Open Access Article
Open Access ArticleSynthesis of stable and phase-adjustable CsPbBr3@Cs4PbBr6 nanocrystals via novel anion–cation reactions†
Leimeng
Xu
,
Jianhai
Li
,
Tao
Fang
,
Yongli
Zhao
,
Shichen
Yuan
,
Yuhui
Dong
 and
Jizhong
Song
and
Jizhong
Song
 *
*
MIIT Key Laboratory of Advanced Display Materials and Devices, Institute of Optoelectronics and Nanomaterials, School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing 210094, China. E-mail: songjizhong@njust.edu.cn
First published on 27th December 2018
Abstract
All-inorganic cesium lead halide perovskites have emerged as promising semiconductor materials due to their preeminent performance in lighting, display, light detecting, and laser fields. However, the applications of lead halide perovskites are limited by the dissatisfactory stability owing to their fragile ionic crystal characteristics and highly dynamic surface-coordinated states. The in situ diphase structure passivation possessing the same chemical constituents (such as passivating CsPbBr3 with Cs4PbBr6) has been proven to be an effective way to improve the stabilities and simultaneously maintain the highly efficient luminescence properties. Herein, for the first time, we report a novel anion–cation reaction method to synthesize the lead halide perovskite NCs with diphase CsPbBr3@Cs4PbBr6 structure. Moreover, we have found that the phase transformation between CsPbBr3 and Cs4PbBr6 is temperature dependent. Thus, we could control the relative composition of the diphase CsPbBr3@Cs4PbBr6 composite by adjusting the temperature. The optimized CsPbBr3@Cs4PbBr6 composite NCs achieve highly light emissive performance and stabilities against atmosphere, moisture and heating. Furthermore, we could obtain 135% of the NTSC color gamut through anion exchange. These highly emissive composite NCs with improved stabilities exhibit great potential in future optoelectronic fields.
Introduction
Lead halide perovskites gained increasing attention in light-emitting diodes (LED),1–8 photodetectors,9–12 and lasers13 in recent years due to their ultrahigh photoluminescence quantum yields (PLQYs), good carrier mobility, multicolor electroluminescence, and low-threshold lasing. The high-performance halide perovskite is commonly used in the form of three-dimensional (3D) cubic or monoclinic phase,14–18 CsPbX3 (X = Cl, Br, I), which is known to be composed of corner-shared PbX64− octahedron and filling Cs+ ions.14 However, this structure is affected by its fragility against moisture, illumination, and heating for its intrinsic ionic characteristics and easy-lost halide atoms.19,20 Agglomeration of NCs,21 polar solvents22 or even the ultraviolet light23 can easily degrade the luminescence or damage the structure, which restricts further application in photonics and optoelectronics. To overcome the above-mentioned concerns, a lot of strategies have been designed to improve the stability of perovskite NCs, for example, Sun et al. wrapped halide perovskite QDs in a cross-linked silica matrix,24 Guarnera et al. improved the stabilities via perovskites coating with Al2O3,25 and some other studies obtained water-resistant CsPbX3 NCs via embedding NCs into polymer materials.26,27 In general, it seems to be an effective strategy that we package the perovskite NCs into tough capsulations to resist the harsh conditions outside. However, the introduced additive may affect the original luminescence of NCs, and also increase the complexity of the process.Recently, the zero-dimensional (0D) Cs4PbBr6, another derived phase of CsPbBr3 NCs,28–31 could provide in situ passivation for CsPbBr3 NCs.15,32–34 Cs4PbBr6 has been reported to be a natural insulator possessing air-stable and robust properties,29,35,36 which shares the same components with CsPbBr3. In addition, Quan et al.29 has proven that the lattice of Cs4PbBr6 matches with that of CsPbBr3 in one direction, which protects a CsPbBr3 NC growing inside a Cs4PbBr6 matrix. The PbBr64− octahedra of Cs4PbBr6 are separated by corner Cs+ without sharing Br− with each other, and the individual PbBr64− octahedron is not connected to the adjacent octahedron leading to the localized excitons in an isolated octahedron, the so-called 0D perovskites. 0D Cs4PbBr6 NCs are generally obtained by adjusting the components or regulating the surface ligands, for instance, the high ratio of Cs/Pb environment was favorable to form Cs4PbBr6,29,37 or directly transforming from CsPbBr3 NCs in a rich Cs+ condition,35 and mediating the surface ligands with more oleylamine could stimulate the formation of Cs4PbBr6.38,39 However, there are only few studies reporting the synthesis of component-controllable CsPbBr3@Cs4PbBr6 composite NCs. Moreover, all these syntheses are based on the re-precipitation method, which is conducted by injecting dimethylformamide (DMF) solution containing CsBr and PbBr2 into the poor solvent. Thus, the production of CsPbBr3@Cs4PbBr6 NCs is limited by the low solubility of CsBr in DMF, particularly for the method needing rich-Cs+ environment. Therefore, exploring a new approach to simultaneously obtain high-yield and phase-adjustable CsPbBr3@Cs4PbBr6 NCs is of great importance for industrialization.
Herein, for the first time, we developed a novel anion–cation method to synthesize phase-adjustable CsPbBr3@Cs4PbBr6 composite NCs under atmospheric conditions. In this method, an active brominated agent, pyridinium tribromide (PDBr), was used as the source of bromine; however, cesium acetate and lead acetate with higher solubility were used to replace CsBr and PbBr2. Besides being used as the source of bromine, PDBr provided highly Br−-rich conditions for the growth of perovskites to obtain high-quality crystalline structures with few surface defects. Furthermore, we found that the phase transformation between CsPbBr3 and Cs4PbBr6 was temperature dependent. Cs4PbBr6 NCs were preferred to be formed at lower temperatures, while increasing the temperature gave rise to more CsPbBr3 NCs. Relative proportions of CsPbBr3 and Cs4PbBr6 were calculated from the XRD pattern at different temperatures and the corresponding optical properties were discussed. The optimized sample showed strong green PL emission with a full width at half maximum (FWHM) of 19 nm. PL attenuation curves and PL lifetime demonstrate the greatly improved stabilities and efficient trap-passivation of CsPbBr3@Cs4PbBr6 NCs compared to naked CsPbBr3 NCs with cubic or monoclinic phase. Furthermore, a wide color gamut of 135% of the NTSC standard was obtained via anion exchange. The as-proposed novel anion–cation method paves the way for the mass production of stable and highly emissive perovskite NCs, which have a huge potential application in lightings, displays, and lasers.
Results and discussion
Emissive 3D CsPbBr3 NCs are usually used in the form of cubic and monoclinic phases, whose structures are presented in Fig. 1a and b, respectively. Cubic and monoclinic CsPbBr3 NCs possess similar lattice arrangements, the difference being that the latter has tilting octahedra compared to the former.40 Cubic and monoclinic CsPbBr3 NCs also exhibit similar optical properties and structural characteristics16 facing the same stability issue. Well, compared to CsPbBr3 with cubic or monoclinic structure, the octahedra of rhombohedral Cs4PbX6 were decoupled completely (Fig. 1c), making it a 0D structure. Thus, Cs4PbBr6 NCs have quite different properties,41,42 such as colorless crystals with wider band gap (3.95 eV)43 and better environmental tolerance,29,38 which make it perfect for capsulation.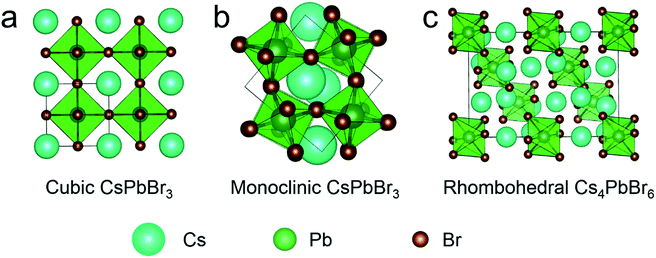 | ||
| Fig. 1 Simulated crystal structure of (a) cubic CsPbBr3, (b) monoclinic CsPbBr3 and (c) rhombohedral Cs4PbBr6. | ||
Cubic NCs are usually synthesized by hot-injection (HI),17,44 and by injecting oleic cesium precursors into high-temperature solvents containing PbBr2 and ligands under inert environments, as shown in Fig. 2. Monoclinic CsPbBr3 NCs (PDF#18-0346) are widely obtained via room-temperature re-precipitation (RP)16 that is conducted by adding the as-prepared CsBr/PbBr2 precursors into poor solvents (Fig. 2) under atmospheric environment. Previous reports30,39 have demonstrated that CsPbBr3 could be converted into Cs4PbBr6, and Cs4PbBr6 could also be converted into CsPbBr3 with excess PbBr2, proving the feasibility of passivating CsPbBr3 with Cs4PbBr6. Now, Cs4PbBr6 NCs are generally obtained via modified RP by using excess Cs+ (ref. 35 and 45) or ligand-mediating method.38,39 Herein, we put forward a novel anion–cation reaction method without forming PbBr64− octahedral precursors in advance to prepare perovskite NCs. The schematic of the anion–cation reaction is shown in Fig. 2, wherein an active brominated agent, pyridinium tribromide (PDBr), was introduced to act as a halogen source. In a typical synthesis, the N,N-dimethyl formamide (DMF) solutions of Pb(Ac)2 and Cs(Ac) were added to toluene containing organic ligands and then, bromine-rich pyridinium tribromide was swiftly added at an appropriate temperature to form perovskite NCs. The adequate Br− of PDBr provides a Br−-rich condition for perovskites to obtain high-quality NCs with less bromine vacancies.46
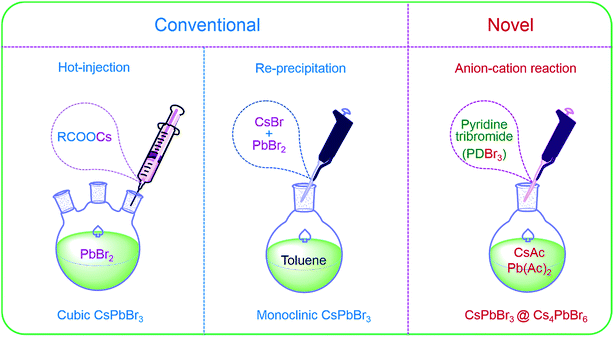 | ||
| Fig. 2 Schematic of the hot-injection, re-precipitation and anion–cation reactions for cubic CsPbBr3, monoclinic CsPbBr3 and CsPbBr3@Cs4PbBr6 NCs. | ||
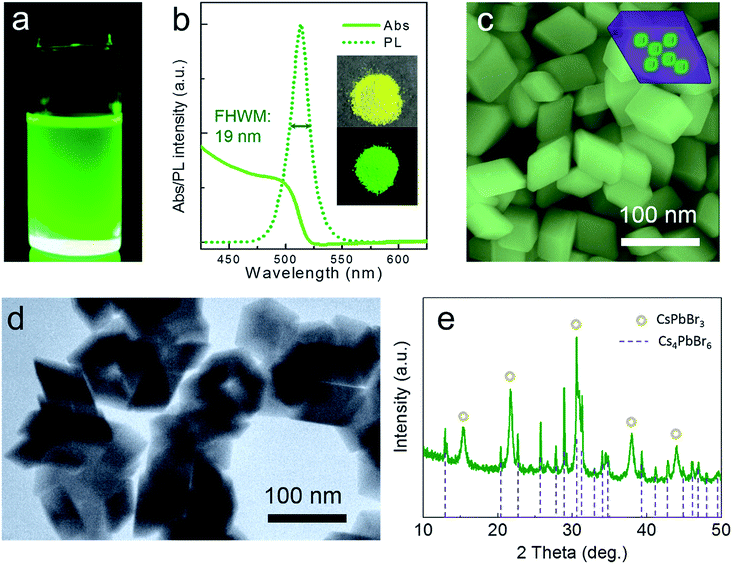
Through this novel anion–cation reaction method, a typical solution sample of CsPbBr3@Cs4PbBr6 NCs was obtained (shown in Fig. 3a), which exhibited bright photoluminescence (PL) at 513 nm with a full width at half maximum (FWHM) of 19 nm (Fig. 3b). The dazzling green emission of CsPbBr3@Cs4PbBr6 came from CsPbBr3 NCs because Cs4PbBr6 NCs owning colorless crystals with wide band gap. Compared to the greatly degenerating PLQY of solid CsPbBr3 NCs,1,29 the powder of CsPbBr3@Cs4PbBr6 NCs (inset photographs in Fig. 3b) could maintain 51% PL QY. The photoluminescence stabilities of different phases will be discussed later. The CsPbBr3@Cs4PbBr6 NCs exhibited the rhombus morphology of rhombohedral Cs4PbBr6 matrix with an average size of 60 nm (Fig. 3c). From the SEM and TEM images in Fig. 3c and d, the morphology of the NCs exhibited consistent and uniform rhombus without cubes; however, the XRD pattern in Fig. 3e reveals the coexistence of CsPbBr3 and Cs4PbBr6. These observations indicate that the CsPbBr3 NCs grew inside the Cs4PbBr6 matrix, which is simplified in the inset diagrammatic figure in Fig. 3c. To demonstrate the capacity of this anion–cation method for volume production, we expanded the scale by 25-fold to 500 mL. The photograph of the expanded manufacture is presented in the inset of Fig. 4a. The PL peak of the obtained NCs is at 513 nm (Fig. 4a), possessing the same peak position with the small scale. The SEM image (Fig. 4b) of the large-scale manufacture also shows the homogeneous rhombus crystals without small particle impurities in a wide range. These results demonstrate the great potential of the anion–cation method for industrialization.
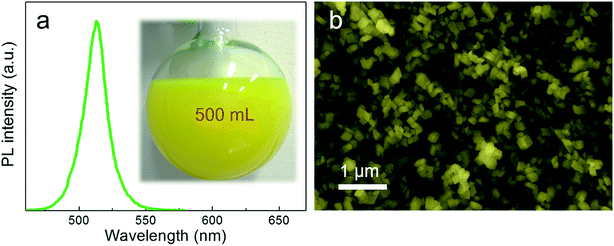 | ||
| Fig. 4 (a) PL spectrum of large-yield CsPbBr3@Cs4PbBr6 NCs, inset is the photograph of large-yield reaction product. (b) Large-scale SEM image of high yields of CsPbBr3@Cs4PbBr6 NCs. | ||
Previous works30,35,41,47 have reported that high ratio of Cs+/Pb2+ over 1 is beneficial for the formation of Cs4PbBr6, and the adjustment of the ratio can yield different proportions of CsPbBr3@Cs4PbBr6 compounds. At the same time, some other reports39 also demonstrated that surface ligands can regulate the phase transformation between CsPbBr3 and Cs4PbBr6. In this system, we found that temperature also played a vital role in the formation of phase structure. The proportion of CsPbBr3 and Cs4PbBr6 were regulated by controlling the temperature from 25 °C to 80 °C (Fig. 5) with a Cs+/Pb2+ ratio of 1. The bottom of the abscissa in Fig. 5e shows the standard peaks of dominant rhombohedral phase (PDF#73-2478), while the opposite shows the standard peaks of cubic phase (PDF#54-0752). At a temperature of 25 °C, the XRD pattern (Fig. 5e) shows that the main peaks all from the rhombohedral and cubic phases are unconspicuous. The corresponding SEM image in Fig. 5a also presents consistent morphological features. With the temperature increasing to 40 °C and 60 °C, new peaks at 15.1°, 21.5°, 30.6°, 37.8°, and 46.7° enhanced gradually, which corresponds to the (100), (110), (200), (211), and (300) lattice planes of cubic CsPbBr3 NCs. Simultaneously, the peak intensities of Cs4PbBr6 become weaker, while view of SEM (Fig. 5b and c) images maintains a uniform rhombic shape without impurities at 40 °C and 60 °C, which demonstrate the effective embedding of cubic CsPbBr3 NCs in Cs4PbBr6. We calculate the relative content of CsPbBr3 and Cs4PbBr6 phases by the area method. Only a tiny amount (2.4%) of cubic phase could be obtained at room temperature and higher proportion of CsPbBr3 (15.4% and 44.5%, respectively) came into being with the increase in temperature. The productions of 44.5% CsPbBr3 under 60 °C almost resulted in the diamond shape without cubes, as shown in Fig. 5c, which confirmed the formation of CsPbBr3@Cs4PbBr6 composite NCs. While with the temperature increasing to 80 °C, CsPbBr3 NCs began to nucleate and grow separated from Cs4PbBr6 matrix into independent bulk alone, as seen from the typical cuboidal NC marked in Fig. 5d. In addition, the XRD pattern in Fig. 5e reveals that only a fraction of Cs4PbBr6 existed in the composites. When the temperature was higher than 100 °C, the cubic phase accounted for the vast majority (more than 90%). The relation between the proportions of these two phases and the temperature is listed in Table 1. Cs4PbBr6 NCs were preferred to form at lower temperature, while higher temperature conditions were more conductive to the formation of cubic phase, and higher temperature also created a more violent reactivity, thereby leading to independent nucleation and growth of CsPbBr3 NCs.
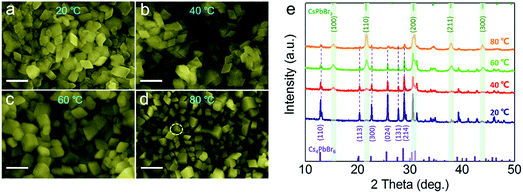 | ||
| Fig. 5 SEM images of products prepared at different temperatures: (a) 20 °C, (b) 40 °C, (c) 60 °C and (d) 80 °C. (e) The corresponding XRD patterns of products prepared at different temperatures. | ||
| T/°C | 20 | 40 | 60 | 80 |
|---|---|---|---|---|
| Ratio/CsPbBr3 | 2.4% | 15.4% | 44.5% | 80.5% |
| Ratio/Cs4PbBr6 | 97.6% | 84.6% | 55.5% | 19.5% |
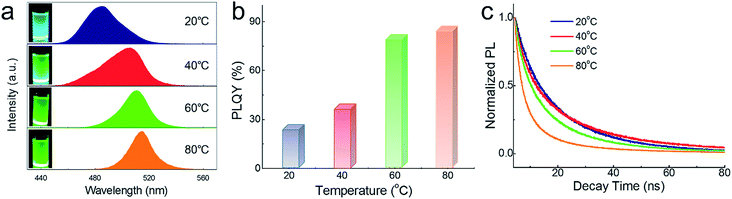
Temperature could not only adjust the phase composition but also influence the optical properties. Fig. 6a exhibits solution samples synthesized at different temperatures and their corresponding PL spectra. Blue emission (480 nm) is observed when reaction temperature is 20 °C, which could be explained by the dimensionality reduction of embedded CsPbBr3 NCs resulted from lower temperature.48,49 With the increase in temperature, the spectra reveal a slight redshift due to the aggregation and size of CsPbBr3 NCs with the increasing proportion. The broader FWHM at 20 °C and 40 °C maybe the result of uneven size of dysgonic CsPbBr3 NCs. The absorption spectra (Fig. S1†) also exhibited the same trend. PLQY gradually improved with the increasing proportion of CsPbBr3 NCs in the system as shown in Fig. 6b. However, owing to the dissociative CsPbBr3 NCs, the PL lifetime of composites obtained at 80 °C was sharply decreased (Fig. 6c). Longer lifetime was detected as the Cs4PbBr6 matrix increased, which is consistent with the previous reports. Detailed parameters of the PL lifetime are listed in Table 2. CsPbBr3@Cs4PbBr6 synthesized at 60 °C (with 44.5% CsPbBr3) shows the best excitons combination performance, which was chosen to conduct the following stability tests.
| T/°C | 20 | 40 | 60 | 80 |
|---|---|---|---|---|
| τ 1/ns | 6.77 | 5.83 | 4.5 | 2.71 |
| τ 2/ns | 22.1 | 28.36 | 18.6 | 14.3 |
| τ aver/ns | 23.1 | 22.3 | 17.7 | 15.6 |
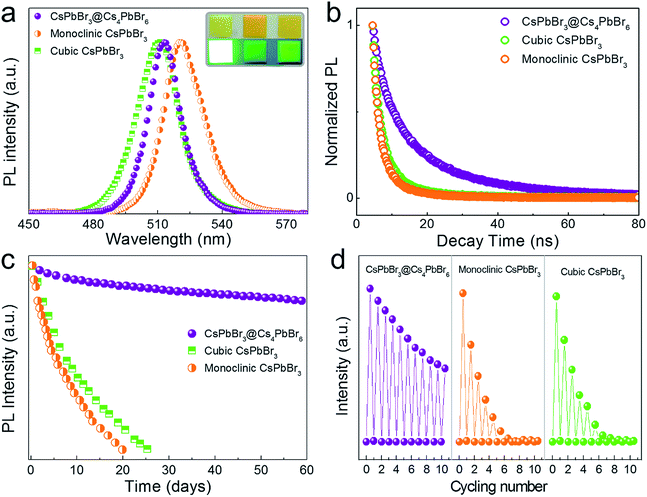
We compared CsPbBr3@Cs4PbBr6 composite NCs with monoclinic CsPbBr3 and cubic CsPbBr3 NCs separately to verify its improved stabilities. The cubic CsPbBr3 NCs were synthesized by traditional hot-injection, whereas the monoclinic CsPbBr3 NCs were synthesized by re-precipitation (see ESI†). For better comparison tests, we mixed the NCs (CsPbBr3@Cs4PbBr6, monoclinic CsPbBr3 and cubic CsPbBr3) with polydimethylsiloxane (PDMS) to form films, respectively, as shown in the inset photographs of Fig. 7a. It could be seen that CsPbBr3@Cs4PbBr6 NCs maintained the best photoluminescence, while severe quenching occurred on both cubic and monoclinic CsPbBr3 NCs. The certain arrangement of CsPbBr3 NCs in Cs4PbBr6 made CsPbBr3@Cs4PbBr6 NCs have a narrower FWHM compared to HI cubic CsPbBr3 NCs. The slight differences in PL spectra (Fig. 7a) were probably caused by the size effect. The PL decay curves of the three are presented in Fig. 7b. The average lifetime of cubic and monoclinic CsPbBr3 were 5.7 ns and 5.5 ns, while an increasing radiative lifetime of 17.7 ns was evidenced in CsPbBr3@Cs4PbBr6 compounds (Table 3). In order to further prove the improving stability of CsPbBr3@Cs4PbBr6 NCs, the storage stability test and thermal stability test were designed based on the luminescence degradation. Fig. 7c shows the PL intensity decays of the three films shown in Fig. 7a under ambient storage conditions with RH 50% for two months. The CsPbBr3@Cs4PbBr6 film showed a decrease of 19%, while cubic and monoclinic CsPbBr3 films completely lost their emission within one month. The thermal cycling tests (Fig. 7d) were performed from room temperature to 150 °C with RH 50% under ambient conditions. After 10 cycles, CsPbBr3@Cs4PbBr6 remained approximately half of the original PL intensity, whereas the cubic CsPbBr3 without Cs4PbBr6 passivation degenerated sharply to baseline only for 6 cycles, and monoclinic CsPbBr3 was even worse. In conclusion, CsPbBr3@Cs4PbBr6 NCs exhibited improved stability, indicating the effective protection of Cs4PbBr6, and this new method will take perovskite NCs towards practical applications in optical and photoelectric fields.
| T/°C | CsPbBr3@Cs4PbBr6 | Cubic CsPbBr3 | Monoclinic CsPbBr3 |
|---|---|---|---|
| τ 1/ns | 4.5 | 1.83 | 1.62 |
| τ 2/ns | 18.6 | 7.37 | 6.33 |
| τ aver/ns | 17.7 | 5.7 | 5.48 |
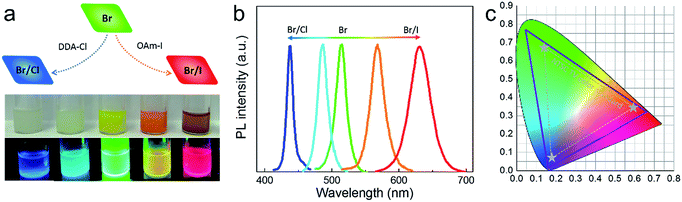
We could obtain blue- and red-emitting NCs based on green emitting CsPbBr3@Cs4PbBr6 NCs via anion exchange. Didodecyldimethylammonium chloride (DDA-Cl) was used for the (Cl/Br)-based blue NCs, and oleylamine iodine (OAm-I) was chosen for red NCs. The schematic of the anion exchange is presented in Fig. 8a, and the amount of DDA-Cl and OAm-I affected the light emissive colors. The photograph of the obtained solution samples under daylight and ultraviolet light is presented in Fig. 8a. Fig. 8b exhibits the corresponding PL spectra, blue to 437 nm and red to 630 nm. We mark the commission Internationale del’Eclairage (CIE) of our spectra using solid line in Fig. 8b; the dashed line area is NTSC standard. It could be seen that our CIE encompasses 135% of the NTSC standard.
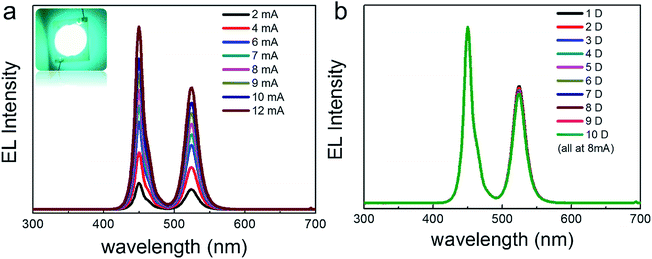
A backlit LED was assembled using the as-prepared CsPbBr3@Cs4PbBr6 powder and a 450 nm blue chip as shown in the insert photograph of Fig. 9a, which showed the EL spectra of the LED under increasing current (2–12 mA). The EL intensity gradually increased with the increase in current. We continuously light the LED for 10 days in ambient environment (under 8 mA), and no obvious attenuation was observed on the LED (Fig. 9b). All these results imply the huge applicable value of CsPbBr3@Cs4PbBr6 NCs in display and lighting fields.
Conclusion
In summary, a novel anion–cation reaction method was proposed to synthesize the highly emissive CsPbBr3 NCs embedded in the air-stable and crystalline Cs4PbBr6 matrix. In this method, temperature was used to adjust the relative proportions of CsPbBr3 and Cs4PbBr6. Besides narrower FWHM and higher PLQY, the as-prepared CsPbBr3@Cs4PbBr6 compound possessed higher optical stability resisting moist ambient condition and heating. The contrast experiments of storage and thermal stability were designed to compare CsPbBr3@Cs4PbBr6 with conventional cubic CsPbBr3 and monoclinic CsPbBr3. Furthermore, the mixed halogen perovskite compound with wide color gamut could be obtained via simple anion exchange. In conclusion, the compound of CsPbX3@Cs4PbX6 provided a new passage to improve the stability of perovskite, which would be of great significance in lightings, displays, backlights, and other optoelectronic applications.Experiment section
Synthesis of CsPbBr3@Cs4PbBr6 nanocrystals
The anion–cation reaction procedure of Cs4PbBr6@CsPbBr3 was conducted by adding the brominating agent into the precursor solution with the presence of cesium ion and lead ion. Typically, the Cs and Pb precursors were formed by dissolving 0.1 mmol cesium acetate and 0.1 mmol lead acetate into 1 mL N,N-dimethyl formamide (DMF), and the brominating agent was prepared by dissolving PDBr into DMF at 0.3 mol L−1 1 mL PDBr/DMF bromide solution was added into 20 mL toluene with 0.5 mL oleyl amine (OAm), 0.5 mL oleic acid (OA) and 1 mL (Cs, Pb)/DMF precursor under atmospheric conditions; the reaction solution was further stirred for 10 min.Purification of Cs4PbBr6@CsPbBr3 nanocrystals
The as-prepared Cs4PbBr6@CsPbBr3 nanocrystals were collected by adding 20 mL acetonitrile and 10 mL toluene into the original solution, and then centrifuging at 8000 rpm for 3 min. The liquid sample was obtained by dispersing the precipitate into 5 mL toluene, and the powder sample was obtained by vacuum drying.Anion exchange reactions
Different amounts of OAm-I dispersed in toluene (0.1 mol L−1) were dropped into 1 mL as-obtained Cs4PbBr6@CsPbBr3 NC solution for red PL emission, while different amounts of DDA-Cl were added for blue emission. The concrete proposal is listed in Table S2.†Characterization
Powder X-ray diffraction (XRD) patterns were recorded using a Bruker D8 Advance X-ray Diffractometer at 40 kV and 40 mA using Cu Kα radiation (λ = 1.5406 Å). The morphologies were investigated using the JEOL JSM-7800F field emission scanning electron microscope (FESEM), and the JEM-2100F and JEM-ARM200F transmission electron microscope (TEM) instruments. PL spectra were recorded using an FLS920P fluorescence spectrometer (Edinburgh Instruments) equipped with a photomultiplier in a thermoelectrically cooled housing (R928P, Hamamatsu) with a 450 W xenon arc lamp used as the excitation source for steady-state spectra. The absolute PL quantum yields were detected using a fluorescence spectrometer with an integrated sphere (Hamamatsu Photonics). The photo-stability measurements were analyzed in a temperature and humidity chamber (25 °C, RH 50%) using a 454 nm LED light (21 mW cm−2) provided by Ocean Optics LS-450.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by NSFC (61604074, 51572128, 51672132, 61725402), the National Key Research and Development Program of China (2016YFB0401701, 2017YFA0305500), the Natural Science Foundation of Jiangsu Province (BK20160827, BK20180020), China Postdoctoral Science Foundation (2016M590455), the Fundamental Research Funds for the Central Universities (No. 30917011202, 30915012205, 30916015106), and PAPD of Jiangsu Higher Education Institutions.References
- J. Song, J. Li, X. Li, L. Xu, Y. Dong and H. Zeng, Adv. Mater., 2015, 27, 7162 CrossRef CAS PubMed.
- J. Song, J. Li, L. Xu, J. Li, F. Zhang, B. Han, Q. Shan and H. Zeng, Adv. Mater., 2018, 30, 1800764 CrossRef PubMed.
- B. Han, B. Cai, Q. Shan, J. Song, J. Li, F. Zhang, J. Chen, T. Fang, Q. Ji, X. Xu and H. Zeng, Adv. Funct. Mater., 2018, 1804285 CrossRef.
- Q. Shan, J. Li, J. Song, Y. Zou, L. Xu, J. Xue, Y. Dong, C. Huo, J. Chen, B. Han and H. Zeng, J. Mater. Chem. C, 2017, 5, 4565 RSC.
- J. Song, T. Fang, J. Li, L. Xu, F. Zhang, B. Han, Q. Shan and H. Zeng, Adv. Mater., 2018, 0, 1805409 CrossRef PubMed.
- F. Zhang, J. Song, B. Han, T. Fang, J. Li and H. Zeng, Small Methods, 2018, 2, 1700382 CrossRef.
- Y. Wei, Z. Cheng and J. Lin, Chem. Soc. Rev., 2019, 48, 310 RSC.
- Q. Shan, J. Song, Y. Zou, J. Li, L. Xu, J. Xue, Y. Dong, B. Han, J. Chen and H. Zeng, Small, 2017, 13, 1701770 CrossRef PubMed.
- J. Song, L. Xu, J. Li, J. Xue, Y. Dong, X. Li and H. Zeng, Adv. Mater., 2016, 28, 4861 CrossRef CAS PubMed.
- S. Zhuo, J. Zhang, Y. Shi, Y. Huang and B. Zhang, Angew. Chem., Int. Ed., 2015, 54, 5693 CrossRef CAS PubMed.
- J. Xue, Z. Zhu, X. Xu, Y. Gu, S. Wang, L. Xu, Y. Zou, J. Song, H. Zeng and Q. Chen, Nano Lett., 2018, 12, 7628 CrossRef PubMed.
- Y. Dong, Y. Gu, Y. Zou, J. Song, L. Xu, J. Li, J. Xue, X. Li and H. Zeng, Small, 2016, 12, 5622 CrossRef CAS PubMed.
- H. Zhu, Y. Fu, F. Meng, X. Wu, Z. Gong, Q. Ding, M. V. Gustafsson, M. T. Trinh, S. Jin and X. Zhu, Nat. Mater., 2015, 14, 636 CrossRef CAS PubMed.
- A. Swarnkar, R. Chulliyil, V. K. Ravi, M. Irfanullah, A. Chowdhury and A. Nag, Angew. Chem., 2015, 127, 15644 CrossRef.
- F. Palazon, C. Urso, L. De Trizio, Q. Akkerman, S. Marras, F. Locardi, I. Nelli, M. Ferretti, M. Prato and L. Manna, ACS Energy Lett., 2017, 2, 2445 CrossRef CAS PubMed.
- X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song and H. Zeng, Adv. Funct. Mater., 2016, 26, 2435 CrossRef CAS.
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nano Lett., 2015, 15, 3692 CrossRef CAS PubMed.
- Z. C. a. J. Lin, CrystEngComm, 2010, 12, 2646 RSC.
- B. Kang and K. Biswas, J. Phys. Chem. Lett., 2018, 9, 830 CrossRef CAS PubMed.
- S. Seth and A. Samanta, J. Phys. Chem. Lett., 2017, 8, 4461 CrossRef CAS PubMed.
- Y. Kim, E. Yassitepe, O. Voznyy, R. Comin, G. Walters, X. Gong, P. Kanjanaboos, A. F. Nogueira and E. H. Sargent, ACS Appl. Mater. Interfaces, 2015, 7, 25007 CrossRef CAS PubMed.
- J. Li, L. Xu, T. Wang, J. Song, J. Chen, J. Xue, Y. Dong, B. Cai, Q. Shan and B. Han, Adv. Mater., 2017, 29, 1603885 CrossRef PubMed.
- L. Xu, J. Chen, J. Song, J. Li, J. Xue, Y. Dong, B. Cai, Q. Shan, B. Han and H. Zeng, ACS Appl. Mater. Interfaces, 2017, 31, 26556 CrossRef PubMed.
- C. Sun, Y. Zhang, C. Ruan, C. Yin, X. Wang, Y. Wang and W. W. Yu, Adv. Mater., 2016, 28, 10088 CrossRef CAS PubMed.
- S. Guarnera, A. Abate, W. Zhang, J. M. Foster, G. Richardson, A. Petrozza and H. J. Snaith, J. Phys. Chem. Lett., 2015, 6, 432 CrossRef CAS PubMed.
- H. Huang, B. Chen, Z. Wang, T. F. Hung, A. S. Susha, H. Zhong and A. L. Rogach, Chem. Sci., 2016, 7, 5699 RSC.
- Q. Zhou, Z. Bai, W. g. Lu, Y. Wang, B. Zou and H. Zhong, Adv. Mater., 2016, 28, 9163 CrossRef CAS PubMed.
- K. H. Wang, L. Wu, L. Li, H. B. Yao, H. S. Qian and S. H. Yu, Angew. Chem., Int. Ed., 2016, 55, 8328 CrossRef CAS PubMed.
- L. N. Quan, R. Quintero-Bermudez, O. Voznyy, G. Walters, A. Jain, J. Z. Fan, X. Zheng, Z. Yang and E. H. Sargent, Adv. Mater., 2017, 29, 1605945 CrossRef PubMed.
- Q. A. Akkerman, S. Park, E. Radicchi, F. Nunzi, E. Mosconi, F. De Angelis, R. Brescia, P. Rastogi, M. Prato and L. Manna, Nano Lett., 2017, 17, 1924 CrossRef CAS PubMed.
- X. Zhang, B. Xu, J. Zhang, Y. Gao, Y. Zheng, K. Wang and X. W. Sun, Adv. Funct. Mater., 2016, 26, 4595 CrossRef CAS.
- X. Chen, F. Zhang, Y. Ge, L. Shi, S. Huang, J. Tang, Z. Lv, L. Zhang, B. Zou and H. Zhong, Adv. Funct. Mater., 2018, 28, 1706567 CrossRef.
- Y. Wang, D. Yu, Z. Wang, X. Li, X. Chen, V. Nalla, H. Zeng and H. Sun, Small, 2017, 13, 1701587 CrossRef PubMed.
- W. H. Junwei Xu, P. Li, D. R. Onken, C. Dun, Y. Guo, C. L. Kamil, B. Ucer, H. Wang, S. M. Geyer, R. T. Williams and a. D. L. Carroll, Adv. Mater., 2017, 29, 1703703 CrossRef PubMed.
- L. Wu, H. Hu, Y. Xu, S. Jiang, M. Chen, Q. Zhong, D. Yang, Q. Liu, Y. Zhao, B. Sun, Q. Zhang and Y. Yin, Nano Lett., 2017, 17, 5799 CrossRef CAS PubMed.
- Y. Zhang, M. I. Saidaminov, I. Dursun, H. Yang, B. Murali, E. Alarousu, E. Yengel, B. A. Alshankiti, O. M. Bakr and O. F. Mohammed, J. Phys. Chem. Lett., 2017, 8, 961 CrossRef CAS PubMed.
- W. H. Junwei Xu, P. Li, D. R. Onken, C. Dun, Y. Guo, K. B. Ucer, C. Lu, H. Wang, S. M. Geyer, R. T. Williams and D. L. Carroll, Adv. Mater., 2017, 1703703 Search PubMed.
- X. Chen, D. Chen, J. Li, G. Fang, H. Sheng and J. Zhong, Dalton Trans., 2018, 47, 5670 RSC.
- Z. Liu, Y. Bekenstein, X. Ye, S. C. Nguyen, J. Swabeck, D. Zhang, S. T. Lee, P. Yang, W. Ma and A. P. Alivisatos, J. Am. Chem. Soc., 2017, 139, 5309 CrossRef CAS PubMed.
- I. Y. Zaitseva, I. Kovaleva and V. Fedorov, Russ. J. Inorg. Chem., 2006, 51, 619 CrossRef.
- S. Kondo, K. Amaya and T. Saito, J. Phys.: Condens. Matter, 2002, 14, 2093 CrossRef CAS.
- S. Kondo, A. Masaki, T. Saito and H. Asada, Solid State Commun., 2002, 124, 211 CrossRef CAS.
- M. Nikl, E. Mihokova, K. Nitsch, F. Somma, C. Giampaolo, G. Pazzi, P. Fabeni and S. Zazubovich, Chem. Phys. Lett., 1999, 306, 280 CrossRef CAS.
- G. G. Huang, C. L. Wang, S. H. Xu, S. F. Zong, J. Lu, Z. Y. Wang, C. G. Lu and Y. P. Cui, Adv. Mater., 2017, 29, 1700095 CrossRef PubMed.
- C. Weerd, J. Lin, L. Gomez, Y. Fujiwara, K. Suenaga and T. Gregorkiewicz, J. Phys. Chem. C Nanomater. Interfaces, 2017, 121, 19490 CrossRef CAS PubMed.
- J. Pan, L. N. Quan, Y. Zhao, W. Peng, B. Murali, S. P. Sarmah, M. Yuan, L. Sinatra, N. M. Alyami and J. Liu, Adv. Mater., 2016, 28, 8718 CrossRef CAS PubMed.
- G. Tong, H. Li, Z. Zhu, Y. Zhang, L. Yu, J. Xu and Y. Jiang, J. Phys. Chem. Lett., 2018, 9, 1592 CrossRef CAS PubMed.
- Y. Bekenstein, B. A. Koscher, S. W. Eaton, P. Yang and A. P. Alivisatos, J. Am. Chem. Soc., 2015, 137, 16008 CrossRef CAS PubMed.
- H. Huang, A. S. Susha, S. V. Kershaw, T. F. Hung and A. L. Rogach, Adv. Sci., 2015, 2, 1500194 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8na00291f |
| This journal is © The Royal Society of Chemistry 2019 |