Open Access Article
Open Access ArticleMolecular bixbyite-like In12-oxo clusters with tunable functionalization sites for lithography patterning applications†
Xiaofeng
Yi
a,
Di
Wang
ab,
Fan
Li
ab,
Jian
Zhang
 a and
Lei
Zhang
a and
Lei
Zhang
 *a
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, P. R. China. E-mail: LZhang@fjirsm.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 22nd September 2021
Abstract
Indium oxides have been widely applied in many technological areas, but their utilization in lithography has not been developed. Herein, we illustrated a family of unprecedented In12-oxo clusters with a general formula [In12(μ4-O)4(μ2-OH)2(OCH2CH2NHCH2CH2O)8(OR)4X4]X2 (where X = Cl or Br; R = CH3, C6H4NO2 or C6H4F), which not only present the largest size record in the family of indium-oxo clusters (InOCs), but also feature the first molecular model of bixbyite-type In2O3. Moreover, through the labile coordination sites of the robust diethanolamine-stabilized In12-oxo core, these InOCs can be accurately functionalized with different halides and alcohol or phenol derivatives, producing tunable solubility. Based on the high solution stability as confirmed by ESI-MS analysis, homogeneous films can be fabricated using these In12-oxo clusters by the spin-coating method, which can be further used for electron beam lithography (EBL) patterning studies. Accordingly, the above structural regulations have significantly influenced their corresponding film quality and patterning performance, with bromide or p-nitrophenol functionalized In12-oxo clusters displaying better performance of sub-50 nm lines. Thus, the here developed bixbyite-type In12-oxo cluster starts the research on indium-based patterning materials and provides a new platform for future lithography radiation mechanism studies.
Introduction
Indium oxides (In2O3), existing in two phases of bixbyite- and corundum-type,1 as n-type semiconductors possess unique electrical and optical properties with great prospects in extensive areas.2–7 For a better structure–property relationship understanding, it is essential to investigate In2O3 materials at the molecular level, for example indium-oxo clusters (InOCs) with a uniform row of structural fabrics and a clear chemical composition. Meanwhile, the exploration of InOCs also provides interesting opportunities for the development of new kinds of indium oxide materials with unprecedented functionalities. The frontier research realm of structurally well-defined InOCs was initiated by Wieghardt Karl and coworkers in 1986.8 In contrast to the remarkable progress in oxo clusters of transition metals9–11 and lanthanides,12 the investigations on InOC chemistry were confined to structural archetypes including star, square, square-pyramid, octahedra, and wheel geometry. And the largest size record in the family of InOCs has been limited to the In10-oxo matrix to date,13 with even much less in developing their applications.14 Therefore, the consecutive exploration of InOCs is highly appealing to provide opportunities to enrich structural diversity and expand potential applications.Nanoscale patterning enables ongoing miniaturization in dense integrated circuit technology to meet expectations predicted by Moore's law,15,16 and extreme ultraviolet (EUV) lithography as a promising next-generation lithography technology requires metal-containing patterning materials with large absorption cross-sectional characteristics for more efficient utilization of EUV photons.17–19 To date, a small number of metal complexes of Sn,20–23 Sb,24 Hf,25 Zr,26 Ti,27 Zn,28 Pt and Pd29 have set foot into this field. Interestingly, in terms of practical applications, the cage-like Sn-oxo cluster is exclusively available for realizing EUV lithography in industry, which indicate that metal-oxo clusters as a patterning material do have significant potential in nanolithography. It is absolutely imperative to facilitate the diversity and to study the radiation mechanism of such metal-containing patterning materials.
Considering the similar strong resonance towards EUV light of indium to the above heavy metals of Sn and Sb, In-based complexes are desperately expected to be promising patterning materials. In order to explore this possibility, InOCs can be an ideal research object for the following reasons: (1) InOCs possess an atomically precise structure favourable for radiation mechanism study; (2) InOCs belong to molecular clusters with excellent solubility, which is conducive to film fabrication; (3) InOCs exhibit uniform distribution in size and composition, which can produce a homogeneous response during radiation; (4) the polynuclear characteristics of InOCs provide more utilization of shorter wavelength photons than mononuclear indium compounds; (5) InOCs can be accurately chemically modified by functionalizing with ligands for further optimization in patterning performance. Thus, research on InOC based patterning materials can fill the blank space of indium in this technologically important field, which might lay a foundation for future patterning materials with improved sensitivity, resolution and line edge roughness.
Results and discussion
Following the above consideration, herein we realized the assembly of In3+ ions with methanol or phenol derivatives under the assistance of diethanol amine as the stabilizing ligand into a series of novel In12-oxo clusters [In12(μ4-O)4(μ2-OH)2(OCH2CH2NHCH2CH2O)8(OR)4X4]X2 (where X = Cl, R = CH3 for InOC-1; X = Br, R = CH3 for InOC-2; X = Cl, R = C6H4NO2 for InOC-3; X = Br, R = C6H4NO2 for InOC-4; X = Cl, R = C6H4F for InOC-5), whose patterning applications have been explored for the first time (Scheme 1). From the perspective of geometry, the obtained In12-oxo clusters present the largest size record in the realm of InOCs. The In–O binding modes of the In12 core are analogous to those of bixbyite-type In2O3, making them the first molecular models of bixbyite-type indium oxide. Meanwhile, there are 8 labile coordination sites in the robust In12-oxo cluster to allow chemical decoration by various halides and functional alcohol or phenol derivatives. Moreover, these In12-oxo clusters display good solubility and solution stability as confirmed by mass spectroscopy (MS), making them potential candidates for spin-coating nanofilm fabrication and further electron beam lithography (EBL) studies. Interestingly, the structural regulations on labile sites play important roles in their patterning applications, with p-nitrophenol or bromide decorated In12-oxo-clusters showing high-performance patterning behaviors for excellent sub-50 nm pitch lines.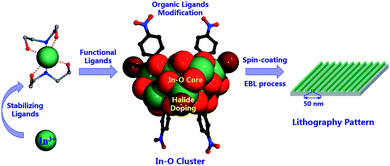 | ||
| Scheme 1 Illustration of the assembly of the atomically precise In12-oxo clusters with chemical modification and patterning evaluation. | ||
To synthesize crystalline indium-oxo clusters, it is crucial to decelerate the hydrolysis process of the In3+ ions for the improvement of crystallization. The recently developed and widely applied coordination delayed hydrolysis (CDH) strategy was hence employed for the growth of InOC crystals.11,30 Diethanolamine was selected as the chelate initiator to protect In3+ ions from violent hydrolysis and also stabilize the produced In–O cores. Accordingly, the self-assembly of InCl3 with diethanolamine in methanol at 100 °C for two days gave rise to block crystals of InOC-1.
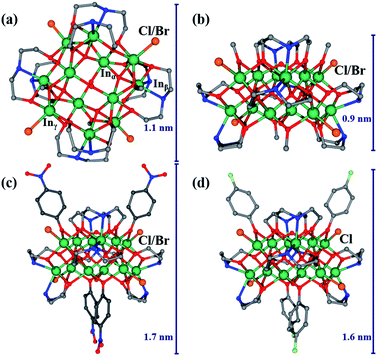
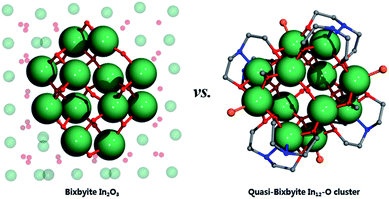
Single-crystal X-ray structural analysis revealed that InOC-1 represented a {In12(μ4-O)4(μ2-OH)2} core, which was further stabilized by eight diethanolamine NH(CH2CH2O)2 moieties accompanied by four chlorides and four functional organic segments OCH3 (Fig. 1a). To the best of our knowledge, this In12-oxo cluster has been the largest InOC reported to date. The 12 In3+ centers reside in the distorted octahedral coordination environment of Inα {InO6}, Inβ {InO5Cl} and Inγ {InO4N2}, and then fused together by four μ4-O and two μ2-OH to form the In12–O core (Fig. S1 and S2†). The two μ2-OH bridges in the In12-oxo skeleton are speculated by the bond valence sum calculation with a BVS value of ca. 1.0 (Table S6†) and recent studies showed that oxygen vacancies also play important roles in catalytic reactions.31 It is interesting to find that the planner-{In6} segments with rectangular geometry can serve as the basic building units, and there exist two such {In6} moieties parallel to each other and linked together via 4 μ4-O to form the In12-core (Fig. S2†). Furthermore, the In–O connectivity in this In12-oxo core is largely consistent with that in bixbyite-type In2O3, making InOC-1 the first ideal model of bixbyite-type In2O3 material at the molecular level (Fig. 2).
In the view of coordination chemistry, the four terminal chlorides in InOC-1 should be quite labile to be replaced by other halides. Moreover, the four functional OCH3 segments could also be modified with their alcohol or phenol derivatives. Therefore, there are 8 possible labile sites on the In12-core in total for further structural functionalization on InOC-1. Indeed, when InBr3 was applied instead of InCl3, bromide functionalized InOC-2 was obtained. It displays the same In12-core as InOC-1 except for the four terminating bromide ions (Fig. S4†). To verify the modification ability on OCH3 sites, methanol in the synthesis of InOC-1 was replaced by p-nitrophenol and tetrahydrofuran (THF) as the organic ligand and solvent, respectively, leading to the formation of InOC-3. As shown in Fig. 1c and S5,† four p-nitrophenol segments were successfully introduced into InOC-3 as the substitute of the four OCH3 in InOC-1. The versatility of these labile sites was further confirmed by the construction of InOC-4 and InOC-5 with –Br/–p-nitrophenol and –Cl/–p-fluorophenol as chemical modification on the In12-core, respectively (Fig. S6 and S7†). These cationic In12-oxo clusters in InOC-1 to InOC-5 display square configuration with similar side lengths of ca. 1.1 nm but different thicknesses (maximum extension of the organic groups OR) ranging from ca. 0.9 to 1.7 nm.
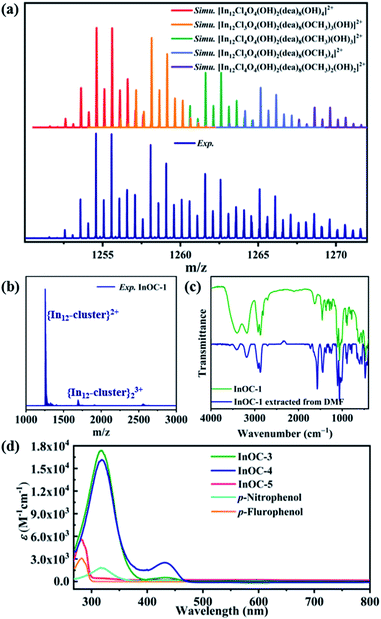
It is known that subtle fluctuations in the material structures or composition dominate their electronic structure and the Hansen solubility parameters (HSPs).19,32 Therefore, we intended to probe the In12-oxo core as a platform for the comparative exploration of their photophysical characteristics as well as HSP values related to solution behaviors. Verified by powder X-ray diffraction analysis (Fig. S13 to S17†), InOC-1 to InOC-5 in the solid state are in a highly pure phase allowing for further inspection. Solution experiments indicated that InOC-1 to InOC-5 could be readily dissolved in dimethyl formamide (DMF). Among them, InOC-1 exhibits relatively poor solubility, which could be improved by ultrasonic treatment and increasing the dissolution time. It is interesting that the implantation of bromide in place of chloride increases the solubility of InOC-2 in DMF. More importantly, the introduction of strong electron withdrawing ligands p-nitrophenol or p-fluorophenol instead of methanol into the In12-oxo backbone endows InOC-3 to InOC-5 with much higher solubility in DMF. Thus, –Br/–p-nitrophenol decorated InOC-4 presents the highest solubility among these In12-oxo clusters. Then electrospray ionization mass spectrometry (ESI-MS) analysis was applied to investigate the stability of the In12-oxo clusters after dissolution. As shown in Fig. 3, the positive ion mode ESI spectrum of InOC-1 in DMF exhibits a unique isotope envelope from m/z 1250 to 1275 of +2 charged species. In comparison of the experimental and simulated patterns, these +2 charged species could be attributed to {In12-cluster}2+ ions based on the intact [In12(μ4-O)4(μ2-OH)2(dea)8(OR)4Cl4]2+ (dea = OCH2CH2NHCH2CH2O) species missing halides or organic OCH3 groups (Table S11†). Furthermore, {In12-cluster} related species are capable of aggregation into a small number of +3 charged dimers in the m/z range between 1680 and 1710. In addition, the In12-oxo clusters dissolved in DMF can be extracted with the assistance of propylene glycol methyl ether acetate (PGMEA), whose FT-IR spectra were in good agreement with those of original InOC-1 to InOC-5 (Fig. 3c and S33 to S36†). Therefore, the MS measurements and FT-IR measurements evidenced the high solution stability of these In12-oxo clusters in the DMF medium. Moreover, the observed aggregation behaviors through their interaction with energetic electrons during the ionization process further support the prospects for their application as patterning materials.
Besides solubility, the functionalized ligands on In12-oxo cores also influence their photophysical properties, as confirmed by solution UV-Vis absorption spectroscopy studies (Fig. 3d). The absorption of colorless InOC-1 and InOC-2 in DMF was almost negligible in the range of 268 to 800 cm−1. Meanwhile the introduction of strong electron withdrawing ligands p-nitrophenol or p-fluorophenol endows the In12-oxo clusters with obvious absorption peaks. Among them, p-nitrophenol decorated InOC-3 or InOC-4 shows a greater absorption coefficient and more significant red-shift adsorption (at 316 nm, ε = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349 L cm−1 mol−1 for InOC-3; at 316 nm, ε = 16
349 L cm−1 mol−1 for InOC-3; at 316 nm, ε = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043 L cm−1 mol−1 for InOC-4) than p-fluorophenol decorated InOC-5 (at 282 nm, ε = 5570 L cm−1 mol−1). More interestingly, the absorption of yellowish InOC-3 and InOC-4 modified with p-nitrophenol can occur in the visible light region, which is consistent with its appearance color. Furthermore, the absorption maxima position in the spectra of InOC-3 to InOC-5 could be compared with the spectral characteristics of free p-nitrophenol or p-flurophenol, but they exhibit a much larger absorption coefficient than their corresponding free decorated ligands. Among them, the yellowish InOC-3 and InOC-4 decorated with four p-nitrophenol ligands display a much larger 4 times higher absorption coefficient than free p-nitrophenol.
043 L cm−1 mol−1 for InOC-4) than p-fluorophenol decorated InOC-5 (at 282 nm, ε = 5570 L cm−1 mol−1). More interestingly, the absorption of yellowish InOC-3 and InOC-4 modified with p-nitrophenol can occur in the visible light region, which is consistent with its appearance color. Furthermore, the absorption maxima position in the spectra of InOC-3 to InOC-5 could be compared with the spectral characteristics of free p-nitrophenol or p-flurophenol, but they exhibit a much larger absorption coefficient than their corresponding free decorated ligands. Among them, the yellowish InOC-3 and InOC-4 decorated with four p-nitrophenol ligands display a much larger 4 times higher absorption coefficient than free p-nitrophenol.
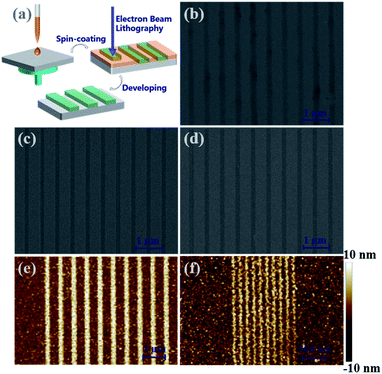
In combination with the above structural and solubility analysis, the obtained In12-oxo clusters can provide molecular candidates for smooth film formation, which might be used as In-containing patterning materials (Fig. 4a). Furthermore, the sophisticated functionalization on the In12-oxo platform with different halides and organic species with various electron-withdrawing groups may influence their film quality and patterning performance, providing unprecedented opportunities to understand the relationship between the chemical modification on In–O cores and their radiolysis attributes. To this end, the patterning performance differences among InOC-1 to InOC-3 have been evaluated by electron beam lithography (EBL), which can produce high-energy electrons during radiation interaction to induce chemical changes in materials for pattern formation. The quite poor solubility of inorganic bixbyite-In2O3 particles usually results in heterogeneous films, preventing further patterning evaluation. In contrast, it is very interesting that clear patterns have been fabricated under electron-beam exposure using these three In12-oxo clusters, which is firstly found for indium-based materials. As shown in Fig. 4 and S43,†InOC-1 with the decoration of chloride and methoxy demonstrated 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 line-to-space patterns with feature sizes from 1000 to 100 nm under a dose of 1000 μC cm−2, which are unfortunately not uniform and present low contrast. In contrary, the electron beam lithographic performance of InOC-2 and InOC-3 based patterning materials was significantly improved with homogeneous lines observed, and there were not any bridging problems or residues observed in the unexposed areas. This might be due to the improved solubility of InOC-2 and InOC-3 compared to InOC-1, giving rise to more homogeneous films with higher quality (Fig. S42†) and better patterns. More importantly, smaller critical dimensions of 50 nm were printed upon radiation with a dose of 1000 μC cm−2 for InOC-2 and InOC-3, as evidenced by scanning electron microscopy (SEM) images (Fig. S44e and S45e†). For InOC-3, atomic force microscopy (AFM) studies indicated that satisfactory 50 nm lines could even be fabricated under a lower radiation energy of 500 μC cm−2 (Fig. 4f). Therefore, these results support a speculated radiolysis process, where the halide and organic ligand sites of the In12-oxo clusters involved in changing the film dissolution behaviors after the EBL treatment. The introduction of bromide instead of chloride, especially p-nitrophenol instead of methoxy, can significantly increase the sensibility of In12-oxo cluster based films upon EBL radiation to produce better patterns. These findings suggest that bixbyite-like In12-oxo clusters not only present competitive advantages in nanolithography, but also may provide a platform for understanding structure–property (e.g. solubility, film quality and patterning ability) relationships in indium oxide materials at the molecular level.
2 line-to-space patterns with feature sizes from 1000 to 100 nm under a dose of 1000 μC cm−2, which are unfortunately not uniform and present low contrast. In contrary, the electron beam lithographic performance of InOC-2 and InOC-3 based patterning materials was significantly improved with homogeneous lines observed, and there were not any bridging problems or residues observed in the unexposed areas. This might be due to the improved solubility of InOC-2 and InOC-3 compared to InOC-1, giving rise to more homogeneous films with higher quality (Fig. S42†) and better patterns. More importantly, smaller critical dimensions of 50 nm were printed upon radiation with a dose of 1000 μC cm−2 for InOC-2 and InOC-3, as evidenced by scanning electron microscopy (SEM) images (Fig. S44e and S45e†). For InOC-3, atomic force microscopy (AFM) studies indicated that satisfactory 50 nm lines could even be fabricated under a lower radiation energy of 500 μC cm−2 (Fig. 4f). Therefore, these results support a speculated radiolysis process, where the halide and organic ligand sites of the In12-oxo clusters involved in changing the film dissolution behaviors after the EBL treatment. The introduction of bromide instead of chloride, especially p-nitrophenol instead of methoxy, can significantly increase the sensibility of In12-oxo cluster based films upon EBL radiation to produce better patterns. These findings suggest that bixbyite-like In12-oxo clusters not only present competitive advantages in nanolithography, but also may provide a platform for understanding structure–property (e.g. solubility, film quality and patterning ability) relationships in indium oxide materials at the molecular level.
Conclusions
In summary, we successfully isolated and characterized a series of atomically precise In12-oxo clusters from the self-assembly of InX3 (X = Cl or Br) with diethanolamine as the chelate initiator. In terms of the structure, these In12-oxo clusters not only record the largest size in the family of InOCs but also elucidate the first molecular model of bixbyite-type indium oxide. The robust diethanolamine-stabilized In12-oxo core presents 8 labile sites on the cluster surface, which allow chemical modification with various terminating halides and bridging methoxy or phenyl derivatives. In addition, such cluster functionalization further leads to variable solubility in DMF, and the solution stability of these In12-oxo clusters has been confirmed by ESI-MS studies. Moreover, their patterning performance was evaluated under the exposure of an electron beam, which is of particular interest because indium-based patterning materials for lithography have never been studied before. Among them, compared to chloride and methoxy functionalized InOC-1, InOC-2 and InOC-3 with bromide or p-nitrophenol moieties exhibited better patterning performance with a feature size of 50 nm lines. Therefore, this work not only provides an interesting In12-oxo model for bixbyite-type indium oxide, but also opens the patterning applications of indium-based materials whose performance can be improved through chemical structure modification.Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Author contributions
L. Z. designed the study and supervised the project; X. Y. implemented materials synthesis and characterization; X. Y and D. W. carried out electron-beam lithography studies; F. L. assisted materials characterization. All authors discussed the results and co-wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research reported in this publication was supported by the National Natural Science Foundation of China (21922111, 91961108, and 21901241), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB20000000), and the Natural Science Foundation of Fujian Province (2020J01117). We appreciate Lv-Bing Yuan for assisting in single-crystal XRD data collection, Ling-Ling Zheng for SEM measurements, Dan-Mei Pan for AFM measurements, and Prof. Li Xu for providing synthetic assistance.Notes and references
- M. Marezio, Acta Crystallogr., 1966, 20, 723–728 CrossRef CAS.
- T. Li, Z. Chen, Y. Wang, J. Tu, X. Deng, Q. Li and Z. Li, ACS Appl. Mater. Interfaces, 2020, 12, 3301–3326 CrossRef CAS PubMed.
- S. Li, M. Tian, Q. Gao, M. Wang, T. Li, Q. Hu, X. Li and Y. Wu, Nat. Mater., 2019, 18, 1091–1097 CrossRef CAS PubMed.
- L. Wang, Y. Dong, T. Yan, Z. Hu, A. A. Jelle, D. M. Meira, P. N. Duchesne, J. Y. Y. Loh, C. Qiu, E. E. Storey, Y. Xu, W. Sun, M. Ghoussoub, N. P. Kherani, A. S. Helmy and G. A. Ozin, Nat. Commun., 2020, 11, 2432 CrossRef CAS PubMed.
- Y. Qi, L. Song, S. Ouyang, X. Liang, S. Ning, Q. Zhang and J. Ye, Adv. Mater., 2019, 1903915, DOI:10.1002/adma.201903915.
- Y. X. Pan, Y. You, S. Xin, Y. Li, G. Fu, Z. Cui, Y. L. Men, F. F. Cao, S. H. Yu and J. B. Goodenough, J. Am. Chem. Soc., 2017, 139, 4123–4129 CrossRef CAS PubMed.
- H. Yan, K. He, I. A. Samek, D. Jing, M. G. Nanda, P. C. Stair and J. M. Notestein, Science, 2021, 371, 1257–1260 CrossRef CAS PubMed.
- K. Wieghardt, M. Kleine-Boymann, B. Nuber and J. Weiss, Inorg. Chem., 1986, 25, 1654–1659 CrossRef CAS.
- Advances in Inorganic Chemistry, Polyoxometalate Chemistry, ed. R. van Eldik and L. Cronin, Zoe Kruze, 1st edn, 2017 Search PubMed.
- P. Yang and U. Kortz, Acc. Chem. Res., 2018, 51, 1599–1608 CrossRef CAS PubMed.
- W. H. Fang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2018, 47, 404–421 RSC.
- X. Y. Zheng, Y. H. Jiang, G. L. Zhuang, D. P. Liu, H. G. Liao, X. J. Kong, L. S. Long and L. S. Zheng, J. Am. Chem. Soc., 2017, 139, 18178–18181 CrossRef CAS PubMed.
- N. N. Chamazi, M. M. Heravi and B. Neumüller, Z. Anorg. Allg. Chem., 2006, 632, 2043–2048 CrossRef CAS.
- Z. L. Mensinger, W. Wang, D. A. Keszler and D. W. Johnson, Chem. Soc. Rev., 2012, 41, 1019–1030 RSC.
- A. Robinson, Frontiers of Nanoscience Materials and Processes for Next Generation Lithography, Elsevier, 2016 Search PubMed.
- M. Tu, B. Xia, D. E. Kravchenko, M. L. Tietze, A. J. Cruz, I. Stassen, T. Hauffman, J. Teyssandier, S. De Feyter, Z. Wang, R. A. Fischer, B. Marmiroli, H. Amenitsch, A. Torvisco, M. J. Velasquez-Hernandez, P. Falcaro and R. Ameloot, Nat. Mater., 2021, 20, 93–99 CrossRef CAS PubMed.
- L. Li, X. Liu, S. Pal, S. Wang, C. K. Ober and E. P. Giannelis, Chem. Soc. Rev., 2017, 46, 4855–4866 RSC.
- N. Mojarad, M. Hojeij, L. Wang, J. Gobrecht and Y. Ekinci, Nanoscale, 2015, 7, 4031–4037 RSC.
- P. D. Ashby, D. L. Olynick, D. F. Ogletree and P. P. Naulleau, Adv. Mater., 2015, 27, 5813–5819 CrossRef CAS PubMed.
- B. Cardineau, R. Del Re, M. Marnell, H. Al-Mashat, M. Vockenhuber, Y. Ekinci, C. Sarma, D. A. Freedman and R. L. Brainard, Microelectron. Eng., 2014, 127, 44–50 CrossRef CAS.
- J. T. Diulus, R. T. Frederick, D. C. Hutchison, I. Lyubinetsky, R. Addou, M. Nyman and G. S. Herman, ACS Appl. Nano Mater., 2020, 3, 2266–2277 CrossRef CAS.
- S. Saha, D. H. Park, D. C. Hutchison, M. R. Olsen, L. N. Zakharov, D. Marsh, S. Goberna-Ferron, R. T. Frederick, J. T. Diulus, N. Kenane, G. S. Herman, D. W. Johnson, D. A. Keszler and M. Nyman, Angew. Chem., Int. Ed., 2017, 56, 10140–10144 CrossRef CAS PubMed.
- I. Bespalov, Y. Zhang, J. Haitjema, R. M. Tromp, S. J. van der Molen, A. M. Brouwer, J. Jobst and S. Castellanos, ACS Appl. Mater. Interfaces, 2020, 12, 9881–9889 CrossRef CAS PubMed.
- J. Passarelli, M. Murphy, R. D. Re, M. Sortland, J. Hotalen, L. Dousharm, R. Fallica, Y. Ekinci, M. Neisser, D. A. Freedman and R. L. Brainard, J. Micro/Nanolithogr., MEMS, MOEMS, 2015, 14 Search PubMed.
- J. Jiang, S. Chakrabarty, M. Yu and C. K. Ober, J. Photopolym. Sci. Technol., 2014, 27, 663–666 CrossRef.
- G. Kickelbick, P. Wiede and U. Schubert, Inorg. Chim. Acta, 1999, 284, 1–7 CrossRef CAS.
- L. Wu, M. Tiekink, A. Giuliani, L. Nahon and S. Castellanos, J. Mater. Chem. C, 2019, 7, 33–37 RSC.
- H. Xu, K. Sakai, K. Kasahara, V. Kosma, K. Yang, H. C. Herbol, J. Odent, P. Clancy, E. P. Giannelis and C. K. Ober, Chem. Mater., 2018, 30, 4124–4133 CrossRef CAS.
- O. R. Wood, E. M. Panning, M. Sortland, R. Del Re, J. Passarelli, J. Hotalen, M. Vockenhuber, Y. Ekinci, M. Neisser, D. Freedman and R. L. Brainard, Extreme Ultraviolet (EUV) Lithography VI, 2015, DOI:10.1117/12.2086598.
- D. Wang, Z.-N. Chen, Q.-R. Ding, C.-C. Feng, S.-T. Wang, W. Zhuang and L. Zhang, CCS Chemistry, 2020, 2607–2616, DOI:10.31635/ccschem.020.202000546.
- A. Tsoukalou, P. M. Abdala, D. Stoian, X. Huang, M. G. Willinger, A. Fedorov and C. R. Muller, J. Am. Chem. Soc., 2019, 141, 13497–13505 CrossRef CAS PubMed.
- M. Larsen, Proceedings of the 17th International Conference on Composite Materials ICCM-17, 2009 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; additional figures and EBL patterns. CCDC 2078614–2078618 for InOC-1 to InOC-5. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc04491e |
| This journal is © The Royal Society of Chemistry 2021 |