Layer-by-layer assembly for photoelectrochemical nanoarchitectonics
Dongseok
Kim†
 a,
Minsu
Gu†
a,
Minsu
Gu†
 a,
Minju
Park†
a,
Minju
Park†
 b,
Taehyung
Kim†
c and
Byeong-Su
Kim
b,
Taehyung
Kim†
c and
Byeong-Su
Kim
 *a
*a
aDepartment of Chemistry, Yonsei University, Seoul 03722, Korea. E-mail: bskim19@yonsei.ac.kr
bDepartment of Chemical Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Korea
cDepartment of Energy Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Korea
First published on 21st November 2018
Abstract
A recent increase in the demand for energy and the environmental issues related to the existing energy production processes have necessitated the production of clean and renewable energies using alternative sources, such as solar energy and the development of high-performance photoactive nanomaterials. To this end, another notable challenge in nanotechnology and molecular engineering for the development of photoactive devices is finding suitable ways to assemble various photoactive nanomaterials into a single platform, while maintaining an efficient photocatalytic activity. In this regard, layer-by-layer (LbL) assembly is one of the most versatile nanoscale blending methods to assemble diverse materials on various surfaces through the sequential adsorption of materials with complementary interactions. The photoactive properties of LbL-assembled hybrid electrodes are highly tunable through coupling with diverse groups of photoactive nanomaterials. Although LbL assembly has often been chosen for designing hybrid nanostructures that combine the advantageous features of each constituent, recent studies on photoactive LbL thin films for use in emerging novel nanomaterials are still limited. Therefore, in this mini-review, we introduce the recent progress made in the field of advanced device applications, with a particular focus on photo-electrochemical devices of LbL-assembled thin film electrodes. We anticipate that this review will offer new insights into the synthesis and assembly of various photoactive nanocomponents toward the development of novel photoelectroactive devices and fundamental analysis of the photo-electrochemical properties of thin film electrodes.
Design, System, ApplicationUnder visible-light irradiation, photoexcited exciton transfer within photoactive materials with suitable band structures is highly important in photoelectrochemical (PEC) cells. Therefore, hybridization of materials and architectural control are important in enhancing the performance and efficiency of devices. This study focuses on the nanoscale architectural control of PEC cells using layer-by-layer (LbL) assembly, which is one of the versatile methods for fabricating multifunctional structures with nanoscale control of the composition and structure. This review explores the hybridization of photoactive materials in multi-layered films and high-performance PEC systems with respect to their various applications. Furthermore, recent research trends in LbL assembly for PEC cells, ranging from previous application fields to emerging and potential application fields, are summarized. |
1. Introduction
1.1 Photoelectrochemistry and semiconductors
Artificial photosynthesis is considered as one of the most promising solutions to the growing energy and environmental concerns, and as an alternative technology for the solar production of clean and renewable energies.1,2 In this regard, photoelectrochemical (PEC) cells such as liquid junction photovoltaics, photoelectron synthesis, and photocatalysis have received significant attention as potential candidates for use in high-performance PECs.3 Efficient photosensitizers and photocatalysts are essential components that absorb photons in PEC cells.4 In principle, the photon absorption by photocatalysts generates an exciton, resulting in the generation of a hole in the highest occupied molecular orbital.5–9 This exciton of an electron–hole pair leads to photophysical and chemical reactions, such as representative water-splitting reaction, including hydrogen evolution reaction (HER) at the photocathode and oxygen evolution reaction (OER) at the photoanode.10–12 To efficiently achieve this PEC catalysis, it is necessary to tune the suitable energy level for efficient carrier transport. Thus, there has been significant progress in the development of various photoactive materials with suitable band gaps and band-edge positions for the desired photochemical reactions.13–16To date, semiconducting materials or organic molecules have been used for solar energy conversion as photoactivators.17–20 Significant advances have recently been made in developing efficient and cost-effective materials, for example, integration with additional catalytic components such as metal nanoparticles, graphene, and co-catalysts. Electrocatalysts are designed not only to facilitate electron–hole transport within semiconducting materials, but also to catalyse electrochemical redox reactions (Fig. 1a).21–23 These strategies can effectively inhibit electron–hole pair recombination and improve electron collection from photogenerated semiconductors, which facilitates the design of nanostructures and the assembly of various photoactive materials within an electrode for high conversion efficiency.24–26
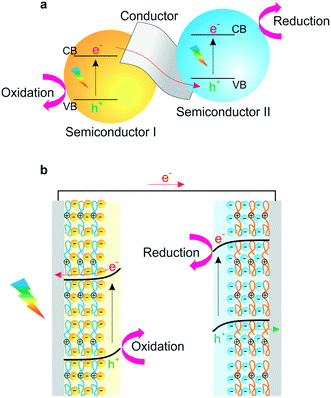 | ||
| Fig. 1 (a) A Z-scheme diagram of hybrid nanocomposites for high conversion efficiency and (b) a schematic representation of an LbL-assembled PEC system. | ||
1.2 Layer-by-layer (LbL) assembly
The word nanoarchitectonics was first coined by the Aono group in 2000 to describe the fabrication of functional hybrid nanomaterials, considering atomic/molecular-level manipulation, chemical reactions, self-assembly and self-organization.27,28 From the perspective of nanoarchitectonics, the utilization of electrode fabrication techniques that allow nanoscale blending of active components is necessary for investigating the effect of nanoscopic hybridization of photoactive materials.29–31 Typically, various photoactive materials fabricated into thin films are applied in energy conversion devices by a simple casting process or other deposition methods such as electrodeposition, chemical vapour deposition, and atomic layer deposition.32 However, these traditional electrode fabrication methods remain challenging to understand the interactions between individual active materials for the overall performance of the electrode.To address these concerns, layer-by-layer (LbL) assembly has been proposed with its advantages such as simplicity, low cost, scalability, and environmentally benign features.33–35 LbL assembly is one of the most versatile tools used for assembling multifunctional nanomaterials with nanoscale control over their composition and structure. LbL-assembled thin films are simply prepared through sequential adsorption of different macromolecular components that exhibit attractive forces such as electrostatic interactions, hydrogen bonding, and van der Waals forces. In that context, we presented a well-established protocol for the assembly of LbL nanocomposites in our previous contribution.36 Specifically, we formulated a detailed protocol to share reliable procedures with other researchers in the field. Furthermore, we described the LbL assembly procedure from preparation methods for the necessary substrates and suspensions of components to fabricate LbL assemblies for potential applications. Readers interested in the detailed protocol for the preparation of a high quality LbL-assembled film are highly encouraged to resort to this protocol.
In addition to their structural advantages, the optical and photoelectrochemical properties of the photoactive materials could be improved through the interactions within the assembled layer. The hybridization of the assembled photoactive materials results in the efficient transfer of photo-induced excitons within the multilayer film with band adjustment for a suitable structure, leading to high performance of PEC devices.37,38 Due to these unique opportunities, there are active research studies on LbL-assembled thin films for various energy storage and conversion applications, including lithium-ion battery, supercapacitor, fuel cell, and electrocatalyst.39–45
1.3 LbL assembly for PEC applications
The most essential factors in PEC cells are the generation of electron–hole pairs and their migration within semiconductors.46,47 Hybridization of semiconductors could improve electron migration to the surface of catalytic materials and engineer the band gap and structure of the redox potentials suitable for specific applications such as water splitting, biodegradation or CO2 reduction.48–51 Though several research studies have been conducted focusing on electron transfer in PEC cells based on suitable band structures, efficient photoelectron transfer has posed a considerable challenge in the field. In that regard, a LbL-assembled multilayer system presents an elegant solution because it could offer advantages in photoelectrochemical or photocatalytic properties through the hybridization of semiconductors in thin films (Fig. 1b).37,52 First, alternative deposition of individual building blocks can attain precisely controllable architectures and enhance structural stability.33 Second, the combination of band structures for each semiconductor can adjust the Z-scheme in heterojunction films for efficient electron transfer and applicable energy level for specific redox reactions.10,53–55 Third, photogenerated carriers can efficiently be transferred inside multilayered films, which would possibly suppress photoexcited electron–hole pair recombinations.38,56–58 These notable advantages of LbL-assembled PEC systems (LbL-PEC hereafter) have spurred vigorous research studies related to LbL-PEC systems, with a gradually increasing number of published papers from 1990 (Fig. 2).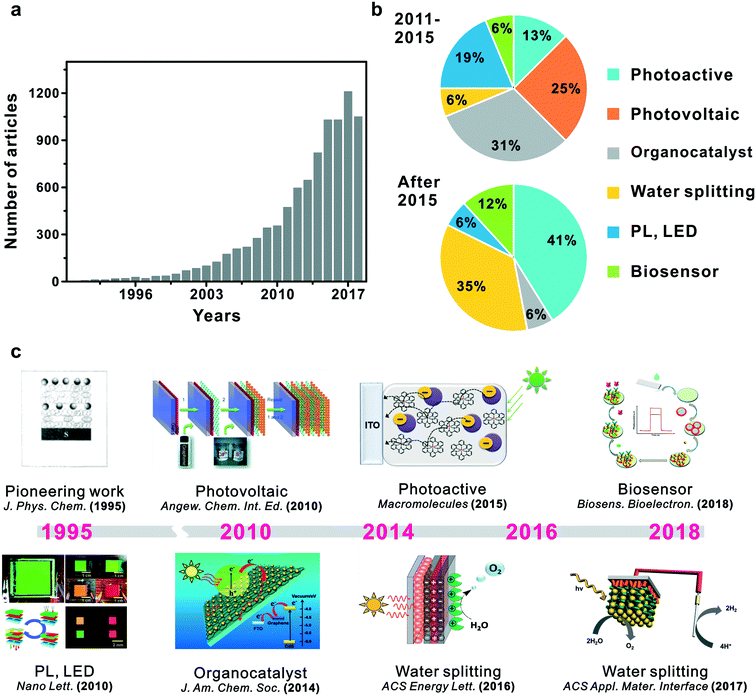 | ||
| Fig. 2 Trend of research in LbL assembly for PEC systems: (a) growth of publications since 1990, (b) types of applications and (c) chronicles with some significant studies in the field since 1995. Reprinted with permission from ref. 59, copyright 1995 American Chemical Society. Reprinted with permission from ref. 64, copyright 2010 John Wiley and Sons. Reprinted with permission from ref. 73, copyright 2010 American Chemical Society. Reprinted with permission from ref. 83, copyright 2014 American Chemical Society. Reprinted with permission from ref. 96, copyright 2015 American Chemical Society. Reprinted with permission from ref. 102, copyright 2016 American Chemical Society. Reprinted with permission from ref. 108, copyright 2017 American Chemical Society. Reprinted with permission from ref. 122, copyright 2018 Elsevier. | ||
As a pioneering work in LbL-based photoelectrochemical applications, Kotov et al. reported ultrathin LbL nanostructured films using the Langmuir–Blodgett technique with semiconducting nanoparticles.59 Lead sulfide (PbS), titanium dioxide (TiO2), and highly fluorescent cadmium sulfide (CdS) were used as semiconductors with polyelectrolytes to investigate the behaviour of photo-generated charge carriers in a multi-layered LbL film. It was found that not only was maximum photocurrent in the LbL photoactive film attainable with respect to the number of bilayers, but it was also possible to transport photogenerated charge carriers based on the position of the active layer within LbL films and the magnitude of the photocurrent. Following this seminar contribution, numerous studies have been reported to apply LbL assembly towards developing highly active photoelectrochemical systems.
Photovoltaic devices, particularly dye-sensitized solar cells (DSSCs), were one of the most popular research topics in LbL-PEC systems, which advanced the progress of development of various photoactive materials.60–63 For example, Li and coworkers combined graphene with CdS quantum dots (QDs) on indium tin oxide (ITO) to construct an efficient electron transfer system.64 Due to the light-harvesting properties of CdS QDs, photoexcited charge carriers could be generated and transported through graphene, which acted as a good charge mediator. Through the analysis of energy level diagrams and photocurrent generation, the mechanism of electron transfer in high-performance LbL-based PEC devices was demonstrated for next-generation solar cells. In another noteworthy endeavour, Kim and coworkers assembled two different QDs on TiO2 to enhance the favourable optoelectronic properties of QDs in multilayered films for efficient solar cells (Fig. 3a).65 The QD bilayer film showed significantly improved photoelectrochemical performance (power conversion efficiency (PCE) of 0.05%) compared to single QD layer films, originating from the efficient transfer of electrons between the QDs. As another promising materials for efficient DSSCs, Hammond and co-workers engineered M13 bacteriophage nanowires into a polymeric network on porous TiO2 photoanodes (Fig. 3b).66 A percolative network created from the inclusion of phage and TiO2 nanowires contributed to the preservation of the electron diffusion length and the improvement of electron transport properties in LbL-assembled DSSC photoanodes, demonstrating large improvement on PCE to 6.21%.
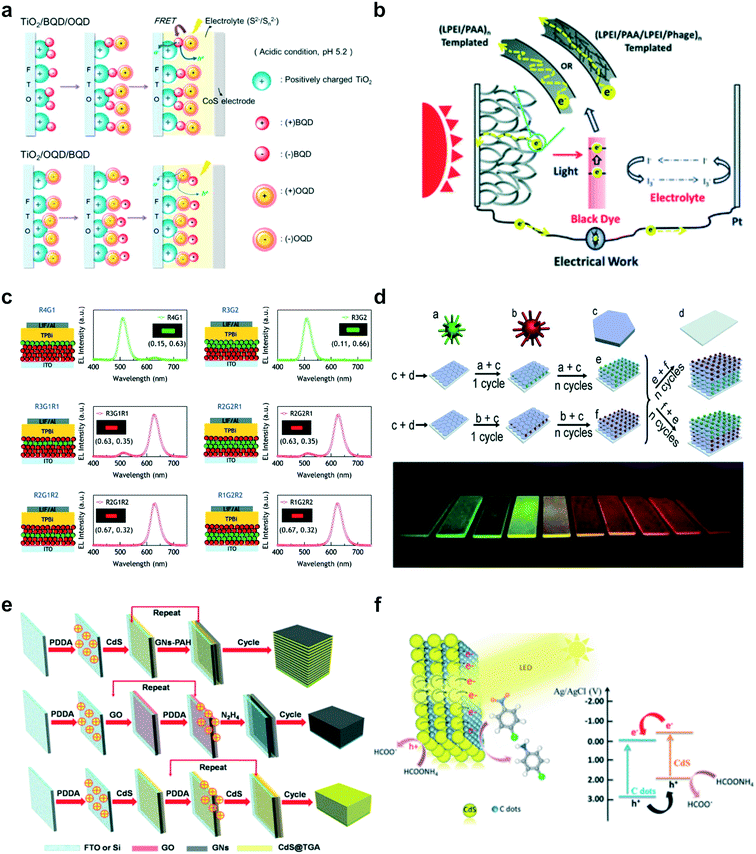 | ||
| Fig. 3 Representative early examples of LbL assemblies for various PEC applications: (a) LbL quantum dot (QD) multilayer assemblies with two different QDs for efficient electron transfer from QD to TiO2. Reprinted with permission from ref. 65, copyright 2012 American Chemical Society. (b) Percolation system of TiO2 nanowires templated by a bacteriophage for dye-sensitized solar cells (DSSCs). Reprinted with permission from ref. 66, copyright 2013 Royal Society of Chemistry. (c) Systematic engineering of an exciton-recombination zone within QD LbL films for highly bright QD-based LEDs. Reprinted with permission from ref. 73, copyright 2010 American Chemical Society. (d) LbL-assembled multicolour luminescent films tunable over the whole red-green region using CdTe QDs and MgAl-layered double hydroxide. Reprinted with permission from ref. 74, copyright 2012 John Wiley and Sons. (e) A hybrid multi-layered film with graphene nanosheets and CdS QDs for photoelectrochemical and photocatalytic activities. Reprinted with permission from ref. 83, copyright 2014 American Chemical Society. (f) Carbon dot (C-dot)/CdS QD heterojunction films for efficient photoelectrochemical and photocatalytic reduction of nitro-aromatic compounds. Reprinted with permission from ref. 84, copyright 2015 Royal Society of Chemistry. | ||
Meanwhile, the development of LED devices based on LbL assembly has been demonstrated with QDs, taking advantage of their superior photoluminescent properties.67–72 In an early example, Lee and coworkers employed various QDs and successfully demonstrated multi-coloured QD-based LED films with high brightness by appropriately arranging various QDs in the LbL-assembled film (Fig. 3c).73 Furthermore, the sensing profile within the QD multilayer films was investigated through electroluminescence emission, and it was discovered that approximately 90% of the total electroluminescence was due to the top QD layer while the remainder originated from the second QD monolayer. In addition, a significantly improved device performance was achieved in terms of its brightness (450 cd m−2 at 50 mA cm−2) and device efficiency (0.3% at 50 mA cm−2) at 2.5 QD bilayers. To improve sensing behaviour with tunable light-emitting QD-containing LbL multilayers, the Duan group assembled negatively charged CdTe QDs with positively charged MgAl-layered double hydroxide (LDH) nanosheets (Fig. 3d).74 By adjusting the size of the QDs, deposition sequence and number of layers, the luminescence colour of the multilayered thin films could easily be controlled over a wide range from red to green, with a strong luminescence intensity and high photostability.
In another application field, photocatalytic degradation of organic pollutants under light irradiation has become an active research area in the LbL-PEC field.20,75,76 The incorporation of various photocatalytic materials into multilayered films can provide significant benefits for efficient photocatalytic performance.77–82 For instance, Liu and coworkers constructed graphene–semiconductor nanocomposites via LbL assembly using polymer-modified graphene nanosheets and negatively charged CdS QDs as efficient photocatalysts for the reduction of nitro-aromatic compounds (Fig. 3e).83 A significant performance enhancement was achieved due to improvement in charge separation and transport within the multilayered film. As another example of the photocatalytic reduction for nitro-aromatic compounds, Zhang and co-workers employed carbon dots with CdS to fabricate heterojunction nanohybrid films (Fig. 3f).84 The combination of carbon dots with CdS resulted in efficient transfer of photoinduced electrons while suppressing charge recombination. These various examples of LbL assembled PEC application are summarized with type of applications and photoactive materials (Table 1).
| Application | LbL assembly | Substrate | Ref. | |
|---|---|---|---|---|
| (+) component | (−) component | |||
| a Electrodeposition. b Successive ionic layer adsorption and reaction (SILAR). c Covalent binding. * NC: nanocrystal/QD: quantum dot/NP: nanoparticle/NS: nanosheet/NF: nanofiber/ITO: indium tin oxide/FTO: fluorine-doped tin oxide. ** PVP: poly(4-vinylpyridine)/PEI: poly(ethyleneimine)/PAA: poly(acrylic acid)/PDDA: poly(diallyldimethylammonium chloride)/PMMA-SH: poly(methyl methacrylate) containing thiol groups/PSS: poly(sodium 4-styrene sulfonate)/PAH-CdTe QDs: poly(allylamine hydrochloride)-CdTe QDs/MMT: montmorillonite/QAICS: quaternary ammonium ion cationic starch/LDH: layered double hydroxide/POSS: polysilsesquioxane/PSEI: poly(dimethylsiloxane-block-etherimide)/PW12: tungsto(molybdo)phosphate/SePEI: organoselenium-modified PEI/b-PEI: branched PEI/PAA-rGO: PAA-modified reduced graphene oxide/TALH titanium(IV) bis(ammoniumlactato)dihydroxide/PAH-GNs: PAH-modified graphene nanosheet. | ||||
| Solar cells | PVP | PbSe NC | ITO | 60 |
| CdSe QDs | CdSe QDs | FTO//TiO2 | 61 | |
| TiO2 NPs | Nb2O5 NPs | FTO | 62 | |
| TiO2 NPs | TiO2 NPs | FTO | 63 | |
| Graphenea | CdSb QDs | ITO | 64 | |
| TiO2, CdSe/CdS/ZnS QDs | CdSe QDs | FTO | 65 | |
| PEI | PAA, M13-bacteriophage | FTO | 66 | |
| Light-emitting diodes | PDDA | CdSe QDs | Glass | 68 |
| CdSe@CdS QDs | PMMA-SH | Glass | 69 | |
| PDDA | CdSe QDs | Glass | 70 | |
| PAH-CdTe QDs | MMT | Quartz glass | 71 | |
| QAICS, PDDA, PAH | ZnSe, CdTe QDs | Glass | 72 | |
| CdSe@ZnS QDs | CdS@ZnS QDs | ITO | 73 | |
| LDH | CdTe QDs | Quartz | 74 | |
| Organocatalyst | POSS | TiO2 NPs | PSEI | 20 |
| PVPc | Na2PdCl4 | Quartz//O3SiC6H4CH2 | 75 | |
| TiO2 NPs | PW12 | ITO | 76 | |
| PAH | PAA, metal (Au, Ag, Pt) colloidal NPs | TiO2 nanotube | 77 | |
| SePEI | Alginate | Quartz | 78 | |
| b-PEI, PAH | PSS, SnS2 NS/TiO2 NF | Textile | 79 | |
| Graphene | Au NPs | PET | 80 | |
| PAA-rGO | TALH | Quartz | 81 | |
| PDDA | Titanate | Quartz | 82 | |
| PAH-GNs | CdS QDs | FTO | 83 | |
| C-dot,a graphene oxide | CdSb QDs | ITO | 84 | |
2. Emerging applications in photoactive LbL electrodes
2.1 Photoactivity of LbL films
Since the importance of renewable energy cannot be overstated with the exhaustion of fossil fuels, various studies on energy conversion, storage, and catalysts for alternative energy production have been reported recently.85–87 Solar energy conversion, especially solar to hydrogen conversion and water splitting, constitutes a large proportion of renewable energy research.12,88,89 Accordingly, various recent research studies in LbL-PEC films have been geared towards the development of solar energy conversion systems.90 As shown in Fig. 2b, the researches associated with solar energy conversion, such as photocurrent generation and photo-water splitting, have increased considerably since 2015, unlike traditional research trends.In general, high photocurrent and photon-to-current efficiencies from specific redox potentials are the main parameters required to achieve efficient solar energy conversion in photoelectrochemical systems. In that regard, John and coworkers showed practical benefits that the LbL technique offers to PEC systems using reduced graphene oxide (rGO) and ZnO (Fig. 4a).91 In the case of rGO–ZnO hybrids, the presence of graphene enhanced the dark conductivity, however, the passivation of oxygen vacancies due to the diffusion of oxygen from rGO to ZnO during the reduction process resulted in depletion of the visible-light photoconductivity. Meanwhile, an improvement in photoinduced charge separation and efficient electron transfer in multilayer systems was demonstrated through the LbL assembly technique, leading to the generation of highly enhanced photocurrent (33.80 μA cm−2 at −0.5 V bias voltage).
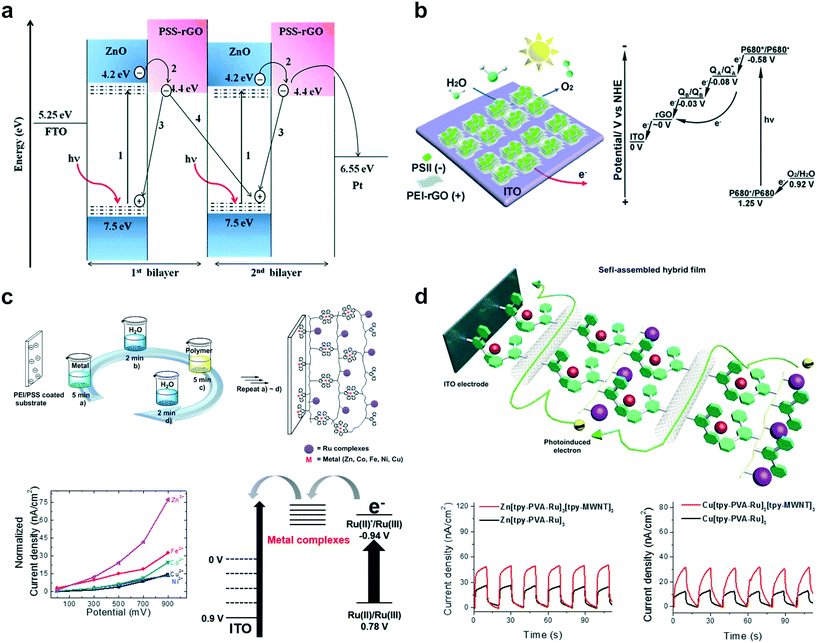 | ||
| Fig. 4 Recent representative examples of LbL assembly for PEC systems in energy conversion applications based on photocurrent generation: (a) photoinduced electron transfer between reduced graphene oxide (rGO) and ZnO in multilayered films. Reprinted with permission from ref. 91, copyright 2015 Royal Society of Chemistry. (b) Multilayered film utilizing photosystem II (PS II) and rGO for efficient transfer of photoexcited electrons in a PEC system. Reprinted with permission from ref. 93, copyright 2015 Royal Society of Chemistry. (c) LbL assembly using various metal–terpyridine coordination complexes for efficient photocurrent generation. Reprinted with permission from ref. 96, copyright 2015 American Chemical Society. (d) LbL assembly employing multi-walled carbon nanotubes (MWNTs) to enhance photoinduced electron hopping rate in multilayered films. Reprinted with permission from ref. 97, copyright 2015 Elsevier. | ||
In addition, various photoactive materials have been used to design efficient photoelectrochemical multilayered films by exploiting the prominent advantages offered by LbL-PEC systems.92 For instance, Li and coworkers utilized photosystem II (PSII) as the photosensitizer and rGO to design an efficient solar energy conversion system. A biomimetic hybrid photoanode displayed a reliable electron pathway by which the photoinduced electron from P680* was transferred to the ITO substrate passing through plastoquinone (QA) and pheophytin (QB), leading to the generation of significant photocurrent (Fig. 4b).93 Moreover, a two-fold enhancement of photocurrent and stability improvement was further demonstrated via an optimal LbL assembly process with graphene materials and photoactive proteins. As another promising photoactive material, a metal–ligand complex was utilized in an LbL-PEC system because of its strong visible-light harvesting characteristics and outstanding photochemical properties.94,95 Consequently, Song and coworkers introduced the Ru–terpyridine (Ru(tpy)2) complex with various metal–terpyridine (M(tpy)2) coordination complexes (M = Fe, Co, Ni, Cu, and Zn) to enable the self-assembly of polymeric thin films. The transfer rates for photoexcited electrons from Ru(tpy)2 inside the multilayered film were compared with different M(tpy)2 linkers to reveal the diffusion rate constant of the generated photoelectrons (Fig. 4c).96 It was observed that the photoinduced electrons were transported to the electrode via M(tpy)2 linkers through an electron hopping process. In addition, Zn(tpy)2 displayed the highest electron diffusion rate among several M(tpy)2 linkers tested. In a subsequent work, the authors additionally employed multi-walled carbon nanotubes (MWNTs) to provide an efficient electron transfer pathway (Fig. 4d).97 The fabricated photoactive polymer–MWNT hybrid thin films achieved 35% enhancement in photocurrent, attributed to the improved electron-hopping rate constants due to inclusion of the MWNTs. These Ru(tpy)2-incorporated photoactive polymer–MWNT hybrid thin films hold huge promise for artificial photosynthesis or solar energy conversion.
2.2 Photo-water splitting of LbL films
Hydrogen gas is a green energy carrier that is considered a key element in the field of future sustainable energy.98,99 In this regard, photocatalytic water splitting offers a promising and competitive technique for producing hydrogen gas to address future environmental and energy concerns.1,100,101 Although most research studies thus far have focused on the development of new materials for photo-water splitting reactions, their integration into photoelectrodes is another critical factor to enhance their photocatalytic performance and stability through the ensemble effect on hybrid photoactive materials. Therefore, LbL assembly can offer a unique and ideal platform for assembling various functional components within the electrode in a highly controllable manner.As a representative example, Schanze and coworkers reported the application of LbL for the construction of chromophore–catalyst assemblies consisting of a cationic polystyrene-based Ru polychromophore and a [Ru(tpy)(2-pyridyl-N-methylbenzimidazole)(OH2)]2+ water oxidation catalyst.102 When the polychromophore/catalyst assembly was deposited onto mesoporous substrates consisting of a SnO2/TiO2 core/shell structure, it could successfully drive water oxidation with a significant photocurrent (18 μA cm−2 at 0.44 V vs. NHE) at the photoanode, coupled with O2 observed at the collector electrode. Dye-sensitized p-type NiO photocathodes for photoreduction were developed with a Ru-based and tetraphosphonated [Ni(P2N2)2]2+ proton reduction catalyst (Fig. 5a).103 The LbL-assembly approach could control the spatial arrangement of individual species while retaining the dye in close proximity to the catalyst and the surface of the semiconductor. It was shown that electron transfer from the excited dye to the catalyst was highly efficient, while the recombination kinetics can be slowed down.
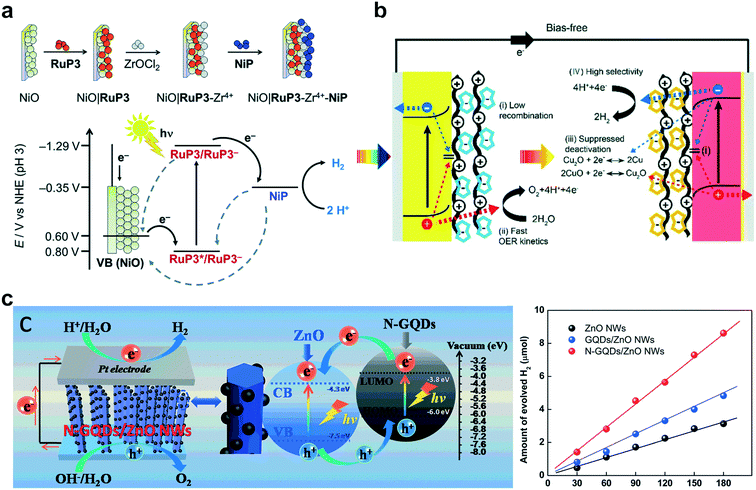 | ||
| Fig. 5 Schematic illustration of LbL multilayers for water splitting reactions: (a) energy diagram of dye–catalyst deposition on p-type NiO and current responses under light irradiation. Reprinted with permission from ref. 103, copyright 2016 Royal Society of Chemistry. (b) PEC cell with LbL-modified electrodes and LSC curves with a functionalized photocathode and photoanode. Reprinted with permission from ref. 52, copyright 2018 Royal Society of Chemistry. (c) Mechanism of N-GQDs/ZnO NW under light irradiation and hydrogen evolution performance. Reprinted with permission from ref. 109, copyright 2016 Royal Society of Chemistry. | ||
Polyoxometalates (POMs) with a transition metal complex have been considered as both fast and reversible hole scavengers and effective water oxidation catalysts.104–106 Moreover, due to their highly negative surface charges, POMs can be used as building blocks for the self-assembly of supramolecular hybrid nanomaterials.107 The Ryu group reported several studies of POM-based photocatalysts for water splitting reactions.52,108 POMs were employed as model molecular water-oxidation catalysts integrated into various electrode surfaces such as α-Fe2O3, TiO2, BiVO4, and fluorine-doped tin oxide (FTO). They demonstrated that photoelectrochemical properties and stability of photoanodes can effectively be improved by controlling the types of polyelectrolytes and the number of bilayers. Another study involved a model PEC cell constructed using a BiVO4 photoanode and Cu2O photocathode (Fig. 5b). The prepared electrodes were further functionalized with catalytic multilayers of anionic Co-based POMs and Ni-based POMs, respectively. Due to the unique catalytic multilayer structures of POMs, the PEC performance was significantly enhanced after the modification. Furthermore, the desired PEC cell for overall water splitting could be conducted under the bias-free conditions with a photocurrent of 0.2 mA cm−2 and 1.46% incident photon-to-electron conversion efficiency (IPCE).
Apart from metal-based substrates, the hybridization of carbon nanomaterials and the nanoscale construction of multilayer LbL films have opened even wider research opportunities. In addition, a facile chemical modification towards the different functionalities of carbon-based materials offers a new platform for hosting and growing functional nanomaterials on the surface of carbon-based materials. Yang Tan and co-workers introduced highly ordered nitrogen-doped graphene quantum dots (N-GQDs) decorated with 1-dimensional ZnO nanowire heterostructures through LbL assembly (Fig. 5c).109 It was revealed that the well-defined LbL-assembled N-GQDs/ZnO nanowire photoanode resulted in significantly enhanced PEC water splitting performances under both simulated solar and visible light irradiations. This result is attributed to the extraordinary photosensitization effect of N-GQDs and the intimate interfacial interactions between N-GQDs and the ZnO nanowire framework afforded by nitrogen doping and LbL assembly. In their subsequent contribution, a series of metal/graphene quantum dot (M/GQDs)n (M = Au, Ag, Pt) multilayers were fabricated by LbL assembly.110 The catalytic performance of the (M/GQDs)n multilayer thin films could effectively be improved and optimized by fine-tuning the number of bilayers and the types of metal nanoparticles. This strategy allows the direct assembly of customized units of positively-charged GQDs and negatively-charged metal nanocrystals, which were integrated in an alternating stacked fashion towards unravelling the architecture-controlled effect of 3D nanoelectrodes, including the prior contribution of our studies towards the development of effective electrocatalysts.42–44
2.3 Potential applications of photoactive LbL films
Although LbL-assembled multilayer systems can offer effective solutions for PEC cells, most of the applications have been focused on water splitting reactions. Water splitting is an environmentally friendly process used to produce hydrogen without by-products. However, direct water splitting requires high over-potential of about 1.23 V due to the sluggish kinetics of oxygen evolution reaction (OER).111,112 In addition, costly gas separation systems are required to prevent the mixing of H2 and O2 due to safety concerns.Alternatively, several reports have recently introduced a new concept of electrochemical reforming, which could replace OER to easily oxidize species such as ethanol, benzyl alcohol, urea, or biomass to improve electrocatalytic performance.113–116 Among them, replacing OER with biomass oxidation reactions could not only improve the energy conversion efficiency, but also generate value-added chemicals instead of oxygen.117–119 Recently, our group discovered the potential of LbL films assembled with various metal nanoparticles to improve electrochemical performance of biomass oxidation reactions. Since this is an emerging field in the production of hydrogen, PEC reforming needs to be explored further.
Moreover, LbL films could be utilized as photoelectrochemical biosensors for the quantitative detection of glucose, DNA aptamer and cancer cells.120–122 While conventional methods for detecting bio-related molecules suffers from time-consuming processes and expensive apparatus, photoelectrochemistry-based analytical methods offer unique opportunities owing to their facile and cost-effective nature, and high sensitivity. LbL multilayers allow the immobilization of photoelectrochemically active sites, which can enhance the sensitivity and selectivity of biosensors. Similarly, this strategy has the potential for use as a drug delivery system through the deposition of photo-responsive therapeutic materials on LbL films.123 Due to the high versatility of the LbL assembly method, it is easy to alter the surface of substrates by adding different polymers or attaching targeting moieties. Moreover, architectures can be manipulated to create multifunctional carrier systems to meet specific requirements. We anticipate a variety of drug delivery systems that could be designed with multiple therapeutic agents for effective dual chemo- and photothermal treatments.
3. Summary and outlook
In this review, we presented research progress in the field of LbL assembly towards PEC applications. The LbL assembly has been demonstrated to be an appealing strategy for the hybridization of various semiconducting nanomaterials into functional nanoelectrodes. As a result, LbL-PEC films can play an important role not only in transferring electron–hole carriers, but also in preventing the recombination of electron–hole pairs to induce charge separation. In particular, this review has focused on emerging applications such as water splitting using photoactive LbL multilayer thin films with the remarkable advantages offered by LbL-PEC systems (Table 2). Despite increasing recent examples in the field of LbL-PEC, there remain unexplored fundamental studies and more advanced design strategies for future developments in LbL-PEC systems (Fig. 6).| Application | LbL assembly | Substrate | Ref. | |
|---|---|---|---|---|
| (+) component | (−) component | |||
| a Metal–ligand coordination binding. * NC: nanocrystal/QD: quantum dot/NP: nanoparticle/ITO: indium tin oxide/FTO: fluorine-doped tin oxide. ** PAM-ZnO: poly(acrylamide)-ZnO/PSS: poly(sodium 4-styrene sulfonate)/PDDA: poly(diallyldimethylammonium chloride)/rGO: reduced graphene oxide/P3TOPA: poly-2-(3-thienyloxy)propyltrimethylammonium/CCG: chemically converted graphene/P3TOPS: poly-2-(3-thienyloxy)propanesulfonate/PEI: poly(ethyleneimine)/PS II: photosystem II/tpy: terpyridine/MWNT(MWCNTs): multi-walled carbon nanotubes/GO: graphene oxide/RuL(ClO4)2 {L = 2-(2,6-di(pyridin-2-yl)pyridine-4-yl)-1H-imidazo[4,5-f]-1,10-phenanthroline}/PVA: poly(vinyl alcohol)/PEI: polyethylenimine/NiPOM: [Ni4(H2O)2(PW9O34)2]10−/CoPOM: [Co4(H2O)2(PW9O34)2]10−/Ps-Ru: polystyrene-based Ru polychromophore/RuC: [Ru(tpy)(2-pyridyl-N-methylbenzimidazole) (OH2)]2+/PAA: poly(acrylic acid)/NiP: tetraphosphonated molecular [Ni(P2N2)2]2+/RuP3: hexaphosphonated Ru(2,2′-bipyridine)3-based dye/b-PEI: branched PEI/N-GQDs: nitrogen-doped graphene quantum dots/MAA-CdSe QDs: mercaptoacetic acid-capped CdSe QDs. | ||||
| Energy conversion | PAM-ZnO | PSS-rGO | FTO | 91 |
| P3TOPA | CCG-P3TOPS | Al | 92 | |
| PEI-rGO | PS II | ITO | 93 | |
| Metal (Ru) iona | tpy-MWCNTs | ITO | 94 | |
| RuL(ClO4)2 | GO | ITO | 95 | |
| Metal iona (Zn, Cu, Co, Fe, Ni) | tpy-PVA-Ru | ITO | 96 | |
| Metal iona (Zn, Cu) | tpy-PVA-Ru, tpy-MWNT | ITO | 97 | |
| Water splitting | PEI | NiPOM, CoPOM | FTO//Cu2O, FTO//BiVO4 | 52 |
| PS-Ru, RuC | PAA | FTO//SnO2/TiO2 | 102 | |
| Metal ion (Zr) | NiP | ITO//NiO/RuP3 | 103 | |
| b-PEI | CoPOM | FTO//Fe2O3, FTO//BiVO4, ITO//TiO2 | 108 | |
| PEI | N-GQDs | FTO//ZnO | 109 | |
| GQD-NH2 | Metal NCs (Au, Ag, Pt) | FTO | 110 | |
| Bioapplication | CdS NPs | Glucose oxidase | Pt//Nafion | 120 |
| PDDA | MAA-CdSe QDs | ITO | 121 | |
| Poly(L-lysine)-GO | GO | Liposome | 123 | |
We anticipate that the following critical issues require further development to advance the field and enrich its wider application for the future and practical implementation in the industry. First, a new design of photoactive components is necessary to overcome the inherent limitations of the existing nanomaterials. It is well known that the PEC properties of semiconducting nanomaterials depend highly on controlling the chemical structure of individual materials such as particle size and dimensions, and light absorption range. In addition, the issue of stability in physical/chemical degradation induced by photoredox reactions during PEC reactions should be improved. Second, individual photoactive nanomaterials can be hybridized with supporting building blocks such as polymer and graphene sheets. This is because the drawbacks of each photoactive nanomaterial can be complementary to enhance the performance and durability through synergy between the coupled photoactive nanomaterials. Third, it should be possible not only to construct hybridized photoactive materials in 3D architectures, but also to maximize the PEC performance with nanoscale control of their composition and architecture.
Furthermore, multicomponent assembly beyond the conventional two-component assembly is expected to assemble multi-functional nanocomposites in the LbL-PEC systems. For example, in a multilayer film, electrocatalysts can be deposited alternately on the photocatalytic layer, or anodic catalysts can serve as bifunctional catalysts by being deposited as layers over cathodic catalysts. Finally, architecture-controlled LbL-PEC electrodes can be employed to construct artificial photosynthetic devices such as optimized high-performance PEC cells. We anticipate that this review will offer researchers new insights into future LbL-PEC systems. Moreover, LbL-PEC systems are not limited to academic research but can advance gradually to industrial-scale applications due to their scalable, solution-processable nature, and their simple and environmentally friendly design process.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF-2017M3A7B4052802 and NRF-2018R1A5A1025208). M. P. acknowledges financial support from the Global Ph.D. Fellowship (GPF) funded by the National Research Foundation of Korea (NRF-2014H1A2A1018269).References
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- A. J. Bard, J. Photochem., 1979, 10, 59–75 CrossRef CAS.
- M. Rahman, F. Tajabadi, L. Shooshtari and N. Taghavinia, ChemPhysChem, 2011, 12, 966–973 CrossRef CAS PubMed.
- K. Takanabe, ACS Catal., 2017, 7, 8006–8022 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- S. Gao, D. Pan and R. Cao, J. Colloid Interface Sci., 2011, 358, 593–597 CrossRef CAS PubMed.
- J. Sun, D. Meng, S. Jiang, G. Wu, S. Yan, J. Geng and Y. Huang, J. Mater. Chem., 2012, 22, 18879–18886 RSC.
- N. Singh, J. Prakash and R. K. Gupta, Mol. Syst. Des. Eng., 2017, 2, 422–439 RSC.
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z. X. Guo and J. Tang, Energy Environ. Sci., 2015, 8, 731–759 RSC.
- C. Zhang, Q. Wu, X. Ke, J. Wang, X. Jin and S. Xue, Int. J. Hydrogen Energy, 2014, 39, 14604–14612 CrossRef CAS.
- K. Sivula, F. Le Formal and M. Grätzel, ChemSusChem, 2011, 4, 432–449 CrossRef CAS PubMed.
- S. Yanagida, A. Nakajima, T. Sasaki, T. Isobe, Y. Kameshima and K. Okada, Appl. Catal., A, 2009, 366, 148–153 CrossRef CAS.
- K. Sivula and R. Van De Krol, Nat. Rev. Mater., 2016, 1, 15010 CrossRef CAS.
- M. Grätzel, Nature, 2001, 414, 338–344 CrossRef PubMed.
- X. Li, X. Zhang, J. Hua and H. Tian, Mol. Syst. Des. Eng., 2017, 2, 98–122 RSC.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- T. Sasaki, Y. Ebina, T. Tanaka, M. Harada, M. Watanabe and G. Decher, Chem. Mater., 2001, 13, 4661–4667 CrossRef CAS.
- T. Sasaki, Y. Ebina, K. Fukuda, T. Tanaka, M. Harada and M. Watanabe, Chem. Mater., 2002, 14, 3524–3530 CrossRef CAS.
- J. A. Lee, Y. S. Nam, G. C. Rutledge and P. T. Hammond, Adv. Funct. Mater., 2010, 20, 2424–2429 CrossRef CAS.
- H. Zhang, G. Chen and D. W. Bahnemann, J. Mater. Chem., 2009, 19, 5089–5121 RSC.
- S. Srivastava and N. A. Kotov, Acc. Chem. Res., 2008, 41, 1831–1841 CrossRef CAS PubMed.
- H. Li, S. Pang, S. Wu, X. Feng, K. Müllen and C. Bubeck, J. Am. Chem. Soc., 2011, 133, 9423–9429 CrossRef CAS PubMed.
- A. T. Nagaraja, A. Sooresh, K. E. Meissner and M. J. McShane, ACS Nano, 2013, 7, 6194–6202 CrossRef CAS PubMed.
- A. O. T. Patrocinio, L. F. Paula, R. M. Paniago, J. Freitag and D. W. Bahnemann, ACS Appl. Mater. Interfaces, 2014, 6, 16859–16866 CrossRef CAS PubMed.
- K. Wang, S. Wan, Q. Liu, N. Yang and J. Zhai, RSC Adv., 2013, 3, 23755–23761 RSC.
- M. Aono, Y. Bando and K. Ariga, Adv. Mater., 2012, 24, 150–151 CrossRef CAS PubMed.
- K. Ariga and M. Aono, Jpn. J. Appl. Phys., 2016, 55, 1102A6 CrossRef.
- F.-X. Xiao, M. Pagliaro, Y.-J. Xu and B. Liu, Chem. Soc. Rev., 2016, 45, 3088–3121 RSC.
- K. Ariga, Y. Yamauchi, G. Rydzek, Q. Ji, Y. Yonamine, K. C.-W. Wu and J. P. Hill, Chem. Lett., 2014, 43, 36–68 CrossRef CAS.
- G. Rydzek, Q. Ji, M. Li, P. Schaaf, J. P. Hill, F. Boulmedais and K. Ariga, Nano Today, 2015, 10, 138–167 CrossRef CAS.
- L. K. Sagar, W. Walravens, Q. Zhao, A. Vantomme, P. Geiregat and Z. Hens, Chem. Mater., 2016, 28, 6953–6959 CrossRef CAS.
- G. Decher, Science, 1997, 277, 1232–1237 CrossRef CAS.
- J. J. Richardson, M. Bjornmalm and F. Caruso, Science, 2015, 348, aaa2491 CrossRef PubMed.
- T. Lee, S. H. Min, M. Gu, Y. K. Jung, W. Lee, J. U. Lee, D. G. Seong and B.-S. Kim, Chem. Mater., 2015, 27, 3785–3796 CrossRef CAS.
- E. Ahn, T. Lee, M. Gu, M. Park, S. H. Min and B.-S. Kim, Chem. Mater., 2017, 29, 69–79 CrossRef CAS.
- B. N. Nunes, L. F. Paula, Í. A. Costa, A. E. H. Machado, L. G. Paterno and A. O. T. Patrocinio, J. Photochem. Photobiol., C, 2017, 32, 1–20 CrossRef CAS.
- J. L. Gunjakar, T. W. Kim, H. N. Kim, I. Y. Kim and S.-J. Hwang, J. Am. Chem. Soc., 2011, 133, 14998–15007 CrossRef CAS PubMed.
- M. Gu, J. Lee, Y. Kim, J. S. Kim, B. Y. Jang, K. T. Lee and B.-S. Kim, RSC Adv., 2014, 4, 46940–46946 RSC.
- T. Lee, T. Yun, B. Park, B. Sharma, H.-K. Song and B.-S. Kim, J. Mater. Chem., 2012, 22, 21092–21099 RSC.
- K. Jo, M. Gu and B.-S. Kim, Chem. Mater., 2015, 27, 7982–7989 CrossRef CAS.
- Y. Choi, M. Gu, J. Park, H.-K. Song and B.-S. Kim, Adv. Energy Mater., 2012, 2, 1510–1518 CrossRef CAS.
- M. Gu and B.-S. Kim, Nano Energy, 2016, 30, 658–666 CrossRef CAS.
- M. Gu, J. Choi, T. Lee, M. Park, I.-S. Shin, J. Hong, H.-W. Lee and B.-S. Kim, Nanoscale, 2018, 10, 16159–16168 RSC.
- A. A. Mamedov, N. A. Kotov, M. Prato, D. M. Guldi, J. P. Wicksted and A. Hirsch, Nat. Mater., 2002, 1, 190–194 CrossRef CAS PubMed.
- B. Ohtani, J. Photochem. Photobiol., C, 2010, 11, 157–178 CrossRef CAS.
- F. Fabregat-Santiago, G. Garcia-Belmonte, I. Mora-Seró and J. Bisquert, Phys. Chem. Chem. Phys., 2011, 13, 9083–9118 RSC.
- W.-J. Ong, L.-L. Tan, Y. H. Ng, S.-T. Yong and S.-P. Chai, Chem. Rev., 2016, 116, 7159–7329 CrossRef CAS PubMed.
- J. Rongé, J. Bets, S. Pattanaik, T. Bosserez, S. Borellini, S. Pulinthanathu Sree, G. Decher and J. A. Martens, Catal. Today, 2015, 246, 28–34 CrossRef.
- W. Tu, Y. Zhou, Q. Liu, Z. Tian, J. Gao, X. Chen, H. Zhang, J. Liu and Z. Zou, Adv. Funct. Mater., 2012, 22, 1215–1221 CrossRef CAS.
- D. N. Priya, J. M. Modak and A. M. Raichur, ACS Appl. Mater. Interfaces, 2009, 1, 2684–2693 CrossRef CAS PubMed.
- H. Kim, S. Bae, D. Jeon and J. Ryu, Green Chem., 2018, 20, 3732–3742 RSC.
- J. Su, L. Guo, N. Bao and C. A. Grimes, Nano Lett., 2011, 11, 1928–1933 CrossRef CAS PubMed.
- S. J. Hong, S. Lee, J. S. Jang and J. S. Lee, Energy Environ. Sci., 2011, 4, 1781–1787 RSC.
- S. Yuan, J. Mu, R. Mao, Y. Li, Q. Zhang and H. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 5719–5725 CrossRef CAS PubMed.
- J. Huang, R. Ma, Y. Ebina, K. Fukuda, K. Takada and T. Sasaki, Chem. Mater., 2010, 22, 2582–2587 CrossRef CAS.
- D. Y. Lee, I. Lim, C. Y. Shin, S. A. Patil, W. Lee, N. K. Shrestha, J. K. Lee and S.-H. Han, J. Mater. Chem. A, 2015, 3, 22669–22676 RSC.
- A. Vuorema, J. J. Walsh, M. Sillanpää, W. Thielemans, R. J. Forster and F. Marken, Electrochim. Acta, 2012, 73, 31–35 CrossRef CAS.
- N. A. Kotov, I. Dekany and J. H. Fendler, J. Phys. Chem., 1995, 99, 13065–13069 CrossRef CAS.
- B. Vercelli, G. Zotti, A. Berlin and M. Natali, Chem. Mater., 2010, 22, 2001–2009 CrossRef CAS.
- H. Jin, S. Choi, R. Velu, S. Kim and H. J. Lee, Langmuir, 2012, 28, 5417–5426 CrossRef CAS PubMed.
- L. F. Paula, R. C. Amaral, N. Y. Murakami Iha, R. M. Paniago, A. E. H. Machado and A. O. T. Patrocinio, RSC Adv., 2014, 4, 10310–10316 RSC.
- K. P. S. Zanoni, R. C. Amaral and N. Y. Murakami Iha, ACS Appl. Mater. Interfaces, 2014, 6, 10421–10428 CrossRef CAS PubMed.
- C. X. Guo, H. Bin Yang, Z. M. Sheng, Z. S. Lu, Q. L. Song and C. M. Li, Angew. Chem., Int. Ed., 2010, 49, 3014–3017 CrossRef CAS PubMed.
- S. Choi, H. Jin, J. Bang and S. Kim, J. Phys. Chem. Lett., 2012, 3, 3442–3447 CrossRef CAS.
- P.-Y. Chen, R. Ladewski, R. Miller, X. Dang, J. Qi, F. Liau, A. M. Belcher and P. T. Hammond, J. Mater. Chem. A, 2013, 1, 2217–2224 RSC.
- S. Jaffar, K. T. Nam, A. Khademhosseini, J. Xing, R. S. Langer and A. M. Belcher, Nano Lett., 2004, 4, 1421–1425 CrossRef CAS.
- X. Zhang, C. Zhou, S. Zang, H. Shen, P. Dai, X. Zhang and L. S. Li, ACS Appl. Mater. Interfaces, 2015, 7, 14770–14777 CrossRef CAS.
- F. Jin, M.-L. Zheng, M.-L. Zhang, Z.-S. Zhao and X.-M. Duan, RSC Adv., 2014, 4, 33206–33214 RSC.
- Y. Chan, J. Chen, Q. Liu, S. E. Wark, D. H. Son and J. D. Batteas, Anal. Chem., 2010, 82, 3671–3678 CrossRef CAS.
- W. Zhou, W. Guan and C. Lu, Chem. Commun., 2014, 50, 11370–11373 RSC.
- J. Zhang, Q. Li, X. Di, Z. Liu and G. Xu, Nanotechnology, 2008, 19, 435606 CrossRef.
- W. K. Bae, J. Kwak, J. Lim, D. Lee, M. K. Nam, K. Char, C. Lee and S. Lee, Nano Lett., 2010, 10, 2368–2373 CrossRef CAS.
- R. Liang, S. Xu, D. Yan, W. Shi, R. Tian, H. Yan, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2012, 22, 4940–4948 CrossRef CAS.
- Z. Ren, H.-L. Wang, Y.-Q. Cai, M. Chen and D.-J. Qian, Mater. Chem. Phys., 2011, 127, 310–315 CrossRef CAS.
- Z. Sun, L. Xu, W. Guo, B. Xu, S. Liu and F. Li, J. Phys. Chem. C, 2010, 114, 5211–5216 CrossRef CAS.
- F. Xiao, J. Phys. Chem. C, 2012, 116, 16487–16498 CrossRef CAS.
- J. Yang, J. L. Welby and M. E. Meyerhoff, Langmuir, 2008, 24, 10265–10272 CrossRef CAS.
- L. Truong-Phuoc, K. C. Christoforidis, F. Vigneron, V. Papaefthimiou, G. Decher, N. Keller and V. Keller, ACS Appl. Mater. Interfaces, 2016, 8, 34438–34445 CrossRef CAS PubMed.
- I. Shakir, Z. Ali and D. J. Kang, J. Alloys Compd., 2014, 617, 707–712 CrossRef CAS.
- Y.-K. Kim, H. Jang and K. Kang, Thin Solid Films, 2017, 636, 359–366 CrossRef CAS.
- M.-C. Sun, J.-B. Liang, W.-Q. Peng, Z.-M. Wang, N. Negishi, K. Koike, Y.-H. Chu and H.-Q. Yin, Mater. Sci. Semicond. Process., 2015, 40, 954–963 CrossRef CAS.
- F.-X. Xiao, J. Miao and B. Liu, J. Am. Chem. Soc., 2014, 136, 1559–1569 CrossRef CAS PubMed.
- N.-N. Chai, H.-X. Wang, C.-X. Hu, Q. Wang and H.-L. Zhang, J. Mater. Chem. A, 2015, 3, 16613–16620 RSC.
- D. Kim, K. K. Sakimoto, D. Hong and P. Yang, Angew. Chem., Int. Ed., 2015, 54, 3259–3266 CrossRef CAS PubMed.
- J. Ryu, D. H. Nam, S. H. Lee and C. B. Park, Chem. – Eur. J., 2014, 20, 12020–12025 CrossRef CAS PubMed.
- K. Sun, S. Shen, Y. Liang, P. E. Burrows, S. S. Mao and D. Wang, Chem. Rev., 2014, 114, 8662–8719 CrossRef CAS PubMed.
- T. Bak, J. Nowotny, M. Rekas and C. Sorrell, Int. J. Hydrogen Energy, 2002, 27, 991–1022 CrossRef CAS.
- K. S. Joya, Y. F. Joya, K. Ocakoglu and R. van de Krol, Angew. Chem., Int. Ed., 2013, 52, 10426–10437 CrossRef CAS PubMed.
- Z.-J. Li, W.-J. Zhao, Y. Shi, Z.-P. Ying, W. Feng and L. Bai, Compos. Interfaces, 2018, 25, 809–821 CrossRef CAS.
- M. K. Kavitha, P. Gopinath and H. John, Phys. Chem. Chem. Phys., 2015, 17, 14647–14655 RSC.
- J. Sun, L. Xiao, D. Meng, J. Geng and Y. Huang, Chem. Commun., 2013, 49, 5538–5540 RSC.
- P. Cai, X. Feng, J. Fei, G. Li, J. Li, J. Huang and J. Li, Nanoscale, 2015, 7, 10908–10911 RSC.
- Y. Pan, B. Tong, J. Shi, W. Zhao, J. Shen, J. Zhi and Y. Dong, J. Phys. Chem. C, 2010, 114, 8040–8047 CrossRef CAS.
- T.-T. Meng, Z.-B. Zheng and K.-Z. Wang, Langmuir, 2013, 29, 14314–14320 CrossRef CAS PubMed.
- D.-C. Jeong, J. Lee, Y. Lee, C. Satheeshkumar and C. Song, Macromolecules, 2015, 48, 1621–1626 CrossRef CAS.
- D.-C. Jeong, S. G. Song, C. Satheeshkumar, Y. Lee, K. Kim and C. Song, Polymer, 2015, 69, 39–44 CrossRef CAS.
- T. R. Cook, D. K. Dogutan, S. Y. Reece, Y. Surendranath, T. S. Teets and D. G. Nocera, Chem. Rev., 2010, 110, 6474–6502 CrossRef CAS PubMed.
- V. S. Thoi, Y. Sun, J. R. Long and C. J. Chang, Chem. Soc. Rev., 2013, 42, 2388–2400 RSC.
- F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC.
- J. Ran, J. Zhang, J. Yu, M. Jaroniec and S. Z. Qiao, Chem. Soc. Rev., 2014, 43, 7787–7812 RSC.
- G. Leem, B. D. Sherman, A. J. Burnett, Z. A. Morseth, K.-R. Wee, J. M. Papanikolas, T. J. Meyer and K. S. Schanze, ACS Energy Lett., 2016, 1, 339–343 CrossRef CAS.
- M. A. Gross, C. E. Creissen, K. L. Orchard and E. Reisner, Chem. Sci., 2016, 7, 5537–5546 RSC.
- I. V. Kozhevnikov, Chem. Rev., 1998, 98, 171–198 CrossRef CAS PubMed.
- M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34–48 CrossRef.
- H. T. Teo and B. Saha, J. Catal., 2004, 228, 174–182 CrossRef CAS.
- Y. Choi, D. Jeon, Y. Choi, J. Ryu and B.-S. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 13434–13441 CrossRef CAS PubMed.
- D. Jeon, H. Kim, C. Lee, Y. Han, M. Gu, B.-S. Kim and J. Ryu, ACS Appl. Mater. Interfaces, 2017, 9, 40151–40161 CrossRef CAS PubMed.
- Z. Zeng, F.-X. Xiao, X. Gui, R. Wang, B. Liu and T. T. Yang Tan, J. Mater. Chem. A, 2016, 4, 16383–16393 RSC.
- Z. Zeng, F.-X. Xiao, H. Phan, S. Chen, Z. Yu, R. Wang, T.-Q. Nguyen and T. T. Yang Tan, J. Mater. Chem. A, 2018, 6, 1700–1713 RSC.
- J. Tang, J. R. Durrant and D. R. Klug, J. Am. Chem. Soc., 2008, 130, 13885–13891 CrossRef CAS PubMed.
- X. Yang and M. B. Hall, J. Am. Chem. Soc., 2010, 132, 120–130 CrossRef CAS PubMed.
- Y. X. Chen, A. Lavacchi, H. A. Miller, M. Bevilacqua, J. Filippi, M. Innocenti, A. Marchionni, W. Oberhauser, L. Wang and F. Vizza, Nat. Commun., 2014, 5, 4036 CrossRef CAS PubMed.
- B. You, N. Jiang, X. Liu and Y. Sun, Angew. Chem., Int. Ed., 2016, 55, 9913–9917 CrossRef CAS PubMed.
- G. Liu, X. Zhang, C. Zhao, Q. Xiong, W. Gong, G. Wang, Y. Zhang, H. Zhang and H. Zhao, New J. Chem., 2018, 42, 6381–6388 RSC.
- Z.-Y. Yu, C.-C. Lang, M.-R. Gao, Y. Chen, Q.-Q. Fu, Y. Duan and S.-H. Yu, Energy Environ. Sci., 2018, 11, 1890–1897 RSC.
- H. G. Cha and K.-S. Choi, Nat. Chem., 2015, 7, 328–333 CrossRef CAS PubMed.
- B. You, X. Liu, N. Jiang and Y. Sun, J. Am. Chem. Soc., 2016, 138, 13639–13646 CrossRef CAS PubMed.
- W.-J. Liu, L. Dang, Z. Xu, H.-Q. Yu, S. Jin and G. W. Huber, ACS Catal., 2018, 8, 5533–5541 CrossRef CAS.
- J. Sun, Y. Zhu, X. Yang and C. Li, Particuology, 2009, 7, 347–352 CrossRef CAS.
- X. Zhang, S. Li, X. Jin and X. Li, Biosens. Bioelectron., 2011, 26, 3674–3678 CrossRef CAS PubMed.
- J. Feng, Y. Li, Z. Gao, H. Lv, X. Zhang, D. Fan and Q. Wei, Biosens. Bioelectron., 2018, 99, 14–20 CrossRef CAS PubMed.
- M. Hashemi, M. Omidi, B. Muralidharan, L. Tayebi, M. J. Herpin, M. A. Mohagheghi, J. Mohammadi, H. D. C. Smyth and T. E. Milner, Acta Biomater., 2018, 65, 376–392 CrossRef CAS PubMed.
Footnote |
| † All authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2019 |