Open Access Article
Open Access ArticleComprehensive screening of quaternary ammonium surfactants and ionic liquids in wastewater effluents and lake sediments†
Sarah G.
Pati‡
 * and
William A.
Arnold
* and
William A.
Arnold
 *
*
Department of Civil, Environmental, and Geo- Engineering, University of Minnesota, 500 Pillsbury Drive SE, Minneapolis, Minnesota 55455-0116, USA. E-mail: arnol032@umn.edu; Fax: +1 612 626 7750; Tel: +1 612 625 8582
First published on 24th January 2020
Abstract
Quaternary ammonium compounds (QACs) are widely applied as surfactants and biocides in cleaning and personal-care products. Because of incomplete removal during wastewater treatment, QACs are present in wastewater effluents, with which they are discharged into natural waters, where they accumulate in sediments. To assess the levels of QACs in aquatic environments, a liquid chromatography high-resolution mass spectrometry method using both target and suspect screening was developed. The water and sediment sample preparation, measurement, and data analysis workflow were optimized for 22 target compounds with a wide range of hydrophobicity, including ionic liquids that have potential use as solvents and QACs common in personal-care and sanitizing products. In wastewater effluents, average concentrations of all target and suspect QACs combined ranged from 0.4 μg L−1 to 6.6 μg L−1. Various homologs of benzylalkyldimethylammonium (BAC) and dialkyldimethylammonium (DADMAC) as well as the ionic liquid butylpyridinium and 15 suspect QACs were detected in at least one wastewater effluent sample. A spatial profile of sediment samples in a lake demonstrated potential inputs from both municipal wastewater effluent and agricultural sources for BACs. In sediment cores, two distinct trends of temporal QAC accumulation were observed. In lakes with large watersheds and mixed domestic and industrial wastewater sources (Lake Pepin and Duluth Harbor), peak concentrations of QACs were found at depths corresponding to deposition in the 1980s and decreases after this time are attributed to improved wastewater treatment and source control. In a smaller lake with predominantly domestic wastewater inputs (Lake Winona), concentrations of QACs increased slowly over time until today.
Environmental significanceQuaternary ammonium compounds (QACs) are widely used as surfactants and biocides and also comprise the cations in ionic liquids. QACs have the potential to reach the environment via household, agricultural, and industrial use, and it is important to understand the distribution and sources of these compounds in aquatic systems. QACs were found in both wastewater effluents and sediments, with compounds containing twelve or more carbons in the side-chain being more prevalent in both matrices. Data from wastewater treatment plants with different unit operations and from sediment cores point to wastewater treatment practices and usage rates being important factors in dictating the environmental prevalence of QACs. |
1. Introduction
Quaternary ammonium compounds (QACs) are a group of organic chemicals containing a positively-charged quaternary amine group and one or two long alkyl chains. Due to their amphiphilic properties, QACs have been used extensively for decades as surfactants and biocides and are found in cleaning products, personal-care products, liquid medical products, and pesticide formulations.1–3 The three most commonly used groups of QACs are benzylalkyldimethylammonium compounds (BACs), dialkyldimethylammonium compounds (DADMACs), and alkyltrimethylammonium compounds (ATMACs). More recently, compounds containing a quaternary amine group in a ring system, such as imidazolium, pyridinium, pyrrolidinium, and piperidinium, have been developed as ionic liquids, which are potential replacements for volatile organic solvents in industrial applications.4 While BACs, DADMACs, and ATMACs are typically applied as homolog mixtures with alkyl chains ranging from 10 to 20 C atoms, ionic liquids often have shorter alkyl chains of 2 to 10 C atoms, making them less hydrophobic and potentially more mobile in aqueous environments.Due to their applications as cleaners, solvents, and as additives to personal-care products, the majority of QACs used end up in domestic and industrial wastewater. While biodegradation of QACs has been shown to occur in laboratory studies,5–8 their removal in wastewater treatment plants (WWTPs) is likely driven by sorption to activated sludge.9–11 Consequently, QACs have been detected world-wide in WWTP influent, effluent, and sludge samples with concentrations typically in the high and low μg L−1 range for influents and effluents, respectively, as well as in the mid-to-high μg g−1 range for sewage sludge.12–15 As evident from these results as well as detection of QACs in river water samples,16–18 QACs are not completely removed during wastewater treatment and are released into the natural environment. Concern about the presence of QACs in the environment arises from the potential of these compounds to promote antibiotic resistance and serve as precursors for disinfection by-products.3,19–21 In addition, the degradation of QACs in the natural environment by both microorganisms and photolysis is slow,4,5,7,8,22–24 resulting in accumulation of significant amounts of these compounds in sediments.25–27
For a complete exposure assessment, quantitative analysis of all QACs present in the environment is desirable yet challenging due to the large number of different homologs that are used for each QAC subgroup. In addition, the hydrophobicity of QACs varies considerably depending on the number of C atoms in the alkyl chain, potentially affecting their recovery during sample preparation and their chromatographic behavior. For example, dioctadecyldimethylammonium (C18-DADAMAC) contains 38 aliphatic C atoms and strongly accumulates in sediments.13,28 The ionic liquid 1-ethyl-3-methylimidazolium (C2-IMI), however, contains a total of 6 C atoms, only 3 of which are aliphatic, and is highly water soluble.29 Additional analytical challenges, including instrument blanks and carry-over, are often associated with quantifying QACs by liquid chromatography coupled to mass spectrometry (LC/MS).28,30 Consequently, previously developed methods have mostly focused on one QAC subgroup and targeted analysis resulting in the successful quantification of BACs, DADAMACs, or ATMACs by LC/MS in water and sediment samples with overall detection limits of 1–200 ng L−1 and 0.1–3 ng g−1, respectively.12–14,16,17,28,31–35 Unlike these main QAC groups, ionic liquids, cetylpyridinium, domiphen, and benzethonium have, to the best of our knowledge, not been included in previous methods.
Given the large number of QACs that are potentially present in the environment, it is often not feasible to have standards on hand for all compounds that could be detected in a sample. For other groups of organic contaminants, suspect screening is being applied increasingly as a tool to maximize the number of compounds detected, at least qualitatively, in a single measurement.36–39 Application of a suspect screening approach holds great potential for detecting homolog series of QACs in environmental samples due to the chemical similarity of these compounds, which simplifies the prediction of retention times and fragmentation patterns for a given family of structures.
The goal of this study was to develop a target and suspect screening method utilizing liquid chromatography coupled to high-resolution mass spectrometry (LC-HRMS) and sample preparation procedures capable of capturing a wide range of QACs in water and sediment samples. The combined analytical and data evaluation workflow was optimized for 22 target compounds ranging from the polar ionic liquid C2-IMI to apolar QACs, such as C18-DADMAC, and it was applied to screen for QAC contamination in effluents from 12 WWTPs and sediment cores from 4 different lakes in the state of Minnesota. The combined results provide a comprehensive overview of current and historic input of QACs into natural aquatic environments.
2. Methods
A detailed description of all chemicals used is given in Section S1 of the ESI.† Abbreviations of the target QACs are listed in Table S1.† In general, compounds will be referred to by the number of C atoms in the longest side-chain as a prefix to the abbreviation of the QAC subgroup, for example C12-BAC for dodecyldimethylbenzylammonium.2.1 Wastewater effluent sample collection and preparation
Thirteen wastewater effluent samples were collected from 12 WWTPs in and around the Minneapolis–Saint Paul metropolitan area in late November 2018. An overview of general water chemistry parameters of the samples, as well as the size and treatment methods of the WWTPs, are listed in Table S2 in the ESI.† Sampling volumes were approximately one liter. Effluents 1–8 are 24 hour composites sampled automatically from 6:00 am on November 26 to 6:00 am on November 27. Effluents 9–13 are grab samples collected on November 28 between 9:00 am and noon. Effluents 12 and 13 were collected from the same WWTP, which operates two parallel treatment trains, one with conventional aeration (sample 12) and one using pure O2 (sample 13). Due to the season, effluents were not disinfected at any of the sampled plants except for effluent 8, which is chlorinated year-round. Samples were collected in glass bottles and kept on ice and in the dark until processed on November 29.Unfiltered samples were processed by solid-phase extraction (SPE) to limit loss of QACs observed during filtration and centrifugation of matrix-free control samples. To remove larger particles, all samples were shaken and allowed to settle overnight on ice before aliquots for SPE were removed from the top portion of the bottle. Final concentrations reported in this study thus reflect total QAC amounts present in the samples after overnight settling, which includes truly dissolved QACs, QACs associated with dissolved organic matter, and QACs bound to remaining small particles. Effluent 8 was amended with 1 g L−1 sodium thiosulfate to quench residual chlorine. For each effluent sample, two sub-samples were processed (one spiked and one unspiked). For each sub-sample, 250 g of effluent was weighed into a clean glass flask and equilibrated to room temperature. One of the sub-samples served as a spike-and-recovery control and was amended with 250 ng of all 22 target compounds (see Table S3 in the ESI†). In addition, all samples were spiked with three surrogate standards: 450 ng L−1 tetraoctylammonium (C8-TAA), 350 ng L−1 C10-ATMAC-d9, and 100 ng L−1 C14-BAC-d7. SPE was performed with 6 mL Oasis WCX cartridges (150 mg, 30 μm, Waters Corporation). Cartridges were conditioned with 5 mL methanol and 5 mL ultrapure water before loading the samples at approximately 5 mL min−1. Afterwards, cartridges were washed with 5 mL ultrapure water and 5 mL methanol and dried under vacuum for 2 min. Samples were eluted with 6 mL of 2% formic acid in acetonitrile. Increasing elution volumes above 6 mL did not have an effect on recoveries. SPE extracts were completely evaporated under a gentle N2 stream in a water bath at 40 °C. Samples were reconstituted in 1 mL acetonitrile, filtered through a 0.2 μm PTFE syringe filter, amended with 50 μg L−1 internal standard mix (see Table S4 in the ESI†), and kept at −20 °C until analysis.
In addition to the effluent samples, two method blanks (MB, ultrapure water) were processed with the same SPE procedure, one MB accounting for sampling bottles and procedures of effluent samples 1–8 and one MB accounting for sampling bottles and procedures of effluent samples 9–13. MB were amended with surrogate standards before SPE and internal standards before analysis identically to the effluent samples. A 100 mL ultrapure water sample, spiked with the same total amount of target QACs as in the spiked effluent samples, was processed as a reference for assessing the matrix effect on compound recoveries through the whole SPE procedure. Evaporation of SPE extracts to dryness and filtration of reconstituted samples did not result in significant loss of target compounds. QAC concentrations in effluent samples are reported as ng L−1 (for quantitative targets) and μg L−1 (for semi-quantitative targets and suspects) without converting sample weights into sample volumes. The effluent temperatures at sampling were between 8 °C and 16 °C, for which neglecting the density conversion introduced an error of no more than 0.1%.
2.2 Sediment sample collection and preparation
Four sediment cores were collected as part of a previous sampling campaign in August and September 2014 in Lake Pepin, Lake Winona, Lake Superior at Duluth Harbor, and Little Wilson Lake.40 Additional surface sediment samples were collected along the bottom of Lake Winona in September 2014.41 The sediment cores were vertically divided into 2–4 cm thick samples and dated by lead-210 methods (see Kerrigan et al.40 for details). All sediment samples analyzed as part of this study were freeze-dried and stored at −20 °C shortly after sampling. Details on sampling procedures and locations, general sample characterization (e.g., organic carbon content), and calculation of sediment accumulation rates are given in previous publications.40–42Sediment extraction procedures for QAC analysis were adapted from Li and Brownawell28 and performed in cleaned glassware. SPE was performed with the same procedure as described above for the effluent samples. For each sample, 250 mg freeze-dried sediment was amended with the three surrogate standards (510 ng g−1 C8-TAA, 380 ng g−1 C10-ATMAC-d9, and 110 ng g−1 C14-BAC-d7). Select samples (at least two from each core) were prepared twice, with one of them additionally spiked with 250 ng of all 22 target compounds. Sediment samples were suspended in 5 mL of a 1 M HCl solution in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 methanol
1 methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, vortexed for 15 s, placed in an ultrasound-bath for 60 min at 50 °C and 40 kHz, and centrifuged for 1 min at 3400 rpm. This ultrasound-assisted extraction was performed twice to yield a combined extract of approximately 10 mL, which was diluted with 100 mL of 250 mM citric acid or 250 mM sodium citrate for SPE. A third ultrasound-assisted extraction step did not result in higher recoveries. Method blanks and a matrix-free, recovery-control sample spiked with all target compounds were prepared by mixing 10 mL of the HCl–methanol–water solution with 100 mL citrate buffer. After addition of the surrogate standards, these samples were processed by SPE identically to all the other samples.
water, vortexed for 15 s, placed in an ultrasound-bath for 60 min at 50 °C and 40 kHz, and centrifuged for 1 min at 3400 rpm. This ultrasound-assisted extraction was performed twice to yield a combined extract of approximately 10 mL, which was diluted with 100 mL of 250 mM citric acid or 250 mM sodium citrate for SPE. A third ultrasound-assisted extraction step did not result in higher recoveries. Method blanks and a matrix-free, recovery-control sample spiked with all target compounds were prepared by mixing 10 mL of the HCl–methanol–water solution with 100 mL citrate buffer. After addition of the surrogate standards, these samples were processed by SPE identically to all the other samples.
Dilution of extracts in citrate buffer proved to be the best option for achieving a solution pH above 5 (pKa of the SPE material) without causing precipitation of matrix components extracted from the sediments. Interactions between the QACs and the SPE material are driven by pH-independent hydrophobic interactions and pH-dependent charge-interactions with negative charge-equivalents of the SPE material increasing with pH. The charge-equivalents of the SPE material at pH 5 (50% of total) already exceed the expected maximum number of positive charge of all QACs combined by one order of magnitude. Therefore, no variations in recoveries were observed despite varying solution pH (5–6) of the extract-citrate buffer mixtures.
After SPE, filtered extracts were amended with internal standards as described above and kept at −20 °C until analysis. Final sediment extracts were diluted again with equal volumes of acetonitrile before analysis to improve peak shape and retention of QACs during liquid chromatography.
2.3 Target and suspect screening
Sample aliquots of 2 μL were analyzed by liquid chromatography high-resolution tandem mass spectrometry (LC-HRMS/MS) with an UltiMate 3000 RSLCnano UPLC coupled to a Thermo LTQ Orbitrap Velos (Thermo Scientific). For analyte separation, hydrophilic interaction liquid chromatography (HILIC) was applied with a manually packed nanoflow column (approx. 75 μm × 200 mm) containing Cogent 4 Diamond Hydride material (4 μm particle size, MicroSolv Technology Corporation). This HILIC column was chosen because it offered the best chromatographic results from all the available stationary phases, despite limited chromatographic resolution of the target QACs. Details on LC-HRMS/MS method parameters are described in Section S3 of the ESI.† Sample composition was an important parameter for stable retention times and good peak shape. In particular, samples containing small amounts of acid or water resulted in the QACs not being retained on the column. Samples containing more than 10% methanol were subject to substantial peak broadening. The candidate list for data-dependent MS/MS scans (see ESI† for details) consisted of 57 unique accurate masses including all the target compounds, surrogates and internal standards listed in Tables S3 and S4 in the ESI† as well as 27 suspect QAC masses. Suspects were included for all QACs groups with side chain length of 2–10 C atoms (ionic liquids), 2–22 C atoms (BACs and DADMACs), and 10–22 C atoms (ATMACs), excluding compounds already listed as target compounds.Data evaluation for target screening was performed with Xcalibur v4.1 (Thermo Fisher Scientific). Chromatograms were extracted from the full-scan acquisition for each target, surrogate, and internal standard based on their accurate mass (±10 ppm). Peaks were integrated manually. Concentration levels of target compounds were quantified with a 7-point, linear regression forced through the origin of area ratios vs. spiked concentrations corresponding to 40–1000 ng L−1 in effluent and 40–1000 ng g−1 in sediment samples, respectively. Area ratios of targets vs. internal standards were used for calculating absolute recoveries and concentrations of the three target DADMACs in all effluent samples. Area ratios were also calculated for targets vs. surrogates and used to quantify relative recoveries and concentrations for all other samples and target compounds. Average recoveries were calculated for the 22 target QACs from the difference in concentration between the 13 spiked and 13 unspiked effluent samples as well as between 11 spiked sediment samples and the corresponding unspiked samples (see Section S3 in the ESI† for equations for limits of detection and quantification, recoveries, final concentrations, and sediment accumulation rates).
Suspect screening was performed with an automated workflow in enviMass v4.0 beta.43 A complete list of relevant setting for the workflow steps is shown in Table S5 in the ESI.† Instrument files were converted to mzXML-files with ProteoWizard v3.0.18282.44 Enabled workflow steps included mass recalibration, blank/blind detection, LOD interpolation, compound screening, and homolog series detection. All surrogate and internal standards as defined in Tables S3 and S4 in the ESI† were labeled as internal standards in the enviMass workflow. All target compounds as well as 41 suspect QACs (Cn-DADMAC with n = 2–22, Cn/n+2-DADMAC with n = 2–20, Cn-BAC with n = 4–22, Cn-ATMAC with n = 10–22, Cn-PYR with n = 2–16, Cn-MPY and Cn-PIP with n = 2–10) were labeled as target compounds in the enviMass workflow. The method blanks were used as the relevant blank/blind samples with a cut-off threshold of 5, meaning that peak areas in samples need to be 5 times larger than in blanks to count as positive hits. Based on the retention times of the target analytes, only peaks between 15 and 30 min were considered. For each sample, the workflow generated a list of all targets and suspects with a positive hit above the cut-off threshold, which was manually compared to results from the target screening to obtain the number of false positive and false negative hits for target analytes (see Tables S9 and S12 in the ESI†). For 26 of the 41 suspect QACs, the enviMass workflow found at least one positive hit in any of the samples, however, only 15 suspects could be confirmed by manual comparison with retention times and MS/MS fragments with the structurally closest target compound (see Table S4 in the ESI†). We semi-quantitatively determined concentrations of these 15 suspects in all unspiked effluent and sediment samples analyzed by integrating peaks from full-scan acquisitions in Xcalibur. For confirmed positive suspect hits, concentrations, LODs, and LOQs were calculated using response factors of the structurally closest target compound and absolute recoveries derived from linear interpolation of target recoveries from the same QAC group (e.g., all BACs). Similar approaches for quantification of QACs without standards have been reported by Li and Brownawell.26,28
3. Results and discussion
3.1 Target screening
Target screening was optimized for 22 QACs (see Table S3 in the ESI†) ranging in hydrophobicity from the small ionic liquid C2-IMI to QACs with long side-chains, such as C18-DADMAC. To best capture this wide range in hydrophobicity, SPE material with both hydrophobic and weak cation exchange interactions was applied for cleaning and pre-concentrating wastewater effluent samples and sediment extracts. The final SPE procedure as described in the Experimental section was tested with two matrix-free control samples spiked with 250 ng of each target QAC. Absolute recoveries (the fraction of targets not lost during SPE) ranged from 58% to 86% for targets spiked in 100 mL ultrapure water (see Table S6 in the ESI†). When spiked into 100 mL citrate buffer containing 10% methanol, absolute recoveries were substantially reduced for the 5 smallest target compounds (C2-IMI, C4-IMI, C4-PYR, C4-MPY, C4-PIP, see Table S6 in the ESI†). Consequently, compounds with side-chains of 4 C atoms or less had very low absolute recoveries for the entire sediment extraction procedure (<10%), impeding reliable quantification. These compounds are, however, sufficiently polar as to not accumulate strongly in sediments and mostly remain in the water phase, where they can be quantified reliably. Reduction in absolute recoveries was also observed for target QACs spiked into samples containing dissolved organic matter (data not shown) and for target QACs spiked into the 13 wastewater effluent samples (see Table S7 in the ESI†). The reduction in absolute recovery was clearly related to the hydrophobicity of the QACs, with the highest and smallest absolute recoveries determined for C4-IMI with 82 ± 25% (mean ± standard deviation) and C18-DADMAC with 5.8 ± 4%, respectively. Recoveries were essentially identical between spiked sediment and effluents samples for all except the 5 smallest target compounds (see Tables S7 and S11 in the ESI†) indicating that the sediment extraction did not contribute substantially to loss of target compounds during sample preparation.To compensate for compound losses observed during SPE, relative recoveries (retention of targets relative to one of the three surrogate standards) were used to quantify target QACs whenever possible. Relative recoveries were between 76% and 109% in spiked ultrapure water and between 67% and 160% in citrate buffer (see Table S6 in the ESI†) suggesting that the sample preparation procedure is, in principal, suitable for all compounds except for the 5 smallest targets in sediments. As shown in Fig. 1, relative recoveries were between 70% and 130% for all targets spiked in wastewater effluent samples except for C16-PYR, C18-BAC, C18-ATMAC, and all 3 DADMACs. Relative recoveries in spiked sediment samples were also between 70% and 130% for all targets except for C16-PYR, C18-ATMAC, C16-DADMAC, C18-DADMAC, and most of the ionic liquids. Because ionic liquids are not expected to be present in sediments, at least at the moment, our target screening method can reliably quantify the majority of relevant QACs irrespective of the type of sample. In the case of C16-PYR, C18-ATMAC, C16-DADMAC, and C18-DADMAC, both absolute and relative recoveries were low in effluent and sediment samples, which results in a less reliable quantification of these targets. To distinguish between more and less reliable quantification, we will refer to concentrations of these targets as semi-quantitative results and report them in μg instead of ng. Similarly, C18-BAC and C12-DADMAC results are only semi-quantitative in effluent samples because their best matching surrogate standard in matrix-free controls and sediment samples could not be used for calculating concentrations in effluent samples. The fact that recoveries were consistent within one type of sample, however, suggest that the concentrations determined in this study for semi-quantitative targets as well as for suspect QACs still represent good approximations of environmental concentrations.
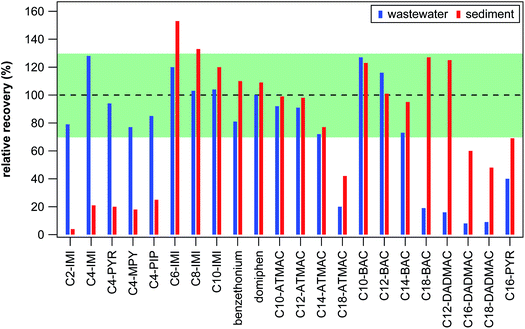 | ||
| Fig. 1 Average relative recoveries of target QACs in spiked wastewater effluents (left blue bar) and in spiked sediment samples (right red bars). The dashed line indicates 100% relative recovery (equal absolute recoveries of a target and its corresponding surrogate) and the green shaded area covers 70–130% relative recovery. See Tables S6, S7, and S11 in the ESI† for all recovery data. | ||
Limit of detection and quantification (LOD and LOQ) were 0.9–179 ng L−1 and 12–338 ng L−1, respectively, for effluent samples (see Table S7 in the ESI†) and 2.8–53 ng g−1 and 12–118 ng g−1, respectively, for sediment samples (see Table S11 in the ESI†). These values account for losses during sample preparation and sample pre-concentration. For most target QACs, the LOD and LOQ values of this method are in the range of previously published methods but they are slightly elevated for the semi-quantitative targets due to low recoveries. Absolute method recoveries for BACs (12–81%) and ATMCAs (13–68%) are comparable to published methods for water14,16,17 and sediment13,31,35 samples, most of which have not included C18-BAC or C18-ATMAC. Martínez-Carballo et al.14 have determined significantly better absolute recoveries for C18-BAC and DADMACs than we were able to achieve, however, in addition to using a different sample preparation (liquid–liquid extraction) that might not be able to capture the most polar QACs included in our study, recovery tests were conducted with 25–250 times higher concentration of spiked QACs. Recoveries of BACs and DADMACs from sediment samples between 98% and 118% were reported by Li and Brownawell,28 however, it is unclear if these are absolute recoveries or recoveries relative to C12-DADMAC, which was used as a surrogate standard. Overall, recoveries of the method used in this study are in the range reported by others, at least for the quantitative targets. The lower recoveries of the semi-quantitative targets and the resulting uncertainties in quantification are compensated by the largest analyte spectrum and a standardized, fast sample preparation procedure.
3.2 Suspect screening
With the automated suspect screening workflow in enviMass, 6298 and 8210 total peaks were detected on average per effluent and sediment sample, respectively, within the given retention time window and above the defined blank-threshold (see Tables S9 and S12 in the ESI†). Between 3 and 11 target compounds were detected in each of the 13 effluent samples with no more then 1 false positive (compounds detected by enviMass workflow but not above LOD in manual target screening) and with 1–9 false negatives (compounds not detected by enviMass workflow but above LOD in manual target screening). The number of suspects detected by the enviMass workflow ranged from 6 to 17 (see Table S9 in the ESI†), however, not all of these hits could be confirmed by MS/MS fragments. In the sediment samples, 4–13 targets were detected by the enviMass workflow with 0–5 false positives and 0–9 false negatives and the number of detected suspects ranged from 3 to 17 (see Table S12 in the ESI†). The total number of peaks did not vary substantially between effluent samples as well as among samples from the Lake Pepin and Duluth Harbor cores, however, a decrease in total peaks was observed with depth in the Lake Winona core and with distance from the WWTP in the surface sediment samples from Lake Winona. Variations in the number of detected homolog series (see Tables S9 and S12 in the ESI†) is difficult to interpret without additional manual data evaluation because, despite selective SPE, homolog series likely comprise both anthropogenic and natural compounds. Overall, the workflow is well suited for a fast screening of samples to reduce the manual work needed to search for suspect compounds as well as providing qualitative measures of the number of total peaks as well as homolog series detected in each sample.Manual inspection of the suspect hits from enviMass resulted in a positive confirmation based on accurate mass, retention time, and MS/MS fragments of 15 suspect QACs in all effluent and sediment samples combined (see Table S4†). Among these compounds were 4 BACs, 7 DADMACs, 2 ATMACs, and 2 pyridinium compounds. BACs, DADMACs, and pyridinium compounds were generally unambiguously identified if present at sufficient levels, due to very consistent MS/MS fragments or fragmentation pattern (see Tables S3 and S4 in the ESI†). For example, all BACs had an intense fragment with an m/z value of 91.05 (benzyl-group) and all DADMACs had an intense fragment resulting from the loss of one large alkyl chain. MS/MS spectra of ATMACs were generally less indicative because of fewer and less intense fragments, thus positive identification was not always possible. Semi-quantitative concentrations were calculated for the confirmed suspect QACs with estimated absolute recoveries (see Tables S10 and S14 in the ESI†) and with response factors of the structurally most similar target compound (see reference targets in Table S4 in the ESI†). In effluent samples, suspects with a semi-quantitative concentration above 0.1 μg L−1 were C2/4-DADMAC, C6-PYR, C10-DADMAC, C14-DADMAC, C16-BAC, and C22-ATMAC (see Table S10 in the ESI†). The most abundant suspects in sediment samples were C4/6-DADMAC, C6-DADMAC, C8-DADMAC, C10-DADMAC, C14-DADMAC, C16-BAC, C16-ATMAC, and C22-ATMAC (see Tables S14, S17, S21, and S25 in the ESI†).
3.3 QACs in wastewater effluents
In the 13 unfiltered wastewater effluent samples analyzed in this study, 1–7 targets and 2–6 suspects were detected above LOQ and 9–13 targets and 10–14 suspects were detected above LOD in each sample (see Tables S8 and S10 in the ESI† for detailed results). For target compounds, LOD and LOQ were generally below 10 and 100 ng L−1, respectively, except for the 3 DADMACs (see Table S7 in the ESI†), which had higher LOD (0.05–0.18 μg L−1) and LOQ (0.24–0.34 μg L−1) due to low recoveries. Among the quantitative target QACs, C14-BAC had the highest number of detections (12 > LOQ and 13 > LOD), the highest average concentration (216 ng L−1), and the highest maximum concentration in a single sample (1386 ng L−1). Other quantitative targets with significant average concentrations were C12-BAC (23 ng L−1) and the ionic liquid C4-PYR (53 ng L−1). Detection of the two BAC homologs in wastewater effluents is expected because they are the most abundantly used QACs in anti-microbial household cleaners and hand soaps. Out of the approximately 195 metric tons of total QAC sold in Minnesota in 2017 for non-agricultural purposes, 56 and 57 metric tons were C12-BAC and C14-BAC, respectively.45 The fact that C4-PYR was detected above LOQ in 10 of the samples was surprising because we could not find any known applications of C4-PYR in household or personal-care products. C4-PYR was the only target compound detected at higher levels in the 24 hour composites than in the grab samples, which suggests a different application and source profile than the other QACs or that it represents a degradation product of another pyridinium compound. Only three effluent samples contained any of the ATMAC homologs above LOQ, resulting in average total ATMAC concentrations of less than 10 ng L−1. Substantially lower ATMAC concentrations compared to BACs and DADMACs are, however, in agreement with previous studies12,14 and the fact that fewer household products are known to contain ATMACs.46 Additionally, benzethonium was detected above LOD in all effluent samples but only in samples 2 and 6 above LOQ with concentrations of 20 and 70 ng L−1, respectively. Benzethonium, domiphen, and cetylpyridinium all have known applications in personal-care products but only benzethonium is known to be used as an industrial surface disinfectant,46 which might explain its presence at higher concentrations.Out of the 6 semi-quantitative target QACs, C18-BAC, C12-DADMAC, C16-DADMAC, and C18-DADMAC were present above LOQ in 3–11 effluent samples with approximate average concentrations of 0.11, 0.12, 0.07, and 1.03 μg L−1, respectively. These concentrations are all in the range of the quantitative targets, except for C18-DADMAC, which, together with the fact that all these compounds have known domestic and industrial applications, suggest that these concentrations are reasonable. The high levels of C18-DADMAC can only partially be attributed to the larger method uncertainties because even without accounting for losses during SPE, average concentrations are 0.07 μg L−1. In addition, Clara et al.12 have reported an average effluent concentration of 0.65 μg L−1 for C18-DADMAC, which is only a factor of 1.58 smaller than our result. Three additional suspect DADMACs were detected with substantial approximate average effluent concentrations: C2/4-DADMAC (0.17 μg L−1), C10-DADMAC (0.20 μg L−1), and C14-DADMAC (0.12 μg L−1). For comparison, approximately 25 metric tons of C10-DADMAC and 0.6 metric tons of C18-DADMAC were sold in Minnesota in 2017 for non-agricultural uses.45 Numbers for other DADMAC homologs were not reported. The similar sales numbers (25–57 tons) and average effluent concentrations (23 ng L−1 to −0.22 μg L−1) of C12-BAC, C14-BAC, and C10-DADMAC, further suggest that the semi-quantitative approach yields reasonable results. The reason for the overall high abundance of the long side-chain DADMACs in our 13 effluent samples is currently unclear, even though similar effluent concentrations of DADMACs were reported previously.12,14 Apart from DADMACs, 3 additional suspect QACs had elevated approximate average concentration in our samples: C16-BAC with 0.28 μg L−1, C6-PYR with 0.10 μg L−1, and C22-ATMAC with 0.82 μg L−1. While C16-BAC has known applications in cleaning products, the origin of C6-PYR remains elusive. Although previously detected in sludge and sediment samples,15,27 to the best of our knowledge no study to date has reported (semi-)quantitative concentrations of C22-ATMAC in wastewater effluent despite its frequent addition to hair care products.46
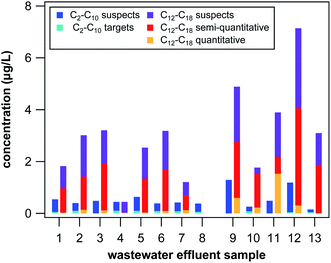
Two general observations can be drawn from the combined target and suspect screening of QACs in the 13 wastewater effluent samples, which is illustrated in Fig. 2. When summing up all targets and suspects in each sample, approximate total QAC concentrations were between 0.4 μg L−1 and 8.3 μg L−1 with concentrations doubled in the grab samples (4.8 μg L−1 on average) compared to the 24 h composites (2.4 μg L−1 on average), which cannot be explained by differences in sample composition (total organic carbon or sample-specific losses during SPE) and are likely the result of fluctuating QAC concentrations in wastewater effluent over the course of a day. Furthermore, average concentration of all QACs with side-chains of at least 12 C atoms were much higher than concentrations of QACs with side-chains of 10 or less C atoms in all samples except in effluent 4 and 8 (see Fig. 2). These two 24 hour composite samples had by far the lowest total QAC concentrations (0.9 and 0.4 μg L−1), which is likely explained by the presence of a membrane bioreactor (effluent 8) or an increased residence time and sludge age (effluent 4) potentially leading to increased removal of QACs through sorption and/or biodegradation. Increased biodegradation of QACs in WWTP 4 and 8 is supported by the fact that predominantly short-chain compounds were found in these two effluents (C2/4-DADMAC, C4-PYR, C6-PYR). In all the other samples, considerable amounts of long-chain BACs and DADMACs were found, which are subject to slow biodegradation.2,6 Comparison between samples 12 and 13 also suggest an effect of biodegradation efficiency on total QAC levels. These two samples receive the same source water but are independently operated treatment trains, one with conventionally aerated activated sludge (sample 12) and one with pure O2 purged activated sludge (sample 13). One would expect the biodegradation efficiency to be increased with additional O2 inputs and, indeed, total QAC levels are substantially lower in sample 13 (3.2 μg L−1) than in sample 12 (8.3 μg L−1). Reduction in total QAC concentrations could also be a result of increased removal by sorption due to higher biomass concentrations, however, as no BAC was detected above LOQ in sample 13 whereas total BAC concentration was 1.8 μg L−1 in sample 12 increased biodegradation likely played an important role in lowering QAC concentrations. Investigation of enhanced removal of other micropollutants by aerating with pure O2 is worth additional study.
3.4 QACs in surface sediments
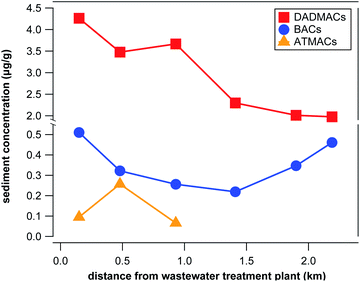
Surface sediment samples from Lake Winona were analyzed to differentiate between wastewater and agricultural inputs of QACs into aquatic environments. Lake Winona is a small (0.75 km2) but long (2.6 km) lake with one WWTP discharging into one end of the lake and with a third of the surrounding area occupied by agricultural fields.41 These conditions make Lake Winona ideal for distinguishing between wastewater-derived and agricultural inputs of organic contaminants that accumulate in sediments. With increasing distance from the WWTP outlet, surface sediment concentrations of wastewater-derived contaminants are expected to decrease while a different concentration pattern indicates (additional) input from agriculture. We applied our target and suspect screening approach for 6 surface sediment samples taken along the bottom of the lake with increasing distance (0.15–2.2 km) from the WWTP outlet. Three BACs (C12, C14, and C16) as well as 6 DADMACs (C4/6, C6, C10, C12, C16, and C18) were detected in all samples above LOQ. C18-BAC, C18-ATMAC, C14-DADMAC, C22-ATMAC, and benzethonium were also detected in at least one of the samples. Concentrations of individual compounds ranged between 14 and 436 ng g(dry weight)−1 for quantitative targets and between 0.1 and 2.7 μg g(dry weight)−1 for semi-quantitative targets and suspects (see Tables S13 and S14 in the ESI†). The spectrum of compounds detected expectantly deviated from the wastewater effluent samples in that no QACs with less than 10 C atoms (combined) in the side-chains were present in surface sediment samples. On average, quantitative targets contributed 16%, semi-quantitative targets contributed 59%, and suspects contributed 24% to the total QAC concentration in surface sediment samples, which ranged from 2.4 to 4.9 μg g(dry weight)−1.As illustrated in Fig. 3, the concentration of total BACs, DADMACs, and ATMACs exhibit very different spatial patterns. ATMACs were only present in the 3 surface sediment samples located closest to the WWTP outlet suggesting that wastewater effluents are the only source of input into Lake Winona for ATMACs. Similarly, total DADMAC concentrations decreased with distance from the WWTP outlet (see Fig. 3) from 4.3 μg g(dry weight)−1 to 2.0 μg g(dry weight)−1. Total BAC concentration, however, decreased from 0.5 μg g(dry weight)−1 at 0.15 km from the WWTP outlet to 0.2 μg g(dry weight)−1 at 1.4 km from the WWTP outlet and then increased again to 0.5 μg g(dry weight)−1 at 2.2 km from the WWTP outlet. This pattern was also reflected in the concentrations of all BAC homologs except for C18-BAC, which was only detected in the two samples closest to the WWTP. While wastewater effluents seem to be the sole or at least dominant source of input into aquatic environments for most QACs, discharge from field runoff cannot be excluded as a source for BACs in Lake Winona sediments. The 2017 sales numbers of BACs in Minnesota for non-agricultural purposes (approximately 134 metric tons) vs. as formulating chemicals in pesticide mixtures (approximately 14 metric tons) support this conclusion.45
The measured average sediment concentrations of Lake Winona can be converted to total QAC deposition rates with the known surface area and sediment accumulation rates.40 Approximately 0.6 kg BACs, 5.2 kg DADMACs, 0.1 kg ATMACs, and 5.9 kg total QACs are deposited at the bottom of Lake Winona in one year. Estimating from the average concentrations determined in the 24 h effluent composites and a daily discharge of the WWTP located at Lake Winona of 3 million gallons, yearly inputs from wastewater into Lake Winona are approximately 0.9 kg BACs, 5.2 kg DADMACs, 3.0 kg ATMACs, and 9.9 kg total QACs. Although these are only rough estimations, the two completely independent measurement and calculation for total inputs from effluent and total accumulation in surface sediments lead to similar results, suggesting that our approach is suited to estimate total QAC levels in water and sediment samples. Furthermore, comparison between total input from effluents and total deposition to sediments suggests that wastewater effluent is the dominant source of QACs in Lake Winona and the fraction removed from the lake by sedimentation increases with hydrophobicity from ATMACs (≈3%) to BACs (≈70%) to DADMAC (≈100%).
Martínez-Carballo et al.13 have analyzed 21 river sediment samples in Austria with average total DADMAC, BAC, and ATMAC concentrations of 0.58 μg g−1, 1.02 μg g−1, and 0.04 μg g−1, respectively. When summing up the same QAC homologs, approximate average surface sediment concentrations in Lake Winona were comparable with 2.6 μg g−1 DADMAC, 0.35 μg g−1 BAC, and 0.05 μg g−1 ATMAC. Surface estuarine sediments taken around New York City were contaminated with high total QAC concentrations of 1–114 μg g−1 and substantial ATMAC concentrations of 0.4–6.7 μg g−1, up to 77% of which was C22-ATMAC.26,27 The range of total QAC concentrations in surface sediments from Lake Winona were in the same range with 2.4–4.9 μg g−1 but ATMAC concentrations were significantly lower (up to 0.3 μg g−1).
3.5 QACs in sediment cores
In addition to analysis of wastewater effluent and surface sediment, which allows assessment of current input and exposure of contaminants in the aquatic environment, measurements of contamination profiles in dated sediment cores additionally provides historic information about the use and fate of organic contaminants that accumulate in sediments. Despite the amount of literature on QAC contamination in aqueous environments including sediments, only few temporal trends of QAC concentrations in dated sediment cores were published to date.25,27 In this study, we have analyzed 4 previously dated sediment cores from Lake Pepin, Lake Winona, Lake Superior at Duluth Harbor, and Little Wilson Lake with sediment deposition dates ranging from 1870 to 2014. Results for individual target and suspect QACs are listed in Tables S15–S27† and illustrated in Fig. S1–S3 in the ESI.† Little Wilson Lake is located in the Superior National Forest and served as a control site due to lack of any major waste inputs. There were only a few detections above LOQ in samples from Little Wilson Lake, which were much lower than levels in the other sediment cores and likely arose from contamination during sample preparation and/or analysis.Lake Pepin is a natural impoundment of the Mississippi River with a large watershed and multiple WWTP outlets in and upstream of the lake. No QACs were detected in the sediment core of Lake Pepin prior to 1929 except for low levels of C18-DADMAC, which is likely due to contamination during sample preparation or an artifact of the low recovery of C18-DADMAC. Apart from BACs, DADMACs, and ATMACs with side-chains of 10–18 C atoms, benzethonium was detected in samples dating from 1949 to 1980. Fig. 4(A) shows temporal trends in total accumulation rates for BACs, DADMACs, and ATMACs in Lake Pepin sediments. Accumulation rates increased for all 3 QAC groups after 1950 up until the 1970s with maximum accumulation rates of approximately 2.9 μg DADMAC, 3.4 μg BAC, and 0.4 μg ATMAC per cm2 and year. After 1980, accumulation rates drastically decreased for all 3 QAC groups until the late 1990s, after which accumulation rates stagnate around 0.3 μg DADMAC, 1.0 μg BAC, and 0.05 μg ATMAC per cm2 and year. This temporal trend is also reflected in accumulation rates of individual QAC homologs except for C10-DADMAC, which was only detected after 1980 (see Fig. S1 in the ESI†). Assuming that the majority of QACs in Lake Pepin sediments are wastewater-derived, changes or reduction in use volumes are unlikely the reason for the observed temporal contamination trend considering that the population served by WWTPs discharging into or upstream of Lake Pepin has almost tripled since 1930. The reduction in QAC accumulation coincides with implementations of secondary wastewater treatment and a federally mandated industrial pretreatment program initiated in 1981, and similar trends in metal pollutants are observed.47 Improved wastewater treatment is possibly the sole reason for the observed reduction in QAC accumulation rates as over 90% of QACs can be removed by current wastewater treatment practices.12,14
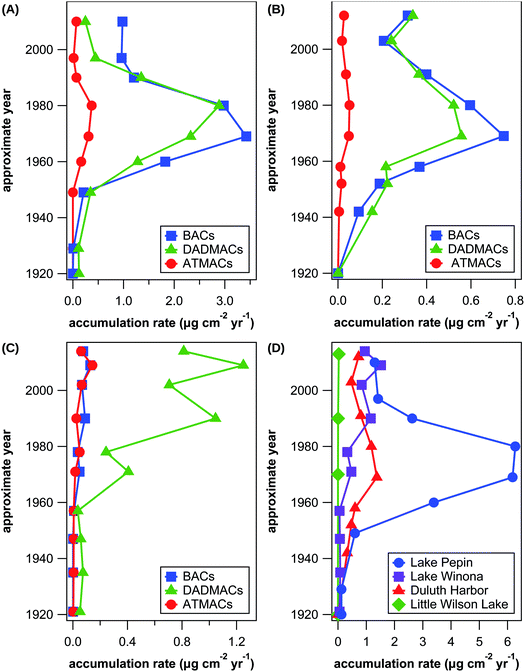 | ||
| Fig. 4 Approximate accumulation rates of all BACs (blue squares), all DADMACs (green triangles), and all ATMACs (red circles) in sediment cores from Lake Pepin (A), Lake Superior at Duluth Harbor (B), and Lake Winona (C) as well as approximate accumulation rates of all target and suspect QACs combined (D) in sediment cores from Lake Pepin (blue circles), Lake Winona (purple squares), Lake Superior at Duluth Harbor (red triangles), and Little Wilson Lake (green diamonds). See Fig. S1–S3 in the ESI† for illustrations of individual compound trends. | ||
Duluth Harbor also receives treated wastewater from multiple discharge locations, but the overall watershed is 3.6 times smaller than that of Lake Pepin. Unlike Lake Pepin, the population served by WWTPs discharging into Duluth Harbor has been constant since 1930 and is today only a tenth of the respective population of Lake Pepin. Expectantly, the temporal trends of QAC accumulation in Duluth Harbor followed the same pattern as in Lake Pepin with maximum accumulation rates around 1970, however, with much lower absolute accumulation rates (see Fig. 4(B)). The maximum total accumulation rates of DADMACs, BACs, and ATMACs were 5.2, 4.6, and 7.1 times lower in Duluth Harbor compared to Lake Pepin. The larger watershed and sediment load of Lake Pepin are likely the main drivers of the observed difference in the magnitude of QAC accumulation rates, as hypothesized previously for similar observations with antibiotic accumulation at the same sites.40 Similar temporal trends of QAC contamination as in Lake Pepin and Duluth Harbor were observed in cores from estuarine sediments in the New York City area for DADMACs, BACs, and ATMACs with maximum concentration approximately corresponding to 1990, 1965, and 1988, respectively.25,27 In the same study area, C10-DADMAC concentrations continually increased over time from 1950 until 2010,25 which is similar to temporal trends of C10-DADMAC in Duluth Harbor (see Fig. S3(A) in the ESI†). Absolute levels of sediment concentrations were comparable between this and previous studies only in the case of BACs with maximum approximate concentrations of 8.1 μg g−1 in Duluth Harbor and 3.6 μg g−1 in Lake Pepin. Maximum DADMAC and ATMAC concentrations were around 60 and 25 times higher in estuarine sediments in the New York City area than in Lake Pepin or Duluth Harbor.
Even though much smaller in terms of catchment area and population than Lake Pepin and Duluth Harbor, Lake Winona has a uniquely high percentage of wastewater effluent (63%) of the average water inflow into the lake. The population served by the WWTP discharging into Lake Winona has doubled since 1930, thus similar temporal trends were expected as observed in Lake Pepin and Duluth Harbor. In a previous study at the same 3 sites, Lake Winona had the highest accumulation rates for various antibiotics.40 As evident from Fig. 4(C), however, maximum QAC accumulation rates were much lower for BACs (0.13 μg cm−2 per year) and about half for ATMACs (0.14 μg cm−2 per year) and DADMACs (1.3 μg cm−2 per year) in Lake Winona compared to Lake Pepin. Even more surprisingly, the temporal trends of QAC contamination were completely different in Lake Winona with all QACs detected increasing in accumulation rates over time. The only conceivable difference between Lake Winona and the other two sites is that Lake Pepin and, to a lesser extent, Duluth Harbor receive significant amount of industrial wastewater discharge in addition to domestic wastewater, while wastewater inputs into Lake Winona are predominantly domestic. In a previous study,14,48 analysis of WWTP effluents with predominantly industrial or domestic sources revealed much higher QAC concentrations in industrial wastewater. This observation supports the hypothesis that industrial QAC inputs into aquatic environments were and are substantially larger than domestic QAC inputs.
4. Conclusion
The average total QAC concentration in wastewater effluents observed in this study was approximately 2.5 μg L−1. While effluent concentrations are significantly lower than reported acute toxicity values of QACs (high μg L−1 to mg L−1 range),1,48 cumulative chronic effects including promotion of antibiotic resistance cannot be excluded and warrants further investigations. Sediments serve as an archive of QAC levels, with a bias toward more hydrophobic compounds with long carbon chains. The total deposition of different QAC structural groups range from grams to kilograms per year, and while the presence in decades old sediments indicates that QACs are strongly sorbed, further assessment of bioavailability is warranted. Although the detected levels of QACs unlikely pose an acute threat to aquatic ecosystems, the overall high concentrations in both effluent and sediment samples might still be relevant for promotion of antibiotic resistance and formation of disinfection by-products.3,19–21 While the extraction and analytical methods developed herein could benefit from adding additional QACs to the target compounds list, especially ATMACs, our combined target and suspect screening approach is well suited for identifying individual QACs as well as providing good approximations of total QAC levels. Specifically, our method was able to detect additional QACs, particularly pyridinium compounds and benzethonium, which were not the focus of previous research, and provides the basis for future monitoring of ionic liquids. Given the small volumes of water and masses of sediment needed, the method offers a fast way to assess total QAC exposure in aquatic systems.5. Data availability
Target screening data are available in the Data Repository for the University of Minnesota (https://doi.org/10.13020/ram6-m093).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Minnesota Environment and Natural Resources Trust Fund as recommended by the Legislative-Citizen Commission on Minnesota Resources (LCCMR). Mass spectrometry was carried out in the Analytical Biochemistry Shared Resource of the Masonic Cancer Center, University of Minnesota, funded in part by Cancer Center Support Grant CA-77598. Xun Ming and Makenzie Pillsbury are thanked for support with method development and analysis. Daniel Engstrom is thanked for his past assistance with sediment collection and dating. Thanks also goes to all the wastewater treatment plant operators for providing effluent samples for this study and to Annika Heaps for help with sample preparation.References
- C. Zhang, F. Cui, G.-M. Zeng, M. Jiang, Z.-Z. Yang, Z.-G. Yu, M.-Y. Zhu and L.-Q. Shen, Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment, Sci. Total Environ., 2015, 518, 352–362 CrossRef PubMed.
- R. S. Boethling, Environmental fate and toxicity in wastewater treatment of quaternary ammonium surfactants, Water Res., 1984, 18, 1061–1076 CrossRef CAS.
- S. Buffet-Bataillon, P. Tattevin, M. Bonnaure-Mallet and A. Jolivet-Gougeon, Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—a critical review, Int. J. Antimicrob. Agents, 2012, 39, 381–389 CrossRef CAS PubMed.
- M. Amde, J.-F. Liu and L. Pang, Environmental application, fate, effects, and concerns of ionic liquids: A review, Environ. Sci. Technol., 2015, 49, 12611–12627 CrossRef CAS PubMed.
- T. Nishihara, T. Okamoto and N. Nishiyama, Biodegradation of didecyldimethylammonium chloride by Pseudomonas fluorescens TN4 isolated from activated sludge, J. Appl. Microbiol., 2000, 88, 641–647 CrossRef CAS PubMed.
- H. Sütterlin, R. Alexy, A. Coker and K. Kümmerer, Mixtures of quaternary ammonium compounds and anionic organic compounds in the aquatic environment: Elimination and biodegradability in the closed bottle test monitored by LC-MS/MS, Chemosphere, 2008, 72, 479–484 CrossRef PubMed.
- A. Liffourrena, F. López, M. Salvano, C. Domenech and G. Lucchesi, Degradation of tetradecyltrimethylammonium by Pseudomonas putida A ATCC 12633 restricted by accumulation of trimethylamine is alleviated by addition of Al3+ ions, J. Appl. Microbiol., 2008, 104, 396–402 CAS.
- E. Ertekin, K. T. Konstantinidis and U. Tezel, A rieske-type oxygenase of Pseudomonas sp. BIOMIG1 converts benzalkonium chlorides to benzyldimethyl amine, Environ. Sci. Technol., 2016, 51, 175–181 CrossRef PubMed.
- L. M. Games, J. E. King and R. J. Larson, Fate and distribution of a quaternary ammonium surfactant, octadecyltrimethylammonium chloride (OTAC), in wastewater treatment, Environ. Sci. Technol., 1982, 16, 483–488 CrossRef CAS.
- Z. Z. Ismail, U. Tezel and S. G. Pavlostathis, Sorption of quaternary ammonium compounds to municipal sludge, Water Res., 2010, 44, 2303–2313 CrossRef CAS PubMed.
- R. Ren, K. Li, C. Zhang, D. Liu and J. Sun, Biosorption of tetradecyl benzyl dimethyl ammonium chloride on activated sludge: Kinetic, thermodynamic and reaction mechanisms, Bioresour. Technol., 2011, 102, 3799–3804 CrossRef CAS PubMed.
- M. Clara, S. Scharf, C. Scheffknecht and O. Gans, Occurrence of selected surfactants in untreated and treated sewage, Water Res., 2007, 41, 4339–4348 CrossRef CAS PubMed.
- E. Martínez-Carballo, C. González-Barreiro, A. Sitka, N. Kreuzinger, S. Scharf and O. Gans, Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part II. Application to sediment and sludge samples in Austria, Environ. Pollut., 2007, 146, 543–547 CrossRef PubMed.
- E. Martínez-Carballo, A. Sitka, C. González-Barreiro, N. Kreuzinger, M. Fürhacker, S. Scharf and O. Gans, Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part I. Application to surface, waste and indirect discharge water samples in Austria, Environ. Pollut., 2007, 145, 489–496 CrossRef PubMed.
- T. Ruan, S. Song, T. Wang, R. Liu, Y. Lin and G. Jiang, Identification and composition of emerging quaternary ammonium compounds in municipal sewage sludge in China, Environ. Sci. Technol., 2014, 48, 4289–4297 CrossRef CAS PubMed.
- I. Ferrer and E. T. Furlong, Identification of alkyl dimethylbenzylammonium surfactants in water samples by solid-phase extraction followed by ion trap LC/MS and LC/MS/MS, Environ. Sci. Technol., 2001, 35, 2583–2588 CrossRef CAS PubMed.
- F. Merino, S. Rubio and D. Pérez-Bendito, Solid-phase extraction of amphiphiles based on mixed hemimicelle/admicelle formation: Application to the concentration of benzalkonium surfactants in sewage and river water, Anal. Chem., 2003, 75, 6799–6806 CrossRef CAS PubMed.
- W.-H. Ding and P.-C. Tsai, Determination of alkyltrimethylammonium chlorides in river water by gas chromatography/ion trap mass spectrometry with electron impact and chemical ionization, Anal. Chem., 2003, 75, 1792–1797 CrossRef CAS PubMed.
- Q. Wang, D. Mao and Y. Luo, Ionic liquid facilitates the conjugative transfer of antibiotic resistance genes mediated by plasmid RP4, Environ. Sci. Technol., 2015, 49, 8731–8740 CrossRef CAS PubMed.
- K. Hegstad, S. Langsrud, B. T. Lunestad, A. A. Scheie, M. Sunde and S. P. Yazdankhah, Does the wide use of quaternary ammonium compounds enhance the selection and spread of antimicrobial resistance and thus threaten our health?, Microb. Drug Resist., 2010, 16, 91–104 CrossRef CAS PubMed.
- T. Wassenaar, D. Ussery, L. Nielsen and H. Ingmer, Review and phylogenetic analysis of qac genes that reduce susceptibility to quaternary ammonium compounds in Staphylococcus species, Eur. J. Microbiol. Immunol., 2015, 5, 44–61 CrossRef CAS PubMed.
- S. G. Pati and W. A. Arnold, Photochemical transformation of four ionic liquid cation structures in aqueous solution, Environ. Sci. Technol., 2017, 51, 11780–11787 CrossRef CAS PubMed.
- M. Garcıa, E. Campos, J. Sanchez-Leal and I. Ribosa, Anaerobic degradation and toxicity of commercial cationic surfactants in anaerobic screening tests, Chemosphere, 2000, 41, 705–710 CrossRef.
- M. Garcıa, I. Ribosa, T. Guindulain, J. Sanchez-Leal and J. Vives-Rego, Fate and effect of monoalkyl quaternary ammonium surfactants in the aquatic environment, Environ. Pollut., 2001, 111, 169–175 CrossRef.
- X. Li, A. C. Doherty, B. Brownawell and P. A. Lara-Martin, Distribution and diagenetic fate of synthetic surfactants and their metabolites in sewage-impacted estuarine sediments, Environ. Pollut., 2018, 242, 209–218 CrossRef CAS PubMed.
- X. Li and B. J. Brownawell, Quaternary ammonium compounds in urban estuarine sediment environments – A class of contaminants in need of increased attention?, Environ. Sci. Technol., 2010, 44, 7561–7568 CrossRef CAS PubMed.
- P. A. Lara-Martin, X. Li, R. F. Bopp and B. J. Brownawell, Occurrence of alkyltrimethylammonium compounds in urban estuarine sediments: Behentrimonium as a new emerging contaminant, Environ. Sci. Technol., 2010, 44, 7569–7575 CrossRef CAS PubMed.
- X. Li and B. J. Brownawell, Analysis of quaternary ammonium compounds in estuarine sediments by LC-ToF-MS: Very high positive mass defects of alkylamine ions as powerful diagnostic tools for identification and structural elucidation, Anal. Chem., 2009, 81, 7926–7935 CrossRef CAS PubMed.
- L. Ropel, L. S. Belvèze, S. N. Aki, M. A. Stadtherr and J. F. Brennecke, Octanol–water partition coefficients of imidazolium-based ionic liquids, Green Chem., 2005, 7, 83–90 RSC.
- M. L. Manier, D. S. Cornett, D. L. Hachey and R. M. Caprioli, Identification of dimethyldioctadecylammonium ion (m/z 550.6) and related species (m/z 522.6, 494.6) as a source of contamination in mass spectrometry, J. Am. Soc. Mass Spectrom., 2008, 19, 666–670 CrossRef CAS PubMed.
- I. Ferrer and E. T. Furlong, Accelerated solvent extraction followed by on-line solid-phase extraction coupled to ion trap LC/MS/MS for analysis of benzalkonium chlorides in sediment samples, Anal. Chem., 2002, 74, 1275–1280 CrossRef CAS PubMed.
- O. Núñez, E. Moyano and M. T. Galceran, Determination of quaternary ammonium biocides by liquid chromatography-mass spectrometry, J. Chromatogr. A, 2004, 1058, 89–95 CrossRef PubMed.
- P. Bassarab, D. Williams, J. Dean, E. Ludkin and J. Perry, Determination of quaternary ammonium compounds in seawater samples by solid-phase extraction and liquid chromatography-mass spectrometry, J. Chromatogr. A, 2011, 1218, 673–677 CrossRef CAS PubMed.
- X.-T. Peng, Z.-G. Shi and Y.-Q. Feng, Rapid and high-throughput determination of cationic surfactants in environmental water samples by automated on-line polymer monolith microextraction coupled to high performance liquid chromatography-mass spectrometry, J. Chromatogr. A, 2011, 1218, 3588–3594 CrossRef CAS PubMed.
- A. Van de Voorde, C. Lorgeoux, M.-C. Gromaire and G. Chebbo, Analysis of quaternary ammonium compounds in urban stormwater samples, Environ. Pollut., 2012, 164, 150–157 CrossRef CAS PubMed.
- K. A. Barzen-Hanson, S. C. Roberts, S. Choyke, K. Oetjen, A. McAlees, N. Riddell, R. McCrindle, P. L. Ferguson, C. P. Higgins and J. A. Field, Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater, Environ. Sci. Technol., 2017, 51, 2047–2057 CrossRef CAS PubMed.
- P. Gago-Ferrero, E. L. Schymanski, A. A. Bletsou, R. Aalizadeh, J. Hollender and N. S. Thomaidis, Extended suspect and non-target strategies to characterize emerging polar organic contaminants in raw wastewater with LC-HRMS/MS, Environ. Sci. Technol., 2015, 49, 12333–12341 CrossRef CAS PubMed.
- E. L. Schymanski, H. P. Singer, P. Longrée, M. Loos, M. Ruff, M. A. Stravs, C. Ripollés Vidal and J. Hollender, Strategies to characterize polar organic contamination in wastewater: Exploring the capability of high resolution mass spectrometry, Environ. Sci. Technol., 2014, 48, 1811–1818 CrossRef CAS PubMed.
- C. M. Carpenter, L. Y. J. Wong, C. A. Johnson and D. E. Helbling, Fall Creek monitoring station: Highly resolved temporal sampling to prioritize the identification of nontarget micropollutants in a small stream, Environ. Sci. Technol., 2018, 53, 77–87 CrossRef PubMed.
- J. F. Kerrigan, K. D. Sandberg, D. R. Engstrom, T. M. LaPara and W. A. Arnold, Sedimentary record of antibiotic accumulation in Minnesota Lakes, Sci. Total Environ., 2018, 621, 970–979 CrossRef CAS PubMed.
- J. F. Kerrigan, K. D. Sandberg, D. R. Engstrom, T. M. LaPara and W. A. Arnold, Small and large-scale distribution of four classes of antibiotics in sediment: Association with metals and antibiotic resistance genes, Environ. Sci.: Processes Impacts, 2018, 20, 1167–1179 RSC.
- C. T. Anger, C. Sueper, D. J. Blumentritt, K. McNeill, D. R. Engstrom and W. A. Arnold, Quantification of triclosan, chlorinated triclosan derivatives, and their dioxin photoproducts in lacustrine sediment cores, Environ. Sci. Technol., 2013, 47, 1833–1843 CrossRef CAS PubMed.
- M. Loos, enviMass version 3.5 LC-HRMS trend detection workflow – R package, Zenodo, 2018 Search PubMed.
- D. Kessner, M. Chambers, R. Burke, D. Agus and P. Mallick, ProteoWizard: Open source software for rapid proteomics tools development, Bioinformatics, 2008, 24, 2534–2536 CrossRef CAS PubMed.
- Minnesota Department of Agriculture, Minnesota Pesticide Sales Information and Database, https://www.mda.state.mn.us/minnesota-pesticide-sales-information, accessed November 2019 Search PubMed.
- U.S. Department of Health & Human Services, Household Product Database, https://householdproducts.nlm.nih.gov, accessed June 2019 Search PubMed.
- S. J. Balogh, D. R. Engstrom, J. E. Almendinger, C. McDermott, J. Hu, Y. H. Nollet, M. L. Meyer and D. K. Johnson, A sediment record of trace metal loadings in the Upper Mississippi River, J. Paleolimnol., 2009, 41, 623–639 CrossRef.
- N. Kreuzinger, M. Fuerhacker, S. Scharf, M. Uhl, O. Gans and B. Grillitsch, Methodological approach towards the environmental significance of uncharacterized substances – quaternary ammonium compounds as an example, Desalination, 2007, 215, 209–222 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9em00554d |
| ‡ Current address: Department of Environmental Sciences, University of Basel, Bernoullistrasse 30, 4056 Basel, Switzerland; E-mail: E-mail: sarah.pati@unibas.ch |
| This journal is © The Royal Society of Chemistry 2020 |