 Open Access Article
Open Access ArticleIlluminating the dark side of indoor oxidants
Cora J.
Young
 *a,
Shan
Zhou
*a,
Shan
Zhou
 b,
Jeffrey A.
Siegel
cd and
Tara F.
Kahan
b,
Jeffrey A.
Siegel
cd and
Tara F.
Kahan
 e
e
aDepartment of Chemistry, York University, Canada. E-mail: youngcj@yorku.ca
bDepartment of Chemistry, Syracuse University, USA
cDepartment of Civil and Mineral Engineering, University of Toronto, Canada
dDalla Lana School of Public Health, University of Toronto, Canada
eDepartment of Chemistry, University of Saskatchewan, Canada
First published on 7th June 2019
Abstract
The chemistry of oxidants and their precursors (oxidants*) plays a central role in outdoor environments but its importance in indoor air remains poorly understood. Ozone (O3) chemistry is important in some indoor environments and, until recently, ozone was thought to be the dominant oxidant indoors. There is now evidence that formation of the hydroxyl radical by photolysis of nitrous acid (HONO) and formaldehyde (HCHO) may be important indoors. In the past few years, high time-resolution measurements of oxidants* indoors have become more common and the importance of event-based release of oxidants* during activities such as cleaning has been proposed. Here we review the current understanding of oxidants* indoors, including drivers of the formation and loss of oxidants*, levels of oxidants* in indoor environments, and important directions for future research.
Environmental significanceA clear understanding of oxidants and their precursors in indoor environments is necessary to constrain much of the chemistry that can occur indoors. Most indoor oxidant studies have focused on ozone, since oxidation indoors was thought to be dominated by ozone reactions. Recent measurements have demonstrated that other oxidants could be of equal or greater importance under many conditions, and that episodic chemistry (e.g., initiated by cooking or cleaning) could be critical to oxidation indoors. This review describes the considerations important for indoor oxidant formation and loss, as well as observations and models of oxidants indoors. Important areas of future research are also identified. |
Introduction
Indoor oxidants and their precursors (the combination of which will be referred to as “oxidants*”) are widely studied in atmospheric science, but are considerably less explored in indoor environments.1 There are many important differences between indoor and outdoor environments including differences in sources of oxidants*, the intensity and wavelength of light available, increased surface area-to-volume ratios, and the increased likelihood of human exposure.1 Photochemistry, initiated by sunlight at wavelengths shorter than ∼320 nm, drives oxidation outdoors. These high energy photons are not generally available indoors, so the oxidizing capacity has been thought to be controlled by physical transport and non-photochemical reactions (also known as “dark chemistry”). This results in very different oxidizing atmospheres indoors and outdoors. While hydroxyl radicals (OH) are the most important oxidants outdoors, their concentrations are generally expected to be very low indoors, and ozone (O3) is the only oxidant that has been widely investigated. Because of the prevalence of O3 and nitrogen dioxide (NO2) in several indoor environments, the importance of the nitrate radical (NO3) in indoor oxidation has also been suspected.2 Recent studies suggest that photolysis of molecules that absorb light at wavelengths longer than 320 nm, such as nitrous acid (HONO) and formaldehyde (HCHO), may lead to higher indoor OH concentrations than previously expected.3–6 It has also recently been shown that human activities including use of bleach can lead to the formation of numerous reactive chlorine species, many of which are photo-labile and could form Cl indoors.7,8 While Cl is not often considered a major oxidant outdoors, recent work has shown that it can contribute significantly to oxidation chemistry under some conditions,6,7 although its importance for the indoor oxidizing capacity is largely unexplored. The objectives of this review are to describe: (i) environmental factors important for oxidation chemistry indoors; (ii) the importance of different oxidant species and their precursors indoors; and (iii) future research directions to understand the extent and impacts on indoor oxidative chemistry.Indoor environmental factors affecting oxidants
Sources of oxidants indoors
Chemicals present outdoors can impact oxidant* levels and chemistry indoors (e.g.ref. 9). The extent of this effect depends on pollutant levels outdoors, the air exchange rate, and penetration through the heating, ventilation, and air-conditioning (HVAC) system (mechanical ventilation) or building enclosure (leakage). Numerous additional sources of particles and gas-phase compounds—both direct and indirect—exist indoors. These have been reported comprehensively in several reviews (e.g.ref. 10) and will be discussed only briefly here in the context of oxidants*. The components and contents of indoor environments, including building materials11–14 and electronics,15 are a direct source of indoor oxidant* emissions. Human and microbial occupants of indoor environments can also act as a direct source of oxidants*, such as acetaldehyde and other carbonyls.12,16,17 Human activities can be a major source of indoor oxidants*. For example, oxidants* have been detected in simulated and real indoor environments after cleaning or disinfection of surfaces7,18,19 and water,20 as well as after the use of air fresheners.21 Cooking is an important source of indoor oxidants*,12,22–24 as is burning of candles or incense,12,25 cigarette smoking,12,26 and vaping.27 Several types of air purifiers can increase indoor O3 levels, either deliberately (e.g., O3 generators) or as a byproduct of their operation (e.g., ion generators, electrostatic precipitators, and some UV-lamp containing air cleaners).28–30 Specific devices may emit oxidants other than O3.31 Most emission sources are constrained only to select chemicals (e.g. reactive chlorine species) under specific conditions (e.g. bleach cleaning) and may depend substantially on the context and environment investigated. As a result, significant uncertainty in primary emissions indoors remains.Secondary oxidant formation
In addition to direct emission and transport from outdoors, oxidants* can be formed chemically indoors. For example, a dominant OH source indoors may be reactions between O3 and alkenes.9 Photochemistry (especially of HONO) is thought to also be an important indoor OH source under some conditions.32–35 Due to the high surface areas encountered indoors (discussed in more detail below), heterogeneous chemistry may be very important. Some reactions of known importance are the disproportionation of NO2 on surfaces to form HONO36 and reactions of O3 with components of skin oil.37 Heterogeneous reactions on indoor surfaces are even less well understood than gas-phase reactions, largely due to the large range of materials present indoors and the unknown, and likely variable, nature of surface soiling.Light conditions indoors
Outdoors, oxidant chemistry is primarily initiated by photons in the 290 to 400 nm range. Indoors, solar radiation at wavelengths shorter than ∼330 nm is completely attenuated by windows (Fig. 1).4,22,33–35 This precludes many photochemical reactions that are important outdoors, including the formation of hydroxyl radicals (OH) by O3 photolysis. Recent studies suggest that OH may be formed photochemically indoors from species such as HONO and HCHO that absorb at longer wavelengths,4,7,22,34,38 but the overall importance of photochemistry in indoor chemistry remains unclear. Different factors govern photochemical reaction rates indoors and outdoors, and different reactions are likely to be important. While the sun is generally the only important light source outdoors, this is not the case indoors. As shown in Fig. 1, artificial lights including fluorescent and incandescent halogen lamps emit at wavelengths short enough to affect photolysis of several chemicals likely to be important oxidants* indoors. Spectral profiles, and therefore photolysis rates, vary depending on the light source and the presence and construction of light coverings. Outdoors, photon fluxes will be uniform within a given volume of air unless the light is attenuated or reflected (e.g. by clouds, trees, or the ground). Solar photon fluxes are also expected to be relatively constant in directly illuminated indoor regions. However, the volume of air illuminated may be small relative to the total volume, and the volume of illuminated air (as well as its location) may change more dramatically over the course of the day than that observed outdoors due to the placement of windows. Photon fluxes from most compact light bulbs decrease as approximately r−2 with increasing distance from the source, which leads to large gradients in photon fluxes.34 Other factors are likely to additionally impact the photon flux, including lamp manufacturer, style, wattage, number of bulbs in a fixture, and coverings such as lamp shades.34 Due to the large anticipated spatial heterogeneity of photon fluxes indoors, mixing ratios of photochemically formed species may also display large spatial heterogeneity.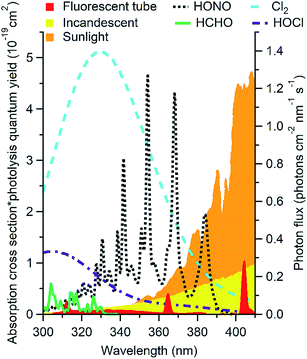 | ||
| Fig. 1 Photon flux of common indoor light sources (right axis) shown with the product of absorption cross section and photolysis quantum yield of likely indoor oxidants* (left axis). Data from ref. 34, 39–42. | ||
Photochemistry follows a clear diurnal pattern outdoors, with additional variability introduced by clouds or particulate matter, as well as by changes in the solar zenith angle (SZA) in different seasons. Temporal trends indoors are much more variable and depend on building construction and human activity. The construction of buildings influences the distribution of windows and artificial lights, which will determine the photon fluxes in the actinic region at different times of the day. Human activity – e.g. closing blinds and turning on lights in occupied rooms – will play a large role in determining the extent of photolysis occurring at any given time. Given the different emission spectra of artificial lights compared to that of sunlight in the ultraviolet region, different photochemical reactions may dominate when sunlight vs. artificial lights is the primary illumination source (e.g. during the day vs. during the evening). Indirect (i.e. diffuse and scattered) light contributes to photon fluxes outdoors, depending on factors such as cloud cover, SZA, and surface albedo. Reflected light may also impact photon fluxes indoors, though this has been examined in only one laboratory study, which observed that reflected light contributed less than 5% of total light in a laboratory illuminated by sunlight filtered through a window.33 It is probable that, as is the case outdoors, the importance of indirect light indoors will depend on variables such as the SZA and the colour and material of surfaces.
Surface area
Indoors, the available surface area is much greater than that outdoors for a given volume of air, and heterogeneous reactions may affect indoor oxidants*. Indoor surfaces are known to be an important sink for O3. The reactive uptake of O3 by indoor surfaces including glass, green building materials, drywall, HVAC ducts, office materials and contents, carpets, and skin oil and skin oil proxies has been investigated by several research groups,37,43–60 but uptake kinetics of many of the diverse surfaces found in indoor environments are not known. The reactive uptake of HOCl by skin oil proxies was recently studied,61 but little literature about the reactive uptake of oxidants other than O3 by indoor surfaces is available. Relationships between indoor surface properties and uptake coefficients of O3 or other oxidants* have not been established.62,63 It is possible that there is little variance in the uptake efficiency of oxidants* by different indoor surfaces due to films that coat indoor surfaces.64,65 These surface coatings are dependent on indoor concentrations of film components and surface properties and have been studied for only a narrow subset of compounds and surfaces, e.g., organic films formed by the deposition of semivolatile organic compounds (SVOCs) on impermeable glass surfaces.65 The effect of the indoor surface coatings relative to the underlying surfaces on gas phase oxidant* levels can be critically important. Further, most investigations of surface chemistry have focused on visible surfaces. However, most indoor surfaces are hidden, including the vast surface areas of indoor textiles and other porous materials,56,58 and unseen surfaces in building interstitial spaces (e.g., wall cavities), buffer zones (e.g., dropped ceiling spaces, attics and crawlspaces), and HVAC systems. The role of oxidant* interaction with hidden spaces has been explored for narrow sets of materials (e.g., ref. 53), often only for O3 and with little acknowledgement of soiling.Physical processes
Exchange with outdoor air is an important driver of indoor oxidant* concentrations. In general, more rapid exchange with outdoor air will lead to diminished indoor concentrations of indoor emitted oxidants* and increased concentrations of oxidants* that originate outdoors. The nature of the air flow is potentially important: in some buildings air leaks through the enclosure (leakage or infiltration) where oxidants* may react or be deposited. The penetration factors for contaminants through building enclosures have generally only been studied for O3 and for particles (e.g., ref. 66 and 67), but are potentially important for other oxidants. Air exchange is also provided by HVAC systems (mechanical ventilation) which can serve as sources and sinks for oxidants* (e.g., ref. 68 and 69). HVAC systems often recirculate some portion of the air in a building which can lead to loss within the system and serve to increase mixing and interzonal transport of oxidants throughout the building. Air exchange and HVAC system operation are also important factors in local indoor air velocities and mixing, which can promote oxidant interaction with surfaces.Which oxidants* are important indoors?
The majority of indoor oxidant* studies have been performed in non-residential buildings such as offices and classrooms. Relatively high air exchange rates (often of the order of 5 h−1) in these environments lead to significant influence of outdoor air on the indoor air composition. Ozone is generally the dominant oxidant* measured in these locations.9 Conversely, we know surprisingly little about the indoor oxidizing capacity in occupied residences. A low AER (often < 0.5 h−1) reduces the influence of outdoor air, and indoor oxidant* sources such as gas appliances can elevate nitrogen oxide levels significantly compared to those detected outdoors and in commercial buildings.5,22Table 1 lists the sources and mixing ratios of some oxidants* that may be important in residential buildings. The mixing ratios are those reported in North American residences to give an idea of the range of possible oxidant* levels. Indoor mixing ratios of some oxidants* may be different in other regions (e.g. indoor HCHO mixing ratios in excess of 100 ppbv have recently been reported14 in Chinese residences). The mixing ratios of all oxidants* listed are subject to high uncertainty due to factors including a lack of measurements (especially highly time-resolved measurements) and a poor understanding of indoor sources and sinks. Reported or predicted ambient mixing ratios for most of the oxidants* in Table 1 span two or more orders of magnitude; this prevents accurate prediction of the oxidizing capacity in general for residences.| Oxidant | Mixing ratios (ppbv) | References | Major indoor sources | Sources of uncertainty |
|---|---|---|---|---|
| Nitrogen dioxide (NO2) | 1–394 | Ref. 22 and references therein | Emission from gas appliances and transport from outdoors | Few highly time-resolved measurements; measurements subject to HONO interference |
| Nitric oxide (NO) | 0.8–400 | Ref. 22 and references therein | Emission from gas appliances and transport from outdoors | Few measurements |
| Nitrous acid (HONO) | <1–35.9 | Ref. 22 and 70 and references therein | Emission from gas appliances and surface reactions of NO2 | Few measurements |
| Ozone (O3) | <1–73 | 22,71–78 | Transport from outdoors | Commonly used levels measured in non-residential environments |
| Formaldehyde (HCHO) | 10–70 | Ref. 12 and references therein | Building and furnishing materials and residential combustion | Few highly time-resolved measurements |
| Nitrate radical (NO3) | <4 × 10−3 | 79 | NO2 + O3 | Only one measurement |
Nitrogen oxides
Nitrogen oxides (NOx = NO + NO2) are not oxidants themselves, but play a critical role in oxidant cycling, as both oxidant sources and sinks. These compounds are emitted from combustion processes, resulting in levels of up to 10s of parts-per-billion-by-volume (ppbv) in polluted and <1 ppbv in remote outdoor environments. Indoor combustion sources (e.g. gas appliances)22,24 and transport from outdoors3,5,80 can contribute to NOx levels indoors. The photolysis of NO2 could contribute to the formation of O3:| NO2 + hν → NO + O (λ < 398 nm) | (R1) |
| O + O2 → O3 | (R2) |
To our knowledge, the importance of this chemistry has never been demonstrated indoors, although Kowal et al. predicted that ozone levels in indoor locations illuminated by sunlight could be 5 times greater than those in shaded regions due to NO2 photolysis.34 In the presence of NO, this O3 can react to re-form NO2:
| O3 + NO → NO2 + O2 | (R3) |
The reduction of O3 levels during use of a gas stove has been attributed to titration by NO (R3).29
Ozone
Photochemistry outdoors generates O3, a major component of photochemical smog. Levels of 10s to 100s of ppbv of O3 are common in polluted outdoor environments. The dominant source of indoor O3 is outdoor air, with O3 levels in many indoor environments closely tracking outdoor levels.9 Direct O3 emissions from human activities, including use of office equipment (e.g., photocopiers81) and devices marketed as air purifiers (e.g., ref. 28 and 30), can occur. Several studies suggest that O3 is an important oxidant in many indoor environments, and the dominant OH source is expected to be dark reactions between O3 and unsaturated organics.9 Indoor-to-outdoor ratios (I/O) of O3 in the absence of major sources or sinks are commonly between 0.2 and 0.7 and change as a function of the air exchange rate (AER).82 Thus, in residences that use natural ventilation or have a high AER, O3 levels are likely to be higher. Similarly, under conditions of direct emission (e.g. office equipment or O3-emitting air cleaners), O3 could be an important oxidant. Some (albeit relatively rare) HVAC systems may also contain activated carbon air filters that can effectively remove O3 from indoor environments.83,84 Furthermore, residences are more likely to contain cooking appliances that can act as a source of NO. As described above, high NO levels titrate O3 from indoor environments through reaction (R3).29Hydroxyl radicals
The hydroxyl radical (OH) is the dominant oxidant in the daytime outdoor atmosphere. Dominant fates of OH include abstraction of a hydrogen from or addition to organic molecules, as well as reaction with NO2. Production of OH outdoors from various sources has been the subject of many studies. The vast majority have been conducted at low latitudes or in mid-high latitude summer, where photolysis of O3, carbonyls (e.g. HCHO), and HONO are the dominant sources (e.g.ref. 85–87):| O3 + hν + H2O → 2OH + O2 (λ < 320 nm)39 | (R4) |
| HC(O)H + hν + 2O2 + 2NO → 2OH + CO + 2NO2 (λ < 340 nm)39 | (R5) |
| HONO + hν → OH + NO (λ < 405 nm)40 | (R6) |
A few studies have examined the impact of OH sources under low-light outdoor conditions that may be more like conditions indoors. For example, during spring in Los Angeles, photolysis of HONO dominates OH production from dawn to mid-morning.87 Similarly, studies examining OH formation during high-latitude winter showed that OH production was dominated by HONO or carbonyl photolysis.88,89 Formation of OH is also possible in the absence of light through oxidation reactions of O3 with unsaturated organics, which are a major source of OH outdoors at night:87
| O3 + alkene → R′ + OH + RO2 | (R7) |
Under the low light conditions indoors, reaction of O3 with organics has been historically considered the dominant oxidation process (e.g., ref. 90). Studies proposing this have primarily focused on high-O3 indoor environments. As described above, in many indoor environments (and especially in residences) O3 levels are low. Under these conditions, production of OH from other sources can compete with or dominate OH production from the reaction of O3 with unsaturated organics.5
Formaldehyde (HCHO) is known to be present in many indoor environments. The levels, sources, and impacts of indoor HCHO have been extensively reviewed by Salthammer et al.12 Indoor HCHO mixing ratios are in the range of tens to hundreds of ppbv and indoor sources include building materials (e.g. pressed-wood products), combustion (e.g. cooking and cigarette burning), human metabolism and heterogeneous or gas-phase oxidation of organics.12 It is possible that HCHO could be photolyzed indoors and generate OH radicals (R5). This has not yet been investigated experimentally, but calculations based on measured photon fluxes and estimated indoor HCHO and NO mixing ratios suggest that photolysis of HCHO initiated by fluorescent tubes could be an important indoor OH source under some conditions.34 Analogously, other volatile carbonyl compounds could be photolyzed and lead to OH formation, including acetaldehyde, acetone, and glyoxal.34 These compounds are emitted from similar sources as HCHO.25–27,91
High levels of HONO have been measured in a few indoor environments, including residences, laboratories, and offices.22,70,92 The few studies performed in residences report indoor HONO levels 5–10 times higher than those outdoors. As discussed above, HONO photolysis could be an important OH source indoors under some conditions. Sunlight filtered through windows will generally be the most important light source, but Kowal et al. have predicted that artificial light sources including halogen, incandescent, and fluorescent bulbs could also initiate photochemistry, especially in close proximity to the source.34 Recent studies have reported HONO levels that vary from <1 ppbv to over 30 ppbv in residences across North America.22,70 More studies are required to better understand indoor HONO levels, especially time-resolved levels. Accurately measuring HONO is challenging, but recent advances in instrumentation present an opportunity for more widespread measurements indoors.
Several modelling studies have targeted OH concentrations indoors. Predicted concentrations typically range from 1 to 5 × 105 molecules per cm3.3,5,90 The first measurement of OH indoors was made by Weschler and Shields using an indirect technique to obtain a time-integrated concentration. They observed OH concentrations of ∼7 × 105 molecules per cm3 in a commercial building that had elevated O3 and alkenes as well as a moderate AER.93 Under most conditions, predicted levels of OH indoors are below the detection limit of the instrument used to quantify OH at high time resolution in the outdoor environment (fluorescence assay by gas expansion, limit of detection 6.5 × 105 molecules per cm3).31 Two high time resolution OH measurements have been made in cases where OH concentrations exceed this value. In the first case, concentrations up to 1.8 × 106 molecules per cm3 were observed in the presence of high HONO levels in a classroom in France in the summer.32 This concentration is comparable to typical outdoor concentrations of OH.24 The in situ technique has also been applied to an office in the presence of a commercially available air purifier.31 Under background conditions, OH concentrations were close to or below the limit of detection of the instrument. During air purifier operation, OH concentrations increased to 2 × 107 molecules per cm3.
Nitrate radicals
Nitrate radicals (NO3) can abstract a hydrogen from or add to organics. These radicals are formed in environments with high O3 and NO2:| NO2 + O3 → NO3 + O2 | (R8) |
Outdoors, accumulation of NO3 can only occur at night because it is rapidly photolyzed:39
| NO3 + hν → NO2 + O (400 nm < λ < 640 nm) | (R9a) |
| → NO + O2 (400 nm < λ < 640 nm) | (R9b) |
The formation of NO3 cannot occur in areas with appreciable NO (e.g. above soil surfaces94), which prevents the accumulation of NO3 radicals in two ways: by (i) titrating O3(R3) and preventing NO3 radical formation through (R8) and (ii) depleting any NO3 radicals formed by conversion to NO2:
| NO3 + NO → 2NO2 | (R10) |
The importance of NO3 radicals in indoor oxidation has long been suspected.2 In the past few years, models have predicted very low NO3 levels indoors. Using a box model, Carslaw observed NO3 levels <0.03 parts-per-trillion-by-volume (pptv).3 Similarly, Waring and Wells used a time-averaged model to show that NO3 radicals could be present at mixing ratios of ∼0.001 to 0.01 pptv in residences.5 Zhou et al. recently predicted steady-state NO3 concentrations in a residence based on measured O3, NO2, and NO mixing ratios.22 Low O3 and high NO levels led to the conclusion that NO3 levels would be negligible in residences under most conditions. A subsequent study made the first direct measurements of NO3 radicals in a residence.79 As predicted, NO3 radical mixing ratios were below the detection limit (1.5 pptv) under most conditions. In scenarios where O3 levels were artificially increased using a commercial O3 air purifier, NO was completely titrated through reaction (R3) and average NO3 mixing ratios of 3–4 pptv were observed. These studies suggest that NO3 can be an important indoor oxidant if O3 levels are high and NO levels are low. The high O3 and low NO conditions required for NO3 levels to be significant are uncommon in residences, but may exist at times, for example under natural ventilation (with significant influence from high O3 and low NO outdoor air), or during the use of ozone air purifiers.
Chlorine atoms
Chlorine atoms (Cl) are 1–2 orders of magnitude more reactive than OH and can abstract a hydrogen atom from or add to organics. The occurrence of Cl outdoors is driven by the formation and/or emission of photolabile chlorine atom precursors such as nitryl chloride (ClNO2) and molecular chlorine (Cl2). These compounds can be photolyzed under illumination by visible light to produce Cl:39,95| ClNO2 + hν → Cl + NO2 (λ < 475 nm) | (R11) |
| Cl2 + hν → 2Cl (λ < 493 nm) | (R12) |
Like HONO described above, Cl2 and ClNO2 are photolabile under low-light outdoor conditions and have been shown to contribute up to half of the primary radicals in an urban area during the morning87 and to be a significant contributor to total radicals in continental mid-latitude winter.88
Only very recently has production of Cl atoms been considered in indoor environments. After application of bleach to a laboratory floor, up to 481 ppbv of Cl2 was observed, along with ClNO2 and other photolabile chlorinated species (e.g. hypochlorous acid (HOCl) and nitrogen trichloride (NCl3)).7,39,96,97
| HOCl + hν → OH + Cl (λ < 420 nm) | (R13) |
| NCl3 + hν → products (λ < 420 nm) | (R14) |
A model incorporating the reactive chlorine species released from mopping with bleach predicted the formation of Cl through photolysis by window-attenuated sunlight. Modelled Cl concentrations were up to ∼2 × 105 molecules per cm3 and were of a similar magnitude to those of predicted OH.7 Typical lights used indoors could also photolyze these reactive chlorine species to form Cl.8,35 These levels cannot be verified through measurement since there is currently no analytical technique capable of measuring atmospheric Cl. Typical levels of chlorinated species are not well-understood indoors and appear to be limited to specific activities or environments, such as bleach use7 and chlorinated swimming pools,20 respectively. The photolability of Cl2, ClNO2, HOCl, and NCl3 under visible wavelengths suggests that Cl atoms could play an important role in indoor oxidation.
An additional oxidation pathway can be derived from the reaction of chlorinated compounds with unsaturated organics. Schwartz-Narbonne et al.61 recently demonstrated the heterogeneous oxidation of skin-oil proxies by HOCl, where reactions could occur fast enough to effectively compete with loss via transport of HOCl. This is consistent with the unidentified loss process of HOCl observed by Wong et al. following bleaching.7
Potential effects of changing policy and human behaviour on indoor oxidant levels
The indoor oxidizing capacity may change substantially in the future, due to new policies or regulations, changing construction practices, or human behaviour. This has been observed in the past through substantially decreased indoor O3 levels in the United States in response to the passing of the Clean Air Act in 1997. Average O3 levels in houses prior and subsequent to 1997 were of the order of 5–73 ppbv (ref. 71, 76 and 78) and <2 ppbv,73,77 respectively. This Act targeted outdoor air quality but had clear effects on indoor oxidant levels. Another example is the current movement by several local and national governments to improve outdoor air quality by restricting wood burning indoors. An example of policy directed toward indoor air quality that could change indoor oxidant* levels is the recent move by some jurisdictions (including Canada and the United States) to regulate HCHO emissions from composite wood products used in building materials. While indoor HCHO levels in North America and western Europe are generally fairly low (∼20 ppbv),12 they can be higher in some cases, such as in newly constructed homes.13 Levels of the order of 80 ppbv and as high as 590 ppbv were measured in emergency trailers supplied by the government of the United States as temporary housing for those displaced by Hurricane Katrina in 2006.98 In addition to reducing people's direct exposure to HCHO, regulating emissions from building materials could reduce photochemical radical formation, since HCHO photolysis has been predicted to be an important OH source under some lighting conditions.34 These effects would be even greater in other areas of the world, such as China, where indoor HCHO levels exceeding 1000 ppbv have been measured in newly constructed buildings.14 Although most indoor O3 comes from outdoors, some jurisdictions have promulgated regulations to limit the O3 emissions from air cleaners. A final example of a policy change that could affect indoor oxidant levels is the recent move in several countries to phase out the use of incandescent lightbulbs. The overall (room-averaged) effect of these lights on photochemical OH production is minimal, but significant amounts of OH can be produced locally. Local OH levels could change substantially depending on what type of replacement is used in place of the incandescent bulb. LEDs do not generally emit at wavelengths shorter than 400 nm,34 so their use will result in decreased local OH levels. Conversely, fluorescent lights can rapidly photolyze HONO and HCHO.34 Therefore, if CFLs are the primary replacement for incandescent bulbs, we might expect local OH levels near lights to increase.Changes in building design and construction will also likely alter the indoor oxidizing capacity. For example, “green” or energy-efficient buildings often reduce the amount of outdoor air brought into the building. However, energy savings can come with air quality tradeoffs. For example, HCHO levels in energy-efficient homes in Colorado were recently reported to be higher than those in conventional homes.99 The increased use of “green” building materials and cleaning products can introduce additional oxidative capacity and such secondary emissions are rarely considered in product evaluation and labeling.
Changing human behaviour patterns may also greatly affect indoor oxidant levels. For example, if people change their stove-type (e.g. from electric to gas or from gas to induction), oxidant levels during cooking events will also change. Changes in window-opening patterns (e.g. decreased use of natural ventilation as wealth and access to air conditioning increases as well as increased frequency of severe urban air pollution events) will have large effects on both the identity and concentration of oxidants indoors.
Future directions
Many of the uncertainties surrounding indoor oxidant* levels can be addressed through additional measurements. Of special importance is obtaining artifact-free measurements under a range of conditions (including, but not limited to, geographic location, building type, AER, exposure to sunlight). Measuring several species simultaneously with high time resolution will elucidate rapid and complex interactions between oxidants* and help quantify their sources and sinks (and thereby explain their concentrations under different conditions).Another area where further study is necessary is the effect of surfaces on indoor gas-phase oxidant levels. Indoor surface interactions and their contribution to the indoor oxidizing capacity remain poorly understood. Given the diversity of indoor surfaces and soiling amounts/compositions, it is also critically important to characterize realistic surfaces from the perspective of elucidating oxidant* interactions. Part of this effort should include surfaces that represent a large surface area in indoor spaces, but are hidden from view, not often/ever cleaned, and may have different soiling patterns compared to visible room surfaces. These include HVAC ducts and components and surfaces in buffer spaces (e.g., attics, crawlspaces, and attached garages), surfaces in interstitial spaces (e.g., wall cavities and dropped ceilings) and in porous materials. The conditions in these hidden spaces may also differ greatly from those in occupied areas. For example, attics and crawl spaces may experience extreme temperature conditions. Similar extreme temperature conditions may exist within HVAC systems, with the additional possibility of high relative humidity and the presence of disinfecting light sources. A final surface type that warrants further study is catalytic surfaces, such as self-cleaning surfaces and surface coatings.
Given the uncertainties around indoor oxidants* and their interactions with building materials, contents, and systems, it is imperative that the research community focuses efforts on well-characterized and high time-resolution measurements of different oxidant* mixtures. Emphasis should be placed on oxidants in addition to O3 and its reaction products as well as on phenomena surrounding extreme oxidant chemistry (e.g., during cleaning activities or the impact of strong UV light sources) and in building locations that are likely to be important for oxidants* but have not been widely studied (e.g., HVAC systems).
Conflicts of interest
There are no conflicts to declare.References
- S. Gligorovski and C. J. Weschler, The oxidative capacity of indoor atmospheres, Environ. Sci. Technol., 2013, 47(24), 13905–13906 CrossRef CAS PubMed.
- C. J. Weschler, B. Michael and K. Petros, Indoor ozone and nitrogen dioxide: A potential pathway to the generation of nitrate radicals, dinitrogen pentoxide, and nitric acid indoors, Environ. Sci. Technol., 1992, 26(1), 179–184 CrossRef CAS.
- N. Carslaw, A new detailed chemical model for indoor air pollution, Atmos. Environ., 2007, 41, 1164–1179 CrossRef CAS.
- E. Gómez Alvarez, D. Amedro, C. Afif, S. Gligorovski, C. Schoemaker, C. Fittschen, J.-F. Doussin and H. Wortham, Unexpectedly high indoor hydroxyl radical concentrations associated with nitrous acid, Proc. Natl. Acad. Sci. U. S. A., 2013, 13294–13299 CrossRef PubMed.
- M. S. Waring and J. R. Wells, Volatile organic compound conversion by ozone, hydroxyl radicals, and nitrate radicals in residential indoor air: magnitudes and impacts of oxidant sources, Atmos. Environ., 2015, 106(3), 382–391 CrossRef CAS PubMed.
- S. F. Kowal, S. R. Allen and T. F. Kahan, Wavelength-resolved photon fluxes of indoor light sources: implications for HOx production, Environ. Sci. Technol., 2017, 51, 10423–10430 CrossRef CAS PubMed.
- J. P. S. Wong, N. Carslaw, R. Zhao, S. Zhou and J. P. D. Abbatt, Observations and impacts of bleach washing on indoor chlorine chemistry, Indoor Air, 2017, 27(6), 1082–1090 CrossRef CAS PubMed.
- K. E. R. Dawe, T. C. Furlani, S. F. Kowal, T. F. Kahan, T. C. Vandenboer and C. J. Young, Formation and emission of hydrogen chloride in indoor air, Indoor Air, 2019, 29, 70–78 CrossRef CAS PubMed.
- C. J. Weschler, Ozone in indoor environments: concentration and chemistry, Indoor Air, 2000, 10(4), 269–288 CrossRef CAS PubMed.
- C. J. Weschler, Changes in indoor pollutants since the 1950s, Atmos. Environ., 2009, 43(1), 153–169 CrossRef CAS.
- C. Yu and D. Crump, Review of the emission of VOCs from polymeric materials used in buildings, Build. Sci., 1998, 33(6), 357–374 Search PubMed.
- T. Salthammer, S. Mentese and R. Marutsky, Formaldehyde in the indoor environment, Chem. Rev., 2010, 110, 2536–2572 CrossRef CAS PubMed.
- E. Hult, H. Willem, P. N. Price, T. Hotchi, M. Russell and B. C. Singer, Formaldehyde and acetaldehyde exposure mitigation in US residences: in-home measurements of ventilation control and source control, Indoor Air, 2015, 25, 523–535 CrossRef CAS PubMed.
- S. Huang, W. Wei, L. B. Weschler, T. Salthammer, H. Kan, Z. Bu and Y. Zhang, Indoor formaldehyde concentrations in urban China: preliminary study of some important influencing factors, Sci. Total Environ., 2017, 590–591, 394–405 CrossRef CAS PubMed.
- H. Destaillats, R. L. Maddalena, B. C. Singer, A. T. Hodgson and T. E. McKone, Indoor pollutants emitted by office equipment: a review of reported data and information needs, Atmos. Environ., 2008, 42(7), 1371–1388 CrossRef CAS.
- C. Stönner, A. Edtbauer and J. Williams, Real-world volatile organic compound emission rates from seated adults and children for use in indoor air studies, Indoor Air, 2018, 28(1), 164–172 CrossRef PubMed.
- P. K. Misztal, D. S. Lymperopoulou, R. I. Adams, R. A. Scott, S. E. Lindow, T. Bruns, J. W. Taylor, J. Uehling, G. Bonito and R. Vilgalys, et al., Emission Factors of Microbial Volatile Organic Compounds from Environmental Bacteria and Fungi, Environ. Sci. Technol., 2018, 52(15), 8272–8282 CrossRef CAS PubMed.
- J. V. Rogers, Y. W. Choi, W. R. Richter, D. C. Rudnicki, D. W. Joseph, C. L. K. Sabourin, M. L. Taylor and J. C. S. Chang, Formaldehyde gas inactivation of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surface materials, J. Appl. Microbiol., 2007, 103(4), 1104–1112 CrossRef CAS PubMed.
- J. V. Rogers, C. L. K. Sabourin, Y. W. Choi, W. R. Richter, D. C. Rudnicki, K. B. Riggs, M. L. Taylor and J. Chang, Decontamination assessment of Bacillus anthracis, Bacillus subtilis, and Geobacillus stearothermophilus spores on indoor surfaces using a hydrogen peroxide gas generator, J. Appl. Microbiol., 2005, 99(4), 739–748 CrossRef CAS PubMed.
- S. C. Weng, W. A. Weaver, M. Zare Afifi, T. N. Blatchley, J. S. Cramer, J. Chen and I. R. Blatchley, Dynamics of gas-phase trichloramine (NCl3) in chlorinated, indoor swimming pool facilities, Indoor Air, 2011, 21(5), 391–399 CrossRef CAS PubMed.
- E. Uhde and N. Schulz, Impact of room fragrance products on indoor air quality, Atmos. Environ., 2015, 106, 492–502 CrossRef CAS.
- S. Zhou, C. J. Young, T. C. Vandenboer, S. F. Kowal and T. F. Kahan, Time-resolved measurements of nitric oxide, nitrogen dioxide, and nitrous acid in an occupied New York home, Environ. Sci. Technol., 2018, 52(15), 8355–8364 CrossRef CAS PubMed.
- M. Brauer, P. Ryan, H. Suh, P. Koutrakis, J. Spengler, N. Leslie and I. Billick, Measurements of nitrous acid inside two research houses, Environ. Sci. Technol., 1990, 24, 1521–1527 CrossRef CAS.
- B. Singer, R. Pass, W. Delp, D. Lorenzetti and R. Maddalena, Pollutant concentrations and emission rates from natural gas cooking burners without and with range hood exhaust in nine California homes, Build. Sci., 2017, 122, 215–229 Search PubMed.
- A. Manoukian, E. Quivet, B. Temime-Roussel, M. Nicolas, F. Maupetit and H. Wortham, Emission characteristics of air pollutants from incense and candle burning in indoor atmospheres, Environ. Sci. Pollut. Res., 2013, 20(7), 4659–4670 CrossRef CAS PubMed.
- W. W. Nazaroff and B. C. Singer, Inhalation of hazardous air pollutants from environmental tobacco smoke in US residences, J. Exposure Sci. Environ. Epidemiol., 2004, 14(suppl. 1), S71–S77 CrossRef CAS PubMed.
- M. Sleiman, J. M. Logue, V. N. Montesinos, M. L. Russell, M. I. Litter, L. A. Gundel and H. Destaillats, Emissions from electronic cigarettes: key parameters affecting the release of harmful chemicals, Environ. Sci. Technol., 2016, 50(17), 9644–9651 CrossRef CAS PubMed.
- N. Britigan, A. Alshawa and S. A. Nizkorodov, Quantification of ozone levels in indoor environments generated by ionization and ozonolysis air purifiers, J. Air Waste Manage. Assoc., 2006, 56(5), 601–610 CrossRef CAS.
- H. Hubbard, B. K. Coleman, G. Sarwar and R. L. Corsi, Effects of an ozone-generating air purifier on indoor secondary particles in three residential dwellings, Atmos. Environ., 2005, 15, 432–444 CAS.
- M. S. Waring and J. A. Siegel, The effect of an ion generator on indoor air quality in a residential room, Indoor Air, 2011, 21(4), 267–276 CrossRef CAS PubMed.
- N. Carslaw, L. Fletcher, D. Heard, T. Ingham and H. Walker, Significant OH production under surface cleaning and air cleaning conditions: impact on indoor air quality, Indoor Air, 2017, 1091–1100 CrossRef CAS PubMed.
- E. Gómez Alvarez, D. Amedro, A. Charbel, S. Gligorovski, C. Schoemacker, C. Fittschen, J. Doussin and H. Wortham, Unexpectedly high indoor hydroxyl radical concentrations associated with nitrous acid, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(33), 13294–13299 CrossRef PubMed.
- A. Gandolfo, V. Gligorovski, V. Bartolomei, S. Tlili, E. Gómez Alvarez, H. Wortham, J. Kleffmann and S. Gligorovski, Spectrally resolved actinic flux and photolysis frequencies of key species within an indoor environment, Build. Sci., 2016, 109, 50–57 Search PubMed.
- S. F. Kowal, S. R. Allen and T. F. Kahan, Wavelength-resolved photon fluxes of indoor light sources: implications for HOx production, Environ. Sci. Technol., 2017, 51(18), 10423–10430 CrossRef CAS PubMed.
- M. Blocquet, F. Guo, M. Mendez, M. Ward, S. Coudert, S. Batut, C. Hecquet, N. Blond, C. Fittschen and C. Schoemaecker, Impact of the spectral and spatial properties of natural light on indoor gas-phase chemistry: experimental and modeling study, Indoor Air, 2018, 28(3), 426–440 CrossRef CAS PubMed.
- B. J. Finlayson-Pitts, L. M. Wingen, A. L. Sumner, D. Syomin and K. A. Ramazan, The heterogeneous hydrolysis of NO2 in laboratory systems and in outdoor and indoor atmospheres: An integrated mechanism, Phys. Chem. Chem. Phys., 2003, 5, 223–242 RSC.
- A. Wisthaler and C. J. Weschler, Reactions of ozone with human skin lipids: Sources of carbonyls, dicarbonyls, and hydroxycarbonyls in indoor air, Proc. Natl. Acad. Sci. U. S. A., 2010, 107(15), 6568–6575 CrossRef CAS PubMed.
- V. Bartolomei, E. Gomez Alvarez, J. Wittmer, S. Tlili, R. Strekowski, B. Temime-Roussel, E. Quivet, H. Wortham, C. Zetzsch and J. Kleffmann, et al., Combustion processes as a source of high levels of indoor hydroxyl radicals through the photolysis of nitrous acid, Environ. Sci. Technol., 2015, 49(11), 6599–6607 CrossRef CAS PubMed.
- J. B. Burkholder, S. P. Sander, J. Abbatt, J. R. Barker, R. E. Huie, C. E. Kolb, M. J. Kurylo, V. L. Orkin, D. M. Wilmouth, P. H. Wine, et al., Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies Evaluation Number 15, JPL Publ 15-10 2006, California, pp. 1–153 Search PubMed.
- J. Stutz, E. S. Kim, U. Platt, P. Bruno, C. Perrino and A. Febo, UV-visible absorption cross sections of nitrous acid, J. Geophys. Res., 2000, 105(D11), 14585–14592 CrossRef CAS.
- R. J. Barnes, A. Sinha and H. A. Michelson, Assessing the contribution of the lowest triplet state to the near-UV absorption spectrum of HOCl, J. Phys. Chem. A, 1998, 102, 8855–8859 CrossRef CAS.
- D. Maric, J. P. Burrows, R. Meller and G. K. Moortgat, A study of the UV-visible absorption spectrum of molecular chlorine, J. Photochem. Photobiol., A, 1993, 70, 205–214 CrossRef CAS.
- S. M. Zhou, M. W. Forbes and J. P. D. Abbatt, Kinetics and products from heterogeneous oxidation of squalene with ozone, Environ. Sci. Technol., 2016, 50, 11688–11697 CrossRef CAS PubMed.
- D. Rim, E. T. Gall, R. L. Maddalena and W. W. Nazaroff, Ozone reaction with interior building materials: influence of diurnal ozone variation, temperature and humidity, Atmos. Environ., 2016, 125, 15–23 CrossRef CAS.
- D. Fu, C. B. Leng, J. Kelley, G. Zeng, Y. H. Zhang and Y. Liu, ATR-IR study of ozone initiated heterogeneous oxidation of squalene in an indoor environment, Environ. Sci. Technol., 2013, 47, 10611–10618 CrossRef CAS PubMed.
- M. Springs, J. R. Wells and G. C. Morrison, Heterogeneous oxidation of squalene film by ozone under various indoor conditions, Indoor Air, 2011, 21, 381–391 CrossRef PubMed.
- L. Petrick and Y. Dubowski, Heterogeneous oxidation of squalene film by ozone under various indoor conditions, Indoor Air, 2009, 19, 319–327 CrossRef PubMed.
- L. S. Pandrangi and G. C. Morrison, Ozone interactions with human hair: Ozone uptake rates and product formation, Atmos. Environ., 2008, 42, 5079–5089 CrossRef CAS.
- B. K. Coleman, H. Destaillats, A. T. Hodgson and W. W. Nazaroff, Ozone consumption and volatile byproduct formation from surface reactions with aircraft cabin materials and clothing fabrics, Atmos. Environ., 2008, 42, 642–654 CrossRef CAS.
- G. C. Morrison and W. W. Nazaroff, The rate of ozone uptake on carpets: Experimental studies, Environ. Sci. Technol., 2000, 34, 4963–4968 CrossRef CAS.
- A. C. Rai, B. Guo, C. H. Lin, J. Zhang, J. Pei and Q. Chen, Ozone reaction with clothing and its initiated VOC emissions in an environmental chamber, Indoor Air, 2014, 24, 49–58 CrossRef CAS PubMed.
- D. Poppendieck, H. Hubbard, M. Ward, C. Weschler and R. Corsi, Ozone reactions with indoor materials during building disinfection, Atmos. Environ., 2007, 41(15), 3166–3176 CrossRef CAS.
- G. C. Morrison, W. W. Nazaroff, J. A. Cano-Ruiz, A. T. Hodgson and M. P. Modera, Indoor air quality impacts of ventilation ducts: ozone removal and emissions of volatile organic compounds, J. Air Waste Manage. Assoc., 1998, 48(10), 941–952 CrossRef CAS.
- O. A. Abbass, D. J. Sailor and E. T. Gall, Effect of fiber material on ozone removal and carbonyl production from carpets, Atmos. Environ., 2017, 148, 42–48 CrossRef CAS.
- D. Rim, E. T. Gall, R. L. Maddalena and W. W. Nazaroff, Ozone reaction with interior building materials: Influence of diurnal ozone variation, temperature and humidity, Atmos. Environ., 2016, 125, 15–23 CrossRef CAS.
- E. T. Gall, R. L. Corsi and J. A. Siegel, Impact of physical properties on ozone removal by several porous materials, Environ. Sci. Technol., 2014, 48(7), 3682–3690 CrossRef CAS PubMed.
- E. Gall, E. Darling, J. A. Siegel, G. C. Morrison and R. L. Corsi, Evaluation of three common green building materials for ozone removal, and primary and secondary emissions of aldehydes, Atmos. Environ., 2013, 77, 910–918 CrossRef CAS.
- E. T. Gall, J. A. Siegel and R. L. Corsi, Modeling ozone removal to indoor materials, including the effects of porosity, pore diameter, and thickness, Environ. Sci. Technol., 2015, 49(7), 4398–4406 CrossRef CAS PubMed.
- N. Oyaro, S. R. Sellevåg and C. J. Nielsen, Study of the OH and Cl-initiated oxidation, IR absorption cross-section, radiative forcing, and global warming potential of four C4-hydrofluoroethers, Environ. Sci. Technol., 2004, 38(21), 5567–5576 CrossRef CAS PubMed.
- C. Cros, G. Morrison, J. Siegel and R. Corsi, Long-term performance of passive materials for removal of ozone from indoor air, Indoor Air, 2012, 22(1), 43–53 CrossRef CAS PubMed.
- H. Schwartz-Narbonne, C. Wang, S. Zhou, J. P. D. Abbatt and J. Faust, Heterogeneous chlorination of squalene and oleic acid, Environ. Sci. Technol., 2018, 53, 1217–1224 CrossRef PubMed.
- A. Gandolfo, L. Rouyer, H. Worthan and S. Gligorovski, The influence of wall temperature on NO2 removal and HONO levels released by indoor photocatalytic paints, Appl. Catal., B, 2017, 209, 429–436 CrossRef CAS.
- A. Gandolfo, V. Bartlomei, E. Gomez Alvarez, S. Tlili, S. Gligorovski, J. Kleffmann and H. Wortham, The effectiveness of indoor photocatalytic paints on NOx and HONO levels, Appl. Catal., B, 2015, 166, 84–90 CrossRef.
- Q. T. Liu, R. Chen, B. E. McCarry, M. L. Diamond and B. Bahavar, Characterization of polar organic compounds in the organic film on indoor and outdoor glass windows, Environ. Sci. Technol., 2003, 37, 2340–2347 CrossRef CAS PubMed.
- C. J. Weschler and W. W. Nazaroff, Growth of organic films on indoor surfaces, Indoor Air, 2017, 1–12 Search PubMed.
- B. Stephens, E. T. Gall and J. A. Siegel, Measuring the penetration of ambient ozone into residential buildings, Environ. Sci. Technol., 2011, 46(2), 929–936 CrossRef PubMed.
- H. Zhao and B. Stephens, A method to measure the ozone penetration factor in residences under infiltration conditions: application in a multifamily apartment unit, Indoor Air, 2016, 26(4), 571–581 CrossRef CAS PubMed.
- M. Hyttinen, P. Pasanen, J. Salo, M. Björkroth, M. Vartiainen and P. Kalliokoski, Reactions of ozone on ventilation filters, Indoor Built Environ., 2003, 12(3), 151–158 CrossRef CAS.
- P. Zhao, J. Siegel and R. Corsi, Ozone removal by HVAC filters, Atmos. Environ., 2007, 41(15), 3151–3160 CrossRef CAS.
- D. B. Collins, R. F. Hems, S. Zhou, C. Wang, E. Grignon, M. Alavy, J. A. Siegel and J. P. D. Abbatt, Evidence for Gas-Surface Equilibrium Control of Indoor Nitrous Acid, Environ. Sci. Technol., 2018, 52(21), 12419–12427 CrossRef CAS PubMed.
- E. Avol, W. Navidi and S. Colome, Modeling ozone levels in and around Southern California homes, Environ. Sci. Technol., 1998, 32(4), 463–468 CrossRef CAS.
- P. N. Breysse, T. J. Buckley, D. Williams, C. M. Beck, S. J. Jo, B. Merriman, S. Kanchanaraksa, L. J. Swartz, K. A. Callahan and A. M. Butz, et al., Indoor exposures to air pollutants and allergens in the homes of asthmatic children in inner-city Baltimore, Environ. Res., 2005, 98, 167–176 CrossRef CAS PubMed.
- G. B. Diette, N. N. Hansel, T. J. Buckley, J. Curtin-Brosnan, P. A. Eggleston, E. C. Matsui, M. C. McCormack, D. L. Williams and P. N. Breysse, Home indoor pollutant exposures among inner-city children with and without asthma, Environ. Health Perspect., 2007, 115, 1665–1669 CrossRef PubMed.
- M.-E. Héroux, N. Clark, K. Van Ryswyk, R. Mallick, N. L. Gilbert, I. Harrison, K. Rispler, D. Wang, A. Anastassopoulos and M. Guay, et al., Home indoor pollutant exposures among inner-city children with and without asthma, Int. J. Environ. Res. Public Health, 2010, 7(8), 3080–3099 CrossRef PubMed.
- K. Lee, W. J. Parkhurst, J. P. Xue, A. H. Ozkaynak, D. Neuberg and J. D. Spengler, Outdoor/indoor/personal ozone exposures of children in Nashville, Tennessee, J. Air Waste Manage. Assoc., 2004, 54, 352–359 CrossRef CAS.
- K. Lee, J. Xue, A. S. Geyh, H. Özkaynak, B. P. Leaderer, C. J. Weschler and J. D. Spengler, Nitrous acid, nitrogen dioxide, and ozone concentrations in residential environments, Environ. Health Perspect., 2002, 110(2), 145–149 CrossRef CAS PubMed.
- E. Simons, J. Curtin-Brosnan, T. Buckley, P. Breysse and P. A. Eggleston, Indoor environmental differences between inner city and suburban homes of children with asthma, J. Urban Health, 2007, 84, 577–590 CrossRef PubMed.
- J. Zhang and P. Lioy, Ozone in residential air - concentrations, I/O ratios, indoor chemistry, and exposures, Indoor Air, 1994, 4, 95–105 CrossRef CAS.
- C. Arata, K. J. Zarzana, P. K. Misztal, Y. Liu, S. S. Brown, W. W. Nazaroff and A. H. Goldstein, Measurement of NO3 and N2O5 in a residential kitchen, Environ. Sci. Technol. Lett., 2018, 3, 6–10 Search PubMed.
- C. Weschler and H. C. Shields, Indoor chemistry involving O3, NO, and NO2 as evidence by 14 months of measurements at a site in Southern California, Environ. Sci. Technol., 1994, 28, 2120–2132 CrossRef CAS PubMed.
- K. W. Leovic, L. S. Sheldon, D. A. Whitaker, R. G. Hetes, J. A. Calcagni and J. N. Baskir, Measurement of indoor air emissions from dry-process photocopy machines, J. Air Waste Manage. Assoc., 1996, 46(9), 821–829 CrossRef CAS PubMed.
- C. J. Weschler, Ozone in indoor environments: concentration and chemistry, Indoor Air, 2000, 10(4), 269–288 CrossRef CAS PubMed.
- M. A. Sidheswaran, H. Destaillats, D. P. Sullivan, S. Cohn and W. J. Fisk, Energy efficient indoor VOC air cleaning with activated carbon fiber (ACF) filters, Build. Sci., 2012, 47, 357–367 Search PubMed.
- J. R. Aldred, E. Darling, G. C. Morrison, J. Siegel and R. Corsi, Benefit-cost analysis of commercially available activated carbon filters for indoor ozone removal in single-family homes, Indoor Air, 2016, 26(3), 501–512 CrossRef CAS PubMed.
- B. Alicke, U. Platt and J. Stutz, Impact of nitrous acid photolysis on the total hydroxyl radical budget during the Limitation of Oxidant Production/Pianura Padana Produzione di Ozono study in Milan, J. Geophys. Res., 2002, 107(D22), 8196, DOI:10.1029/2000JD000075.
- R. Volkamer, P. Sheehy, L. T. Molina and M. J. Molina, Oxidative capacity of the Mexico City atmosphere - Part 1: A radical source perspective, Atmos. Chem. Phys., 2010, 10(14), 6969–6991 CrossRef CAS.
- C. J. Young, R. A. Washenfelder, J. M. Roberts, L. H. Mielke, H. D. Osthoff, C. Tsai, O. Pikelnaya, J. Stutz, P. R. Veres and A. K. Cochran, et al., Vertically resolved measurements of nighttime radical reservoirs in Los Angeles and their contribution to the urban radical budget, Environ. Sci. Technol., 2012, 46, 10965–10973 CrossRef CAS PubMed.
- S. Kim, T. C. VandenBoer, C. J. Young, T. P. Riedel, J. A. Thornton, B. Swarthout, B. Sive, B. Lerner, J. B. Gilman and C. Warneke, et al., The primary and recycling sources of OH during the NACHTT-2011 campaign: HONO as an important OH primary source in the wintertime, J. Geophys. Res.: Atmos., 2014, 119, 6886–6896 CAS.
- P. M. Edwards, C. J. Young, K. Aikin, J. DeGouw, W. P. Dubé, F. Geiger, J. Gilman, D. Helmig, J. S. Holloway and J. Kercher, et al., Ozone photochemistry in an oil and natural gas extraction region during winter: simulations of a snow-free season in the Uintah Basin, Utah, Atmos. Chem. Phys., 2013, 13, 8955–8971 CrossRef.
- C. J. Weschler and H. C. Shields, Production of the hydroxyl radical in indoor air, Environ. Sci. Technol., 1996, 30(11), 3250–3258 CrossRef CAS.
- T. Salthammer, Very volatile organic compounds: an understudied class of indoor air pollutants, Indoor Air, 2016, 26, 25–38 CrossRef CAS PubMed.
- K. Lee, J. Xue, A. S. Geyh, H. Ozkaynak, B. P. Leaderer, C. J. Weschler and J. D. Spengler, Nitrous acid, nitrogen dioxide, and ozone concentrations in residential environments, Environ. Health Perspect., 2002, 110, 145–150 CrossRef CAS PubMed.
- C. J. Weschler and H. C. Shields, Measurements of the hydroxyl radical in a manipulated but realistic indoor environment, Environ. Sci. Technol., 1997, 31(12), 3719–3722 CrossRef CAS.
- S. S. Brown, W. P. Dube, H. D. Osthoff, D. E. Wolfe, W. M. Angevine and A. R. Ravishankara, High resolution vertical distributions of NO3 and N2O5 through the nocturnal boundary layer, Atmos. Chem. Phys., 2007, 7(1), 139–149 CrossRef CAS.
- B. Ghosh, D. K. Papanastasiou, R. K. Talukdar, J. M. Roberts and J. B. Burkholder, Nitryl chloride (ClNO2): UV/vis absorption spectrum between 210 and 296 K and O(3P) quantum yield at 193 and 248 nm, J. Phys. Chem. A, 2012, 116(24), 5796–5805 CrossRef CAS PubMed.
- T. Clark and M. Clyne, Kinetic mechanisms in nitrogen-chlorine systems. Part 1. - The formation and detection of NCl2 and N3 free radicals using time-resolved absorption spectrophotometry, Trans. Faraday Soc., 1969, 65, 2994–3004 RSC.
- A. Briggs and R. Norrish, The decomposition of nitrogen trichloride photosensitized by chlorine, Proc. R. Soc. London, 1964, 264(1372), 27–34 Search PubMed.
- M. Murphy, J. Lando, S. Kieszak, M. Sutter, G. Noonan, J. Brunkard and M. McGeehin, Formaldehyde levels in FEMA-supplied travel trailers, park models, and mobile homes in Louisiana and Mississippi, Indoor Air, 2013, 23, 134–141 CrossRef CAS PubMed.
- R. E. Militello-Hourigan and S. L. Miller, The impacts of cooking and an assessment of indoor air quality in Colorado passive and tightly constructed homes, Build. Sci., 2018, 144(June), 573–582 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |




