Open Access Article
Open Access ArticleMicrowave assisted synthesis of five membered nitrogen heterocycles
Gopinadh Meera
a,
K. R. Rohit
a,
Salim Saranya
a and
Gopinathan Anilkumar
 *abc
*abc
aSchool of Chemical Sciences, Mahatma Gandhi University, Priyadarsini Hills P O., Kottayam, Kerala 686 560, India. E-mail: anilgi1@yahoo.com; anil@mgu.ac.in; Fax: +91-481-273 1036
bAdvanced Molecular Materials Research Centre (AMMRC), Mahatma Gandhi University, Priyadarsini Hills P O., Kottayam, Kerala 686 560, India
cInstitute for Integrated Programmes and Research in Basic Sciences (IIRBS), Mahatma Gandhi University, Priyadarsini Hills P O., Kottayam, Kerala 686 560, India
First published on 30th September 2020
Abstract
Our continuously changing environment demands sensible and sustainable chemistry. Consequently, organic synthesis started to follow green chemistry principles in recent years. It is observed that microwave (MW) radiation has been widely used as a source of energy in organic synthesis in the past decade. The MW heating approach has evolved into a new green method in organic synthesis since it provides short reaction time, high yields, and high product purities along with a decrease in the rate of by-product formation. Solvent-free reaction protocols worked well under MW irradiation. All these features make MW assisted organic synthesis an environment-friendly approach. In organic synthesis, heterocycles are vital targets especially nitrogen-containing ones because of their prominent presence in natural products and widespread applications in pharmaceutical industries. Five membered nitrogen heterocycles include pyrroles, oxazoles, pyrrolidones, etc. among which pyrroles are the most important ones due to their potent biological properties. Even though there are a variety of reaction protocols for the synthesis of pyrroles, a significant development materialized in MW assisted synthesis of pyrroles in the past few years. In this review, we focus on the developments in MW assisted synthesis of pyrroles and other five-membered nitrogen heterocycles.
1 Introduction
Heterocycles are vital targets in organic synthesis especially nitrogen containing ones because of their notable presence in natural products and their wide variety of applications in pharmaceutical industries. Nitrogen containing five membered heterocycles include pyrroles, pyrrolidines, oxazoles, indoles, pyrazoles etc. Among which pyrrole was found to be the most important one.Pyrroles are nitrogen containing five membered heterocycles whose structural moiety appears in a variety of pharmaceuticals and a large number of biologically active natural products, and also acts as the key factor throughout the total synthesis of these molecules.1 The major part of porphyrin rings is pyrrole and its derivatives which act as building blocks in chlorophyll, heme, vitamin B12, and bile pigments.2 Pyrrole containing pharmaceutical compounds acts as fungicides, antibiotics, anti-inflammatory drugs,3 cholesterol reducing drugs, anti-tumor agents,4 anti-microbial5 and many more. Not only in pharmaceuticals but also in polymer chemistry, pyrroles are used as an efficient catalyst for polymerization process, used as corrosion inhibitor,6 preservative, solvent for resin, terpenes, in metallurgical process.7 In recent years pyrroles and its derivatives are successfully implemented as organic conducting materials also.8
There are a variety of protocols available for the synthesis of pyrroles. Paal–Knorr synthesis, Knorr synthesis, Hantzsch pyrrole synthesis are some of them. The major disadvantages regarding these protocols are they are carried out in presence of acid catalyst in organic solvent medium and the protocols are time consuming ones. Since the constantly changing environment demands a sensible and sustainable chemistry organic synthesis also started to follow green principles. As part of that microwave irradiation introduced in place of conventional heating technique.
Microwave irradiation, one of the most effective nonconventional activation methods, has been illuminating organic synthesis over the last 30 years.9 Substantial deceleration of reaction time (hours to minutes) and greater yield rendered this alternative heating source an attractive chemical synthesis method. This thermal control provides fine tuning of the parameters of the reaction and less chemical waste. Advantages such as elimination of side reactions and cost-effective response have also increased the popularity of microwave-assisted reactions. The first microwave-irradiated synthetic reaction was reported in 1969, but experiments performed by Gedye and Giguere made it more popular.10 The interaction of microwave energy with organic molecule is due to dielectric heating and the efficient interaction of polar molecules such as ethanol, water, acetonitrile, etc. with microwaves leads to rapid heat generation. The convection mode of microwave heating increases the reaction rate whereas the external heating source of traditional heating slows down the transfer of energy and hence the reaction rate.11 Usual domestic ovens were used in the early stages of microwave irradiated chemical synthesis while nowadays we have sophisticated monomods and multimodes.
The major advantages of implementing microwave heating over conventional heating technique are shorter reaction time, higher yield under milder reaction conditions, neat reactions worked well under microwave irradiation, higher purity of the products formed, and suppression in the rate of by-product formation. All these features make microwave assisted reactions more towards a greener and environment-friendly process.
In the past few years microwave irradiation is found to be applied in the synthesis of nitrogen containing five membered heterocycles in a large scale. And it is observed that most of these protocols were carried out either in water or under solvent-free conditions. Here, in this review we summarize the developments in microwave assisted synthesis of pyrroles, pyrrolidine, indoles, quinoline and its derivatives in the past five years.
2. Microwave assisted synthesis of pyrrole and derivatives
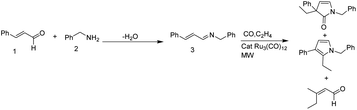
Feller and Imhof developed a microwave mediated Ru catalyzed four component (primary amine 2 and α,β-unsaturated aldehyde 1 with ethylene and carbon monoxide) reaction pathway for the synthesis of substituted pyrroles and chiral γ-lactams (Scheme 1).12 As compared to the conventional heating method, this protocol took lesser reaction time and the precatalyst (Ru3(CO)12) loading was also found to be lower.A new methodology for microwave assisted multicomponent reaction for the synthesis of substituted pyrrole derivatives 6 from sodium diethyl oxaloacetate 5, aromatic aldehydes 4 and primary amines 2 was reported by Komiotis and coworkers (Scheme 2).13 They studied the scope of this reaction in a variety of substrate molecules under optimized reaction conditions (sodium diethyl oxaloacetate (1 equiv.), amine (1 equiv.) and aldehyde (1 equiv.) in ethanol under MW, 100 W) and obtained average to good yields. From the pharmacological studies conducted, they found that some of the substituted pyrroles show excellent cytostatic and antiviral activities.
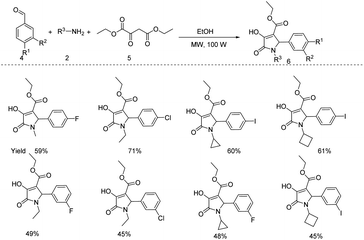 | ||
| Scheme 2 Three-component reaction between sodium diethyl oxaloacetate, amines and aromatic aldehydes under MW irradiation for the synthesis of pyrrole. | ||
Vyankatesh and coworkers designed a new microwave assisted protocol for the synthesis of 2-amino-4,5-diphenylpyrrole-3-carbonitriles 10 from a heterocyclic compound with substituted anilines 8 (Scheme 3).14 Under optimized reaction conditions (benzoin 7 (2.12 g, 0.01 mol), 8 and conc. HCl (6–8 drops), ethanol (40 mL), malononitrile (1.66 mg, 0.01 mol), pyridine (1.5 mL), MW, 240 W, 25 min), they explored the applications and limitations of this protocol and obtained good yields. From the pharmacological studies they found that some of the pyrrole derivatives show excellent in vitro anti-inflammatory activity.
 | ||
| Scheme 3 Microwave assisted protocol for the synthesis of 2-amino-4,5-diphenylpyrrole-3-carbonitriles. | ||
There are few reports on synthesis of pyrrole using enaminones (in situ generated) with phenacyl bromide 12, recently in 2018 Chawla et al. designed a new methodology for the microwave assisted synthesis of imidazole substituted pyrroles through a one pot synthesis (Scheme 4).15 Under the optimized reaction conditions they studied a number of substrates and obtained good to excellent yields.
In 2015 Zhang and coworkers developed an efficient microwave assisted three component (2, α-bromoacetophenone 15 and ethyl acetoacetate 16) one-pot synthesis of N-substituted 2-methyl-1H-pyrrole-3-carboxylate derivatives 17 (Scheme 5).16 Here substituted phenacyl bromides are used for studying the scope of the reaction. This protocol is both catalyst- and solvent-free and the substrate scope studies are carried out under the optimized reaction conditions (15 (1.0 mmol), 16 (2.5 mmol) and 2 (1.0 mmol), MW, 450 W, Neat) in various amines, different α-bromoacetophenone and ethylacetoacetate and obtained good yields.
 | ||
| Scheme 5 Microwave assisted one-pot three component (amine, α-bromoacetophenone and ethyl acetoacetate) synthesis of N-substituted 2-methyl-1H-pyrrole-3-carboxylate derivatives. | ||
In that same year similar kind of a protocol was reported by Reddy and coworkers. They proposed a new protocol for microwave assisted synthesis of trisubstituted pyrroles 20 from substituted β-amino unsaturated ketone 19 and substituted phenacyl bromide 18 (Scheme 6).17 The applications and limitations of this protocol was studied in a variety of substrate molecules under the optimized reaction conditions (19 (1 mmol) and boron trifluoride diethyl etherate (10 mol%), DCM (5 mL), 18 (1 mmol), MW, 10–16 min, 130 °C, 250 W) and obtained good to excellent yields.
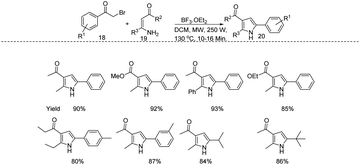 | ||
| Scheme 6 Microwave assisted synthesis of trisubstituted pyrroles from substituted β-amino unsaturated ketone and substituted phenacyl bromide. | ||
A new microwave assisted protocol for the synthesis of 6-(pyrrolyl)coumarin/quinolone derivatives 24 through an indium(III) catalysed one pot three component reaction (6-amino coumarin/quinolones 21, dialkyl acetylene dicarboxylates 23, and β-nitrostyrenes 22) (Scheme 7) was reported by Ansary et al.18 They applied this protocol under optimized reaction condition (21 (100 mg, 1.0 equiv.), 22 (1.2 equiv.), 23 (1.2 equiv.), EtOH (2 mL) and InCl3 (30 mol%), MW 100 W, 1 h) in a number of different substrates and obtained good yields.
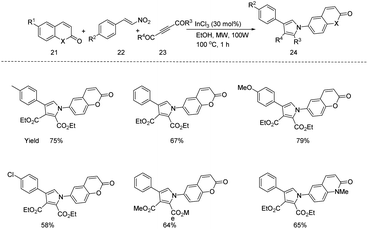 | ||
| Scheme 7 Microwave assisted protocol for the synthesis of 6-(pyrrolyl) coumarin/quinolone derivatives through an indium(III) catalysed one pot three component reaction. | ||
The proposed mechanism evolves nucleophilic addition 21 to the 23 leading to the formation of the enamine followed by a indium(III) catalysed Michael type reaction between enamine and β-nitrostyrene, which leads to the formation of an intermediate (Scheme 8). This would be followed by an intermolecular cyclization and aromatization generating the desired 6-(pyrrolyl) coumarin/quinolone derivatives.
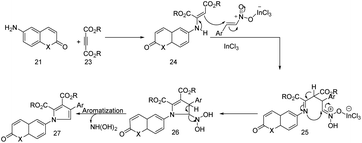 | ||
| Scheme 8 Proposed mechanism for the formation of 6-(pyrrolyl) coumarin/quinolones. This figure has been adapted from ref. 18 with permission from John Wiley and Sons, copyright 2020. | ||
Younis et al. designed an efficient synthesis of pyrrole derivatives via solvent-free reaction of chalcones 28 with different aldehydes 29 and ammonium acetate in the presence of sodium cyanide in a one-pot under microwave irradiation (Scheme 9).19 In comparison to the conventional methods, the major advantages of this protocol are high yield, short reaction time and easy purification of product by non-chromatographic methods. Some of the synthesized compounds showed significant antitumor activities. They have studied the possibility and limitations of this protocol in a variety of molecules under optimized conditions and obtained excellent yields.
 | ||
| Scheme 9 Microwave assisted synthesis of pyrroles from chalcones with different aldehydes and ammonium acetate in the presence of sodium cyanide. | ||
Perumal and coworkers proposed a new microwave assisted one pot stereoselective synthesis of dihydro-2′H-spiro[indene-2,1′-pyrrolo[3,4-c]pyrrole]-tetraone derivatives 35 (Scheme 10).20 They explored the scope of this three component 1,3-dipolar cycloaddition reaction under the optimized reaction conditions (3-(4-1-(aryl/alkyl)-1H-pyrrole-2,5-dione) 33 (1 mmol), ninhydrin 32 (1 mmol), sarcosine 34 in methanol at 100 °C, 120 W and 1 bar pressure) employing a series of maleimides differing in the aryl part and obtained good yields. From the pharmacological studies, they found that some of these compounds show AChE inhibition and antimycobacterial activities.
 | ||
| Scheme 10 Microwave assisted one pot stereoselective synthesis of dihydro-2′H-spiro[indene-2,1′-pyrrolo[3,4-c]pyrrole]-tetraone derivatives. | ||
The proposed mechanism goes through the generation of azomethine ylides in situ from 32 and 34, followed by 1,3-dipolar cycloaddition with a number of 33 (Scheme 11).
 | ||
| Scheme 11 Proposed mechanism for the formation of dihydro-2′H-spiro[indene-2,1′-pyrrolo[3,4-c]pyrrole]-tetraone derivatives. This figure has been reproduced from ref. 20 with permission from Elsevier, copyright 2020 | ||
Dürüst and coworkers designed a new protocol for the microwave assisted synthesis of benzothiophene-fused pyrrole derivatives 38 through cycloaddition and metal-free Pummerer-type sulfone deoxygenation pathway (Scheme 12).21 The applications and limitations of this protocol was explored under the optimized reaction condition (N-acylated amino acid derivatives 36 (0.500 mmol), benzo[b]thiophene 1,1-dioxide 37 (0.167 mmol), Ac2O (0.2 mL), toluene (3 mL), MW, 125 °C, 1.5 h) and obtained moderate yields in a variety of substrate molecules.
Zanatta and coworkers developed a new microwave assisted regioselective synthesis of polyfunctionalized 3-ferrocenyl-1H pyrroles 39 from pentane-2,4-dione or non-symmetrical 1,3-dicarbonyl compounds 38 and ferrocenyl vinyl azide 37 (Scheme 13).22 The scope of this reaction was explored under the optimized conditions (37 (0.5 equiv.) and 38 (0.55 equiv.), DCE (5 mL), MW, 100 °C, 30 min) and obtained moderate yields (32–75%) of the products. When compared to the acidic, basic or metal-catalyzed methodologies for polysubstituted pyrrole synthesis, the present synthetic route is mild, quick, simple, and it furnishes new functionalized tetrasubstituted-pyrroles in short reaction time (30 min).
 | ||
| Scheme 13 Microwave assisted regioselective synthesis of polyfunctionalized 3-ferrocenyl-1H pyrroles from pentane-2,4-dione or non-symmetrical 1,3-dicarbonyl compounds and ferrocenyl vinyl azide. | ||
Dumitrescu et al. developed a new simple and clean strategy for the synthesis of the derivatives of pyrrolo[1,2-c]quinazolines which follows a three component, one pot synthesis protocol under microwave irradiation(Scheme 14).23 The three components are 15, quinazolines 40 and electron deficient nonsymmetrically substituted alkynes 41. The synthesis was carried out through a 1,3-dipolar cycloaddition of quinazolinium N-ylides. The applicability of the reaction was studied under optimized conditions (15 (2 mmol), 40 (2 mmol) 41 (2.2 mmol), and 1,2-epoxybutane (18 mL) under MW irradiation) in a number of substrates and obtained moderate to good yields of the product.
3. Microwave assisted synthesis of pyrrolidines
Narayanarao and coworkers designed a new protocol for the microwave mediated synthesis of regioselective diisospiropyrrolidine analogs (Scheme 15).24 The protocol was carried out through an efficient one-pot four-component tandem reaction in the presence of four common reactants (substituted benzaldehyde 44, N,N-dimethylbarbituric acid 43, ninhydrin 32, sarcosine 34) and magnesium silicate nanoparticles (NPs) in ethanol under microwave irradiation. Some of the diisospiropyrrolidines synthesized show antiproliferative and antibacterial activity. A wide range of diisospiropyrrolidine analogs were synthesized under the optimized condition (32 (1 mmol), 34 (1.2 mmol), 43 (1.2 mmol), MgSiO3 NPs (10 mol%), ethanol (10 mL) treated under MW irradiation at 120 °C for 60–90 min)Nayak et al. worked on synthesising different spiro pyrrole derivatives through cycloaddition of various dipolarophiles with azomethine ylides generated from methyl glycine or related amino acids.
In 2019 they designed a new autocatalytic, simple, clean, one pot, three component (indenoquinoxalone 47, L-proline 49/L-phenyl alanine 50 and 3-nitro-2H-chromene 48) microwave assisted reaction protocol for the synthesis of derivatives of spiroindenoquinoxaline pyrrolidine fused nitrochromenes (Scheme 16).25 Compared to the conventional heating protocol, the microwave assisted pathway is much better affording high yields of the products, mild reaction condition, high regioselectivity, and operational simplicity to assemble complex structural entity in a single operation with good to excellent yield. The reaction mechanism involves a 1,3-dipolar cycloaddition of azomethine ylides generated in situ by the condensation of α-amino acids (49 and 50) and 47 with 48 as dipolarophile. The scope of this protocol was investigated in a variety of substrates under optimized conditions and compared with the conventional heating method which revealed that the microwave assisted reactions offered excellent yields.
 | ||
| Scheme 16 Microwave assisted reaction protocol for the synthesis of derivatives of spiroindenoquinoxaline pyrrolidine fused nitrochromenes. | ||
Before that in the same year they proposed a microwave assisted reaction protocol for the synthesis of spiro indanone pyrrolidine/piperidine fused nitrochromene derivatives 54 (Scheme 17).26 It is a one pot, three component (2-phenyl-3-nitrochromenes 53, indane-1,3-dione 32, and proline/pipecolic acid 49) reaction and from the substrate scope studies under optimized condition (49 (1 mmol), 53 (1 mmol), 32 (1 mmol) in EtOH (2 mL), MW irradiation (100 W power) at 80 °C for 5–10 min) it is clear that microwave assisted reaction is more regiospecific and diastereospecific, affording high yield in less reaction time as compared to the conventional heating method.
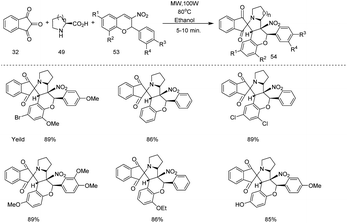 | ||
| Scheme 17 Microwave assisted synthesis of spiro indanone pyrrolidine/piperidine fused nitrochromene derivatives. | ||
The proposed mechanism goes through a 1,3-dipolar cycloaddition in which 2-phenyl-nitrochromene dipolarophiles react with azomethine ylides 55, generated in situ by the condensation of dicarbonyl compound 32 and secondary amino acid 49 (Scheme 18).
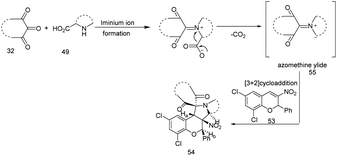 | ||
| Scheme 18 Proposed plausible mechanism. This figure has been adapted/reproduced from ref. 26 with permission from John Wiley and Sons, copyright 2020. | ||
Hakimi et al. developed a new microwave assisted solvent-free methodology for the synthesis of different antibacterial fluoroquinolone compounds 57 from 7-halo-6-fluoroquinolone-3-carboxylic acids 56 with a variety of amines 58 through direct amination (Scheme 19).27 From the substrate scope studies carried out under the optimized reaction conditions (56 (1 g, 3.5 mmol), 58 (0.6 g, 5.25 mmol), MW, 25 min, 150 °C, Neat) the products were formed in excellent yields.
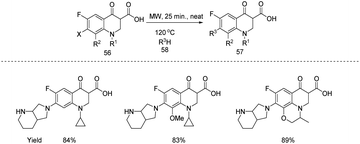 | ||
| Scheme 19 Microwave assisted solvent-free methodology for the synthesis of different antibacterial fluoroquinolone compounds from 7-halo-6-fluoroquinolone-3-carboxylic acids with a variety of amines. | ||
Trivedi et al. designed a four component (ninhydrin 32, phenylenediamine 59, proline 49, and nitrostyrene derivatives 22) reaction for the synthesis of spiroindeno[1,2-b]quinoxaline-11,3′-pyrrolizines 60 through a microwave assisted protocol and studied their AChE inhibitory activity (Scheme 20).28 Under the optimized reaction condition (32 (0.2 mmol), 59 (0.2 mmol), 49 (0.2 mmol) and 22 (0.2 mmol) in EtOH (2 mL), MW irradiation, 80 °C, 150 W) the scope of the one pot four-component 1,3-dipolar cycloaddition was explored which afforded the products in excellent yields as compared to the conventional method. Pharmacological studies revealed that some of the compounds synthesized could be used as potential AChE inhibitors.
 | ||
| Scheme 20 Four component reaction for the synthesis of spiroindeno[1,2-b]quinoxaline-11,3′-pyrrolizines. | ||
Rajput et al. designed a new solvent-free protocol for the microwave assisted synthesis of a number of malononitrile derivatives of substituted N-phenylpyrrolidine-2,5-diones 63 from substituted N-phenylpyrrolidine-2,5-dione 61 and dicyanomethane 64 in presence of succinic anhydride, benzene and acetyl chloride (Scheme 21).29 They studied the scope of the reaction under the optimized reaction conditions (malononitrile derivatives (5 mmol), N-phenyl succinimides 62 (10 mmol), 64 (2 g) in neutral Al2O3 (2 g), MW, 640 W, 4–7 min, Neat) and obtained average yields. Pharmacological studies showed that most of the synthesised molecules exhibit antimicrobial activities.
 | ||
| Scheme 21 Microwave assisted synthesis of a number of malononitrile derivatives of substituted N-phenylpyrrolidine-2,5-diones. | ||
Joseph and coworkers developed a microwave assisted protocol for the synthesis of a series of 2,5-disubstituted pyrrolidines 68 through an efficient telescoped cross-metathesis/cyclizing aza-Michael addition involving N-heteroaromatic olefinic derivatives (Scheme 22).30 This strategy was successfully applied for the preparation of original nicotine–lobeline, nicotine–pelletierine and lobeline–nicotine–epibatidine hybrids under optimized reaction conditions and obtained 50–70% yields.
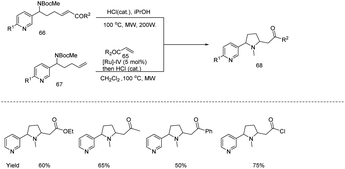 | ||
| Scheme 22 Microwave assisted protocol for the synthesis of a series of 2,5-disubstituted pyrrolidines. | ||
4. Microwave assisted synthesis of indoles
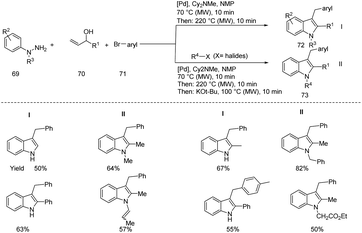
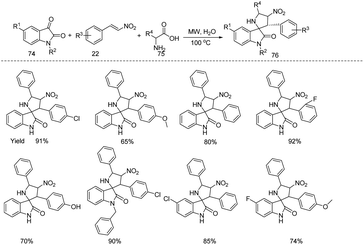
Müller and coworkers designed a microwave assisted three-component Heck isomerization–Fischer indolization (HIFI) and the four-component Heck isomerization–Fischer indolization–alkylation (HIFIA) for the synthesis of 3-arylmethylindoles 73 and 1-alkyl-3-benzylindoles 72 respectively from (hetero)aryl bromides 71, allyl alcohols 70, hydrazines 69 and alkyl bromides (Scheme 23).31 They studied the applications and limitations of this protocol under the optimized reaction conditions (Three-Component Heck Isomerization–Fischer Indolization (HIFI) for the synthesis of 3-arylmethylindoles: 71 (1 mmol), 70 (1.1 equiv.), Pd2(dba)3 (0.5 mol%) CataCXium Ptb (2 mol%), Cy2NMe (1.1 equiv.), NMP, 70 °C, 10 min then hydrazines (1 equiv.), 220 °C MW, 10 min. Four-Component Heck Isomerization–Fischer Indolization Alkylation (HIFIA) for the synthesis of 1-alkyl-3-benzylindoles: bromobenzene (1 mmol), Pd2(dba)3 (0.5 mol%), CataCXium Ptb (2.0 mol%), allyl alcohol (1.1 equiv.), Cy2NMe (1.1 equiv.), NMP (0.5 mL), 70 °C, MW, 10 min then PhNHNH3Cl (1 equiv.), 220 °C, MW, 10 min followed by addition of t-BuOK (5 equiv.), RX 7 (3 equiv.), then heat at 100 °C, MW, 10 min) and obtained moderate to good yields.Meshram et al. developed an ecofriendly one-pot three-component (isatin 74, β-nitrostyrene 22 & benzyl amine/α-amino acids 75) microwave assisted, aqueous phase, catalyst-free protocol for the synthesis of functionalized spirooxindoles 76 (Scheme 24).32 The protocol allows easy construction of a variety of spirooxindoles in moderate to good yields under the optimised reaction conditions (74 (1 mmol), 75 (1.2 mmol), 22 (1 mmol), water (2 mL), MW, 100 °C, 10 min) with good diastereoselectivity starting from readily available precursors. The newly synthesised molecules showed antimicrobial activity against Escherichia coli, Candida tropicalis, Staphylococcus aureus and Pseudomonas aeruginosa.
A simple nature friendly, cleaner, faster, and better yielding, multicomponent reaction protocol for the synthesis of a series of 3-(3′-pyrrolyl)-2-oxindoles containing phenothiazine, pyrrole or triazole like heterocyclic moieties under both conventional and microwave heating conditions has been reported.33 Comparison of both conventional and microwave heating methodologies revealed that the microwave assisted protocol gave the highest yields.
Sondhi et al. designed a simple and eco-friendly reaction protocol for the synthesis of azomethine and amidine derivatives of isoindole and pyrrolopyrazine from different dicarboxylic acids 77 (cis cyclohexane 1,2-dicarboxylic acid, phthalic acid and pyrazine 2,3-dicarboxylic acid) and hydrazine hydrate (Scheme 25).34 First of all 2-aminohexahydro-1H-isoindole-1,3(2H)-dione, 2-amino-1H-isoindole-1,3(2H)-dione and 6-amino-5H-pyrrolo[3,4-b]pyrazine-5,7(6H)-dione were generated from dicarboxylic acids and hydrazine hydrate through grinding followed by microwave irradiation with aldehydes and different pyridines to generate the corresponding azomethine and amidine derivatives of isoindole and pyrrolopyrazine with excellent yields. Pharmacological studies revealed that the newly synthesised compounds possess both good anti-inflammatory and anti-cancer activity.
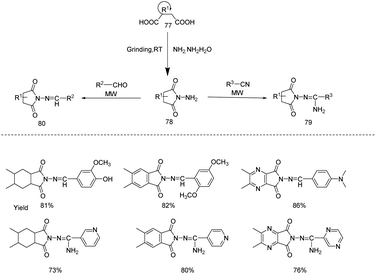 | ||
| Scheme 25 Microwave assisted synthesis of azomethine and amidine derivatives of isoindole and pyrrolopyrazine from different dicarboxylic acids. | ||
5. Microwave assisted synthesis of fused pyrazoles and isoxazoles
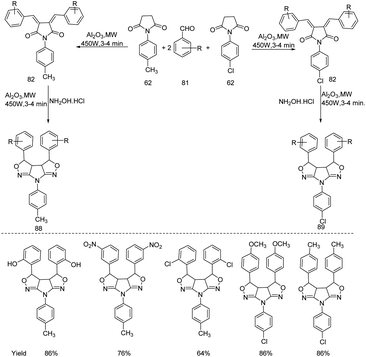
Patel and Rajput successfully conducted synthesis and microbial evaluation of hexahydro-2H-pyrrolo[2,3-c:5,4-c′]dipyrazole derivatives 83 from chalcone through a microwave mediated protocol (Scheme 26).35 The methodology is a one pot solvent-free synthetic pathway in which 1-p-tolylpyrolodine-2,5-dione and 1-p-chloropyrolodine-2,5-dione 62 react with substituted benzaldehydes 81 upon microwave irradiation in presence of neutral alumina affording both (3Z,4Z)-3,4-bis(benzylidene)-1-(4-chlorophenyl)pyrrolidine-2,5-dione and (3Z,4Z)-3,4-bis(benzylidene)-1-(p-tolylphenyl)pyrrolidine-2,5-dione. Further treatment with hydrazine hydrate in presence of neutral alumina upon irradiation under microwave results in the formation of dipyrazole derivatives. From the microbial evaluation it is clear that 7-(4-chlorophenyl)-3,3a,3b,4,5,7-hexahydro-2H-pyrrolo [2,3-c:5,4-c′] dipyrazole-3,4-diyl-diphenol exhibited bacterial activity against P. aeruginosa.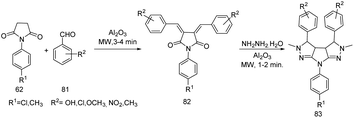 | ||
| Scheme 26 Synthesis of hexahydro-2H-pyrrolo[2,3-c:5,4-c′]dipyrazoles from chalcone through a microwave mediated protocol. | ||
Rajput et al. developed a new series of bis-isoxazoles 87 by the reaction of N-5-methyl pyridine succinimide 84 and substituted benzaldehyde under microwave irradiation in presence of neutral alumina under neat conditions (Scheme 27).36 First, chalcones were synthesized as an intermediate that undergo ring closure with hydroxyl amine hydrochloride under microwave irradiation in presence of neutral alumina resulting in the formation of bis-isoxazole derivatives. The substrate scope studies carried out under optimized conditions (bis-chalcone (0.1 mol) and hydroxyl amine hydrochloride (0.2 mol) in neutral Al2O3 (1 g) MW, 600 W, 3–5 min) on a wide variety of substrates showed that the products were formed in good to excellent yields.
 | ||
| Scheme 27 Microwave assisted synthesis of bis-isoxazoles by the reaction of N-5-methyl pyridine succinimide and substituted benzaldehyde. | ||
Patel and Rajput successfully synthesized 7H-pyrrolo[2,3-c:5,4-c′]diisoxazole derivatives using a microwave oven in minimum time compared to the conventional methods through a solvent-free methodology (Scheme 28).37 From the microbial studies carried out on the substrates obtained under the optimized conditions (for synthesis of chalcone: 62 (10 mmol), neutral alumina (2–2.5 grams) 81 (20 mmol), MW 450 W, 3–4 min), for the synthesis of 7H-pyrrolo[2,3-c:5,4-c′]diisoxazole derivatives: bis-chalcone derivatives (10 mmol), neutral alumina (2–2.5 grams), hydroxylamine hydrochloride (2 mol), MW, 450 W, 4–6 min, it was found that some of the synthesized compounds showed antibacterial and antifungal activity.
6 Conclusions
The short review based on the recent developments in the area of microwave assisted synthesis of nitrogen containing five membered heterocycles summarizes the synthesis of pyrroles, pyrrolidines, fused pyrazoles, fused isoxazoles and indoles. Some of these syntheses occurred in aqueous medium while some others were carried out under solvent-free conditions. As compared to other synthetic protocols, microwave assisted ones were found to be cleaner with short reaction time. The high purity and higher yield of products under milder reaction conditions make these protocols environmentally friendly.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SS and KRR thank the Council of Scientific and Industrial Research (CSIR), New Delhi-India and the University Grants Commission (UGC), New Delhi-India for the CSIR and UGC research fellowships, respectively. GA thanks the Kerala State Council for Science, Technology and Environment (KSCSTE-Trivandrum), for financial support in the form of research grant (Order No. 341/2013/KSCSTE dated 15.03.2013). The authors are also thankful to EVONIK Industries, Germany for financial support (ECRP 2016 dated 6.10.2016).References
- B. Zhang and A. Studer, Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors, Chem. Soc. Rev., 2015, 44, 3505–3521 RSC.
- V. Bhardwaj, D. Gumber, V. Abbot, S. Dhiman and P. Sharma, Pyrrole: A resourceful small molecule in key medicinal hetero-aromatics, RSC Adv., 2015, 5, 15233–15266 RSC.
- L. Yurttaş, Y. Özkay, Z. A. Kaplancikli, Y. Tunali and H. Karaca, Synthesis and antimicrobial activity of some new hydrazone-bridged thiazole-pyrrole derivatives, J. Enzyme Inhib. Med. Chem., 2013, 28, 830–835 CrossRef.
- G. La Regina, A. Coluccia, R. Bai, V. Famiglini, S. Pelliccia, S. Passacantilli, C. Mazzoccoli, V. Ruggieri, L. Sisinni, A. Bolognesi, W. Maria Rensen, A. Miele, M. Nalli, R. Alfonsi, L. Di Marcotullio, A. Gulino, A. Brancale, E. Novellino, G. Dondio, S. Vultaggio, M. Varasi, C. Mercurio, E. Hamel, P. Lavia and R. Silvestri, New pyrrole derivatives with potent tubulin polymerization inhibiting activity as anticancer agents including hedgehog-dependent cancer, J. Med. Chem., 2014, 57, 6531–6552 CrossRef CAS.
- C. Battilocchio, G. Poce, S. Alfonso, G. Cesare Porretta, S. Consalvi, L. Sautebin, S. Pace, A. Rossi, C. Ghelardini, L. Di Cesare Mannelli, S. Schenone, A. Giordani, L. Di Francesco, P. Patrignani and M. Bava, A class of pyrrole derivatives endowed with analgesic/anti-inflammatory activity, Bioorg. Med. Chem., 2013, 21, 3695–3701 CrossRef CAS.
- V. J. Gelling, M. M. Wiest, D. E. Tallman, G. P. Bierwagen and G. G. Wallace, Electroactive-conducting polymers for corrosion control: 4. Studies of poly(3-octyl pyrrole) and poly(3-octadecyl pyrrole) on aluminum 2024-T3 alloy, Prog. Org. Coat., 2001, 43, 149–157 CrossRef CAS.
- R. D. Rieth, N. P. Mankad, E. Calimano and J. P. Sadighi, Palladium-catalyzed cross-coupling of pyrrole anions with aryl chlorides, bromides, and iodides, Org. Lett., 2004, 6, 3981–3983 CrossRef CAS.
- A. Facchetti, A. Abbotto, L. Beverina, M. E. van der Boom, P. Dutta, G. Evmenenko, G. A. Pagani and T. J. Marks, Layer-by-layer self-assembled pyrrole-based donor-acceptor chromophores as electro-optic materials, Chem. Mater., 2003, 15, 1064–1072 CrossRef CAS.
- S. Manta, T. Niki, K. Nikolaos, A. Pelagia, M. Marili, P. Aggeliki, P. Loannis, M. Christos, T. Andrew, S. Dominique and K. Dimitri, Polyfunctionalized pyrrole derivatives: Easy three-component microwave-assisted synthesis, cytostatic and antiviral evaluation, Curr. Microw. Chem., 2018, 05, 23–31 CrossRef CAS.
- M. C. Bagley and M. C. Lubinu, Microwave-Assisted Multicomponent Reactions for the Synthesis of Heterocycles, 2006, pp. 31–58, DOI:10.1007/7081_004.
- M. Abid, B. Trk and X. Huang, Microwave-assisted tandem processes for the synthesis of N-heterocycles, Aust. J. Chem., 2009, 62, 208–222 CrossRef CAS.
- N. Feller and W. Imhof, Microwave-assisted ruthenium catalysed high-pressure synthesis of N-heterocyclic compounds, Monatsh. Chem., 2019, 150, 1289–1296 CrossRef CAS.
- S. Manta, et al., Polyfunctionalized pyrrole derivatives: Easy three-component microwave-assisted synthesis, cytostatic and antiviral evaluation, Curr. Microw. Chem., 2018, 05, 23–31 CrossRef CAS.
- D. Vyankatesh, T. Swapnali, A. Rahul, M. Shrinivas and M. Chandrakant, Microwave Assisted Synthesis of 2 Amino-4,5-diphenyl-1-Substituted-1H-Pyrrole-3-Carbonitrile for Anti-Inflammatory and Antifungal Activity, Der Pharm. Lett., 2017, 9, 51–58 CAS.
- A. Chawla, Microwave Assisted One Pot Synthesis and Antimicrobial Activity of 2-(3′-acetyl-2′-methyl-5′-phenyl)-pyrrol-1-yl-1,4,5-triphenyl-1H-imidazole Derivatives, Der Pharm. Chem., 2018, 10, 27–31 CAS.
- W. Kan, T. Jing, X. Zhang, Y. Zheng, L. Chen and B. Zhao, Microwave-assisted one pot synthesis of N-substituted 2-methyl-1H-pyrrole-3-carboxylate derivatives without catalyst and solvent, Heterocycles, 2015, 91, 2367–2376 CrossRef CAS.
- V. Hanuman Reddy, G. Mallikarjuna Reddy, M. Thirupalu Reddy and Y. V. Rami Reddy, Microwave-assisted facile synthesis of trisubstituted pyrrole derivatives, Res. Chem. Intermed., 2015, 41, 9805–9815 CrossRef CAS.
- A. Das, H. Roy and I. Ansary, Microwave-Assisted, One-Pot Three-Component Synthesis of 6-(Pyrrolyl) Coumarin/Quinolone Derivatives Catalyzed by In(III) Chloride, ChemistrySelect, 2018, 3, 9592–9595 CrossRef CAS.
- A. Younis, et al., Microwave-Assisted One-Pot Synthesis of Novel Polyarylpyrrole Derivatives of Expected Anticancer Activity, Der Pharm. Chem., 2017, 9, 33–44 CAS.
- C. Bharkavi, S. Vivek Kumar, M. A. Ali, H. Osman, S. Muthusubramaniam and S. Perumal, One-pot microwave assisted stereoselective synthesis of novel dihydro-2′H-spiro[indene-2,1′-pyrrolo-[3,4-c]pyrrole]-tetraones and evaluation of their antimycobacterial activity and inhibition of AChE, Bioorg. Med. Chem. Lett., 2017, 27, 3071–3075 CrossRef CAS.
- H. Karakuş and Y. Dürüst, Novel benzothiophene 1,1-dioxide deoxygenation path for the microwave-assisted synthesis of substituted benzothiophene-fused pyrrole derivatives, Mol. Diversity, 2017, 21, 53–60 CrossRef.
- H. G. Bonacorso, F. M. Libero, G. M. Dal Forno, E. P. Pittaluga, D. F. Back, M. Hörner, M. A. P. Martins and N. Zanatta, New regioselective synthesis of polyfunctionalized 3-ferrocenyl-1H-pyrroles under microwave irradiation, Tetrahedron Lett, 2016, 57, 4568–4573 CrossRef CAS.
- E. Georgescu, F. Dumitrascu, F. Georgescu, C. Draghici and D. Dumitrescu, Microwave-Assisted synthesis of a library of pyrrolo[1,2-c]quinazolines, Rev. Chim., 2019, 70, 3094–3099 CrossRef CAS.
- S. Gopal Hegde, L. Koodlur and M. Narayanarao, Regioselective synthesis and biological evaluation of novel dispiropyrrolidine derivatives via one-pot four-component reaction, Synth. Commun., 2019, 49, 3453–3464 CrossRef CAS.
- S. Nayak, P. Pattanaik, S. Mohapatra, D. R. Mishra, P. Panda, B. Raiguru, N. P. Mishra, S. Jena and H. S. Biswal, One pot, three component synthesis of spiroindenoquinoxaline pyrrolidine fused nitrochromene derivatives following 1,3-dipolar cycloaddition, Synth. Commun., 2019, 49, 1823–1835 CrossRef CAS.
- S. Nayak, P. Panda, S. Mohapatra, B. Raiguru and N. Baral, Microwave-assisted One-pot, Three-component Regiospecific and Sterospecific Synthesis of Spiro Indanone Pyrrolidine/Piperidine Fused Nitrochromene Derivatives Through 1,3-Dipolar Cycloaddition Reactions, J. Heterocycl. Chem., 2019, 56, 1757–1770 CrossRef CAS.
- Y. Mirzaie, J. Lari, H. Vahedi and M. Hakimi, Conventional and microwave-assisted synthesis of quinolone carboxylic acid derivatives, Russ. J. Gen. Chem., 2016, 86, 2865–2869 CrossRef CAS.
- A. M. Akondi, S. Mekala, M. L. Kantam, R. Trivedi, L. R. Chowhan and A. Das, An expedient microwave assisted regio- and stereoselective synthesis of spiroquinoxaline pyrrolizine derivatives and their AChE inhibitory activity, New J. Chem., 2017, 41, 873–878 RSC.
- R. S. Dhivare and S. S. Rajput, Synthesis and antimicrobial evaluation of some novel malononitrile derivatives from N-phenylpyrrolidine-2, 5-diones under microwave irradiation, Der Pharm. Chem., 2016, 8, 257–262 CAS.
- E. Drège, J. Oko, P. E. Venot, N. Gigant and D. Joseph, Microwave-assisted telescoped cross metathesis-ring closing aza-Michael reaction sequence: step-economical access to nicotine-lobeline hybrid analogues, RSC Adv., 2015, 5, 96720–96724 RSC.
- J. Panther and T. J. J. Müller, Three- and Four-Component Syntheses of 3-Arylmethylindoles by Microwave-Assisted One-Pot Heck Isomerization-Fischer Indolization (Alkylation) (HIFI and HIFIA) Sequences, Synthesis, 2016, 48, 974–986 CrossRef CAS.
- P. R. Mali, L. C. Rao, V. M. Bangade, P. K. Shirsat, S. A. George, N. J. Babu and H. M. Meshram, A convenient and rapid microwave-assisted synthesis of spirooxindoles in aqueous medium and their antimicrobial activities, New J. Chem., 2016, 40, 2225–2232 RSC.
- A. S. Al-Bogami and A. S. El-Ahl, Microwave-assisted, multicomponent, ecofriendly synthesis of 3-bihetaryl-2-oxindole derivatives grafted with phenothiazine moiety, Synth. Commun., 2015, 45, 2462–2472 CrossRef CAS.
- S. Kumar, A. Kumar, N. Kumar, P. Roy and S. M. Sondhi, Grinding and Microwave-assisted Synthesis of Heterocyclic Molecules in High Yields and Their Biological Evaluation, J. Heterocycl. Chem., 2016, 53, 1761–1770 CrossRef CAS.
- J. Patel, Microwave Assisted Synthesis , Characterization and Antimicrobial Evaluation of Pyrazolyl and Pyrazoline, Int. J. Res. Anal. Rev., 2019, 6, 385–404 Search PubMed.
- S. S. Rajput, S. N. Patel and N. B. Jadhav, Synthesis And Charecterization Of Some Novel Bis-Isoxazole From 3,4-Di((Z)-Benzylidene)-1-(5-Methylpyridin-2-Yl)Pyrrolidine-2,5-Dione Under Microwave Irradiation, Eur. J. Biomed. Pharm. Sci., 2018, 5, 254–258 CAS.
- S. S. Patole and S. S. Rajput, Microwave Assisted Solid Phase Synthesis And Characterization Of Microbially Potent 7H-Pyrrolo[2,3-C:5,4-C′] Diisoxazole Derivatives, Eur. J. Biomed. Pharm. Sci., 2017, 4, 601–607 CAS.
| This journal is © The Royal Society of Chemistry 2020 |