Open Access Article
Open Access ArticleAdvances in the green chemistry of coordination polymer materials
Emile R.
Engel
 *ab and
Janet L.
Scott
*ab and
Janet L.
Scott
 a
a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: ee380@bath.ac.uk
bDepartment of Fibre and Polymer Technology, KTH Royal Institute of Technology, SE-100 44 Stockholm, Sweden
First published on 19th May 2020
Abstract
Coordination polymers, including metal–organic frameworks, are a diverse class of materials with myriad properties and potential applications. However, a number of drawbacks have hindered the scaling up of such materials towards commercial applications. These include health and safety risks, environmental hazards, poor cost efficiency and sustainability shortfalls. In contrast to the systematic progress of organic green chemistry, which has contributed to improvements in the sustainability of chemical processing, the development of green chemistry in the context of coordination polymers has been fragmented and sporadic. This review describes advances in the use of green components: benign sustainable ligands and non-hazardous earth abundant metals. Additionally, solvent considerations, synthesis strategies for improved sustainability and the performance of coordination polymers relative to alternative competing materials are discussed.
Introduction
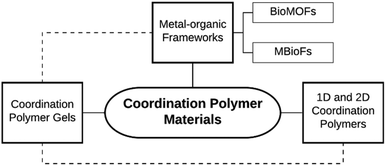
Coordination polymers (CPs) are metal–ligand coordination compounds that form one-dimensional (1D) extended chains, two-dimensional (2D) sheets or three-dimensional (3D) frameworks. There are two recognised sub-classes: “coordination networks”, which are 2D, 3D or crosslinked 1D CPs, and “metal–organic frameworks” (MOFs) (also referred to as “porous coordination polymers”), which are coordination networks that contain potential voids.1 Metallopolymers are a distinct class of organic polymers incorporating metal ions into the backbone or side chains.2 “Inorganic–organic hybrid materials” is a related term, which may be appropriate when referring to CPs that resemble extended inorganic compounds;3 however, in accordance with the approach of Biradha et al.,4 we have disregarded this term to avoid unnecessary confusion and use a simplified taxonomy as illustrated in Fig. 1.Metal biomolecule frameworks (MBioFs) has been proposed as a sub-class of MOFs, where the MOF incorporates “at least one biomolecule” as organic linker and where “biomolecule” refers to naturally-occurring molecules found in living organisms, such as amino acids, nucleobases or sugars.5 To be clear, under strict application of this definition, a MBioF may incorporate any metal, including hazardous metal ions, and additional ligands that are not biomolecules. A similar term, BioMOFs, is defined as “metal–organic frameworks for biological and medical applications”.6,7 Here the focus is on biocompatibility such that these can be used for applications involving living organisms, including humans. Concern for biological safety does not explicitly include all aspects relating to the principles of green chemistry, such as the minimisation of waste or the elimination of hazardous solvents used during synthesis. Nevertheless, CPs that qualify as BioMOFs tend to comprise environmentally benign components, such as MIL-88A, which is constructed from Fe(III) and fumaric acid.8 The prefix “MIL” is used for a variety of porous 3D CPs discovered by researchers at Materials Institute Lavoisier.9 “Bio-based MOFs” is an emerging category that has not yet been explicitly defined.
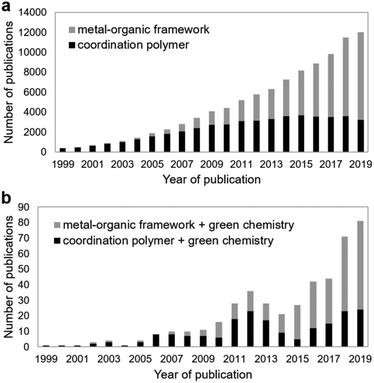
Within the field of CP materials, MOFs have received the bulk of research attention in recent years. Fig. 2a shows the number of publications in a given year containing the term “coordination polymer” and those containing “metal–organic framework” in the title, abstract or keywords. The term “metal–organic framework” surpassed “coordination polymer” in 2014 and is now clearly dominant.
In addition to (usually) crystalline and structurally well-defined solid MOFs and CPs, CP gels are an important class of metal–organic materials. A CP may form a network of interacting particles or crystallites within a solvent medium, immobilising the solvent to form a gel.10–12 The solvent usually makes up over 95 wt% of the gel and CP gels may be classified as hydrogels or organogels for water and organic solvent-containing materials, respectively.
Green chemistry was initially developed with organic synthesis and industrial chemical processes in mind.13 In comparison, sustainable approaches to the chemistry of metal–organic materials have emerged rather indirectly. Fig. 2b shows publications that include the term “green chemistry” alongside “coordination polymer” or “metal–organic framework”. There were only 81 such articles in 2019, which is a mere 0.7% of all CP and MOF articles published that year. Systematic, rigorous endeavours to develop the green chemistry of CPs have been limited, which may explain why progress in this area has been fragmented and sporadic. However, if CPs are to move from laboratory curiosities to functional materials enabling sustainable technologies a focus on green CP chemistry is critical.
Numerous applications have been proposed for CPs, including catalysis,14,15 gas capture,16,17 molecular separations18–21 and sensing.22,23 Given their wide-ranging potential, and considering the imperative to incorporate sustainability into the design of new materials, the development of green CPs is essential as are sustainable approaches to their preparation. An obvious approach would be to design CPs according to the 12 Principles of Green Chemistry. Despite this, an authoritative 1999 review “Green Chemistry: Challenges and Opportunities” contained no mention of CPs or metal–organic products.24 Similarly, the 2009 review “Green Chemistry: Principles and Practice”, included no references to CPs.25 Even recently, major aspects of green chemistry have been reviewed,26,27 where the authors did not cite any examples of metal–organic or CP materials. A number of reviews have addressed environmental sustainability and biocompatibility aspects of CPs. These have focussed on specific aspects, such as the green synthesis of well-known MOFs exclusively28 and ionic liquids and supercritical CO2 as green solvents in MOF synthesis.29 Friščić et al. have reviewed mechanochemistry as a green alternative to conventional solution-based synthesis of CPs,30 while Chen et al. addressed the green chemistry of MOFs in the context of sustainable catalysis.15 The prospects for application of MOFs in various “environmentally conscious applications” have been discussed by Ajoyan et al.31 Very recently, cyclodextrin-based MOFs, considered inherently sustainable, were covered by Rajkumar and co-workers.32 The present review is an attempt to consolidate advances in the green chemistry of CP materials in general.
In determining how “green” a CP material is, the following factors should be considered:
• What hazards are associated with the ligand? Ideally the ligand should be non-hazardous to the environment and to human health, and should not pose any safety risks (e.g. explosivity).
• Is the ligand obtainable from renewable and sustainable resources via green processes? Non-fossil fuel-based ligands are generally preferable.
• What hazards are associated with the metal ion? Ideally the metal ion should be non-hazardous to the environment and to human health, and should not pose any safety risks.
• How earth abundant is the metal? Earth abundant metals are preferred.
• Can the CP be prepared by a green chemistry procedure?
Sustainable approaches to the production and use of CPs span a variety of strategies. In this review we consider aspects of the most common strategies, including: (1) appropriate ligand selection; (2) appropriate metal ion selection; (3) solvent minimisation or the use of benign solvents and (4) specific techniques for green synthesis.
Ligand selection
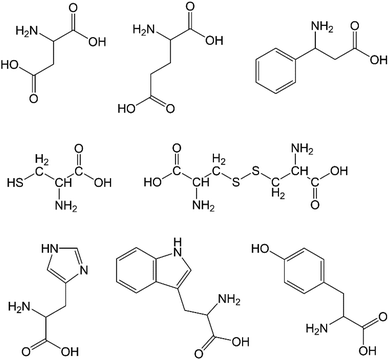
CPs incorporate a variety of synthetic and naturally-occurring ligands. The earliest high surface area, thermally stable MOFs employed rigid ligands bearing carboxylate, imidazolate or pyridyl moieties, often incorporating aromatic groups or polyaromatic spacers.33 Such rigid aromatic ligands are usually fossil carbon-based and some may be harmful to humans and the environment, but are useful for preparing rigid 3D networks with permanent porosity and high internal surface areas. Engineering permanent porosity has been a major focus of MOF research; however, research on “soft porous crystals”34 has grown, where ligand flexibility and labile coordination bonds are of interest, and there remains huge potential for materials based on 1D and 2D CPs.Naturally-occurring and bio-based ligands for CPs have received growing attention as green chemistry approaches have gained traction and as researchers have broken with the received wisdom that only rigid 3D MOFs have potential as new technologies. ‘Green ligands’ should be obtainable from renewable sources (e.g. biomass), and be environmentally benign and biodegradable, unless recycling of the CP is feasible. Among the most widely exploited are aliphatic diacids, amino acids and nucleobases. Beobide and co-authors have produced a useful review of metal–carboxylato–nucleobase architectures.44 Here we focus on selected aliphatic diacids, amino acids and cyclodextrins as examples of important green ligands (Table 1). We highlight a range of existing CPs based on these ligands to illustrate their utility. However, many of these examples incorporate unfavourable metal ions and would not be considered green CPs. A great deal more work is required to match sustainable ligands and metals. Metal ion selection will be discussed separately.
| Type | Ligand | Commercial availability | Safety | Comments |
|---|---|---|---|---|
| Aliphatic diacids | Succinic acid | Large scale production by industrial fermentation35,36 | Readily biodegradable; causes serious eye damage (H318) | Good water solubility lends itself to CP synthesis in aqueous medium |
| Adipic acid | Not yet available in large quantities from bio-based production but considerable research efforts are underway36–38 | Harmful to aquatic life but readily biodegradable; causes serious eye damage (H318) | The leading industrial aliphatic dicarboxylic acid because of its use in Nylon 6,6 production | |
| Itaconic acid | Considered a niche commodity chemical but available at large scale via fermentation36,39 | Harmful to aquatic life but readily biodegradable; causes serious eye damage (H318) | Underexplored as a CP ligand. Only limited examples have been reported | |
| 2,5-Furandicarboxylic acid | A leading bio-based platform chemical produced in very large quantities from biomass but not via green synthesis40 | Readily biodegradable; causes serious eye irritation (H319) | Employed as a terephthalic acid alternative because of its structural similarity | |
| Oxalic acid | Large scale production from biomass but not via green synthesis41 | Readily biodegradable; harmful if swallowed (H302) or in contact with skin (H312) and causes serious eye irritation (H319) | Mostly present in mixed ligand CPs | |
| Amino acids | Aspartic acid | Available from industrial enzymatic methods42 | Not a hazardous substance | Used in the production of aspartame and polyaspartic acid, a biodegradable polymer |
| Glutamic acid | The most important industrial amino acid produced in very large quantities as L-glutamate by fermentation42 | Not a hazardous substance | Widely used as a food additive | |
| Phenylalanine | Available from industrial fermentation42 | Not a hazardous substance | Used in nutritional applications, such as aspartame production | |
| Cysteine | Available from industrial enzymatic methods42 | Harmful if swallowed (H302) | Dimerises to cystine in alkaline medium | |
| Cystine | Available from industrial enzymatic methods42 | Not a hazardous substance | — | |
| Histidine | Available from industrial fermentation42 | Not a hazardous substance | — | |
| Tryptophan | Available from industrial fermentation and enzymatic methods42 | Not a hazardous substance | — | |
| Tyrosine | Available from industrial fermentation42 | Not a hazardous substance | — | |
| Cyclodextrins | α-Cyclodextrin | Large scale production by enzymatic conversion43 | Not a hazardous substance | Good water solubility:43 145 g L−1 |
| β-Cyclodextrin | Large scale production by enzymatic conversion43 | Not a hazardous substance | Poor water solubility:43 18.5 g L−1 | |
| γ-Cyclodextrin | Large scale production by enzymatic conversion43 | Not a hazardous substance | Good water solubility:43 232 g L−1 |
Aliphatic diacids
Key aliphatic diacids employed as green ligands in CP materials are shown in Fig. 3. The unsaturated linear aliphatic dicarboxylic acids are the series with general formula HOOC–[CH2]n–COOH. Those that are readily available as commodity or fine chemicals range from the smallest, oxalic acid, with n = 0 to hexadecanedioic acid, with n = 14. Most are considered to be of low risk, are biodegradable and some are already available by fermentation from bio-based feedstocks as commodity chemicals.36 Adipic acid and oxalic acid are important CP ligands that are candidates for industrial production by fermentation. Industrial fermentation processes for adipic acid production36,37 are advanced as compared with fermentation-based oxalic acid, which is not yet commercially viable.41 Beyond the HOOC–[CH2]n–COOH series, itaconic acid and 2,5-furandicarboxylic acid are bio-based ligands that are produced at large scale and have been explored for the construction of CPs. Large quantities of itaconic acid are produced by fermentation.36,39 2,5-Furandicarboxylic acid is obtainable from biomass at scale but not yet by green synthesis.40 | ||
| Fig. 3 Important aliphatic diacids employed as green ligands, from left to right: oxalic acid, succinic acid, itaconic acid (2-methylenesuccinic acid), adipic acid and 2,5-furandicarboxylic acid. | ||
Rare earth (mainly lanthanide) metal succinate CPs are particularly prevalent in literature and are well documented in the recent review by Bernini et al., which includes mixed ligand CPs.46 Worth noting are the Ho(III) succinate CPs prepared from succinylsalicylic acid, which was hydrolysed in situ47 and a broad series of lanthanide succinate CPs, including 2D architectures and 3D architectures with relatively large calculated solvent-accessible volumes, synthesised with 5-sulfosalicylate as templating agent.48 The near-infrared luminescence of lanthanide succinate and lanthanide p-toluylate-acetate CPs has been explored, with enhancement of luminescence noted for p-toluylate-acetate only, confirming that succinate itself tends not to contribute to electromagnetic properties.49
Of course, transition metal succinate CPs are also common. A Cu(II) succinate 1D CP, reported as a 3D framework based on hydrogen bonding, has been subjected to low temperature magnetic studies.50 Several Co(II) succinate CPs are known, with thirteen unique Cambridge Structural Database (CSD) entries at the time of writing containing Co(II) as the only metal and succinate as the only organic ligand (CSD reference codes: 221317, 830032, 620466, 138985, 257268, 157785, 161392, 165917, 268305, 830034, 156110, 1050327, 1050331). Analysis of results of high throughput screening experiments showed that varying temperature, pH, concentration and time of hydrothermal reactions could produce seven unique Co(II) succinate CPs, two of which were novel at the time.51 Reversible dehydration, where crystallinity is preserved, accompanied by colour change has been demonstrated for a Co(II) succinate CP.52 A systematic study of mixed metal succinates containing Co(II), Fe(II) and Mn(II) found evidence of partial ordering of the different metal cations across the crystallographic metallic nodes of the framework.53 In a separate mixed metal study, by varying the Co/Ni ratio in the reaction mixture, four different metal succinate CP structures (including mixed metal CPs) were isolated from constant reaction conditions (Fig. 4).54 Unusual low temperature magnetic transitions were observed for a Mn(II) CP.55 However, while many succinates have been explored for their magnetic properties, mostly modest results are reported. CPs based on Li, succinic acid, methyl succinic acid and malic acid were prepared by a solvent-free method.56 A rare example of an Ag(I)-based succinate CP was reported in 1993,57 while spontaneous symmetry breaking was achieved to obtain a rare homochiral succinate CP with a uranyl metal centre.58
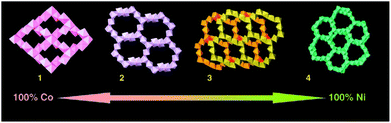 | ||
| Fig. 4 Four crystal architectures of mixed metal succinates based on Co(II) and Ni(II), starting from Co succinate Co5(OH)2(C4H4O4)4 (1) with increasing substitution by Ni: M4(OH)2(H2O)2(C4H4O4)3·2H2O (2), M7(OH)2(H2O)2(C4H4O4)6·3.5H2O (3), and Ni7(OH)2(H2O)2-(C4H4O4)6·2H2O (4). Only the metal polyhedral are represented. Reprinted from ref. 54 with permission from John Wiley and Sons. | ||
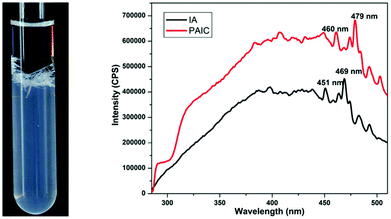 | ||
| Fig. 5 Crystals of a luminescent CP based on Ca and itaconic acid, and solid state luminescence spectra of the ligand (IA) and CP (PAIC) at room temperature with λmax = 285 nm. Reprinted from ref. 67 with permission from Elsevier. | ||
By varying reaction conditions, non-porous 2 mm-sized pale blue crystals and porous turquoise microcrystals of different Cu(II) FDC CPs have been synthesised. Alongside these, CP organogels of Fe(II) FDC and Al(III) FDC were also obtained.68
A number of Zn(II) FDC CPs have been identified as potential drug delivery materials with evidence of nontoxicity. Aqueous stability and 17.9 wt% loading of the chemotherapy drug 5-fluorouracil has been demonstrated for a Zn(II) FDC 3D CP with rectangular channels,69 while for a different Zn(II) FDC 3D CP 22.5 wt% encapsulation of 5-fluorouracil, along with selective CO2 sorption, was reported.70 5-Fluorouracil is of interest because its targeting of cancer cells may be improved by developing innovative controlled release drug delivery strategies. Recently a Zn(II) FDC 2D CP was been described exhibiting 11.4 wt% 5-fluorouracil uptake.71
An unusual cage-within-cage structure based on Zn(II) and FDC was prepared by solvothermal synthesis72 and photoluminescent Zn(II) FDC CPs have been reported.73
Zhai et al. prepared several Mg-based MOFs, including one with FDC as linker, called CPM-203. Of the series of five MOFs tested for gas sorption capacity and selectivity, CPM-203 performed best with relatively high CO2 uptake of 53.1 cm3 g−1 at 273 K and 1 atm, and negligible adsorption of CH4 under the same conditions.74
An impressive series of thirty-four different robust Fe-based CPs were synthesised by a rational approach using [Fe2M(μ3-O)(CH3COO)6] as starting material.76 Among these was PCN-233, based on Fe(III) and FDC, which is also described in a patent covering “iron metal organic framework materials”.77
The formation of crown ether-type secondary building units based on K+ and FDC was observed as part of an investigation of lanthanide FDC CPs.75 These unusual secondary building units resemble 16-crown-6 (Fig. 6) and 23-crown-9. The cation-dependence of crown ether type metallolinkers based on FDC has been systematically investigated by Einkauf and co-workers.79
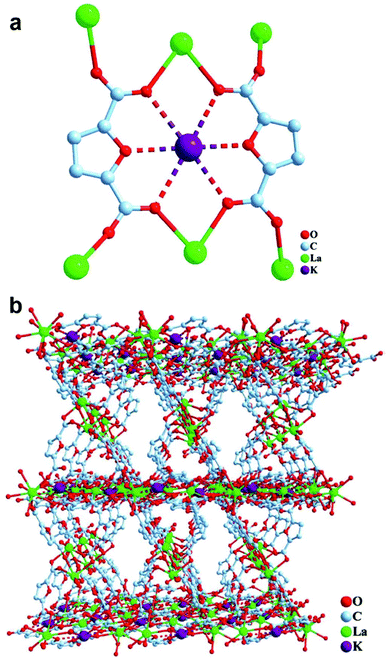 | ||
| Fig. 6 Single-crystal X-ray structure of a 3D lanthanide FDC CP containing K+ ions: (a) secondary building unit resembling 16-crown-6 and (b) packing diagram viewed along [100]. Reprinted from ref. 75 with permission from John Wiley and Sons. | ||
Li et al. investigated two homo- and two heterometallic FDC CPs, based on Co(II), Gd(III) and Dy(III), including magnetic studies and photoluminescence studies.80 Three Sc(III) fumarate MOFs have been described by Barsukova et al. and one of these is thermally stable up to 300 °C and remarkably hydrolytically stable in aqueous medium across the pH range 1–13.81
MIL-160 (Fig. 7) is an Al(III) FDC CP that can be prepared via scalable green synthesis by refluxing in aqueous medium and performs well as a potential water sorbent for heat exchange applications, with superior performance 313 K and 1.2 kPa when compared with other important water sorbents.78 The crystal structure of MIL-160 has been established by synchrotron PXRD.82 The framework exhibits high water sorption capacity and potential use in heat exchange applications with its relatively high energy storage capacity of 343 W h kg−1.78,83
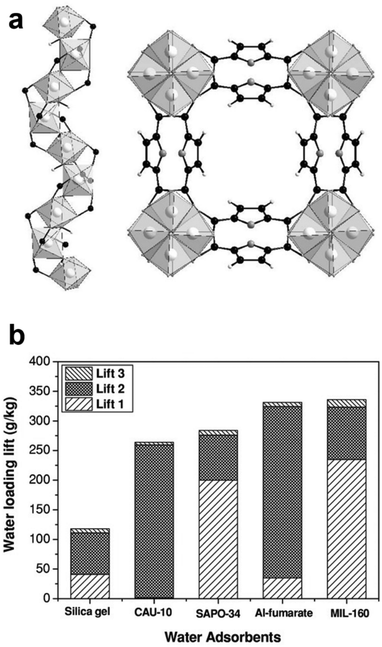 | ||
| Fig. 7 MIL-160, an Al(III) FDC CP. (a) Partial X-ray single crystal structure of MIL-160 with C, H and O atoms represented in black, white and grey, respectively. (b) Gravimetric water loading (three “lifts” of different cycle conditions) for MIL-160 compared with other important water sorbents, showing superior sorption of MIL-160 under lift 1 conditions, 313 K and 1.2 kPa. Reprinted from ref. 78 with permission from John Wiley and Sons. | ||
Amino acids
Natural amino acids, the building blocks of proteins, are a potentially rich source of bio-based ligands. The amino acids represented in Fig. 8 have been important in CP synthesis and are available in varying quantities from industrial fermentation or enzymatic synthesis. L-Glutamic acid is the most important amino acid produced by industrial fermentation, as L-glutamate, at well over 3 million tons per year.42 By far the most widely explored amino acids for CP synthesis are the dicarboxylic acids aspartic acid and glutamic acid. Cysteine poses a challenge due to its propensity to dimerise in alkaline media, but its dimer, cysteine, is also structurally well-suited to CP formation. Phenylalanine, histidine, tyrosine and tryptophan have been employed to a lesser extent.Chiral nanofibres of a Cu(II) aspartate CP (Fig. 9) can be prepared rapidly at room temperature by combining a solution of Cu(NO3)2 and alkaline solution of L- or D-aspartic acid.89 The authors refer to these nanofibres as “infinite CP particles”, which readily form aqueous gels. At a minimum concentration of 0.07 M of the reactants, Cu(NO3)2 and L- or D-aspartic acid, a blue aqueous gel was formed; the gel remained stable for months at ambient temperature. Cu(II) and racemic glutamic acid have also been shown to form CP nanofibres from aqueous solutions at room temperature. When Cu(II) was combined with enantiomerically pure glutamic acid, nanoparticles of varying morphologies were obtained. However; employing racemic glutamic acid as ligand produced relatively uniform nanofibres, regardless of whether the metal source was Cu(NO3)2, CuSO4 or CuCl2. Sorption of Congo red from aqueous solutions and anti-bacterial properties have been demonstrated for this Cu(II) glutamate CP.90 In a later publication, authors described the effect of stereochemistry on the rate of self-assembly of the Cu(II) aspartate and Cu(II) glutamate nanofibres along with a new material, Cu(II) aminoadipate. The presence of both enantiomers of aspartic acid reduced the rate of self-assembly of the Cu(II) aspartate CP compared to the single enantiomer system. As ligand, DL-aspartic acid resolved to form a mixture of chiral CP crystallites of opposite handedness. Achiral nanofibre crystallites were formed when Cu(II) was combined with DL-glutamic acid at ligand concentrations below 0.35 mM, while plate-shaped microcrystals were obtained at ligand concentrations above 0.35 mM. Aqueous gels of this Cu(II)-glutamate could be prepared at relatively high concentrations (280 mM) based on the plate-shaped microcrystals but these gels were weak, and were easily disassembled by agitation.91
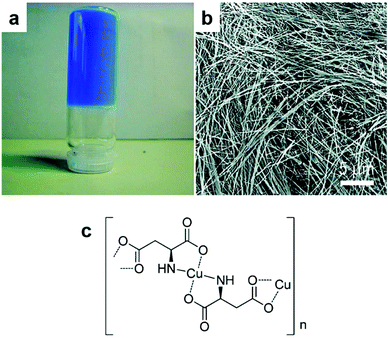 | ||
| Fig. 9 Cu(II)-Aspartate chiral CP: (a) photograph of the CP aqueous gel, (b) FESEM image of the CP nanofibres and (c) proposed molecular structure of the CP. Reprinted with permission from ref. 89. Copyright (2009) American Chemical Society. | ||
Cu(II) also forms chiral CPs and stereochemically-dependent aqueous gels with D- and L-phenylalanine.92 The enantiomeric ratio affects the kinetics of CP self-assembly and gelation. As in the case of Cu(II) L-adipate and Cu-D-adipate, the presence of both enantiomers of phenylalanine inhibits CP self-assembly. The Cu(II) D-phenylalanine and Cu(II) L-phenylalanine CPs have a minimum gelator concentration of 0.35 wt% in water. Phenylalanine CP nanofibres have also been prepared at room temperature by combining an aqueous solution of Zn(NO3) with an aqueous alkaline solution of the enantiopure ligand.93 The nanofibres of Zn(II) D-phenylalinine and Zn(II)-L-phenylalanine were approximately 700–900 nm in diameter, hundreds of micrometers in length and appeared, from SEM images, to have smooth surfaces. When Zn(NO3)2 was combined with racemic phenylaniline, self-assembly resulted in plate-shaped microcrystals. Unfortunately, these nanofibres and microcrystals have typically been too small to allow for the elucidation of single-crystal X-ray structures.
Self-assembly of Ag(I) with enantiomerically pure cysteine yields fibrous “nanobelts” with right- (L-cysteine) and left-handed (D-cysteine) helicity (Fig. 10), while the reaction of Ag(I) with racemic cysteine gives rise to two-dimensional achiral nanosheets.94 While the authors have represented the ligand as cysteine, the CP self-assembly reactions were carried out in alkaline medium and cysteine is well-known to be highly susceptible to oxidative dimerisation to cystine under such conditions. Elsewhere ZnCl2 and L-cystine were combined in aqueous solution to yield well-defined spherical microspheres (Fig. 12). The size and morphology of the crystalline particles was concentration dependent: mixing 1 mM ZnCl2 and 1 mM L-cystine at pH 8 produced microspheres of 5 μm diameter, made up of rod-like crystallites of 50 nm in width and 200 nm in length. These Zn(II) cystine particles were developed into hybrid microspheres by incorporating a porphyrin (TTPS) and alcohol dehydrogenase (ADH). The Zn(II) cystine/TTPS/ADH microspheres exhibit a combination of photocatalytic and biocatalytic activity that mimics the photosynthetic activity of chloroplasts. Replacing Zn(II) with other divalent transition metals yielded alternative morphologies.95
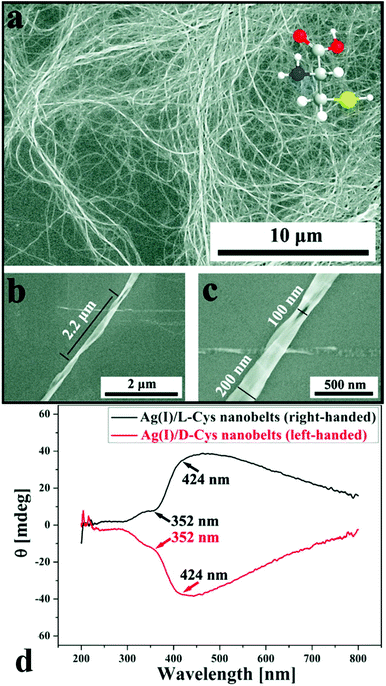 | ||
| Fig. 10 Self-assembled Ag(I) cysteine helical “nanobelts” with homochirality: (a) large-area SEM image and (b, c) high-resolution SEM image of the right-handed (L-cysteine) version. (d) Circular dichroism spectra for the right-handed (L-cysteine) and left-handed (D-cysteine) versions. Reprinted with permission from ref. 94. Copyright (2010) American Chemical Society. | ||
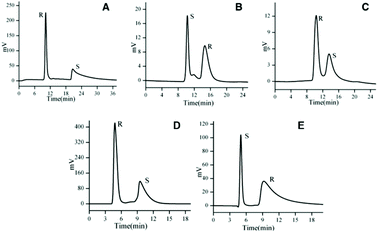 | ||
| Fig. 11 Representative chromatograms showing effective chiral resolution on MOF-based HPLC columns. (A–C) Chromatograms for 1,2-diphenyl-1,2-ethanediol, praziquantel and propranolol, respectively, obtained on a column with MOF-1, based on Zn(II) and L-tyrosine, as stationery phase. (D–E) Chromatograms for 1,2-diphenyl-1,2-ethanediol and chlorprophenpyridamine obtained on a column with MOF-6, based on Co(II) and L-glutamic acid, as stationery phase. MOF-1 and MOF-6 were judged to have outperformed the other 4 MOFs tested. Reprinted from ref. 99 with permission from John Wiley and Sons. | ||
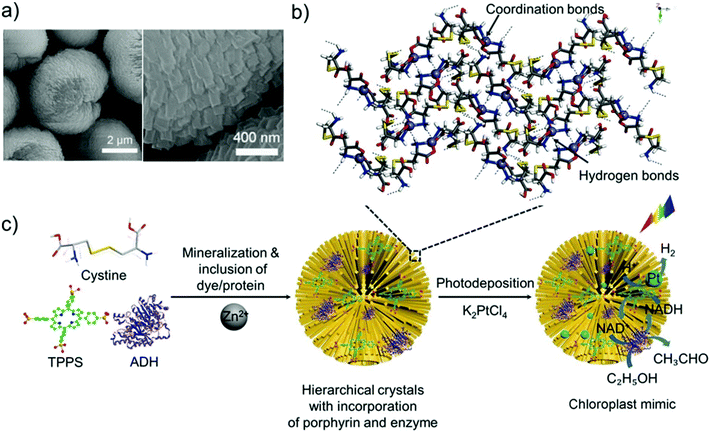 | ||
| Fig. 12 Zn(II) cystine CP microspheres: (a) SEM images. (b) Suggested molecular packing based on density functional theory geometry optimisation and crystal structure refinement from powder X-ray diffraction. (c) Schematic representation of the hierarchical chloroplast mimics, prepared by self-assembly of the CP microspheres, followed by encapsulation of porphyrin and enzyme molecules for photoenzymatic reactions. Reprinted from ref. 95 with permission from John Wiley and Sons. | ||
Histidine–metal coordination (specifically imidazolate–metal coordination) has been exploited towards developing materials with self-healing properties because of the reversible nature of histidine–metal coordination bonds. These materials have typically been metallopolymers, not classic CPs.96 Metallopolymers comprise long organic polymer chains interspersed by coordinated metal ions. Antimicrobial activity was demonstrated for an Ag(I)–histidine coordination compound where meso-form D–D and L–L dimer units are connected via silver–silver interactions.97 This is also strictly not a CP but the material is of interest because it exhibited antimicrobial activity and was prepared at room temperature by simple slow diffusion of aqueous solutions of D-histidine and L-histidine silver complexes. During the same study an Ag(I) D-histidine silver 1D CP (mirror image of the previously reported L-version98) was also obtained from aqueous self-assembly at room temperature.
L-Tyrosine, L-histidine, L-tryptophan and L-glutamic acid were combined with Co(II) and Zn(II) to prepare homochiral CP stationary phases for HPLC enantiomeric separations.99 Satisfactory resolution of a variety of organic small molecule enantiomers was achieved (Fig. 11). MOF-1 and MOF-6, based on Zn(II) and Co(II), respectively, with only amino acids as ligands and where the amino acids act as bridging ligands, were found to outperform the other MOFs, where the amino acids were coordinated as end groups. In a separate study of CP-based enantiomeric separations, a Cu(II) 3D CP was synthesised with the tripeptide Gly-L-His-Gly (GHG) as ligand, in ethanol at room temperature.100 Methamphetamine and ephedrine were chosen as model compounds for HPLC separation because of their prevalence in clinical research, forensics and toxicology. With the Cu(II) GHG CP as stationary phase, more efficient separation was achieved for ephedrine, by trapping of (+)-ephedrine, than for methamphetamine. The HPLC results were consistent with Monte Carlo simulations, where the calculated adsorption energy was greatest for the (+)-ephedrine enantiomer. Previous work had explored the sponge-like “amorphous-to-crystalline” sorption behaviour of Cu(II)–GHG and a similar porous CP based on Cu(II) and Gly-L-His-L-Lyc.101
A number of other CPs based on transition metals with aspartic acid102–105 or glutamic acid106,107 as ligand are known but, beyond crystallographic analysis, most of these materials have not been extensively investigated, suggesting a possible rich seam for future exploration.
Cyclodextrins
Cyclodextrins (CDs) are naturally-occurring cyclic oligosaccharides that have been used extensively in pharmaceutical industry, as well as in household cleaning products, agrochemical formulations and molecular separations.108 The readily available native CDs are α-, β- and γ-CD, comprising 6, 7 and 8 α-D-glucopyranoside units, respectively. Aqueous solubility is an important consideration in the use of native CDs as ligands. α-CD and γ-CD have good water solubilities of 232 g L−1 and 145 g L−1, respectively, but the aqueous solubility of β-CD is much lower at 18.5 g L−1.43Cyclodextrin-based MOFs (CD-MOFs) are based on the native cyclodextrins as green ligands with alkali metals as metallic nodes. The first CD-MOFs were based on γ-CD with Na, K, Rb and Cs. They were referred to as CD-MOF-1 (K and γ-CD), CD-MOF-2 (Rb and γ-CD) and CD-MOF-3 (Cs and γ-CD), their single-crystal X-ray crystal structures were elucidated (Fig. 13) and it was emphasised that the potassium version could be prepared from edible natural products.109 The synthesis of CD-MOF-1 and CD-MOF-2 has been improved upon and single-crystal X-ray structures for new γ-CD-MOFs based on NaOH and SrBr2 metallic nodes as well as a polymorph comprising Cs and γ-CD have since been reported.110 After the original publications involving γ-CD exclusively, the first α-CD-MOF was discovered in 2012, exhibiting chiral helices, and crystallising as hollow needles.111 The first β-CD-MOF was then reported in 2015.112 Subsequently, Sha and co-workers reported two β-CD-MOFs113 and one α-CD-MOF,114 which are the first examples crystallising in the monoclinic space group.
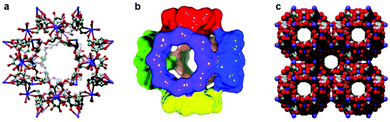 | ||
| Fig. 13 Single-crystal X-ray structure representations of the first reported CD-MOF, based on K and γ-CD. (a) Ball-and-stick representation of the cubic (γ-CD)6 repeating motif (C grey, O red, K purple). (b) Cuboidal orientation of the six γ-CD tori, illustrating the 1.7 nm sized pore at the centre of each (γ-CD)6 repeating motif, where K+ ions have been omitted for clarity. (c) Space-filling representation of the extended solid-state structure, with body-cantered cubic packing arrangement (C grey, O red, K purple). Reprinted from ref. 109 with permission from John Wiley and Sons. | ||
The CD-MOFs typically encapsulate small hydrophobic organic molecules without alteration of the overall MOF crystal structure. Encapsulation of curcumin from a methanol solution was demonstrated by UV-vis spectrophotometry.115 Formaldehyde capture from air by a CD-MOF based on K and γ-CD has been achieved with a nine-fold improvement in adsorption capacity and rate of adsorption over activated carbon.116 CD-MOFs have already been extensively explored for pharmaceutical applications.117 As potential drug delivery materials, CD-MOFs have been used to encapsulate the chemotherapy drug 5-fluorouracil. Examples of Na- and K-γ-CD-MOFs, and an Na-α-CD-MOF have been reported with 5-fluorouracil incorporated as guest molecule.113,118
The CD-MOFs are typically soluble in water; the ligand and metal cation readily dissociate when exposed to moisture but their moisture stability has been improved by the functionalisation of crystal surfaces by covalent grafting of cholesterol. The cholesterol-grafted CD-MOF crystals largely maintained morphological integrity and crystallinity even after immersion in water for 24 h. However; microporosity was compromised, with a reduction in almost 50% in BET surface area for the cholesterol-grafted material as compared with the unmodified CD-MOF.119
Holcroft et al. have demonstrated the versatility of CD-MOF-1 (K and γ-CD) and CD-MOF-2 (Rb and γ-CD) for separating mixtures of BTEX (benzene, toluene, ethylbenzene and xylene isomers), regioisomers of ethyltoluene and cymene, and the purification of cumene.120 Hartlieb et al. extended molecular separations using CD-MOFs to a wide variety of organic mixtures, including ethylbenzene from styrene, haloaromatics, terpinenes, pinenes and other chiral compounds.121
Metal ion selection
When considering the choice of metal ions for construction of CPs, a number of factors could be important. In general, non-toxic, earth abundant metals are preferred. However, the “green” credentials of CPs can depend significantly on the application of the material. For example, use of a CP that is an effective CO2 sorption material, facilitating carbon capture and storage (or even transformation into useful chemicals, if it is also a catalyst), may provide significant sustainability gains, even if the metal salt starting material, or the synthesised CP, exhibits some mammalian toxicity when ingested. In such a bulk application, where the CP is contained and recoverable, effective recovery and recycling strategies, keeping the metal ions and ligands in use in keeping with the concepts of the Circular Economy, may mitigate any potential hazards associated with toxicity and provide a suitable sustainable technology. Earth abundant metals in chemistry are most frequently discussed in the context of organometallic catalysis,122 but the wider concepts of “critical” metals (and metalloids)123 should also be considered when designing and building new CPs. In this more considered analysis, “earth abundance” proves to be a good proxy for the critical factors defining metals of highest concern; metals that are: derived as by-products from production of others; used in small quantities and in highly specialised applications, and possessing no effective substitutes.123 Metal salts are usually the source of metal ions for CP synthesis and the counterions of these reagents should be chosen to minimise safety and toxicity problems. Nitrates and perchlorates should generally be avoided because of the potential for explosive oxidation under certain conditions.28 Further, if these materials are to be prepared industrially in large quantities, metal salt anions that are removed during the process, constitute a source of waste and thus, avoidance of large molecular weight and/or waste stream contaminating anions is advised.As with all “wicked” problems, complex interdependencies make simple analysis impossible and almost always context-dependent. Full coverage of selection of “green” metal ion building blocks and starting salts for CP construction would require an extensive analysis of source (including energy costs and pollution resulting from mining and refining), in-use hazards and end-of-life fate, so here we confine ourselves to representative examples (Table 2). To avoid the use of terms that such as “heavy metal” that are poorly defined and can lead to confusion124 we group metal ions based on the position of the metal in the periodic table only. Certain CPs mentioned in this section would not be considered green CP systems because of ligand or synthesis failures but are mentioned to highlight the potential versatility of these favourable metal ions.
| Type | Metal ion | Earth abundancea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 125,126 125,126 |
Notable CPs |
|---|---|---|---|
| a Abundance in the earth's crust, ignoring O and H. | |||
| s-Block | Ca2+ | Calcium is the 5th most abundant element | MOF-1201 and MOF-1203;127 BioMIL-4;128 Ca(H2O)3(H2PXBP)129 |
| Mg2+ | Magnesium is the 7th most abundant element | Mg-MOF-74151 | |
| d-Block | Fe3+ | Iron is the 3rd most abundant element | BioMIL-1;130 Fe(III) BTC gels;159–161 Fe2BTC3 (Basolite F300)131 |
| Zn2+ | Zinc is present in moderate abundance but is heavily mined and there is some concern about resource depletion | MOF-5;132 Zn(II) dicarboxylate catalysts;133 Zn(II) cystine/guanine microparticles134 | |
| Ti4+ | Titanium is the 8th most abundant element | MIP-207135 | |
| Zr4+ | Zirconium is moderately abundant but not easily accessible compared to other ores | UiO-66;136 UiO-66-NO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 137 137 |
|
| p-Block | Al3+ | Aluminium is the most earth abundant metal | MIL-160![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78 78 |
s-Block
While the low formula weight alkaline metal- and alkaline earth metal-based CPs are attractive from a green chemistry perspective, frequently these CPs exhibit poor aqueous and thermal stability. However, a number of remarkably stable Ca CPs have been discovered.Single-crystal X-ray crystal structures were reported for isoskeletal CPs of Ca adipate monohydrate,138 Ca glutarate monohydrate139 and Ca succinate monohydrate140 without investigation of possible functional properties. Ca succinate 2D CP crystals were prepared by hydrothermal synthesis and were “washed repeatedly with distilled water”, suggesting these are not readily water soluble.141 De Lill et al., achieved remarkable synthesis of 3D Ca adipate CPs for which the crystals were washed with water (possibly water stable), possessing infinite channels occupied by the templating agent 4,4′-bipyridyl or 4,4′-bipyridylethane.63
Ca14(L-lactate)20(acetate)8(C2H5OH)(H2O) designated MOF-1201 and Ca6(L-lactate)3(acetate)9(H2O) designated MOF-1203,127 have been demonstrated to encapsulate and slowly release the agricultural fumigant cis-1,3-dichloropropene (DCP) used as a pesticide in a wide range of crops. DCP contained in MOF-1201 was released at a rate 100 times slower than from the liquid and the MOF hydrolyses in water making it a degradable solid carrier. Here the use of both metal ion and linker that are acceptable when distributed onto agricultural lands is key and the potential for reduction of worker exposure to the fumigant an improvement on current practice (the US permissible exposure level for DCP is 1 ppm).142 Further MOF-1201 was synthesised in ethanol making all organic components potentially bioderived from renewable sources. MOFs 1201 and 1203, along with another MOF based on Zn(II), L-lactic acid and terephthalic acid, have since been prepared from polylactic acid, as part of a strategy for upcycling polylactic acid waste.143
The incorporation of biocompatible linkers and Ca2+ ions allows construction of “biomineralisation” agents for potential restoration of bone. A MOF with formula Ca(H2O)3(H2PXBP), where PXBP is p-xylylenebisphosphonate, was described as nontoxic and able to stimulate in vitro bone formation.144 Generation of mixed cation Sr2+/Ca2+ forms of the MOF, in hydrothermal syntheses, allowed control of the rate of dissolution in simulated body fluids (Fig. 14).129
Building on many reports of MOFs able to separate, to varying degrees, light hydrocarbons such as C2H2/C2H4 and C2H4/C2H6, Li et al. described a calcium squarate MOF, synthesised in water from CaCO3 and squaric acid.145 The air stable and water stable product exhibited 1D channels that effectively “sieved” light C2 hydrocarbons with good adsorption selectivity of 8.1 for a C2H2/C2H4 mixture (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v).
50, v/v).
In a food-based application, a Ca fumarate CP (described as a MOF although no structural data was provided), prepared from Ca acetate and fumaric acid in water, was shown to absorb fluoride ions from brick tea, offering a potential treatment to reduce the risk of fluorosis from this source.146
A Ca glutarate CP was denoted BioMIL-2 and crystal structures of both the anhydrous and hydrate versions have been elucidated.147 The authors classified glutaric acid as therapeutically active based on its status as an “experimental drug”. The anhydrous phase is a 3D CP and was the first example of a Ca CP exhibiting permanent porosity, demonstrated by N2 sorption. Upon hydration, the 3D porous phase transforms to a non-porous “1D inorganic sub-network phase”. BioMIL-3 is based on Ca and 3,3′,5,5′-azobenzenetetracarboxylate as ligand. The ligand is expected to be biocompatible and exhibit antimicrobial activity, as is common among azobenzene compounds, and the CP has been investigated for controlled delivery of NO under biologically relevant conditions.148 BioMIL-4 is also based on Ca, incorporates alendronate as ligand and was synthesised hydrothermally.128 Alendronate is a bisphosphonate that has been used in the treatment of various bone diseases.
In addition to BioMIL-4,128 a number of other promising Ca(II) bisphosphonate CPs have been discovered. The nitrogen-containing bisphosphonates pamidronate and zoledronate are cytotoxic against cancer cells but have unfavourable pharmacokinetic properties. These drugs have been successfully incorporated into nanoscale Ca CPs as ligands.149 The CPs were coated with single lipid bilayers to improve their stability in biologically relevant media. When combined with lipid coating, the nanoscale CPs proved highly effective against human lung and pancreatic cancer cells, with vastly superior performance over the free bisphosphonates. Ca CPs with p-xylylenebisphosphonate have been prepared with only water as solvent.144 The reported synthetic procedure involved autoclaving the reaction mixtures at 180 °C for 3 days but this can likely be substituted with an alternative method, especially where well-defined single crystals are not required. One of these Ca p-xylylenebisphosphonate CPs stimulated bone mineralization in experiments with MG63 osteoblast-like cells.
Mg-MOF-74, with open metal sites and infinite hexagonal pores, has been extensively studied because of its potential gas sorption applications, with a dynamic (flowing gas) CO2 sorption capacity of 8.9%.151 The adsorbed CO2 can be released at 80 °C, which is relatively low compared with other competing systems. Isoreticular to Mg-MOF-74 is a 3D porous CP based on the anti-inflammatory drug olsalazine as ligand (a BioMOF according to the definition by McKinlay et al.6). Analogues with Fe, Co, Ni, Zn were also prepared but the Mg version is of particular interest because of its excellent biocompatibility. Multicomponent drug release was demonstrated under simulated physiological conditions with olsalazine being the first drug and phenethylamine, a central nervous system stimulant chosen as model second drug, encapsulated as guest.152
Mg2+ has been combined with a carboxylate–phosphonate ligand in water and acetonitrile to prepare a 2D CP capable of Fe3+ luminescent sensing in aqueous medium. The pure Mg CP emits blue light in aqueous medium. This CP emission intensity is much greater than that of the free ligand. In the presence of various di- and trivalent metal ions in aqueous solutions, the emission was dampened and, in the case of Fe3+ at approximately 600 μM, the luminescence was fully quenched.153
A 1D Mg imidazolate CP has been prepared by solvent-free synthesis directly from the ligand melt. Mg metal and solid imidazole were combined in a sealed vessel and heated to 90 °C (ligand melting point) for 42 h, yielding crystals of the CP upon cooling.154 A similar result was achieved for Ca, resulting in a 2D Ca imidazolate CP, although for the Ca CP, higher temperature (as much as 145 °C) and much longer reaction time were required.154
A plethora of further CPs with Mg2+ and Ca2+ ion metal ion nodes have been reported. Consideration must be given to synthetic routes and the ligands selected, both with regards to source and end-of-life fate, but the significant number of syntheses conducted in water, in benign solvents, or in solvent-free conditions, and the increasing range of bio-based ligands reported indicates that these Group II metal ion CPs may provide a rich source of potentially green materials for a wide range of applications.
d-Block
A wide range of d-block metals are employed in CP synthesis, but many are contraindicated, particularly where the CP may be ingested by animals or humans. As with all metal complexes, solubility often defines toxicity.The Fe-MIL series has been extensively studied, particularly towards gas storage, drug delivery and catalysis applications. Some important compounds in the series are: MIL-88A with fumarate as linker; MIL-53, MIL-88B and MIL-101 with terephthalate as linker; and MIL-100 with 1,3,5-benzene tricarboxylate as linker. It has been noted that the Fe(III) fumarate 3D porous CP MIL-88A has the same chemical composition as the commercially available salt iron fumarate, which is approved for medical treatment.6 An in vitro toxicity study of MIL-100(Fe) found that this CP exhibited good biocompatibility and low cytotoxicity. The safe dosage level was determined to be 80 μg mL−1.156 Recently a range of bimetallic CPs based on MIL-100(Fe,M) have been prepared by green synthesis in water at room temperature, using Na3BTC, the sodium salt of trimesic acid, as ligand source.157
The related BioMIL series is a set of CPs that incorporate both a therapeutically active ligand and a metal ion that is benign or beneficial to human health. First in the series is an Fe(III)-based CP called BioMIL-1 (Fig. 15), which incorporates vitamin B3 (also known as niacin of nicotinic acid) as ligand and was reported as the first therapeutically active MOF.130 Biological delivery of the active component is based on degradation of the MOF. The vitamin B3 release rate was estimated under simulated physiological conditions. Elsewhere, other vitamin B3 porous CPs were investigated as carriers for the delivery of therapeutic NO.158 However, these later versions were based on Ni(II) and Co(II), which are far less attractive than Fe(III) in the context of biomedical applications.
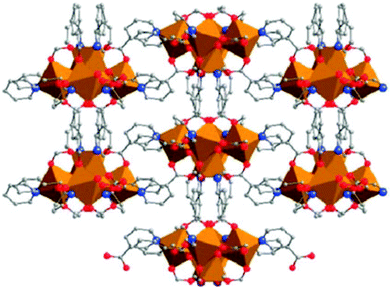 | ||
| Fig. 15 Projection along [001] for the single-crystal X-ray structure of BioMIL-1, based on Fe(III) and vitamin B3. Iron octahedra, and O, C and N atoms are represented in orange, red, grey and blue, respectively. H atoms are omitted for clarity. Reprinted from ref. 130 with permission from the Royal Society of Chemistry. | ||
Fe(III) readily forms metal–organic gels with a variety of carboxylate-based ligands. Fe(III) forms CP gels with 1,3,5-benzenetricarboxylic (BTC) via conventional solvothermal synthesis and by green synthetic routes. Fe(III) BTC gels were reported as aerogels based on a precursor CP gel prepared by mixing ethanolic solutions of the Fe(NO3)3 and the ligand. Unreacted reagents were removed by Soxhlet extraction and the molecular formula, [Fe3O(C6H3(COO)3)2NO3]n, determined by elemental analysis.159 In a separate study Fe(III) BTC gels were prepared from Fe(NO3)3 as well as from FeCl3. The FeCl3-derived material gelled at a slower rate.160 The CP gels were evacuated under various conditions and one of the resultant aerogels exhibited CO2 sorption capacities of 5.9 wt% at 1.1 bar and 33 wt% at 30 bar. Fe(III) BTC gels have been investigated for sorption of As5+ ions from aqueous solution (Fig. 16) with vastly superior sorption capacity over commercially available iron oxide powders, although the CP gel was prepared by an unsatisfactory method using DMF at 150 °C for 24 h.161 The CP gel absorbed 12.3 mg g−1 of As5+ as compared with 6.4 mg g−1 for Fe2O3 nanoparticles and 1.1 mg g−1 for bulky Fe2O3 powders. Similarly Fe(III) and 1,4-napthalenedicarboxylic acid form a CP gel with a far superior As5+ sorption capacity of 144 mg g−1.162
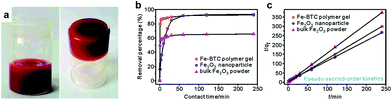 | ||
| Fig. 16 (a) Fe(III) BTC (1,3,5-benzenetricarboxylic) CP gel. (b) Percentage of As(V) adsorption with increasing contact time. (c) Pseudo-second-order kinetic plots for As(V) adsorption. Adsorption plots are given for the Fe(III) BTC CP gel, Fe2O3 nanoparticles and bulky Fe2O3 powder at pH 4, 5.0 g L−1, 298 K. Reprinted with permission from ref. 161. Copyright 2012 American Chemical Society. | ||
Marquez et al. prepared the MOF Fe3(BTC)2, which is commercially available as Basolite F300 (a fine chemical produced by BASF), by a green and scalable aerosol route called “evaporation induced self-assembly”. The group obtained their best results using low concentrations of cetyltrimethyl ammonium bromide as a templating agent.131
The so-called Zn(II) “paddle–wheel” secondary building unit has been extensively exploited in the preparation of mixed-ligand MOFs, such as those based on carboxylate- and pyridyl-bearing ligands.164
BioMIL-5 is a CP incorporating Zn(II) and azelaic acid that was originally prepared by hydrothermal synthesis. The antimicrobial activity of BioMIL-5 has been demonstrated against Staphilococcus bacteria.165
Zn(II) CP hydrogels and flower-like microparticles (Fig. 17) were synthesised by combining Zn(NO3)2 with guanine and cytosine as ligands. The hydrogels, based on nanofibre particles, were obtained from single-ligand reactions with pure guanine or cytosine and the flower-like microparticles were obtained from mixed ligand reactions that combined guanine with cytosine. The materials were effective towards photocatalytic degradation of methylene blue and methyl orange, with the single-ligand hydrogels outperforming the mixed-ligand microparticles in terms of catalytic activity.134
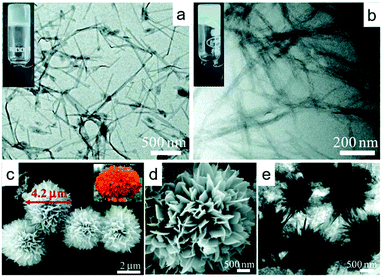 | ||
| Fig. 17 TEM images of hydrogels: (a) Zn(II) cytosine CP and (b) Zn(II) guanine CP. Flower-like microparticles of Zn(II) cystine/guanine microparticles: (c) SEM image with inset of a marigold flower, (d) magnified SEM image, (e) TEM image. Reprinted from ref. 134 with permission from the Royal Society of Chemistry. | ||
A variety of Zn dicarboxylates are well-known heterogeneous catalysts for copolymerisation of epoxides with CO2. These include CPs based on succinic, adipic, glutaric and pimelic acid.133
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 172 and photocatalytic chemical fixation of CO2.173
172 and photocatalytic chemical fixation of CO2.173
A systematic approach, based on Ti(IV) isopropoxide clusters [(Ti3O)(iPrO)8]2+ and [(Ti4O2)(iPrO)6]6+ and various carboxylate linkers, was employed to prepare a range of Ti(IV) CPs, including 1D and 2D structures and a rare structure referred to as a “2D → 3D polycatenation framework”.174 Copper iodide dopants were incorporated to impart photoluminescence properties.
A 3D porous Ti(IV) CP, MIP-177-LT with 3,3′,5,5′-tetracarboxydiphenylmethane as ligand was prepared under mild reflux of the reagents, without the need for any added solvents. Upon calcining, MIP-177-LT underwent an irreversible phase transformation to the high temperature phase MIP-177-HT, which comprises ultrathin Ti–O nanowires connected via the organic ligand.175 Later, exceptional proton conductivity of 2.6 × 10−2 S cm−1 at 298 K and 95% RH was demonstrated for a related compound, MIP-177-SO4H-LT, where an HSO4− ion is coordinated to the metal node as an additional component. Coordinated HSO4− is introduced post-synthetically, via treatment of MIP-177-LT with H2SO4.176
Green scalable synthesis has been reported for a Ti(IV) MOF designated MIP-207. A strategy based on a Ti8O8 cluster precursor, combined with ligand exchange, was used to demonstrate rational design of a series of Ti(IV) CPs, which is known to be challenging because of the complex chemistry of Ti in solution.135
p-Block
An Al(III) fumarate CP, known as MIL-53-FA and commercially available as Basolite A520, produced by BASF at ton scale.178 This MOF exhibits exceptional hydrothermal stability and is used in natural gas storage applications. Scalable production of MIL-53-FA has been achieved at a space time yield of 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 159 kg m−3 day−1 and a rate of 5.6 kg h−1.179 Nanoflakes of MIL-53-FA have been synthesised by combining sodium aluminate with fumaric acid in water at 90 °C with the resultant material showing relatively good volatile organic solvent sorption capacity and selective sorption of dichloromethane over trichloromethane.180
159 kg m−3 day−1 and a rate of 5.6 kg h−1.179 Nanoflakes of MIL-53-FA have been synthesised by combining sodium aluminate with fumaric acid in water at 90 °C with the resultant material showing relatively good volatile organic solvent sorption capacity and selective sorption of dichloromethane over trichloromethane.180
In the section covering ligand selection we highlighted MIL-160, well-known Al(III) CP that has been explored as a water sorbent for heat exchange applications.78
An extensive series of Al(III) CPs, with aliphatic dicarboxylic acids as ligands, has been prepared by Mani and co-workers. These CPs were tested for efficiency towards enhancing nucleation of isotactic polypropylene (iPP), which is important for controlling iPP crystallisation during polymer processing. Favourable results were obtained, particularly for the Al(III) succinate CP.181
f-Block
Despite lanthanide compounds being explored as therapeutic agents,182 lanthanides have moderate to high toxicity, depending on their chemical form, while the actinides are generally toxic to humans.183 Additionally, the lanthanides and actinides are not earth abundant (the lanthanides, along with Sc and Y, are classified as rare-earth elements) and pose environmental hazards, making them unfavourable as metal centres for green CPs. Among others, pulmonary,184,185 liver186 and blood-related toxicity have been investigated. In a study of their effect on hemoglobin, lanthanide and actinide ions disrupted the alpha-helix conformation at lower concentrations, while above 75 μM, interaction with heme interfered with oxygen binding.187Solvents used in synthesis
Among the most common solvents employed in CP synthesis are hazardous solvents such as N,N′-dimethylformamide (DMF) and N,N′-dimethylacetamide (DMAc), which are both toxic by inhalation (H332) and via skin contact (H312) and may be harmful to unborn children (H360D), and methanol, which is also toxic by inhalation (H331) and dermal exposure (H311). As Reinsch has argued,28 the ideal solvent for CP synthesis is water, followed by ethanol and acetic acid (both obtainable from renewable feedstocks). Recyclable organic solvents, that are stable under the reaction conditions, are to be considered as a last resort.There are examples of MOFs where the original synthesis under harsh conditions has been replaced with a reaction in more sustainable solvent medium. ZIF-8 is an example where organic solvents were originally employed, but efficient synthesis can now be achieved in water. ZIF-8 was originally synthesised by dissolving 2-methylimidazole in methanol and layering onto a solution of Zn(OH)2 in 25% aqueous ammonia to yield crystals after one month.188 The procedure was improved upon to obtain the product after 24 h by combining Zn(NO3)2·4H2O with 2-methylimidazole in DMF at 140 °C for 24 h.189 In contrast to the harsh conditions of solvothermal synthesis, a straightforward green synthesis produced ZIF-8 as nanocrystals within several minutes by combining Zn(NO3)2·6H2O and 2-methylimidazole in water at room temperature, although a large excess of ligand was required.190 In many cases the original synthetic routes and methods were chosen for the preparation of large crystalline particles amenable to analysis by single crystal X-ray diffractometry, but more readily scaled methods using benign solvents and lower (frequently even ambient) temperatures are possible. In addition, a move away from hydro- or solvothermal methods mitigates the requirement for high pressure apparatus.
Low solvent and solvent-free methods
Mechanochemistry is a synthetic approach involving intimate mixing of reagents by techniques such as ball-milling, grinding or extrusion. Its primary advantage over conventional synthetic methods is the minimisation or elimination of (typically hazardous) organic solvents.Friščić and co-workers have developed several low-solvent and solvent-free approaches to the synthesis of metal–organic frameworks based on grinding and milling.30 CP synthesis by ball-milling has been investigated by in situ X-ray powder diffraction using synchrotron radiation, shedding light on mechanistic aspects of these reactions.191–193In situ monitoring of the mechanochemical synthesis of HKUST-1 revealed that the reaction proceeds either directly (without a discernible intermediate phase) or via one of two previously unidentified CP intermediates. A crystal structure could be determined for one of these intermediates, which is a mononuclear CP.191 By a similar analytical method it was discovered that the synthesis of MOF-74 from ZnO, an excellent ‘green’ metal source, and 2,5-dihydroxyterephthalic acid by milling in the presence of water, proceeds via a non-porous intermediate CP.192 The Friščić group has also been able to demonstrate successful synthesis of several close-packed ZIFs (zinc imidazolate frameworks) by exposing the reactants to high humidity at moderate temperatures of up to 45 °C in the presence of a salt catalyst; a process referred to as “accelerated ageing” (Fig. 18).194 One of the close-packed ZIFs could be converted to the well-known ZIF-8 by exposure to methanol vapour. The accelerated aging approach was improved to achieve multi-gram synthesis of ZIF-8, its Co(II) analogue ZIF-67 and a related ZIF based on Zn and 2-ethylimidazole,195 and extended to the synthesis of other 2D and 3D CPs based on Zn(II), Ni(II) and Co(II).196 Recently accelerated aging has been referred to as a ‘geomimetic’ approach, inspired by geology and mimicking the natural process of mineral formation.197
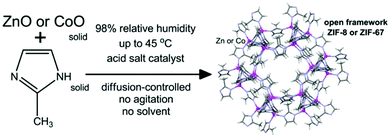 | ||
| Fig. 18 Reaction scheme for the synthesis of ZIFs by the “accelerated aging” approach. Reprinted from ref. 194 with permission from the Royal Society of Chemistry. | ||
Efficient continuous production of MOFs by extrusion, with little to no solvent, was first achieved by James and co-workers using single- and twin-screw extruders, demonstrating the method on archetypal MOFs including HKUST-1 and ZIF-8. For ZIF-8 the synthesis was achieved completely free of additional solvent by extruding at elevated temperature (melt phase synthesis).198
Mechanochemistry methods have frequently been investigated for the synthesis of UiO-66, based on terephthalic acid as linker, alongside its analogue UiO-66-NH2, based on 2-aminoterephthalic acid as linker. An effective procedure for UiO-66-NH2 and UiO-66 was first reported by Užarević et al., who used a Zr(IV) methacrylate cluster, Zr6O4(OH)4(C2H3CO2)12, as preorganised metal source, although small proportions of methanol were required.199 Scalable mechanochemical synthesis, by twin-screw extrusion, of UiO–NH2 in the presence of only water, eliminating organic solvents, was demonstrated by Karadeniz and co-workers. The method is based on the acetate cluster, [Zr6O4(OH)4(CH3COO)12]2, as precursor, prepared in a preliminary step from zirconium propoxide and acetic acid. In the same study UiO-66 synthesis was achieved by smaller scale milling only and triethylamine was required in addition to water. The greater solubility of 2-aminoterephthalic acid over terephthalic acid is a critical factor.200 A truly green procedure for the preparation of UiO-66 remains elusive.
The landmark 3D porous CP, MOF-5 has been targeted for green synthesis using alternative solvents. However, as yet no procedure has been reported that is completely free of hazardous organic solvents and yields MOF-5 of excellent crystallinity. Perhaps the best green method reported is a mechanochemical procedure where an oxozinc carboxylate complex201 is milled for 60 min with terephthalic acid.202 While MOF-5 could be prepare by solvent-free milling, the product was of poor crystallinity. Improved crystallinity was achieved by milling in the presence of THF and washing with THF. Thus, although cleaner methods have been developed for MOF-5, this example illustrates the challenge posed in achieving entirely green synthetic procedures for certain architectures.
Some examples of solvent-free melt phase synthesis have been reported.154,203 In general, a mixture of the solid ligand and metal is heated, in a sealed vial, to the ligand melting point and held at this temperature for an extended period of time before slow cooling. These procedures require long reaction times of several days and the metal is introduced in its elemental form, which would be extremely dangerous at scale. Melt phase extrusion, such as the earlier example of ZIF-8 synthesis,198 is likely to be more feasible.
Ionic liquids and deep eutectic solvents (ionothermal synthesis)
Ionic liquids (ILs) comprise ionic species in the liquid state. Examples of common IL ions are shown in Fig. 19. They are sometimes defined as salts with melting points below 100 °C, although this is a somewhat arbitrary definition. Deep eutectic solvents (DESs) are a special class of ILs. DESs are eutectic mixtures of Lewis or Brønsted acids and bases, which can contain a variety of anionic and/or cationic species. ILs and DESs have received attention for their potential as sustainable alternative solvents. They are attractive as green solvents particularly because of their low vapour pressures. This translates to reactions that can be carried out at elevated temperature without generating the autogenous pressure that is usually associated with typical solvothermal reactions used in CP synthesis. They can also play a structure-directing role, which has been exploited in MOF synthesis.204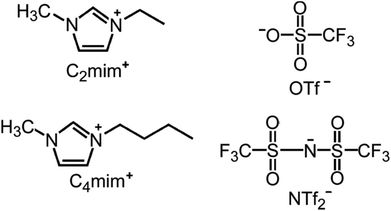 | ||
| Fig. 19 Molecular structures of common ions in ionothermal synthesis: 1,3-dialkylimidazolium cations [C2mim]+ and [C4mim]+, and the anions OTf− and NTf2−. Reprinted from ref. 204 with permission from the IUCr. | ||
A variety of ZIFs have been synthesised using ionothermal synthesis. One of the earliest examples is the synthesis of two previously reported ZIFs along with two novel ZIFs using the IL 1-ethyl-3-methylimidazolium bis[(trifluoro-methyl)sulfonyl]imide as solvent.205 A related early example is the synthesis of the first Zn(II)–B(III)-imidazolate porous CP, using the DES choline chloride/N,N′-dimethylurea (m-urea).206 Rapid synthesis of ZIFs has been achieved using microwave-assisted ionothermal synthesis. ZIF-8 was synthesised in 60 min at 140 °C using microwave-assisted ionothermal synthesis with 1-butyl-3-methyl-imidazolium tetra-fluoroborate as solvent.207 In a very similar microwave procedure, also using 1-butyl-3-methylimidazoliumtetrafluoroborate, ZIF-61 was prepared in 40 min at 140 °C.208
Polyoxometalate MOFs have been successfully synthesised under harsh ionothermal conditions of 170 °C for 5 days.209 The replacement of hazardous organic solvents with ILs is in this case undermined by the long reaction time and high temperature.
Zottnick et al. were able to prepare La(III) tetracyanoborate CPs by ionothermal reaction where the ionic liquid, 1-ethyl-3-methylimidazolium tetracyanoborate serves as both solvent and ligand source.210
UiO-66 was originally synthesised solvothermally in DMF at 120 °C for 24 h.211 This highly stable CP has been successfully synthesised at room temperature in the ionic liquids 1-hexyl-, 1-octyl and 3-methylimidazolium chloride. Yields were excellent and the reaction time was reduced to as short as 30 min, while in DMF at room temperature UiO-66 may only precipitate after 120 h.212
Despite the promise of ionic liquids as green synthesis medium, in many cases the conditions for CP reactions tend to involve relatively high temperatures and long reaction times. Interesting benefits seems to be the possibility of templating structures that difficult to obtain otherwise, and the simultaneous role of ILs as solvent medium and ligand. Another benefit is the tunability of ILs. Different ILs with slightly different molecular structures can be tested to optimise desired CP structures. Furthermore, the probability of accessing MOFs bearing a net charge is improved because of the IL ions can play a counterbalancing role.
Alternative techniques for green synthesis
As discussed earlier, there have been major advances in the development of mechanochemistry for low solvent or solvent-free synthesis of CPs. Additionally, approaches exploiting ultrasound and electrochemistry have been used as alternative techniques for green synthesis of CPs.Sonochemical synthesis
Cintas and Luche provided a detailed perspective on the sonochemical approach as it relates to green chemistry.213 The potential advantages of this method include energy savings, increased reaction rates, and improved or alternative selectivity towards reduced waste. In practice, however, there have been limited gains in terms of more sustainable synthesis. Many reported sonochemical procedures for CP preparation still rely on unfavourable solvents.Son et al. were the first to specifically employ sonochemical synthesis in MOF research, preparing MOF-5 by a scalable method.214 However, their method relied on 1-methyl-2-pyrrolidone as reaction solvent, which poses risks to human health and the environment. Under ultrasonic treatment the reaction mixture reached 129–164 °C. Higher power and its associated higher temperature yielded a higher rate of precipitation of the crystalline product.
In aqueous medium, Cu(II) was combined with 4,4′-(hexafluoroisopropylidene)-bis(benzoic acid) to prepare sub-micron sized MOF crystals by sonochemical synthesis. To modulate crystal morphology from high aspect ratio (needle-like) to more isotropic crystallites, a small quantity of 2-propanol was added to the reaction mixture.215
The extensively researched Mg-MOF-74 has been prepared sonochemically in as little as one hour, although DMF was used as solvent and triethylamine was required for ligand deprotonation.216
Scalability has been demonstrated by the preparation of well over 100 g of ZIF-8 at a yield of 85% in a 1 L sonochemical reaction using DMF as solvent.217 While the solvent system is unfavourable, the prospect of sonochemical synthesis of CPs at larger scales is promising.
Electrochemical synthesis
Electrochemical synthesis of CPs is relatively underdeveloped compared with other methods. A number of proof-of-concept experiments have been based on the well-known material HKUST-1. Researchers at BASF first demonstrated electrochemical synthesis of 3D porous CPs by preparing HKUST-1 with Cu electrodes in a solution of 1,3,5-benzentricarboxylic acid in methanol at 12–19 V and 1.3 A for 150 min to produce crystals of 0.5–5 μm in size.218 Densely-packed patterned CP layers have been grown on Cu surfaces by electrochemical synthesis of HKUST-1.219 MOF-5 has been synthesised electrochemically in an IL medium, although DMF was still used. The reaction yielded unusual flower-like crystallites of MOF-5.220 A number of other archetypal 3D CPs, including ZIF-8 and MIL-100(Al) have also been prepared by electrochemical synthesis, requiring relatively short reaction times under mild conditions.221Competing with alternative materials
The advantages of CPs over alternative competing materials include diversity, tunability, high surface area (porous CPs) and periodicity. However, many CPs suffer from poor thermal or chemical stability, which hinders further development towards industrial applications. Several reviews have addressed the subject of thermal, chemical and mechanical stability of MOFs.222–225 Additionally, considerable efforts have been dedicated specifically to understanding and improving MOF stability in aqueous media.226–228MOFs tend to be compared with zeolites and mesoporous silica as competing alternative materials. In heterogeneous catalysis zeolites are cost effective and tend to outperform both MOFs and mesoporous silica because of their superior thermal and chemical stability.229 Howarth et al. have argued and that MOFs can now be routinely designed for thermal and chemical stability but that “it is difficult to envisage MOF structures with stabilities that greatly exceed the thermal, mechanical and chemical stabilities of the most robust existing MOFs”.222 Among the most robust MOFs are UiO-66 and its analogues136,137 and Ni3(BTP)2.230 Some features of MOFs, including their distinct pore size range, higher diffusivity and ability to act as “single-site catalysts”, present potential advantages over zeolites, particularly for liquid phase synthesis of fine chemicals under mild conditions and asymmetric catalysis.229
CPs are typically highly crystalline and brittle. To compete with alternative materials that may be structurally tougher, more flexible or more easily shaped, deposition or in situ synthesis on supports is a common strategy. Common applications for such hybrid CP materials include heterogeneous catalysis,231 functional textiles232,233 and chromatographic separations.234 Polymer films have been used as supports for the preparation of mixed matrix membranes incorporating MOFs.235 As a sustainable support for CPs, cellulose-based substrates have also been extensively explored.236
Various additional challenges need to be overcome to improve the industrial uptake of CPs. With respect to CPs for electrochemical energy storage and conversion applications, stability under electrochemical storage conditions, cost efficiency and scalable synthesis are key challenges that have been identified.237 Among CP gels, an emerging sub-class CP materials, detailed characterisation is a challenge that may necessitate the development of innovative characterisation techniques.10
Conclusion
Progress in the development of green CP materials has accelerated in recent years. The emergence of new CPs incorporating bio-based ligands and earth abundant metal ions is very encouraging. Remarkable materials such as CD-MOFs and the BioMIL series demonstrate that entirely benign, sustainable CPs are possible. However, despite environmental, energy and other sustainability applications being the target for many new CP materials, most CP research demonstrates little consideration for the sustainability of the novel materials themselves. There is great scope and a strong societal imperative for more extensive, direct research into CPs based on sustainable components and green synthetic procedures.Researchers investigating CP materials should be more intentional about their selection of reagents and their approach to synthesis, particularly where large-scale applications of bulk materials are envisioned. While elaborate ligands can be designed to impart specific properties, scalability will prove challenging if these are challenging to synthesise. As we have shown, a number of naturally-occurring and bio-based ligands, some of which are already available as commodity chemicals, hold promise as useful ligands for green CPs. Selecting these increases the chances of good scalability of the final material. Every effort should be made to avoid harsh reaction conditions, involving hazardous organic solvents, which have been common in CP research. Sonochemical and electrochemical synthesis present some specific advantages over conventional solvothermal methods. However; the replacement of hazardous organic solvents with green alternatives or switching to solvent-free or low-solvent synthesis by continuous processing techniques like twin-screw extrusion is arguably a far more promising strategy for efficient scalable green synthesis.
It will not be sufficient to focus solely on improving the sustainability of CP materials. Cost is another important factor. One strategy has been to concentrate on bio-based ligands that are becoming increasingly important as commodity chemicals, such as lactic acid and 2,5-furandicarboxylic acid, because such chemicals are likely to become more affordable as demand increases and production efficiencies improve.
Furthermore, it will be important to demonstrate that sustainable CPs can at least match or, better yet, outperform existing alternative materials. This is a major challenge. Thousands of CPs have been discovered but many have proven to be appealing only as curiosities. While some have broken through to industrial testing, particularly in gas storage, textiles and catalysis,238,239 CPs have arguably not yet lived up to their promise as novel materials for the numerous potential applications envisioned.
It is worthwhile for researchers to revisit known CPs that fulfil the requirements of green chemistry but have not yet been thoroughly investigated towards potential applications. There is already an enormous library of known CPs. For many of these, single crystal X-ray structures have already been determined and are readily available from the Cambridge Structural Database. Existing green CP materials should be explored for previously overlooked properties and potential new applications.
Generally, 1D and 2D CPs have been neglected compared to 3D CPs. Constructing inherently 3D porous CPs (MOFs) usually requires rigid synthetic organic ligands and is frequently challenging without resorting to templating agents and undesirable reaction conditions. Even the archetypal MOFs, such as MOF-5, HKUST-1, ZIF-8 and UiO-66, despite their favourable attributes, present limitations that have made scaled-up applications difficult. 1D and 2D CPs may be comparatively better suited to green synthesis and can be constructed using a wide variety of benign, sustainable metal ions and ligands.
Conflicts of interest
There are no conflicts to declare.References
- S. R. Batten, N. R. Champness, X.-M. Chen, J. Garcia-Martinez, S. Kitagawa, L. Öhrström, M. O'Keeffe, M. Paik Suh and J. Reedijk, Pure Appl. Chem., 2013, 85, 1715–1724 CAS.
- Y. Wang, D. Astruc and A. S. Abd-El-Aziz, Chem. Soc. Rev., 2019, 48, 558–636 RSC.
- A. K. Cheetham, C. N. R. Rao and R. K. Feller, Chem. Commun., 2006, 4780–4795 RSC.
- K. Biradha, A. Ramanan and J. J. Vittal, Cryst. Growth Des., 2009, 9, 8–9 CrossRef.
- I. Imaz, M. Rubio-Martínez, J. An, I. Solé-Font, N. L. Rosi and D. Maspoch, Chem. Commun., 2011, 47, 7287–7302 RSC.
- A. C. McKinlay, R. E. Morris, P. Horcajada, G. Férey, R. Gref, P. Couvreur and C. Serre, Angew. Chem., Int. Ed., 2010, 49, 6260–6266 CrossRef CAS PubMed.
- H. Cai, Y. L. Huang and D. Li, Coord. Chem. Rev., 2019, 378, 207–221 CrossRef CAS.
- C. Serre, F. Millange, S. Surblé and G. Férey, Angew. Chem., Int. Ed., 2004, 43, 6286–6289 CrossRef CAS PubMed.
- F. Millange, C. Serre, N. Guillou, G. Férey and R. I. Walton, Angew. Chem., Int. Ed., 2008, 47, 4100–4105 CrossRef CAS PubMed.
- P. Sutar and T. K. Maji, Chem. Commun., 2016, 52, 8055–8074 RSC.
- J. Zhang, X. Feng and P. Liao, in Gel Chemistry: Interactions, Structures and Properties, ed. J. Zhang, Y. Hu and Y. Li, Springer Nature, Singapore, 2018, pp. 61–118 Search PubMed.
- J. Hou, A. F. Sapnik and T. D. Bennett, Chem. Sci., 2020, 11, 310–323 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- E. Loukopoulos and G. E. Kostakis, J. Coord. Chem., 2018, 71, 371–410 CrossRef CAS.
- J. Chen, K. Shen and Y. Li, ChemSusChem, 2017, 10, 3165–3187 CrossRef CAS PubMed.
- S. Mukherjee, A. Kumar and M. J. Zaworotko, in Metal-Organic Frameworks (MOFs) for Environmental Applications, Elsevier, 2019, pp. 5–61 Search PubMed.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed.
- J. Duan, W. Jin and S. Kitagawa, Coord. Chem. Rev., 2017, 332, 48–74 CrossRef CAS.
- Y. Peng and W. Yang, Adv. Mater. Interfaces, 2020, 7, 1–30 Search PubMed.
- R. B. Lin, S. Xiang, H. Xing, W. Zhou and B. Chen, Coord. Chem. Rev., 2019, 378, 87–103 CrossRef CAS.
- Y. Zhang, X. Cheng, X. Jiang, J. J. Urban, C. H. Lau, S. Liu and L. Shao, Mater. Today, 2020 DOI:10.1016/j.mattod.2020.02.002.
- J. Deng, F. Wu, P. Yu and L. Mao, Appl. Mater. Today, 2018, 11, 338–351 CrossRef.
- J. Q. Liu, Z. D. Luo, Y. Pan, A. Kumar Singh, M. Trivedi and A. Kumar, Coord. Chem. Rev., 2020, 406, 213145 CrossRef CAS.
- J. H. Clark, Green Chem., 1999, 1, 1–8 RSC.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- L. Rogers and K. F. Jensen, Green Chem., 2019, 21, 3481–3498 RSC.
- H. Reinsch, Eur. J. Inorg. Chem., 2016, 2016, 4290–4299 CrossRef CAS.
- B. Zhang, J. Zhang and B. Han, Chem. – Asian J., 2016, 11, 2610–2619 CrossRef CAS PubMed.
- T. Friščić, I. Halasz, V. Štrukil, M. Eckert-Maksić and R. E. Dinnebier, Croat. Chem. Acta, 2012, 85, 367–378 CrossRef.
- Z. Ajoyan, P. Marino and A. J. Howarth, CrystEngComm, 2018, 20, 5899–5912 RSC.
- T. Rajkumar, D. Kukkar, K. H. Kim, J. R. Sohn and A. Deep, J. Ind. Eng. Chem., 2019, 72, 50–66 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed.
- M. L. A. Jansen and W. M. van Gulik, Curr. Opin. Biotechnol., 2014, 30, 190–197 CrossRef CAS PubMed.
- J. Becker, A. Lange, J. Fabarius and C. Wittmann, Curr. Opin. Biotechnol., 2015, 36, 168–175 CrossRef CAS PubMed.
- E. Skoog, J. H. Shin, V. Saez-Jimenez, V. Mapelli and L. Olsson, Biotechnol. Adv., 2018, 36, 2248–2263 CrossRef CAS PubMed.
- Y. Deng, L. Ma and Y. Mao, Biochem. Eng. J., 2016, 105, 16–26 CrossRef CAS.
- J. Cunha da Cruz, A. Machado de Castro and E. F. Camporese Sérvulo, 3 Biotech, 2018, 8, 138 CrossRef PubMed.
- M. M. Cajnko, U. Novak, M. Grilc and B. Likozar, Biotechnol. Biofuels, 2020, 13, 66 CrossRef CAS PubMed.
- K. Kobayashi, T. Hattori, Y. Honda and K. Kirimura, J. Ind. Microbiol. Biotechnol., 2014, 41, 749–756 CrossRef CAS PubMed.
- S. Sanchez, R. Rodríguez-Sanoja, A. Ramos and A. L. Demain, J. Antibiot., 2018, 71, 26–36 CrossRef CAS PubMed.
- T. Loftsson and D. Duchêne, Int. J. Pharm., 2007, 329, 1–11 CrossRef CAS PubMed.
- G. Beobide, O. Castillo, J. Cepeda, A. Luque, S. Pérez-Yáñez, P. Román and J. Thomas-Gipson, Coord. Chem. Rev., 2013, 257, 2716–2736 CrossRef CAS.
- Q. Yu, S. Black and H. Wei, J. Chem. Eng. Data, 2009, 54, 2123–2125 CrossRef CAS.
- M. C. Bernini, G. E. Gomez, E. V. Brusau and G. E. Narda, Isr. J. Chem., 2018, 58, 1044–1061 CrossRef CAS.
- M. C. Bernini, N. Snejko, E. Gutierrez-Puebla, E. V. Brusau, G. E. Narda and M. Á. Monge, Inorg. Chem., 2011, 50, 5958–5968 CrossRef CAS PubMed.
- R. F. D′vries, I. Camps and J. Ellena, Cryst. Growth Des., 2015, 15, 3015–3023 CrossRef.
- Y. X. Chi, T. S. Liu, J. Jin, G. N. Zhang and S. Y. Niu, J. Phys. Chem. Solids, 2013, 74, 1745–1750 CrossRef CAS.
- D. Ghoshal, A. Kumar Ghosh, G. Mostafa, J. Ribas and N. Ray Chaudhuri, Inorg. Chim. Acta, 2007, 360, 1771–1775 CrossRef CAS.
- P. M. Forster, N. Stock and A. K. Cheetham, Angew. Chem., Int. Ed., 2005, 44, 7608–7611 CrossRef CAS PubMed.
- S. Demir, G. K. Kantar, Y. Topcu and Q. Li, Transition Met. Chem., 2012, 37, 257–263 CrossRef CAS.
- R. Sibille, T. Mazet, B. Malaman, Q. Wang, E. Didelot and M. François, Chem. Mater., 2015, 27, 133–140 CrossRef CAS.
- C. Livage, P. M. Forster, N. Guillou, M. M. Tafoya, A. K. Cheetham and G. Férey, Angew. Chem., Int. Ed., 2007, 46, 5877–5879 CrossRef CAS PubMed.
- P. J. Saines, P. Jain and A. K. Cheetham, Chem. Sci., 2011, 2, 1929–1939 RSC.
- H. H. M. Yeung, W. Li, P. J. Saines, T. K. J. Köster, C. P. Grey and A. K. Cheetham, Angew. Chem., Int. Ed., 2013, 52, 5544–5547 CrossRef CAS PubMed.
- A. Michaelides, V. Kiritsis, S. Skoulika and A. Aubry, Angew. Chem., Int. Ed. Engl., 1993, 32, 1495–1497 CrossRef.
- J. Wang, Z. Wei, F. Guo, C. Li, P. Zhu and W. Zhu, Dalton Trans., 2015, 44, 13809–13813 RSC.
- Y. Q. Zheng, H. L. Zhu, X. X. Guo and J. Y. Liu, Solid State Sci., 2013, 18, 42–49 CrossRef CAS.
- Y. J. Kim and D. Y. Jung, Inorg. Chem., 2000, 39, 1470–1475 CrossRef CAS PubMed.
- Z. G. Sun, Y. P. Ren, L. S. Long, R. Bin Huang and L. S. Zheng, Inorg. Chem. Commun., 2002, 5, 629–632 CrossRef CAS.
- D. T. De Lill, N. S. Gunning and C. L. Cahill, Inorg. Chem., 2005, 44, 258–266 CrossRef CAS PubMed.
- D. T. De Lill, D. J. Bozzuto and C. L. Cahill, Dalton Trans., 2005, 2111–2115 RSC.
- P. J. Saines, P. T. Barton, M. Jura, K. S. Knight and A. K. Cheetham, Mater. Horiz., 2014, 1, 332–337 RSC.
- Z. Y. Li, B. Zhai, S. Z. Li, G. X. Cao, F. Q. Zhang, X. F. Zhang, F. L. Zhang and C. Zhang, Cryst. Growth Des., 2016, 16, 4574–4581 CrossRef CAS.
- Z. Y. Li, C. Zhang, B. Zhai, J. C. Han, M. C. Pei, J. J. Zhang, F. L. Zhang, S. Z. Li and G. X. Cao, CrystEngComm, 2017, 19, 2702–2708 RSC.
- R. M. Nair, M. R. Sudarsanakumar, S. Suma and M. R. Prathapachandra Kurup, J. Mol. Struct., 2016, 1105, 316–321 CrossRef CAS.
- M. Rose, D. Weber, B. V. Lotsch, R. K. Kremer, R. Goddard and R. Palkovits, Microporous Mesoporous Mater., 2013, 181, 217–221 CrossRef CAS.
- J. Wang, D. Ma, W. Liao, S. Li, M. Huang, H. Liu, Y. Wang, R. Xie and J. Xu, CrystEngComm, 2017, 19, 5244–5250 RSC.
- D. Y. Ma, J. Xie, Z. Zhu, H. Huang, Y. Chen, R. Su and H. Zhu, Inorg. Chem. Commun., 2017, 86, 128–132 CrossRef CAS.
- Y. Zhang and J. Wang, Inorg. Chim. Acta, 2018, 477, 8–14 CrossRef CAS.
- F. Bu, Q. Lin, Q. Zhai, L. Wang, T. Wu, S. T. Zheng, X. Bu and P. Feng, Angew. Chem., Int. Ed., 2012, 51, 8538–8541 CrossRef CAS PubMed.
- H. H. Li, Z. Niu, L. Chen, H. Bin Jiang, Y. P. Wang and P. Cheng, CrystEngComm, 2015, 17, 5101–5109 RSC.
- Q. G. Zhai, X. Bu, X. Zhao, C. Mao, F. Bu, X. Chen and P. Feng, Cryst. Growth Des., 2016, 16, 1261–1267 CrossRef CAS.
- H. Wang, R. M. Wen and T. L. Hu, Eur. J. Inorg. Chem., 2014, 1185–1191 CrossRef CAS.
- D. Feng, K. Wang, Z. Wei, Y. P. Chen, C. M. Simon, R. K. Arvapally, R. L. Martin, M. Bosch, T. F. Liu, S. Fordham, D. Yuan, M. A. Omary, M. Haranczyk, B. Smit and H. C. Zhou, Nat. Commun., 2014, 5, 5723 CrossRef CAS PubMed.
- H. Zhou, D. Feng and K. Wang, US Pat, 9724668B2, 2017 Search PubMed.
- A. Cadiau, J. S. Lee, D. Damasceno Borges, P. Fabry, T. Devic, M. T. Wharmby, C. Martineau, D. Foucher, F. Taulelle, C. H. Jun, Y. K. Hwang, N. Stock, M. F. De Lange, F. Kapteijn, J. Gascon, G. Maurin, J. S. Chang and C. Serre, Adv. Mater., 2015, 27, 4775–4780 CrossRef CAS PubMed.
- J. D. Einkauf, J. P. Karram, N. E. Greig, B. C. Chan and D. T. De Lill, Eur. J. Inorg. Chem., 2016, 1085–1092 CrossRef CAS.
- H. H. Li, W. Shi, N. Xu, Z. J. Zhang, Z. Niu, T. Han and P. Cheng, Cryst. Growth Des., 2012, 12, 2602–2612 CrossRef CAS.
- M. O. Barsukova, D. G. Samsonenko, A. A. Sapianik, S. A. Sapchenko and V. P. Fedin, Polyhedron, 2018, 144, 219–224 CrossRef CAS.
- M. Wahiduzzaman, D. Lenzen, G. Maurin, N. Stock and M. T. Wharmby, Eur. J. Inorg. Chem., 2018, 2018, 3626–3632 CrossRef CAS.
- A. Permyakova, O. Skrylnyk, E. Courbon, M. Affram, S. Wang, U. H. Lee, A. H. Valekar, F. Nouar, G. Mouchaham, T. Devic, G. De Weireld, J. S. Chang, N. Steunou, M. Frère and C. Serre, ChemSusChem, 2017, 10, 1419–1426 CrossRef CAS PubMed.
- N. Scales, Y. Zhang, M. Bhadbhade, I. Karatchevtseva, L. Kong, G. R. Lumpkin and F. Li, Polyhedron, 2015, 102, 130–136 CrossRef CAS.
- H. Wang, S. J. Liu, D. Tian, J. M. Jia and T. L. Hu, Cryst. Growth Des., 2012, 12, 3263–3270 CrossRef CAS.
- F. N. Shi, D. Ananias, T. H. Yang and J. Rocha, J. Solid State Chem., 2013, 204, 321–328 CrossRef CAS.
- M. Sadakiyo, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 9906–9907 CrossRef CAS PubMed.
- M. Sadakiyo, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2014, 136, 13166–13169 CrossRef CAS PubMed.
- I. Imaz, M. Rubio-Martínez, W. J. Saletra, D. B. Amabilino and D. Maspoch, J. Am. Chem. Soc., 2009, 131, 18222–18223 CrossRef CAS.
- F. Pu, X. Liu, B. Xu, J. Ren and X. Qu, Chem. – Eur. J., 2012, 18, 4322–4328 CrossRef CAS PubMed.
- H. Wu, C. Tian, Y. Zhang, C. Yang, S. Zhang and Z. Jiang, Chem. Commun., 2015, 51, 6329–6332 RSC.
- J. S. Shen, G. J. Mao, Y. H. Zhou, Y. B. Jiang and H. W. Zhang, Dalton Trans., 2010, 39, 7054–7058 RSC.
- E. Chen, B. Guo, B. Zhang, L. H. Gan and J. R. Gong, J. Nanosci. Nanotechnol., 2011, 11, 7682–7686 CrossRef CAS PubMed.
- C. Li, K. Deng, Z. Tang and L. Jiang, J. Am. Chem. Soc., 2010, 132, 8202–8209 CrossRef CAS PubMed.
- K. Liu, C. Yuan, Q. Zou, Z. Xie and X. Yan, Angew. Chem., Int. Ed., 2017, 56, 7876–7880 CrossRef CAS PubMed.
- S. Zechel, M. Hager, T. Priemel and M. Harrington, Biomimetics, 2019, 4, 20 CrossRef CAS PubMed.
- N. C. Kasuga, Y. Takagi, S. I. Tsuruta, W. Kuwana, R. Yoshikawa and K. Nomiya, Inorg. Chim. Acta, 2011, 368, 44–48 CrossRef CAS.
- K. Nomiya, S. Takahashi, R. Noguchi, S. Nemoto, T. Takayama and M. Oda, Inorg. Chem., 2000, 39, 3301–3311 CrossRef CAS PubMed.
- J.-H. Zhang, R.-Y. Nong, S.-M. Xie, B.-J. Wang, P. Ai and L.-M. Yuan, Electrophoresis, 2017, 38, 2513–2520 CrossRef CAS PubMed.
- J. Navarro-Sánchez, A. I. Argente-García, Y. Moliner-Martínez, D. Roca-Sanjuán, D. Antypov, P. Campíns-Falcó, M. J. Rosseinsky and C. Martí-Gastaldo, J. Am. Chem. Soc., 2017, 139, 4294–4297 CrossRef PubMed.
- C. Martí-Gastaldo, J. E. Warren, M. E. Briggs, J. A. Armstrong, K. M. Thomas and M. J. Rosseinsky, Chem. – Eur. J., 2015, 21, 16027–16034 CrossRef PubMed.
- E. V. Anokhina and A. J. Jacobson, J. Am. Chem. Soc., 2004, 126, 3044–3045 CrossRef CAS PubMed.
- E. V. Anokhina, Y. B. Go, Y. Lee, T. Vogt and A. J. Jacobson, J. Am. Chem. Soc., 2006, 128, 9957–9962 CrossRef CAS PubMed.
- L. Antolini, L. Menabue, G. C. Pellacani and G. Marcotrigiano, J. Chem. Soc., Dalton Trans., 1982, 2541–2543 RSC.
- H. Schmidbaur, I. Bach, J. Riede, G. Müller, J. Helbig and G. Hopf, Chem. Ber., 1988, 121, 795–797 CrossRef CAS.
- Y. Zhang, M. K. Saha and I. Bernal, CrystEngComm, 2003, 5, 34–37 RSC.
- M. Mizutani, N. Maejima, K. Jitsukawa, H. Masuda and H. Einaga, Inorg. Chim. Acta, 1998, 283, 105–110 CrossRef CAS.
- A. R. Hedges, Chem. Rev., 1998, 98, 2035–2044 CrossRef CAS PubMed.
- R. A. Smaldone, R. S. Forgan, H. Furukawa, J. J. Gassensmith, A. M. Z. Slawin, O. M. Yaghi and J. F. Stoddart, Angew. Chem., Int. Ed., 2010, 49, 8630–8634 CrossRef CAS PubMed.
- R. S. Forgan, R. A. Smaldone, J. J. Gassensmith, H. Furukawa, D. B. Cordes, Q. Li, C. E. Wilmer, Y. Y. Botros, R. Q. Snurr, A. M. Z. Slawin and J. F. Stoddart, J. Am. Chem. Soc., 2012, 134, 406–417 CrossRef CAS PubMed.
- J. J. Gassensmith, R. A. Smaldone, R. S. Forgan, C. E. Wilmer, D. B. Cordes, Y. Y. Botros, A. M. Z. Slawin, R. Q. Snurr and J. F. Stoddart, Org. Lett., 2012, 14, 1460–1463 CrossRef CAS PubMed.
- H. Lu, X. Yang, S. Li, Y. Zhang, J. Sha, C. Li and J. Sun, Inorg. Chem. Commun., 2015, 61, 48–52 CrossRef CAS.
- J. Sha, L. Wu, S. Li, X. Yang, Y. Zhang, Q. Zhang and P. Zhu, J. Mol. Struct., 2015, 1101, 14–20 CrossRef CAS.
- J. Q. Sha, X. H. Zhong, L. H. Wu, G. D. Liu and N. Sheng, RSC Adv., 2016, 6, 82977–82983 RSC.
- Z. Moussa, M. Hmadeh, M. G. Abiad, O. H. Dib and D. Patra, Food Chem., 2016, 212, 485–494 CrossRef CAS PubMed.
- L. Wang, X. Liang, Z. Chang, L. Ding, S. Zhang and B. Li, ACS Appl. Mater. Interfaces, 2018, 10, 42–46 CrossRef CAS PubMed.
- X. Li, T. Guo, L. Lachmanski, F. Manoli, M. Menendez-Miranda, I. Manet, Z. Guo, L. Wu, J. Zhang and R. Gref, Int. J. Pharm., 2017, 531, 424–432 CrossRef CAS PubMed.
- J. Sha, X. Yang, L. Sun, X. Zhang, S. Li, J. Li and N. Sheng, Polyhedron, 2017, 127, 396–402 CrossRef CAS.
- V. Singh, T. Guo, H. Xu, L. Wu, J. Gu, C. Wu, R. Gref and J. Zhang, Chem. Commun., 2017, 53, 9246–9249 RSC.
- J. M. Holcroft, K. J. Hartlieb, P. Z. Moghadam, J. G. Bell, G. Barin, D. P. Ferris, E. D. Bloch, M. M. Algaradah, M. S. Nassar, Y. Y. Botros, K. M. Thomas, J. R. Long, R. Q. Snurr and J. F. Stoddart, J. Am. Chem. Soc., 2015, 137, 5706–5719 CrossRef CAS PubMed.
- K. J. Hartlieb, J. M. Holcroft, P. Z. Moghadam, N. A. Vermeulen, M. M. Algaradah, M. S. Nassar, Y. Y. Botros, R. Q. Snurr and J. F. Stoddart, J. Am. Chem. Soc., 2016, 138, 2292–2301 CrossRef CAS PubMed.
- P. Chirik and R. Morris, Acc. Chem. Res., 2015, 48, 2495 CrossRef CAS PubMed.
- T. E. Graedel, E. M. Harper, N. T. Nassar, P. Nuss, B. K. Reck and B. L. Turner, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 4257–4262 CrossRef CAS PubMed.
- J. H. Duffus, Pure Appl. Chem., 2002, 74, 793–807 CAS.
- R. M. Izatt, S. R. Izatt, R. L. Bruening, E. Izatt and B. A. Moyer, Chem. Soc. Rev., 2014, 43, 2451–2475 RSC.
- J. R. Rumble, D. R. Lide and T. J. Bruno, CRC handbook of chemistry and physics : a ready-reference book of chemical and physical data, CRC Press, Boca Raton, 2019 Search PubMed.
- J. Yang, C. A. Trickett, S. B. Alahmadi, A. S. Alshammari and O. M. Yaghi, J. Am. Chem. Soc., 2017, 139, 8118–8121 CrossRef CAS PubMed.
- E. Alvarez, A. G. Marquez, T. Devic, N. Steunou, C. Serre, C. Bonhomme, C. Gervais, I. Izquierdo-Barba, M. Vallet-Regi, D. Laurencin, F. Mauri and P. Horcajada, CrystEngComm, 2013, 15, 9899–9905 RSC.
- M. A. Matlinska, M. Ha, B. Hughton, A. O. Oliynyk, A. K. Iyer, G. M. Bernard, G. Lambkin, M. C. Lawrence, M. J. Katz, A. Mar and V. K. Michaelis, ACS Appl. Mater. Interfaces, 2019, 11, 32739–32745 CrossRef CAS PubMed.
- S. R. Miller, D. Heurtaux, T. Baati, P. Horcajada, J.-M. Grenèche and C. Serre, Chem. Commun., 2010, 46, 4526 RSC.
- A. G. Marquez, P. Horcajada, D. Grosso, G. Ferey, C. Serre, C. Sanchez and C. Boissiere, Chem. Commun., 2013, 49, 3848–3850 RSC.
- O. M. Yaghi, M. O'Keeffe, M. Eddaoudi and H. Li, Nature, 1999, 402, 276–279 CrossRef.
- S. Klaus, M. W. Lehenmeier, E. Herdtweck, P. Deglmann, A. K. Ott and B. Rieger, J. Am. Chem. Soc., 2011, 133, 13151–13161 CrossRef CAS PubMed.
- B. Sharma, A. Mahata, S. Mandani, N. Thakur, B. Pathak and T. K. Sarma, New J. Chem., 2018, 42, 17983–17990 RSC.
- S. Wang, H. Reinsch, N. Heymans, M. Wahiduzzaman, C. Martineau-Corcos, G. De Weireld, G. Maurin and C. Serre, Matter, 2020, 2, 440–450 CrossRef.
- M. J. Katz, Z. J. Brown, Y. J. Colón, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449–9451 RSC.
- M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS.
- M. Mathew, S. Takagi and H. L. Ammon, J. Crystallogr. Spectrosc. Res., 1993, 23, 617–621 CrossRef CAS.
- M. Mathew and S. Takagi, Z. Kristallogr. - Cryst. Mater., 1995, 210, 199–201 CrossRef CAS.
- M. Mathew, S. Takagi, B. O. Fowler and M. Markovic, J. Chem. Crystallogr., 1994, 24, 437–440 CrossRef CAS.
- M. Mazaj, V. Kaucic, A. Golobic and N. Zabukovec Logar, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2012, 68, 4–6 CrossRef PubMed.
- R. L. Metcalf and A. R. Horowitz, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2014, DOI:10.1002/14356007.s14_s01.
- B. Slater, S. O. Wong, A. Duckworth, A. J. P. White, M. R. Hill and B. P. Ladewig, Chem. Commun., 2019, 55, 7319–7322 RSC.
- F. N. Shi, J. C. Almeida, L. A. Helguero, M. H. V. Fernandes, J. C. Knowles and J. Rocha, Inorg. Chem., 2015, 54, 9929–9935 CrossRef CAS PubMed.
- L. Li, L. Guo, S. Pu, J. Wang, Q. Yang, Z. Zhang, Y. Yang, Q. Ren, S. Alnemrat and Z. Bao, Chem. Eng. J., 2019, 358, 446–455 CrossRef CAS.
- F. Ke, C. Peng, T. Zhang, M. Zhang, C. Zhou, H. Cai, J. Zhu and X. Wan, Sci. Rep., 2018, 8, 939 CrossRef PubMed.
- S. R. Miller, P. Horcajada and C. Serre, CrystEngComm, 2011, 13, 1894–1898 RSC.
- S. R. Miller, E. Alvarez, L. Fradcourt, T. Devic, S. Wuttke, P. S. Wheatley, N. Steunou, C. Bonhomme, C. Gervais, D. Laurencin, R. E. Morris, A. Vimont, M. Daturi, P. Horcajada and C. Serre, Chem. Commun., 2013, 49, 7773–7775 RSC.
- D. Liu, S. A. Kramer, R. C. Huxford-Phillips, S. Wang, J. Della Rocca and W. Lin, Chem. Commun., 2012, 48, 2668–2670 RSC.
- Z. F. Wu, B. Tan, W. P. Lustig, E. Velasco, H. Wang, X. Y. Huang and J. Li, Coord. Chem. Rev., 2019, 399, 213025 CrossRef CAS.
- D. Britt, H. Furukawa, B. Wang, T. G. Glover and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 20637–20640 CrossRef CAS PubMed.
- D. J. Levine, T. Runčevski, M. T. Kapelewski, B. K. Keitz, J. Oktawiec, D. A. Reed, J. A. Mason, H. Z. H. Jiang, K. A. Colwell, C. M. Legendre, S. A. Fitzgerald and J. R. Long, J. Am. Chem. Soc., 2016, 138, 10143–10150 CrossRef CAS PubMed.
- Y. Liu, J. Xu, X. Tang, Y. Ma, R. Yuan and R. Matsuda, Chem. Lett., 2019, 48, 156–158 CrossRef CAS.
- A. Zurawski, J. C. Rybak, L. V. Meyer, P. R. Matthes, V. Stepanenko, N. Dannenbauer, F. Würthner and K. Müller-Buschbaum, Dalton Trans., 2012, 41, 4067–4078 RSC.
- X. Liu, Y. Zhou, J. Zhang, L. Tang, L. Luo and G. Zeng, ACS Appl. Mater. Interfaces, 2017, 9, 20255–20275 CrossRef CAS PubMed.
- G. Chen, X. Leng, J. Luo, L. You, C. Qu, X. Dong, H. Huang, X. Yin and J. Ni, Molecules, 2019, 24, 1211 CrossRef PubMed.
- T. Steenhaut, S. Hermans and Y. Filinchuk, New J. Chem., 2020, 44, 3847–3855 RSC.
- R. V. Pinto, F. Antunes, J. Pires, V. Graça, P. Brandão and M. L. Pinto, Acta Biomater., 2017, 51, 66–74 CrossRef CAS PubMed.
- M. R. Lohe, M. Rose and S. Kaskel, Chem. Commun., 2009, 6056–6058 RSC.
- S. K. Nune, P. K. Thallapally and B. P. McGrail, J. Mater. Chem., 2010, 20, 7623–7625 RSC.
- B. J. Zhu, X. Y. Yu, Y. Jia, F. M. Peng, B. Sun, M. Y. Zhang, T. Luo, J. H. Liu and X. J. Huang, J. Phys. Chem. C, 2012, 116, 8601–8607 CrossRef CAS.
- J. Sui, L. Wang, W. Zhao and J. Hao, Chem. Commun., 2016, 52, 6993–6996 RSC.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- B. Q. Ma, K. L. Mulfort and J. T. Hupp, Inorg. Chem., 2005, 44, 4912–4914 CrossRef CAS PubMed.
- C. Tamames-Tabar, E. Imbuluzqueta, N. Guillou, C. Serre, S. R. Miller, E. Elkaïm, P. Horcajada and M. J. Blanco-Prieto, CrystEngComm, 2015, 17, 456–462 RSC.
- H. Assi, G. Mouchaham, N. Steunou, T. Devic and C. Serre, Chem. Soc. Rev., 2017, 46, 3431–3452 RSC.
- H. L. Nguyen, New J. Chem., 2017, 41, 14030–14043 RSC.
- C. Serre, J. A. Groves, P. Lightfoot, A. M. Z. Slawin, P. A. Wright, N. Stock, T. Bein, M. Haouas, F. Taulelle and G. Férey, Chem. Mater., 2006, 18, 1451–1457 CrossRef CAS.
- V. Benoit, R. S. Pillai, A. Orsi, P. Normand, H. Jobic, F. Nouar, P. Billemont, E. Bloch, S. Bourrelly, T. Devic, P. A. Wright, G. De Weireld, C. Serre, G. Maurin and P. L. Llewellyn, J. Mater. Chem. A, 2016, 4, 1383–1389 RSC.
- M. Dan-Hardi, C. Serre, T. Frot, L. Rozes, G. Maurin, C. Sanchez and G. Férey, J. Am. Chem. Soc., 2009, 131, 10857–10859 CrossRef CAS PubMed.
- C. Zlotea, D. Phanon, M. Mazaj, D. Heurtaux, V. Guillerm, C. Serre, P. Horcajada, T. Devic, E. Magnier, F. Cuevas, G. Férey, P. L. Llewellyn and M. Latroche, J. Chem. Soc., Dalton Trans., 2011, 40, 4879–4881 RSC.
- J. Ding, M. Chen, X. Du, R. Shang, M. Xia, J. Hu and Q. Zhong, Catal. Lett., 2019, 149, 3287–3295 CrossRef CAS.
- S. Wu, X. Xing, D. Wang, J. Zhang, J. Chu, C. Yu, Z. Wei, M. Hu, X. Zhang and Z. Li, ACS Sustainable Chem. Eng., 2020, 8, 148–153 CrossRef CAS.
- C. Wang, C. Liu, H. R. Tian, L. J. Li and Z. M. Sun, Chem. – Eur. J., 2018, 24, 2952–2961 CrossRef CAS PubMed.
- S. Wang, T. Kitao, N. Guillou, M. Wahiduzzaman, C. Martineau-Corcos, F. Nouar, A. Tissot, L. Binet, N. Ramsahye, S. Devautour-Vinot, S. Kitagawa, S. Seki, Y. Tsutsui, V. Briois, N. Steunou, G. Maurin, T. Uemura and C. Serre, Nat. Commun., 2018, 9, 1–9 CrossRef PubMed.
- M. Wahiduzzaman, S. Wang, J. Schnee, A. Vimont, V. Ortiz, P. G. Yot, R. Retoux, M. Daturi, J. S. Lee, J. S. Chang, C. Serre, G. Maurin and S. Devautour-Vinot, ACS Sustainable Chem. Eng., 2019, 7, 5776–5783 CrossRef CAS.
- J. Santos-Lorenzo, R. San José-Velado, J. Albo, G. Beobide, P. Castaño, O. Castillo, A. Luque and S. Pérez-Yáñez, Microporous Mesoporous Mater., 2019, 284, 128–132 CrossRef CAS.
- M. Gaab, N. Trukhan, S. Maurer, R. Gummaraju and U. Müller, Microporous Mesoporous Mater., 2012, 157, 131–136 CrossRef CAS.
- M. Rubio-Martinez, T. D. Hadley, M. P. Batten, K. Constanti-Carey, T. Barton, D. Marley, A. Mönch, K. S. Lim and M. R. Hill, ChemSusChem, 2016, 9, 938–941 CrossRef CAS PubMed.
- L. Zhou, X. Zhang and Y. Chen, Mater. Lett., 2017, 197, 224–227 CrossRef CAS.
- M. R. Mani, R. Chellaswamy, Y. N. Marathe and V. K. Pillai, RSC Adv., 2016, 6, 1907–1912 RSC.
- K. Wang, R. Li, Y. Cheng and B. Zhu, Coord. Chem. Rev., 1999, 190–192, 297–308 CrossRef CAS.
- R. S. Ripa, J. Kastrup, A. Ekblond, C. Stem, N. Medicine, T. H. Centre and C. Catheterization, in Encyclopedia of Metalloproteins, Springer, New York, 2013, pp. 1098–1103 Search PubMed.
- C. Lizon and P. Fritsch, Int. J. Radiat. Biol., 1999, 75, 1459–1471 CrossRef CAS PubMed.
- P. J. Haley, Health Phys., 1991, 61, 809–820 CrossRef CAS PubMed.
- H. I. Sarkander and W. P. Brade, Arch. Toxicol., 1976, 36, 1–17 CrossRef CAS PubMed.
- A. Kumar, M. Ali, R. S. Ningthoujam, P. Gaikwad, M. Kumar, B. B. Nath and B. N. Pandey, J. Hazard. Mater., 2016, 307, 281–293 CrossRef CAS PubMed.
- X.-C. Huang, Y.-Y. Lin, J.-P. Zhang and X.-M. Chen, Angew. Chem., Int. Ed., 2006, 45, 1557–1559 CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- Y. Pan, Y. Liu, G. Zeng, L. Zhao and Z. Lai, Chem. Commun., 2011, 47, 2071–2073 RSC.
- T. Stolar, L. Batzdorf, S. Lukin, D. Žilić, C. Motillo, T. Friščić, F. Emmerling, I. Halasz and K. Užarević, Inorg. Chem., 2017, 56, 6599–6608 CrossRef CAS PubMed.
- P. A. Julien, K. Užarević, A. D. Katsenis, S. A. J. Kimber, T. Wang, O. K. Farha, Y. Zhang, J. Casaban, L. S. Germann, M. Etter, R. E. Dinnebier, S. L. James, I. Halasz and T. Friščić, J. Am. Chem. Soc., 2016, 138, 2929–2932 CrossRef CAS PubMed.
- L. S. Germann, A. D. Katsenis, I. Huskić, P. A. Julien, K. Užarević, M. Etter, O. K. Farha, T. Friščić and R. E. Dinnebier, Cryst. Growth Des., 2020, 20, 49–54 CrossRef CAS.
- M. J. Cliffe, C. Mottillo, R. S. Stein, D. K. Bučar and T. Friščić, Chem. Sci., 2012, 3, 2495–2500 RSC.
- C. Mottillo, Y. Lu, M.-H. Pham, M. J. Cliffe, T.-O. Do and T. Friščić, Green Chem., 2013, 15, 2121 RSC.
- F. Qi, R. S. Stein and T. Friščić, Green Chem., 2014, 16, 121–132 RSC.
- I. Huskić and T. Friščić, Philos. Trans. R. Soc., A, 2019, 377, 20180221 CrossRef PubMed.
- D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Chem. Sci., 2015, 6, 1645–1649 RSC.
- K. Užarević, T. C. Wang, S. Y. Moon, A. M. Fidelli, J. T. Hupp, O. K. Farha and T. Friščić, Chem. Commun., 2016, 52, 2133–2136 RSC.
- B. Karadeniz, A. J. Howarth, T. Stolar, T. Islamoglu, I. Dejanović, M. Tireli, M. C. Wasson, S. Y. Moon, O. K. Farha, T. Friščić and K. Užarević, ACS Sustainable Chem. Eng., 2018, 6, 15841–15849 CrossRef CAS.
- W. Bury, I. Justyniak, D. Prochowicz, A. Rola-Noworyta and J. Lewiński, Inorg. Chem., 2012, 51, 7410–7414 CrossRef CAS PubMed.
- D. Prochowicz, K. Sokołowski, I. Justyniak, A. Kornowicz, D. Fairen-Jimenez, T. Friščić and J. Lewiński, Chem. Commun., 2015, 51, 4032–4035 RSC.
- J. C. Rybak and K. Müller-Buschbaum, Z. Anorg. Allg. Chem., 2010, 636, 126–131 CrossRef CAS.
- T. P. Vaid, S. P. Kelley and R. D. Rogers, IUCrJ, 2017, 4, 380–392 CrossRef CAS PubMed.
- G. A. V. Martins, P. J. Byrne, P. Allan, S. J. Teat, A. M. Z. Slawin, Y. Li and R. E. Morris, Dalton Trans., 2010, 39, 1758–1762 RSC.
- S. Chen, J. Zhang, T. Wu, P. Feng and X. Bu, Dalton Trans., 2010, 39, 697–699 RSC.
- L. Yang and H. Lu, Chin. J. Chem., 2012, 30, 1040–1044 CrossRef CAS.
- Z. Lian, L. Huimin and Y. Lisha, Mater. Lett., 2014, 125, 59–62 CrossRef.
- K. Lu, A. Liebman Peláez, L. C. Wu, Y. Cao, C. H. Zhu and H. Fu, Inorg. Chem., 2019, 58, 1794–1805 CrossRef CAS PubMed.
- S. H. Zottnick, M. Finze and K. Müller-Buschbaum, Chem. Commun., 2017, 53, 5193–5195 RSC.
- J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- X. Sang, J. Zhang, J. Xiang, J. Cui, L. Zheng, J. Zhang, Z. Wu, Z. Li, G. Mo, Y. Xu, J. Song, C. Liu, X. Tan, T. Luo, B. Zhang and B. Han, Nat. Commun., 2017, 8, 175 CrossRef PubMed.
- P. Cintas and J.-L. Luche, Green Chem., 1999, 1, 115–125 RSC.
- W. J. Son, J. Kim, J. Kim and W. S. Ahn, Chem. Commun., 2008, 6336–6338 RSC.
- C. G. Carson, A. J. Brown, D. S. Sholl and S. Nair, Cryst. Growth Des., 2011, 11, 4505–4510 CrossRef CAS.
- D. A. Yang, H. Y. Cho, J. Kim, S. T. Yang and W. S. Ahn, Energy Environ. Sci., 2012, 5, 6465–6473 RSC.
- H. Y. Cho, J. Kim, S. N. Kim and W. S. Ahn, Microporous Mesoporous Mater., 2013, 169, 180–184 CrossRef CAS.
- U. Mueller, M. Schubert, F. Teich, H. Puetter, K. Schierle-Arndt and J. Pastré, J. Mater. Chem., 2006, 16, 626–636 RSC.
- R. Ameloot, L. Stappers, J. Fransaer, L. Alaerts, B. F. Sels and D. E. De Vos, Chem. Mater., 2009, 21, 2580–2582 CrossRef CAS.
- H. M. Yang, X. L. Song, T. L. Yang, Z. H. Liang, C. M. Fan and X. G. Hao, RSC Adv., 2014, 4, 15720–15726 RSC.
- A. Martinez Joaristi, J. Juan-Alcañiz, P. Serra-Crespo, F. Kapteijn and J. Gascon, Cryst. Growth Des., 2012, 12, 3489–3498 CrossRef CAS.
- A. J. Howarth, Y. Liu, P. Li, Z. Li, T. C. Wang, J. T. Hupp and O. K. Farha, Nat. Rev. Mater., 2016, 1, 15018 CrossRef CAS.
- M. Bosch, M. Zhang and H.-C. Zhou, Adv. Chem., 2014, 2014, 182327 Search PubMed.
- I. J. Kang, N. A. Khan, E. Haque and S. H. Jhung, Chem. – Eur. J., 2011, 17, 6437–6442 CrossRef CAS PubMed.
- N. Li, J. Xu, R. Feng, T. L. Hu and X. H. Bu, Chem. Commun., 2016, 52, 8501–8513 RSC.
- N. U. Qadir, S. A. M. Said and H. M. Bahaidarah, Microporous Mesoporous Mater., 2015, 201, 61–90 CrossRef CAS.
- M. E. A. Safy, M. Amin, R. R. Haikal, B. Elshazly, J. Wang, Y. Wang, C. Wöll and M. H. Alkordi, Chem. Eur. J., 2020, 26 DOI:10.1002/chem.202000207.
- B. S. Gelfand and G. K. H. Shimizu, Dalton Trans., 2016, 45, 3668–3678 RSC.
- J. Liang, Z. Liang, R. Zou and Y. Zhao, Adv. Mater., 2017, 29, 1–21 Search PubMed.
- V. Colombo, S. Galli, H. J. Choi, G. D. Han, A. Maspero, G. Palmisano, N. Masciocchi and J. R. Long, Chem. Sci., 2011, 2, 1311–1319 RSC.
- E. V. Ramos-Fernandez, M. Garcia-Domingos, J. Juan-Alcañiz, J. Gascon and F. Kapteijn, Appl. Catal., A, 2011, 391, 261–267 CrossRef CAS.
- M. Rose, B. Böhringer, M. Jolly, R. Fischer and S. Kaskel, Adv. Eng. Mater., 2011, 13, 356–360 CrossRef CAS.
- M. K. Smith and K. A. Mirica, J. Am. Chem. Soc., 2017, 139, 16759–16767 CrossRef CAS PubMed.
- F. Maya, C. Palomino Cabello, A. Figuerola, G. Turnes Palomino and V. Cerdà, Chromatographia, 2019, 82, 361–375 CrossRef CAS.
- M. Kalaj, K. C. Bentz, S. Ayala, J. M. Palomba, K. S. Barcus, Y. Katayama and S. M. Cohen, Chem. Rev., 2020 DOI:10.1021/acs.chemrev.9b00575.
- M. L. Kim, E. H. Otal and J. P. Hinestroza, Cellulose, 2019, 26, 123–137 CrossRef CAS.
- H. N. Wang, X. Meng, L. Z. Dong, Y. Chen, S. L. Li and Y. Q. Lan, J. Mater. Chem. A, 2019, 7, 24059–24091 RSC.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Chem. Commun., 2012, 48, 11275–11288 RSC.
- P. Silva, S. M. F. Vilela, J. P. C. Tomé and F. A. Almeida Paz, Chem. Soc. Rev., 2015, 44, 6774–6803 RSC.
| This journal is © The Royal Society of Chemistry 2020 |