Open Access Article
Open Access ArticleA more sustainable and highly practicable synthesis of aliphatic isocyanides†
K. A.
Waibel‡
a,
R.
Nickisch‡
b,
N.
Möhl
b,
R.
Seim
b and
M. A. R.
Meier
 *ab
*ab
aInstitute of Biological and Chemical Systems – Functional Molecular Systems (IBCS-FMS), Karlsruhe Institute of Technology (KIT), Straße am Forum 7, 76131 Karlsruhe, Germany. E-mail: m.a.r.meier@kit.edu; Web: http://www.meier-michael.com
bInstitute of Organic Chemistry, Karlsruhe Institute of Technology (KIT), Straße am Forum 7, 76131 Karlsruhe, Germany
First published on 16th January 2020
Abstract
Synthesis protocols to convert N-formamides into isocyanides using three different dehydration reagents (i.e. p-toluenesulfonyl chloride (p-TsCl), phosphoryl trichloride (POCl3) and the combination of triphenylphosphane (PPh3) and iodine) were investigated and optimized, while considering the principles of green chemistry. Comparison of the yield and the E-factors of the different synthesis procedures revealed that, in contrast to the typically applied POCl3 or phosgene derivatives, p-TsCl was the reagent of choice for non sterically demanding aliphatic mono- or di-N-formamides (yields up to 98% and lowest E-factor 6.45). Apart from a significantly reduced E-factor, p-TsCl is cheap, offers a simplified reaction protocol and work-up, and is less toxic compared to other dehydration reagents. Thus, this procedure offers easier and greener access to aliphatic isocyanide functionalities.
Introduction
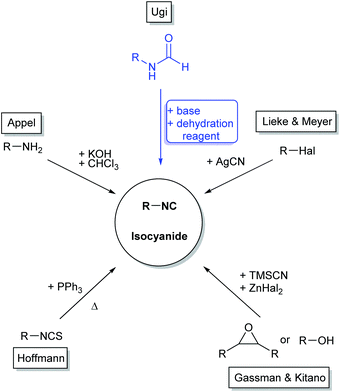
Isocyanides represent a unique type of functional group due to their α-acidity and their ability to perform α-addition as well as radical reactions.1 Except for a few examples, most derivatives exhibit low toxicity and interestingly, many natural isocyanides show antibiotic, fungicidal, antineoplastic, or antifouling effects.1,2 Most commonly, they are used in isocyanide-based multicomponent reactions (IMCRs),1,3–5 which have manifold applications ranging from organic synthesis1 to drug discovery6,7 and polymer science.8–11 As multicomponent reactions (MCRs) are generally considered as sustainable synthesis tools, as they fulfil many of the twelve principles of green chemistry,5 it is important to further consider the synthesis of their starting materials in the scope of green chemistry.Since the first known isocyanide synthesis by Lieke in 1859, many synthesis routes starting from different precursors were described. While Lieke and Meyer were able to obtain isocyanides by reacting allyl or sugar halides with silver cyanide, Hoffmann obtained them by converting amines with in situ formed carbenes of chloroform and potassium hydroxide, or by heating isothiocyanates with PPh3.12–16 Gassman and Kitano introduced trimethylsilyl cyanide as cyanide transfer reagent, which forms isocyanides with alcohols and epoxides in the presence of zinc salts.17,18 However, these procedures suffer from major drawbacks, for instance low to moderate yields and the lack of general applicability, since they are restricted to specific moieties. Nowadays, N-formamides are most often used as starting materials to form isocyanides by addition of a dehydration reagent under basic conditions. Ugi first described this procedure using phosgene and later its surrogates (di- and triphosgene) as dehydration reagents.6,19–23 Afterwards, other reagents were introduced, for instance the Burgess reagent, Appel reagent, trifluoromethyl sulfonic acid anhydride, or p-TsCl.24–27 Nowadays, the commonly used reagent is POCl3 due to its suitability for different structural motifs.28–32 Generally, isocyanide synthesis still heavily relies on laboratory preparation, since the number of commercially available isocyanides is limited to a few examples, and even small amounts are relatively expensive.33 Bienaymé, Bossio and Armstrong focused their isocyanide synthesis work on feasible derivatisation routes, which eventually led to more easily accessible isocyanides.23,34,35 However, most of the dehydration reagents, which are used for converting the formamides into the targeted isocyanide, are either highly toxic or were synthesized by employing toxic precursors (Fig. 1).
In addition, large amounts of waste are produced during the synthesis and thus, typical isocyanide syntheses cannot be considered as sustainable. Recently, Wang et al. introduced a less toxic dehydration reagent using PPh3 and iodine obtaining good yields of up to 90% for mainly aromatic formamides.36 Porcheddu et al. were able to improve the approach initially reported by Hoffmann to a more sustainable procedure by applying mechanochemical activation via ball-milling, reducing the required amount of chloroform to a stoichiometric amount. Thus, they were able to obtain isocyanides with a broad spectrum of aliphatic, benzylic and aromatic moieties in yields up to 71%.37
In this work, we investigated various synthesis procedures in order to develop a more sustainable and generally applicable route to convert aliphatic N-formamides into isocyanides. Therefore, we optimized the isocyanide syntheses employing POCl3, p-TsCl, and the combination of PPh3 with iodine and compared not only the yields but also the E-factors as well as several other parameters such as waste in purification steps and energy consumption.
Results and discussion
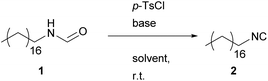
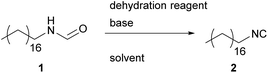
Regarding the aforementioned syntheses of isocyanides, we focused our work on the dehydration of N-formamides to improve the sustainability of these procedures. For this purpose, we investigated three commonly applied dehydration reagents, namely POCl3, PPh3/iodine, and p-TsCl.19,27,38In our investigations, the focus was furthermore laid on suitable, more sustainable solvents since typically dichloromethane (DCM) is used for the dehydration of N-formamides, which is considered as hazardous, as are many other halogenated solvents. Therefore, we chose several candidates by following the respective guidelines for greener solvents.39–41 The synthesis of isocyanides is usually carried out by using highly reactive reagents like POCl3, phosgene, phosgene surrogates, or p-TsCl. Thus, the solvents have to be chemically inert in the reaction, which excludes alcohols, ketones, water and amines, yet leaves a range of different sustainable solvents suitable, which were used in the optimization study (see Tables 1–3). The solvent tests were carried out for each dehydration reagent individually, using N-octadecyl formamide 1 as model substance, because of an easy handling and the absence of other functional groups that could interfere during the reaction (see Tables 1–4).
| Entry | Solvent | Base | c/mol | t/h | Yield/%a | E-factor |
|---|---|---|---|---|---|---|
| a Yields calculated by GC using a calibration curve of product 2. b The corresponding solvent, 5.00 mmol formamide (1.00 eq.), p-TsCl (1.30 eq.) and the base (2.60 eq.) were applied. c The corresponding solvent, 5.00 mmol formamide (1.00 eq.), p-TsCl (1.50 eq.) and the base (3.00 eq.) were applied. d Isolated yield after work-up. | ||||||
| 1 | DCMa | DIPA | 0.330 | 21 | 35b | 51.3 |
| 2 | DCMa | DIPEA | 0.330 | 7.80 | 14b | 132 |
| 3 | DCMa | TEA | 0.330 | 1.50 | 25b | 72.2 |
| 4 | DCMa | Py | 0.330 | 2 | 66b | 26.4 |
| 5 | Me-THFa | Py | 0.330 | 2 | 12c | 106 |
| 6 | DMCa | Py | 0.330 | 2/18 | 7/85c | 217/31.8 |
| 7 | ACNa | Py | 1.00 | 4/18 | 70/56c | 8.20/10.5 |
| 8 | Cyrene™![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
Py | 1.00 | 1/2 | 10/2.4c | 80.2/324 |
| 9 | GBLa | Py | 1.00 | 1/2 | 28/22c | 26.5/34.8 |
| 10 | DCM | Py | 1.00 | 2 | 96c,d | 7.76 |
| 11 | DMC | Py | 1.00 | 18 | 89c,d | 7.41 |
| Entry | Solvent | Yield/%a | E-factor |
|---|---|---|---|
| a The corresponding solvent, formamide (0.33 M in solvent, 3.00 mmol, 1.00 eq.), POCl3 (1.30 eq.) and base (2.60 eq.) were utilized under ice-bath cooling and the reaction was stirred for two hours at room temperature. | |||
| 1 | DCM | 96 | 17.8 |
| 2 | EA | 90 | 13.9 |
| 3 | Me-THF | 94 | 12.6 |
| 4 | DMC | 90 | 15.9 |
In addition, E-factors were calculated according to Sheldon,42 considering all reactants, including the solvent used for the reaction as well as the reagents and solutions applied for quenching, respectively. Please note that the work-up of crude reaction mixtures (i.e. solvent used for extraction and washing or column chromatography) was not taken into account for the herein reported E-factors. Therefore, a synthesis E-factor was exclusively calculated and used herein for comparison reasons.
Formamide dehydration utilizing p-TsCl
In 1979, a procedure to synthesize isocyanides involving quinoline and p-TsCl as dehydrating agent was published by Schuster and collegues.27 They stated that this protocol, involving a simultaneous distillation, is superior to the Ugi-approach for smaller isocyanides (methyl and ethyl isocyanide), as these substrates show decent water solubility and therefore exclude an aqueous work-up of the reaction mixture.The advantageous properties of p-TsCl, if compared to POCl3, are particularly the easier operation, and the significantly lower toxicity. In addition, it has to be emphasized that it is a waste product of the industrial saccharine synthesis by the Remsen–Fahlberg procedure, making the use of this reagent even more sustainable and also economically attractive.43,44
In a first approach, the reaction conditions of the Ugi-approach were applied.19 With regard to sustainability, p-TsCl possesses the highest potential, thus the conditions for this particular reaction were optimized utilizing common GC-screening techniques.
GC-screening for optimized conditions utilizing p-TsCl
The generally accepted mechanism for the dehydration proceeds via the formation of a formamide-dehydrating agent adduct, which subsequently undergoes elimination. Both reaction steps are induced by a base and thus, at least two equivalents of a base are needed to ensure full conversion. Thus, we started our investigations by varying different bases. In general, most amine bases are rated toxic by the globally harmonized system, with pyridine being the only exception as it is only rated health hazardous. The sustainability and toxicity of different bases are studied in detail, confirming this classification.45 Therefore, we chose diisopropylamine (DIPA), diisopropyl ethylamine (DIPEA), pyridine (Py) and triethylamine (TEA), which are all commercially available and can be produced sustainably (see Table 1).46–51The screening results for the optimal base showed significant differences, which are presented in Table 1. The two tertiary amines gave the lowest yields (14% and 25%, see Table 1 entries 2 and 3), whereas DIPA yielded 35%. The most promising result were obtained using pyridine, leading to 66% yield after only two hours of reaction.
It seems that strong bases are not necessarily required to convert formamide 1. Rather steric influences seemed to be dominant, as the obtained yield increases while the steric demand of the applied base decreases.
Having found a suitable base for the dehydration, we investigated different solvents (see Table 1, both sustainable and conventional solvents were tested). We also increased the stochiometric amount of dehydrating agent/base from 1.30/2.60 to 1.50/3.00 equivalents and later increased the concentration of the starting material to 1.00 mol L−1. The latter reduces the amount of solvent, which omits waste while increasing the reaction rate. The further excess of dehydration agent was applied in order to compensate the loss of p-TsCl due to hydrolysis to p-toluenesulfonic acid (PTSA) during the reaction. Consequently, the amount of base was also increased to 3.00 equivalents to ensure that the reaction mixture remains basic throughout the process, as isocyanides decompose in acidic media.
Dihydrolevoglucosenone (Cyrene™) and γ-butyro lactone (GBL) were the two least promising solvent candidates, yielding 2.42% and 21.5% yield after two hours reaction time in 1 M solution (see Table 1 entries 8 and 9). Yields even decreased while the reaction proceeded, indicating side-reactions. 2-Methyltetrahydrofuran (Me-THF) allowed the precipitation of ammonium salts, which should foster the shift of the equilibrium to the product side; however, a low yield of 12% was obtained after two hours. Acetonitrile gave a surprisingly high yield after four hours (70%), but the yield started to decrease afterwards, thus only 56% product remained after 18 hours. However, the least toxic solvent dimethyl carbonate (DMC), which performed poorly on first sight (7% of yield after two hours), convinces with a continuously increasing yield over time, resulting in up to 85% after 18 hours, and resulting in an E-factor of 7.71. Therefore, we repeated the reactions in DCM, the commonly used solvent, and DMC with increased concentration to obtain a valid comparison between non-sustainable and sustainable solvents and observed 96% and 89% of yield, respectively. The reaction in DCM proceeds faster, has a higher yield and shows a slightly higher E-factor. In terms of sustainability, DMC has clear advantages in being non-toxic compared to DCM and the possibility to be produced sustainably from various renewable sources.52,53 Furthermore, we investigated several methods for the reaction work-up (washing and column chromatography). Yet, we stayed with quenching with sodium carbonate solution and aqueous work-up, which led to the most promising results.
Also during purification, DMC was often superior to DCM, since DCM tended to produce quite stable emulsions during the aqueous work-up, requiring more time for the separation process.
The results obtained in this approach were very encouraging, since p-TsCl is the least toxic reagent among the reported dehydration reagents for an isocyanide synthesis. Furthermore, dehydration with p-TsCl is less exothermic, which allows a safer handling and an intense water cooling is only required for reactions in larger scale (batches of up to 100 mmol with isocyanide 6 were conducted).
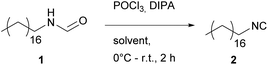
Ugi-approach utilizing (POCl3)
The Ugi procedure utilizing POCl3 is already well-established and is known to give consistently good yields for a wide variety of substrates.9,54–58 As expected, the yields of the first dehydration reaction were quite satisfactory (see Table 2).Further, the replacement of DCM with other solvents also led to consistently good yields ranging from 90% in the case of ethyl acetate (EA) up to 94% in Me-THF. It is noteworthy that POCl3 is highly reactive, which requires consistent cooling while the reactant is added and thus makes the handling of the reaction more challenging. With regard to sustainability, energy intensive consuming processes like cooling or heating should be avoided whenever possible.
The overall E-factors ranged from 12.6 to 17.8, which is in the range of fine chemicals (mostly valued with 5–50).
However, this E-factor does not include the fact that POCl3 is a quite hazardous chemical because of its high reactivity, corrosivity, and toxic properties. Thus, omitting such chemicals should be the privileged task of future syntheses.
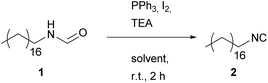
Wang procedure utilizing (PPh3) and iodine (I2)
In 2015, Wang et al.38 published a procedure using PPh3 and iodine, acting as dehydrating agents similar to the Appel reagent. They stated that their investigations were driven by convenience, as POCl3 is not easily available in China. However, both PPh3 and iodine exhibit a lower toxicity compared to POCl3.Moreover, PPh3 is a stable solid, which is reactive with oxidizing agents and can thus be handled more easily. However, iodine slowly sublimes at room temperature and is hazardous when inhaled as well as skin irritating. The procedure reported by Wang was adapted and applied to formamide 1. Similarly as for the other two procedures, a solvent evaluation was performed and the results were compared with the other approaches (see Table 3).
The obtained results of the solvent screening showed significant differences for this reaction (see Table 3), ranging from 33% yield using DMC up to 93% for Me-THF. Interestingly, high yields were not achieved with DCM as solvent.
However, this combination of reactants exhibiting a lower toxicity compared to POCl3 led to a significant increase of the E-factor (18.4 up to 60.8).
Comparison of the three approaches
The results obtained by optimizing the three approaches were compared in a comprehensive comparative study in order to evaluate the effectiveness and E-factor (see Table 4).POCl3 is the most hazardous chemical of all three dehydration reagents. It is toxic, corrosive and reacts highly exothermic. The lower reactivity of p-TsCl is one of its advantages, as it is easier to handle and can be applied in smaller amounts of solvents, thus higher concentration (1 mol L−1 concentration of the starting material). Consequently, less solvent waste is produced.
Moreover, the reaction with p-TsCl only has to be cooled for the quenching process even in large scales (up to 100 mmol with isocyanide 5 were tested), whereas addition of POCl3 always requires active cooling to 0 °C or lower (see Fig. 2).
Triphenylphosphine and iodine are of lower toxicity if compared to POCl3, yet the method has its limitations in the E-factor and the further purification.
The approaches by Ugi and Wang achieve their lowest E-factor using Me-THF as solvent. In case of POCl3 in combination with DIPA, the value amounts to 12.7 kg of waste per kilogram isocyanide, whereas applying triphenylphosphine, iodine and diisopropylethylamine amine results in 18.4 kg waste per kg product. To sum up, the advantage of using less toxic reagents for the isocyanide synthesis of substrate 2 is diminished by producing an additional 5.70 kg of waste per kg product. Another aspect to be considered is the product work-up. Avoiding column chromatography as purification method was one of the main goals in this study, as the amount of solvent and silica used add significantly to the overall E-factor. The reaction with POCl3 yields the product in reasonable purity after simple extraction, as all by-products are water soluble compounds, which is not possible in case of the Wang procedure. In this process, triphenylphosphine oxide is obtained as side product, which, in contrast to the phosphorus acid derivatives, is poorly soluble in water and thus cannot be extracted. Therefore, column chromatography cannot be omitted in this approach, which adds to the E-factor.
The utilization of p-TsCl on the other hand allows to avoid column chromatography, since the formed pyridinium salts can be easily removed by water extraction (vide infra).
Within this comparative study, the best overall results were obtained using DMC as solvent for p-TsCl, which further increases the sustainability of the reaction, as both DMC and Me-THF are renewable, but DMC is non-toxic, whereas Me-THF is health hazardous. Remarkably, this approach led an E-factor of 7.41 and a yield of 89%. It is noteworthy that the more sustainable and less toxic reagent p-TsCl gives a comparable yield for formamide 1, while being easy to handle. Consequently, this approach revealed itself to be the most promising one both in terms of sustainability and in terms of practicability and was thus used in the subsequent investigations.
Isocyanide syntheses with optimized reaction conditions
To prove the versatility of the optimized reaction conditions of the most promising reaction with p-TsCl (see Scheme 1), we decided to synthesize a library of isocyanide compounds (see Table 5). Due to the faster reaction rate of DCM (average reaction time of two hours) compared to DMC, we performed each synthesis in both the conventional solvent and the more sustainable alternative.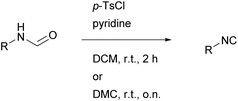 | ||
| Scheme 1 Optimized reaction conditions of the isocyanide synthesis using p-TsCl and pyridine at room temperature in DCM for two hours or in DMC overnight (o.n.). | ||
| Entry | Substrate | Procedure Aa – yield/% | E-factor A | Procedure Bb – yield/% | E-factor B | Literature – yield/% | E-factor literaturec |
|---|---|---|---|---|---|---|---|
| n.L. = no literature available.a Formamide (5.00 mmol, 1.00 eq.) in DCM (1 M), 1.50 (3.00)/3.00 (6.00) eq. p-TsCl/pyridine at r.t. for 2 h.b Formamide (5.00 mmol, 1.00 eq.) in DMC (1 M), 1.50 (3.00)/3.00 (6.00) eq. p-TsCl/pyridine at r.t. overnight.c E-factors were calculated using the values in the respective literature.d Adjusted equivalents: Formamide (5.00 mmol, 1.00 eq.) in DCM (1M), 1.70/3.40 eq. p-TsCl/pyridine at r.t. for 2 h. | |||||||
| 1 |
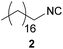
|
96 | 7.76 | 89 | 7.40 | 8754 | 36.9 |
| 2 |
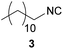
|
90 | 11.9 | 94 | 9.93 | 9454 | 48.8 |
| 3 |
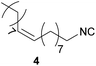
|
97 | 7.73 | 98 | 6.68 | n.L. | — |
| 4 |
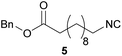
|
97 | 7.11 | 97 | 6.45 | 6660 | 22.3 |
| 5 |
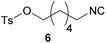
|
53 | 15.0 | 68 | 11.0 | n.L. | — |
| 6 |
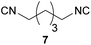
|
48 | 49.0 | 82 | 25.7 | n.L. | — |
| 7 |
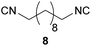
|
93 | 15.8 | 89 | 15.0 | 7159 | 33.6 |
| 8 |
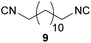
|
87 | 14.8 | 97 | 12.0 | 7556 | 33.6 |
| 9 |
|
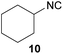
67 | 28.8 | 68 | 24.9 | 7661 | 62.0 |
| 10 |
|
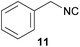
44 | 41.5 | 62 | 25.6 | 649 | 22.2 |
| 11 |
|
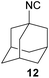
79 | 16.5 | 78 | 14.7 | 9362 | 28.9 |
| 12 |
|
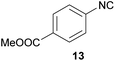
13d | 108.6 | — | — | 9657 | 12.9 |
To compare our results, we have chosen several isocyanides, which either were already synthesized by the Ugi-approach and are reported in the literature, or were commercially available (isocyanides 10, 11 and 12).
In case of the long-chain alkyl isocyanides 2, 3 and 4, the yields obtained in DCM (96/90/97%) as well as in DMC (89/94/98%) are similar to the ones reported in the literature (87/94/no literature (n.L.)%54), but in comparison with literature the E-factor is 75% lower for compound 2 and 80% lower for compound 3, which is a remarkable improvement toward a more sustainable process for these two isocyanides.
Overall, the yields and the E-factors for the di-isocyanides 8/9 as well as the benzylated isocyanide 5 were improved by using the new and more sustainable procedure.56,59,60 It has to be highlighted that isocyanide 4 as well as the di-isocyanides 8/9 are of special interest, as all three originate from renewable feedstock.
Isocyanide 5 was chosen as an example, since it can be used for the synthesis of sequence-defined macromolecules.31 Please note that we decided to revise the reported three-step synthesis in terms of sustainability (compare ESI†). Our new synthesis protocol gives an overall yield of 94% with an E-factor of 16.8, whereas the old procedure exhibits an overall yield of 63.4% with an E-factor of 33.2,60 effectively halving the value of the E-factor of the reported synthesis and also omitting the hazardous chemicals sulfurous dichloride and POCl3, which are applied in the first and third step of the previous synthesis protocol, respectively.
Dehydration of a hydroxy-functionalized formamide to isocyanide 6 was possible in moderate yields, while activating the beforementioned functionality with simultaneous tosylation, which enables post-functionalization.
However, the three commercially available isocyanides (10, 11, 12) reveal the limitations of the presented approach. The yield seems to be related to both water solubility and steric hindrance and is also confirmed considering benzylic or aromatic formamides as reactants (see. 11 and 13). While the new synthesis for the compounds 10 and 12 was more sustainable in terms of the E-factor compared to the literature procedures (62.0–24.9/28.9–14.7),61,62 this is not the case for compound 11, yet the yields were comparable (22.2 to 25.6).9
In 2013, for example, Kim et al. published a convenient synthesis protocol for isocyanides in a continuous-flow microreactor with excellent yields for 10 and 11.58 Yet, such microreactors are not generally available and Kim et al. still relied on POCl3 in DCM, which we tried to omit for reasons discussed above. Finally, the aromatic isocyanide 13 underlined the limitations of our procedure, which led only to 13% yield and an E-factor of 108.6.
This compound variation clearly reveals that the newly developed procedure offers very promising results for the synthesis of non-sterically hindered aliphatic isocyanides. We used column chromatography to determine the yields of the synthesized isocyanides, but we observed that flash column chromatography was sufficient to isolate the pure products. However, as mentioned before, many of the products could be obtained in satisfying purity by simply increasing the number of washing steps. Exemplary, the 1H-NMR spectra of compound 9 after purification by washing and by flash column chromatography are depicted in Fig. 3 and they show a comparable purity.
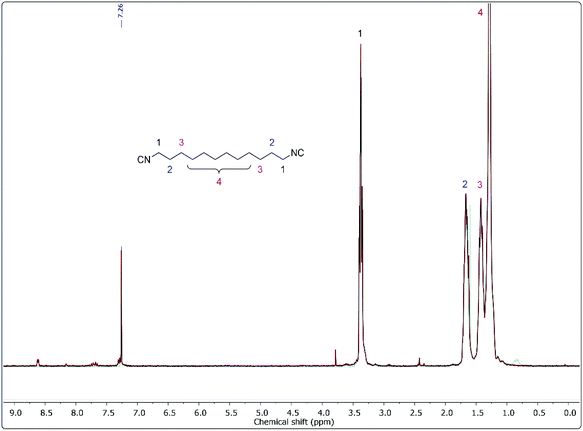 | ||
| Fig. 3 1H-NMR spectrum of compound 9 after several washing step (red line), and after purification by flash column chromatography (blue line), both measured in CDCl3. | ||
Passerini-three-compontent polymerization reaction (P-3CPR)
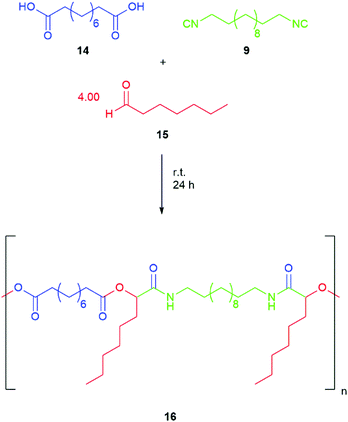
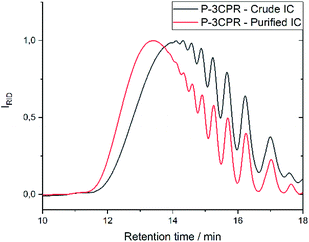
In order to verify that the herein obtained isocyanides can be used without further purification by column chromatography, we have chosen the step-growth polymerization reaction via P-3CR. Since high molecular weights can only be obtained if pure reagents in an equimolar amount are used, which is crucial in step-growth polymerizations (see Scheme 2), even small impurities alter yield and conversion in those polymerizations greatly. Since bifunctional acids and monofunctional aldehydes are often available from renewable feedstocks, we decided to use sebacic acid 14 and heptanal 15, as both are available from castor oil63 and converted them with 1,12-diisocyanododecane 9 to Passerini-polymer 16 in bulk to underline the sustainable character of the test polymerization. For this purpose, the reaction was performed twice, first with the isocyanide 9 purified by flash column chromatography and another time using compound 9 purified by washing steps (compare Scheme 2). After precipitation, the samples were analyzed and compared by SEC (see Fig. 4). The number averaged molecular weight (Mn) of the obtained polymer 16, which was synthesized with pure isocyanide 9 (previously purified by column chromatography), exhibited a Mn value of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1, whereas the crude isocyanide led to a polymer with a slightly lower Mn value of 8350 g mol−1, which is still satisfying and underlines the advantage of the simplified and more sustainable purification process. Both polymers were waxy solids, yet the polymer obtained utilizing the crude isocyanide exhibited a darker color.
500 g mol−1, whereas the crude isocyanide led to a polymer with a slightly lower Mn value of 8350 g mol−1, which is still satisfying and underlines the advantage of the simplified and more sustainable purification process. Both polymers were waxy solids, yet the polymer obtained utilizing the crude isocyanide exhibited a darker color.
Conclusions
The dehydration of formamides utilizing POCl3, PPh3 in combination with iodine, and p-TsCl to yield isocyanides was evaluated in terms of sustainability. We observed that p-TsCl was able to give the highest yields for non-sterically demanding aliphatic N-formamides (up to 98%), while also resulting in the lowest E-factors (down to 6.45). In addition, we introduced DMC as a sustainable alternative to dichloromethane and employed it in the dehydration process of ten different aliphatic formamides. Thus, the aforementioned products were isolated in high yields and excellent purity, while obtaining lower E-factors compared to already established synthesis protocols. Furthermore, we showed that isocyanides of sufficient purity were obtained by simple extraction, and subsequently successfully employed in the P-3CPR to obtain polymers with satisfying molecular weights. However, some limitations of the new procedure were observed, as low conversions for sterically more demanding or aromatic compounds like benzylic or aromatic formamides were obtained. Nonetheless, a straightforward and in terms of sustainability significantly improved synthesis procedure for aliphatic isocyanides was established. The procedure provides a notable advantage for sustainable isocyanide-based chemistries, such as IMCRs.Experimental
General isocyanide synthesis with p-TsCl in DCM (5.00 mmol scale)
The formamide (5.00 mmol, 1.00 eq.) was dissolved in 5 mL DCM and pyridine (15.0 mmol, 3.00 eq.) was added. Subsequently, p-TsCl (7.50 mmol, 1.50 eq.) was added under cooling with a water bath. The cooling was removed, and the reaction mixture was stirred until full conversion (average reaction time of two hours). Afterwards, the mixture was cooled to 0 °C and subsequently aqueous sodium carbonate solution (5 mL, 20 wt%) was added and the biphasic mixture was stirred for another 30 minutes. 10 mL of water and DCM were added, and the organic phase was separated. The aqueous phase was extracted with DCM (3 × 5 mL), the organic extracts were combined and washed with water (3 × 5 mL) and saturated sodium chloride solution (2 × 5 mL). The organic extract was dried over sodium sulfate, filtered off and the solvent removed under reduced pressure. Further purification is not necessary in many cases. Nevertheless, purification by flash column chromatography (mixture of cyclohexane and ethyl acetate) was applied to obtain the product in highest possible purity.General isocyanide synthesis with p-TsCl in DMC (5.00 mmol scale)
The formamide (5.00 mmol, 1.00 eq.) was dissolved in 5 mL DMC and pyridine (15.0 mmol, 3.00 eq.) was added. Subsequently, p-TsCl (7.50 mmol, 1.50 eq.) was added under cooling with a water bath. The cooling was removed, and the reaction mixture was stirred until full conversion was reached (determined by TLC, average reaction time of 18 hours). Afterwards, the mixture was cooled to 0 °C and subsequently aqueous sodium carbonate solution (5 mL, 20 wt%) was added under ice bath cooling and the biphasic mixture was stirred for another 30 minutes at room temperature. 10 mL of water and DMC were added, and the organic phase was separated. The aqueous phase was extracted with DMC (3 × 5 mL), the organic extracts were combined and washed with water (3 × 5 mL) and saturated sodium chloride solution (2 × 5 mL). The organic extract was dried over sodium sulfate, filtered and the solvent was removed under reduced pressure. Further purification is not necessary in many cases. Nevertheless, purification by flash column chromatography (mixture of cyclohexane and ethyl acetate was applied to obtain the product in highest possible purity).Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We would like to acknowledge support from the Karlsruhe Institute of Technology (KIT) and the Helmholtz association. Additionally, we would like to thank Camilla Rieker and Nicola Seul, who also contributed to this work.Notes and references
- A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3168 CrossRef.
- N. Fusetani, Curr. Org. Chem., 1997, 1, 127 CAS.
- M. Passerini, Gazz. Chim. Ital., 1923, 53, 331 CAS.
- I. Ugi, R. Meyr, U. Fetzer and C. Steinbrückner, Angew. Chem., 1959, 71, 386 Search PubMed.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958 RSC.
- T. Yamaguchi, Y. Miyake, A. Miyamura, N. Ishiwata and K. Tatsuta, J. Antibiot., 2006, 59, 729 CrossRef CAS PubMed.
- I. Akritopoulou-Zanze, Curr. Opin. Chem. Biol., 2008, 12, 324 CrossRef CAS PubMed.
- A. Sehlinger, O. Kreye and M. A. R. Meier, Macromolecules, 2013, 46, 6031 CrossRef CAS.
- A. Sehlinger, L. M. De Espinosa and M. A. R. Meier, Macromol. Chem. Phys., 2013, 214, 2821 CrossRef CAS.
- R. Kakuchi, Angew. Chem., Int. Ed., 2014, 53, 46 CrossRef CAS PubMed.
- Z. Zhang, Y.-Z. You, D.-C. Wu and C.-Y. Hong, Macromolecules, 2015, 48, 3414 CrossRef CAS.
- W. Lieke, Liebigs Ann. Chem., 1859, 112, 316 CrossRef.
- E. Meyer, J. Prakt. Chem., 1866, 147 Search PubMed.
- P. Boullanger and G. Descotes, Tetrahedron Lett., 1976, 17, 3427 CrossRef.
- A. W. Hoffmann, Ber. Dtsch. Chem. Ges., 1867, 144, 114 Search PubMed.
- W. P. Weber, G. W. Gokel and I. K. Ugi, Angew. Chem., Int. Ed. Engl., 1972, 11, 530 CrossRef CAS.
- P. G. Gassman and T. L. Guggenheim, J. Am. Chem. Soc., 1982, 104, 5849 CrossRef CAS.
- Y. Kitano, K. Chiba and M. Tada, Tetrahedron Lett., 1998, 39, 1911 CrossRef CAS.
- I. Ugi and R. Meyr, Angew. Chem., 1958, 70, 702 CrossRef CAS.
- G. Skorna and I. Ugi, Angew. Chem., Int. Ed. Engl., 1977, 16, 259 CrossRef.
- A. Efraty, I. Feinstein, L. Wackerle and A. Goldman, J. Org. Chem., 1980, 45, 4059 CrossRef CAS.
- K. Katayama, K. Nakagawa, H. Takeda, A. Matsuda and S. Ichikawa, Org. Lett., 2014, 16, 428 CrossRef CAS PubMed.
- T. A. Keating and R. W. Armstrong, J. Am. Chem. Soc., 1996, 118, 2574 CrossRef CAS.
- S. M. Creedon, H. K. Crowley and D. G. McCarthy, J. Chem. Soc., Perkin Trans. 1, 1998, 1015 RSC.
- R. Appel, R. Kleinstück and K.-D. Ziehn, Angew. Chem., Int. Ed. Engl., 1971, 10, 132 CrossRef CAS.
- J. E. Baldwin and I. A. O'Neil, Synlett, 1990, 603 CrossRef CAS.
- R. E. Schuster, J. E. Scott and J. Casanova Jr., J. Mol. Spectrosc., 1979, 76, 55 CrossRef.
- S. Abou-Shehada, P. Mampuys, B. U. W. Maes, J. H. Clark and L. Summerton, Green Chem., 2017, 19, 249 RSC.
- S. C. Solleder, K. S. Wetzel and M. A. R. Meier, Polym. Chem., 2015, 6, 3201 RSC.
- R. Obrecht, R. Herrmann and I. Ugi, Synthesis, 1985, 400 CrossRef CAS.
- S. C. Solleder, D. Zengel, K. S. Wetzel and M. A. R. Meier, Angew. Chem., Int. Ed., 2016, 55, 1204 CrossRef CAS PubMed.
- D. H. R. Barton, T. Bowles, S. Husinec, J. E. Forbes, A. Llobera, A. E. A. Porter and S. Z. Zard, Tetrahedron Lett., 1988, 29, 3343 CrossRef CAS.
- 23 isocyanides and one diisocyanide are offered on Sigma-Aldrich (purity 94–99% in smaller gram scales) 59 isocyanides and one diisocyanide are offered on abcr (in smaller gram scales, many only on request) date accessed 25.09.19.
- H. Bienaymé, Tetrahedron Lett., 1998, 35, 640 Search PubMed.
- R. Bossio, S. Marcaccini, R. Pepino, U. Schiff, U. Firenze and V. G. Capponi, Liebigs Ann. Chem., 1990, 935 CrossRef CAS.
- X. Wang and Q. Wang, Synthesis, 2015, 47, 49 CAS.
- R. Mocci, S. Murgia, L. De Luca, E. Colacino, F. Delogu and A. Porcheddu, Org. Chem. Front., 2018, 5, 531 RSC.
- X. Wang, Q. G. Wang and Q. L. Luo, Synthesis, 2015, 47, 49 CAS.
- K. Alfonsi, J. Colberg, P. J. Dunn, T. Fevig, S. Jennings, T. A. Johnson, H. P. Kleine, C. Knight, M. A. Nagy, D. A. Perry and M. Stefaniak, Green Chem., 2008, 31 RSC.
- R. K. Henderson, C. Jimenez-Gonzalez, D. J. C. Constable, S. R. Alston, G. G. A. Inglis, G. Fisher, J. Sherwood, S. P. Binks and A. D. Curzons, Green Chem., 2011, 854 RSC.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546 RSC.
- R. A. Sheldon, Chem. Ind., 1992, 903 CAS.
- C. Fahlberg and I. Remsen, Ber. Dtsch. Chem. Ges., 1879, 12, 469 CrossRef.
- D. J. Ager, D. P. Pantaleone, S. A. Henderson, A. R. Katritzky, I. Prakash and D. E. Walters, Angew. Chem., Int. Ed., 1998, 37, 1802 CrossRef CAS.
- R. K. Henderson, A. P. Hill, A. M. Redman and H. F. Sneddon, Green Chem., 2015, 17, 945 RSC.
- W. Bull, One-step process for preparing diisopropylamine, US2686811, 1954.
- F. Haese, J. Wulff-Döring, U. Köhler, P. Gaa, F.-F. Pape, J.-P. Melder and M. Julius, BASF SE, Method for the continuous production of an amine, WO2006136571A1, 2006.
- J. Eberhardt, H. Meissner, B. W. Hoffer, J.-P. Melder and E. Schwab, BASF SE, Process for the preparation of an amine, US20100267948A1, 2010.
- S. Shimizu, N. Watanabe, T. Kataoka, T. Shoji, N. Abe, S. Morishita and H. Ichimura, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed.
- W. Reppe and W. J. Schweckendiek, Justus Liebigs Ann. Chem., 1948, 560, 104 CrossRef CAS.
- K. Eller, E. Henkes, R. Rossbacher and H. Höke, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed.
- P. Tundo and M. Selva, Acc. Chem. Res., 2002, 35, 706 CrossRef CAS PubMed.
- H.-J. Buysch, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed.
- K. Pérez-Labrada, I. Brouard, I. Méndez and D. G. Rivera, J. Org. Chem., 2012, 77, 4660 CrossRef PubMed.
- S. C. Solleder, K. S. Wetzel and M. A. R. Meier, Polym. Chem., 2015, 6, 3201 RSC.
- A. Sehlinger, P. K. Dannecker, O. Kreye and M. A. R. Meier, Macromolecules, 2014, 47, 2774 CrossRef CAS.
- M. K. W. Mackwitz, A. Hamacher, J. D. Osko, J. Held, A. Schöler, D. W. Christianson, M. U. Kassack and F. K. Hansen, Org. Lett., 2018, 20, 3255 CrossRef CAS PubMed.
- S. Sharma, R. A. Maurya, K. I. Min, G. Y. Jeong and D. P. Kim, Angew. Chem., Int. Ed., 2013, 52, 7564 CrossRef CAS PubMed.
- A. V. Gulevich, L. S. Koroleva, O. V. Morozova, V. N. Bakhvalova, V. N. Silnikov and V. G. Nenajdenko, Beilstein J. Org. Chem., 2011, 7, 1135 CrossRef CAS PubMed.
- S. C. Solleder, K. S. Wetzel and M. A. R. Meier, Polym. Chem., 2015, 6, 3201 RSC.
- J. G. Polisar, L. Li and J. R. Norton, Tetrahedron Lett., 2011, 52, 2933 CrossRef CAS.
- K. Škoch, I. Císařová and P. Štěpnička, Chem. – Eur. J., 2018, 24, 13788 CrossRef PubMed.
- H. Mutlu and M. A. R. Meier, Eur. J. Lipid Sci. Technol., 2010, 112, 10 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and Methods. Synthesis procedures and characterization of products. See DOI: 10.1039/c9gc04070f |
| ‡ Both authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |