Gold redox catalysis for cyclization/arylation of allylic oximes: synthesis of isoxazoline derivatives†
Abiola Azeez
Jimoh
a,
Seyedmorteza
Hosseyni
a,
Xiaohan
Ye
a,
Lukasz
Wojtas
a,
Yong
Hu
*b and
Xiaodong
Shi
 *a
*a
aDepartment of Chemistry, University of South Florida, Tampa, FL 33620, USA. E-mail: xmshi@usf.edu
bDepartment of Neonatology, Shanghai Children's Hospital, Shanghai Jiao Tong University, Shanghai, Shanghai 200040, China
First published on 18th June 2019
Abstract
Base-assisted diazonium activation has been employed to promote gold(I)/(III) redox catalysis toward allylic oxime cyclization/aryl coupling. Functional isoxazolines were prepared with good to excellent yields, while the alternative photoactivation method provided trace amounts of the isoxazoline products. This study further broadens the scope of gold redox chemistry.
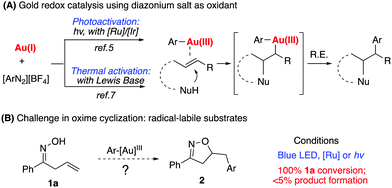
Homogeneous gold catalysis has been successfully applied in the areas of alkyne, allene, and alkene activation for effective C–C and C–heteroatom bond formation.1 However, due to the challenge associated with a high oxidation potential of gold, gold redox catalysis has not been well explored until recently.2 Typically, strong oxidants such as PIDA or selectfluor have been used in oxidizing Au(I) to Au(III).3 The discovery of diazonium salts as oxidizing agents for gold redox catalysis has recently stimulated the interest of many researchers.4 As initially reported by Glorius in 2013, followed by Toste, Shin, Alcaide, Hashimi, and others, photoactivation of diazonium salts leads to the oxidation of Au(I) to Ar–Au(III) intermediates, which promotes sequential C–C multiple bond activation followed by reductive elimination (Scheme 1A).5 It is generally believed that the resulting aryl radical from photoactivated diazonium salts initiates gold oxidation.6 These seminal studies significantly advanced gold catalysis research since they offered effective reactive species (aryl diazonium) as oxidants in promoting the gold-redox cycle.†
Our group recently reported a Lewis base-promoted gold oxidation process using diazonium salts.7 In these reports, inorganic bases were found to be adequate to facilitate diazonium salt activation under thermal conditions (40–60 °C). Herein, we report the successful cyclization of allylic oximes for the preparation of isoxazoline derivatives under mild reaction conditions. On the other hand, allylic oximes were found to undergo significant decomposition under photoconditions, giving trace amounts of the desired product.
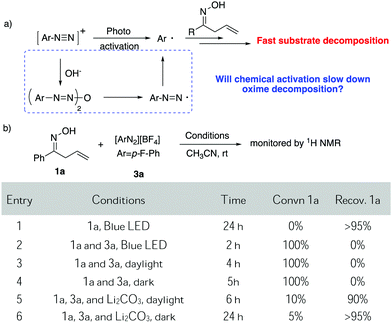
Due to the strong interest in developing a new strategy for heterocycle synthesis under mild conditions, we were curious about the possibility of using the gold-redox strategy for constructing the isoxazoline motif through an allylic oxime cyclization method (Scheme 1B). Notably, isoxazolines and their derivatives are a versatile class of compounds that can be found in numerous natural products and biologically active compounds.8 However, due to the poor substrate stability toward transition metal catalysts, there are only limited successful examples reported regarding oxime cyclization.9 In 2010, Loh's group reported the first example of Pd-catalyzed allylic oxime cyclization for the dioxygenation of an alkene (O2 as both oxidant and the second hydroxyl source).10 Later, Chen's group reported Pd catalyzed allylic oxime cyclization with aryl halides.11 More recently, Zhu and coworkers reported the Pd-catalyzed homoallylic oxime cyclization for the synthesis of 5-methylisoxazoles.12 Unlike Pd catalysis, gold redox chemistry with aryldiazonium salts does not undergo oxidative addition through an aryl halide pathway. Therefore, gold redox catalysis will offer greater orthogonal functional group tolerance compared to traditional Pd catalysis. One general concern is whether oxime could survive under gold redox conditions with radical labile N–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds. In fact, when treating allyl oxime 1a with diazonium 3a under photocatalytic conditions, no desired product 2a was observed although 1a was completely consumed within two hours. With an interest in exploring this useful transformation with gold-redox catalysis, we further evaluated the reactivity of 1a in the presence of diazonium salt 3a. The results are summarized in Fig. 1.
N bonds. In fact, when treating allyl oxime 1a with diazonium 3a under photocatalytic conditions, no desired product 2a was observed although 1a was completely consumed within two hours. With an interest in exploring this useful transformation with gold-redox catalysis, we further evaluated the reactivity of 1a in the presence of diazonium salt 3a. The results are summarized in Fig. 1.
Although with reactive N–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, oxime 1a showed good stability while exposed to light, giving less than 5% decomposition under blue LED initiation. However, upon treatment with diazonium salt 3a (1
C bonds, oxime 1a showed good stability while exposed to light, giving less than 5% decomposition under blue LED initiation. However, upon treatment with diazonium salt 3a (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1a and 3a), rapid decomposition occurred at room temperature and 100% of 1a was consumed with no clear identifiable products observed when exposed to either blue LED or daylight. Slower decomposition was observed (100% decomposition, 5 h) when treating the mixture under darkness. Notably, 19F NMR studies showed that most of the diazonium salt was consumed (entries 1–4). Unfortunately, these results ruled out the possibility of applying diazonium salt in gold redox catalysis under photo-activation conditions.
1 mixture of 1a and 3a), rapid decomposition occurred at room temperature and 100% of 1a was consumed with no clear identifiable products observed when exposed to either blue LED or daylight. Slower decomposition was observed (100% decomposition, 5 h) when treating the mixture under darkness. Notably, 19F NMR studies showed that most of the diazonium salt was consumed (entries 1–4). Unfortunately, these results ruled out the possibility of applying diazonium salt in gold redox catalysis under photo-activation conditions.
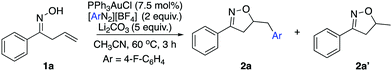
As discussed above, our group had initiated a chemical activation approach as an alternative for diazonium salt promoted gold redox catalysis. We argued that a base will assist the in situ N2 extrusion process to promote the oxidation of Au(I) to Au(III)–Ar, thereby, slowing down the radical generation which decomposes the oxime.7,13 With this in mind, we found that addition of 2 eq. Li2CO3, did slow down 1a decomposition: 10% conversion of 1a in 6 h under daylight (entry 5). Meanwhile, 19F NMR studies showed that only a little amount of diazonium salt was consumed in the presence of Li2CO3. This further suggests that Li2CO3 could potentially prevent the diazonium salt from decomposition to the aryl radical. Encouraged by this result, we evaluated the proposed cyclization–arylation under various conditions, including the choices of catalysts, solvents, bases, and reaction temperatures. Eventually, 7.5% PPh3AuCl, 5 eq. Li2CO3 in CH3CN as a solvent was identified as the optimal conditions, giving the desired product 2a in 78% NMR yield (74% isolated yield). Comparison of various reaction conditions is summarized in Table 1.
| Entry | Reaction conditions varies from standarda | Convn 1a (%) | Yieldb2a (%) | Yieldb2a′ (%) |
|---|---|---|---|---|
| a General reaction conditions: 1a (0.33 mmol), [Au] (0.025 mmol) diazonium salt (0.66 mmol) and base (1.65 mmol) in CH3CN (0.4 M), 60 °C. b Yield was based on 1HNMR with 1,3,5-trimethoxybenzene as an internal standard. c Solvents were degassed using the freeze–pump–thaw method. | ||||
| 1 | None | 100 | 78 | 0 |
| 2 | No [Au] | 0 | — | — |
| 3 | No [ArN2][BF4] | 18 | — | — |
| 4 | No base | 100 | Trace | — |
| 5 | NaHCO3 2 eq. | 95 | 58 | 0 |
| 6 | Li2CO3 2 eq. | 100 | 74 | 0 |
| 7 | 5% PPh3AuCl | 90 | 51 | 0 |
| 8 | 5 eq. [ArN2][BF4] | 100 | 65 | — |
| 9c | No [Au], UVA or Blue LED, degassed MeOH | 100 | 0 | 0 |
| 10c | 10% PPh3AuCl, degassed MeOH or MeCN, 12 W blue LEDs, RT | 100 | <5 | 0 |
| 11c | 10% PPh3AuCl, 2.5% [Ru(bpy)3](PF6)2 degassed MeOH or MeCN, 12 W blue LEDs, RT | 100 | <5 | 0 |
As shown above, the gold catalyst was found to be crucial for the allylic oxime cyclization since no conversion of the substrate was observed without gold (entry 2). Conducting the reaction in the absence of a base gave complete conversion with no appreciable amount of the desired product. Li2CO3 (5 eq.) was identified as the optimal choice for this transformation (entries 5 and 6). Notably, although crude 1H NMR of the reaction mixture indicated a very ‘clean’ reaction, the overall yield was around 75%. Meanwhile, the addition of excess diazonium salt was found to lead to lower yield (entry 9). Also, acetonitrile was the only solvent suitable for this transformation; switching to other solvents like 1,2-dichloroethane or toluene gave no desired product. This is due to the poor solubility of the diazonium salt in these solvents, even at increased temperature. To emphasize the uniqueness of our chemical activation method, photochemical conditions were used for the cyclization in the presence of diazonium salt. The substrate readily decomposed in the presence of diazonium salts under this method. The standard photoactivation conditions using gold and [Ru(bpy)3](PF6)2 in degassed methanol did not provide a significant amount of desired isoxazoline as confirmed by 1H NMR analysis of the crude reaction mixture. This result suggests that the photoactivation condition is unsuitable for the oxime substrates in the presence of diazonium salt. With the optimal reaction conditions in hand, we applied it to a series of allylic oximes (Scheme 2).
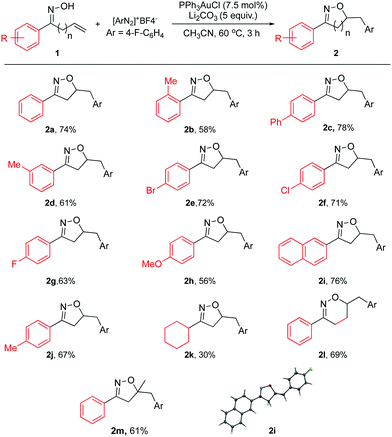 | ||
| Scheme 2 Substrate scope for allylic oxime. General reaction conditions: 1a (0.33 mmol), [Au] (0.025 mmol), diazonium salt (0.66 mmol), and base (1.65 mmol) in CH3CN (0.4 M), 60 °C; isolated yield. | ||
As shown in Scheme 2, both aromatic and aliphatic substrates were evaluated for the reaction. Good yields and high reaction efficiency could be observed when aryl groups were used (2a–2j). The presence of electron withdrawing groups appeared to be more favorable with respect to the isolated yield (2e–2g). Also, the naphthyl group afforded a good yield (2i). In the case of electron donating substituent 2h, a modest yield was achieved. Compared to 2j, the more sterically hindered 2b provided a lower yield, suggesting that sterics played a role. However, the aliphatic substrate gave a much lower yield for this reaction (2k). Also, the homoallylic oxime was found to be viable for this reaction giving a good yield of 5,6-dihydro-4H-1,2-oxazine derivative (2l). This is a significant step towards broadening the scope of this reaction; owing to the fact that oxazines are also known to be a highly desirable class of heterocycles. The 7 or 8 membered-ring analogs could not be observed under the reported reaction conditions.
Furthermore, a series of diazonium salts were investigated (Scheme 3); electron poor aryl diazonium salts could also be enlisted, giving the desired products in high yields (4a–h). The iodo-substituted diazonium salt 4e was also successfully applied with modest yield. This result highlights the orthogonal reactivity under gold catalysis, compared with previously reported Pd catalysis conditions. However, electron rich diazonium salts failed to give the desired product due to the instability of the diazonium species.
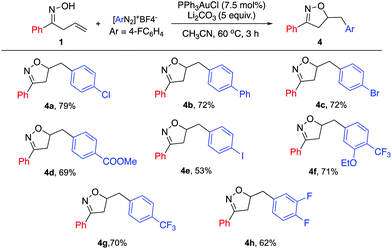 | ||
| Scheme 3 Substrate scope for diazonium salt. General reaction conditions: 1a (0.33 mmol), [Au] (0.025 mmol), diazonium salt (0.66 mmol), and base (1.65 mmol) in CH3CN (0.4 M), 60 °C; isolated yield. | ||
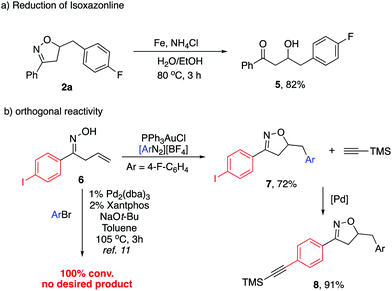
Although the substituted isoxazolines are in themselves useful compounds, we carried out their reduction to the corresponding hydroxyl ketones (Scheme 4a). Isoxazoline 2a was subjected to reductive N–O bond cleavage followed by subsequent hydrolysis of the resulting imine. This reductive ring opening was achieved with up to 82% yield. To further demonstrate the synthetic utility, we synthesized the p-iodo substituted aromatic substrate 6, which was further subjected to our reaction conditions. The desired isoxazoline 7 was obtained with 72% yield (Scheme 4b). Notably, when treating 6 under previously reported Pd-catalyzed conditions, no desired product was observed with 100% conversion. The fast oxidative addition of the C–I bond led to the side reaction pathway. This result clearly indicated the orthogonal catalytic reactivity between Pd and Au. The following Sonogashira reaction of 7 afforded alkyne substituted aromatic isoxazoline 8, allowing an extended synthetic handle.
In conclusion, gold-redox chemistry has been applied for the cyclization/functionalization of allylic oximes in the presence of aryl diazonium salts. This transformation gives access to aryl functionalized 2-isoxazolines in good to excellent yields. Moreover, we believe that the apparent compatibility of Au redox chemistry with oximes will provide an alternate route towards the synthesis of isoxazoline derivatives.
We are grateful to the NSF (CHE-1665122), NIH (1R01GM120240-01) and NSFC (21629201) for financial support.
Conflicts of interest
There are no conflicts to declare.References
-
(a) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef PubMed
; (b) R. A. Widenhoefer and X. Q. Han, Eur. J. Org. Chem., 2006, 4555 CrossRef CAS
; (c) D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395 CrossRef CAS PubMed
; (d) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS PubMed
; (e) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232 CrossRef CAS PubMed
; (f) S. Sengupta and X. D. Shi, ChemCatChem, 2010, 2, 609 CrossRef CAS
; (g) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954 CrossRef CAS PubMed
; (h) H. A. Wegner and M. Auzias, Angew. Chem., Int. Ed., 2011, 50, 8236 CrossRef CAS PubMed
; (i) A. S. K. Hashmi, Acc. Chem. Res., 2014, 47, 864 CrossRef CAS PubMed
; (j) Y. M. Wang, A. D. Lackner and F. D. Toste, Acc. Chem. Res., 2014, 47, 889 CrossRef CAS PubMed
; (k) L. M. Zhang, Acc. Chem. Res., 2014, 47, 877 CrossRef CAS PubMed
; (l) M. Q. Jia and M. Bandini, ACS Catal., 2015, 5, 1638 CrossRef CAS
; (m) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028 CrossRef CAS PubMed
; (n) A. M. Asiri and A. S. K. Hashmi, Chem. Soc. Rev., 2016, 45, 4471 RSC
.
- S. G. Bratsch, J. Phys. Chem. Ref. Data, 1989, 18, 1 CrossRef CAS
.
- For review, see:
(a) M. N. Hopkinson, A. D. Gee and V. Gouverneur, Chem. – Eur. J., 2011, 17, 8248 CrossRef CAS PubMed
; (b) M. Joost, A. Amgoune and D. Bourissou, Angew. Chem., Int. Ed., 2015, 54, 15022 CrossRef CAS PubMed
; (c) J. Miró and C. del Pozo, Chem. Rev., 2016, 116, 11924 CrossRef PubMed
; (d) F. Silva, A. F. Tierno and S. E. Wengryniuk, Molecules, 2017, 22 Search PubMed
. For selected examples, see: ; (e) G. Zhang, Y. Peng, L. Cui and L. Zhang, Angew. Chem., Int. Ed., 2009, 48, 3112 CrossRef CAS PubMed
; (f) E. Tkatchouk, N. P. Mankad, D. Benitez, W. A. Goddard and F. D. Toste, J. Am. Chem. Soc., 2011, 133, 14293 CrossRef CAS PubMed
; (g) A. Leyva-Perez, A. Domenech, S. I. Al-Resayes and A. Corma, ACS Catal., 2012, 2, 121 CrossRef CAS
; (h) H. Peng, Y. Xi, N. Ronaghi, B. Dong, N. G. Akhmedov and X. Shi, J. Am. Chem. Soc., 2014, 136, 13174 CrossRef CAS PubMed
; (i) M. D. Levin and F. D. Toste, Angew. Chem., Int. Ed., 2014, 53, 6211 CrossRef CAS PubMed
; (j) M. Hofer, A. Genoux, R. Kumar and C. Nevado, Angew. Chem., Int. Ed., 2017, 56, 1021 CrossRef CAS PubMed
; (k) J. Wang, S. Zhang, C. Xu, L. Wojtas, N. G. Akhmedov, H. Chen and X. Shi, Angew. Chem., Int. Ed., 2018, 57, 6915 CrossRef CAS PubMed
.
- For review, see:
(a) M. N. Hopkinson, A. Tlahuext-Aca and F. Glorius, Acc. Chem. Res., 2016, 49, 2261 CrossRef CAS PubMed
; (b) M. O. Akram, S. Banerjee, S. S. Saswade, V. Bedi and N. T. Patil, Chem. Commun., 2018, 54, 11069 RSC
.
- For selected examples see:
(a) B. Sahoo, M. N. Hopkinson and F. Glorius, J. Am. Chem. Soc., 2013, 135, 5505 CrossRef CAS PubMed
; (b) A. Tlahuext-Aca, M. N. Hopkinson, B. Sahoo and F. Glorius, Chem. Sci., 2016, 7, 89 RSC
; (c) X. Z. Shu, M. Zhang, Y. He, H. Frei and F. D. Toste, J. Am. Chem. Soc., 2014, 136, 5844 CrossRef CAS PubMed
; (d) H. Kawai, W. J. Wolf, A. G. DiPasquale, M. S. Winston and F. D. Toste, J. Am. Chem. Soc., 2016, 138, 587 CrossRef CAS PubMed
; (e) S. Kim, J. Rojas-Martin and F. D. Toste, Chem. Sci., 2016, 7, 85 RSC
; (f) L. Huang, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 4808 CrossRef CAS PubMed
; (g) J. Xie, K. Sekine, S. Witzel, P. Krämer, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2018, 57, 16648 CrossRef CAS PubMed
; (h) D. V. Patil, H. Yun and S. Shin, Adv. Synth. Catal., 2015, 357, 2622 CrossRef CAS
; (i) T. Cornilleau, P. Hermange and E. Fouquet, Chem. Commun., 2016, 52, 10040 RSC
; (j) C. H. Qu, S. L. Zhang, H. B. Du and C. J. Zhu, Chem. Commun., 2016, 52, 14400 RSC
; (k) J. Um, H. Yun and S. Shin, Org. Lett., 2016, 18, 484 CrossRef CAS PubMed
; (l) M. O. Akram, P. S. Mali and N. T. Patil, Org. Lett., 2017, 19, 3075 CrossRef CAS PubMed
; (m) V. Gauchot, D. R. Sutherland and A. L. Lee, Chem. Sci., 2017, 8, 2885 RSC
; (n) B. Alcaide, P. Almendros, E. Busto and A. Luna, Adv. Synth. Catal., 2016, 358, 1526 CrossRef CAS
; (o) B. Alcaide, P. Almendros, E. Busto and C. Lázaro-Milla, J. Org. Chem., 2017, 82, 2177 CrossRef CAS PubMed
; (p) B. Alcaide, P. Almendros, B. Aparicio, C. Lázaro-Milla, A. Luna and O. N. Faza, Adv. Synth. Catal., 2017, 359, 2789 CrossRef CAS
.
- L. Huang, F. Rominger, M. Rudolph and A. S. K. Hashmi, Chem. Commun., 2016, 52, 6435 RSC
.
-
(a) R. Cai, M. Lu, E. Y. Aguilera, Y. Xi, N. G. Akhmedov, J. L. Petersen, H. Chen and X. Shi, Angew. Chem., Int. Ed., 2015, 54, 8772 CrossRef CAS PubMed
; (b) H. Peng, R. Cai, C. Xu, H. Chen and X. Shi, Chem. Sci., 2016, 7, 6190 RSC
; (c) B. Dong, H. Peng, S. E. Motika and X. Shi, Chem. – Eur. J., 2017, 23, 11093 CrossRef CAS PubMed
.
-
(a) A. Pohjakallio, P. M. Pihko and U. M. Laitinen, Chem. – Eur. J., 2010, 16, 11325 CrossRef CAS PubMed
; (b) Y. Landais, F. Robert, E. Godineau, L. Huet and N. Likhite, Tetrahedron, 2013, 69, 10073 CrossRef CAS
; (c) M. Gassel, C. Wolf, S. Noack, H. Williams and T. Ilg, Insect Biochem. Mol. Biol., 2014, 45, 111 CrossRef CAS PubMed
; (d) W. L. Shoop, E. J. Hartline, B. R. Gould, M. E. Waddell, R. G. McDowell, J. B. Kinney, G. P. Lahm, J. K. Long, M. Xu, T. Wagerle, G. S. Jones, R. F. Dietrich, D. Cordova, M. E. Schroeder, D. F. Rhoades, E. A. Benner and P. N. Confalone, Vet. Parasitol., 2014, 201, 179 CrossRef CAS PubMed
; (e) T. Ismail, S. Shafi, S. Swam, T. Sidiq, A. Khajuria, A. Rouf, M. Yadav, V. Saikam, P. P. Singh, M. S. Alam, N. Islam, K. Sharma and H. M. S. Kumar, Eur. J. Med. Chem., 2016, 123, 90 CrossRef CAS PubMed
; (f) N. Puttaswamy, G. S. P. Kumar, M. Al-Ghorbani, V. Vigneshwaran, B. T. Prabhakar and S. A. Khanum, Eur. J. Med. Chem., 2016, 114, 153 CrossRef CAS PubMed
; (g) H. Ma, V. N. Stone, H. Q. Wang, G. E. Kellogg, P. Xu and Y. Zhang, Bioorg. Med. Chem. Lett., 2017, 27, 3840 CrossRef CAS PubMed
; (h) S. W. Park, S. H. Kim, J. Song, G. Y. Park, D. Kim, T. G. Nam and K. B. Hong, Beilstein J. Org. Chem., 2018, 14, 1028 CrossRef CAS PubMed
; (i) S. S. Prasad and S. Baskaran, J. Org. Chem., 2018, 83, 1558 CrossRef CAS PubMed
.
- For selected examples, see:
(a) H. O. House, D. T. Manning, D. G. Melillo, L. F. Lee, O. R. Haynes and B. E. Wilkes, J. Org. Chem., 1976, 41, 855 CrossRef CAS
; (b) R. W. Bates and K. Sa-Ei, Org. Lett., 2002, 4, 4225 CrossRef CAS PubMed
; (c) K. Moriyama, Y. Izumisawa and H. Togo, J. Org. Chem., 2011, 76, 7249 CrossRef CAS PubMed
; (d) W. Zhang, Y. Su, K.-H. Wang, L. Wu, B. Chang, Y. Shi, D. Huang and Y. Hu, Org. Lett., 2017, 19, 37 Search PubMed
.
- M.-K. Zhu, J.-F. Zhao and T.-P. Loh, J. Am. Chem. Soc., 2010, 132, 6284 CrossRef CAS PubMed
.
- D. Jiang, J. Peng and Y. Chen, Org. Lett., 2008, 10, 1695 CrossRef CAS PubMed
.
- K.-Y. Dong, H.-T. Qin, X.-X. Bao, F. Liu and C. Zhu, Org. Lett., 2014, 16, 5266 CrossRef CAS PubMed
.
-
(a) S. Kindt, K. Wicht and M. R. Heinrich, Org. Lett., 2015, 17, 6122 CrossRef CAS PubMed
; (b) Z. Xia and Q. Zhu, Org. Lett., 2013, 15, 4110 CrossRef CAS PubMed
; (c) M. Hartmann, Y. Li and A. Studer, J. Am. Chem. Soc., 2012, 134, 16516 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data of new compounds. CCDC 1882657. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9cc02830g |
| This journal is © The Royal Society of Chemistry 2019 |