Open Access Article
Open Access ArticleEmerging investigator series: use of behavioural endpoints in the regulation of chemicals
Marlene
Ågerstrand
 *a,
Kathryn
Arnold
b,
Sigal
Balshine
c,
Tomas
Brodin
d,
Bryan W.
Brooks
*a,
Kathryn
Arnold
b,
Sigal
Balshine
c,
Tomas
Brodin
d,
Bryan W.
Brooks
 ef,
Gerd
Maack
g,
Erin S.
McCallum
ef,
Gerd
Maack
g,
Erin S.
McCallum
 d,
Greg
Pyle
h,
Minna
Saaristo
i and
Alex T.
Ford
d,
Greg
Pyle
h,
Minna
Saaristo
i and
Alex T.
Ford
 j
j
aDepartment of Environmental Science (ACES), Stockholm University, Stockholm, Sweden. E-mail: marlene.agerstrand@aces.su.se; Tel: +468164021
bDepartment of Environment and Geography, University of York, York, UK
cDepartment of Psychology, Neuroscience & Behaviour, McMaster University, Hamilton, Canada
dDepartment of Wildlife, Fish and Environmental Studies, Swedish University of Agricultural Sciences (SLU), Umeå, Sweden
eDepartment of Environmental Science, Institute of Biomedical Studies, Baylor University, Waco, TX, USA
fSchool of Environment, Jinan University, Guangzhou, China
gDepartment of Pharmaceuticals, German Environment Agency (UBA), Dessau, Germany
hDepartment of Biological Sciences, University of Lethbridge, Lethbridge, AB, Canada
iSchool of Biological Sciences, Monash University, Victoria, Australia
jInstitute of Marine Sciences, University of Portsmouth, Portsmouth, UK
First published on 16th December 2019
Abstract
Interest in behavioural ecotoxicology is growing, partly due to technological and computational advances in recording behaviours but also because of improvements of detection capacity facilitating reporting effects at environmentally relevant concentrations. The peer-reviewed literature now contains studies investigating the effects of chemicals, including pesticides and pharmaceuticals, on migration, dispersal, aggression, sociability, reproduction, feeding and anti-predator behaviours in vertebrates and invertebrates. To understand how behavioural studies could be used in regulatory decision-making we: (1) assessed the legal obstacles to using behavioural endpoints in EU chemicals regulation; (2) analysed the known cases of use of behavioural endpoints in EU chemicals regulation; and (3) provided examples of behavioural endpoints of relevance for population level effects. We conclude that the only legal obstacle to the use of behavioural endpoints in EU chemicals regulation is whether an endpoint is considered to be relevant at the population level or not. We also conclude that ecotoxicity studies investigating behavioural endpoints are occasionally used in the EU chemicals regulation, and underscore that behavioural endpoints can be relevant at the population level. To improve the current use of behavioural studies in regulatory decision-making contribution from all relevant stakeholders is required. We have the following recommendations: (1) researchers should conduct robust, well-designed and transparent studies that emphasize the relevance of the study for regulation of chemicals; (2) editors and scientific journals should promote detailed, reliable and clearly reported studies; (3) regulatory agencies and the chemical industry need to embrace new behavioural endpoints of relevance at the population level.
Environmental significanceThe peer-reviewed literature contains ecotoxicity studies investigating behavioural effects such as migration, dispersal, aggression, grouping, reproduction and feeding in vertebrates and invertebrates. However, little is known about the studies' contribution to regulatory decision-making. This study concludes that it is possible to use behavioural endpoints in EU chemicals regulation if the endpoint is assessed as relevant at the population level, but that there are few examples of such use. Recommendations for researchers, regulators, risk assessors and scientific journals who strive to improve the use of behavioural endpoints in environmental risk assessment of chemicals are provided. |
Introduction
In recent years there has been growing interest surrounding behavioural ecotoxicology. For example, there has been a steady increase in behavioural effect measurements in the US EPA ECOTOX database,1 currently adding up to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 324 measurements for the aquatic environment and 13
324 measurements for the aquatic environment and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 809 for the terrestrial environment. An area of particular rapid expansion is the use of fish models for drug discovery and design.2,3 This interest in behavioural endpoints is partly driven by technological and computational advances in recording behaviours, but also due to an increasing number of laboratory studies recording behavioural effects at environmentally relevant concentrations.4–6 What was in the past quite a laborious process of careful observations and potentially watching hours of video footage is now high-throughput computer recorded endpoints without human subjectivity. Such advances are important because behavioural ecotoxicology has been previously criticized for concerns over observational errors. Technological advances, for example in electronic tagging, have also allowed the monitoring of behaviour in situ during field studies, which is helping scientists and regulators gain confidence in laboratory derived endpoints. In fact, changes in behaviour are increasingly considered valuable for advancing the next generation of ecotoxicology research.7
809 for the terrestrial environment. An area of particular rapid expansion is the use of fish models for drug discovery and design.2,3 This interest in behavioural endpoints is partly driven by technological and computational advances in recording behaviours, but also due to an increasing number of laboratory studies recording behavioural effects at environmentally relevant concentrations.4–6 What was in the past quite a laborious process of careful observations and potentially watching hours of video footage is now high-throughput computer recorded endpoints without human subjectivity. Such advances are important because behavioural ecotoxicology has been previously criticized for concerns over observational errors. Technological advances, for example in electronic tagging, have also allowed the monitoring of behaviour in situ during field studies, which is helping scientists and regulators gain confidence in laboratory derived endpoints. In fact, changes in behaviour are increasingly considered valuable for advancing the next generation of ecotoxicology research.7
Traditionally the regulatory evaluation of chemicals, including pesticides and pharmaceuticals, has not included behavioural endpoints, even though such endpoints have several advantages. These advantages include that behaviour: (1) provides a connection from molecular and physiological processes to population level processes; (2) is a sensitive ‘early warning signal’ of chemical contamination since behavioural responses can occur at lower levels of contamination than more traditional endpoints; (3) improves the ecological relevance of environmental risk assessments due to the well-established theoretical framework and suite of fitness-related endpoints underpinning behavioural studies; and (4) provides a high throughput identification of potential underlying chemical mode of actions.8–14 In addition, it can be argued that from a resource and animal welfare perspective, behavioural endpoints of sufficient reliability (i.e. inherent quality and repeatability) and relevance (i.e. appropriateness for a particular hazard identification15), should be used in chemicals regulation, particularly considering that public health and environment research is routinely funded by public funds.
Hazard and risk assessments to decide on management measures for chemicals are common for national and international regulation agencies. Ecotoxicity studies are used to determine the potentially environmentally hazardous properties of a chemical and to support establishment of acceptable exposure levels in environmental matrices. Traditionally, regulatory assessments have been based on ecotoxicity studies measuring mortality, growth, reproduction, and development. These individual-level endpoints have been described as having a clear connection to the persistence of a population.16 To facilitate the assessment process and promote use of studies across jurisdictions, standard studies investigating these population relevant endpoints have been developed by international and national organizations, such as OECD and US Environmental Protection Agency.17,18 Inclusion of other endpoints, such as behaviour, are often seen in higher tier assessments, and/or in what is called a “weight of evidence” assessment. Ecotoxicity studies are evaluated for their reliability and relevance when deciding which studies to include in the regulatory assessment.19 Following standard procedures in ecotoxicity studies has led to reliable results applicable for chemicals regulation due to robust test protocols and detailed reporting requirements. In addition, partly due to the use of endpoints considered to be important on a population level, standard studies have been assessed as relevant for chemicals regulation. Ecotoxicity studies that are not performed according to a standard procedure and include alternative endpoints, such as behaviour, run the risk of being disregarded or seen as evidence of lower weight. Often, standard studies are performed by, or on behalf of, the regulated party, while non-standard studies are performed by researchers working in academia.20
Aim and methodology
Given the abundance of information available in the peer-reviewed literature on the impact of chemicals on the behaviour of non-target organisms, this study aimed to:(1) Assess the legal obstacles to using behavioural endpoints in EU chemicals regulation.
Regulatory guidance documents that set the scope for assessment of chemicals were analysed with the purpose to understand if, how and where ecotoxicity studies investigating behavioural endpoints could be used in the assessment of chemicals. Guidance documents for the following six EU policy and regulatory areas were analysed: the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation; Classification, Labelling and Packaging of chemical substances and mixtures (i.e. the CLP regulation); the Biocidal Products Regulation; the Plant Protection Products regulation; Environmental assessment for human and veterinary products; and Derivation of Environmental Quality Standards (within the Water Framework Directive). The following aspects were investigated:
• If the guidance document prohibits use of behavioural endpoints.
• If the guidance document recommends or require tests using behavioural endpoints.
• What weight behavioural endpoints are given in chemical assessments.
• How population level effect is defined.
• Other aspects relevant for use of behavioural endpoints in chemicals regulation.
(2) Analyse the known cases of behavioural endpoints used in EU chemicals regulation.
Due to lack of transparency in EU chemicals regulation and lack of searchable regulatory databases, it is not possible to, in an accessible way, get an overview of current use of behavioural endpoints. Instead we chose to analyse the six known, to us, cases in EU chemicals regulation. These six cases were known to us because of public discussions or due to our personal discussions with regulators and risk assessors at research institutes and national regulatory agencies. The regulatory use was examined to clarify the role of ecotoxicity studies investigating behavioural endpoints for decision-making in these specific cases. The following aspects were investigated:
• Type of behaviour endpoint used.
• Which regulatory framework it was used in.
• The weight the study was given in the chemical assessment by the risk assessors (as key, supportive or low) and how this rank was justified. Key studies are studies used when setting guideline values such as an environmental quality standard (EQS), supportive studies are considered important evidence but are not considered to be key study (e.g. due to issues related to reliability and relevance of the study, or other studies showing toxicity at lower concentrations), and studies with low weight are the ones that are discussed but do not contribute to the overall conclusion of the assessment.
A comparison with the use in the US, and a comparison with human health assessment in EU, was made.
(3) Provide examples of behavioural endpoints with relevance for population level effects.
From a regulatory perspective, contaminant exposure only matters if it influences survival, development and reproduction because these measures can cause population decline. However, there are a number of fitness related behaviours that result in population change. Examples from both invertebrates and vertebrates were provided for different behavioural categories where there are clear links to population dynamics. These behavioural categories include migration, dispersal, aggression, sociability, reproduction, feeding, and anti-predator behaviours, and were selected because of their strong link to population growth and health. We describe how the behavioural effects of chemical contaminants, which are observed at the individual level, can have important cascading impacts and ultimately detrimental effects on population level processes.
Results
Regulatory guidance on the use of behavioural studies in EU chemical assessments
None of the analysed EU guidance documents prohibited use of behavioural endpoints when assessing environmental effects (Table 1). In four of the policy areas, behaviour endpoints were not mentioned as examples of endpoints of interest. Instead, there was a general focus on traditional endpoints such as survival, reproduction, and development. Two of the guidance documents for the REACH regulation stated that behavioural studies could be used as supportive evidence, but not without backing from studies using more traditional endpoints. Examples of such behavioural endpoints included sediment avoidance or burrowing activity.| Responsible agency and policy/regulatory area | Guidance document(s) | Recommendations from the guidance documents | Example text from the guidance document (the number represent guidance document in column 2) |
|---|---|---|---|
| European Chemicals Agency. REACH regulation | (1) Part B hazard assessment | Behavioural endpoints mentioned in two documents, where behavioural studies can be used as supportive evidence. Standard studies are recommended when performing new studies. All available studies are recommended for evaluation | (3) “Behavioural endpoints like sediment avoidance or burrowing activity have not been standardised. Such endpoints can give indications on toxic effects but should not be interpreted in isolation” |
| (2) Chapter R.2 information requirements | |||
| (3) Chapter R.4 evaluation of available information | (4) “Reproduction tests include parental and reproductive endpoints. An endpoint relating to overall reproductive success should normally be selected to define the long-term NOEC. Depending on the individual case and the availability of data, this could be the reproduction rate, the survival or growth rate of the offspring, or behavioural parameters in adults or young”. “Screening endpoints such as behavioural responses, i.e. avoidance testing should not be interpreted in isolation” | ||
| (4) Chapter R.7b + R.7c Endpoint specific guidance | |||
| European Chemicals Agency. Classification, Labelling and Packaging (CLP) regulation | Guidance on the application of the CLP criteria | Behavioural endpoints not mentioned. All available studies are recommended for evaluation | — |
| European Chemicals Agency. Biocidal Products Regulation | (1) Volume IV: environment. Part A: information requirements | Behavioural endpoints not mentioned. Standard studies are recommended when performing new studies. All available studies are recommended for evaluation | — |
| (2) Volume IV: environment – assessment and evaluation (parts B + C) | |||
| European Food Safety Authority, the European Parliament and Council. Plant Protection Products Regulation | (1) Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge-of-field surface waters | Behavioural endpoints mentioned for bees, birds and mammals. Standard studies are recommended when performing new studies. All available studies are recommended for evaluation | (2) “Key parameters which may be considered in a field trial include: mortality (assessed via the use of dead bee traps), behaviour (including foraging behaviour in the crop and around the hive), honey crop (assessed via weighing the hive at appropriate intervals) and state of colony (including an assessment of brood)” |
| (2) Guidance document on terrestrial ecotoxicology, SANCO 2002 | |||
| (3) Guidance document on risk assessment for birds and mammals, EFSA journal 2009 | (3) “Granivorous birds and mammals may be able to distinguish treated seeds from non-treated seeds and may show a preference for either treated or untreated seeds in their diet. This may be influenced by various factors including appearance, taste or surface texture of the treated seed, and aversive reactions to the active substance. Information on such preferences/avoidance behaviour can, in combination with data on the availability of treated and non-treated seeds on the soil surface, be used to refine the risk assessment. No standard guideline for testing avoidance is as yet available”. “…the one-generation avian reproduction study does not include exposure during all relevant stages of the bird's development or the measurement of other relevant endocrine-sensitive endpoints such as behaviour (e.g. parental care, nesting behaviour, territoriality and mounting behaviour)” | ||
| (5) Draft EFSA guidance document on the risk assessment of plant protection products on bees | |||
| European Medicines Agency. Medicinal Products Regulations | (1) Guideline on the environmental risk assessment of medicinal products for human use | Behavioural endpoints not mentioned. Standard studies are recommended when performing new studies. All available studies are recommended for evaluation | — |
| (2) Guideline on environmental impact assessment (EIAS) for veterinary medicinal products – phase I and II | |||
| (3) Guideline on environmental impact assessment for veterinary medicinal products in support of the VICH guidelines GL6 and GL38 | |||
| European Commission. Deriving Environmental Quality Standards | Technical Guidance for Deriving Environmental Quality Standards. Guidance document no. 27 | Behavioural endpoints mentioned, some can be used. All available studies are recommended for evaluation | “…the assessor may be faced with data from studies describing endpoints that do not include direct measurements of survival, development or reproduction but, rather, describe e.g. behavioural effects, anatomical differences between control and treatment groups, effects at the tissue or sub-cellular level, such as changes in enzyme induction or gene expression. Generally these are unsuitable as the basis for EQS derivation. However, some other endpoints are relevant. For example, anatomical changes to gonad development that would prevent successful reproduction, or changes in behaviour if the effect described would impair competitive fitness may be relevant. Avoidance reactions may also be relevant if populations are likely to avoid a contaminated habitat where they would normally be present” |
The guidance document for deriving EQS states that behavioural endpoints are unsuitable as the basis for EQS derivation since the endpoints “do not include direct measurements of survival, development or reproduction”. However, the guidance document makes an exception for changes in behaviour resulting in impaired competitive fitness and avoidance reactions that would make individuals avoid contaminated habitats where otherwise they normally would be present. In contrast to the guidance document for deriving EQS, the guidance documents for Plant Protection Products regarded behavioural endpoints as key evidence. Examples of this are the assessment of field data for bees, and acknowledgement of avoidance and behaviour related to reproduction as important endpoints when assessing risks for birds and mammals.
For the four policy areas where the regulated party is obliged to perform new ecotoxicity studies if existing studies are not sufficient to fulfil the data demands, it is recommended to use standard studies, primarily from OECD. Currently, observed changes in behaviour need to be reported according to several OECD standard studies. For example, the OECD TG 222 Earthworm Reproduction Test states that an inability to dig into the soil should be reported. In the OECD TG 246 Acute Contact Toxicity Test for bumblebees, signs of reduced coordination should be reported. However, very few standard studies are designed to directly investigate effects on specific behaviours; the behavioural observations made are instead an additional measure for the primary endpoints studied, i.e. effects on mortality, growth, reproduction and development.1 The clearest examples of situations where behavioural endpoints are considered important in OECD standard studies, together with other endpoints, are when assessing effects on bees using OECD TG 213, 214 or 245. In contrary, there are two standard studies from ASTM International specifically addressing behaviour: E1604 for testing aquatic organisms, and E1768 for testing freshwater fish. For human health assessment, the OECD TG 426 Developmental Neurotoxicity Study on rats is used to investigate behavioural endpoints such as motor activity, learning and memory, anxiety, and social behaviour.
Several of the EU regulatory documents that we analysed mentioned that there is a need to develop new standard studies with behavioural endpoints. For Plant Protection Products, standard studies for birds that include endpoints such as avoidance behaviour, parental care and nesting behaviour, are requested. For bees, a standard study investigating chronic toxicity for larvae is missing, the guidance document recommends using an extended version of OECD TG 213 where behavioural endpoints are included. Currently, the OECD is developing the standard study “Homing flight test on honeybee after single exposure to sublethal doses”.21
A principle within regulatory assessments of chemicals has been to base decisions on toxicity and ecotoxicity studies investigating adverse effects. WHO defines adverse effects as “Change in the morphology, physiology, growth, development, reproduction or lifespan of an organism, system or (sub)population that results in an impairment of functional capacity to compensate for additional stress or an increase in susceptibility to other influences”.22 What separates human health assessments from environmental assessments is that population relevant effects and not individual effects are considered in the latter. The only known exception to this level of a protection goal in EU is a recommendation from EFSA to protect aquatic vertebrates (fish and amphibians) at the individual level in acute risk assessments to avoid visible mortality.23
In the outdated and no longer used Guidance Document on Risk Assessment for Birds and Mammals,24 the focus on population relevant effects was interpreted as direct effects on survival, development or reproduction, and therefore guidance like the following can be found: “One aim of the ecological risk assessment is to predict effects on the population level, although this is difficult or impossible to measure directly. The usual approach is based on the consideration that effects on populations will not occur if the survival rate, reproduction rate and development of individuals are not affected. Therefore, in principle, only endpoints in toxicity tests which are related to these key factors of population dynamics are ecotoxicologically relevant”. However, in more recently developed guidance documents populations have been linked to abundance/biomass and individuals have been linked to behaviour/survival/growth.23
There are two guidance documents explicitly stating that behavioural endpoints are relevant to the protection goal. The EFSA's guidance document on the risk assessment of plant protection products on bees list behaviour as key factor, and it does so already in the regulation (EC 1107/2009).25 The new EFSA guidance for the identification of endocrine disruptors stresses that behavioural endpoints are implicitly covered by the WHO definition of adversity since these type of effects will have implications on reproduction and development.26
In comparison to the EU, the Canadian Council of Ministers of Environment and some provincial jurisdictions, such as the British Columbia Ministry of Environment and Climate Change Strategy (BCENV) have established water quality guideline-development protocols that allow for behavioural endpoints if ecological relevance can be established. Such endpoints include predator avoidance, swimming ability, or certain olfactory-mediated behaviours that can be linked to ecological relevance. Ecological relevance, then, is defined as follows: “Ecological relevance pertains to whether physical abilities (e.g., swimming speed, orientation ability, and migratory fitness), physical traits (e.g., fin size/shape), physiological traits (e.g., production of a certain enzyme), and/or behavioural tendencies (e.g., swimming in groups) of organisms are important enough to influence a species' ecological competitiveness. Characteristics that are of high ecological relevance are those that have a strong positive or negative influence on survival, reproductive ability, and growth (e.g., stunting, high fertility, and organ failure)”.27 In addition, BCENV continues to monitor the literature for contaminant-induced olfactory impairment (including sensitive olfactory-mediated behaviours) to ensure that existing water quality guidelines remain protective against these sublethal effects (A. Azizishirazi, Pers. Comm., 2019). Thus, there are protocols and precedents that could be exported to other jurisdictions in order to facilitate the integration of behavioural endpoints into both established and newly developed regulatory frameworks for chemicals.
Known cases of behavioural endpoints used in EU chemicals regulation
The total number of cases where behavioural endpoint has been used in EU chemicals regulation is not known, and due to lack of transparency and searchable databases in the EU chemicals regulation it is not possible to make a quantitative analysis in an assessable way. Based on public and personal discussions with regulators and risk assessors we conclude that the overall regulatory use of behaviour endpoints seems to be rare. The six cases presented below are the only known publicly available cases to us (Table 2). From the analysis below we could conclude that there is spatial, temporal and product-specific variation in how these behavioural studies have been used in chemical registration and pre-market assessments in the different EU regulations.| Substance & regulation | Reference | Behaviour endpoint & test animal | Importance for the regulatory decision |
|---|---|---|---|
| Methyl tertiary-butyl ether (MTBE) in existing substances regulation | 28 | Avoidance (attraction) in fish (A. anguilla) | Key study |
| Methyl tertiary-butyl ether (MTBE) in Danish national EQS-derivation | 28 | Key study | |
| 4-Nonylphenol in REACH (substance evaluation, SVHC) | 32 | Spawning in fish (P. promelas) | Supportive evidence but not key endpoints for concluding that the substance is an endocrine disrupting chemical |
| 33 | Feeding, social and aggression in fish (O. mykiss) | ||
| 34 | Sexual in fish (P. reticulata). | ||
| Lambda-cyhalothrin in the Plant Protection Product Regulation | 36 | Drifting in insect species (B. rhodani & L. cf. Fusca) | Supportive evidence but not key endpoints |
| 37 | Pre-copulatory behaviour in amphipod (G. pulex) | ||
| 38 | Drifting in invertebrates (L. nigra, G. pulex, H. sulphurea) | ||
| Alpha-cypermethrin | Field study from chemical company | Foraging activity in honeybee (A. mellifera) | Low weight, other endpoints showing limited or low effects were considered more important |
| Di-2-ethylhexyl phthalate (DEHP) in the Existing Substances Regulation | 41 | Predation efficiency in dragon fly larvae (Aeshna) | Low weight, due to low reliability |
| Zinc pyrithione in the Biocidal Products Directive | 43 | Avoidance in amphipod (M. affinis) | Low weight, due to insufficient reporting of results. However, used to justify selection of assessment factor |
In the assessment for the insecticide lambda-cyhalothrin in the Plant Protection Product Regulation three non-standard studies investigating behaviour endpoints were used.36–38 The behavioural studies were considered to have relevant results but due to a number of factors the studies were only used as supporting evidence. Factors mentioned in the assessment were: the tested formulation was not identical to the assessed formulation; effects were observed at all treatments and hence no NOEC value could be obtained; unclear reporting of methodology; concerns regarding the study reliability; the study was a non-standard study.39
The difficulties interpreting the results from OECD TG 426 was acknowledged in a study aimed at identifying areas of improvement in this standard study. The OECD TG 426 offers several possible options in the test design when assessing “learning and memory”. For example, active avoidance of an unpleasant action can be tested, or the ability to find a way through a maze. These two test designs assess different types of behaviour. Experienced evaluators and thorough guidance are needed to be able to detect study designs that likely will result in negative results. The flexibility in the test design was considered the main reason for the interpretation difficulties, however, the flexibility was also considered necessary due to the large variation in tested substances.51 An analysis of neurotoxicity studies for bisphenol A showed that effects were more often observed in the behavioural endpoints not required (e.g. social and sexual behaviour) according to OECD TG 426, compared to the endpoints required (e.g. motor activity).52 This was seen especially at low doses, and exemplifies the importance of non-standard behavioural endpoints in regulation of chemicals.
Non-standard studies investigating behavioural endpoints have also contributed to regulatory decisions. When the restriction proposal for bisphenol A in thermal paper (used for cash register receipts) was adopted by the European Commission effects on behaviour (alteration of spatial memory and learning functions) was one of four identified key risks for the unborn child, together with effects on the female reproductive system, effects on the mammary gland, and risk for obesity. However, since the available studies, including the behavioural studies, did not allow for a quantification of the dose–response relationship, the studies could not be used directly for the derived no-effect level (DNEL, i.e. the level of exposure to a substance above which humans should not be exposed). Instead, a study investigating kidney effects in mice was used as key study together with the standard assessment factor recommended in the regulation and an additional assessment factor (of six) to take into consideration the effects seen in the behavioural studies.53
Relevance of behavioural endpoints for population level effects
The behaviour of an organism is intrinsically linked to its ‘fitness’ in a variety of ways through its capacity to communicate, find food, evade predation or catch prey, find mates, defend territories, or undergo large scale migrations. Ecologists have been linking behaviour with population level effects for decades54 but within the field of ecotoxicology the need for incorporating ecologically important behavioural measures has only been articulated more frequently in recent years.6 Behavioural ecologists have studied how behavioural variation among animals leads to differential fitness, measured as effects on life-history characters (e.g. growth rate, development rate, survival). For example, reduced mobility (i.e. activity) has repeatedly been linked with reduced growth and development of a wide range of organisms.55–58 Another behaviour where variation has been tightly linked to fitness is sociality, an individual's tendency to associate spatially with conspecifics. More social individuals generally experience increased likelihood of survival in the presence of lethal predators.59–62 These are just two types of behaviours (out of several) where trait-variation has been tightly linked to changes in life-history characters and as such translates into fitness (i.e. individual-level) and population effects. However, identifying which specific behaviours within or among various species are concretely linked to changes at population level due to chemical exposure has not received extensive attention. Below are examples where behavioural effects on individual level in ecotoxicology have been linked with effects and responses of population level importance.In the wild, disruption of reproductive behaviours as a result of exposure to endocrine disrupting compounds has been documented in a range of species. For example, exposures to organochlorine pesticides reduced herring gull (Larus smithsonianus) and merlin (Falco columbarius) offspring incubation time, nest attendance, and defence behaviours as a result of the complex interactions of these pesticides with acetylcholine and estrogen signalling.83–86 More recently, male fish (Pimephales promelas; Gasterosteus aculeatus) exposed to municipal wastewater treatment plant effluents with high concentrations of estrogen-active compounds were less likely to secure a breeding site and performed fewer courtship behaviours.87–89 Sources of contamination in the wild, such as wastewater effluents, are often complex mixtures of compounds that may have many mechanisms of action beyond the endocrine system. As such, researchers have also documented reduced aggression90 and increased courtship behaviours91 following exposure to wastewater.
Furthermore, neurologically active contaminants, such as the antidepressant fluoxetine, which modulate adaptive stress or fear responses to environmental threats also have great potential to impact anti-predator responses. Indeed, several studies have found that guppies (Poecilia reticulata) exposed to environmentally relevant concentrations of fluoxetine show delayed escape responses to a predator, froze for longer and spent more time under cover.99–101 Similarly, Brodin and colleagues102 found that European perch (Perca fluviatilis) exposed to the psychoactive pharmaceutical oxazepam were more active and bold in a novel environment, potentially increasing their susceptibility to predation. Interestingly, using a multi-stressor approach, Saaristo and colleagues6 found the initial movements of European perch to be significantly affected, and fish became bolder (i.e. entered the white background) in the oxazepam, high temperature, and predation treatments. When internal plasma doses of the antidepressant sertraline exceeded human therapeutic plasma levels, serotonin reuptake transporter binding, an anchor 1 molecular initiation event, and anxiety behaviour, and anchor 2 responses with an adverse outcome pathway context, of adult male fathead minnows were significantly altered.103
Contaminants that interfere with the ability to detect chemical cues will also alter predator–prey behaviours by inhibiting the ability to sense prey, detect predators, or group with conspecifics to avoid threat. For example, fathead minnows (Pimephales promelas) exposed to copper and goldfish (Carassius auratus) exposed to the herbicide atrazine both failed to detect and react to conspecific skin-based alarm cues.104,105 Also pharmaceuticals have been found to disrupt info-chemicals, for example the pharmaceutical propranolol lowering the anti-predator response of amphipods to predator cues, albeit at rather high concentrations.106 In addition, the antidepressant fluoxetine was shown to cause elevated alarm responses in Arabian killifish (Aphanius dispar)107 and slower predator avoidance response in larval fathead minnows (Pimephales promelas).108 McPherson and colleagues109 demonstrated that Iowa darter (Etheostoma exile) could detect and avoid traps treated with a conspecific alarm cue in a clean lake, but failed to avoid similarly treated traps in a contaminated lake. This result demonstrates that some behavioural endpoints that are observed under the controlled conditions of the laboratory also occur under natural conditions. Finally, a recent study showed that the chemosensory perception of predators by the gray tree frog (Dryophytes versicolor) was reduced by 50% when tadpoles were housed in polluted stream water and wastewater effluent compared to clean tap water.110
Discussion
Chemicals regulation is based on a combination of scientific knowledge, pragmatic considerations and policy decisions. With advancements in science and changes in community values comes new regulations, albeit with the delay that can be expected in a democratic society where legal certainty is important.Some might argue that the precautionary principle, a cornerstone in the EU chemicals regulation, could be used to justify the use of behavioural endpoints in environmental risk assessments. According to the European Commission, the precautionary principle should be used when there is evidence of potential harm, but there is not enough information to determine the risk with sufficient certainty.117 However, according to a recent review of the operation of the REACH regulation, risk management actions evoking the precautionary principle have been limited. This is because scientific uncertainties are not assessed to the extent needed for decisions based on the precautionary principle.118,119
This study concludes that the only legal obstacle to the use of behavioural endpoints in EU chemicals regulation is whether an endpoint could be considered to be relevant at the population level or not. Since there is no agreed method for establishing this connection, this decision must currently be taken case-by-case. Here, the individual assessor and/or the responsible institution can play a large role. A broader interpretation of what is relevant to populations may result in more behavioural studies being considered for chemicals regulation. A consequence of allowing for additional studies is that more resources are needed for literature search, evaluation and assessment. Since the majority of studies investigating behavioural effects are non-standard studies with possibly new test designs, methodologies and test species, increased complexity in the study evaluations is expected. Absence of standardized methods likely prevents assessors/institutions from including behavioural studies in chemical assessments.
This study also concludes that the current use of ecotoxicity studies investigating behavioural endpoints in regulation of chemicals is low. We do not claim to provide a complete overview of the current use of behavioural endpoints but since many regulators and risk assessors we discussed the matter with had difficulties remembering if behavioural studies had been used in chemicals regulation, stating that they are rarely used is appropriate. The available examples show that behavioural studies have been evaluated for use in several different regulatory frameworks, and that studies have been used as both key and supporting evidence. The available examples also show that studies have been disregarded due to low reliability as well as insufficient reporting. This is a problem that applies to peer-reviewed studies in general, and not behavioural studies in particular, and it can be improved by scientific journals introducing reporting requirements and by increased awareness of the regulatory system among academic researchers.20,120,121 What cannot be captured by this study are the studies that were disregarded in the early steps of the assessment process, e.g. because they were not searched for, or they were disregarded because the endpoint was not considered relevant.
Interestingly, behavioural studies are used in the regulation of pesticides as evidence of the efficiency of the substance. For example, behavioural endpoints are used when assessing repellents for ticks, mosquitoes, and birds.122 This appears important because pesticides are designed to be biologically active compounds that elicit effects through specific mechanisms of action. For plant production products, the most common use of animal behaviour studies is as evidence that the natural behaviour of animals will prevent them from being exposed to chemicals. For example, studies showing that certain birds and rodents prefer to avoid short grass due to lack of food and hiding places are used by chemical companies as evidence that the animals will not be exposed to pesticides used on greens at golf courses.123 This use of behavioural studies shows that these endpoints are accepted as evidence in pesticide assessments, both by regulators and by the chemical industry.
Finally, this study concludes that there is evidence that behavioural endpoints are relevant for population level effects. This conclusion is supported by a recent theme issue of the Philosophical Transactions of the Royal Society B where the connection between animal behaviour and dynamics of populations and communities was highlighted. The editorial stated that “Behavioural ecology has accumulated a rich toolbox for quantifying how the main behaviours of animals relating to foraging, predation, mating, parental care, communication and sociality are affected by the current threats to biodiversity, notably habitat loss and fragmentation, overexploitation, climate change, pollution, disease, and invasive species. This provides a firm foundation for a bottom-up approach to understanding human impacts on the natural world”.124
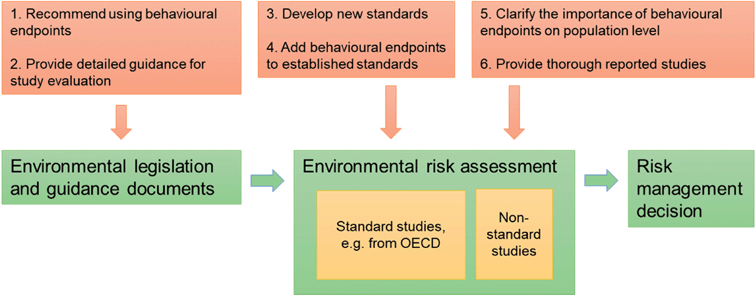
In summary, there are three principal obstacles to improved use of behavioural endpoints in environmental risk assessments of chemicals. Below these obstacles are discussed together with possible ways forward (Fig. 1).
(1) Lack of promotion of behavioural endpoints in chemicals legislation
In EU chemicals regulation, the chemical industry is obliged or recommended by legislators to submit particular types of ecotoxicological studies. The regulatory guidance documents mention either specific standard studies, which currently lack behavioural endpoints (with a few exceptions), or specific effects, focusing on survival rate, reproduction rate and developmental effects. Consequently, the chemical industry is not encouraged to submit studies investigating behavioural endpoints, even though such endpoints might be equally or more relevant for a particular chemical. A way forward is to adjust the guidance regarding the recommended effects of relevance, whenever justified (see obstacle 3).
Future efforts are warranted to develop standard behavioural studies for regulatory applications, or to add behavioural endpoints to already established standard studies. To accomplish this, it will be important to catalogue historical and more contemporary laboratory behavioural responses to contaminants. From this, information on specific behaviours of representative taxa should be identified that are predictive of ecologically important adverse outcomes in the field. Research will be needed to accomplish this goal. These efforts should initially employ specifically acting chemicals that elicit, or are anticipated to cause, behaviour changes through specific pathways because such observations could provide diagnostic positive controls for future studies of other chemicals. Laboratory studies aimed at uncovering the degree and nature of variability should be conducted to ensure that experimental design considerations (e.g., statistical power), repeatability and practices are adequate for regulatory adoption.
(2) Low use of non-standard studies in chemicals regulation
Since very few standard studies measure behavioural effects, an immediate increased use of behavioural endpoints in environmental risk assessments would depend on the use of non-standard studies. To increase the use of non-standard studies legislation must encourage or demand inclusion of such approaches, when available. One example of this practice is reflected in the draft of the new guideline on the environmental risk assessment of medicinal products for human use: “Behaviour is an example of an ecotoxicological endpoint not yet established as a reliable and standardised endpoint. It may however be very relevant for neuro-active substances and when standardised guidelines become available, be taken up in a tailored risk assessment scheme for neuro-active substances”.125 However, it should be noted that adding non-standard studies to environmental risk assessments puts additional demand on those evaluating studies at chemicals companies or regulatory agencies due to the variety of test designs and test organisms used. Here regulatory guidance that promotes thorough and robust evaluations is needed.126
Non-standard studies are primarily performed by academic researchers, during curiosity driven or exploratory research, as opposed to standard studies performed by, or on behalf of, the chemical industry to comply with test demands in chemicals regulations. Recent debated cases show that the use of non-standard studies in chemicals regulation have not been without obstacles, often resulting in disqualification of studies.127 Common reasons why non-standard studies have been assessed as “not reliable” are due to shortages in the test design or in information about how it was performed. For example, some studies are considered unreliable because of a lack of measured tested concentrations, too few replicates, or too few tested concentrations. But other studies have also been assessed as “not assignable” due to a lack of transparency in the description of the methodology and results. However, these problems have a workable solution that does not necessary request that the original research idea is compromised. In fact, the scientific quality of the study can even benefit from it. Researchers can adjust the methodology, performance and presentation of the study in minor ways thereby ensuring that the regulatory requirements are fulfilled, e.g. by increasing the statistical power by adjusting the number of replicates and by improving the transparency of the reporting.128
(3) Lack of clarification of the importance of behavioural endpoints at the population level
When a behavioural study is disqualified for regulatory use because the endpoint used is not considered relevant at the population level, the possibility for the individual researcher to influence the potential regulatory impact of the study is low. Theoretically, a detailed scientific explanation of how a behavioural change in an individual can result in effects at the population and/or community level would be sufficient. A conceptual framework for this has been developed, intended for researchers that would like to expand their investigation from individual level to community level.6 Tools like Systematic Reviews and Systematic Maps has been suggested to facilitate use of behavioural studies in conservation management and policy.129 In EU and the U.S. there is a growing interest for such methodology in chemicals regulation.130
Currently, to claim that behavioural endpoints are relevant at the population level (according to the current EU regulation), their connection to survival rate, reproduction rate and developmental effects needs to be established. Consequently, there may be a lower regulatory threshold for behavioural endpoints closely related to these effects, e.g. as shown in the use of behavioural endpoints in two standard toxicity studies used in human health risk assessments to assess reproductive and developmental effects (OECD TG 416 and 426). The results in the present study show that what is considered relevant at the population level may change between jurisdictions. For example, some legislation has opened up for use of some behavioural endpoints while others only recommend the more traditional endpoints. What is considered population relevant may also change over time. As one example, adverse histopathological effects on the kidney was recently used as key evidence when an EQS for the pharmaceutical diclofenac was set.131 Harmonisation among regulatory frameworks represents a useful goal.
Conclusions and recommendations
There are three main conclusions from this study:(1) Behavioural endpoints representing a wide range of behaviours are relevant at the population level and should therefore be used in chemicals regulation.
(2) There is currently a low use of ecotoxicity studies investigating behavioural endpoints in chemicals regulation.
(3) There are no apparent legal obstacles to be able to use behavioural studies in EU chemicals regulation, except that the endpoints need to be considered relevant at the population level.
In order to improve the current use of behavioural studies in regulation of chemicals, all relevant stakeholders are recommended to contribute:
• Researchers need to write robust and reliable papers, and also carefully explain the relevance of the study for regulation of chemicals.
• Editors and scientific journals need to promote and demand detailed study reports and the highest level of study reliability and transparency, including responsible data sharing.
• Regulatory agencies and the chemical industry need to embrace new behavioural endpoints and develop standardized methods for behaviours when relevant at the population level.
Disclaimer
This paper is the work of the authors and the opinions herein are those of the authors alone and do not necessarily reflect the view or opinions of their institutions.Authors contributions
Ågerstrand devised the study and drafted the manuscript. All authors contributed to the manuscript and approved the final manuscript. All data is available in the manuscript.Conflicts of interests
The authors have no competing financial interests to declare.Acknowledgements
Thanks to researchers, risk assessors and regulators working at the Swiss Ecotox Centre, the Netherlands National Institute for Public Health and the Environment, the UK Environment Agency, the US Environmental Protection Agency, the Danish Environmental Protection Agency, the German Environment Agency and the Swedish Chemicals Agency for valuable discussions.References
- J. F. Postma and C. M. Keijzers, Behavior as response parameter a literature review on the relevance for population sustainability, Ecofide, 2014, http://www.ecofide.nl/uploads/files/2014-behavior_as_response_parameter-_final_report.pdf Search PubMed.
- A. V. Kalueff, A. M. Stewart and R. Gerlai, Zebrafish as an emerging model for studying complex brain disorders, Trends Pharmacol. Sci., 2014, 35(2), 63–75 CrossRef CAS PubMed.
- G. Pyle and A. T. Ford, Behaviour revised: contaminant effects on aquatic animal behaviour, Aquat. Toxicol., 2017, 182, 226–228 CrossRef CAS PubMed.
- A. Gerhardt, Aquatic Behavioral Ecotoxicology—Prospects and Limitations, Hum. Ecol. Risk Assess. Int. J., 2007, 13(3), 481–491 CrossRef.
- G. Pyle and A. T. Ford, Behaviour revised: contaminant effects on aquatic animal behaviour, Aquat. Toxicol., 2017, 182, 226–228 CrossRef CAS PubMed.
- M. Saaristo, B. Tomas, B. Sigal, G. Bertram Michael, W. Brooks Bryan and M. Ehlman Sean, et al., Direct and indirect effects of chemical contaminants on the behaviour, ecology and evolution of wildlife, Proc. R. Soc. B, 2018, 285(1885), 20181297 CrossRef PubMed.
- X. Zhang, Environmental DNA Shaping a New Era of Ecotoxicological Research, Environ. Sci. Technol., 2019, 53(10), 5605–5612 CrossRef CAS PubMed.
- C. Amiard-Triquet, Behavioral Disturbances: The Missing Link between Sub-Organismal and Supra-Organismal Responses to Stress? Prospects Based on Aquatic Research, Hum. Ecol. Risk Assess. Int. J., 2009, 15(1), 87–110 CrossRef CAS.
- S. Brander, S. Hecht and K. Kuivila, The challenge: “Bridging the gap” with fish: advances in assessing exposure and effects across biological scales, Environ. Toxicol. Chem., 2015, 34(3), 459 CrossRef CAS PubMed.
- S. D. Melvin and S. P. Wilson, The utility of behavioral studies for aquatic toxicology testing: a meta-analysis, Chemosphere, 2013, 93(10), 2217–2223 CrossRef CAS PubMed.
- B. B. M. Wong and U. Candolin, Behavioral responses to changing environments, Behav. Ecol., 2015, 26(3), 665–673 CrossRef.
- P. D. Robinson, Behavioural toxicity of organic chemical contaminants in fish: application to ecological risk assessments (ERAs), Can. J. Fish. Aquat. Sci., 2009, 66(7), 1179–1188 CrossRef CAS.
- J. S. Weis, G. Smith, T. Zhou, C. Santiago-Bass and P. Weis, Effects of Contaminants on Behavior: Biochemical Mechanisms and Ecological Consequences, BioScience, 2001, 51(3), 209–217 CrossRef.
- W. B. Steele, L. A. Kristofco, J. Corrales, G. N. Saari, S. P. Haddad and E. P. Gallagher, et al., Comparative behavioral toxicology with two common larval fish models: exploring relationships among modes of action and locomotor responses, Sci. Total Environ., 2018, 640–641, 1587–1600 CrossRef CAS PubMed.
- European Chemicals Agency, REACH Guidance documents, Helsinki, Finland, 2011, https://echa.europa.eu/guidance-documents/guidance-on-reach Search PubMed.
- European Chemicals Agency, Guidance on information requirements and chemical safety assessment. Part B: Hazard assessment, Version 2.1, 2011, http://echa.europa.eu/documents/10162/13643/information_requirements_part_b_en.pdf Search PubMed.
- OECD, OECD Guideline for testing of chemicals. Daphnia sp., Acute immobilisation test, OECD 202, Paris, France, 2004 Search PubMed.
- US EPA, Ecological Effects Test Guidelines OPPTS 850.1400, Fish Early-Life Stage Toxicity Test, 1996 Search PubMed.
- European Chemicals Agency, REACH Guidance documents, Helsinki, Finland, 2011, https://echa.europa.eu/guidance-documents/guidance-on-reach Search PubMed.
- M. Ågerstrand, A. Sobek, K. Lilja, M. Linderoth, L. Wendt-Rasch and A.-S. Wernersson, et al., An academic researcher's guide to increased impact on regulatory assessment of chemicals, Environ. Sci.: Processes Impacts, 2017, 19(5), 644–655 RSC.
- OECD, Work plan for the Test Guidelines Programme (TGP), 2016, https://www.oecd.org/env/ehs/testing/TGPworkplan_July2016-forpublication.pdf Search PubMed.
- WHO, Principles and methods for the risk assessment of chemicals in food, WHO. 2009, http://www.who.int/foodsafety/publications/chemical-food/en/ Search PubMed.
- European Food Safety Authority, Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge-of-field surface waters, 2013, http://www.efsa.europa.eu/en/efsajournal/doc/3290.pdf Search PubMed.
- European Commission, Guidance Document on Risk Assessment for Birds and Mammals Under Council Directive 91/414/EEC, 2002, http://ec.europa.eu/food/plant/pesticides/guidance_documents/docs/wrkdoc19_en.pdf Search PubMed.
- European Food Safety Authority, Guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees), EFSA J., 2013, 11(7), 3295 Search PubMed.
- European Food Safety Authority, Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009, EFSA J., 2018, 16(6), 5311 Search PubMed.
- Canadian Council of Ministers of Environment, A protocol for the derivation of water quality guidelines for the protection of aquatic life, 2007, p. 37, [Guidance Document]. Retrieved from Canadian Council of Ministers of the Environment website: http://www.ccme.ca/files/Resources/supporting_scientific_documents/protocol_aql_2007e.pdf Search PubMed.
- G. I. Petersen, Eels (Anguilla anguilla) avoidance test with MTBE, Report to EFOA – European Fuel Oxygenates Association, Brussels, Belgium, 2003 Search PubMed.
- European Chemicals Bureau, European Union Risk Assessment Report. Tert-butyl methyl ether, Joint Research Center, European Communities, 2002 Search PubMed.
- European Chemicals Bureau, Addendum to the Environmental Risk Assessment for tert-butyl methyl ether (MTBE) Search PubMed.
- Danish Environmental Protection Agency, Determining quality standards for the aquatic environment, in Methyl tertiary-Butyl Ether (MTBE), (CAS no. 1634-04-4), Danish, 2009 Search PubMed.
- H. L. Schoenfuss, S. E. Bartell, T. B. Bistodeau, R. A. Cediel, K. J. Grove and L. Zintek, et al., Impairment of the reproductive potential of male fathead minnows by environmentally relevant exposures to 4-nonylphenol, Aquat. Toxicol., 2008, 86(1), 91–98 CrossRef CAS PubMed.
- A. J. Ward, A. J. Duff and S. Currie, The effects of the endocrine disrupter 4-nonylphenol on the behaviour of juvenile rainbow trout (Oncorhynchus mykiss), Can. J. Fish. Aquat. Sci., 2006, 63(2), 377–382 CrossRef CAS.
- M. Cardinali, F. Maradonna, I. Olivotto, G. Bortoluzzi, G. Mosconi and A. M. Polzonetti-Magni, et al., Temporary impairment of reproduction in freshwater teleost exposed to nonylphenol, Reprod. Toxicol., 2004, 18(4), 597–604 CrossRef CAS PubMed.
- European Chemicals Agency, Support document for identification of 4-nonylphenol, branched and linear, as substances of very high concern because due to their endocrine disrupting properties they cause probable serious effects to the environment which give rise to an equivalent level of concern to those of CMRs and PBT/vPvBs, 2012 Search PubMed.
- R. B. Lauridsen and N. Friberg, Stream macroinvertebrate drift response to pulsed exposure of the synthetic pyrethroid lambda-cyhalothrin, Environ. Toxicol., 2005, 20(5), 513–521 CrossRef CAS PubMed.
- L.-H. Heckmann, N. Friberg and H. W. Ravn, Relationship between biochemical biomarkers and pre-copulatory behaviour and mortality in Gammarus pulex following pulse-exposure to lambda-cyhalothrin, Pest Manage. Sci., 2005, 61(7), 627–635 CrossRef CAS PubMed.
- U. Nørum, N. Friberg, M. R. Jensen, J. M. Pedersen and P. Bjerregaard, Behavioural changes in three species of freshwater macroinvertebrates exposed to the pyrethroid lambda-cyhalothrin: laboratory and stream microcosm studies, Aquat. Toxicol., 2010, 98(4), 328–335 CrossRef PubMed.
- Swedish Chemicals Agency, Renewal Assessment Report under Regulation (EC) 1107/2009. lambda-cyhalothrin. Active substance data, 2013 Search PubMed.
- Swedish Chemicals Agency, Fastac 50, product report, 2009 Search PubMed.
- P. Woin and P. Larsson, Phthalate esters reduce predation efficiency of dragonfly larvae, Odonata; Aeshna, Bull. Environ. Contam. Toxicol., 1987, 38(2), 220–225 CrossRef CAS PubMed.
- European Chemicals Bureau, European Union Risk Assessment Report bis(2-ethylhexyl) phthalate (DEHP), 2008, https://echa.europa.eu/documents/10162/e614617d-58e7-42d9-b7fb-d7bab8f26feb Search PubMed.
- A.-K. Eriksson Wiklund, T. Börjesson and S. J. Wiklund, Avoidance response of sediment living amphipods to zinc pyrithione as a measure of sediment toxicity, Mar. Pollut. Bull., 2006, 52(1), 96–99 CrossRef CAS PubMed.
- Swedish Chemicals Agency, Competent Authority Report för Copper Pyrithione (PT 21); Document II A: Effect assessment for the active substance, Final CAR, 2014 Search PubMed.
- H.-J. Klimisch, M. Andreae and U. Tillmann, A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data, Regul. Toxicol. Pharmacol., 1997, 25(1), 1–5 CrossRef CAS PubMed.
- US EPA, Biological Evaluation under the Endangered Species Act for chlorpyrifos, malathion, and diazinon, 2015, https://www.epa.gov/endangered-species/implementing-nas-report-recommendations-ecological-risk-assessment-endangered-and Search PubMed.
- European Commission, Commission regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances, 2011, https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:155:0001:0066:EN:PDF Search PubMed.
- European Commission, Regulation (EU) No 528/2012 of the European Parliament and of the council of 22 May 2012 concerning the making available on the market and use of biocidal products, 2012, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02012R0528-20140425%26from=SV Search PubMed.
- European Chemicals Agency, Directive 98/8/EC concerning the placing biocidal products on the market Inclusion of active substances in Annex I or IA to Directive 98/8/EC. Assessment Report lambda-cyhalothrin, 2011, https://echa.europa.eu/documents/10162/0c9351c8-a352-6a0e-0ae9-9db17768b70f Search PubMed.
- European Chemicals Agency, Directive 98/8/EC concerning the placing biocidal products on the market Inclusion of active substances in Annex I or IA to Directive 9818/EC Assessment Report Deltamethrin, 2011, https://echa.europa.eu/documents/10162/56abada9-eb4d-ee0e-9426-134d23c6eab1 Search PubMed.
- A. Beronius, A. Hanberg, R. Heimier and H. Håkansson, Risk assessment of developmental neurotoxicity: Evaluation of the OECD TG 426 test guideline and guidance documents, Karolinska Institutet, 2013, https://ki.se/sites/default/files/2013-1.pdf Search PubMed.
- A. Beronius, N. Johansson, C. Rudén and A. Hanberg, The influence of study design and sex-differences on results from developmental neurotoxicity studies of bisphenol A: implications for toxicity testing, Toxicology, 2013, 311(1–2), 13–26 CrossRef CAS PubMed.
- European Chemicals Agency, Opinion on an Annex XV dossier proposing restrictions on bisphenol A, 2015, https://echa.europa.eu/documents/10162/209030fc-ca4b-4745-97b6-98bfc4d6bdd3 Search PubMed.
- Behavioural Ecology: An Evolutionary Approach, ed. J. R. Krebs, and N. B. Davies, 4th edn, 1997, https://www.wiley.com/en-us/Behavioural+Ecology%3A+An+Evolutionary+Approach%2C+4th+Edition-p-9780865427310 Search PubMed.
- B. R. Anholt and E. E. Werner, Predictable changes in predation mortality as a consequence of changes in food availability and predation risk, Evol. Ecol., 1998, 12(6), 729–738 CrossRef.
- S. L. Ball and R. L. Baker, Predator–Induced Life History Changes: Antipredator Behavior Costs or Facultative Life History Shifts?, Ecology, 1996, 77(4), 1116–1124 CrossRef.
- T. A. Crowl and A. P. Covich, Predator-Induced Life-History Shifts in a Freshwater Snail, Science, 1990, 247(4945), 949–951 CrossRef CAS PubMed.
- B. L. Peckarsky, C. A. Cowan, M. A. Penton and C. Anderson, Sublethal Consequences of Stream-Dwelling Predatory Stoneflies on Mayfly Growth and Fecundity, Ecology, 1993, 74(6), 1836–1846 CrossRef.
- R. C. Miller, The Significance of the Gregarious Habit, Ecology, 1922, 3(2), 122–126 CrossRef.
- D. C. Krakauer, Groups confuse predators by exploiting perceptual bottlenecks: a connectionist model of the confusion effect, Behav. Ecol. Sociobiol., 1995, 36(6), 421–429 CrossRef.
- J. Krause and G. D. Ruxton, Living in Groups, Oxford University Press, Oxford, New York, USA, 2002, p. 224 Search PubMed.
- T. Brodin, S. Fogarty, A. Sih and J. Cote, Personality-dependent survival of the invasive mosquitofish: being social can be deadly, Aquatic Invasions, 2019, 14(3), 465–477 CrossRef.
- E. E. Little and S. E. Finger, Swimming behavior as an indicator of sublethal toxicity in fish, Environ. Toxicol. Chem., 1990, 9(1), 13–19 CrossRef CAS.
- P. D. Robinson, Behavioural toxicity of organic chemical contaminants in fish: application to ecological risk assessments (ERAs), Can. J. Fish. Aquat. Sci., 2009, 66(7), 1179–1188 CrossRef CAS.
- G. Hellström, J. Klaminder, M. Jonsson, J. Fick and T. Brodin, Upscaling behavioural studies to the field using acoustic telemetry, Aquat. Toxicol., 2016, 170, 384–389 CrossRef PubMed.
- J. Klaminder, M. Jonsson, J. Leander, J. Fahlman, T. Brodin and J. Fick, et al., Less anxious salmon smolt become easy prey during downstream migration, Sci. Total Environ., 2019, 687, 488–493 CrossRef CAS PubMed.
- S. G. Woodman, D. Steinkey, W. A. Dew, S. R. Burket, B. W. Brooks and G. G. Pyle, Effects of sertraline on behavioral indices of crayfish Orconectes virilis, Ecotoxicol. Environ. Saf., 2016, 134, 31–37 CrossRef CAS PubMed.
- J. R. Marentette, S. Tong, G. Wang, N. M. Sopinka, M. D. Taves and M. A. Koops, et al., Behavior as biomarker? Laboratory versus field movement in round goby (Neogobius melanostomus) from highly contaminated habitats, Ecotoxicology, 2012, 21(4), 1003–1012 CrossRef CAS PubMed.
- B. B. Chapman, C. Brönmark, J.-Å. Nilsson and L.-A. Hansson, The ecology and evolution of partial migration, Oikos, 2011, 120(12), 1764–1775 CrossRef.
- T. Avgar, G. Street and J. M. Fryxell, On the adaptive benefits of mammal migration, Can. J. Zool., 2013, 92(6), 481–490 CrossRef.
- F. Pulido, Evolutionary genetics of partial migration – the threshold model of migration revis(it)ed, Oikos, 2011, 120(12), 1776–1783 CrossRef.
- L. Ellis, Dominance and reproductive success among nonhuman animals: a cross-species comparison, Ethol. Sociobiol., 1995, 16(4), 257–333 CrossRef.
- A. J. W. Ward, J. Duff Alison, S. Horsfall Jennifer and S. Currie, Scents and scents-ability: pollution disrupts chemical social recognition and shoaling in fish, Proc. R. Soc. B, 2008, 275(1630), 101–105 CrossRef PubMed.
- J. A. Mennigen, W. E. Lado, J. M. Zamora, P. Duarte-Guterman, V. S. Langlois and C. D. Metcalfe, et al., Waterborne fluoxetine disrupts the reproductive axis in sexually mature male goldfish, Carassius auratus, Aquat. Toxicol., 2010, 100(4), 354–364 CrossRef CAS PubMed.
- K. A. Sloman, Effects of trace metals on salmonid fish: the role of social hierarchies, Appl. Anim. Behav. Sci., 2007, 104(3), 326–345 CrossRef.
- K. A. Sloman, D. W. Baker, C. G. Ho, D. G. McDonald and C. M. Wood, The effects of trace metal exposure on agonistic encounters in juvenile rainbow trout, Oncorhynchus mykiss, Aquat. Toxicol., 2003, 63(2), 187–196 CrossRef CAS PubMed.
- K. A. Sloman, G. R. Scott, Z. Diao, C. Rouleau, C. M. Wood and D. G. McDonald, Cadmium affects the social behaviour of rainbow trout, Oncorhynchus mykiss, Aquat. Toxicol., 2003, 65(2), 171–185 CrossRef CAS PubMed.
- K. A. Sloman, O. Lepage, J. T. Rogers, C. M. Wood and S. Winberg, Socially-mediated differences in brain monoamines in rainbow trout: effects of trace metal contaminants, Aquat. Toxicol., 2005, 71(3), 237–247 CrossRef CAS PubMed.
- N. M. Sopinka, J. R. Marentette and S. Balshine, Impact of contaminant exposure on resource contests in an invasive fish, Behav. Ecol. Sociobiol., 2010, 64(12), 1947–1958 CrossRef.
- M. Saaristo, J. A. Craft, K. K. Lehtonen and K. Lindström, Sand goby (Pomatoschistus minutus) males exposed to an endocrine disrupting chemical fail in nest and mate competition, Horm. Behav., 2009, 56(3), 315–321 CrossRef CAS PubMed.
- M. Saaristo, J. A. Craft, K. K. Lehtonen and K. Lindström, Exposure to 17α-ethinyl estradiol impairs courtship and aggressive behaviour of male sand gobies (Pomatoschistus minutus), Chemosphere, 2010, 79(5), 541–546 CrossRef CAS PubMed.
- F. Hoffmann and W. Kloas, Estrogens Can Disrupt Amphibian Mating Behavior, PLoS One, 2012, 7(2), e32097 CrossRef CAS PubMed.
- C. E. Grue, P. L. Gibert and M. E. Seeley, Neurophysiological and Behavioral Changes in Non-Target Wildlife Exposed to Organophosphate and Carbamate Pesticides: Thermoregulation, Food Consumption, and Reproduction, Am. Zool., 1997, 37(4), 369–388 CrossRef CAS.
- D. M. Fry, Reproductive Effects in Birds Exposed to Pesticides and Industrial Chemicals, Environ. Health Perspect., 1995, 103, 165–171 CAS.
- D. B. Peakall, Behavioral responses of birds to pesticides and other contaminants, in Residue Reviews, ed. F. A. Gunther, Springer, New York, 1985, pp. 45–77 Search PubMed.
- G. A. Fox and T. Donald, Organochlorine Pollutants, Nest-Defense Behavior and Reproductive Success in Merlins, Condor, 1980, 82, 81–84 CrossRef.
- N. Garcia-Reyero, C. M. Lavelle, B. L. Escalon, D. Martinović, K. J. Kroll and P. W. Sorensen, et al., Behavioral and genomic impacts of a wastewater effluent on the fathead minnow, Aquat. Toxicol., 2011, 101(1), 38–48 CrossRef CAS PubMed.
- D. Martinović, W. T. Hogarth, R. E. Jones and P. W. Sorensen, Environmental estrogens suppress hormones, behavior, and reproductive fitness in male fathead minnows, Environ. Toxicol. Chem., 2007, 26(2), 271–278 CrossRef PubMed.
- M. Sebire, I. Katsiadaki, N. G. H. Taylor, G. Maack and C. R. Tyler, Short-term exposure to a treated sewage effluent alters reproductive behaviour in the three-spined stickleback (Gasterosteus aculeatus), Aquat. Toxicol., 2011, 105(1), 78–88 CrossRef CAS PubMed.
- E. S. McCallum, E. Krutzelmann, T. Brodin, J. Fick, A. Sundelin and S. Balshine, Exposure to wastewater effluent affects fish behaviour and tissue-specific uptake of pharmaceuticals, Sci. Total Environ., 2017, 605–606, 578–588 CrossRef CAS PubMed.
- M. Saaristo, J. Myers, R. Jacques-Hamilton, M. Allinson, A. Yamamoto and G. Allinson, et al., Altered reproductive behaviours in male mosquito fish living downstream from a sewage treatment plant, Aquat. Toxicol., 2014, 149, 58–64 CrossRef CAS PubMed.
- C. Amiard-Triquet, Behavioral Disturbances: The Missing Link between Sub-Organismal and Supra-Organismal Responses to Stress? Prospects Based on Aquatic Research, Hum. Ecol. Risk Assess. Int. J., 2009, 15(1), 87–110 CrossRef CAS.
- T. Brodin, S. Piovano, J. Fick, J. Klaminder, M. Heynen and M. Jonsson, Ecological effects of pharmaceuticals in aquatic systems—impacts through behavioural alterations, Philos. Trans. R. Soc., B, 2014, 369, 20130580 CrossRef PubMed , https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4213591/.
- Behavioural Ecology: An Evolutionary Approach, ed. J. R. Krebs and N. B. Davies, 4th edn, 1997, https://www.wiley.com/en-us/Behavioural+Ecology%3A+An+Evolutionary+Approach%2C+4th+Edition-p-9780865427310 Search PubMed.
- S. E. DuRant, W. A. Hopkins and L. G. Talent, Impaired terrestrial and arboreal locomotor performance in the western fence lizard (Sceloporus occidentalis) after exposure to an AChE-inhibiting pesticide, Environ. Pollut., 2007, 149(1), 18–24 CrossRef CAS PubMed.
- J. K. Stanley, A. J. Ramirez, C. K. Chambliss and B. W. Brooks, Enantiospecific sublethal effects of the antidepressant fluoxetine to a model aquatic vertebrate and invertebrate, Chemosphere, 2007, 69(1), 9–16 CrossRef CAS PubMed.
- T. W. Valenti, P. Perez-Hurtado, C. K. Chambliss and B. W. Brooks, Aquatic toxicity of sertraline to Pimephales promelas at environmentally relevant surface water pH, Environ. Toxicol. Chem., 2009, 28(12), 2685–2694 CrossRef CAS PubMed.
- J. P. Berninger, B. Du, K. A. Connors, S. A. Eytcheson, M. A. Kolkmeier and K. N. Prosser, et al., Effects of the antihistamine diphenhydramine on selected aquatic organisms, Environ. Toxicol. Chem., 2011, 30(9), 2065–2072 CrossRef CAS PubMed.
- M. Pelli and V. P. Connaughton, Chronic exposure to environmentally-relevant concentrations of fluoxetine (Prozac) decreases survival, increases abnormal behaviors, and delays predator escape responses in guppies, Chemosphere, 2015, 139, 202–209 CrossRef CAS PubMed.
- M. Saaristo, A. McLennan, C. P. Johnstone, B. O. Clarke and B. B. M. Wong, Impacts of the antidepressant fluoxetine on the anti-predator behaviours of wild guppies (Poecilia reticulata), Aquat. Toxicol., 2017, 183, 38–45 CrossRef CAS PubMed.
- J. M. Martin, M. Saaristo, M. G. Bertram, P. J. Lewis, T. L. Coggan and B. O. Clarke, et al., The psychoactive pollutant fluoxetine compromises antipredator behaviour in fish, Environ. Pollut., 2017, 222, 592–599 CrossRef CAS PubMed.
- T. Brodin, J. Fick, M. Jonsson and J. Klaminder, Dilute Concentrations of a Psychiatric Drug Alter Behavior of Fish from Natural Populations, Science, 2013, 339(6121), 814–815 CrossRef CAS PubMed.
- T. W. Valenti, G. G. Gould, J. P. Berninger, K. A. Connors, N. B. Keele and K. N. Prosser, et al., Human Therapeutic Plasma Levels of the Selective Serotonin Reuptake Inhibitor (SSRI) Sertraline Decrease Serotonin Reuptake Transporter Binding and Shelter-Seeking Behavior in Adult Male Fathead Minnows, Environ. Sci. Technol., 2012, 46(4), 2427–2435 CrossRef CAS PubMed.
- W. A. Dew, A. Azizishirazi and G. G. Pyle, Contaminant-specific targeting of olfactory sensory neuron classes: Connecting neuron class impairment with behavioural deficits, Chemosphere, 2014, 112, 519–525 CrossRef CAS PubMed.
- P. Saglio and S. Trijasse, Behavioral responses to atrazine and diuron in goldfish, Arch. Environ. Contam. Toxicol., 1998, 35(3), 484–491 CrossRef CAS PubMed.
- A.-K. E. Wiklund, H. Oskarsson, G. Thorsén and L. Kumblad, Behavioural and physiological responses to -pharmaceutical exposure in macroalgae and -grazers from a Baltic Sea littoral community, Aquat. Biol., 2011, 14(1), 29–39 CrossRef.
- M. J. Barry, Effects of fluoxetine on the swimming and behavioural responses of the Arabian killifish, Ecotoxicology, 2013, 22(2), 425–432 CrossRef CAS PubMed.
- M. M. Painter, M. A. Buerkley, M. L. Julius, A. M. Vajda, D. O. Norris and L. B. Barber, et al., Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas), Environ. Toxicol. Chem., 2009, 28(12), 2677–2684 CrossRef CAS PubMed.
- T. D. McPherson, R. S. Mirza and G. G. Pyle, Responses of wild fishes to alarm chemicals in pristine and metal-contaminated lakes, Can. J. Zool., 2004, 82(5), 694–700 CrossRef CAS.
- R. R. Troyer and A. M. Turner, Chemosensory Perception of Predators by Larval Amphibians Depends on Water Quality, PLoS One, 2015, 10(6), e0131516 CrossRef PubMed.
- A. Rodriguez, H. Zhang, J. Klaminder, T. Brodin, P. L. Andersson and M. Andersson, ToxTrac: A fast and robust software for tracking organisms, Methods Ecol. Evol., 2018, 9(3), 460–464 CrossRef.
- Z. Ren, J. Zha, M. Ma, Z. Wang and A. Gerhardt, The early warning of aquatic organophosphorus pesticide contamination by on-line monitoring behavioral changes of Daphnia magna, Environ. Monit. Assess., 2007, 134(1), 373 CrossRef CAS PubMed.
- M. O. Parker, A. J. Brock, A. Sudwarts and C. H. Brennan, Atomoxetine reduces anticipatory responding in a 5-choice serial reaction time task for adult zebrafish, Psychopharmacology, 2014, 231(13), 2671–2679 CrossRef CAS PubMed.
- J. C. Chiu, K. H. Low, D. H. Pike, E. Yildirim and I. Edery, Assaying Locomotor Activity to Study Circadian Rhythms and Sleep Parameters in Drosophila, J. Visualized Exp., 2010,(43), 2157 Search PubMed , https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3229366/.
- R. Cartlidge, D. Nugegoda and D. Wlodkowic, Millifluidic Lab-on-a-Chip technology for automated toxicity tests using the marine amphipod Allorchestes compressa, Sens. Actuators, B, 2017, 239, 660–670 CrossRef CAS.
- B. W. Brooks and W. B. Steele, Ecotoxicological Perspectives on Healthcare and the Environment, in Healthcare and Environmental Contaminants, ed. A. B. A. Boxall and R. S. Kookana, Elsevier, London, UK; Amsterdam, 2018, ch. 4, pp. 41–67, available from: http://www.sciencedirect.com/science/article/pii/B9780444638571000048 Search PubMed.
- European Commission, Communication from the Commission of 2 February 2000 on the precautionary principle, 2000, http://ec.europa.eu/dgs/health_consumer/library/pub/pub07_en.pdf Search PubMed.
- European Commission, Communication from the Commission to the European Parliament, the council and the European economic and social committee, Commission General Report on the operation of REACH and review of certain elements, 2018, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52018DC0116%26from=EN Search PubMed.
- European Commission, Commission staff document. Accompanying the document: Communication from the Commission to the European Parliament, the council and the European economic and social committee, Commission General Report on the operation of REACH and review of certain elements, 2018, https://eur-lex.europa.eu/resource.html?uri=cellar:2834985c-2083-11e8-ac73-01aa75ed71a1.0001.02/DOC_1%26format=PDF Search PubMed.
- M. Ågerstrand, S. Christiansen, A. Hanberg, C. Rudén, L. Andersson and S. Andersen, et al., A call for action: Improve reporting of research studies to increase the scientific basis for regulatory decision-making, J. Appl. Toxicol., 2018, 38(5), 783–785 CrossRef PubMed.
- M. Ågerstrand, L. Edvardsson and C. Rudén, Bad Reporting or Bad Science? Systematic Data Evaluation as a Means to Improve the Use of Peer-Reviewed Studies in Risk Assessments of Chemicals, Hum. Ecol. Risk Assess. Int. J., 2013, 20(6), 1427–1445 CrossRef.
- European Chemicals Regulation, Guidance on the Biocidal Products Regulation. Volume II Efficacy – Assessment and Evaluation, (Parts B+C), 2018 Search PubMed.
- European Food Safety Authority, Risk Assessment for Birds and Mammals, 2009, http://www.efsa.europa.eu/en/efsajournal/doc/1438.pdf Search PubMed.
- J. Bro-Jørgensen, D. W. Franks and K. Meise, Linking behaviour to dynamics of populations and communities: application of novel approaches in behavioural ecology to conservation, Philos. Trans. R. Soc., B, 2019, 374(1781), 20190008 CrossRef PubMed.
- European Medicine Agency, EMEA/CHMP/SWP/4447/00 Rev. 1 2 Committee for Medicinal Products for Human Use (CHMP) 3 Guideline on the environmental risk assessment of medicinal products for human use – Draft, 2019 Search PubMed.
- R. Kase, M. Korkaric, I. Werner and M. Ågerstrand, Criteria for Reporting and Evaluating ecotoxicity Data (CRED): comparison and perception of the Klimisch and CRED methods for evaluating reliability and relevance of ecotoxicity studies, Environ. Sci. Eur., 2016, 28(1), 7 CrossRef PubMed , http://www.enveurope.com/content/28/1/7.
- R. E. Alcock, B. H. MacGillivray and J. S. Busby, Understanding the mismatch between the demands of risk assessment and practice of scientists – the case of Deca-BDE, Environ. Int., 2011, 37(1), 216–225 CrossRef CAS PubMed.
- C. T. A. Moermond, R. Kase, M. Korkaric and M. Ågerstrand, CRED: Criteria for reporting and evaluating ecotoxicity data, Environ. Toxicol. Chem., 2016, 35(5), 1297–1309 CrossRef CAS.
- O. Berger-Tal, A. L. Greggor, B. Macura, C. A. Adams, A. Blumenthal and A. Bouskila, et al., Systematic reviews and maps as tools for applying behavioral ecology to management and policy, Behav. Ecol., 2019, 30(1), 1–8 CrossRef.
- A. A. Rooney, A. L. Boyles, M. S. Wolfe, J. R. Bucher and K. A. Thayer, Systematic Review and Evidence Integration for Literature-Based Environmental Health Science Assessments, Environ. Health Perspect., 2014, 711–718 CrossRef PubMed , http://ehp.niehs.nih.gov/1307972.
- C. T. A. Moermond and C. E. Smit, Derivation of water quality standards for carbamazepine, metoprolol, and metformin and comparison with monitoring data, Environ. Toxicol. Chem., 2016, 35(4), 882–888 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |