Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green ammonia as a spatial energy vector: a review
Nicholas
Salmon
 and
René
Bañares-Alcántara
and
René
Bañares-Alcántara
 *
*
Department of Engineering Science, University of Oxford, Parks Road, Oxford, OX1 3PJ, UK. E-mail: rene.banares@eng.ox.ac.uk
First published on 7th May 2021
Abstract
Green hydrogen is considered a highly promising vector for deep decarbonisation of energy systems and is forecast to represent 20% of global energy use by 2050. In order to secure access to this resource, Japan, Germany and South Korea have announced plans to import hydrogen; other major energy consumers are sure to follow. Ammonia, a promising hydrogen derivative, may enable this energy transport, by densifying hydrogen at relatively low cost using well-understood technologies. This review seeks to describe a global green ammonia import/export market: it identifies benefits and limitations of ammonia relative to other hydrogen carriers, the costs of ammonia production and transport, and the constraints on both supply and demand. We find that green ammonia as an energy vector is likely to be critical to future energy systems, but that gaps remain in the literature. In particular, rigorous analysis of production and transport costs are rarely paired, preventing realistic assessments of the delivered cost of energy, or the selection of optimum import/export partners to minimise the delivered cost of ammonia. Filling these gaps in the literature is a prerequisite to the development of robust hydrogen and ammonia strategies, and to enable the formation of global import and export markets of green fuel.
1. Introduction
Modern energy systems rely on large scale transport of fossil fuels to supply primary demand in energy-importing nations. As many nations transition towards net zero carbon emissions by the middle of the century, there may be some trend towards local energy generation; however, to a significant extent, importing of energy will remain necessary to continue to meet local demand1,2 in some countries, particularly in order to provide affordable options for deep decarbonisation.3,4Green chemical energy vectors are considered the best technology to enable this transport in a sustainable manner and have the capacity to operate as a global reserve fuel. Compared to other energy storage technologies such as batteries, compressed air energy storage (CAES) and pumped hydro, they are comparatively easy to store in industrial quantities, to transport over very large distances and to deploy over large time scales.5 They can also be used in difficult-to-abate sectors, such as the steel and cement industries, or as a source of high-grade heat.6
Green hydrogen is the foundation of most chemical energy vectors. Although it has a high gravimetric energy density, its volumetric energy density is very poor; even in the liquid state under cryogenic conditions it carries only 2.4 kW h L−1 (ref. 7) (compared to gasoline, whose liquid energy density is ∼9 kW h L−1 (ref. 8)). Chemical derivatives of hydrogen are therefore considered a promising option in order to make it more easily portable; transport cost reductions of at least a factor of three are forecast.9,10
Green ammonia is one such chemical derivative; its liquid energy density is 3.5 kW h L−1.7 Ammonia requires only water, air and power for its production, and it does not release carbon emissions on combustion. A schematic demonstrating the production of green ammonia is shown in Fig. 1. It can be stored at relatively mild conditions (−33 °C at atmospheric pressure, or room temperature at ∼10 bar (ref. 5)) compared to liquid hydrogen (−253 °C (ref. 7)). Global systems for ammonia transport are well established and understood. At present ammonia has application mainly as a fertilizer; however, if adopted as an energy vector, it can be used directly, or can be cracked back into hydrogen.
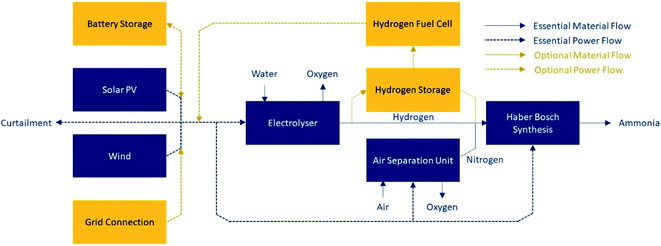 | ||
| Fig. 1 Schematic of green ammonia production. Essential equipment is included in dark blue. Optional equipment to achieve the required process flexibility is shown in orange; at least one of the optional units must be present, or it will not be possible to maintain stable operation of the Haber–Bosch loop. Only one of solar PV or wind is required, although both may be used (adapted from Armijo and Philibert11). | ||
Despite these promising properties, the energy produced from green ammonia in most circumstances exceeds the cost of liquid fossil fuels; this high cost is the largest barrier to widespread adoption of ammonia as an energy vector.10 While reductions in cost are expected through technical improvements in renewable energy generation and electrolyser cells, rigorous system-wide optimisation will be required to ensure availability of dependable and affordable renewable energy.
A number of reviews have recently been published investigating the role of green ammonia in a renewable energy economy. Yapicioglu et al.12 investigated a range of ammonia production and consumption technologies. Rouwenhorst et al.13 focussed on plants between 1 and 10 MW, reviewing various recent technological advances, and designing an optimised production facility. Valera-Medina et al.10 specifically researched ammonia to power pathways, explaining the many technical considerations required in using ammonia as an energy source. Elishav et al.14 provide a comprehensive study of nitrogen based fuels across their life-cycle.
However, despite widespread analysis of ammonia production, and the growing global intention to export ammonia internationally,15,16 there have been no reviews into the true cost and capacity of using ammonia as a spatial energy vector between continents. Other works, e.g. Elishav et al.,14 report the levelized cost of ammonia production, but they do not provide a rigorous comparison of the literature to understand the cause of variability in ammonia production costs. The purpose of this review is to consider the existing body of literature researching the cost of ammonia production and transport on a global scale, in order to assess the realistic cost of energy in an ammonia economy. In doing so, it will identify the major constraints on ammonia supply in energy exporting regions, and on ammonia demand in energy importing regions.
1.1 Scope of review
Hydrogen is often ascribed a label, which refers to the feedstock used and emissions released in its production. When hydrogen is reacted with nitrogen in a Haber–Bosch (HB) loop to produce ammonia, that ammonia is referred to with the same label as the hydrogen from which it was synthesised. At present, ammonia is produced mostly from fossil fuels: it is labelled as brown if hydrogen is made using coal gasification, or grey if hydrogen is made using natural gas reforming. These fossil fuel processes are also referred to as conventional ammonia production. Blue ammonia uses the same feedstock as brown and grey ammonia, but includes a carbon capture and storage (CCS) unit. To be truly ‘blue’, this CCS unit must capture the CO2 in both flue gas streams: the concentrated stream leftover from the water-gas-shift reactor after hydrogen is removed, and the comparatively dilute stream released from the furnace. Green ammonia is not widely produced at present and is made entirely from electricity, water and air; the hydrogen for its synthesis is generated from electrolyser stacks. The term ‘green’ ammonia implies that the electricity is renewably sourced, although much of the literature at present includes a grid connection; the suitability of this approach is discussed in Section 3. Future technologies which produce ammonia directly from raw feed materials without an interim hydrogen generation step will also be considered green if they use only renewable electricity for energy input and have no other associated carbon emissions.This paper focusses on green ammonia. Brown and grey ammonia are unsustainable, and while blue ammonia has comparatively low emissions, its production cost will always be higher than conventional technologies because it requires additional processing. Since green ammonia has no emissions, and is expected to fall below the cost of conventional production at some point before 2050, it is likely to become the dominant mode of ammonia production by the middle of the century.17 The German government considers green hydrogen to be the only sustainable production technology in the long-term;15 the logical extension of this approach excludes all ammonia which is not green.
Downstream of hydrogen production, the HB loop used to produce ammonia remains broadly unchanged regardless of the ‘colour’ of ammonia produced, but there are three minor differences. Firstly, all currently commercially available electrolysers operate at low temperature, so cannot recycle heat as useful energy. Conventional and blue ammonia plants normally recycle heat from the exothermic Haber–Bosch reactor into the endothermic hydrogen reformer; green ammonia plants remove this heat using cooling air or water. Secondly, removal of oxygen from air to obtain the nitrogen required for Haber–Bosch synthesis is integrated into the hydrogen production in conventional ammonia production, usually in an autothermal reformer. Green ammonia plants require a dedicated air separation unit to produce this nitrogen. Thirdly, green ammonia plants drive compressors using electricity, rather than steam.18
This review focusses only on ammonia synthesised using a HB loop. It does describe improvements which may be made to the HB process to increase efficiency and reduce capital costs; however, it does not consider novel technologies which may be more profitable for ammonia production in the future, such as the direct electrolysis of ammonia from water and air,19 or concentrated solar thermal technologies.20 This focus will provide conservative estimates for ammonia costs and production constraints; in case of a major technological breakthrough, these costs are expected to fall.
2. Energy carriers
This section of the review compares different hydrogen carriers throughout their life cycle, from production using green hydrogen at 30 bar to consumption in an energy-importing region. It begins with a description of each of the options, before comparing the cost estimates available for each in the literature. In doing so, it will identify both the competitors to ammonia as an energy transport vector, and the approximate cost of energy transport.2.1 Production and storage
A range of options are available for long-distance energy transport and are surveyed in detail in the literature. These are summarised in Table 1.| Carrier | Abbreviation/chemical formula | Higher heating value (kW h kg−1)8 | Volumetric energy density (kW h L−1) | Hydrogen wt% | Synthesis energy efficiency(i) | Storage conditions | Renewable production technologies(ii) |
|---|---|---|---|---|---|---|---|
| a Notes: (i) synthesis efficiency is presented as the ratio of the HHV of the product fuel to the input energy (calculated as synthesis electricity/heat duty plus the HHV of input hydrogen, adjusted for stoichiometry). It does not include the efficiency of hydrogen generation, which is assumed to be equal in all cases. (ii) Although some novel technologies are emerging for hydrogen generation and carrier synthesis these are not considered here. The scope of this analysis is limited to processes with a high technology readiness level (TRL). (iii) A range of synthetic hydrocarbons is considered in the literature; data is provided for renewable methanol, the most frequently considered option. (iv) Production energy demand is 0.642 kW h kg−1 NH3 for compression and air separation.17 Theoretically, the exothermic heat of reaction (0.75 kW h kg−1 NH3)8 can be recovered and converted to useful energy, although this is not considered here. (v) For liquefaction energy demand, 6 kW h kg−1 H2 may be possible in future applications; at present 10–12 kW h kg−1 H2 is required.24 The efficiency at both points is reported. (vi) The HHV of the product fuel is taken as 39.4 kW h kg−1 for this calculation (i.e. the HHV of the carried hydrogen). A small energy demand of <0.1 kW h kg−1 (ref. 25) is assumed to operate the synthesis unit. As for ammonia, energy which may be recoverable from the exothermic heat of reaction is neglected. The majority of energy consumption (between 8–10 kW h kg−1 H2)24 for this option occurs during the endothermic dehydrogenation process. (vii) Based on analysis in Hank et al.:24 includes ∼1.2 kW h kg−1 MeOH for compression and ∼9.2 kW h kg−1 MeOH for DAC. (viii) CO2 capture can be achieved using DAC, or from a point emissions source from industry, such as flue-gas from a coal fired power plant. Biomass as a CO2 source is excluded as the land-use efficiency of DAC is ∼100 times higher than that of biomass,1 and this paper is considering very large-scale production, meaning low land efficiencies are assumed to be impractical. | |||||||
| Ammonia | NH3 | 6.25 | 3.50 (ref. 7) | 17.6 | 85%(iv) | −33 °C, 1 bar (ref. 7) | Electrolysis + Haber–Bosch |
| Liquid hydrogen | LH2 | 39.4 | 2.36 (ref. 7) | 100 | 74–85%(v) | −253 °C, 2 bar (ref. 7) | Electrolysis + liquefaction |
| Liquid organic hydrogen carriers | LOHC | — | 1.07–2.77 (ref. 7 and 21) | 3.2–7.3 (ref. 21) | >99.9%(vi) | Ambient | Electrolysis + hydrogenation |
| Synthetic hydrocarbons (e.g. MeOH, syn-LNG)(iii) | MeOH | 6.31 | 4.94 (ref. 8) | 12.5 | 62%(vii) | Ambient | Electrolysis + CO2 capture + methanol synthesis(viii) |
Of the five technologies discussed in the literature, four are chemical storage technologies. The exception is HVDC, which is excluded because it is not efficient across very large distances (i.e. >5000 km) due to the energy losses associated with cable resistance and high capital costs.1 Approximately 4.9% of energy is lost per 1000 km of cable.23 In addition, it cannot provide all the benefits associated with chemical fuels, including energy storage or the provision of high-grade heat.
The major economic costs of the remaining four technologies occur at different points of production and use. Liquid hydrogen requires significant energy input for liquefaction. Because it is a cryogenic liquid, the storage equipment required has very high CAPEX. Additionally, some daily boil-off is inevitable regardless of the quality of storage equipment. The rate of boil off is a function of tank design, but is typically reported to be between 0.2–0.3% per day,26 meaning storage delays in shipping or receiving ports are costly. Very large costs are also forecast for the unloading and loading equipment required to transfer LH2; the IEA estimates a CAPEX of 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 USD per t of liquid hydrogen storage capacity, compared to only 11
000 USD per t of liquid hydrogen storage capacity, compared to only 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 USD per t H2 if ammonia is used as a storage medium.25 Salt caverns for gaseous hydrogen are often discussed as a storage alternative to liquid hydrogen;27 although these may offer storage cost reductions, they are not advantageous for energy transport because the hydrogen remains at low density.
000 USD per t H2 if ammonia is used as a storage medium.25 Salt caverns for gaseous hydrogen are often discussed as a storage alternative to liquid hydrogen;27 although these may offer storage cost reductions, they are not advantageous for energy transport because the hydrogen remains at low density.
Liquid organic hydrogen carriers use molecules that are typically liquid at ambient conditions which can be loaded with hydrogen by the energy supplier, and unloaded by the importer, processes referred to as hydrogenation and dehydrogenation respectively. A range of liquid organic carriers have been considered, including toluene, di-benzyltoluene, methanol and naphthalene.7 Shipping and storage of LOHCs can be done under ambient conditions using existing systems for hydrocarbons. However, the volumetric hydrogen density is poor,7 the most efficient carriers themselves can have very high capital costs,24 and additional shipping costs are accrued as the unloaded molecule must be returned to the energy supplier for hydrogenation.
Synthetic hydrocarbons are produced by reacting electrolytic hydrogen with a carbon source. The affordability of this technology depends strongly on the availability of carbon; if a concentrated stream of CO2 is not available, then the energy costs of obtaining carbon via direct air capture are very high.24 Decarbonised energy systems will not have concentrated streams of CO2 available; even if they were available, chemicals produced using CO2 captured from fossil sources face regulatory barriers to being considered carbon neutral.28 Once synthetic hydrocarbons are produced, shipping and transport is straightforward, and can be performed using existing technologies.
The main inefficiency in producing ammonia is the exothermic synthesis reaction in which energy is lost to heat at the production site. Although some energy is recovered to pre-heat reactants, most of the excess is removed as waste heat from the electrified Haber–Bosch process using cooling water. However, the energy loss during ammonia synthesis is much less than hydrocarbon synthesis (as per Table 1), and, like synthetic hydrocarbons, its transport is straightforward given its comparatively high energy density and mild storage conditions.
2.2 Distribution and consumption
Having been delivered to the energy-importer, there are a range of options for the consumption of each of the hydrogen carriers, summarised in Table 2.| Carrier | Additional processing steps | Additional energy consumption (kW h kg−1 H2) | Distribution method | End-use | End-use efficiency (%) |
|---|---|---|---|---|---|
| a Notes: (i) in some cases, it may be possible to generate some power from the evaporation of liquid hydrogen, by using the hydrogen as a cold sink. It is assumed that this is negligible in comparison to the power which can be generated by using hydrogen in a fuel cell or combustion turbine. | |||||
| NH3 | None | 0 | Trucking/pipeline | Fertilizer | N/A |
| None | 0 | Trucking/pipeline | SOFC/CCGT/shipping fuel | 60 (ref. 29) | |
| Ammonia cracking | 8.52 | Trucking/pipeline | CCGT/hydrogen fuel cell | 50–60 (ref. 30) | |
| LH2 | Evaporation | 0(i) | Trucking (liquid)/pipeline (compressed gas) | CCGT/hydrogen fuel cell | 50–60 (ref. 30) |
| LOHC | Dehydrogenation | 8.25 (ref. 24) | Pipeline (after dehydrogenation) | CCGT/hydrogen fuel cell | 50–60 (ref. 30) |
| MeOH/syn-LNG | None | 0 | Trucking/pipeline | SOFC/CCGT/shipping fuel/chemical feedstock | 60 (ref. 29) |
Pipeline distribution of hydrogen is possible regardless of the carrier selected; its cost is expected to be low relative to hydrogen production for very large systems. Existing natural gas grids can tolerate a small percentage of hydrogen (typically 4–6% depending on national regulation25), but for large scale hydrogen economies, new pipeline and compressor systems will be required given the potential for hydrogen embrittlement of steel in existing pipelines.31 Ammonia pipelines are forecast to be cheaper than hydrogen pipelines due to increased carrier density and reduced costs of pumping compared to compression.32
Trucking may also be a useful option for distribution over short distances; this option is better for fuels which can be used directly (i.e. liquid hydrogen, synthetic hydrocarbons, and ammonia if combustion/SOFCs are available). In general, existing infrastructure can be used, although specially designed trucks are required for the transport of liquid hydrogen. Focussing specifically on distribution of hydrogen, Yang and Ogden33 identified trucking of liquids as the best option for moderate distances at small hydrogen distribution rates; for larger distribution rates, pipelines were preferable at all distances.
In some applications, ammonia may also be used directly without further processing. The clearest example is in the fertiliser industry. While ammonia can be directly used as fertiliser,34 it is typically upgraded into urea, ammonium nitrate (AN) or calcium ammonium nitrate (CAN).35 While this may be helpful to create early supply chains for green ammonia, the scope of this report is focussed on the long-term future of ammonia as an energy vector, in which its consumption as fertiliser will be a relatively small fraction of its use. It is also likely to play a substantial role in a decarbonised shipping industry,36 where its only substantial competitor is methanol.37
Alternatively, ammonia can be used directly in a fuel cell; solid oxide fuel cells (SOFCs) offer the highest efficiencies.35,38 When ammonia is used in an SOFC, it can be fed directly to the anode, because the high temperature of these cells effectively cracks the ammonia into its constituent elements before the hydrogen is oxidised into water.19 Recent developments have substantially increased cell durability and efficiency, which is comparable to a hydrogen fuel cell;39 further growth of this market is expected.10 Because of their limited usage, the cost of these fuel cells is not widely published; however, it is expected that their price will fall rapidly in coming years.
Traditionally, combustion of ammonia has been challenging due to its low burning velocity and high minimum ignition energy. However, because of renewed interest in the field, ammonia turbines are likely be commercialised in the medium term and are currently being used at a pilot scale of 50 kW e.40 To the extent that pure ammonia combustion is difficult, partial cracking of ammonia and the combustion of a hydrogen/ammonia blend can overcome the challenges of ammonia combustion with comparatively small energy losses in the endothermic cracking process; a 70/30 mixture of ammonia and hydrogen by volume has been identified as a viable operating point.29,41
If ammonia cannot be directly combusted, then it requires substantial energy input to crack it back into hydrogen. Similar requirements also exist for LOHCs, although the energy demand for this technology is a function of the specific molecule selected for energy transport. The process for ammonia cracking occurs at high temperatures (>550 °C), and resembles steam methane reforming;29 the typical dehydrogenation temperature for an LOHC is comparatively low (∼300 °C).42 While some estimates assume that the energy for these cracking reactions can be supplied using waste heat,43 it is unlikely that a large number of applications will have waste heat available at such high temperatures. In certain applications, therefore, significant energy loss may be observed for ammonia or LOHC consumption in the energy-importing nation. The approximate cost of ammonia cracking is estimated to add ∼1 € per kg to the cost of produced hydrogen,44 assuming that no waste heat is available. The cost of cracking is likely to fall over time as novel membrane technologies allow for the single-step ammonia cracking and its subsequent purification; this will enable simpler conversion of ammonia to high-purity hydrogen for use in proton exchange membrane (PEM) fuel cells.45,46
2.3 Carbon neutrality
Each of the carriers described can be carbon neutral if appropriate technologies are used. In general, true carbon neutrality requires no greenhouse emissions at any point in the supply chain: hydrogen production, carrier synthesis, shipping fuel, distribution by pipeline or truck, and cracking/dehydrogenation must all emit no carbon. In the case of synthetic hydrocarbons, the carbon source must be direct air capture or biomass (although due to land efficiency constraints, biomass availability may be limited).1 If CCS is used when these synthetic fuels are combusted, then the fraction of CO2 captured from the combustion gases may be considered carbon negative.At present, the only scheme which exists to certify hydrogen as “carbon neutral” is the EU program CertifyHy;47 its 2019 specifications required that CO2 equivalent emissions of hydrogen production be less than 36 g CO2-e per MJ (based on LHV), which amounts to a 60% reduction compared to production by steam methane reforming. Under this scheme, producers can exclude emissions caused during transport. It is therefore possible under this scheme to use some non-green grid electricity in production, and to use transport technologies which emit CO2; however, in the long term, this scheme is likely to tighten its requirements, and the true carbon neutrality described above will become the industry standard. The development of more wide-reaching schemes is a prerequisite for exporting chemicals as energy vectors in order to guarantee their origin.28
One challenge for ammonia if it is directly combusted or used in a fuel cell is its comparatively high NOx emissions. Beyond the harms of NOx as a local and regional pollutant, it is also a potent greenhouse gas.10 Bicer and Dincer,48 for instance, identified that an ammonia powered car could be responsible for almost twice the emissions of acid gases as one powered by diesel, mainly due to NOx. Emissions of NOx from ammonia based energy generation can be controlled by using unburned ammonia for catalytic reduction of exhaust gasses,49,50 or with novel burner designs.41 Similarly, use of the SOFC-H type fuel cell (in which a hydrogen proton is transported through the electrolyte, rather than an oxygen ion in an SOFC-O fuel cell) will enable efficient electricity generation without NOx formation.38,51
2.4 Economic comparison of different energy carriers
| Category | Trigger words |
|---|---|
| Ammonia | Ammonia, Haber–Bosch |
| Renewable | Renewable, green |
| Transport | Transport, shipping, inter-continental, export, import |
| Cost | Cost, techno-economic, LCOA, levelised |
Further literature was identified in the citations of the papers located using the Scopus search. Papers were eliminated from this search if they did not provide an estimate of transport costs over a large distance (>300 km). Since this section considers ammonia as a carrier, papers were included even if the ammonia was blue or grey (unlike Section 3, which considers only green ammonia). In order to provide comparison to other carriers, papers were also included which considered other chemical energy storage vectors.
Despite its high hydrogen density and comparatively straightforward storage requirements, ammonia has received limited attention as an energy vector. In the literature surveyed, only 9 authors provided an economic assessment of the cost of international ammonia transport (compared to at least 17 who analysed liquid hydrogen).
All studies considered were at industrial scale, with either pipeline or shipping as the main mode of transport. The shipping volume ranged from 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 to 160
000 m3 to 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3. Authors using 160
000 m3. Authors using 160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 ships are forecasting significant growth in the industry; at present ammonia is shipped in the same vessels used for LPG, only a small fraction of which are greater than 90
000 m3 ships are forecasting significant growth in the industry; at present ammonia is shipped in the same vessels used for LPG, only a small fraction of which are greater than 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3.52
000 m3.52
| Author | Year | Discount rate | Hydrogen production method | H2 carrier | Produced in | Delivered to | Ocean transport distance (km) | Transport mechanism | Production cost/GJ (USD) | Terrestrial transport cost(i) (USD per GJ) | Ocean transport cost(i) (USD per GJ) | Delivered costs of carrier2 (USD per GJ) | Delivered costs of carrier(ii) (USD per t) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Notes: (i) transport costs include special storage/load out equipment required at the shipping and receiving ports. They do not include costs of ammonia synthesis/liquefaction/hydrogenation. Fuel energies are reported on an HHV basis. (ii) The delivered costs are a full life-cycle cost estimate, including renewable electricity, hydrogen production, any carrier synthesis costs and transport. Dehydrogenation costs are included for LOHCs, since they are a prerequisite for usage; cracking costs are not included for ammonia, except in the case of DNV GL.22 (iii) Values are best estimates from other data reported in the paper. | |||||||||||||
| Fúnez Guerra et al.58 | 2020 | 14.67 | Solar (7000 h per year) | NH3 | Chile | Japan | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
Ship | 14.67(iii) | — | 2.44 | 16.89 | 380(iii) |
| Hydrogen import coalition57 (cost estimates are forward projections to 2030) | 2020 | 4.3 | Wind/solar; storage/curtailment details not specified | NH3 | Morocco | Belgium | 3000 | Ship | 17.42 | — | 1.53 | 18.94 | 426 |
| LH2 | 16.81 | 10.69 | 27.50 | 3905 | |||||||||
| MeOH | 22.00 | 1.53 | 23.53 | 541 | |||||||||
| CH4 | 23.22 | 2.44 | 25.67 | 1425 | |||||||||
| LOHC | 15.58 | 11.31 | 26.89 | 3818 | |||||||||
| Hank et al.24 | 2020 | 5 | Wind/solar; salt cavern for H2 storage | NH3 | Morocco | Germany | 4000 | Ship | 35.75 | — | 2.14 | 37.89 | 853 |
| LH2 | 36.36 | 2.14 | 38.50 | 5467 | |||||||||
| MeOH | 39.11 | 0.92 | 40.03 | 921 | |||||||||
| CH4 | 39.11 | 5.19 | 44.31 | 2459 | |||||||||
| LOHC | 27.50 | 20.17 | 47.67 | 6769 | |||||||||
| IEA59 | 2020 | 8 | — | LH2 | Australia | Japan | 9000 | Ship | 38.73 | — | 10.56 | 49.30 | 7000 |
| NH3 | 32.16 | 1.57 | 33.73 | 759 | |||||||||
| LOHC | 29.58 | 11.97 | 41.55 | 5900 | |||||||||
| Ishimoto et al.60 | 2020 | 7.5 | NG & CCS (80%) + wind (20%) | LH2 | Norway | Japan | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Ship | 24.63 | — | 29.59 | 54.23 | 7700 |
| NH3 | 37.01 | 11.56 | 48.57 | 1093 | |||||||||
| Heuser et al.61 | 2019 | 8 | Wind | LH2 | Patagonia | Japan | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
Pipeline/ship | 128.28 | 4.29 | 10.38 | 34.95 | 4963 |
| Kawakami et al.62 | 2019 | 10 | NG + CCS | NH3 | Middle East | Japan | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Ship | 15.94 | — | 2.96 | 18.87 | 425 |
| Wijayanta et al.7 | 2019 | — | — | NH3 | Australia | Japan | 9000 | Ship | 14.60 | 1.22 | 4.05 | 19.83 | 446 |
| LH2 | 12.60 | 1.09 | 12.36 | 26.02 | 3695 | ||||||||
| LOHC | 11.65 | 1.09 | 4.00 | 22.89 | 3251 | ||||||||
| Babarit et al.63 | 2018 | — | Wind | LH2 | Offshore | Land | 1000 | Truck/ship | 27.10 | 4.56 | 3.73 | 35.27 | 5008 |
| DNV GL22 | 2018 | 7 | Greened grid electricity | LH2 | — | — | 1000 | Ship | 52.40(iii) | — | 27.21 | 79.40 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275 275 |
| NH3 | 63.95(iii) | 0.42 | 64.12 | 1443 | |||||||||
| LOHC | 49.25(iii) | 3.14 | 52.19 | 7412 | |||||||||
| Kamiya et al.54 | 2015 | — | Coal + CCS | LH2 | Australia | Japan | 9000 | Pipeline/ship | 18.67 | 0.24 | 4.68 | 23.40 | 3323 |
| Teichmann et al.43 | 2012 | 6 | Solar | LOHC | Africa | Germany | 5050 | Ship/truck | 31.48(iii) | 1.51 | 2.04 | 34.52 | 4902 |
| LH2 | 34.99(iii) | 1.27 | 9.06 | 44.76 | 6356 | ||||||||
| Watanabe et al.64 | 2010 | — | Wind | LH2 | Patagonia | Japan | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
Pipeline/ship | 23.47 | 0.00 | 13.60 | 36.61 | 5199 |
| Stiller et al.23 | 2008 | 8 | Onshore wind | LH2 | Northern Norway | Northern Germany | 2300 | Ship | 35.30(iii) | — | 5.62 | 40.11 | 5695 |
| Wietschel and Hasenauer55 | 2007 | — | Hydro/geothermal | LH2 | Iceland | UK | 1300 | Ship | 22.02 | — | 0.93 | 22.40 | 3181 |
| Solar | LH2 | Algeria | Italy | 1000 | Ship | 40.21 | 0.93 | 40.13 | 5699 | ||||
| Hijikata26 | 2002 | — | Hydro | LH2 | — | — | 5000 | Ship | — | — | — | 95.99 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 631 631 |
| NH3 | 99.38 | 2236 | |||||||||||
| MeOH | 96.75 | 2225 | |||||||||||
| Gretz et al.65 | 1993 | 8 | Hydro | LH2 | Quebec | Hamburg | 6000 | Ship | 54.81(iii) | — | 32.41 | 84.41 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 986 986 |
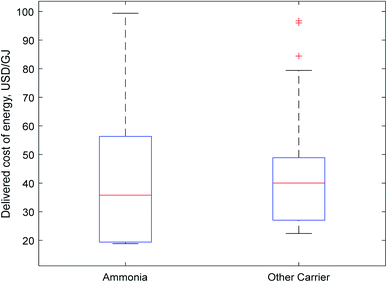 | ||
| Fig. 2 Boxplot of carrier costs. Non-ammonia carriers were clustered together as a comparatively small number of papers were considered for each individual carrier compared to ammonia. | ||
The results for both transport and delivered costs have a large range, indicating that there is little consensus in the literature as to the true cost of energy delivered using one of these carriers. There are wide variations both within and between different carriers.
Fig. 2 represents this wide range in predicted costs of delivered energy; there is a long tail at the upper end of costs for both ammonia and other hydrogen carriers. These tails represent older papers which relied on less-developed technology. However, more recent papers indicate a growing consensus in the literature that ammonia will be the cheapest carrier, as indicated by its more affordable median and lower quartile prices. Although this initial data visualisation is promising for ammonia, direct comparison of the delivered price of energy calculated by different authors is not entirely representative, as different input values (e.g. for the cost of renewable electricity) may skew results between different authors. A more rigorous analysis as is provided in Section 2.4.3 is required for meaningful comparison of the suitability of different carriers.
Teichmann et al.43 are bullish about the prospects of LOHCs, estimating them to have very low transport and storage costs; their delivered cost would register as one of the lowest, except for their assumption of high electricity prices during production. Other authors were far less ambitious about the prospects of this technology; Hank et al.24 identified it as the most expensive option of the five considered because of the high capital costs of the carriers themselves. Wijayanta et al.7 similarly estimated LOHCs to be an expensive option due to the high costs of dehydrogenation.
No paper identified synthetic hydrocarbons as the best option because of the high costs and inefficiencies of DAC. Even where relatively concentrated streams of CO2 were assumed to be available in a land-transport study by Tso et al.,56 ammonia still outperformed methanol. For very long transport distances, the Hydrogen Import Coalition57 preferred methanol to ammonia in some cases, although they assumed a large 50% reduction in the price of direct air capture compared to present state of the art technology.
Seven authors directly compared ammonia to another medium for international transport purposes. In four of these cases,7,24,25,57 ammonia was identified as the cheapest option for international energy transport. Of the remaining authors, Ishimoto et al.60 and Hijikata26 preferred LH2, and DNV GL22 preferred LOHCs. Hijikata observed only very small differences between various energy carriers and used data from 2002 which no longer provides an accurate measure of production costs. Ishimoto et al. only preferred liquid hydrogen over very short distances, used ambitious forecasts for hydrogen liquefaction costs, and used transport costs that were inconsistent with other literature: approximately 6 USD per GJ of ammonia over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km, compared to 2–3 USD per GJ from most other sources over comparable distances. DNV GL estimates the cracking energy demand for ammonia to be much higher than the dehydrogenation energy for an LOHC; they also do not clearly factor the capital costs of the LOHC itself, which Hank et al.24 and the Hydrogen Import Coalition57 report to be very large. The shipping distance considered by DNV GL is only 1000 km; over intercontinental distances (∼10
000 km, compared to 2–3 USD per GJ from most other sources over comparable distances. DNV GL estimates the cracking energy demand for ammonia to be much higher than the dehydrogenation energy for an LOHC; they also do not clearly factor the capital costs of the LOHC itself, which Hank et al.24 and the Hydrogen Import Coalition57 report to be very large. The shipping distance considered by DNV GL is only 1000 km; over intercontinental distances (∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km) the lower volumetric and gravimetric energy density of LOHCs will substantially increase their relative transport costs compared to ammonia. In both the cases of DNV GL and Ishimoto et al., if the energy associated with cracking were not considered, because ammonia could be directly combusted or used in a fuel cell, then ammonia would be the preferred option.
000 km) the lower volumetric and gravimetric energy density of LOHCs will substantially increase their relative transport costs compared to ammonia. In both the cases of DNV GL and Ishimoto et al., if the energy associated with cracking were not considered, because ammonia could be directly combusted or used in a fuel cell, then ammonia would be the preferred option.
Authors tended to consider hydrogen export from one energy exporting nation/region to an energy importing nation/region. The energy exporters included: Algeria, Argentina, Australia, Chile, Iceland, Morocco, Norway, Oman, Saudi Arabia and the US. The only specific energy importers considered were Germany, Belgium, and Japan, although Europe as a general region was considered by Wietschel and Hasenauer.55 In some cases,25,55,60 multiple importer–exporter pairs were considered, and Kawakami et al.62 performed a simple optimisation to select shipping size, and to determine which of the Middle East and the US would be more suitable energy exporters to Japan. The Hydrogen Import Coalition57 estimate the costs of shipping various hydrogen derivatives to Belgium from five locations; their results showed it was cheaper to import ammonia from Morocco than Chile, even though production was cheaper in Chile, because of the impact of transport costs. No paper considered the general optimisation problem of which nations would be best placed to export, and to which regions they could most economically ship their product.
The cost of transport will depend on the financial arrangements of exporters and importers. The most likely export model is comparable to those currently used in the oil and gas industries, where it is rare for energy producers to own gas carrier ships. The norm is to charter vessels at a day rate, enabling more market flexibility; additional costs accrue for fuel, berthing, and canal use if required on the nominated route. The charter rates of energy carriers are highly volatile based on market conditions and energy demands; the Covid-19 pandemic, for instance, caused the charter rate of very large LNG ships to fall from 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 20
000 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 USD per day. Even under less extreme economic conditions, the charter cost can be highly variable.66
000 USD per day. Even under less extreme economic conditions, the charter cost can be highly variable.66
Rogers67 accurately predicts the mean charter rate for LNG ships based on the capital cost of the carrier and a 5% O&M cost. Using a similar approach, and ship prices stated in Kawakami,62 the average charter rate for an 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 very large gas carrier (VLGC) for ammonia would be ∼22
000 m3 very large gas carrier (VLGC) for ammonia would be ∼22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 USD per day. Using ship fuel consumption from the IEA25 and berthing fees from Rogers,67 the transport costs for ammonia were estimated. It is assumed that green ammonia itself is used as shipping fuel; the opportunity cost of its use is estimated at 500 USD per t, which is on the upper end of spot prices for ammonia in the current market, and a realistic LCOA for 2020 production in good locations.11 The results are summarised in Fig. 3.
000 USD per day. Using ship fuel consumption from the IEA25 and berthing fees from Rogers,67 the transport costs for ammonia were estimated. It is assumed that green ammonia itself is used as shipping fuel; the opportunity cost of its use is estimated at 500 USD per t, which is on the upper end of spot prices for ammonia in the current market, and a realistic LCOA for 2020 production in good locations.11 The results are summarised in Fig. 3.
 | ||
Fig. 3 Estimated green ammonia shipping cost (using a fuel cost of 500 USD per t ammonia) compared to literature values. The dashed lines represent estimated shipping costs for a low (300 USD per t) and high (700 USD per t) case. The data from Ishimoto et al.60 are not displayed; they estimate a cost of 11 USD per GJ over 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km. 000 km. | ||
This high-level estimate is not a precise representation of shipping costs, due to the significant uncertainty surrounding the charter rate and fuel price. Berthing fees could also be substantially higher if an ammonia plant cannot take advantage of an existing nearby port. At an intercontinental scale, the model estimates a price of 1.5–3 USD per GJ; as a benchmark, a typical cost for shipping LNG on this scale is 1 USD per GJ.68 Ammonia's energy density is 50% lower than LNG, and the fuel component of the shipping cost is larger because of the high cost of green ammonia. These two factors collectively account for the increased shipping costs of ammonia compared to LNG. If a conventional fuel were to be used to power the ship, the transport cost would be significantly reduced as per the lower bound on the estimate in Fig. 3.
This estimate demonstrates a trend which is not clear in the literature, and contrary to the perspective of Ishimoto et al.:60 it appears the price of shipping is a strong function of shipping distance, because of the impact of daily charter costs, and the significant contribution of shipping fuel to transport costs. Only 4 of the 8 authors which provided ammonia shipping costs fall within the plausible range identified by this estimate. This is caused predominantly by oversimplified or vague assumptions,7,57,58 or because authors price shipping as a capital investment, rather than using the cheaper charter model that is the norm in the existing energy transport industry.24,60,62
Beyond the cost of transport, authors also used a range of approaches to estimate the cost of production. As described in Section 3, estimating ammonia production costs is a complex process which requires consideration of the specific nature of the available renewable resource; simply estimating a number of annual operating hours is overly simplistic. Failing to make this consideration can underestimate ammonia production cost by ∼40%.17 Even if ammonia synthesis is substituted with liquefaction or any other carrier synthesis process, similar complexities are expected to increase the production cost. Some works greatly simplified this complexity,23,33,58,64 either ignoring the variability of the resource altogether, or failing to make specific provision for intermediate hydrogen storage. Other authors relied on grid electricity,22 or on conditions very specific to certain locations, such as excellent hydroelectric or geothermal resources,26,55 or salt caverns.24 Kawakami et al.60 and Ishimoto et al.62 assume that hydrogen is produced using natural gas and CCS. No author who considered transport costs provided as robust an estimate of production costs as is available in other literature.11,17,69
By contrast, Haber–Bosch synthesis and LOHC hydrogenation are already well understood and their costs are not expected to fall substantially. It is possible for the green ammonia cost to fall if new synthesis technologies are designed which are superior to Haber–Bosch synthesis, but these receive little attention in the literature due to their low technology readiness level, as discussed in Section 1.1.6,17,70
One key development which may significantly reduce the cost of green ammonia, LOHCs and synthetic hydrocarbons is the technical readiness of solid oxide electrolyser cells (SOECs) for fuel production. Many papers consider SOECs to have a low technology readiness level and to be unable to handle dynamic load variations; however, Hauch et al.71 and Posdziech et al.72 both report substantial technological growth in the area in the past two years and indicate that dynamic load flexibility may be possible. These cells have higher efficiencies, but also are able to use heat as an energy supply to the hydrogen production process (as opposed to low temperature cells, which can only use electricity). Therefore, useful work can be performed using the heat released from the exothermic reactions which occur when hydrogen is synthesised into a carrier; in low temperature cells that energy is wasted. Integration of the solid oxide electrolyser cell and ammonia synthesis loop also removes the requirement for a dedicated air separation unit, reducing capital investment and increasing air separation energy efficiency (see Hauch et al.71 for an explanation of this concept).
2.5 Energy carriers summary
There are a range of transport carriers available for energy transport, each with different strengths and weaknesses that will make them more or less economic based on specific circumstances.The literature has not achieved a unanimous consensus on a single best chemical energy vector. Although liquid hydrogen is often discussed, there is limited evidence that it will be the most affordable carrier. It is more likely that LOHCs, ammonia or methanol will be preferable depending on the intended use case. However, there is clear evidence that ammonia is the best option if it is being used as fertilizer, or if it can be used directly without cracking, (i.e. in a fuel cell, as a shipping fuel, or in a direct combustion engine). Even if none of those conditions are met, many authors still consider it to be the best available hydrogen carrier. For that reason, the rest of this document will focus solely on ammonia: at worst, it will be an important vector for a small but significant group of sectors, and at best, it will be the dominant chemical for intercontinental energy transport in a future decarbonised economy.
Further work is required to extend the existing transport cost analysis to incorporate more rigorous models of production cost, and to consider ammonia import and export that is multilateral, rather than bilateral.
3. Ammonia production
As is clear from Table 4, it is expected that the most substantial contributor to the cost of delivered green ammonia in an energy-importing nation will be the cost of production. Because of significant interest in chemical energy storage, a slew of recent techno-economic analyses (TEAs) have attempted to provide a clear description of the costs of green ammonia production, yielding a range of cost estimates.Techno-economic analyses of green ammonia production typically report a levelised cost of ammonia (LCOA), which refers to the minimum cost of green ammonia required to provide a project with a positive net present value at a nominated discount rate.
Papers tended to consider both current and future production costs, due to the expected fall in renewable and electrolyser costs in the next decade. For those papers which reported LCOAs using technology currently available, the minimum reported values of the LCOA are ∼480 USD per t,11,17 comparable to high spot prices of ammonia which have been observed in the last decade.11 Other literature, however, reports far higher prices in the order of 1000 USD per t.73–75
Looking to the future, most of the literature is in agreement that the cost of green ammonia will trend downwards, but there are inconsistencies between the date at which it is expected to reach parity with conventional ammonia. More ambitious estimates believe parity will be possible in some locations in 2030;17 others do not expect this point will be reached until 2040 or even 2050.73
This section analyses the cause of the difference between LCOAs in order to identify which works have produced the most reliable assessments of green ammonia cost, and to identify the key factors which impact the LCOA. The wide range of LCOAs reported in the literature can be attributed to variance in technical approach, project financial considerations, renewable energy resource, and technological inputs.
While parity with the cost of fossil fuels is a useful benchmark for the cost of green ammonia, reductions below this cost are expected and necessary to drive uptake of green ammonia as a clean energy vector in the long term. This is because, as observed in Wijayanta et al.,7 ammonia's chemical role as a fertiliser increases its market price well above its value as an energy vector. The current price of ammonia fluctuates between 350 and 550 USD per t.36,76 Even at the minimum value, 350 USD per t, the value of energy provided by ammonia on a HHV basis is 15.55 USD per GJ, which compares unfavourably to natural gas in the US, whose price ranges between 3 and 8 USD per GJ, depending on location and market conditions.35,77 For that reason, green ammonia needs to be significantly cheaper than fossil-fuel based ammonia to provide very cheap energy (although this direct comparison does not factor in efficiency of usage, which may be marginally higher for ammonia using SOFCs than for natural gas).
3.1 Methodology for compiling literature on ammonia production
In order to obtain an initial short-list of publications in this field, a keyword search was performed on Scopus for literature containing the trigger words shown in Table 5.| Category | Trigger words |
|---|---|
| Ammonia | Ammonia, Haber–Bosch |
| Renewable | Renewable, green |
| Price | Price, techno-economic, LCOA, levelised |
Literature was excluded if it relied upon a technology that was not yet commercially available (e.g. photocatalytic ammonia production), if the ammonia production technology was brown, grey or blue, or if the author did not provide a detailed cost assessment of hydrogen or ammonia production. After reviewing the list generated by Scopus, 21 of these papers were found to contain a detailed assessment of the LCOA. A further 8 papers were identified in the citations of the shortlisted literature, leading to a total of 29 papers considered. All papers considered are summarised in Table 6. Using the same approach as Section 2.4.2, the LCOA was adjusted to 2020 prices using an inflation rate of 2.3%.
| Author | Category | Year | Location | Electrolyser energy efficiency (kW h kg−1) | Discount rate | Electrolyser size (MW) | Electrolyser CAPEX (USD per kW) | Energy cost (USD per MW h) | LCOA(i) (USD per t) |
|---|---|---|---|---|---|---|---|---|---|
| a (i) Where literature reported multiple LCOAs, the lowest value reported was selected. Where multiple time horizons were considered, data for the shortest time horizon was selected (i.e. 2020 estimates were preferred to 2030 estimates). | |||||||||
| Armijo et al.11 | Relevant | 2020 | Argentina/Chile | 47.6 | 8.5 | 1 | 600 | 30 | — |
| Gomez et al.89 | Did not use HB ammonia | 2020 | — | — | — | — | — | — | — |
| Fúnez Guerra et al.58 | Lack of clarity for handling VRE | 2020 | Chile | 47.6 | 8 | 150 | 495 | 20 | — |
| Lin et al.44 | Relies heavily on grid electricity | 2020 | Minnesota, USA | 50.0 | 7 | 20.1 | 995 | 41 | 955 |
| Nayak-Luke, R et al.17 | Relevant | 2020 | Multiple | 49.0 | 3.3–18 | 100 | 700 | 19 | 470 |
| Osman et al.69 | Relevant | 2020 | UAE | 47.6 | 4 | 1300 | 652 | 25 | 617 |
| Palys et al.78 | LCOA subsidized by selling electricity | 2020 | USA | — | 10 | 1 | 800 | — | 527 |
| Wang et al.90 | Relies heavily on grid electricity | 2020 | Germany | 35.1 | 5 | — | 100 | 71 | 580 |
| Zhang et al.85 | Relies heavily on grid electricity | 2020 | — | — | 10 | 43 | — | 73 | 544 |
| Allman et al.91 | Wind farm also sells to grid; subsidises NH3 | 2019 | Minnesota, USA | 60.0 | 8.3 | 0.25 | 2347 | — | — |
| Rivarolo et al.76 | Relies heavily on grid electricity | 2019 | Paraguay | 52.7 | — | 200 | 784 | 17 | 373 |
| Tso et al.56 | Lack of clarity for handling VRE | 2019 | Texas, USA | — | — | 607 | 45 | 2106 | |
| Zhao et al.27 | Relies heavily on salt cavern | 2019 | USA Gulf Coast | — | 6 | — | 433 | 51 | 360 |
| Demirhan et al.92 | Lack of clarity for handling VRE | 2018 | USA (various states) | — | — | — | 622 | 46 | 823 |
| Eichhammer84 | Lack of clarity for handling VRE | 2018 | Morocco | 49.0 | 6.12 | 700 | 708 | 38 | 705 |
| Ikäheimo et al.88 | Wind farm also sells to grid; subsidises NH3 | 2018 | Northern Europe | 53.0 | 7.00 | — | 462 | — | 496 |
| Nayak-Luke, R et al.93 | Out of date inputs/assumptions | 2018 | Scotland, UK | 53.0 | Not used | 196 | 1202 | 92 | 1361 |
| Palys et al.94 | Relies heavily on grid electricity | 2018 | USA Midwest | 66.1 | 7 | — | 1313 | 32 | 639 |
| ISPT95 | Out of date inputs/assumptions | 2017 | Netherlands | 53.0 | 7 | 40 | 1421 | — | — |
| Morgan et al.73 | Relies heavily on grid electricity | 2017 | USA | 53.8 | 7 | 135 | — | — | 1310 |
| Pfromm87 | Lack of clarity for handling VRE | 2017 | — | 54.0 | — | — | — | 25 | 273 |
| Sánchez et al.83 | Lack of clarity for handling VRE | 2017 | Southern Europe | 53.2 | — | — | — | — | — |
| Wang et al.79 | Lack of clarity for handling VRE | 2017 | — | — | 7.50 | 100 | 275 | 32 | 1017 |
| Bañares-Alcántara et al.96 | Out of date inputs/assumptions | 2015 | Victoria, Australia | 47.0 | 8 | 12 | 1353 | 57 | 1460 |
| Beerbühl et al.80 | Out of date inputs/assumptions | 2015 | Germany | 49.3 | — | 34.5 | — | 45 | 641 |
| Matzen et al.86 | Lack of clarity for handling VRE | 2015 | — | 54.8 | — | — | 735 | 51 | 742 |
| Trop97 | Relies heavily on hydro electricity | 2015 | Iceland | 63.5 | 90 | 600 | 162 | 34 | 490 |
| Morgan et al.75 | Lack of clarity for handling VRE | 2014 | Maine, USA | 53.6 | 7 | — | — | — | 1471 |
| Tunå et al.74 | Out of date inputs/assumptions | 2014 | General | 47.6 | 8.5 | 10 | 669 | 46 | 1163 |
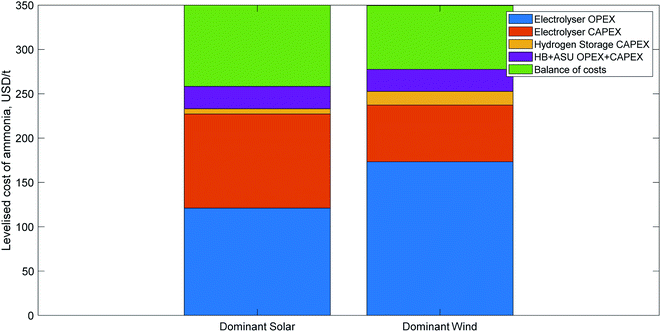 | ||
| Fig. 4 Indicative cost breakdown of green ammonia production for two different renewable energy profiles, adapted from Nayak-Luke and Bañares-Alcántara,17 with the LCOA normalised to 350 USD per t, which that paper found to be a realistic price in several locations by 2030 with good project finance. The first cost breakdown is typical of locations with a very good solar resource; the second is indicative of very good wind resources. The balance of costs segment includes operations and maintenance, a hydrogen fuel cell for back-up power, water, and curtailed electricity. | ||
3.2 Variance in technical approach
Managing flexibility is a prerequisite in the production of green ammonia powered by variable renewable energy (VRE). Operational flexibility must smooth out short term power variations (i.e. on an hour-to-day timescale caused by the rising and setting of the sun), and longer term variation (i.e. on a month-to-year timescale caused by variation in the wind profile).78Hydrogen electrolysers are highly flexible;79 PEM type electrolysers, in particular, can reduce their operation to 5% of rated capacity,3 increasing efficiency as they do so.80 However, the ammonia synthesis plant presents the major complexity to flexible operation, because it operates at high temperatures and pressures (400–650 °C and 200–400 bar),10,81 and because frequent cycling of production rate may damage catalysts and equipment.80 Managing the ammonia plant when renewable energy is not available is therefore a major challenge.
In some literature, it is assumed that the Haber–Bosch loop is entirely inflexible, and can only operate at its maximum rate of 100%;69,73 other research has forecast a theoretical minimum operating rate of 20%,80 with remaining authors falling between these values.11,49 Lower operating rates may be facilitated by technology developments, such as the use of advanced catalysts and ammonia separation using absorbents, which enable ammonia synthesis to be conducted at low temperatures and pressures (∼275 °C and 8 bar).13,82 These would be substantially useful modifications in order to mitigate hydrogen storage costs; however, these modifications alone cannot entirely smooth out the variability of wind and solar farms.
There are a range of approaches taken in the literature to manage the challenge of operating the Haber–Bosch loop in periods without enough available renewable energy, which are represented by the orange boxes and power flows on Fig. 1. However, in much of the literature,58,75,83–87 this challenge is not discussed in enough detail to confirm if the proposed design is operable. In other cases79,88 flexibility is neglected in order to simplify process modelling; because of this simplification, these cases provide limited insight into specific process design.
A third approach is the use of grid73,74,85,90,98 or hydro76,97 electricity to entirely supply the electrolyser and ammonia plant at almost all times, or whenever renewables are not available. Although this approach enables maximum value to be extracted from the installed capital equipment, and no costs to be allocated to hydrogen storage, it demands the use of either of hydroelectricity, whose global potential is far less than global demand,99 or of prohibitively expensive grid electricity, which is not generally decarbonized. In addition, relying on highly renewable-dependent grids is not likely to be possible for green ammonia plants when wind or solar farms are not operating, because those times will be correlated with periods when grid demand exceeds supply. At best, drawing on the grid at these times will be expensive; at worst it will be prohibited by regulators.
In the remaining papers limited to islanded VREs (i.e. without grid connection), several authors11,17,69,78 include a hydrogen buffer which can store excess hydrogen produced during periods of high electricity generation and therefore maintain the required supply of raw material to the ammonia plant. The size of the buffer required depends on the renewable energy profile, and the expected flexibility of the ammonia plant. An additional energy source (e.g. non-variable renewables, batteries, or cannibalisation of some hydrogen) may be used to provide continuous electricity to the ammonia compressor and air separation unit.
Interestingly, although flexibility is a major complicating factor in plant design, plants which fail to give due consideration to flexibility do not always have the lowest calculated LCOA. This is typically because of the use of more conservative financial estimates, but also because of the more sophisticated models used in papers considering islanded systems.
3.3 Variance in project financial parameters
The literature proposes a range of financial parameters to amortise the large capital costs of green ammonia plants caused by the significant investment required in the electrolysis unit. This range of finance options can distort the LCOA and therefore conceal underlying project strengths or weaknesses.The simplest financial approach to reducing the LCOA is to reduce the discount rate, but reductions can also be achieved using more favourable loan conditions (e.g. high debt/equity ratios, long loan terms, and low interest rates on debt). In contrast to traditional engineering applications, changing these financial parameters can have a major impact on LCOA because green ammonia production is highly capital intensive.100
Excluding Nayak-Luke et al.,93 in which the discount rate was set to 0 to specifically exclude it from consideration, then the range of discount rates considered in the literature surveyed spanned from 4% (ref. 69) to 12%.74 Nayak-Luke and Bañares-Alcántara17 varied the discount rate based on location, sector, and the nature of the investor (domestic or multinational); that study used a minimum discount rate of 3.3% for a multinational corporation investing in a renewably powered utility, and a maximum of 18.74% for a Venezuelan domestic company investing in an ammonia plant. This range of discount rates may be reflective of actual difference in the cost of capital available to projects in different regions. For instance, Steffen100 estimated the discount rate considered for a large number of renewable projects using different techniques, finding values as low as 2.5% in Germany, and in excess of 10% in India for both solar and wind installations.
This wide range in discount rates can significantly impact the LCOA. Consider an islanded ammonia plant which is entirely funded by equity capital (i.e. renewable energy, electrolyser and Haber–Bosch synthesis are all purchased using CAPEX in year 0). The only OPEX costs will be operations and maintenance, which the IEA estimates to be 1.5% of capital costs.25 If this islanded plant achieved an LCOA of 500 USD per t under a discount rate of 10%, then an identical plant constructed with a rate of 2.5% would achieve an LCOA of 278 USD per t (using a plant lifetime of 25 years).
With the exception of Nayak-Luke and Bañares-Alcántara17 and Fúnez Guerra et al.,58 few papers provided clear detail as to the selection of the discount rate, despite its significant impact on the LCOA. Future works should be more specific about how discount rates are selected; corporations considering creating green ammonia projects should consider financial parameters to be as important as geographical ones in selecting a facility location.
3.4 Variance in renewable energy resource
Of the studies which plausibly considered process flexibility using an islanded production facility, the most significant source of variance in LCOA was caused by the quality of the renewable energy resource. Armijo et al.,11 for instance, found very low ammonia costs (<500 USD per t) using the excellent renewable energy sources available in Patagonia. Nayak-Luke and Bañares-Alcántara17 applied the same optimisation approach and discount rates to over 500 renewable energy locations around the globe, demonstrating the optimum cost could range from USD 470 per t in the best location to almost 2000 USD per t in less suitable locations (using technology available in 2018). A study comparing the use of ammonia for power-to-ammonia-to-power in American cities78 found the LCOE using ammonia as energy storage was a strong function of resource quality.Low costs were typically found using a combination of wind and solar resources to supply power, with better results where the wind and solar profiles were anti-correlated. In studies which used only wind73 or solar,69 the minimum costs were much higher (>1000 USD per t for wind only, and 641 USD per t for solar only, using comparable assumptions to Nayak-Luke and Bañares-Alcántara17).
Additionally, Armijo et al.11 were able to achieve cost reductions by allowing the ammonia plant to perform a “cold stop” if weather inconducive to ammonia production was forecasted for an extended period of time. Nayak-Luke and Bañares-Alcántara17 reduced costs by performing system maintenance during the two worst-performing renewable power weeks over a given year. In both cases, accurate forecasting would improve the efficacy of this technique; locations in which weather is highly predictable may be able to achieve cost reductions that are not possible where weather is comparatively hard to forecast.
3.5 Variance in technology forecast
The most substantial contributors to the cost of green ammonia are renewable electricity and electrolyser CAPEX. These two contributions also represent the components of the cost which are most likely to fall in the future. Although the capital cost of ammonia synthesis is substantial, it is small compared to electrolyser costs, and is a mature technology that is unlikely to see significant cost reduction. For that reason, accurate estimates of electricity prices and electrolyser CAPEX are critical to accurate LCOA forecasting, but the selection of suitable values often receives limited attention in the literature.Comparison of renewable energy price in the literature is complicated by the varied approaches taken by different authors. In some instances, electricity was purchased by a power purchasing agreement (PPA);69,86,87 in others, it was supplied by onsite energy farms.11,17,96 In some cases, renewable power was also sold directly onto the grid, cross-subsidizing its costs,78 or was supplied from an existing windfarm which was being curtailed.91 Some authors did not specify a renewable energy cost.80 The total amount of energy available from wind and solar farms was also not typically reported, making it impossible to convert between the capital and amortized costs of renewable energy. For those reasons, a comparison of renewable energy inputs to the model used across the literature was not possible. However, it should be noted that forecasts for renewable energy prices are widely available,3,30,101 so it is expected that broadly similar inputs were used.
It is easier to compare the electrolyser capital cost used in the model. Using an excessively low electrolyser CAPEX will not only underestimate project costs, but will also lead to a non-optimal design, favouring lower load factor renewable sources if they provide cheaper power. International energy bodies are divided over the expected costs of electrolysers for both today and in 2030. Bloomberg NEF,101 for instance, forecasts a price of just 200 USD per kW for a Chinese electrolyser purchased in 2020, falling to 135 USD per kW by 2030. Both IRENA3 and the IEA25 disagree with this forecast, reporting prices of 840 USD per kW and 900 USD per kW for electrolysers purchased today. IRENA suggests that a price of 200 USD per kW will not be possible until 2050; the IEA's forecast ends in 2030 at a minimum price of 450 USD per kW.
The green ammonia literature is similarly divided over the price of electrolysers, with minimum prices of 143 USD per kW (ref. 97) compared with maximum prices in excess of 1000 USD per kW, which tend to be found in papers published in 2015 or earlier.74,96 However, the papers identified as most robust in earlier sections,11,17,69 with rigorously optimised designs, tended to use prices between 600 and 1000 USD per kW, which are plausible estimates given the data available.
Forecasting of electrolyser price is complicated by variations in the electrolyser scope. For instance, Wang et al.90 specifies a very low price of 100 USD per kW for a solid oxide cell (typically the most capital intensive cell type71); however, this only includes the stack and excludes all balance of plant, as well as installation costs, which they do not specify. Lin et al.98 and Palys et al.94 both use a bare module cost (327 USD per kW and 637 USD per kW respectively) alongside published installation factors to estimate a project CAPEX (994 USD per kW and 1248 USD per kW). IRENA3 and Nayak-Luke et al.17 specify that their electrolyser costs include installation and balance of plant costs. In general, though, authors are not specific as to the scope of what is included within their electrolyser cost.
Equating the bare material cost of electrolysers to their installed cost is risky and is likely to result in a large underestimation of total project costs. Electrolyser vendors typically provide the stack and some electrical equipment; they do not provide installation or the entire balance of plant, which may include additional electrical work, pipework, and civil/structural engineering. Those costs must be borne separately by the project owner. The US National Renewable Energy Laboratory provides a complete cost breakdown of the installation of electrolysis units, concluding that the electrolyser stack alone may represent only 1/6th of the total project CAPEX; installation costs may be as high as 1/3rd of these costs.102
3.6 Production cost summary
All literature agrees that the LCOA is expected to fall over time, and it is gradually expected to become increasingly competitive with fossil fuels, particularly if aided by a carbon tax (based on current performance as reported by the IEA,25 a 100 USD per t carbon tax would increase the competitiveness of green ammonia compared to grey ammonia by 235 USD per t). Despite this general consensus, only a relatively small number of authors provide useful estimates of the LCOA, as identified in the categorisation of literature shown in Table 7. Although the work of only three authors was categorised as relevant, useful insights are available in other papers.| Literature category | References |
|---|---|
| Out of date inputs/assumptions | 74, 80, 93, 95 and 96 |
| Lack of clarity for handling variability of renewable energy source in ammonia plant | 56, 58, 79, 83, 84, 86, 87 and 92 |
| Relies heavily on grid electricity when renewables are not available | 73, 79, 85, 97 and 98 |
| Used electrochemical rather than HB ammonia synthesis | 89 |
| LCOA subsidised by sale of excess electricity/hydrogen | 78, 88 and 91 |
| Relevant | 11, 17 and 69 |
Table 8 lists the five key root causes of variance in the LCOA, and how they impact the cost of green ammonia. Three of those factors can be considered exogenous to a green ammonia facility, i.e. they will be the same for all state-of-the-art green ammonia plants constructed in a given year. The other two factors are endogenous to the facility, and therefore will differ between plants constructed using the same technology based on their location. An estimation of the parameter's impact on the cost breakdown of an ammonia plant is provided for both the dominant solar and dominant wind profiles shown in Fig. 4.
| Parameter | Exogenous/endogenous | Significance | Indicative impact (dominant solar) | Indicative impact (dominant wind) |
|---|---|---|---|---|
| Electrolyser CAPEX/efficiency | Exogenous | Low CAPEX and high efficiency reduce LCOA | Moderate: electrolysis is ∼30% of LCOA | Small: electrolysis is ∼20% of LCOA |
| Solar/wind CAPEX | Exogenous | Low energy prices reduce LCOA | High: power is ∼35% of LCOA | Very high: power is ∼50% of LCOA |
| Ammonia plant flexibility/hydrogen storage costs | Exogenous | High ammonia flexibility or low storage costs reduce the cost impact of an adaptable process and reduce LCOA | Moderate: more flexible equipment requires less oversizing | Small: wind profiles are more steady than solar so require less oversizing even with inflexible ammonia |
| Renewable energy profile | Endogenous | High solar radiation/wind strength reduces energy costs; reliable energy increases equipment capacity factors and reduces process flexibility costs | High: power is ∼35% of LCOA | Very high: power is ∼50% of LCOA |
| Financial parameters (e.g. discount rate, debt/equity ratio, loan term, interest rate) | Endogenous | Because ammonia plants are capital intensive, favourable financial conditions can substantially reduce the LCOA | Very high: the variation in discount rates can impact the LCOA by a factor of 2 (see Section 3.3) | High: the impact is the same as for solar, but if a PPA is used to buy electricity, the impact on wind will be smaller, since it is less CAPEX intensive |
Critically, the presence of these endogenous factors makes it clear that the location of a facility dictates the cost of ammonia at a given point in time; it is this behaviour that drives the opportunity for ‘spatial arbitrage’ in the ammonia market, which is to say that it is possible to profit by producing energy in one country and exporting it to another. The arbitrage is justified economically if the costs of energy conversion to ammonia and transport are less than the energy cost difference between the two locations at which energy is produced and consumed.
4. Ammonia supply capacity
Ammonia supply capacity depends on both the number and size of ammonia facilities. While smaller ammonia systems are often touted as a solution which can decentralise energy grids, for intercontinental energy transport it is more likely that a comparatively small number of large ammonia facilities will be used given the economies of scale associated with port and shipping infrastructure. This section therefore aims to identify constraints on both individual project size and domestic production capacity. Four major factors are identified which may constrain the production of ammonia, both at the level of individual projects and global markets. The factors considered are the availability of materials, availability of land, availability of capital, and public perception.4.1 Size of current projects
In the literature surveyed in Section 3, a range of project sizes were assumed. In older papers, electrolysers tended to be ∼10 MW;74 in more recent papers, project scale has generally increased to between ∼100 MW (ref. 7, 17 and 60) and several gigawatts.69 Some production papers do not specify a production scale and work on a per MW basis;11 while this may be appropriate for electrolysers, it is expected that the cost per unit production of ammonia will decrease as scale increases because of the economies of scale achievable in the Haber–Bosch process.34The standard literature scale of 100 MW is larger than any completed green hydrogen project, even those which are not producing feedstock for ammonia plants. However, industry in recent years has been highly active, and a number of projects in gigawatt scale have been announced which will be commissioned between 2020 and 2025. Table 9 provides a summary of existing and announced projects for which substantial detail is publicly available, demonstrating the rapid increase in project scale which is forecast over the next decade.
| Country | Company/organisation | Phase | Renewable source | Targeted first production | Total electrolyser capacity (MW) | Ammonia capacity (t per year) | CAPEX (106 USD) |
|---|---|---|---|---|---|---|---|
| Japan104 | SIP | — | — | 2018 | — | 7 | — |
| UK104 | Siemens | — | Wind | 2018 | — | 10 | — |
| Australia105 | Yara & Engie | 0 | Solar | 2022 | 10 | 3500 | 43.75 |
| 1 | Wind/solar | 2026 | 500 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | ||
| 2 | Wind/solar | 2028 | 1000 | 480![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | ||
| 3 | Wind/solar | 2030 | 1500 | 720![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | ||
| Denmark106 | HaldorTopsoe | — | Wind/solar | 2022 | 10 | 8500 | — |
| Norway107 | NEL & Yara | 1 | Hydro | 2022 | 5 | 5000 | — |
| 2 | Hydro | 2026 | — | 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | ||
| US108 | CF Industries | — | — | 2023 | — | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
<450 |
| Chile109 | Engie & Enaex | 1 | Solar | 2024 | 26 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
| 2 | Solar | 2030 | 1600 | 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | ||
| Australia103 | AREH | — | Wind/solar | 2025 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Australia110 | Origin Energy | — | Hydro | 2025 | 500 | 420![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
| Netherlands107 | Ørsted & Yara | — | Wind | 2025 | 100 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
| Saudi Arabia111 | NEOM | — | Wind/solar | 2025 | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5000 |
| Oman112 | ACME | — | — | — | — | 803![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2500 |
| Chile113 | Ökowind EE GmbH | — | Wind | — | — | 850![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
— |
Of particular note is the recently announced Asian Renewable Energy Hub (AREH) in Western Australia, which intends to use 15 GW of installed electrolyser capacity to produce ammonia by the middle of the decade;103 based on their published data, this will correspond to between and 8 and 9 million tons of ammonia per year.
It is likely that the list of projects shown in Table 9 will grow quickly in the coming years, often stimulated by governments attempting to develop local hydrogen and ammonia industries. The Indian government, for instance, has announced its intention seek bids from companies wishing to produce green ammonia using a local solar resource,114 and an associated project is expected to be announced soon.
4.2 Availability of materials
There are five major components required for green ammonia export facilities: renewable energy supply, hydrogen electrolysis, water supply, ammonia synthesis, and ammonia storage and transport. The former two components are novel technologies, and their capacity for scale-up is to be determined. The latter two components are already carried out at a global scale and are constructed mostly from structural materials (e.g. concrete and steel) which are expected to be available in adequate quantities. The capacity to provide very large quantities of water to renewable ammonia facilities may also impact supply in the future.An investigation into the availability of these materials in the EU115 found that in a complete decarbonisation scenario, significant pressure was expected on the supply chains of dysprosium and terbium for wind, and germanium, tellurium, indium and selenium for Solar PV. Weng et al.116 agreed with the assessment that supply chains of rare earth metals required for renewable energy technology are likely to be strained. They performed an assessment of all rare earth metals globally, and concluded that supply chain limitations were not caused by a lack of geological availability (for which there are adequate deposits for supply until 2100 assuming a 5% growth rate in demand). Rather, supply chain restrictions are caused by China's monopoly of the current rare earth metal market, for which it is historically responsible for >95% of global production. This can create distortive effects on global trade; for instance, in 2006, China placed export limits on three rare earth metals in order to build domestic industry, causing a spike in prices globally.
However, to avoid this constraint, it may be possible to expand access to rare earth metals: Weng et al.116 found that although China accounts for the vast majority of rare earth metal production today, more than 57% of known deposits globally are outside China, located predominantly in Australia, Russia, Canada and Brazil. Diversifying production to include these deposits would provide a more robust supply chain. The size of the deposits outside of China is likely an underestimate caused by a lack of geological exploration for these metals.
Therefore, it is very likely that there is an adequate amount of to produce very large-scale wind and solar farms; obtaining those materials will require geopolitical stability to prevent market distortions, and diversification of rare earth metal mining efforts in a range of countries.
Overall, availability of materials may inform technology selection of renewable electricity generators and electrolysis cell types; however, it should not significantly limit the size of large-scale green ammonia plant installations. It may encourage the production of ammonia in nations which have access to certain materials, or who have stable relationships with countries that produce those materials.
The availability of water is often raised as a concern for hydrogen electrolysers which are heavily reliant on solar energy; these are often in arid regions with limited rainfall and may therefore be challenged by water scarcity. In these circumstances, desalination is the only viable technology for water production.69 However, even the energy demands of desalination are relatively low compared to the power input required by the electrolyser, and desalination would contribute at most ∼0.02 USD per kg to the cost of hydrogen.4 The majority of this cost is energy demand, the upper limit of which using modern desalination techniques is 4.5 kW h m3 of water produced.119 Since electrolysis is almost stoichiometric, this equates to 0.0405 kW h kg−1 of hydrogen, which is in the order of 0.1% of the total energy consumed during electrolytic hydrogen production.
In theory, therefore, water supply should not be a major problem to the establishment of a global ammonia industry; however, it may constrain production away from areas with significant water stress, or encourage operation near coastal areas to reduce the costs of desalinated water.
4.3 Availability of land
The vast majority of land requirements for green ammonia plants pertain to the renewable energy farm. The largest (conventional) ammonia plant in the world, SAFCO,120 has a footprint of approximately 1 km2, and a production which exceeds 1 MTPA. At this land intensity, the entire global production of ammonia could be produced in around 200 km2, which is a negligible fraction of the land available for chemical production. However, gathering energy to supply these plants with renewable electricity will require significantly more land.In general, land availability should not limit total global production of renewable energy. For instance, Moriarty and Honnery121 found that the solar insolation on the Sahara desert alone would be adequate to supply the global population in 2050, even assuming limited energy efficiency innovations. Similarly, Babarit et al.63 found the offshore wind potential at shallow and intermediate depths alone could theoretically supply 75% of global energy demand in 2050, and that the capacity of floating windfarms in deep water could meet energy demand almost 10-fold.
In practice, land limitations can be substantial; collecting all of the solar irradiation on the Sahara, for instance, is not practical due to the difficulty of installing panels on sloping dunes. This can be particularly problematic in specific regions: in Japan and Korea, which have low levels of agricultural self-sufficiency, installation of significant solar infrastructure would displace land usually used for food production.122,123 Moreover, the land-use change associated with conversion of land from crops to energy generation can impact atmospheric CO2 levels. In Japan and Korea, the emissions intensity of electricity from solar panels which have displaced agriculture could be as high as 10% of emissions intensity of electricity from natural gas.122
By contrast, other countries are not land-limited. The recently announced 100 TW h per year Asian Renewable Energy hub in Australia has a land area of 6500 km2; factoring a 60% conversion efficiency to ammonia, this facility would generate 3.5% of Australia's annual energy consumption on just 0.08% of the land.124 In other words, renewable energy harvested at this rate on less than 2.5% of Australia's land mass could produce its entire energy demand. In practice, as panel efficiency improves, this forecast area may fall. Because the land in question is not agricultural land, and because of the higher solar insolation in Australia compared to Japan and Korea, the land-use change effects are not likely to be significant.122
It is theoretically possible to produce enough renewable energy to supply global demand in 2050; however, the distribution of energy production will not enable all nations to be self-sufficient. Countries with low land-availability (either because of population density, highly sloping terrains, or substantial agricultural demands) will need to import energy from countries with comparatively high land availability.
4.4 Availability of capital
As discussed in Section 3.3, green ammonia production is highly capital intensive; this is particularly true if a facility includes capital investment on a wind or solar farm, rather than purchasing electricity through a PPA. The presence of significant capital investment is therefore a prerequisite for large-scale ammonia production at the global level, and at the level of individual projects.The IEA125 reports that investment in clean energy technology has largely been stable over the past 5 years, at approximately 600 billion USD per annum (including investment in power, nuclear, energy efficiency and energy storage technologies). For reference, the AREH has an expected CAPEX of 36 billion USD,126 or 6% of the current global annual investment in clean energy. Using the same assumptions for its conversion efficiency described in the previous section, it will produce only ∼1% of Japan's annual primary energy demand;127 in other words, converting energy systems to make substantial use of ammonia will require capital investment on a very large scale over a sustained period of time.
Compared to other forms of energy, investment in renewables has been relatively resilient to the Covid-19 pandemic. Despite this resilience, the path forwards for renewables is not clear, and the IEA imagines two pathways to economic recovery in the first half of this decade.30 On one path, a slow economic recovery with limited government support causes consumers and producers to extend the life of existing fossil-fuel based assets, with little investment in new technologies. On the other path, government support and renewed public commitment to decarbonisation stimulates rapid investment into renewables, with total investment doubling by 2030 compared to current levels.
Investment in renewables comes predominantly from the private sector, although often is stimulated by government incentives, or sold at a premium to state-owned utilities.125,128 Both energy importing and exporting governments can stimulate capital investment in green ammonia projects. Energy exporting governments can use public finance to encourage institutional investors. They can achieve this by creating supply chains (e.g. by supporting pilot projects, building capacity, and facilitating international trade), and by providing risk mitigation to projects in difficult sectors (e.g. providing innovative financing instruments, guarantees, and promoting a green bond market).128 Meanwhile, energy importing governments can create demand for hydrogen that enables offtake agreements with large projects, de-risking major capital investments. The current approach of energy importing nations to building demand is discussed in Section 5.1.
Overall, the availability of capital finance for green ammonia projects is expected to be a significant constraint on individual project size and global ammonia availability. Governments have opportunities to create virtuous cycles, in which supply of green technologies stimulate demand and vice versa, with targeted market interventions.5 This intervention will dictate the size and scope of future ammonia markets, and which countries will be successful in establishing major export industries.
4.5 Public perception
In democratic nations, the public, voting either directly or with their feet, can shape the success or otherwise of major projects. The capacity of a country to export energy on a very large scale therefore depends on a supportive public that is comfortable to allocate large areas of land to produce energy for a different country. Similarly, importing chemical fuels relies on a public that is willing and able to use those fuels safely.Because of perceived opposition, the literature on public resistance to energy projects focusses particularly on wind, although here too, both local communities and the public at large are broadly supportive.130 The perception of opposition is created by highly vocal minorities who receive disproportionate media air time.131
Although support is generally high, there are some grounds upon which local communities have objected to windfarms, such as aesthetic and noise complaints. Rand and Hoen130 find that these concerns are all manageable by taking small tangible steps in a project which alleviate community angst, by providing the public with detailed information about projects, and by taking the public's concerns seriously.
Some objections reported in the literature are unlikely to materialise if a project intends to export chemically stored energy (e.g. concerns that a project will increase local electricity prices,130 or that renewable energy is unreliable132). On the other hand, other objections may become more severe (e.g. fear that profits from a project are being delivered to multi-national companies rather than local communities130).
To the extent that research has been conducted, Ono and Tsunemi133 identified that 66% of the Japanese public was supportive of implementing hydrogen fuel stations. Schmidt and Donsbach134 found that attitudes towards hydrogen were broadly positive, and could be improved by reframing arguments in favour of hydrogen to focus on energy independence and decentralisation of energy grids. Lambert and Ashworth135 specifically asked Australian respondents about their support for hydrogen export, with 78% in support, although only 38% were supportive of a hydrogen port if it were located close to their home. The main concern identified in all surveys was safety.
Even where there is some public opposition to green hydrogen, localised energy projects are rarely a source of objection from the public; they are more often opposed to transmission and distribution of electrical power, usually for aesthetic reasons.129 Therefore, to the extent that the public may object to elements of chemical energy storage projects, it is a relatively favourable method of energy transport.
It was consistently observed that the public's knowledge of hydrogen was relatively poor. Ashworth and Lambert135 asked five simple questions pertaining to hydrogen; only 7% of survey respondents answered all questions correctly; these respondents were almost three times as likely to be supportive or very supportive of a hydrogen industry in Australia. Itaoka et al.136 asked similar questions; less than 30% correctly answered all of them, and there was only a marginal improvement in public knowledge observed between 2008 and 2015.
The majority of research focusses on public attitudes to hydrogen, and there may be unique concerns associated with ammonia that are not yet examined in the literature. Given the public's lack of understanding of hydrogen as an energy vector, Guati-Rojo et al.137 find that it is unlikely that the public has yet formed opinions of hydrogen derivatives such as ammonia. In their small study in Mexico, focus groups were generally supportive of ammonia as an energy vector once the concept had been explained to them, although they raised concerns about NOx emissions and water consumption. In the same way that these challenges can be managed technically, concerns about NOx emissions and water consumption should be manageable with quality public education and consultation on the benefits and risks of ammonia.
In another focus-group study focussing on expert perspectives, the most substantial concern for green ammonia use pertained to its toxicity.137 Safety concerns may be exacerbated in the public domain by the explosion of more than 2500 t of ammonium nitrate in Beirut in 2020;138 although liquid ammonia is non-explosive, the public may still form an association between the chemicals. Ammonia has been used as an industrial chemical since the 1920s; therefore, while safe handling of ammonia is a critical engineering challenge, it is not a novel one. Although it is toxic to humans above 25 ppm, it has a strong odour at lower concentrations (5 ppm) which can provide warning of its presence. Several studies have found it to be as safe or safer than similar hydrocarbons, because it is less flammable.19
Overall, it appears that there is a reasonable level of public support for the hydrogen industry, and a shortage of research into support for the ammonia industry. If the public's concerns are taken seriously, and an effort is made to educate the public about green fuels, then social acceptance should not be a constraint to a hydrogen or ammonia export economy. High levels of public support such as those observed in Australia may stimulate the government action described in Section 4.4, which in turn incentivises the capital investment required for ammonia plants.
4.6 Probable energy exporters
In summary, high-quality renewable resources alone are not sufficient to facilitate a large-scale hydrogen or ammonia industry. For international trade of industrial quantities of green hydrogen and ammonia, projects require an abundance of land, and governments which are able to stimulate capital investment. In some circumstances, the availability of key materials required for green ammonia production, and public opposition, may constrain domestic supply capability. However, on a global scale, materials are generally available, and the public is generally supportive.Because government support to provide public finance is so critical, investment is likely to be concentrated in nations which have announced intentions to support the hydrogen industry. In particular, the literature focusses on Australia, Morocco, Chile, and Norway as countries with a high likelihood of exporting hydrogen as energy. Australia and Morocco benefit from abundant land, a high-quality solar resource, and proximity to demand markets (East Asia and Europe respectively). Chile and Norway both have excellent wind resources; the latter may also benefit from proximity to Germany. New players like Scotland are emerging as governments increasingly publish hydrogen strategies in an attempt to form the basis of this future energy market.139
5. Forecast ammonia demand
Because of the limited focus on ammonia in the literature on green hydrogen, there is scarce data on the future demand for green ammonia. Most forecasts for chemical energy storage technologies focus on predicting the demand for hydrogen. However, since ammonia is a highly effective vector for hydrogen transport, an increase in hydrogen demand is a good proxy for ammonia demand.The literature is generally in agreement that hydrogen will supply ∼20% of global energy demands by 2050.140 However, the distribution of this hydrogen across different sectors and regions is variable in different sources. For instance, Japan and Korea have similar economies and energy systems, yet the fraction of hydrogen forecast for use in the mobility industry varies from 4% in Japan141 to 32% in Korea.142 Additionally, hydrogen and ammonia production and consumption will vary seasonally; Palys and Daoutidis78 found this is caused by seasonal change in both renewable energy supply to the ammonia plant, and seasonal change in energy demand.
At least in the short term, hydrogen demand is stimulated by government policy. Without a ‘nudge’ from governments, it will not be possible to create end-users, and therefore hydrogen demand.5,143 For that reason, hydrogen consumption forecasts are a strong function of national policy, which can change rapidly. Recent announcements by Japan and China to target complete decarbonisation in 2050 and 2060 respectively are likely to impact hydrogen markets.144
The purpose of this section is to collect predictions of hydrogen demand in 2030 and 2050 from a range of sources, and to subsequently understand the extent of hydrogen and ammonia production that will be required in the long-term.
5.1 Countries with hydrogen import strategies
National energy strategies typically envisage a simultaneous deployment of green hydrogen production and investment in technologies which stimulate hydrogen demand, such as fuel-cell vehicles,145 and combined heat and power systems.15 There is comparatively little focus on ammonia for use in the power sector, even though this may be a major source of consumption in the future.4 In general, green hydrogen production lags hydrogen demand. For instance, the French hydrogen strategy predicts 6.5 GW of installed electrolyser capacity in 2030, which is equivalent to ∼700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year (ambitiously assuming a 50% load factor);146 meanwhile, a French not-for-profit organisation supporting the hydrogen industry, Afhypac, estimates French hydrogen consumption will be almost triple that value.147 Similarly, Korea intends to consume almost 2 million t per year of hydrogen by 2030, but will not start importing substantial quantities until the following decade,145 implying a substantial differential between production and demand.
000 t per year (ambitiously assuming a 50% load factor);146 meanwhile, a French not-for-profit organisation supporting the hydrogen industry, Afhypac, estimates French hydrogen consumption will be almost triple that value.147 Similarly, Korea intends to consume almost 2 million t per year of hydrogen by 2030, but will not start importing substantial quantities until the following decade,145 implying a substantial differential between production and demand.
The difference between a nation's renewable production capacity and its demand will be made up by blue or grey hydrogen domestically, or through hydrogen import. Japan, Germany and Korea are the only countries whose national hydrogen strategies specifically include an intention to import hydrogen; the targets they have set are summarised in Table 10.
| Country | 2030 domestic green hydrogen production target(i) (kt per year) | 2030 domestic hydrogen consumption forecast (kt per year) | Long term domestic hydrogen consumption forecast (kt per year) | Equivalent long-term ammonia demand(ii) (MTPA) |
|---|---|---|---|---|
| a Notes: (i) where production was specified in GW of electrolyser capacity, an 82% efficiency was assumed on the HHV, with 4000 load hours per year, to convert between electrolyser capacity and hydrogen production. (ii) Equivalence based on HHV. For reference, existing global demand for ammonia is in the order of 200 MTPA.105 | ||||
| Germany15 | 415 | 3000 | 3000–11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
19–72 |
| Japan16 | — | 300 | 5000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
32–63 |
| Korea145 | 300 | 1940 | 5260 | 33 |
Japan is unique among these nations, in that it intends to import significant quantities of hydrogen by 2030. By contrast, in Germany and Korea, the transition to imported green hydrogen will not commence until 2030; conventional and blue hydrogen will fill the gaps until an import/export industry is created.
The challenge for green hydrogen producers in the next decade, therefore, is not to find hydrogen consumers in a general sense; rather, it is to find consumers who are willing to pay a premium for a green product. Their capacity to do so will dictate the balance between green and conventional ammonia in 2030.
The trend of renewable supply lagging global demand for hydrogen is also observable for ammonia. The proposed Asian Renewable Energy Hub is an order of magnitude larger than any previously announced ammonia facility; using a 60% conversion efficiency, we estimate its annual production will be ∼8.5 MTPA. This is less than 5% of current global consumption of ammonia as fertiliser. If it were to be used for energy, 8.5 MTPA of ammonia is approximately equivalent to 1.3 MTPA of hydrogen on an HHV basis. Although this is a very small quantity of energy when compared to Japan's total primary energy demand, the AREH could supply the entirety of Japan's hydrogen import target with just 23% of its annual production.
Again, there is no shortage of ammonia demand; merely a shortage of publicised appetite for a carbon-neutral product used as an energy vector. Mega-projects of the magnitude of the AREH may crowd out other smaller projects with less access to capital, or may aggressively stimulate demand for green ammonia as an energy vector, and encourage other similarly-scaled projects.
5.2 Countries without hydrogen import strategies
Although they have not publicly announced a specific intention to do so, four large markets for hydrogen import may also exist beyond 2030: China, India, Singapore and the EU (excluding Germany). The shipping industry may also contribute significantly to synthetic fuel demand.Although a collection of Chinese companies known as the Chinese Hydrogen Alliance have published a white paper outlining the forecast developments in the industry,148 China has not published an official hydrogen strategy. China is forecast to have very high energy demands caused by a rapidly growing economy, but it also has announced its intention to fully decarbonise by 2060 and will require chemical energy storage to do so. China dominates global production of solar panels, and some market outlooks suggest that it has the capacity to produce much cheaper electrolysers than Europe,101 although these figures are disputed by the IEA.25 Therefore, China will have significant capacity to manufacture its own hydrogen, and it is not likely that energy import will be necessary to meet demand.
India has access to a high-quality solar resource and its current electricity use per capita is very low. That said, India has a very large population; in order to maintain its agricultural self-sufficiency, substantial land may need to be allocated to food production, reducing its capacity for energy independence.122 An analysis of available land (including barren and scrublands) in India indicates that its total renewable energy capacity is approximately three times its forecast energy demand. This factor of three is relatively small compared to most nations, which could create pressure to import energy.4 In general, though, Indian markets are highly dependent on government policy and estimated economic growth, which creates forecasting uncertainty. Therefore, India could, like China, play a role as both an energy importer or exporter in the future.
Singapore also has no official hydrogen strategy. At present, 95% of Singapore's energy comes from imported natural gas. Singapore envisages decarbonisation through a three-pronged approach: increasing the supply of local solar, interconnecting with grid electricity of other ASEAN nations, and the use of low carbon fuels, either through CCS or the importing of green hydrogen.149 Detail in the final prong remains scarce. Compared to Japan, Singapore is well positioned to import large scale grid electricity because of its location and close geopolitical ties to regional allies; however, even large-scale integration of national energy systems may not be suitable to provide significant grid firming. Similarly, solar energy alone is unlikely to provide adequate energy for Singapore; its 2030 plans to install 2 GW of capacity represents only ∼4% of national energy demand.150 It is therefore likely that Singapore will need to import a substantial fraction of its energy; the extent to which it will require ammonia depends on its success in connecting to an ASEAN grid.
It is possible that other European countries (including the UK) may also engage in hydrogen trade; Scotland, for instance, announced that it intends to export hydrogen, although using ammonia to do so is not discussed in its strategy.139 Many European countries have announced ambitious hydrogen strategies as part of the EU's plan to increase hydrogen production within the region to 40 GW by 2020. This increase in production is to be combined with technology investments in fuel cell vehicles, and the use of green hydrogen in natural gas pipelines and other industries. However, in general, the strategies of most nations (France, Spain, Portugal, Norway, and the Netherlands) do not foresee or discuss importing of hydrogen in detail.
The majority of the EU's hydrogen trade is likely to be internal. For instance, there is already trade between Belgium, the Netherlands and France facilitated by a pipe constructed by Air Liquide.146 However, to some extent it is expected that the EU will need to engage in energy import: Hank et al.24 report that the EU's forecast electricity consumption in 2030 will be ∼16 PW h; its maximum energy generation capacity from renewables is estimated at between 9 and 14 PW h. For that reason, some energy import is essential, although it is possible that any shortfall may be supplied by HVDC from nearby regions (e.g. Morocco to Spain), rather than by chemical energy transport, unless the EU requires external stabilisation of its energy grid.
Although it is not a country, the shipping industry is a large consumer of energy. IRENA estimates it consumed 2472 TW h of bunker fuel in 2017;151 if it were a country, it would be the world's sixth largest emitter of carbon dioxide.152 In 2018, the International Maritime Organisation (IMO) set a target of reducing total shipping emissions by 50% by 2050, which will require an emissions intensity reduction of more than 70%.153 As discussed in Section 2, ammonia is a strong competitor for use as a renewable fuel to enable emissions reduction in this sector. If the entirety of the IMO's target emissions reduction were achieved by fuel substitution, approximately 2800 TW h of renewable fuels would be required. The IEA, however, argues it will be possible to achieve some emissions reductions through operational modification (e.g. reducing speed limits), and by electrification (for very short routes only), meaning a more realistic estimate of renewable fuel demand is ∼1400 TW h (224 MTPA ammonia on a HHV basis).153 To meet even this demand, 27 projects of equivalent size to the Asian Renewable Energy Hub would be required; constructing them would more than double current global ammonia production.105
Overall, there is significant demand for imported hydrogen and ammonia as a driver for decarbonisation in some of the world's largest economies. In the medium term, green fuel demand is capped by cost, as it is competing against grey hydrogen. In the longer term, demand is capped by energy consumption, although the consumption of the shipping industry alone could be very large. Fig. 5 summarises the ammonia demand in the countries listed in Table 10, compared to the size of the announced projects listed in Table 9. At present, supply is much less than the prospective demand, although the demand shown will not manifest until the middle of the century.
To some extent, energy consumption will be shaped by hydrogen availability. Very cheap chemical storage would favour uptake of fuel cell vehicles and global energy trade. Comparatively expensive chemical storage would drive electrification and demand-response smart grids, with hydrogen and ammonia only used where absolutely necessary.
6. Conclusions
There is a growing body of literature recommending ammonia as an important contributor to global decarbonisation; in particular, as its price falls over the next three decades, it will become highly useful as a global reserve fuel when it is used as a spatial energy vector. Most authors concur that it outperforms other chemical energy vectors because of its promising hydrogen density and ease of distribution and storage.Despite an abundance of literature which approximates the LCOA, only a small number of authors present a plausible green ammonia plant design which is robust to the inherent variability of renewable energy and uses plausible input data. The range of ammonia costs from these authors using estimates for 2020 is between 470 and 617 USD per t; ammonia produced at the lower end of this range would occasionally be cheaper than ammonia available in the spot market in the last decade.
The largest constraints on ammonia supply are land availability and finance, although these will tend to encourage production in certain countries, rather than limiting the overall size of an ammonia economy. There is growing demand for hydrogen import in some countries; ammonia is well placed to satisfy that demand. Possible demand by 2050 is very large in comparison to existing demand; the shipping industry alone will require ammonia production to double in order to meet its 2050 goals. Similarly, even considering only the three countries with national strategies to import hydrogen could increase ammonia demand by between 40 and 80% of existing demand.
Despite these promising findings, more rigorous consideration of ammonia as a spatial energy vector is still merited. There is a lack of detailed consideration of the relationship between ammonia production and ammonia transport. Typically, the papers which provide a detailed solution to the complexities of production neglect to consider how they may interact with the complexities of transport, and vice versa. Additionally, research to date has focussed on the bilateral exchange of energy. This contrasts with the multilateral nature of fossil-fuel transport and is therefore likely to be an oversimplification of future energy systems.
Ideally, future work will optimise a complete system for ammonia delivery, including all aspects of production, transport, and consumption. These include:
• Selection of ammonia production location for the purpose of energy export, considering both the quality of the local resource and the transport costs to the intended destination or destinations.
• Management of process flexibility on a short-term basis to ensure continuous and safe operation of the Haber–Bosch process.
• Management of process flexibility on a long-term basis to ensure seasonal supply in exporting nations matches seasonal demand for ammonia in importing nations.
• Understanding of the constraints on ammonia supply and demand, including physical limits (e.g. land, materials), project-specific factors (e.g. availability of capital, public support) and maximum consumption rates in energy-importing nations.
• Consideration of possible synergies between production and transport requirements.
Collectively, these gaps in the literature represent a risk to the optimisation of what is likely to be a major energy supply chain of the future. By optimising this supply chain, we can:
• Enable efficient allocation of finite capital.125
• Enable investors to identify lowest-cost options for large scale ammonia development, which is necessary in the short term to stimulate demand3 and demonstrate feasibility.
• Prevent wasteful allocation of large capital subsidies offered by governments (renewable subsidies were greater than 60 billion USD in 2016 in the EU alone140).
• Enable energy importers to identify appropriate export partners, and to facilitate international relationships.
• Enable energy exporters to identify possible future markets, and tailor policy and regulation to encourage investment and growth of new renewable export industries.
List of acronyms
| AREH | Asian Renewable Energy Hub |
| CAES | Compressed air energy storage |
| CCGT | Combined cycle gas turbine |
| CCS | Carbon capture and storage |
| DAC | Direct air capture (of carbon dioxide) |
| HB | Haber–Bosch |
| HVDC | High voltage direct current |
| LCOA | Levelised cost of ammonia |
| LNG | Liquid natural gas |
| LOHC | Liquid organic hydrogen carrier |
| IEA | International Energy Agency |
| IMO | International Maritime Organisation |
| IRENA | International Renewable Energy Agency |
| PEM(FC) | Proton exchange membrane (fuel cell) |
| PPA | Power purchasing agreement |
| SOEC | Solid oxide electrolyser cell |
| SOFC | Solid oxide fuel cell |
| TEA | Techno-economic analysis |
| VRE | Variable renewable energy |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work presented here was supported financially by the Rhodes Trust.References
- J. Schmidt, K. Gruber, M. Klingler, C. Klöckl, L. Ramirez Camargo, P. Regner, O. Turkovska, S. Wehrle and E. Wetterlund, Energy Environ. Sci., 2019, 12, 2022–2029 RSC
.
- Institute of Energy Economics Japan, IIEJ Outlook – Energy transition and a thorny path for 3E challenges, https://eneken.ieej.or.jp/data/8122.pdf, accessed February 2021 Search PubMed.
- International Renewable Energy Agency, Hydrogen: A Renewable Energy Perspective, https://www.irena.org/publications/2019/Sep/Hydrogen-A-renewable-energy-perspective, accessed December 2020 Search PubMed.
- W. Hall, T. Spencer, G. Renjith and S. Dayal, Report on The Potential Role of Hydrogen in India – 'Harnessing the Hype', https://www.teriin.org/event/potential-role-hydrogen-india-harnessing-hype-teri-report-launch-event, accessed January 2021 Search PubMed.
- W. L. Ahlgren, Proc. IEEE, 2012, 100, 3001–3052 Search PubMed
.
- V. Srinivasan, M. Temminghoff, S. Chanrnock and P. Hartley, Hydrogen Research, Development and Demonstration: Priorities and Opportunities for Australia, CSIRO, https://www.csiro.au/en/Do-business/Futures/Reports/Energy-and-Resources/Hydrogen-Research, accessed December 2020 Search PubMed.
- A. T. Wijayanta, T. Oda, C. W. Purnomo, T. Kashiwagi and M. Aziz, Int. J. Hydrogen Energy, 2019, 44, 15026–15044 CrossRef CAS
.
- National Institute for Standards and Technology, Chemistry Webbook, https://webbook.nist.gov/chemistry/, accessed November 2020 Search PubMed.
-
J. Bartels, PhD Thesis, Iowa State University, 2008
.
- A. Valera-Medina, H. Xiao, M. Owen-Jones, W. I. F. David and P. J. Bowen, Prog. Energy Combust. Sci., 2018, 69, 63–102 CrossRef
.
- J. Armijo and C. Philibert, Int. J. Hydrogen Energy, 2020, 45, 1541–1558 CrossRef CAS
.
- A. Yapicioglu and I. Dincer, Renewable Sustainable Energy Rev., 2019, 103, 96–108 CrossRef CAS
.
- K. H. Rouwenhorst, A. G. J. Van Der Ham, G. Mul and S. R. A. Kersten, Renewable Sustainable Energy Rev., 2019, 114, 109339 CrossRef CAS
.
- O. Elishav, B. Mosevitzky Lis, E. M. Miller, D. J. Arent, A. Valera-Medina, A. Grinberg Dana, G. E. Shter and G. S. Grader, Chem. Rev., 2020, 120, 5352–5436 CrossRef CAS PubMed
.
- German Federal Ministry for Economic Affairs and Energy, The National Hydrogen Strategy, https://www.bmbf.de/files/bmwi_NationaleWasserstoffstrategie_Eng_s01.pdf, accessed February 2021 Search PubMed.
- Ministry of Economy Trade and Industry, Basic Hydrogen Strategy, https://www.meti.go.jp/english/press/2017/pdf/ 1226_003a.pdf, accessed November 2020 Search PubMed.
- R. Nayak-Luke and R. Bañares-Alcántara, Energy Environ. Sci., 2020, 13, 2957–2966 RSC
.
- C. Smith, A. K. Hill and L. Torrente-Murciano, Energy Environ. Sci., 2020, 13, 331–344 RSC
.
- D. R. MacFarlane, P. V. Cherepanov, J. Choi, B. H. R. Suryanto, R. Y. Hodgetts, J. M. Bakker, F. M. Ferrero Vallana and A. N. Simonov, Joule, 2020, 4, 1186–1205 CrossRef CAS
.
-
J. T. Hinkley, R. K. McNaughton, A. J. Hayward and K. Lovegrove, presented in part at the International Conference on Concentrating Solar Power and Chemical Energy Systems, Abu Dhabi, 2017 Search PubMed
.
- P. T. Aakko-Saksa, C. Cook, J. Kiviaho and T. Repo, J. Power Sources, 2018, 396, 803–823 CrossRef CAS
.
- DNV GL, Hydrogen as an energy carrier, https://www.dnvgl.com/oilgas/download/hydrogen-as-an-energy-carrier.html, accessed November 2020 Search PubMed.
- C. Stiller, A. M. Svensson, S. Møller-Holst, U. Bünger, K. A. Espegren, Ø. B. Holm and A. Tomasgård, Energy, 2008, 33, 1623–1633 CrossRef CAS
.
- C. Hank, A. Sternberg, N. Köppel, M. Holst, T. Smolinka, A. Schaadt, C. Hebling and H.-M. Henning, Sustainable Energy Fuels, 2020, 4, 2256–2273 RSC
.
- International Energy Agency, The future of Hydrogen, https://www.iea.org/reports/hydrogen, accessed January 2021 Search PubMed.
- T. Hijikata, Int. J. Hydrogen Energy, 2002, 27, 115–129 CrossRef CAS
.
- Y. Zhao, B. P. Setzler, J. Wang, J. Nash, T. Wang, B. Xu and Y. Yan, Joule, 2019, 3, 2472–2484 CrossRef CAS
.
- C. Hank, S. Gelpke, A. Schnabl, R. J. White, J. Full, N. Wiebe, T. Smolinka, A. Schaadt, H.-M. Henning and C. Hebling, Sustainable Energy Fuels, 2018, 2, 1244–1261 RSC
.
- Z. Cesaro, M. Ives, R. Nayak-Luke, M. Mason and R. Bañares-Alcántara, Appl. Energy, 2021, 282, 116009 CrossRef CAS
.
- International Energy Agency, IEA World Energy Outlook 2020, https://www.iea.org/reports/world-energy-outlook-2020, accessed January 2020 Search PubMed.
- Z. Hafsi, M. Mishra and S. Elaoud, Procedia Struct. Integr., 2018, 13, 210–217 CrossRef
.
-
W. C. Leighty and J. H. Holbrook, presented in part at the World Hydrogen Energy Conference, Toronto, 2012 Search PubMed
.
- C. Yang and J. Ogden, Int. J. Hydrogen Energy, 2007, 32, 268–286 CrossRef CAS
.
- M. J. Palys, A. Kuznetsov, J. Tallaksen, M. Reese and P. Daoutidis, Chem. Eng. Process., 2019, 140, 11–21 CrossRef CAS
.
- A. Afif, N. Radenahmad, Q. Cheok, S. Shams, J. H. Kim and A. K. Azad, Renewable Sustainable Energy Rev., 2016, 60, 822–835 CrossRef CAS
.
-
H. Kwon, M. Ryu and S.-K. An, presented in part at the AIChE Annual Meeting Conference, Orlando, 2019 Search PubMed
.
- T. Ben Brahim, F. Wiese and M. Münster, Energy, 2019, 188, 116009 CrossRef CAS
.
- R. Lan and S. Tao, Front. Energy Res., 2014, 2, 1–4 Search PubMed
.
-
G. Cinti, L. Barelli and G. Bidini, presented in part at the Second International Conference on material science, smart structures and applications, Erode, India, 2019 Search PubMed
.
- O. Kurata, N. Iki, T. Inoue, T. Matsunuma, T. Tsujimura, H. Furutani, M. Kawano, K. Arai, E. C. Okafor, A. Hayakawa and H. Kobayashi, Proc. Combust. Inst., 2019, 37, 4587–4595 CrossRef CAS
.
- A. Valera-Medina, M. Gutesa, H. Xiao, D. Pugh, A. Giles, B. Goktepe, R. Marsh and P. Bowen, Int. J. Hydrogen Energy, 2019, 44, 8615–8626 CrossRef CAS
.
- Hydrogenious, LOHC Product Technologies, https://www.hydrogenious.net/wp-content/uploads/2018/08/Hydrogenious-LOHC-Technologies-Product-Brochure_short.pdf, accessed December 2020.
- D. Teichmann, W. Arlt and P. Wasserscheid, Int. J. Hydrogen Energy, 2012, 37, 18118–18132 CrossRef CAS
.
- L. Lin, Y. Tian, W. Su, Y. Luo, C. Chen and L. Jiang, Sustainable Energy Fuels, 2020, 4, 3006–3017 RSC
.
- G. Ondrey, Chem. Eng., 2017, 124, 8 Search PubMed
.
- CSIRO, Metal membrane for hydrogen separation, https://www.csiro.au/en/Research/EF/Areas/Renewable-and-low-emission-tech/Hydrogen/Hydrogen-membrane, accessed December 2020 Search PubMed.
- CertifHy, https://www.certifhy.eu/, accessed November 2020.
- Y. Bicer and I. Dincer, J. Cleaner Prod., 2018, 170, 1594–1601 CrossRef CAS
.
-
J. Fuhrmann, M. Hülsebrock and U. Krewer, in Transition to Renewable Energy Systems, 2013, pp. 691–706, DOI:10.1002/9783527673872.ch33
.
-
Y. Bicer and I. Dincer, in Towards 100% Renewable Energy, 2017, ch. 7, pp. 69–86, DOI:10.1007/978-3-319-45659-1_7
.
- O. Siddiqui and I. Dincer, Therm. Sci. Eng. Prog., 2018, 5, 568–578 CrossRef
.
- N. Roussanoglou, Once a niche segment, LPG carrier market is now a boon for ship owners, https://www.hellenicshippingnews.com/once-a-niche-segment-lpg-carrier-market-is-now-a-boon-for-ship-owners/, accessed January 2020 Search PubMed.
- U.S. Bureau of Labor Statistics, CPI Inflation Calculator, https://www.bls.gov/data/inflation_calculator.htm, accessed April 2021 Search PubMed.
- S. Kamiya, M. Nishimura and E. Harada, Phys. Procedia, 2015, 67, 11–19 CrossRef CAS
.
- M. Wietschel and U. Hasenauer, Renewable Energy, 2007, 32, 2129–2146 CrossRef
.
-
W. W. Tso, C. D. Demirhan, S. Lee, H. Song, J. B. Powell and E. N. Pistikopoulos, in Proceedings of the 9th International Conference on Foundations of Computer-Aided Process Design, 2019, pp. 1–6, DOI:10.1016/b978-0-12-818597-1.50001-1
.
- Hydrogen Import Coalition, Shipping sun and wind to Belgium is key in climate neutral economy, https://www.portofantwerp.com/sites/default/files/HydrogenImportCoalition.pdf, accessed February 2021 Search PubMed.
- C. Fúnez Guerra, L. Reyes-Bozo, E. Vyhmeister, M. Jaén Caparrós, J. Luis Salazar and C. Clemente-Jul, Renewable Energy, 2020, 157, 404–414 CrossRef
.
- International Energy Agency, Hydrogen, https://www.iea.org/reports/hydrogen, accessed January 2020 Search PubMed.
- Y. Ishimoto, M. Voldsund, P. Nekså, S. Roussanaly, D. Berstad and S. O. Gardarsdottir, Int. J. Hydrogen Energy, 2020, 45(58), 32865–32883 CrossRef CAS
.
- P.-M. Heuser, D. S. Ryberg, T. Grube, M. Robinius and D. Stolten, Int. J. Hydrogen Energy, 2019, 44, 12733–12747 CrossRef CAS
.
- Y. Kawakami, S. Endo and H. Hirai, A feasibility study on the supply chain of CO2-Free Ammonia, https://eneken.ieej.or.jp/data/8371.pdf, accessed January 2021 Search PubMed.
- A. Babarit, J.-C. Gilloteaux, G. Clodic, M. Duchet, A. Simoneau and M. F. Platzer, Int. J. Hydrogen Energy, 2018, 43, 7266–7289 CrossRef CAS
.
-
T. Watanabe, K. Murata, S. Kamiya and K.-I. Ota, presented in part at the 18th World Hydrogen Energy Conference 2010, Essen, 2010 Search PubMed
.
- J. Gretz, B. Drolet, D. Kluyskens, F. Sandmann and O. Ullman, Int. J. Hydrogen Energy, 1993, 19(2), 169–174 CrossRef
.
- H. Homan and C. Klass, Argus LNG Freight Rates, https://www.argusmedia.com/en/hubs/lng, accessed January 2021 Search PubMed.
- H. Rogers, The LNG Shipping Forecast: Costs rebounding, outlook uncertain, https://www.oxfordenergy.org/wpcms/wp-content/uploads/2018/02/The-LNG-Shipping-Forecast-costs-rebounding-outlook-uncertain-Insight-27.pdf, accessed January 2021 Search PubMed.
-
K. Engblom, LNG to Power in remote locations-the optimal way, 2016 Search PubMed
.
- O. Osman, S. Sgouridis and A. Sleptchenko, J. Cleaner Prod., 2020, 271, 121627 CrossRef CAS
.
- D. Kim, D. Shin, J. Heo, H. Lim, J.-A. Lim, H. M. Jeong, B.-S. Kim, I. Heo, I. Oh, B. Lee, M. Sharma, H. Lim, H. Kim and Y. Kwon, ACS Energy Lett., 2020, 5, 3647–3656 CrossRef CAS
.
- A. Hauch, R. Kungas, P. Blennow, A. B. Hansen, J. B. Hansen, B. V. Mathiesen and M. B. Mogensen, Science, 2020, 370, 6513 CrossRef PubMed
.
- O. Posdziech, K. Schwarze and J. Brabandt, Int. J. Hydrogen Energy, 2019, 44, 19089–19101 CrossRef CAS
.
- E. R. Morgan, J. F. Manwell and J. G. McGowan, ACS Sustainable Chem. Eng., 2017, 5, 9554–9567 CrossRef CAS
.
- P. Tunå, C. Hulteberg and S. Ahlgren, Environ. Prog. Sustainable Energy, 2014, 33, 1290–1297 CrossRef
.
- E. Morgan, J. Manwell and J. Mcgowan, Renewable Energy, 2014, 72, 51–61 CrossRef CAS
.
- M. Rivarolo, G. Riveros-Godoy, L. Magistri and A. F. Massardo, ChemEngineering, 2019, 3, 87 CrossRef CAS
.
- J. Moore and N. Meeks, Clean Energy, 2020, 4, 270–287 CrossRef
.
- M. J. Palys and P. Daoutidis, Comput. Chem. Eng., 2020, 136 Search PubMed
.
- G. Wang, A. Mitsos and W. Marquardt, AIChE J., 2017, 63, 1620–1637 CrossRef CAS
.
- S. S. Beerbühl, M. Fröhling and F. Schultmann, Eur. J. Oper. Res., 2015, 241, 851–862 CrossRef
.
- S. S. Beerbühl, B. Kolbe, C. Roosen and F. Schultmann, Chem. Ing. Tech., 2014, 86, 649–657 CrossRef
.
- M. Malmali, G. Le, J. Hendrickson, J. Prince, A. V. McCormick and E. L. Cussler, ACS Sustainable Chem. Eng., 2018, 6, 6536–6546 CrossRef CAS
.
- A. Sánchez and M. Martín, J. Cleaner Prod., 2018, 178, 325–342 CrossRef
.
- W. Eichhammer, Study on the opportunities of Power to X in Morocco, https://www.isi.fraunhofer.de/en/presse/2020/presseinfo-26-policy-brief-wasserstoff.html, accessed November 2020 Search PubMed.
- H. Zhang, L. Wang, J. van Herle, F. Maréchal and U. Desideri, Appl. Energy, 2020, 259 CAS
.
- M. Matzen, M. Alhajji and Y. Demirel, J. Adv. Chem. Eng., 2015, 5(3), 1000128 Search PubMed
.
- P. H. Pfromm, J. Renewable Sustainable Energy, 2017, 9, 034702 CrossRef
.
- J. Ikäheimo, J. Kiviluoma, R. Weiss and H. Holttinen, Int. J. Hydrogen Energy, 2018, 43, 17295–17308 CrossRef
.
- J. R. Gomez, J. Baca and F. Garzon, Int. J. Hydrogen Energy, 2020, 45, 721–737 CrossRef CAS
.
- G. Wang, A. Mitsos and W. Marquardt, AIChE J., 2020, 66(6), e16947 CrossRef CAS
.
- A. Allman, M. J. Palys and P. Daoutidis, AIChE J., 2019, 65(7), e16434 CrossRef
.
- C. D. Demirhan, W. W. Tso, J. B. Powell and E. N. Pistikopoulos, AIChE J., 2019, 65(7), e16498 CrossRef
.
- R. Nayak-Luke, R. Bañares-Alcántara and I. Wilkinson, Ind. Eng. Chem. Res., 2018, 57, 14607–14616 CrossRef CAS
.
- M. J. Palys, A. Allman and P. Daoutidis, Ind. Eng. Chem. Res., 2018, 58, 5898–5908 CrossRef
.
- Institute for Sustainable Process Technology, Power to Ammonia, https://ispt.eu/news/power-ammonia-renewable-energy-co2-free-ammonia-chemical-feedstock-fuel/, accessed November 2020 Search PubMed.
- R. Bañares-Alcántara, G. Dericks, M. Fiaschetti, P. Grünewald, J. M. Lopez, E. Tsang, A. Yang, L. Ye and S. Zhao, Analysis of Islanded Ammonia-based Energy Storage Systems, http://www2.eng.ox.ac.uk/systemseng/publications/Ammonia-based_ESS.pdf, accessed October 2020 Search PubMed.
- P. Trop and D. Goricanec, Energy, 2016, 108, 155–161 CrossRef CAS
.
- B. Lin, T. Wiesner and M. Malmali, ACS Sustainable Chem. Eng., 2020, 8, 15517–15531 CrossRef CAS
.
- O. A. C. Hoes, L. J. J. Meijer, R. J. van der Ent and N. C. van de Giesen, PLoS One, 2017, 12, e0171844 CrossRef PubMed
.
- B. Steffen, Energy Econ., 2020, 88, 104783 CrossRef
.
- Bloomberg NEF, Hydrogen Economy Outlook Key Messages, https://data.bloomberglp.com/professional/sites/24/BNEF-Hydrogen-Economy-Outlook-Key-Messages-30-Mar-2020.pdf, accessed December 2020 Search PubMed.
- A. Mayyas, M. Ruth, B. Pivovar, G. Bender and K. Wipke, Manufacturing cost analysis for PEM water electrolysers, 2018, https://www.nrel.gov/docs/fy19osti/72740.pdf, accessed January 2021 Search PubMed.
- Asian Renewable Energy Hub, Asian Renewable Energy Hub, https://asianrehub.com/about/, accessed February 2021 Search PubMed.
- T. Brown, Green ammonia demonstration plants now operational in Oxford and Fukushima, https://www.ammoniaenergy.org/articles/green-ammonia-demonstration-plants-now-operational-in-oxford-and-fukushima/, accessed February 2021 Search PubMed.
- Yara and Engie, ENGIE-YARA Renewable hydrogen and ammonia deployment in Pilbara, https://arena.gov.au/assets/2020/11/engie-yara-renewable-hydrogen-and-ammonia-deployment-in-pilbara.pdf, accessed February 2020 Search PubMed.
- S. Ravn, Danish partnership sets out to build world's first commercial scale green ammonia plant, https://blog.topsoe.com/danish-partnership-sets-out-to-build-worlds-first-commercial-scale-green-ammonia-plant, accessed February 2021 Search PubMed.
- Yara, Yara News and Media, https://www.yara.com/news-and-media/, accessed February 2021 Search PubMed.
- C. Close, CF Industries Announces Commitment to Clean Energy Economy, https://cfindustries.q4ir.com/news-market-information/press-releases/news-details/2020/CF-Industries-Announces-Commitment-to-Clean-Energy-Economy/default.aspx, accessed February 2021 Search PubMed.
- J. S. Jones, First green hydrogen projects emerge in Chile, https://www.powerengineeringint.com/hydrogen/first-green-hydrogen-projects-emerge-in-chile/, accessed February 2021 Search PubMed.
- T. Ong, Origin to investigate export scale green hydrogen project in Tasmania, https://www.originenergy.com.au/about/investors-media/media-centre/origin_to_investigate_export_scale_green_hydrogen_project_in_tasmania.html, accessed Febraury 2021 Search PubMed.
- J. Parnell, World's Largest Green Hydrogen Project Unveiled in Saudi Arabia, https://www.greentechmedia.com/articles/read/us-firm-unveils-worlds-largest-green-hydrogen-project, accessed February 2021 Search PubMed.
- S. Jai, ACME Solar plans to invest $2.5 billion in Oman green ammonia unit, https://www.business-standard.com/article/companies/acme-solar-plans-to-invest-2-5-billion-in-oman-green-ammonia-unit-121032301509_1.html, accessed April 2021 Search PubMed.
- Austria Energy, Green Hydrogen, https://www.austriaenergy.com/en/green-hydrogen/, accessed April 2021 Search PubMed.
- A. Gupta, SECI to seek bids for green hydrogen units, https://www.eqmagpro.com/seci-to-seek-bids-for-green-hydrogen-units/, accessed February 2021 Search PubMed.
- S. Carrara, P. Alves Dias, B. Plazzotta and C. Pavel, Raw materials demand for wind and solar PV technologies in the transition towards a decarbonised energy system, DOI:10.2760/160859, https://ec.europa.eu/jrc/en/publication/raw-materials-demand-wind-and-solar-pv-technologies-transition-towards-decarbonised-energy-system, accessed December 2020.
- Z. Weng, S. M. Jowitt, G. M. Mudd and N. Haque, Econ. Geol., 2015, 110, 1925–1952 CrossRef
.
- J. Brauns and T. Turek, Processes, 2020, 8, 248 CrossRef CAS
.
- F. Hegge, F. Lombeck, E. Cruz Ortiz, L. Bohn, M. Von Holst, M. Kroschel, J. Hübner, M. Breitwieser, P. Strasser and S. Vierrath, ACS Appl. Energy Mater., 2020, 3, 8276–8284 CrossRef CAS
.
- J. Kim, K. Park, D. Yang and S. Hong, Appl. Energy, 2019, 254, 113652 CrossRef CAS
.
- ThyssenKrupp Industrial Solutions, Making the world's largest ammonia plant even larger, https://insights.thyssenkrupp-industrial-solutions.com/story/making-the-worlds-largest-ammonia-plant-even-larger/, accessed March 2021 Search PubMed.
- P. Moriarty and D. Honnery, Renewable Sustainable Energy Rev., 2012, 16, 244–252 CrossRef
.
- D. van de Ven, M. Gonzalez-Equino and I. Arto, Land-use impacts from renewable energy policies, http://transrisk-project.eu/sites/default/files/Documents/4.4.6_Land-useimpactsfromrenewableenergypolicies.pdf, accessed December 2020 Search PubMed.
- E. Rowan, L. Seabrook and C. Mcalpine, J. Geogr. Sci., 2018, 28, 1567–1579 CrossRef
.
- Australian Energy Market Operator, Australian Energy Statistics 2020 Energy Update Report, https://www.energy.gov.au/publications/australian-energy-update-2020, accessed December 2020 Search PubMed.
- W. Michael, A. Lucila, B. Simon, G. Pablo, G. Tim, N. Yoko, P. Ryszard, S. Rebecca and T. Eleni, World Energy Investment 2020, https://webstore.iea.org/download/direct/3003, accessed January 2020 Search PubMed.
- S. Jamie and H. Robin, Will Australia's ‘hydrogen road’ to Japan cut emissions?, https://www.ft.com/content/5a18dc92-ae8c-4b27-98fc-ed7f1465980e, accessed January 2021 Search PubMed.
- International Energy Agency, Japan – countries and regions, https://www.iea.org/countries/japan, accessed January 2020 Search PubMed.
- International Renewable Energy Agency and Climate Policy Initiative, Global landscape of renewable energy finance 2020, https://irena.org/publications/2020/Nov/Global-Landscape-of-Renewable-Energy-Finance-2020, accessed December 2020 Search PubMed.
- S. Carley, D. M. Konisky, Z. Atiq and N. Land, Environ. Res. Lett., 2020, 15, 093007 CrossRef
.
- J. Rand and B. Hoen, Energy Res. Soc. Sci., 2017, 29, 135–148 CrossRef
.
-
S. Chapman and F. Crichton, in Wind Turbine Syndrome, Sydney University Press, 2017, pp. 179–224, DOI:10.2307/j.ctt1zrvhrt.12
.
- H. Gavin, Integrating renewable energy: opportunities and challenges, https://www.research.ox.ac.uk/Article/2019-12-13-integrating-renewable-energy-opportunities-and-challenges, accessed November 2020 Search PubMed.
- K. Ono and K. Tsunemi, Int. J. Hydrogen Energy, 2017, 42, 10697–10707 CrossRef CAS
.
- A. Schmidt and W. Donsbach, Int. J. Hydrogen Energy, 2016, 41(8), 4509–4520 CrossRef CAS
.
- V. Lambert and P. Ashworth, The Australian public's perception of hydrogen for energy, https://arena.gov.au/assets/2018/12/the-australian-publics-perception-of-hydrogen-for-energy.pdf, accessed December 2020 Search PubMed.
- K. Itaoka, A. Saito and K. Sasaki, Int. J. Hydrogen Energy, 2017, 42, 7290–7296 CrossRef CAS
.
-
A. Guati-Rojo, C. Demski and A. Valera-Medina, in Techno-Economic Challenges of Green Ammonia as an Energy Vector, ed. A. Valera-Medina and R. Banares-Alcantara, Academic Press, 2021, pp. 277–302, DOI:10.1016/B978-0-12-820560-0.00012-6
.
- G. Guglielmi, Why Beirut's ammonia nitrate blast was so devastating, https://www.nature.com/articles/d41586-020-02361-x, accessed January 2021 Search PubMed.
- Government of Scotland, Scottish Hydrogen Strategy, https://www.gov.scot/binaries/content/documents/govscot/publications/research-and-analysis/2020/12/scottish-hydrogen-assessment-report/documents/scottish-hydrogen-assessment/scottish-hydrogen-assessment/govscot%3Adocument/scottish-hydrogen-assessment.pdf?forceDownload=true, accessed January 2021 Search PubMed.
- The Hydrogen Council, Path to Hydrogen Competitiveness – a cost perspective, https://hydrogencouncil.com/en/path-to-hydrogen-competitiveness-a-cost-perspective/, accessed February 2021 Search PubMed.
- M. Nagashima, Japan's hydrogen strategy and its economic and geopolitical implications, https://www.ifri.org/sites/default/files/atoms/files/nagashima_japan_hydrogen_2018_.pdf, accessed February 2021 Search PubMed.
- Korean Hydrogen Study Task Force, Korean Hydrogen Strategy, http://www.h2eva.org/download.php?file=20181112_visualization_of_key_results.pdf, accessed November 2020 Search PubMed.
- P. Cedric, Perspectives on a hydrogen strategy for the EU, https://www.ifri.org/sites/default/files/atoms/files/philibert_hydrogen_strategy_2020.pdf, accessed December 2020 Search PubMed.
- B. Dooley, Japan's New Leader Sets Ambitious Goal of Carbon Neutrality by 2050, https://www.nytimes.com/2020/10/26/business/japan-carbon-neutral.html, accessed December 2020 Search PubMed.
- Government of Korea, Hydrogen economy road map of Korea, http://english.motie.go.kr/www/main.do, accessed November 2020 Search PubMed.
- Hydrogen Council, Clean hydrogen monitor 2020, https://hydrogeneurope.eu/sites/default/files/2020-10/CleanHydrogenMonitor2020_0.pdf, accessed November 2020 Search PubMed.
- Afhypac, Developing hydrogen for the French economy, https://www.afhypac.org/documents/publications/rapports/Afhypac_EtudeH2FceGB_def.pdf, accessed November 2020 Search PubMed.
- China Hydrogen Alliance, White paper on Chinese Hydrogen, http://www.h2cn.org/en/publication.html, accessed December 2020 Search PubMed.
- Singapore Energy Market Authority, Singapore's energy story, https://www.ema.gov.sg/ourenergystory Search PubMed.
- A. H. Min, Singapore sets solar energy target for 2030 that would provide enough power for 350,000 homes, https://www.channelnewsasia.com/news/singapore/solar-power-target-energy-350000-homes-2030-hdb-rooftops-12042228, accessed December 2020 Search PubMed.
- International Renewable Energy Agency, Navigating the way to a renewable future: Solutions to decarbonise shipping, Preliminary Findings, https://www.irena.org/publications/2019/Sep/Navigating-the-way-to-a-renewable-future, accessed February 2021 Search PubMed.
- L. Van Hoecke, L. Laffineur, R. Campe, P. Perreault, S. W. Verbruggen and S. Lenaerts, Energy Environ. Sci., 2021, 14, 815–843 RSC
.
- J. Tattini and J. Teter, International Shipping – IEA Tracking Report, https://www.iea.org/reports/international-shipping#resources, accessed February 2021 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |