Mesoporous carbon host material for stable lithium metal anode†
Jooyoung
Jeong
ab,
Jinyoung
Chun
c,
Won-Gwang
Lim
ab,
Won Bae
Kim
 b,
Changshin
Jo
*d and
Jinwoo
Lee
b,
Changshin
Jo
*d and
Jinwoo
Lee
 *a
*a
aDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), 291 Daehak-ro, Yuseong-gu, Daejeon 34141, Republic of Korea. E-mail: jwlee1@kaist.ac.kr
bDepartment of Chemical Engineering, Pohang University of Science and Technology (POSTECH), 77 Cheongam-ro, Nam-gu, Pohang 37673, Republic of Korea
cKorea Institute of Ceramic Engineering and Technology (KICET), 101 Soho-ro, Jinju 52851, Republic of Korea
dSchool of Chemical Engineering & Materials Science, Chung-Ang University (CAU), 84 Heukseok-ro, Dongjak-gu, Seoul, 06974, Republic of Korea. E-mail: changshin@cau.ac.kr
First published on 20th May 2020
Abstract
Lithium (Li) metal is a promising anode material for next-generation batteries because of its low standard reduction potential (−3.04 V vs. SHE) and high specific capacity (3860 mA h g−1). However, it is still challenging to directly use Li metal as anode material in commercial batteries because of unstable Li dendrite formation and accumulated solid–electrolyte interphase. Possible methods that can suppress the unwanted formation of Li dendrites are (i) by increasing the electrode surface area and (ii) formation of porosity for confining Li. Here, we tested microporous (<2 nm) carbon and mesoporous (2–50 nm) carbon as host materials for the Li metal anode to avoid their degradation during cycling of lithium metal batteries (LMBs). Mesoporous carbon was more effective than microporous carbon as a host material to confine the Li metal and the lifetime of mesoporous carbon was more than twice as long as those of the Cu foil and microporous carbon. After confirmed better anode performance of mesoporous carbon host material, we applied Li-plated mesoporous carbon as an anode in a lithium–sulfur battery (Li–S) full cell. This research work suggests that mesopores, in spite of their low specific surface area, are better than micropores in stabilizing the Li metal and that a mesoporous host material can be applied to Li metal anodes for use in next-generation battery applications.
Lithium-ion batteries (LIBs) have a high operation voltage, high energy density, low self-discharge, and no memory effect,1–3 so they have become the main energy-storage devices for portable electronics. Besides, the LIB market is getting bigger since an enormous size of LIBs are required to run an electric vehicle (EV) or energy storage system (ESS). However, despite the high energy density of LIBs, it is difficult to make the EV or ESS lighter, smaller, and cheaper with commercial LIBs. Next-generation batteries such as sodium ion batteries,4 lithium sulfur (Li–S) batteries5,6 and lithium oxygen (Li–O2) batteries5 may provide a solution to this problem. Lithium metal batteries (LMBs) can be applied in commercial LIBs, Li–S and Li–O2 battery systems, because a lithium (Li) metal anode has high specific capacity (3860 mA h g−1) and low redox potential (−3.04 V vs. SHE).7,8
However, use of Li metal as an anode active material in batteries is impeded by its hazardous nature, short cycle life, loss of coulombic efficiency (CE), and poor safety because of the formation of Li dendrites.9–11 When micrometer-scale Li dendrites grow during Li plating, they can penetrate the separator and thereby cause a short circuit that can cause cell failure or battery explosion.9 Furthermore, formation of Li dendrites consumes a large amount of liquid electrolyte, leading to the development of a solid electrolyte interphase (SEI) on the surface of the Li metal.10 This SEI layer is not mechanically strong enough to suppress the formation and growth of Li dendrites and is easily cracked by Li dendrites formed during subsequent cycles, leading to the additional decomposition of electrolyte to make new SEI.
As a result, a thick SEI layer with electrically isolated Li forms on the Li metal surface.10,11 Therefore, the repetitive charge/discharge cycle wastes Li ions (Li+) and impedes Li+ diffusion into the active Li domain, so the overpotential increases and the cell can fail.
Attempts have been made to suppress the formation of Li dendrites and to apply Li metal as an anode material for commercial battery systems.12,13 For instance, researchers have considered the modification of various battery components including the electrolyte, separator, and electrode layer. To achieve a stable SEI layer, additives such as lithium nitrate (LiNO3),14 fluoroethylene carbonate (FEC),15,16 and cesium ion (Cs+)17,18 have been introduced into the traditional electrolyte and have extended battery cycle life. Techniques to deposit an artificial SEI (h-BN,19,20 LiPON-polymer composite,21etc.) were also effective. To make a physically strong separator or electrolyte, separators coated with inorganic materials (SiO2,22,23 metal–organic framework,24,25 NbN,26etc.) and polymer electrolytes with inorganic materials (g-C3N4,27 SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28,29) have also been evaluated. To improve stability at the electrode level, lithiophilic additives30–33 and 3D structured host material approaches have been applied.32,34–36
28,29) have also been evaluated. To improve stability at the electrode level, lithiophilic additives30–33 and 3D structured host material approaches have been applied.32,34–36
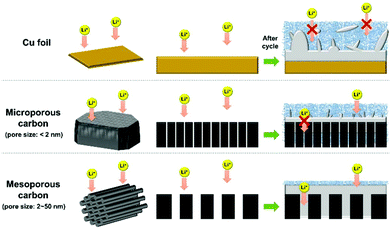
Recently, carbonaceous materials are widely studied as host materials for Li metal anodes. Graphene oxide (GO) and carbon nanotubes (CNTs) showed lithiophilic property and Li metal can be impregnated into these carbon based hosts by the simple melt infusion method.37–39 Graphite is lithiophilic after it is fully reduced into LiC6.40 In contrast, amorphous carbons have less lithiophilic properties but are known as good candidates for host material applications because of the easy structuring, high surface area, defect sites, and surface functionalities.41–44 Nanostructured carbons have high conductivity and surface area (∼1000 m2 g−1), which effectively distribute electrons in the electrode layer (low areal current density), resulting in the stable formation of small-sized Li metal.42 Structured carbon materials that have pore sizes of less than a few hundred nanometers to less than a few micrometers have been applied as Li host materials. However, the effects of nanometer-scale pore size have not been well studied. Much of the surface area of carbon is contributed by micropores (<2 nm) and mesopores (2–50 nm); the relative importance of these pore categories45 in Li plating/stripping applications should be determined.
In this work, we evaluated the pore-size effects of nanoporous carbon on stabilizing the Li metal anode. We selected two kinds of carbon materials as Li metal hosts: microporous carbon (MSP-20) and mesoporous carbon (CMK-3). Even though CMK-3 has a lower specific surface area than MSP-20, the electrode composed of CMK-3 showed better cycling stability, less polarization, and less Li dendrite formation than that composed of MSP-20, because the large pores and well aligned porosity of CMK-3 increased the accessibility of Li ions and space for the formation of Li metal. Furthermore, the CMK-3 electrode plated with the Li metal exhibited improved electrochemical performance as the anode in a Li–S full-cell system.
In general, low current density yields relatively stable cycling of Li metal; this relationship can be explained by the equation of Sand's time (τ):34
| τ = πDe2C02(1 + μc/μa)2/(4Jeff2) | (1) |
Here, to increase τ, we chose MSP-20 and CMK-3 porous carbons, which have a high surface area (Fig. S1†), as host material and then compared the effect of nanoporous structure on the electrochemical performance of LMB. MSP-20 is a well-known commercial microporous carbon and is widely used as electrode material in supercapacitors and batteries.49,50 CMK-3 has been actively investigated for use in various Li rechargeable battery applications,51–53 and it is an ordered mesoporous carbon with hexagonal arrays of channel-like pores which have pore size <5 nm.
To measure the surface area and pore volume, we conducted nitrogen (N2) physisorption analysis (Fig. S1a and b†). The pore volume and surface area are 1 cm3 g−1 and 2110 m2 g−1 in MSP-20 and 1.3 cm3 g−1 and 1120 m2 g−1 in CMK-3 and the pore size distribution shows that MSP-20 has micropores (<2 nm) and CMK-3 has mesopores (3.4 nm). Scanning electron microscopy (SEM) and transmission electron electroscopy (TEM) clearly showed well-formed channel-like mesopores in CMK-3, while showing randomly formed micropores in MSP-20 (Fig. S1c–h†).
For further characterization of the two carbon host materials, MSP-20 and CMK-3 were identified by Raman spectroscopy and X-ray photoelectron spectroscopy (XPS) to estimate the lithiophilic properties. Considering that graphites show lithiophilicity after lithiation to LiC6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and several studies reported that lithiophilic properties of carbons can be controlled by functional groups,41,54–56 the degree of graphitization and surface functionality difference of MSP-20 and CMK-3 materials could affect the cycle life.
40 and several studies reported that lithiophilic properties of carbons can be controlled by functional groups,41,54–56 the degree of graphitization and surface functionality difference of MSP-20 and CMK-3 materials could affect the cycle life.
In Raman spectra (Fig. S2†), we calculated the ID/IG ratio in each carbon, where the D-band at ∼1350 cm−1 corresponds to sp3 carbons and the G-band at ∼1600 cm−2 corresponds to sp2 carbons.57,58 The ID/IG ratios were ∼1.02 in MSP-20 and ∼1.04 in CMK-3, respectively. This result indicates that the synthesized CMK-3 was controlled to have a similar degree of graphitization to that of MSP-20. Therefore, the degree of graphitization in both carbons does not affect the lithiophilic properties in carbon host materials much.
Oxygen functionalities can also increase the lithiophilicity of carbon materials.41 From the XPS data (Fig. S3†), oxygen contents were ∼17 at% in MSP-20 and ∼11 at% in CMK-3, respectively, indicating that MSP-20 might have higher lithiophilicity and better Li impregnation inside the structures.
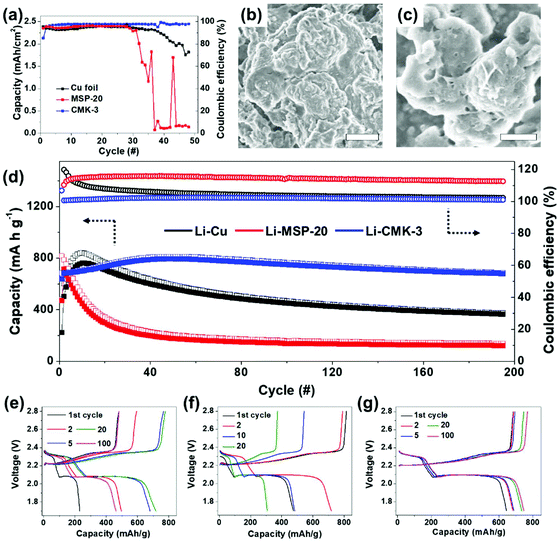
To investigate the effects of different pore sizes on carbon hosts, we fabricated [carbon host electrode]/[Li foil] and [Cu foil]/[Li foil] half-cells, and then electrochemically lithiated the host materials (Fig. 1). As an electrolyte, an electrolyte that contains FEC was used to stabilize the Li metal counter-electrode. All half-cells were cycled under 1 mA cm−2 current density for 1 h. During the initial cycle, the carbon electrodes showed poor CEs because most of the Li ions (Li+) were inserted into the carbon matrix by intercalation (open-circuit voltage to 0 V range) and were consumed to form the SEI (Fig. S4†). Generally, amorphous carbon materials have poor initial CE.44,46 Starting in the second cycle (Fig. 2a), the CE was higher for the CMK-3 electrode (82%) than for the MSP-20 electrode (70%). In contrast, the Cu foil half-cell showed initial CE = 94%, but it started to decrease after the 60th cycle and the half-cell finally failed after the 110th cycle. MSP-20 electrodes reached CE = 90% at the 5th cycle, and CMK-3 electrodes achieved CE = 90% at the 2nd cycle. The CE of the MSP-20 electrode decreased slowly starting at the 80th cycle and the half-cell failed around the 110th cycle, like for the Cu foil electrode. In contrast, the CMK-3 electrode achieved CE > 95% and maintained high CE up to the 200th cycle; at that point, the CE started to decrease, and the half-cell failed after the 240th cycle, i.e., its lifetime was more than twice as long as the Cu foil and MSP-20.
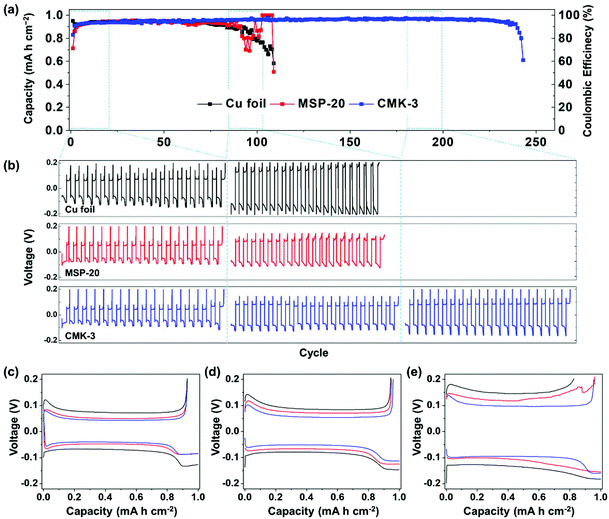 | ||
Fig. 2 Electrochemical test result of Cu foil, MSP-20, and CMK-3 at rate = 1 mA cm−2, capacity cut = 1 mA h cm−2 with 1 M LiPF6 in EMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FEC = 7 FEC = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 electrolyte. (a) Cycling stability from the second cycle (initial cycle is in ESI†), voltage profiles at different ranges of cycles (b) 1st–20th, 85th–105th, and 180th–200th. Voltage profiles of specific cycle (c) at 10th, (d) at 50th, and (e) at 100th. 3 electrolyte. (a) Cycling stability from the second cycle (initial cycle is in ESI†), voltage profiles at different ranges of cycles (b) 1st–20th, 85th–105th, and 180th–200th. Voltage profiles of specific cycle (c) at 10th, (d) at 50th, and (e) at 100th. | ||
The overpotential of each half-cell increased from the 1st to 20th cycle to the 85th to 105th cycle as a result of the accumulation of an SEI layer on the host material (Fig. 2b–e). At the 10th cycle, in detail, the overpotential was ∼80 mV in Cu foil, ∼50 mV in MSP-20, and ∼40 mV in CMK-3 (Fig. 2c). Both carbon electrodes showed lower overpotential than the Cu foil electrode; the difference may be a result of the high surface area of the carbon host materials. The CMK-3 host showed a lower overpotential than MSP-20; this difference may be a result of more facile ion diffusion in CMK-3 than in MSP-20 due to the larger pore size, so CMK-3 could trap Li metals in the electrode layer. At the 100th cycle, the overpotential increased to ∼130 mV in Cu foil, ∼110 mV in MSP-20, and ∼95 mV in CMK-3 (Fig. 2d). After the Cu foil and MSP-20 half-cells failed, the overpotential of CMK-3 remained at ∼100 mV even at the 200th cycle (Fig. S5†).
According to the studies on the relationship between voltage profile and Li dendrite growth,11,32 Li plating curve is based on (1) Li nucleation under the preformed SEI layer (peak at capacity = 0–0.2 mA h cm−2), (2) Li+ diffusion through the SEI layer (1st plateau around capacity = 0.2–0.8 mA h cm−2), and (3) the plateau at new potential range, contributed by the additional Li stripping from the counter-electrode after exhaustion of the deposited Li metal in previous Li plating (2nd plateau around capacity = 0.8–1.0 mA h cm−2). Charge–discharge profiles in Fig. 2e show that the 2nd plateau forms in earlier cycles in Cu foil and MSP-20 electrodes at the 100th cycle. This result indicates that CMK-3 has effectively suppressed the Li dendrite formation and minimized the loss of Li metal, while Cu foil and MSP-20 could not.
We initially expected that a high surface area could increase τ and stabilize Li metal formation. However, the cycle test showed that cycling stability was higher in the CMK-3 (surface area: 1120 m2 g−1) half-cell than in the MSP-20 (surface area: 2110 m2 g−1) and Cu foil half-cells. This result suggests that τ is not the only factor that affects Li metal plating and striping; i.e., that morphologies and pore size also affect the electrochemical performance of Li metal anodes.
The reason for the poor cycle life of MSP-20 might be due to the formation of a large amount of SEI on the wide surface of MSP-20. The surface area of MSP-20 is ∼2200 m2 g−1, which is about twice larger than the surface area of CMK-3. SEI formation on the large surface of MSP-20 particles can lower the electrical conductivity of electrode. During subsequent cycles, electrical conductivity of MSP-20 might decrease more than Cu foil and CMK-3 through a larger amount of electrolyte decomposition and SEI accumulation. Also, clogging of micropores can lower the active surface area of MSP-20 for Li metal plating by inducing the Li dendrite formation.
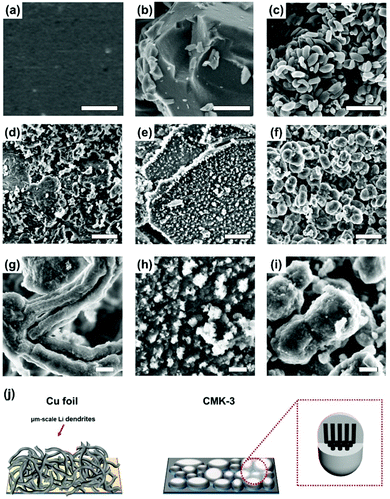
To further investigate the Li metal formation on different host materials, we captured the SEM images of each electrode after its 50th lithiation (Fig. 3). In the Cu foil half-cell, Li dendrites with their micrometer size covered the entire foil, as has been reported previously.19,34 In the SEM images of porous carbon hosts, micrometer-sized Li dendrites were not observed; this result means that the porous structure of amorphous carbons effectively suppressed the growth of Li dendrites so that they did not exceed the nanometer scale.
Li dendrites grew differently on the two types of porous carbon. From the SEM images of the MSP-20 electrode, after its 50th lithiation, a large portion of Li metal formed a number of nanosized weed-like Li dendrites on the surfaces of MSP-20 particles (Fig. 3e and h). This may be a result of the restricted ability of Li+ to penetrate the small pores (<2 nm) of MSP-20 particles due to the small sized pores and accumulating SEI layer. In contrast, very few nanosized weed-like Li dendrites were formed on the CMK-3 particles (Fig. 3f and i). Also, the size of CMK-3 particles after 50 cycles increased to 500 nm–1 μm (initial size: 300–600 nm) whose size is larger than that of the bare CMK-3 particles (Fig. 3c and S1f–h†); this indicates that Li metal was formed not only inside of the channel-type pores (3.4 nm) but also outside of the particles of CMK-3, smoothly. This result indicates that introducing micropores into the host material can decrease Jeff, but is not effective in extending cycling life, because the pores become easily clogged. Therefore, this SEM study suggests that the nanostructure is an important factor for the design of stable Li metal host materials.
To quantify the cycling stability at high Li metal loading, we increased the capacity cut to 2.5 mA h cm−2 for 50 cycles while fixing the current density to 1 mA cm−2 (Fig. 4a, S6, and S7†). The result was similar to the cycling stability at 1 mA h cm−2 capacity cut (Fig. 2a). The MSP-20 and CMK-3 half-cells had a low CE in the first cycle because of Li intercalation into carbon and SEI formation, but they increased to 90% after the initial cycles. The CEs of the Cu foil and MSP-20 half-cells started to decrease after the 30th cycle, whereas those of CMK-3 remained stable even at the 50th cycle. Also, under high Li loading, CMK-3 showed a lower overpotential than Cu foil and MSP-20 (Fig. S5†). The SEM images of CMK-3 after 50 cycles showed that its surface was still smooth, implying that excess Li metal was smoothly plated between particles, and thereby the formation of micrometer-scale Li dendrites was suppressed (Fig. 4b and c).
To investigate whether CMK-3 can work as an effective host material even at a higher Li plating rate, we tested the Cu foil, MSP-20, and CMK-3 half-cells at rate = 3 mA cm−2 and capacity cut = 1 mA h cm−2 for 100 cycles (Fig. S8†). Similar to Fig. S4 and S6,† the initial cycle showed a low CE because of Li+ intercalation into carbon matrix and SEI formation (Fig. S8b†). The CEs of the Cu foil and MSP-20 faded rapidly and reached CE = 60% at ∼43rd cycle and the half-cells failed before the 60th cycle. In contrast, the CE of CMK-3 faded much slowly and the half-cell became unstable at ∼87th cycle.
The overpotential of each half-cell after the 2nd cycle (Fig. S8c–e†) at rate = 3 mA cm−2 was also checked to see the trend of overpotential change by subsequent cycles. At the 10th cycle, the overpotential was ∼85 mV in Cu foil, ∼70 mV in MSP-20, and ∼65 mV in CMK-3 (Fig. S8c†), similar to the trend at the 10th cycle at rate = 1 mA cm−2. The overpotential of Cu foil and MSP-20 rapidly increased to >110 mV from the 25th cycle, while CMK-3 showed ∼75 mV even after the 50th cycle (Fig. S8d and e†).
To further investigate the Li metal morphologies at a high rate condition (3 mA cm−2), the SEM images of each cell after the 50th Li plating were captured (Fig. S9†). Compared to those of the 1 mA cm−2 condition, images of Cu foil and MSP-20 electrodes show more severe SEI formation on the electrodes (Fig. S9a, b, d, and e†). The SEI layer on MSP-20 covered all MSP-20 particles and shows dendrite-shaped SEI formation after cycles, which is different from the shape of SEI at slow rate conditions (Fig. 3e and h). Contrary to Cu foil and MSP-20, Li metal was smoothly plated on CMK-3 particles without forming any Li dendrites (Fig. S9c and f†). The Li metal seems to be plated in the pores of CMK-3 and also between CMK-3 particles, linking the particles together.
To check the anode capability in a full-cell system, we prepared Li-plated Cu foil (Li-Cu), Li-plated MSP-20 (Li-MSP-20) and Li-plated CMK-3 (Li-CMK-3) electrodes (2.5 mA h cm−2 metal loading). For the realization of Li–S battery, a sulfur based cathode was prepared by mixing sulfur and reduced graphene oxide (rGO) in 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 wt%. At 0.5C rate, the capacity increase of all electrodes at the initial stage is attributed to the insulating nature of sulfur in the rGO framework that requires an activation process to gradually utilize the active material (Fig. 4d–g).59 After 200 cycles, the discharge capacities of Li-Cu and Li-MSP-20 based cells are 380 and 140 mA h g−1, respectively. Compared to the maximum discharge capacities, only 55.5% and 17.2% of the capacity are maintained in Li-Cu and Li-MSP-20 based cells. On the contrary, the cell with the Li-CMK-3 anode exhibits a discharge capacity of 700 mA h g−1 even at the 200th cycle, with a retention ratio of 86% and a capacity decay per cycle of 0.07%. Also, the CEs of Li-CMK-3 were ∼100% in most cycles even at the discharge capacity >700 mA h g−1. The superior cycling stability of Li–S cell using Li-CMK-3 anode compared to using Li-Cu and Li-MSP-20 anodes is mainly attributed to the stabilized Li plating/stripping without the formation of uncontrolled Li dendrite in the anode.
30 wt%. At 0.5C rate, the capacity increase of all electrodes at the initial stage is attributed to the insulating nature of sulfur in the rGO framework that requires an activation process to gradually utilize the active material (Fig. 4d–g).59 After 200 cycles, the discharge capacities of Li-Cu and Li-MSP-20 based cells are 380 and 140 mA h g−1, respectively. Compared to the maximum discharge capacities, only 55.5% and 17.2% of the capacity are maintained in Li-Cu and Li-MSP-20 based cells. On the contrary, the cell with the Li-CMK-3 anode exhibits a discharge capacity of 700 mA h g−1 even at the 200th cycle, with a retention ratio of 86% and a capacity decay per cycle of 0.07%. Also, the CEs of Li-CMK-3 were ∼100% in most cycles even at the discharge capacity >700 mA h g−1. The superior cycling stability of Li–S cell using Li-CMK-3 anode compared to using Li-Cu and Li-MSP-20 anodes is mainly attributed to the stabilized Li plating/stripping without the formation of uncontrolled Li dendrite in the anode.
For further investigation of the Li-CMK-3 full cell, rate performance of Li-CMK-3 from 0.1C to 2C was tested (Fig. S10†). The initial capacity was ∼1100 mA h g−1 and became lower as the rate increased, showing ∼670 mA h g−1 at 2C rate. Capacity at 0.5C was ∼810 mA h g−1 and maintained ∼800 mA h g−1 at the 25th cycle where 2C rate cycle ended, which shows a similar result of capacity in 0.5C cycle test (Fig. 4d).
The electrochemical performance of Li metal in CMK-3 can be further optimized by changing the electrolyte system. Therefore, we plated the Li metal into CMK-3 by using 1 M LiNO3 in the tetraethyleneglycol dimethylether (TEGDME) electrolyte (Fig. S11†). LiNO3 is a well-known additive or Li salt for LMB; it effectively suppresses the growth of Li dendrites and yields round and smooth Li metal deposits.14 With this electrolyte, the CE of CMK-3 after the 3rd cycle was ∼99.9% and the overpotential was maintained at ∼22 mV even at the 50th cycle. The SEM images of a CMK-3 particle after plating Li metal under 2.5 mA h cm−2 (Fig. S11c and d†) indicate that Li metal was well confined in the mesopores and smoothly covered the particles of CMK-3. In addition, nanosized weed-like Li dendrites were not observed on the surface of Li plated CMK-3.
In summary, we applied two different nanoporous carbons to check the surface area and pore size effects on Li metal plating and stripping. Even though the large surface area of microporous carbon can extend the Sand's time compared to that of mesoporous carbon, CMK-3 showed more improved electrochemical performance, possibly because the large pores and well aligned porosity increased the accessibility of Li ions and increased space for the formation of Li metal. The Li metal smoothly covered the CMK-3 particles without forming micrometer-scale Li dendrites, and the excess Li metal was smoothly plated between CMK-3 particles even when metal loading was high. Due to the morphological merits, the CMK-3 electrode plated with Li metal exhibited improved electrochemical performance as the anode in a Li–S battery. Furthermore, optimization using an appropriate electrolyte may further suppress the growth of Li dendrite on CMK-3 and thereby improve the electrochemical performance in next-generation battery systems.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT, Korea) (2020R1A2C3004146) and supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) (No. 20182010600430).Notes and references
- G. E. Blomgren, J. Electrochem. Soc., 2016, 164, A5019 CrossRef.
- K. Liu, Y. Liu, D. Lin, A. Pei and Y. Cui, Sci. Adv., 2018, 4, eaas9820 CrossRef PubMed.
- Y. Nishi, Chem. Rec., 2001, 1, 406–413 CrossRef CAS PubMed.
- D. Kundu, E. Talaie, V. Duffort and L. F. Nazar, Angew. Chem., Int. Ed., 2015, 54, 3431–3448 CrossRef CAS PubMed.
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J.-M. Tarascon, Nat. Mater., 2012, 11, 19–29 CrossRef CAS PubMed.
- H. J. Peng, J. Q. Huang, X. B. Cheng and Q. Zhang, Adv. Energy Mater., 2017, 7, 1700260 CrossRef.
- W. Xu, J. Wang, F. Ding, X. Chen, E. Nasybulin, Y. Zhang and J.-G. Zhang, Energy Environ. Sci., 2014, 7, 513–537 RSC.
- Y. Zhang, W. Luo, C. Wang, Y. Li, C. Chen, J. Song, J. Dai, E. M. Hitz, S. Xu and C. Yang, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 3584–3589 CrossRef CAS PubMed.
- Q. Wang, P. Ping, X. Zhao, G. Chu, J. Sun and C. Chen, J. Power Sources, 2012, 208, 210–224 CrossRef CAS.
- D. Aurbach, E. Zinigrad, Y. Cohen and H. Teller, Solid State Ionics, 2002, 148, 405–416 CrossRef CAS.
- K.-H. Chen, K. N. Wood, E. Kazyak, W. S. LePage, A. L. Davis, A. J. Sanchez and N. P. Dasgupta, J. Mater. Chem. A, 2017, 5, 11671–11681 RSC.
- C. Niu, H. Lee, S. Chen, Q. Li, J. Du, W. Xu, J.-G. Zhang, M. S. Whittingham, J. Xiao and J. Liu, Nat. Energy, 2019, 4, 551–559 CrossRef CAS.
- J. Zhao, G. Zhou, K. Yan, J. Xie, Y. Li, L. Liao, Y. Jin, K. Liu, P.-C. Hsu and J. Wang, Nat. Nanotechnol., 2017, 12, 993–999 CrossRef CAS PubMed.
- Y. Liu, D. Lin, Y. Li, G. Chen, A. Pei, O. Nix, Y. Li and Y. Cui, Nat. Commun., 2018, 9, 1–10 CrossRef PubMed.
- X. Q. Zhang, X. B. Cheng, X. Chen, C. Yan and Q. Zhang, Adv. Funct. Mater., 2017, 27, 1605989 CrossRef.
- E. Markevich, G. Salitra, F. Chesneau, M. Schmidt and D. Aurbach, ACS Energy Lett., 2017, 2, 1321–1326 CrossRef CAS.
- F. Ding, W. Xu, G. L. Graff, J. Zhang, M. L. Sushko, X. Chen, Y. Shao, M. H. Engelhard, Z. Nie and J. Xiao, J. Am. Chem. Soc., 2013, 135, 4450–4456 CrossRef CAS PubMed.
- J.-S. Kim, D. W. Kim, H. T. Jung and J. W. Choi, Chem. Mater., 2015, 27, 2780–2787 CrossRef CAS.
- J. Xie, L. Liao, Y. Gong, Y. Li, F. Shi, A. Pei, J. Sun, R. Zhang, B. Kong and R. Subbaraman, Sci. Adv., 2017, 3, eaao3170 CrossRef PubMed.
- L. Shi, A. Xu and T. Zhao, ACS Appl. Mater. Interfaces, 2017, 9, 1987–1994 CrossRef CAS PubMed.
- A. C. Kozen, C.-F. Lin, O. Zhao, S. B. Lee, G. W. Rubloff and M. Noked, Chem. Mater., 2017, 29, 6298–6307 CrossRef CAS.
- K. Liu, D. Zhuo, H. W. Lee, W. Liu, D. Lin, Y. Lu and Y. Cui, Adv. Mater., 2017, 29, 1603987 CrossRef PubMed.
- N. Zhang, B. Li, S. Li and S. Yang, Adv. Energy Mater., 2018, 8, 1703124 CrossRef.
- S. Bai, X. Liu, K. Zhu, S. Wu and H. Zhou, Nat. Energy, 2016, 1, 1–6 Search PubMed.
- Y. He, Z. Chang, S. Wu, Y. Qiao, S. Bai, K. Jiang, P. He and H. Zhou, Adv. Energy Mater., 2018, 8, 1802130 CrossRef.
- S. Kim, W.-G. Lim, A. Cho, J. Jeong, C. Jo, D. Kang, S. M. Han, J. W. Han and J. Lee, ACS Appl. Energy Mater., 2020, 3, 2643–2652 CrossRef CAS.
- J. Hu, J. Tian and C. Li, ACS Appl. Mater. Interfaces, 2017, 9, 11615–11625 CrossRef CAS PubMed.
- Y.-S. Lee, S. H. Ju, J.-H. Kim, S. S. Hwang, J.-M. Choi, Y.-K. Sun, H. Kim, B. Scrosati and D.-W. Kim, Electrochem. Commun., 2012, 17, 18–21 CrossRef CAS.
- G. Jiang, S. Maeda, H. Yang, Y. Saito, S. Tanase and T. Sakai, J. Power Sources, 2005, 141, 143–148 CrossRef CAS.
- Z. Liang, G. Zheng, C. Liu, N. Liu, W. Li, K. Yan, H. Yao, P.-C. Hsu, S. Chu and Y. Cui, Nano Lett., 2015, 15, 2910–2916 CrossRef CAS PubMed.
- K. Yan, Z. Lu, H.-W. Lee, F. Xiong, P.-C. Hsu, Y. Li, J. Zhao, S. Chu and Y. Cui, Nat. Energy, 2016, 1, 1–8 Search PubMed.
- Z. Liang, D. Lin, J. Zhao, Z. Lu, Y. Liu, C. Liu, Y. Lu, H. Wang, K. Yan and X. Tao, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 2862–2867 CrossRef CAS PubMed.
- H. Yuan, J. Nai, H. Tian, Z. Ju, W. Zhang, Y. Liu, X. Tao and X. W. Lou, Sci. Adv., 2020, 6, eaaz3112 CrossRef PubMed.
- K. Xie, W. Wei, K. Yuan, W. Lu, M. Guo, Z. Li, Q. Song, X. Liu, J.-G. Wang and C. Shen, ACS Appl. Mater. Interfaces, 2016, 8, 26091–26097 CrossRef CAS PubMed.
- S. S. Chi, Y. Liu, W. L. Song, L. Z. Fan and Q. Zhang, Adv. Funct. Mater., 2017, 27, 1700348 CrossRef.
- H. Zheng, Q. Zhang, Q. Chen, W. Xu, Q. Xie, Y. Cai, Y. Ma, Z. Qiao, Q. Luo, J. Lin, L. Wang, B. Qu, B. Sa and D.-L. Peng, J. Mater. Chem. A, 2020, 8, 313–322 RSC.
- P. Yao, Q. Chen, Y. Mu, J. Liang, X. Li, X. Liu, Y. Wang, B. Zhu and J. Zhu, Mater. Chem. Front., 2019, 3, 339–343 RSC.
- D. Lin, Y. Liu, Z. Liang, H.-W. Lee, J. Sun, H. Wang, K. Yan, J. Xie and Y. Cui, Nat. Nanotechnol., 2016, 11, 626–632 CrossRef CAS PubMed.
- F. Liu, R. Xu, Z. Hu, S. Ye, S. Zeng, Y. Yao, S. Li and Y. Yu, Small, 2019, 15, 1803734 CrossRef PubMed.
- J. Duan, Y. Zheng, W. Luo, W. Wu, T. Wang, Y. Xie, S. Li, J. Li and Y. Huang, Natl. Sci. Rev., 2020 DOI:10.1093/nsr/nwz222.
- X. Chen, X.-R. Chen, T.-Z. Hou, B.-Q. Li, X.-B. Cheng, R. Zhang and Q. Zhang, Sci. Adv., 2019, 5, eaau7728 CrossRef CAS PubMed.
- D. Cao, Y. Jiao, Q. Cai, D. Han, Q. Zhang, Y. Ma, A. Dong and H. Zhu, J. Mater. Chem. A, 2019, 7, 3289–3297 RSC.
- G. Zheng, S. W. Lee, Z. Liang, H.-W. Lee, K. Yan, H. Yao, H. Wang, W. Li, S. Chu and Y. Cui, Nat. Nanotechnol., 2014, 9, 618–623 CrossRef CAS PubMed.
- J. Lang, Y. Jin, X. Luo, Z. Liu, J. Song, Y. Long, L. Qi, M. Fang, Z. Li and H. Wu, J. Mater. Chem. A, 2017, 5, 19168–19174 RSC.
- C. Jo, Y. Park, J. Jeong, K. T. Lee and J. Lee, ACS Appl. Mater. Interfaces, 2015, 7, 11748–11754 CrossRef CAS PubMed.
- C. Brissot, M. Rosso, J.-N. Chazalviel, P. Baudry and S. Lascaud, Electrochim. Acta, 1998, 43, 1569–1574 CrossRef CAS.
- J. Qian, W. A. Henderson, W. Xu, P. Bhattacharya, M. Engelhard, O. Borodin and J.-G. Zhang, Nat. Commun., 2015, 6, 1–9 Search PubMed.
- Y. Lee, J. Lee, J. Lee, K. Kim, A. Cha, S. Kang, T. Wi, S. J. Kang, H.-W. Lee and N.-S. Choi, ACS Appl. Mater. Interfaces, 2018, 10, 15270–15280 CrossRef CAS PubMed.
- E. Lim, C. Jo and J. Lee, Nanoscale, 2016, 8, 7827–7833 RSC.
- T. Kim, C. Jo, W.-G. Lim, J. Lee, J. Lee and K.-H. Lee, Carbon, 2016, 104, 106–111 CrossRef CAS.
- X. Zhou, L.-J. Wan and Y.-G. Guo, Nanoscale, 2012, 4, 5868–5871 RSC.
- X. Xu, Z. Fan, X. Yu, S. Ding, D. Yu and X. W. Lou, Adv. Energy Mater., 2014, 4, 1400902 CrossRef.
- W.-G. Lim, C. Jo, J. Lee and D. S. Hwang, Korean J. Chem. Eng., 2018, 35, 579–586 CrossRef CAS.
- H. Liu, X. Chen, X.-B. Cheng, B.-Q. Li, R. Zhang, B. Wang, X. Chen and Q. Zhang, Small Methods, 2019, 3, 1800354 CrossRef.
- H. Zhang, X. Liao, Y. Guan, Y. Xiang, M. Li, W. Zhang, X. Zhu, H. Ming, L. Lu, J. Qiu, Y. Huang, G. Cao, Y. Yang, L. Mai, Y. Zhao and H. Zhang, Nat. Commun., 2018, 9, 3729 CrossRef PubMed.
- N. Zhang, S.-H. Yu and H. D. Abruña, Chem. Commun., 2019, 55, 10124–10127 RSC.
- J. Schwan, S. Ulrich, V. Batori, H. Ehrhardt and S. Silva, J. Appl. Phys., 1996, 80, 440–447 CrossRef CAS.
- A. Cuesta, P. Dhamelincourt, J. Laureyns, A. Martinez-Alonso and J. D. Tascón, Carbon, 1994, 32, 1523–1532 CrossRef CAS.
- S. Lu, Y. Chen, X. Wu, Z. Wang and Y. Li, Sci. Rep., 2014, 4, 4629 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental process and supplementary figures. See DOI: 10.1039/d0nr02258f |
| This journal is © The Royal Society of Chemistry 2020 |