Modulation of ligand conjugation for efficient FAPbBr3 based green light-emitting diodes†
Feng
Zhang
 ab,
Mengna
Sun
ab,
Xiyu
Luo
ab,
Dongdong
Zhang
*ab and
Lian
Duan
ab,
Mengna
Sun
ab,
Xiyu
Luo
ab,
Dongdong
Zhang
*ab and
Lian
Duan
 *ab
*ab
aKey Lab of Organic Optoelectronics and Molecular Engineering of Ministry of Education, Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: ddzhang@mail.tsinghua.edu.cn; duanl@mail.tsinghua.edu.cn
bCenter for Flexible Electronics Technology, Tsinghua University, Beijing 100084, P. R. China
First published on 3rd March 2020
Abstract
Organic ligands that capped the surfaces of perovskite nanocrystals (PeNCs) strongly influence the optical and electrical properties of the obtained PeNC films, which are fundamental for efficient perovskite light-emitting diodes (PeLEDs). Here, we systematically investigate the ligand effect through introduction of a ligand with strong π conjugation, 1-(1-naphthyl)ethylamine bromide (NEABr), for the fabrication of FAPbBr3 PeNCs. Compared to the widely applied ligand phenylethylamine bromide (PEABr), NEABr molecules containing naphthalene rings have more delocalized electrons and better conductivity, which is beneficial for charge injection and transportation between interfaces. By varying the ligand ratio, high quality three dimensional FAPbBr3 PeNC films with photoluminescence quantum yields up to 80% are fabricated. Based on the optimized PeNC films, electroluminescent (EL) devices achieve a maximum external quantum efficiency of 8.6%, which is about three times higher than that of EL devices based on PEABr. Importantly, by understanding the ligand effect, we demonstrate that the enhanced device efficiency is attributed to the lower defect densities and decreased interfacial resistance in the NEABr derived EL devices. Our findings about the effect of ligand conjugation on device performance may benefit other perovskite based optoelectronic devices.
Introduction
Halide perovskites with the structure ABX3, where A is CH3NH3+ (methylammonium, MA), CH2(NH2)2+ (formamidinium, FA) or Cs+, B is Pb2+ or Sn2+, X is Cl−, Br− or I−, show high luminous efficiency, narrow emission line widths and ease of solution processability.1–3 These superior features make them promising candidates for light emitting applications including phosphor converted light-emitting diodes (LEDs),4–6 lasers,7–9 and electroluminescent (EL) devices.10–13 Compared to inorganic quantum dot based LEDs (QDLEDs) and organic LEDs (OLEDs), perovskite based LEDs (PeLEDs) exhibit superiority in terms of higher color saturation, relatively low cost and simple fabrication processes. Since the first realization of room temperature operated PeLEDs by Tan and Friend et al. in 2014, the external quantum efficiency (EQE) of PeLEDs has witnessed rapid improvement from 0.76% to exceeding 20%,10,11,14–17 showing great potential in next generation display technologies.13,18–21 However, precise control of crystal growth and long-term stability are main challenges for further development of PeLEDs.It has been well recognized that smooth and uniform perovskite nanocrystal (PeNC) films with high PLQYs are prerequisites for highly efficient EL devices.22,23 In the fabrication of PeNC films, ligands are known to be key factors in determining their morphology, phase and luminescence properties.24–26 For example, Xiao and Rand et al. reported highly efficient PeLEDs through the formation of nanometer sized crystals by using n-butylammonium halides as surfactants.27 Yuan and Sargent et al. constructed a quasi-two-dimensional (2D) structure based on phenylethylamine (PEA) to improve the PLQY of perovskite films.28 Although ligand engineering has been intensively investigated to fabricate perovskite films with high quality, the ligands reported in the literature are always aliphatic amines or PEA.27–30 The lack in charge conductivity of these ligands limits charge injection and transportation to a certain degree.31–34 The replacement of these ligands with those with better conductivity is an alternative way to enhance the charge injection and transportation properties of PeLEDs. Compared to the widely applied aliphatic amines or PEA, ligands containing naphthalene rings exhibit delocalized electrons and higher electrical conductivity, which is expected to improve the charge transport between interfaces.35–37 Although naphthalene derived ligands have been used in a few papers, almost all these investigations were based on quasi-2D perovskites with complicated components.38,39 Meanwhile, little attention was paid to the influence of ligand conjugation on charge injection and transportation.
In this work, small molecule 1-(1-naphthyl)ethylamine bromide (NEABr) with enhanced conjugation is introduced as the ligand for the fabrication of FAPbBr3 PeNC films. By varying the amount of NEABr, flat and uniform FAPbBr3 PeNC films with PLQYs up to 80% are fabricated. X-ray diffraction (XRD) analysis combined with UV-vis absorption and PL spectra demonstrate that the obtained PeNC films are 3D structures with high phase purity. Based on the as fabricated FAPbBr3 PeNC films, EL devices with a luminance of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 cd m−2 and a maximum EQE of 8.6% are achieved. Moreover, compared with PEABr derived EL devices, lower trap-state densities and optimized interface properties with decreased interface resistance are demonstrated. Our findings of enhancing device performance through modulation of ligand conjugation can be easily applied to blue and red emitting PeLEDs and extended to other perovskite based devices such as solar cells and photodetectors.
630 cd m−2 and a maximum EQE of 8.6% are achieved. Moreover, compared with PEABr derived EL devices, lower trap-state densities and optimized interface properties with decreased interface resistance are demonstrated. Our findings of enhancing device performance through modulation of ligand conjugation can be easily applied to blue and red emitting PeLEDs and extended to other perovskite based devices such as solar cells and photodetectors.
Results and discussion
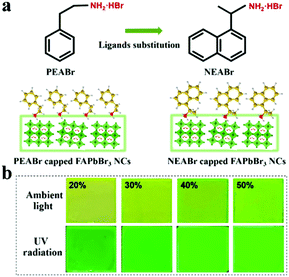
FAPbBr3 films were deposited through an in situ fabrication strategy with the widely applied ligand PEABr substituted with NEABr, as schematically illustrated in Fig. 1a. Compared to PEABr consisting of the benzene ring, NEABr molecules containing the naphthalene ring have more delocalized electrons with higher electrical conductivity. In a typical fabrication process, PbBr2, FABr and NEABr were mixed with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) x and dissolved in N,N-dimethylformamide (DMF) to form a precursor solution. The molar fraction of NEABr (x) was adjusted to 20%, 30%, 40% and 50%. Then the precursor solution was spread out on top of pretreated ITO substrates and spin cast into thin films. During the spin casting process, a liquid film first formed on top of the substrates. With solvent evaporation, the liquid film experienced an obvious color change from colorless to yellow green, indicating the nucleation of perovskite precursors. Previous studies have investigated the important role of dripping antisolvent before nucleation of perovskite crystals.40,41 In our cases, toluene was selected as the antisolvent for fast crystallization of perovskite crystals. Toluene was dripped one second earlier before the color change. Fig. 1b shows the photos of the PeNC films fabricated with NEABr molecules. These obtained perovskite films are uniform and transparent and their color turns from yellow green to bright green under ambient light. Brighter green emissions are also observed from sample 20% to 50% under UV radiation.
x and dissolved in N,N-dimethylformamide (DMF) to form a precursor solution. The molar fraction of NEABr (x) was adjusted to 20%, 30%, 40% and 50%. Then the precursor solution was spread out on top of pretreated ITO substrates and spin cast into thin films. During the spin casting process, a liquid film first formed on top of the substrates. With solvent evaporation, the liquid film experienced an obvious color change from colorless to yellow green, indicating the nucleation of perovskite precursors. Previous studies have investigated the important role of dripping antisolvent before nucleation of perovskite crystals.40,41 In our cases, toluene was selected as the antisolvent for fast crystallization of perovskite crystals. Toluene was dripped one second earlier before the color change. Fig. 1b shows the photos of the PeNC films fabricated with NEABr molecules. These obtained perovskite films are uniform and transparent and their color turns from yellow green to bright green under ambient light. Brighter green emissions are also observed from sample 20% to 50% under UV radiation.
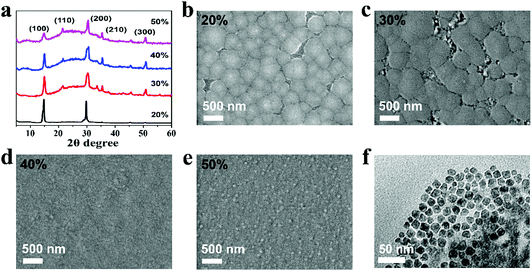
We first investigate the morphologies and structures of the as-fabricated FAPbBr3 PeNC films by applying X-ray diffraction (XRD), and scanning electron microscope (SEM) and transmission electron microscope (TEM) measurements. As shown in Fig. 2a, the XRD patterns of all these four films show characteristic diffraction peaks, which can be indexed to FAPbBr3 perovskite in the cubic phase (Pm3m).42 It should be noted that no obvious diffraction peaks are observed below 10 degrees, illustrating the formation of pure 3D structured FAPbBr3 without any 2D phases. Upon increasing the amount of ligand, significant peak broadening is observed in the XRD patterns owing to the decreased crystal size. As shown in the SEM images in Fig. 2b–e, obvious pin holes are observed in samples 20% and 30%, while the pin holes disappear in samples 40% and 50%. However, the SEM image of sample 50% exhibits many raised small particles, which lead to a relatively rough surface. Besides, greatly decreased crystal sizes from hundreds of nanometers to smaller than 100 nm can also be identified with increased amounts of ligand. A typical TEM image of sample 40% is shown Fig. 2f with an average size of 15.2 nm (see Fig. S1 in the ESI†). By combining the XRD patterns, and SEM and TEM results, it can be concluded that the NEABr ratio of 40% results in nanometer sized FAPbBr3 crystals with the best film quality, which is most suitable for application in PeLEDs.
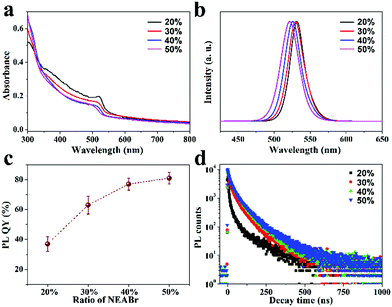
Steady state and time resolved PL (TRPL) spectra, UV-vis absorption spectra, and absolute PLQY measurements are applied to investigate the influence of ligands on optical properties. Fig. 3a and b show the UV-vis absorption and PL spectra of the as-fabricated FAPbBr3 PeNC films. The absorption spectra of all the samples exhibit typical absorption features of 3D structured FAPbBr3 with an absorption edge close to 510 nm.43 In the PL spectra, sharp emission peaks around 525 nm with a full width at half maximum (FWHM) of about 21 nm are detected for all the samples, indicating green emissions with high color purity. Obvious blue shift can also be observed in the UV absorption and PL spectra from samples 20% to 50%, which can be well explained by the size reduction due to the increasing amount of NEABr.44,45 We further determine the absolute PLQYs of these samples through an integrated sphere, with results of 36%, 62%, 76% and 80% for samples 20%, 30%, 40% and 50%, as shown in Fig. 3c. In order to understand the differences in PLQY, TRPL is applied to further reveal the detailed carrier recombination process. As shown in Fig. 3d, the decay curves can be well fitted using a tri-exponential function (eqn (1)) and the average PL lifetime can be calculated through eqn (2).
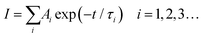 | (1) |
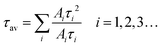 | (2) |
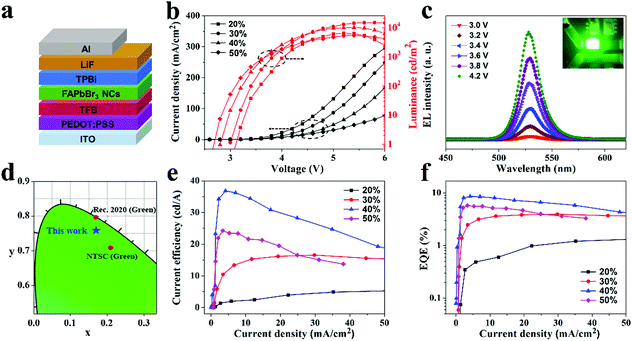
Based on the obtained FAPbBr3 PeNC films, EL devices are fabricated with an ITO/PEDOT:PSS (40 nm)/TFB (30 nm)/FAPbBr3 (50 nm)/TPBi (25 nm)/LiF (1 nm)/Al (80 nm) structure, as depicted in Fig. 4a. All the layers can be distinguished from the cross sectional SEM image shown in Fig. S2 (ESI†). In this device structure, PEDOT:PSS is used to enhance hole injection. TFB is employed as a hole-transporting and electron-blocking layer due to its high hole mobility and low electron affinity, whereas TPBi serves as an electron transport layer and a hole blocking layer. Fig. 4b shows the current density–luminance–voltage characteristics of the resultant EL devices. Detailed operation parameters of these EL devices are summarized in Table S2 in the ESI.† Upon increasing the molar fraction of NEABr, the current density decreases from nearly 150 mA cm−2 to below 50 mA cm−2 (under 5 V) from sample 20% to 50%, while the turn on voltage decreases from 3.1 V to 2.6 V. It is known that the turn on voltage is closely related to the inner defect states of PeNC films.29,40 The reduction of the turn on voltage can be explained by lower defect states achieved upon increasing the amount of NEABr, which has been demonstrated by the prolonged PL lifetime. The maximum luminance reaches 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 cd m−2 for sample 30% under 5 V. Fig. 4c presents the EL spectra of a typical device (based on sample 40%) operated at varied voltages. It can be seen that the corresponding EL intensity rapidly increases upon increasing the voltage. No obvious peak shift can be observed from the EL spectra, which demonstrates that the EL emissions totally originate from the FAPbBr3 layer with balanced electron and hole injection at varied voltages. The EL spectra show emission peaks located at 526 nm with an FWHM of about 20 nm, which is consistent with the corresponding PL spectrum. As shown in Fig. 4d, this detected pure green EL emission is related to color coordinates of (0.17, 0.76), as labeled in the Commission Internationale de l’Eclairage (CIE) diagram. It can be seen that the resulting color saturation is superior to the National Television System Committee (NTSC) standard and close to the standard of ITU-R Recommendation BT.2020 (Rec. 2020). Fig. 4e and f show the resultant current efficiency and EQE curves. Both the current efficiency and EQE increase upon increasing the NEABr molar ratio from 20% to 40%. A maximum current efficiency of 36.8 cd A−1 and a maximum EQE of 8.6% are achieved at a current density of 4 mA cm−2. When the NEABr molar ratio further increases to 50%, the maximum luminance of the resultant EL devices substantially decreases compared to the NEABr molar ratio of 40%, which results in moderate current efficiency and EQE. Although sample 50% shows the highest PLQY, the excess amount of ligand may lead to uncoordinated NEABr molecules remaining in the FAPbBr3 PeNC films, which partly hinders the carrier transportation and results in a relatively lower efficiency. Above all, by varying the amount of NEABr, the best NEABr ratio is determined to achieve highly efficient PeLEDs.
630 cd m−2 for sample 30% under 5 V. Fig. 4c presents the EL spectra of a typical device (based on sample 40%) operated at varied voltages. It can be seen that the corresponding EL intensity rapidly increases upon increasing the voltage. No obvious peak shift can be observed from the EL spectra, which demonstrates that the EL emissions totally originate from the FAPbBr3 layer with balanced electron and hole injection at varied voltages. The EL spectra show emission peaks located at 526 nm with an FWHM of about 20 nm, which is consistent with the corresponding PL spectrum. As shown in Fig. 4d, this detected pure green EL emission is related to color coordinates of (0.17, 0.76), as labeled in the Commission Internationale de l’Eclairage (CIE) diagram. It can be seen that the resulting color saturation is superior to the National Television System Committee (NTSC) standard and close to the standard of ITU-R Recommendation BT.2020 (Rec. 2020). Fig. 4e and f show the resultant current efficiency and EQE curves. Both the current efficiency and EQE increase upon increasing the NEABr molar ratio from 20% to 40%. A maximum current efficiency of 36.8 cd A−1 and a maximum EQE of 8.6% are achieved at a current density of 4 mA cm−2. When the NEABr molar ratio further increases to 50%, the maximum luminance of the resultant EL devices substantially decreases compared to the NEABr molar ratio of 40%, which results in moderate current efficiency and EQE. Although sample 50% shows the highest PLQY, the excess amount of ligand may lead to uncoordinated NEABr molecules remaining in the FAPbBr3 PeNC films, which partly hinders the carrier transportation and results in a relatively lower efficiency. Above all, by varying the amount of NEABr, the best NEABr ratio is determined to achieve highly efficient PeLEDs.
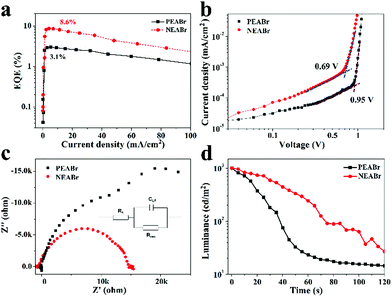
In order to address the important effect of ligand conjugation on optical and electrical properties, FAPbBr3 based PeLEDs fabricated using NEABr and PEABr are compared. PEABr molecules are widely applied as surface ligands in PeNC films for the fabrication of efficient PeLEDs.28–30 Here, the molar fraction of PEABr is controlled to 40% (PEABr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PbBr2 = 0.4
PbBr2 = 0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which is the best condition demonstrated by NEABr. As shown in Fig. S3 and S4 in the ESI,† the as fabricated FAPbBr3 PeNC films capped with PEABr molecules share similar structures and luminescent properties with the NEABr capped FAPbBr3 PeNC films. Fig. 5a shows the comparison of the EQE curves of the PEABr and NEABr derived EL devices with the same structure. The corresponding current density–voltage–luminance characteristics of the PEABr derived devices are shown in Fig. S5 in the ESI.† It can be seen that the optimized EL device based on PEABr exhibits a much lower EQE of 3.1% than that based on NEABr. We note that Qin and Adachi et al. also compared EL devices based on PEABr and 1-naphthylmethylamine bromide (NMABr) and concluded that PEABr results in more efficient PeLEDs.39 However, their investigations are based on quasi-2D perovskites with low dimensional components (n = 1, n = 2, etc.). In our cases, the fabricated FAPbBr3 NCs are 3D structures with an excited state energy of 2.36 eV, which is lower than the triplet state energy of the naphthalene ring (E = 2.6 eV). So the energy transfer to ligands can be negligible in our systems. To further reveal the ligand effect on device performance, we first use the space charge limited current (SCLC) method to evaluate the trap-state densities with an ITO/perovskite/Au structure. As can be seen in Fig. 5b, the trap-filled limiting voltages (VTFL) of the FAPbBr3 films capped with NEABr and PEABr are 0.69 V and 0.95 V, respectively. The relatively smaller VTFL indicates lower trap-state densities in the NEABr capped FAPbBr3 PeNC films,48,49 which is also consistent with the observation of TRPL results of PeNC films capped with PEABr and NEABr (see Fig. S6 in the ESI†). The detected lower trap-state densities may be attributed to the more rigid structure of the PeNC films formed under strong π–π interactions between NEABr molecules. Furthermore, impedance spectroscopy (IS) is also applied to analyze the charge injection and transportation. Nyquist plots of these EL devices are depicted in Fig. 5c. For each device, a semi-circle can be observed in the low frequency range, from which the recombination resistance (Rrec) and low frequency capacitance (CLF) can be calculated through the equivalent circuit as shown in the inset of Fig. 5c. As per the fitting results presented in Table S3 (ESI†), EL devices based on NEABr molecules exhibit a lower recombination resistance than those based on PEABr molecules. The decreased resistance is considered to have originated from the increased conjugation of NEABr compared to PEABr. According to the literature, the recombination resistance can reflect the recombination rate of injected electrons and holes.50,51 The lower resistance of EL devices based on NEABr molecules implies more feasibility for charge injection through charge transport layers and finally recombinations in the FAPbBr3 layer, which leads to higher EL efficiency. Besides, we also estimate the operating lifetimes of EL devices based on PEABr and NEABr. The devices are measured at an initial luminance of 1000 cd m−2 without encapsulation at 303 K and a humidity of 60%. As shown in Fig. 5d, both the EL devices based on PEABr and NEABr experience fast degradation. The device based on NEABr can maintain 20% of the initial luminance after being illuminated for 60 s, which is much better than that of the EL device based on PEABr.
1), which is the best condition demonstrated by NEABr. As shown in Fig. S3 and S4 in the ESI,† the as fabricated FAPbBr3 PeNC films capped with PEABr molecules share similar structures and luminescent properties with the NEABr capped FAPbBr3 PeNC films. Fig. 5a shows the comparison of the EQE curves of the PEABr and NEABr derived EL devices with the same structure. The corresponding current density–voltage–luminance characteristics of the PEABr derived devices are shown in Fig. S5 in the ESI.† It can be seen that the optimized EL device based on PEABr exhibits a much lower EQE of 3.1% than that based on NEABr. We note that Qin and Adachi et al. also compared EL devices based on PEABr and 1-naphthylmethylamine bromide (NMABr) and concluded that PEABr results in more efficient PeLEDs.39 However, their investigations are based on quasi-2D perovskites with low dimensional components (n = 1, n = 2, etc.). In our cases, the fabricated FAPbBr3 NCs are 3D structures with an excited state energy of 2.36 eV, which is lower than the triplet state energy of the naphthalene ring (E = 2.6 eV). So the energy transfer to ligands can be negligible in our systems. To further reveal the ligand effect on device performance, we first use the space charge limited current (SCLC) method to evaluate the trap-state densities with an ITO/perovskite/Au structure. As can be seen in Fig. 5b, the trap-filled limiting voltages (VTFL) of the FAPbBr3 films capped with NEABr and PEABr are 0.69 V and 0.95 V, respectively. The relatively smaller VTFL indicates lower trap-state densities in the NEABr capped FAPbBr3 PeNC films,48,49 which is also consistent with the observation of TRPL results of PeNC films capped with PEABr and NEABr (see Fig. S6 in the ESI†). The detected lower trap-state densities may be attributed to the more rigid structure of the PeNC films formed under strong π–π interactions between NEABr molecules. Furthermore, impedance spectroscopy (IS) is also applied to analyze the charge injection and transportation. Nyquist plots of these EL devices are depicted in Fig. 5c. For each device, a semi-circle can be observed in the low frequency range, from which the recombination resistance (Rrec) and low frequency capacitance (CLF) can be calculated through the equivalent circuit as shown in the inset of Fig. 5c. As per the fitting results presented in Table S3 (ESI†), EL devices based on NEABr molecules exhibit a lower recombination resistance than those based on PEABr molecules. The decreased resistance is considered to have originated from the increased conjugation of NEABr compared to PEABr. According to the literature, the recombination resistance can reflect the recombination rate of injected electrons and holes.50,51 The lower resistance of EL devices based on NEABr molecules implies more feasibility for charge injection through charge transport layers and finally recombinations in the FAPbBr3 layer, which leads to higher EL efficiency. Besides, we also estimate the operating lifetimes of EL devices based on PEABr and NEABr. The devices are measured at an initial luminance of 1000 cd m−2 without encapsulation at 303 K and a humidity of 60%. As shown in Fig. 5d, both the EL devices based on PEABr and NEABr experience fast degradation. The device based on NEABr can maintain 20% of the initial luminance after being illuminated for 60 s, which is much better than that of the EL device based on PEABr.
Conclusions
In summary, we have systematically investigated the influence of ligand conjugation on performance optimization in FAPbBr3 based PeLEDs. The introduction of a ligand (NEABr) containing the naphthalene ring results in 3D structured FAPbBr3 PeNCs with high phase purity. Besides, the increasing amount of NEABr in FAPbBr3 precursor solution contributes to nanocrystalline FAPbBr3 films with a more uniform morphology and higher PLQYs up to 80%. Based on the obtained FAPbBr3 PeNC films, EL devices are fabricated with a luminance of 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 cd m−2 and a peak EQE of 8.6%. The EL emission of typical EL devices corresponds to color coordinates of (0.17, 0.76), which is very close to the standard of Rec. 2020. Furthermore, compared with EL devices based on PEABr, lower trap state densities and decreased interfacial resistance are demonstrated, which is responsible for the improved efficiency in the NEABr derived PeLEDs. We reveal the effect of ligand conjugation on device performance in PeLEDs, which can be easily adapted to enhance the performance of red and blue PeLEDs as well as other perovskite based optoelectronic devices such as solar cells and photodetectors.
630 cd m−2 and a peak EQE of 8.6%. The EL emission of typical EL devices corresponds to color coordinates of (0.17, 0.76), which is very close to the standard of Rec. 2020. Furthermore, compared with EL devices based on PEABr, lower trap state densities and decreased interfacial resistance are demonstrated, which is responsible for the improved efficiency in the NEABr derived PeLEDs. We reveal the effect of ligand conjugation on device performance in PeLEDs, which can be easily adapted to enhance the performance of red and blue PeLEDs as well as other perovskite based optoelectronic devices such as solar cells and photodetectors.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We acknowledge the support from the National Key Basic Research and Development Program of China (Grant Number: 2016YFB0400702 and 2017YFA0204501), the National Science Fund of China (Grant Number: U1601651 and 61890942), and the China Postdoctoral Science Foundation (Grant Number: 2019M650627).Notes and references
- D. B. Mitzi, Synthesis, Structure, and Properties of Organic-Inorganic Perovskites and Related Materials, Prog. Inorg. Chem., 1999, 48, 1–121 CAS.
- Q. Chen, N. De Marco, Y. M. Yang, T. B. Song, C. C. Chen, H. Zhao, Z. Hong, H. Zhou and Y. Yang, Under the Spotlight: The Organic-Inorganic Hybrid Halide Perovskite for Optoelectronic Applications, Nano Today, 2015, 10, 355–396 CrossRef CAS.
- S. D. Stranks and H. J. Snaith, Metal-Halide Perovskites for Photovoltaic and Light-Emitting Devices, Nat. Nanotechnol., 2015, 10, 391–402 CrossRef CAS PubMed.
- F. Zhang, H. Zhong, C. Chen, X. G. Wu, X. Hu, H. Huang, J. Han, B. Zou and Y. Dong, Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology, ACS Nano, 2015, 9, 4533–4542 CrossRef CAS PubMed.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS PubMed.
- Q. Zhou, Z. Bai, W. G. Lu, Y. Wang, B. Zou and H. Zhong, In Situ Fabrication of Halide Perovskite Nanocrystal-Embedded Polymer Composite Films with Enhanced Photoluminescence for Display Backlights, Adv. Mater., 2016, 28, 9163–9168 CrossRef CAS PubMed.
- G. Xing, N. Mathews, S. S. Lim, N. Yantara, X. Liu, D. Sabba, M. Grätzel, S. Mhaisalkar and T. C. Sum, Low-Temperature Solution-Processed Wavelength-Tunable Perovskites for Lasing, Nat. Mater., 2014, 13, 476–480 CrossRef CAS PubMed.
- S. Yakunin, L. Protesescu, F. Krieg, M. I. Bodnarchuk, G. Nedelcu, M. Humer, G. De Luca, M. Fiebig, W. Heiss and M. V. Kovalenko, Low-Threshold Amplified Spontaneous Emission and Lasing from Colloidal Nanocrystals of Caesium Lead Halide Perovskites, Nat. Commun., 2015, 6, 8056 CrossRef CAS PubMed.
- H. Zhu, Y. Fu, F. Meng, X. Wu, Z. Gong, Q. Ding, M. V. Gustafsson, M. T. Trinh, S. Jin and X. Zhu, Lead Halide Perovskite Nanowire Lasers with Low Lasing Thresholds and High Quality Factors, Nat. Mater., 2015, 14, 636–642 CrossRef CAS PubMed.
- Z. K. Tan, R. S. Moghaddam, M. L. Lai, P. Docampo, R. Higler, F. Deschler, M. Price, A. Sadhanala, L. M. Pazos, D. Credgington, F. Hanusch, T. Bein, H. J. Snaith and R. H. Friend, Bright Light-Emitting Diodes based on Organometal Halide Perovskite, Nat. Nanotechnol., 2014, 9, 687–692 CrossRef CAS PubMed.
- Y. H. Kim, H. Cho, J. H. Heo, T. S. Kim, N. Myoung, C. L. Lee, S. H. Im and T. W. Lee, Multicolored Organic/Inorganic Hybrid Perovskite Light-Emitting Diodes, Adv. Mater., 2015, 27, 1248–1254 CrossRef CAS PubMed.
- Y. H. Kim, H. Cho and T. W. Lee, Metal Halide Perovskite Light Emitters, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11694–11702 CrossRef CAS PubMed.
- F. Yan, S. T. Tan, X. Li and H. V. Demir, Light Generation in Lead Halide Perovskite Nanocrystals: LEDs, Color Converters, Lasers, and Other Applications, Small, 2019, 15, 1902079 CrossRef CAS PubMed.
- Y. Cao, N. Wang, H. Tian, J. Guo, Y. Wei, H. Chen, Y. Miao, W. Zou, K. Pan, Y. He, H. Cao, Y. Ke, M. Xu, Y. Wang, M. Yang, K. Du, Z. Fu, D. Kong, D. Dai, Y. Jin, G. Li, H. Li, Q. Peng, J. Wang and W. Huang, Perovskite Light-Emitting Diodes based on Spontaneously Formed Submicrometre-Scale Structures, Nature, 2018, 562, 249–253 CrossRef CAS PubMed.
- K. Lin, J. Xing, L. N. Quan, F. P. G. De Arquer, X. Gong, J. Lu, L. Xie, W. Zhao, D. Zhang, C. Yan, W. Li, X. Liu, Y. Lu, J. Kirman, E. H. Sargent, Q. Xiong and Z. Wei, Perovskite Light-Emitting Diodes with External Quantum Efficiency Exceeding 20 Percent, Nature, 2018, 562, 245–248 CrossRef CAS PubMed.
- T. Chiba, Y. Hayashi, H. Ebe, K. Hoshi, J. Sato, S. Sato, Y. J. Pu, S. Ohisa and J. Kido, Anion-Exchange Red Perovskite Quantum Dots with Ammonium Iodine Salts for Highly Efficient Light-Emitting Devices, Nat. Photonics, 2018, 12, 681–687 CrossRef CAS.
- Y. Shen, L. P. Cheng, Y. Q. Li, W. Li, J. D. Chen, S. T. Lee and J. X. Tang, High-Efficiency Perovskite Light-Emitting Diodes with Synergetic Outcoupling Enhancement, Adv. Mater., 2019, 31, 1901517 CrossRef PubMed.
- R. H. Friend, D. Di, S. Lilliu and B. Zhao, Perovskite LEDs, Sci. Video Protoc., 2019, 1, 1–5 Search PubMed.
- Z. Wei and J. J. Xing, The Rise of Perovskite Light-Emitting Diodes, J. Phys. Chem. Lett., 2019, 10, 3035–3042 CrossRef CAS PubMed.
- F. Yan and H. V. Demir, LEDs Using Halide Perovskite Nanocrystal Emitters, Nanoscale, 2019, 11, 11402–11412 RSC.
- L. N. Quan, B. P. Rand, R. H. Friend, S. G. Mhaisalkar, T. W. Lee and E. H. Sargent, Perovskites for Next-Generation Optical Sources, Chem. Rev., 2019, 119, 7444–7477 CrossRef CAS PubMed.
- H. Cho, S. H. Jeong, M. H. Park, Y. H. Kim, C. Wolf, C. L. Lee, J. H. Heo, A. Sadhanala, N. Myoung, S. Yoo, S. H. Im, R. H. Friend and T. W. Lee, Overcoming the Electroluminescence Efficiency Limitations of Perovskite Light-Emitting Diodes, Science, 2015, 350, 1222–1225 CrossRef CAS PubMed.
- G. Li, Z. K. Tan, D. Di, M. L. Lai, L. Jiang, J. H. W. Lim, R. H. Friend and N. C. Greenham, Efficient Light-Emitting Diodes based on Nanocrystalline Perovskite in a Dielectric Polymer Matrix, Nano Lett., 2015, 15, 2640–2644 CrossRef CAS PubMed.
- M. V. Kovalenko, L. Manna, A. Cabot, Z. Hens, D. V. Talapin, C. R. Kagan, V. I. Klimov, A. L. Rogach, P. Reiss and D. J. Milliron, Prospects of Nanoscience with Nanocrystals, ACS Nano, 2015, 9, 1012–1057 CrossRef CAS PubMed.
- N. K. Noel, A. Abate, S. D. Stranks, E. S. Parrott, V. M. Burlakov, A. Goriely and H. J. Snaith, Enhanced Photoluminescence and Solar Cell Performance via Lewis Base Passivation of Organic-Inorganic Lead Halide Perovskites, ACS Nano, 2014, 8, 9815–9821 CrossRef CAS PubMed.
- Y. Shirasaki, G. J. Supran, M. G. Bawendi and V. Bulovic, Emergence of Colloidal Quantum-Dot Light-Emitting Technologies, Nat. Photonics, 2013, 7, 13–23 CrossRef CAS.
- Z. Xiao, R. A. Kerner, L. Zhao, N. L. Tran, K. M. Lee, T. W. Koh, G. D. Scholes and B. P. Rand, Efficient Perovskite Light-Emitting Diodes Featuring Nanometre-Sized Crystallites, Nat. Photonics, 2017, 11, 108–115 CrossRef CAS.
- M. Yuan, L. N. Quan, R. Comin, G. Walters, R. Sabatini, O. Voznyy, S. Hoogland, Y. Zhao, E. M. Beauregard, P. Kanjanaboos, Z. Lu, D. H. Kim and E. H. Sargent, Perovskite Energy Funnels for Efficient Light-Emitting Diodes, Nat. Nanotechnol., 2016, 11, 872–877 CrossRef CAS PubMed.
- Z. Li, Z. Chen, Y. Yang, Q. Xue, H. L. Yip and Y. Cao, Modulation of Recombination Zone Position for Quasi-Two-Dimensional Blue Perovskite Light-Emitting Diodes with Efficiency Exceeding 5%, Nat. Commun., 2019, 10, 1027 CrossRef PubMed.
- X. Yang, X. Zhang, J. Deng, Z. Chu, Q. Jiang, J. Meng, P. Wang, L. Zhang, Z. Yin and J. You, Efficient Green Light-Emitting Diodes based on Quasi-Two-Dimensional Composition and Phase Engineered Perovskite with Surface Passivation, Nat. Commun., 2018, 9, 570 CrossRef PubMed.
- H. Huang, F. Zhao, L. Liu, F. Zhang, X. G. Wu, L. Shi, B. Zou, Q. Pei and H. Zhong, Emulsion Synthesis of Size-Tunable CH3NH3PbBr3 Quantum Dots: An Alternative Route toward Efficient Light-Emitting Diodes, ACS Appl. Mater. Interfaces, 2015, 7, 28128–28133 CrossRef CAS PubMed.
- J. Li, L. Xu, T. Wang, J. Song, J. Chen, J. Xue, Y. Dong, B. Cai, Q. Shan, B. Han and H. Zeng, 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control, Adv. Mater., 2017, 29, 1603885 CrossRef PubMed.
- J. Song, J. Li, L. Xu, J. Li, F. Zhang, B. Han, Q. Shan and H. Zeng, Room-Temperature Triple-Ligand Surface Engineering Synergistically Boosts Ink Stability, Recombination Dynamics, and Charge Injection toward EQE-11.6% Perovskite QLEDs, Adv. Mater., 2018, 30, 1800764 CrossRef PubMed.
- G. Li, M. Price and F. Deschler, Research Update: Challenges for High-Efficiency Hybrid Lead-Halide Perovskite LEDs and the Path Towards Electrically Pumped Lasing, APL Mater., 2016, 4, 091507 CrossRef.
- T. H. Han, S. Tan, J. Xue, L. Meng, J. W. Lee and Y. Yang, Interface and Defect Engineering for Metal Halide Perovskite Optoelectronic Devices, Adv. Mater., 2019, 31, 1803515 CrossRef CAS PubMed.
- M. R. Molla, D. Gehrig, L. Roy, V. Kamm, A. Paul, F. Laquai and S. Ghosh, Self Assembly of Carboxylic Acid Appended Naphthalene Diimide Derivatives with Tunable Luminescent Color and Electrical Conductivity, Chem. – Eur. J., 2014, 20, 760–771 CrossRef CAS PubMed.
- H. Kar, M. R. Molla and S. Ghosh, Two-Component Gelation and Morphology-Dependent Conductivity of a Naphthalene-Diimide (NDI) π-System by Orthogonal Hydrogen Bonding, Chem. Commun., 2013, 49, 4220–4222 RSC.
- N. Wang, L. Cheng, R. Ge, S. Zhang, Y. Miao, W. Zou, C. Yi, Y. Sun, Y. Cao, R. Yang, Y. Wei, Q. Guo, Y. Ke, M. Yu, Y. Jin, Y. Liu, Q. Ding, D. Di, L. Yang, G. Xing, H. Tian, C. Jin, F. Gao, R. H. Friend, J. Wang and W. Huang, Perovskite Light-Emitting Diodes based on Solution-Processed Self-Organized Multiple Quantum Wells, Nat. Photonics, 2016, 10, 699–704 CrossRef CAS.
- C. Qin, T. Matsushima, W. J. Potscavage, A. S. Sandanayaka, M. R. Leyden, F. Bencheikh, K. Goushi, F. Mathevet, B. Heinrich, G. Yumoto, Y. Kanemitsu and C. Adachi, Triplet Management for Efficient Perovskite Light-Emitting Diodes, Nat. Photonics, 2020, 14, 70–75 CrossRef CAS.
- D. Han, M. Imran, M. Zhang, S. Chang, X. G. Wu, X. Zhang, J. Tang, M. Wang, S. Ali, X. Li, G. Yu, J. Han, L. Wang, B. Zou and H. Zhong, Efficient Light-Emitting Diodes based on In Situ Fabricated FAPbBr3 Nanocrystals: The Enhancing Role of the Ligand-Assisted Reprecipitation Process, ACS Nano, 2018, 12, 8808–8816 CrossRef CAS PubMed.
- X. Zhang, D. Han, C. Wang, I. Muhammad, F. Zhang, A. Shmshad, X. Xue, W. Ji, S. Chang and H. Zhong, Highly Efficient Light Emitting Diodes based on In Situ Fabricated FAPbI3 Nanocrystals: Solvent Effects of On-Chip Crystallization, Adv. Opt. Mater., 2019, 7, 1900774 CrossRef CAS.
- M. I. Saidaminov, A. L. Abdelhady, G. Maculan and O. M. Bakr, Retrograde Solubility of Formamidinium and Methylammonium Lead Halide Perovskites Enabling Rapid Single Crystal Growth, Chem. Commun., 2015, 51, 17658–17661 RSC.
- I. Levchuk, A. Osvet, X. Tang, M. Brandl, J. D. Perea, F. Hoegl, G. J. Matt, R. Hock, M. Batentschuk and C. J. Brabec, Brightly Luminescent and Color-Tunable Formamidinium Lead Halide Perovskite FAPbX3 (X = Cl, Br, I) Colloidal Nanocrystals, Nano Lett., 2017, 17, 2765–2770 CrossRef CAS PubMed.
- F. Zhang, S. Huang, P. Wang, X. Chen, S. Zhao, Y. Dong and H. Zhong, Colloidal Synthesis of Air-Stable CH3NH3PbI3 Quantum Dots by Gaining Chemical Insight into the Solvent Effects, Chem. Mater., 2017, 29, 3793–3799 CrossRef CAS.
- J. A. Sichert, Y. Tong, N. Mutz, M. Vollmer, S. Fischer, K. Z. Milowska, R. García Cortadella, B. Nickel, C. Cardenas-Daw, J. K. Stolarczyk, A. S. Urban and J. Feldmann, Quantum Size Effect in Organometal Halide Perovskite Nanoplatelets, Nano Lett., 2015, 15, 6521–6527 CrossRef CAS PubMed.
- L. Zhang, X. Yang, Q. Jiang, P. Wang, Z. Yin, X. Zhang, H. Tan, Y. M. Yang, M. Wei, B. R. Sutherland, E. H. Sargent and J. You, Ultra-Bright and Highly Efficient Inorganic based Perovskite Light-Emitting Diodes, Nat. Commun., 2017, 8, 15640 CrossRef CAS PubMed.
- S. D. Stranks, V. M. Burlakov, T. Leijtens, J. M. Ball, A. Goriely and H. J. Snaith, Recombination Kinetics in Organic-Inorganic Perovskites: Excitons, Free Charge, and Subgap States, J. Phys. Rev. Appl., 2014, 2, 034007 CrossRef.
- F. Schauer, R. Novotny and S. J. Nešpůrek, Space-Charge-Limited-Current Spectroscopy: Possibilities and Limitations, J. Appl. Phys., 1997, 81, 1244–1249 CrossRef CAS.
- R. H. J. Bube, Trap Density Determination by Space-Charge-Limited-Currents, J. Appl. Phys., 1962, 33, 1733–1737 CrossRef CAS.
- S. Ali, S. Chang, M. Imran, Q. Shi, Y. Chen and H. Zhong, Impedance Spectroscopy: A Versatile Technique to Understand Solution-Processed Optoelectronic Devices, Phys. Status Solidi RRL, 2019, 13, 1800580 CrossRef.
- S. C. Chang, Y. Yang, F. Wudl, G. He and Y. Li, AC Impedance Characteristics and Modeling of Polymer Solution Light-Emitting Devices, J. Phys. Chem. B, 2001, 105, 11419–11423 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fabrication process of FAPbBr3 PeNC films and EL devices, characterization of the obtained films and devices, analysis of size distributions, cross-sectional SEM images, XRD patterns of the FAPbBr3 films fabricated using PEABr, device performance of EL devices based on PEABr, detailed fitting results of PL lifetimes, fitting results of resistance and capacitance, etc. See DOI: 10.1039/c9qm00768g |
| This journal is © the Partner Organisations 2020 |