspond to rapid ripening and senescence, resulting in low durability and a more perishable product. Ncama et al. suggested that coatings’ application limits the transpiration rate because they partially block the stoma of vegetables.142 The respiration rate is usually evaluated by measuring the change in the concentration of headspace gas (CO2 and O2) when coated samples are placed in closed trays during the experiments for the determination of the maximum storage time.125,144 For example, the respiration rate of peaches coated by immersion during 10 minutes in a mango peel flour (MPF) and mango peel flour with the antioxidant extract of mango seed (MPFS) aqueous solution was evaluated and compared with peaches immersed in distilled water.125 Results showed that MPF coating did not present significant differences with the control, but the MPFS coating slowed down the gas exchange up to 29% and 39% for CO2 and O2, respectively.125 In the work of Panoth Abhirami et al., the respiration rates of uncoated tomatoes (control) and dip-coated tomatoes treated with a rice bran wax coating emulsion prepared with polysorbate-80 in water were measured. Results indicated that the oxygen level underwent an important decline in uncoated tomatoes, while tomatoes coated with rice bran wax exhibited lower variation. At the end of the experiment (122 hours), uncoated tomatoes consumed three times more oxygen than tomatoes with rice bran wax.144Interestingly, when the oxygen level decays below 3%, anaerobic respiration takes place. Anaerobiosis and fermentation processes lead to ethanol and acetaldehyde production, characterized by its unpleasant flavor.145 Another ripening indicator is the release of ethylene from the fruit, especially in climacteric fruit such as apple, avocado, or tomato.146,147 In Torres-León et al., the ethylene emission was reduced by 64% for peach samples coated by immersion into mango peel flour solution prepared in citrate buffer solution (pH 4.5) after 8 days of storage.125
4.2 Coatings for texture preservation and reduced water transpiration of fruits and vegetables
Fruit softening is the major indicator of the shelf-life, and consequently, of the commercial value of the fruits and vegetables.148 The loss of turgor in fruits is strongly related to the ripening, which triggers many physicochemical changes that provide to the fruit the characteristic texture, color, aroma, and taste upon maturation.149 The problem appears when fruits overcome the maturation stage and overripe, leading to loss of firmness, senescence, and over-soften flesh. During ripening, starch degrades into smaller saccharides such as glucose, fructose, or sucrose, increasing the sweetness of the fruit, while the concentration of organic acids decreases, being the most critical losses those of maleic and citric acids. Thus, most fruits become softer during ripening due to the disaggregation of cell walls and starch degradation, which promote lower values of firmness and increase the transpiration of water.149 The increase in water transpiration causes weight loss, a reliable parameter to determine plant degradation.150 For example, uncoated tomatoes and tomatoes coated with a rice bran wax emulsion by dip-coating underwent a different rate of decay of firmness and weight during the storage. The application of the coating increased the shelf-life of the fruit, obtaining the same weight-loss (−10%) and firmness (−62.5%) values of the uncoated fruit but nine days later.144It is well-known that reducing fruit and vegetable water loss prevents the softening of plant tissue. Several techniques have been developed with this aim, including the fruit dipping into calcium salt solution to cross-link pectin present in the plant tissue and reinforce the cell wall's structural integrity.148,151 For example, in the work of Parsa et al., the application of a coating composed of 1 wt% calcium sulfate and 200 or 400 ppm pomegranate peel extract reduced the weight loss and firmness of sweet cherry fruits by 50% and 25%, respectively.148
The incorporation of lipophilic compounds, such as proteins, waxes, or EOs, into fruit coatings has significant effects on water transpiration and tissue firmness.152,153 Hydrophobic sesame protein has been used to prepare a bilayer coating system to improve the shelf-life of mango and pineapple fruit.152,153 The preservation properties, especially firmness, were improved when sesame protein was cross-linked with different organic acids (succinic, malic, and citric acid) before the coating application. The pineapple fruit was first immersed in a cross-linked sesame protein water solution, and then a second layer consisting of a water solution of mango puree and calcium chloride was applied. Coated pineapples exhibited up to 20% more firmness than uncoated fruit after 15 days of storage at 5 °C.153 The weight loss associated with the water transpiration was reduced in Satsuma mandarin, after dip-coating during 30 seconds into an emulsion based on grapefruit seed extract and carnauba wax prepared with Tween 80 as emulsifier. After seven days of storage at 25 °C, the weight loss of coated mandarins was reduced by ∼3 times in comparison to the uncoated mandarins.154
4.3 Coatings to avoid undesirable color changes in food
The enzymatic browning is one of the most important color reactions in nature that occurs to fruits, vegetables, and seafood, which harmfully affects the attractiveness and the consumption of these products.155,156 The enzymatic browning starts due to the breaking down of the cell walls (cutting, peeling-off). Next, some environmental factors, such as temperature, pH, and oxygen concentration, induce the activation of Polyphenol oxidase (PPO) enzyme.155,156 PPO enzyme catalyzes the first polymerization of phenols to produce quinones, which undergo further polymerization into dark pigments called melanoidin, represented in Fig. 5,156 causing the brown coloration during fruit storage. Plant-based coatings, mainly composed of biomolecules with high polarity, may reduce non-polar molecules’ transpiration like oxygen.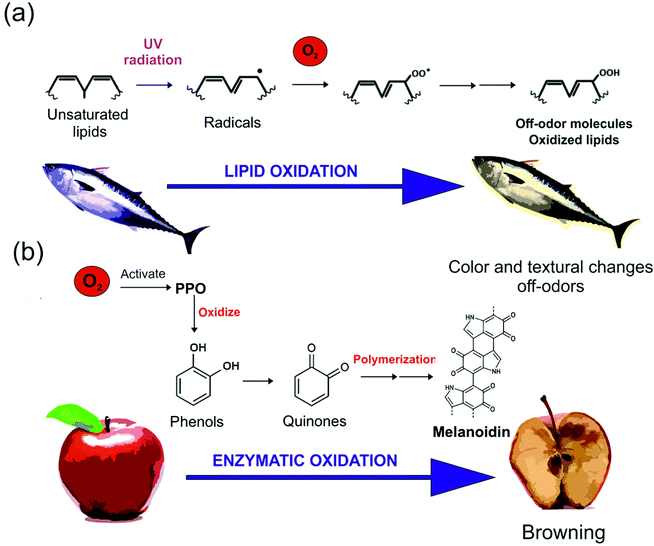 | ||
| Fig. 5 Schematic representation of the process involved in (a) lipid oxidation and (b) enzymatic oxidation. PPO corresponds to the enzyme polyphenol oxidase. | ||
Moreover, vegetable and fruit wastes are rich in antioxidants, which avoid oxygen activity by scavenging.141 In the work of Wang et al., the PPO activity and total phenols were evaluated by spectrometry and monitored in fresh-cut carrots dip-coated during 5 minutes in a 30 wt% carrot puree-based water solution containing also 1 wt% corn starch, 0.5 wt% gelatin, 1.5 vol% glycerol, 1 wt% chitosan, and 1 vol% cinnamaldehyde. As expected, coated samples showed lower values of total polyphenols (∼10 OD280 per g) and PPO activity (∼160 U g−1) compared to uncoated carrots (∼22 OD280 per g and ∼220 U g−1, respectively).157
Besides the enzymatic oxidation, other factors also induce undesirable color changes in fruits, including cutting operation, surface dehydration, and non-enzymatic oxidation. The application of coatings on fruits and vegetables may prevent the tissue damage generated by these environmental conditions (changes in the temperature, pH, and relative humidity, for example).141 Furthermore, the coating plays a fundamental role in preserving the natural color of the fruit, which is fundamental for its commercialization. The color changes can be evaluated during storage by colorimetric methods. The darkening of the fruit due to the undesirable browning process can be measured with the light (L*) values or with the whiteness index (WI) over the storage period157 and the results found in the literature are summarized in Table 2.
4.4 Coatings with antimicrobial activity for fruits and vegetables
Vegetables and fruits present an outer protective waxy cuticle layer that avoids microbial growth. Besides, most of the fruits’ characteristic acidity acts as a natural defense against the spoilage microbes. The manipulation and transportation of these foods usually result in the breakdown of this external layer that drives rapid colonization and development of several microorganisms.158 Incorporating coatings may prevent external damage such as cracks and punctures while including antimicrobial agents into the coating can also limit microbial growth. EOs have demonstrated bactericide properties due to the hydrophobic constituents, capable of breaking down the microbial cell wall of the pathogens.159 For instance, the incorporation of 0.5 wt% oregano EO into a coating based on grapefruit seed extract and carnauba wax was demonstrated to protect Satsuma mandarin against the fungus Penicillium italicum.154 The diseases’ effects were measured in coated and uncoated samples at two different temperatures (25 and 4 °C), showing less incidence at lower temperatures. The application of the previously described coating retarded the appearance of the symptoms caused by the fungi to 21 days, while uncoated samples manifested the disease after five days at 4 °C of storage temperature. Furthermore, the protective features of coatings derived from several vegetables and fruits residue flours (orange, passion fruit, watermelon, lettuce, among others) were evaluated by spraying or dipping onto fresh-cut carrots.160 Results showed that coating by dipping prevented more effectively the growth of microorganisms (mesophiles, yeast, and phychrophiles) after 15 days at 5 °C.4.5 Antioxidant and antimicrobial coatings for meat and seafood
Meat and seafood provide high-quality proteins and valuable micronutrients essential for a healthy human diet. Red meat is an important source of iron while fish meat supplies healthy fatty acids and vitamin D. However, their chemical composition is easily prone to oxidative deterioration, especially during the post-mortem handling and storage, resulting in off-odors, rancidity, and modification of the texture, color and the characteristic brightness of a fresh product. Most of these reactions are linked to the natural oxidation of lipids and iron present in hemoglobin and myoglobin.161 During lipid oxidation, poly-unsaturated fatty acids produce secondary products such as malondialdehyde (MDA), known to be cytotoxic and genotoxic, and small volatile ketones and aldehydes that cause rancidity and unpleasant odors. UV-light, metals, and oxygen molecules play an essential role in lipid oxidation as activators of the reactive oxygen species (ROS), which are involved in the oxidation mechanism.162 The oxidative damage of food is determined by quantifying the amount of peroxide, ketone, and aldehyde products generated, with PV or quantifying the MDA concentration represented in thiobarbituric acid reactive substances (TBARS).163,164 The PV scale for freshness suggests that foods with values above 8–10 mmol of O2 per kg are classified as spoiled.165Plant-based coatings may prevent lipid oxidation by reducing the food contact with atmospheric oxygen.166 Furthermore, the protection against oxidation can be reinforced by incorporating natural antioxidants, such as EOs. In the work of Alotaibi and Tahergorabi, several shrimps were coated with thyme EO (2 wt%) incorporated as emulsion into a sweet potato starch-based aqueous solution by immersion during 15 minutes. The coating kept the TBARS value lower than 0.5 mg MDA per kg of shrimp after eight days of storage, while the uncoated shrimps exhibited TBARS value close to 3 mg MDA per kg of shrimp after the same storage period.159 The potential of EOs to prevent radicals’ formation was demonstrated in Mohammad Noshad et al.167 Incorporating 2 wt% of citrus lemon essential oil in a Plantago major seed mucilage-based emulsion suppressed radicals’ formation by 86% less meq O2 per kg in dip-coated buffalo meat, due to the strong scavenging activity.167
As stated before, the high water activity, as well as the amount of lipids and proteins present in meat and seafood, make them prone to the growth of bacteria and other pathogenic microorganisms responsible for food spoilage. The main indicator of degradation and low quality of seafood is the putrefactive odor caused by spoilage microorganisms, which release small volatile organic bases, called biogenic amines.168 The reduction of oxygen concentration in the package, by modification of its atmosphere or by vacuum, and the freezing conditions, can only inhibit part of the development of microorganisms in food.166 In particular, psychotropic bacteria grow within a temperature range of −21 to −14 °C, while in anaerobic environments, psychotropic bacteria and anaerobic lactic acid bacteria survive, generating off-odors metabolites and severe acidification.169 The microbial activity is usually defined as a total viable count (TVC) or total plate count, which includes the concentration of microorganisms such as bacteria, yeast, or mold spores in food in terms of colony-forming units (CFU) per weight, volume or surface of the sample. For freshwater and marine species, the microbial limit recommended by the International Commission on Microbiological Specifications for Foods (ICMSF) is 7 log CFU per g, while humans are capable of noticing the food spoilage with TVC in the range of 6 to 8 log CFU per g of the product.165,170 The low effectivity of sensory indicators underlines the high importance of preventing bacterial spoilage by edible antimicrobial coating. Shrimp dip-coated with sweet potato starch loaded with thyme EO has been demonstrated to inhibit completely aerobic bacteria during eight days of storage when the thyme EO concentration overcomes 4 wt%.159
Few articles include only vegetable and fruit by-products as a matrix of meat coatings; in their place, chitosan appears as the main component because of its widely known antimicrobial activity.74 Licciardello et al. have studied the preservation effect of chitosan-based and locust bean gum-based edible coatings incorporating 7, 18, or 36 wt% pomegranate peel extract on shrimps during cold storage.170 Results showed that chitosan coating exhibited a better inhibition response against psychotropic bacteria than locust bean gum coating. As expected, chitosan films loaded with pomegranate extract showed the best antimicrobial results against psychotropic bacteria and Pseudomonas spp., while LGB with pomegranate peel extract showed the same bacterial inhibition of the control chitosan coating.170
5 Summary and perspectives
Non-edible agro-food wastes, such as industrial discards from processing fruits, vegetables or grains, represent a promising source of raw materials that is currently underutilized. These resources, which include structural biopolymers (such as polysaccharides, lignin and proteins), and a wide variety of bioactive molecules (such as the antioxidant carotenoids and anthocyanins and antibacterial essential oils), are the subject of research that aims to incorporate them back into new added-value products according to a circular economy model. The review covers upcycling of such biomass from agricultural plant residues to films or coatings, intended for food packaging to improve food safety, extending shelf life and reducing spoilage. Active food packaging was also addressed, when plant biomolecules with peculiar antimicrobial and antioxidant activities were incorporated into the developed films or coatings.Approximately 2 billion tons of only lignocellulosic agricultural waste, such as corncob, rice husk, rice straw, sugarcane bagasse, wheat straw, etc., are produced annually worldwide.173 Although estimations of the total industrial fruit and vegetable non-edible discards are missing in the literature, such agrowaste biomass quantities are much higher compared to the 147 million tons of plastic produced annually for packaging.5 Therefore, they could be the ideal raw materials to be upcycled into plastic alternatives. This approach would also prevent competition between the food industry and the material industry. For this reason, this review focused on the scientific publications that used non edible agricultural biomass that is currently discarded or underutilized, to develop methods to convert such biomass into materials, without the use or generation of harmful substances, in accordance to the principles of green chemistry. Few literature examples that have used potentially unsafe substances or fossil-derived non-biodegradable polymers have been included, to highlight the effort towards a greener chemistry. When that was the case, it was always highlighted.
The processing with green chemicals or low energy methods of different agrowaste biomasses for their partial or preferably complete conversion into bioplastic materials or active coatings for food preservation, is an area of research with enormous potential and opportunities, but also great challenges.
The first challenges arise from the high variability of raw materials’ composition, which require processing strategies to be mitigated. As shown in the review, the composition of the starting biomass largely determines the properties of the developed materials, and therefore, standardized methods need to be developed to obtain bioplastics with specific properties from a wide range of diverse plant resources. In the article, a distinction was made between biomass that was fully converted into films or coatings, and biomass components that were used as fillers in biocomposites. The former strategy has the ambition of producing packaging materials made entirely of plant biomass, whereas the latter uses a lower percentage of plant waste (typically not higher than 50 wt%). Despite the attractiveness of using materials made entirely of plant constituents, another challenge to address in this case is the moisture sensitivity and poor mechanical properties that such biocomposites occasionally demonstrate. Indeed, we have discussed the main physicochemical properties of the developed materials, as well as the reported methods to improve their mechanical properties and humidity resistance. In the case of the use of biomass components as fillers for the development of packaging films, some of them were developed with the traditional methods of processing of thermoplastic polymers, as melting processing, that were adapted to plant-originated biocomposites. Such materials usually have better mechanical properties and are less moisture sensitive than the ones made using entirely plant biomass and solvent processing methods, but can face the challenge of most difficult biodegradation.
The development of such materials needs to be supported by life cycle assessments (LCA), to quantify how the new materials can improve sustainability at a system scale, to determine the most sustainable transformation or conversion processes and identify possible process shortcomings. Unfortunately, LCA of the proposed packaging is almost never considered in the research articles. LCA is important since it highlights the best circular pathways that convert by-products derived from biomass processing back into the Market that generated them or into other external ones. Unfortunately, the literature reviewed here did not show a significant focus on examining and quantifying environmental impact parameters for the different circular scenarios. However, it is noted that greater efforts are still needed to broaden and deepen the vision of real opportunities and viable challenges. Most of the processes reviewed are still on a laboratory scale, requiring major efforts to convert them to an industrial scale.
In addition, it is still necessary to delve into issues related to the biodegradability of such materials. Materials prepared from plant residues have the advantage to be prone to rapid biodegradation,104 but at the same time it has to be assured that when they are used for food packaging they maintain the structure and functionality at least during the food shelf life and ideally even for longer, so that recycle or reuse can be an option. Upon disposal, the biodegradation process should be clearly distinguished between industrial and home compost, the first requiring dedicated facilities and the second household compost conditions. In this way, the consumer will have all the necessary information for an easy and correct disposal. Pushbacks that are seen with the disposal and separation of some commercially available bioplastics, like PLA,174,175 should be overcome by new focused investments but also support actions from government organizations and decision making centers. It is expected that once some numerous promising materials and packaging alternatives will be developed, new plants to handle their biodegradability will be developed, as is currently happening with polymers such as PLA and PHAs.
A strong connection between academia and policymaking is sorely needed. Currently, the scientific community continues to focus its efforts on developing solutions and improvements for new bioplastics based on agrowaste biomass. However, this initiative must be accompanied by governmental actions that promote the valorization this waste, or rather “new raw materials”, on a large scale. In addition, it is still necessary to focus on the economic analysis and the social impact of the production, use, and disposal of these materials. Undoubtedly, the joint and integrated work between these sectors will lead to the changes that our society demands for the coming decades.
Abbreviations
| AAE | Ascorbic acid equivalent |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid radical |
| a w | Water activity |
| CNC | Cellulose nanocrystals |
| CNFs | Cellulose nanofibers |
| CNPs | Cellulose nanoparticles |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl radical |
| Eb | Elongation at break |
| EOs | Essential oils |
| FAO | Food and agriculture organization of the United Nations |
| FRAP | Ferric reducing antioxidant power |
| GAE | Gallic acid equivalent |
| GRAS | Generally recognized as safe |
| HDPE | High-density polyethylene |
| HG | Homogalacturonan |
| ICMSF | International commission on microbiological specifications for foods |
| MCC | Microcrystalline cellulose |
| MDA | Malondialdehyde |
| MPF | Mango peel flour |
| MPFS | Mango peel flour with the antioxidant extract of mango seed |
| O2P | Oxygen permeability |
| PE | Polyethylene |
| PEAs | Polyesteramides |
| PET | Polyethylene terephthalate |
| PHAs | Polyhydroxyalkanoates |
| PLA | Polylactic acid |
| PP | Polypropylene |
| PPO | Polyphenol oxidase |
| PV | Peroxide value |
| PVC | Polyvinyl chloride |
| RBW | Rice bran wax coating |
| RG I | Rhamnogalacturonan I |
| RG II | Rhamnogalacturonan II |
| ROS | Reactive oxygen species |
| TBARS | Thiobarbituric acid reactive substances |
| TFA | Trifluoroacetic acid |
| TPC | Total polyphenol content |
| TS | Tensile strength |
| TVC | Total viable count |
| WG | Wheat gluten |
| WVP | Water vapor permeability |
| YM | Young's modulus |
Author contributions
Danila Merino: Conceptualization, project administration, supervision, writing – original draft and writing – review & editing. Ana Isabel Quilez-Molina: Writing – original draft and writing – review & editing. Giovanni Perotto: Writing – original draft and writing – review & editing. Andrea Bassani: Writing – original draft and writing – review & editing. Giorgia Spigno: Writing – original draft. Athanassia Athanassiou: Funding acquisition, writing – review & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to thank the Industrial Designer Ezequiel Macri for designing the Fig. 2.References
- C. G. Otoni, R. J. Avena-Bustillos, H. M. C. Azeredo, M. V. Lorevice, M. R. Moura, L. H. C. Mattoso and T. H. McHugh, Compr. Rev. Food Sci. Food Saf., 2017, 16, 1151–1169 CrossRef PubMed.
- A. R. V. Ferreira, V. D. Alves and I. M. Coelhoso, Membranes, 2016, 6, 1–17 CrossRef PubMed.
- C. G. Otoni, R. J. Avena-Bustillos, H. M. C. Azeredo, M. V. Lorevice, M. R. Moura, L. H. C. Mattoso and T. H. McHugh, Compr. Rev. Food Sci. Food Saf., 2017, 16, 1151–1169 CrossRef PubMed.
- A. A. Jahnke, Reinf. Plast., 2020, 64, 54–56 CrossRef.
- PlasticsEurope, Plastics – The Facts 2020, 2020 Search PubMed.
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, 3–8 Search PubMed.
- B. D. Vogt, K. K. Stokes and S. K. Kumar, ACS Appl. Polym. Mater., 2021, 3, 4325–4346 CrossRef CAS.
- I. Wojnowska-Baryła, D. Kulikowska and K. Bernat, Sustainability, 2020, 12, 2088 CrossRef.
- The European Parliament and the Council of the European Union, Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the reduction of the impact of certain plastic products on the environment, 2019.
- European Commission, Commission guidelines on single-use plastic products in accordance with Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the reduction of the impact of certain plastic products on the environment, 2021.
- European Bioplastics e.V., Do bioplastics have a lower carbon footprint than fossil based plastics? How is this measured?, https://www.european-bioplastics.org/faq-items/do-bioplastic-have-a-lower-carbon-footprint-than-fossil-based-plastics-how-is-this-measured/.
- G. Perotto, L. Ceseracciu, R. Simonutti, U. C. Paul, S. Guzman-Puyol, T. N. Tran, I. S. Bayer and A. Athanassiou, Green Chem., 2018, 20, 894–902 RSC.
- K. W. Meereboer, M. Misra and A. K. Mohanty, Green Chem., 2020, 22, 5519–5558 RSC.
- J. W. R. Chong, K. S. Khoo, G. Y. Yew, W. H. Leong, J. W. Lim, M. K. Lam, Y. C. Ho, H. S. Ng, H. S. H. Munawaroh and P. L. Show, Bioresour. Technol., 2021, 342, 125947 CrossRef CAS PubMed.
- C. Zhang, C. Wang, G. Cao, D. Wang and S. H. Ho, J. Hazard. Mater., 2020, 388, 121773 CrossRef CAS PubMed.
- S. Guzman-Puyol, D. Russo, I. Penna, L. Ceseracciu, F. Palazon, A. Scarpellini, R. Cingolani, R. Bertorelli, I. S. Bayer, J. A. Heredia-Guerrero and A. Athanassiou, Algal Res., 2017, 27, 1–11 CrossRef.
- Food and Agriculture Organization of the United Nations, Tracking progress on food and agriculture-related SDG indicators 2020, 2020 Search PubMed.
- EU Fusions, Food Waste Definition, https://www.eu-fusions.org/index.php/about-food-waste/280-food-waste-definition.
- Å. Stenmarck, C. Jensen, T. Quested, G. Moates, M. Buksti, B. Cseh, S. Juul, A. Parry, A. Politano, B. Redlingshofer, S. Scherhaufer, K. Silvennoinen, H. Soethoudt, C. Zübert and K. Östergren, Estimates of European food waste levels. Reducing food waste through social innovation, 2016 Search PubMed.
- S. Dahiya, A. N. Kumar, J. Shanthi Sravan, S. Chatterjee, O. Sarkar and S. V. Mohan, Bioresour. Technol., 2018, 248, 2–12 CrossRef CAS PubMed.
- R. Ravindran and A. K. Jaiswal, Trends Biotechnol., 2016, 34, 58–69 CrossRef CAS PubMed.
- J. A. Cecilia, C. Garcia-Sancho, P. J. Maireles-Torres and R. Luque, in Biorefinery, ed. J.-R. Bastidas-Oyanedel and J. E. Schmidt, Springer Nature Switzerland AG, 2019, pp. XIV, 763 Search PubMed.
- K. L. Johnson, M. J. Gidley, A. Bacic and M. S. Doblin, Curr. Opin. Biotechnol., 2018, 49, 163–171 CrossRef CAS PubMed.
- D. Mohnen, Curr. Opin. Plant Biol., 2008, 11, 266–277 CrossRef CAS PubMed.
- P. Wettstein-Knowles, eLS, 2016, pp. 1–13 Search PubMed.
- C. Voiniciuc, M. Pauly and B. Usadel, Plant Physiol., 2018, 176, 2590–2600 CrossRef CAS PubMed.
- L. Taiz and E. Zeiger, in Vegetal Physiology, Universitat Jaume I, 3rd edn, 2006, pp. 587–610 Search PubMed.
- R. A. Ilyas, S. M. Sapuan, R. Ibrahim, M. S. N. Atikah, A. Atiqah, M. N. M. Ansari and M. N. F. Norrrahim, Nanocryst. Mater., 2019, 3–22 Search PubMed.
- C. Loix, M. Huybrechts, J. Vangronsveld, M. Gielen, E. Keunen and A. Cuypers, Front. Plant Sci., 2017, 8, 1–19 Search PubMed.
- M. Ochoa-Villarreal, E. Aispuro-Hernandez, I. Vargas-Aispuro and M. A. Martinez-Tellez, in Polymerization, InTech, 2012, vol. 4, pp. 63–86 Search PubMed.
- F. G. Malinovsky, J. U. Fangel and W. G. T. Willats, Front. Plant Sci., 2014, 5, 178 Search PubMed.
- E. R. Lampugnani, G. A. Khan, M. Somssich and S. Persson, J. Cell Sci., 2018, 131, jcs207373 CrossRef PubMed.
- S. Thakur, J. Chaudhary, V. Kumar and V. K. Thakur, J. Environ. Manage., 2019, 238, 210–223 CrossRef CAS PubMed.
- E. G. Andriotis, G. K. Eleftheriadis, C. Karavasili and D. G. Fatouros, Pharmaceutics, 2020, 12(1), 56 CrossRef CAS PubMed.
- A. Valdés, N. Burgos, A. Jiménez and M. C. Garrigós, Coatings, 2015, 5, 865–886 CrossRef.
- C. Mellinas, M. Ramos, A. Jiménez and M. C. Garrigós, Materials, 2020, 13, 673 CrossRef CAS PubMed.
- A. C. S. de Oliveira, L. F. Ferreira, D. de Oliveira Begali, J. C. Ugucioni, A. R. de Sena Neto, M. I. Yoshida and S. V. Borges, J. Polym. Environ., 2021, 29, 2546–2556 CrossRef CAS.
- O. M. Terrett and P. Dupree, Curr. Opin. Biotechnol., 2019, 56, 97–104 CrossRef CAS PubMed.
- E. Bertoft, Agronomy, 2017, 7, 56 CrossRef.
- M. P. Guarás, L. N. Ludueña and V. A. Alvarez, Handb. Nanomater. Nanocomposites Energy Environ. Appl, 2020, pp. 1–24 Search PubMed.
- B. Khan, M. Bilal Khan Niazi, G. Samin and Z. Jahan, J. Food Process Eng., 2017, 40(3), e12447 CrossRef.
- P. R. Day, Crit. Rev. Food Sci. Nutr., 1996, 36, 39–47 CrossRef PubMed.
- T. Osborne, Nature, 1924, 114, 822–822 Search PubMed.
- Z. Berk, Technology of production of edible flours and protein products from soybeans, https://www.fao.org/3/t0532e/t0532e00.htm#con (accessed 3 March 2021).
- T. J. Anderson and B. P. Lamsal, Cereal Chem. J., 2011, 88, 159–173 CrossRef CAS.
- J. C. Arthur, Adv. Protein Chem., 1953, 8, 393–414 CrossRef CAS.
- G. M. Weisz, L. Schneider, U. Schweiggert, D. R. Kammerer and R. Carle, Innovative Food Sci. Emerging Technol., 2010, 11, 733–741 CrossRef CAS.
- C. J. R. Verbeek and L. E. van den Berg, Macromol. Mater. Eng., 2010, 295, 10–21 CrossRef CAS.
- P. R. Shewry, N. G. Halford, P. S. Belton and A. S. Tatham, Philos. Trans. R. Soc. London, Ser. B, 2002, 357, 133–142 CrossRef CAS PubMed.
- H. Wieser, Food Microbiol., 2007, 24, 115–119 CrossRef CAS PubMed.
- N. Singh, S. Singh, A. Kaur and M. Bakshi, in Natural Polymers, Royal Society of Chemistry, 2012, vol. 1, pp. 204–218 Search PubMed.
- W. Huo, D. Wei, W. Zhu, Z. Li and Y. Jiang, J. Cereal Sci., 2018, 79, 354–361 CrossRef CAS.
- J. Liang, Q. Xia, S. Wang, J. Li, Q. Huang and R. D. Ludescher, Food Hydrocolloids, 2015, 44, 94–100 CrossRef CAS.
- P. S. Belton, I. Delgadillo, N. G. Halford and P. R. Shewry, J. Cereal Sci., 2006, 44, 272–286 CrossRef CAS.
- J. Taylor, J. O. Anyango, P. J. Muhiwa, S. I. Oguntoyinbo and J. R. N. Taylor, Food Chem., 2018, 245, 178–188 CrossRef CAS PubMed.
- J. Taylor and J. R. N. Taylor, J. Am. Oil Chem. Soc., 2018, 95, 969–990 CrossRef CAS.
- J. Taylor, K. Zhang and D. Wang, in Sorghum and Millets: Chemistry, Technology, and Nutritional Attributes, Elsevier, 2018, pp. 393–420 Search PubMed.
- C. J. Dillard and J. B. German, J. Sci. Food Agric., 2000, 80(12), 1744–1756 CrossRef CAS.
- A. I. Quilez-Molina, J. A. Heredia-Guerrero, A. Armirotti, U. C. Paul, A. Athanassiou and I. S. Bayer, Food Packag. Shelf Life, 2020, 23, 100445 CrossRef.
- P. Cazón, G. Velazquez, J. A. Ramírez and M. Vázquez, Food Hydrocolloids, 2017, 68, 136–148 CrossRef.
- N. A. Al-Tayyar, A. M. Youssef and R. Al-hindi, Food Chem., 2020, 310, 125915 CrossRef CAS PubMed.
- R. Irkin and O. K. Esmer, J. Food Sci. Technol., 2015, 52, 6095–6111 CrossRef CAS PubMed.
- T. Hintz, K. K. Matthews and R. Di, BioMed Res. Int., 2015, 10, 77–80 Search PubMed.
- I. Gutiérrez-del-Río, J. Fernández and F. Lombó, Int. J. Antimicrob. Agents, 2018, 25, 309–315 CrossRef PubMed.
- B. K. Tiwari, N. P. Brunton and C. S. Brennan, Handbook of Plant Food Phytochemicals, John Wiley & Sons, 2013 Search PubMed.
- D. Merino, L. Bertolacci, U. C. Paul, R. Simonutti and A. Athanassiou, ACS Appl. Mater. Interfaces, 2021, 13, 38688–38699 CrossRef CAS PubMed.
- G. Tedeschi, S. Guzman-Puyol, L. Ceseracciu, U. C. Paul, P. Picone, M. Di Carlo, A. Athanassiou and J. A. Heredia-Guerrero, Biomacromolecules, 2020, 21, 910–920 CrossRef CAS PubMed.
- S. Guzman-Puyol, L. Ceseracciu, G. Tedeschi, S. Marras, A. Scarpellini, J. Benítez, A. Athanassiou and J. Heredia-Guerrero, Nanomaterials, 2019, 9, 368 CrossRef CAS PubMed.
- G. Scoponi, S. Guzman-Puyol, G. Caputo, L. Ceseracciu, A. Athanassiou and J. A. Heredia-Guerrero, Polymer, 2020, 193, 122371 CrossRef CAS.
- R. Gune, A. Sawant and N. Joglekar, Formation of Bio-Based Polymer (Poly-Lactic Acid) From Potato Peel Waste and Blending with Chitosan Extracted from Fish Scales, 2021, vol. 4 Search PubMed.
- G. Scoponi, N. Francini and A. Athanassiou, Macromol. Mater. Eng., 2021, 306, 1–10 CrossRef.
- D. Merino, T. J. Gutiérrez and V. A. Alvarez, Starch/Staerke, 2019, 71, 1800341 CrossRef.
- D. Merino, T. J. Gutiérrez, A. Y. Mansilla, C. Casalongué and V. A. Alvarez, ACS Sustainable Chem. Eng., 2018, 6, 15662–15672 CrossRef CAS.
- A. Valdés, M. Ramos, A. Beltrán, A. Jiménez and M. C. Garrigós, Coatings, 2017, 7, 1–23 CrossRef.
- H. M. C. De Azeredo, M. F. Rosa, M. De Sá, M. Souza Filho and K. W. Waldron, The use of biomass for packaging films and coatings, Advances in biorefineries, 2014, pp. 819–874 Search PubMed.
- V. Vadivel, A. Moncalvo, R. Dordoni and G. Spigno, Waste Manage., 2017, 64, 305–314 CrossRef CAS PubMed.
- F. Vásquez-Garay, I. Carrillo-Varela, C. Vidal, P. Reyes-Contreras, M. Faccini and R. T. Mendonça, Sustainability, 2021, 13, 1–15 CrossRef.
- T. Wang and Y. Zhao, Carbohydr. Polym., 2021, 253, 117225 CrossRef CAS PubMed.
- P. Methacanon, J. Krongsin and C. Gamonpilas, Food Hydrocolloids, 2014, 35, 383–391 CrossRef CAS.
- C. He, I. Sampers and K. Raes, ACS Sustainable Chem. Eng., 2021, 9, 833–843 CrossRef CAS.
- A. Bassani, C. Fiorentini, V. Vadivel, A. Moncalvo and G. Spigno, Appl. Sci., 2020, 10, 6112 CrossRef CAS.
- C. Fiorentini, A. Bassani, G. D. Garrido, D. Merino, G. Perotto, A. Athanassiou, J. Peräntie, N. Halonen and G. Spigno, Biocatal. Agric. Biotechnol., 2022, 39, 102282 CrossRef CAS.
- I. M. Rodrigues, J. F. J. Coelho and M. G. V. S. Carvalho, J. Food Eng., 2012, 109(3), 337–346 CrossRef CAS.
- L. E. Alca, E. G. Tovar-pe and T. P. Castro-ja, BioRes. Open Access, 2020, 9, 198–208 CrossRef PubMed.
- T. Tesfaye, M. Ayele, E. Ferede, M. Gibril, F. Kong and B. Sithole, Clean Technol. Environ. Policy, 2021, 23, 581–595 CrossRef.
- I. Majid, G. A. Nayik and V. Nanda, Cogent Food Agric., 2015, 1, 1071022 CrossRef.
- A. Díaz, R. Katsarava and J. Puiggalí, Int. J. Mol. Sci., 2014, 15, 7064–7123 CrossRef PubMed.
- S. Biswas, P. Ghosh, A. Dutta, M. Biswas and S. Chatterjee, Curr. Res. Nutr. Food Sci., 2021, 9, 62–74 Search PubMed.
- C. Caldeira, A. Vlysidis, G. Fiore, V. De Laurentiis, G. Vignali and S. Sala, Bioresour. Technol., 2020, 312, 123575 CrossRef CAS PubMed.
- G. S. da Rosa, T. R. Martiny, G. L. Dotto, S. K. Vanga, D. Parrine, Y. Gariepy, M. Lefsrud and V. Raghavan, Sustainable Mater. Technol., 2021, 28, e00276 CrossRef.
- D. Rente, A. Paiva and A. R. Duarte, Molecules, 2021, 26, 2336 CrossRef CAS PubMed.
- M. Gagneten, G. Leiva, D. Salvatori, C. Schebor and N. Olaiz, Food Bioprocess Technol., 2019, 12, 1102–1109 CrossRef CAS.
- P. Sharma, V. K. Gaur, R. Sirohi, S. Varjani, S. H. Kim and J. W. Wong, Bioresour. Technol., 2021, 325, 124684 CrossRef CAS PubMed.
- J. R. Costa, R. V. Tonon, L. Cabral, L. Gottschalk, L. Pastrana and M. E. Pintado, ACS Sustainable Chem. Eng., 2020, 8, 13112–13125 CrossRef CAS.
- O. Vega-Castro, J. Contreras-Calderon, E. León, A. Segura, M. Arias, L. Pérez and P. J. A. Sobral, J. Biotechnol., 2016, 231, 232–238 CrossRef CAS PubMed.
- T. M. M. M. Amaro, D. Rosa, G. Comi and L. Iacumin, Front. Microbiol., 2019, 10, 992 CrossRef PubMed.
- A. Kovalcik, D. Kucera, P. Matouskova, I. Pernicova, S. Obruca, M. Kalina, V. Enev and I. Marova, J. Environ. Chem. Eng., 2018, 6, 3495–3501 CrossRef CAS.
- R. Davis, R. Kataria, F. Cerrone, T. Woods, S. Kenny, A. O'Donovan, M. Guzik, H. Shaikh, G. Duane, V. K. Gupta, M. G. Tuohy, R. B. Padamatti, E. Casey and K. E. O'Connor, Bioresour. Technol., 2013, 150, 202–209 CrossRef CAS PubMed.
- M. Matos, R. A. P. Cruz, P. Cardoso, F. Silva, E. B. Freitas, G. Carvalho and M. A. M. Reis, ACS Sustainable Chem. Eng., 2021, 9, 8270–8279 CrossRef CAS.
- H. M. C. Azeredo, H. Barud, C. S. Farinas, V. M. Vasconcellos and A. M. Claro, Front. Sustainable Food Syst., 2019, 3, 7 CrossRef.
- D. Lin, Z. Liu, R. Shen, S. Chen and X. Yang, Int. J. Biol. Macromol., 2020, 158, 1007–1019 CrossRef CAS PubMed.
- R. A. Rodríguez Galán, M. L. Franco García and J. Puiggalí Bellalta, Biodegradable poly(ester amide)s: synthesis and applications, in Biodegradable Polymers: Processing, Degradation and Applications, ed. G. P. Felton, Nova Publishers, 2011, pp. 207–272 Search PubMed.
- G. Perotto, R. Simonutti, L. Ceseracciu, M. Mauri, D. Besghini and A. Athanassiou, Polymer, 2020, 200, 122598 CrossRef CAS.
- D. Merino, R. Simonutti, G. Perotto and A. Athanassiou, Green Chem., 2021, 23, 5956–5971 RSC.
- D. Merino and A. Athanassiou, Green Chem., 2022 Search PubMed , Under Review.
- D. Merino, U. C. Paul and A. Athanassiou, Food Packag. Shelf Life, 2021, 29, 100707 CrossRef.
- R. Ruan, Y. Zhang, P. Chen, S. Liu, L. Fan, N. Zhou, K. Ding, P. Peng, M. Addy, Y. Cheng, E. Anderson, Y. Wang, Y. Liu, H. Lei and B. Li, Biofuels: Introduction, 2019 Search PubMed.
- M. Jawaid, P. M. Tahir and N. Saba, Lignocellulosic fibre and biomass-based composite materials: processing, properties and applications, Woodhead Publishing, 2017 Search PubMed.
- G. C. Lenhani, D. F. dos Santos, D. L. Koester, B. Biduski, V. G. Deon, M. Machado Junior and V. Z. Pinto, J. Polym. Environ., 2021, 29(9), 2813–2824 CrossRef CAS.
- S. Collazo-Bigliardi, R. Ortega-Toro and A. Chiralt, Food Packag. Shelf Life, 2019, 22, 100383 CrossRef.
- M. Mehdikhani, L. Gorbatikh, I. Verpoest and S. V. Lomov, J. Compos. Mater., 2019, 53, 1579–1669 CrossRef CAS.
- T. de Moraes Crizel, T. M. H. Costa, A. de Oliveira Rios and S. H. Flôres, Ind. Crops Prod., 2016, 87, 218–228 CrossRef.
- N. H. Farah, H. Salmah and M. Marliza, Procedia Chem., 2016, 19, 335–339 CrossRef CAS.
- U. Qasim, A. I. Osman, A. H. Al-Muhtaseb, C. Farrell, M. Al-Abri, M. Ali, D. V. N. Vo, F. Jamil and D. W. Rooney, Environ. Chem. Lett., 2021, 19, 613–641 CrossRef CAS.
- H. Kargarzadeh, N. Johar and I. Ahmad, Compos. Sci. Technol., 2017, 151, 147–155 CrossRef CAS.
- G. P. Bruni, J. P. de Oliveira, L. M. Fonseca, F. T. da Silva, A. R. G. Dias and E. da Rosa Zavareze, Starch – Stärke, 2020, 72, 1900051 CrossRef CAS.
- R. A. Ilyas, S. M. Sapuan, M. R. Ishak and E. S. Zainudin, Carbohydr. Polym., 2018, 202, 186–202 CrossRef CAS PubMed.
- T. T. de Barros-Alexandrino, M. M. Tosi and O. B. G. Assis, Polym. Eng. Sci., 2019, 59, E287–E292 CrossRef CAS.
- J. Huang, L. Zhang and F. Chen, J. Appl. Polym. Sci., 2003, 88, 3284–3290 CrossRef CAS.
- J. Li, F. Ye, L. Lei and G. Zhao, Int. J. Biol. Macromol., 2018, 115, 547–553 CrossRef CAS PubMed.
- J. S. Lee, E. S. Lee and J. Han, Sci. Rep., 2020, 10(1), 1–15 CrossRef CAS PubMed.
- R. Priyadarshi, B. Kumar, F. Deeba, A. Kulshreshtha and Y. S. Negi, Food Hydrocolloids, 2018, 85, 158–166 CrossRef CAS.
- S. Tulamandi, V. Rangarajan, S. S. H. Rizvi, R. S. Singhal, S. K. Chattopadhyay and N. C. Saha, Food Packag. Shelf Life, 2016, 10, 60–71 CrossRef.
- G. Tedeschi, J. J. Benitez, L. Ceseracciu, K. Dastmalchi, B. Itin, R. E. Stark, A. Heredia, A. Athanassiou and J. A. Heredia-Guerrero, ACS Sustainable Chem. Eng., 2018, 6, 14955–14966 CrossRef CAS.
- C. Torres-León, A. A. Vicente, M. L. Flores-López, R. Rojas, L. Serna-Cock, O. B. Alvarez-Pérez and C. N. Aguilar, LWT, 2018, 97, 624–631 CrossRef.
- M. Menossi, M. Cisneros, V. A. Alvarez and C. Casalongué, Agron. Sustainable Dev., 2021, 41(4), 1–27 Search PubMed.
- H. Wu, Y. Lei, R. Zhu, M. Zhao, J. Lu, D. Xiao, C. Jiao, Z. Zhang, G. Shen and S. Li, Food Hydrocolloids, 2019, 90, 41–49 CrossRef CAS.
- Z. N. Hanani, A. A. Husna, S. N. Syahida, M. N. Khaizura and B. Jamilah, Food Packag. Shelf Life, 2018, 18, 201–211 CrossRef.
- J. C. P. Santos, R. C. S. Sousa, C. G. Otoni, A. R. F. Moraes, V. G. L. Souza, E. A. A. Medeiros, P. J. P. Espitia, A. C. S. Pires, J. S. R. Coimbra and N. F. F. Soares, Innovative Food Sci. Emerging Technol, 2018, 48, 179–194 CrossRef CAS.
- W. X. Du, C. W. Olsen, R. J. Avena-Bustillos, T. H. McHugh, C. E. Levin and M. Friedman, J. Food Sci., 2009, 74, M372–M378 CrossRef CAS PubMed.
- P. Rodsamran and R. Sothornvit, Food Hydrocolloids, 2019, 97, 105173 CrossRef CAS.
- M. Kurek, L. Hlupić, I. E. Garofulić, E. Descours, M. Ščetar and K. Galić, Food Packag. Shelf Life, 2019, 20, 100315 CrossRef.
- T. de Moraes Crizel, A. de Oliveira Rios, V. D. Alves, N. Bandarra, M. Moldão-Martins and S. H. Flôres, Food Hydrocolloids, 2018, 74, 139–150 CrossRef.
- H. Almasi, S. Forghani and M. Moradi, Food Packag. Shelf Life, 2022, 32, 100839 CrossRef.
- J. G. de Oliveira Filho, A. R. C. Braga, B. R. de Oliveira, F. P. Gomes, V. L. Moreira, V. A. C. Pereira and M. B. Egea, Food Res. Int., 2021, 142, 110202–110202 CrossRef PubMed.
- L. A. Toro-Márquez, D. Merino and T. J. Gutiérrez, Food Bioprocess Technol., 2018, 11, 1955–1973 CrossRef.
- K. Halász and L. Csóka, Food Packag. Shelf Life, 2018, 16, 185–193 CrossRef.
- M. M. Goodarzi, M. Moradi, H. Tajik, M. Forough, P. Ezati and B. Kuswandi, Int. J. Biol. Macromol., 2020, 153, 240–247 CrossRef PubMed.
- J. Zia, G. Mancini, M. Bustreo, A. Zych, R. Donno, A. Athanassiou and D. Fragouli, Chem. Eng. J., 2021, 403, 126373 CrossRef CAS.
- N. Jirukkakul, Int. Food Res. J., 2016, 23, 95–101 CAS.
- A. T. Petkoska, D. Daniloski, N. M. D'Cunha, N. Naumovski and A. T. Broach, Food Res. Int., 2021, 140, 109981 CrossRef.
- K. Ncama, L. S. Magwaza, A. Mditshwa and S. Z. Tesfay, Food Packag. Shelf Life, 2018, 16, 157–167 CrossRef.
- T. A. Comunian, M. P. Silva and C. J. F. Souza, Trends Food Sci. Technol., 2021, 108, 269–280 CrossRef CAS.
- P. Abhirami, N. Modupalli and V. Natarajan, J. Food Process. Preserv., 2020, 44, 1–11 Search PubMed.
- U. Tylewicz, R. Inchingolo and M. T. Rodriguez-Estrada, in Nutraceutical and Functional Food Components: Effects of Innovative Processing Techniques, Elsevier Inc., 2017, pp. 297–334 Search PubMed.
- S. Brizzolara, G. A. Manganaris, V. Fotopoulos, C. B. Watkins and P. Tonutti, Front. Plant Sci., 2020, 11, 1–16 CrossRef PubMed.
- H. Wei, F. Seidi, T. Zhang, Y. Jin and H. Xiao, Food Chem., 2021, 337, 127750 CrossRef CAS PubMed.
- Z. Parsa, S. Roozbehi, M. Hosseinifarahi, M. Radi and S. Amiri, J. Food Process. Preserv., 2021, 45, 1–15 Search PubMed.
- G. B. Seymour, J. E. Taylor and G. A. Tucker, Biochemistry of fruit ripening, Springer Science & Business Media, 2012 Search PubMed.
- B. Maringgal, N. Hashim, I. S. M. A. Tawakkal and M. T. M. Mohamed, Trends Food Sci. Technol., 2020, 96, 253–267 CrossRef CAS.
- S. Yildirim, B. Röcker, M. K. Pettersen, J. Nilsen-Nygaard, Z. Ayhan, R. Rutkaite, T. Radusin, P. Suminska, B. Marcos and V. Coma, Compr. Rev. Food Sci. Food Saf., 2018, 17, 165–199 CrossRef PubMed.
- L. Sharma, C. S. Saini, H. K. Sharma and K. S. Sandhu, J. Food Process Eng., 2019, 42, 1–9 Search PubMed.
- L. Sharma, C. Singh Saini and H. K. Sharma, J. Food Process. Preserv., 2018, 42(2), e13527 CrossRef.
- M. Y. Won and S. C. Min, Food Sci. Biotechnol., 2018, 27, 1649–1658 CrossRef CAS PubMed.
- M. R. Marshall, J. Kim and C. Wei, Food Agric. Organ., 2000, 41, 259–312 Search PubMed.
- K. M. Moon, E. B. Kwon, B. Lee and C. Y. Kim, Molecules, 2020, 25(12), 2754 CrossRef CAS PubMed.
- X. Wang, D. Kong, Z. Ma and R. Zhao, Ir. J. Agric. Food Res., 2015, 54, 64–71 Search PubMed.
- M. Barth, T. R. Hankinson, H. Zhuang and F. Breidt, Compendium of the Microbiological Spoilage of Foods and Beverages, 2009 Search PubMed.
- S. Alotaibi and R. Tahergorabi, LWT – Food Sci. Technol., 2018, 88, 203–209 CrossRef CAS.
- A. E. C. Fai, M. R. A. de Souza, S. T. de Barros, N. V. Bruno, M. S. L. Ferreira and É. C. B. de Andrade Gonçalves, Postharvest Biol. Technol., 2016, 112, 194–204 CrossRef CAS.
- S. Gorelik and J. Kanner, J. Agric. Food Chem., 2001, 49, 5945–5950 CrossRef CAS PubMed.
- F. Tian, E. A. Decker and J. M. Goddard, Food Funct., 2013, 4, 669–680 RSC.
- S. C. M. Burri, A. Ekholm, U. Bleive, T. Püssa, M. Jensen, J. Hellström, S. Mäkinen, R. Korpinen, P. H. Mattila, V. Radenkovs, D. Segliņa, Å. Håkansson, K. Rumpunen and E. Tornberg, Meat Sci., 2020, 162, 108033 CrossRef CAS PubMed.
- D. R. Johnson and E. A. Decker, Annu. Rev. Food Sci. Technol., 2015, 6, 171–190 CrossRef CAS PubMed.
- Y. Alparslan and T. Baygar, Food Bioprocess Technol., 2017, 10, 842–853 CrossRef CAS.
- V. COMA, Meat Sci., 2008, 78, 90–103 CrossRef CAS PubMed.
- M. Noshad, B. Alizadeh Behbahani, H. Jooyandeh, M. Rahmati-Joneidabad, M. E. Hemmati Kaykha and M. Ghodsi Sheikhjan, Food Sci. Nutr., 2021, 9(3), 1625–1639 CrossRef CAS PubMed.
- K. B. Biji, C. N. Ravishankar, R. Venkateswarlu, C. O. Mohan and T. K. S. Gopal, J. Food Sci. Technol., 2016, 53, 2210–2218 CrossRef CAS PubMed.
- V. Pothakos, F. Devlieghere, F. Villani, J. Björkroth and D. Ercolini, Meat Sci., 2015, 109, 66–74 CrossRef CAS PubMed.
- F. Licciardello, S. Kharchoufi, G. Muratore and C. Restuccia, Food Packag. Shelf Life, 2018, 17, 114–119 CrossRef.
- A. Nawab, F. Alam and A. Hasnain, Int. J. Biol. Macromol., 2017, 103, 581–586 CrossRef CAS PubMed.
- F. Riazi, F. Zeynali, E. Hoseini, H. Behmadi and S. Savadkoohi, Meat Sci., 2016, 121, 350–358 CrossRef CAS PubMed.
- R. Millati, R. B. Cahyono, T. Ariyanto, I. Nafi, A. Stp, R. Utami, P. Stp and M. J. Taherzadeh, Agricultural, Industrial, Municipal, and Forest Wastes: An Overview, Elsevier B.V., 2019 Search PubMed.
- S. Kakadellis and G. Rosetto, Science, 2021, 373, 49–50 CrossRef CAS PubMed.
- K. Ghosh and B. H. Jones, ACS Sustainable Chem. Eng., 2021, 9, 6170–6187 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |