Open Access Article
Open Access ArticleMetallo-supramolecular polymers derived from benzothiadiazole-based platinum acetylide complexes for fluorescent security application†
Ming Yuanac,
Feng Wang a and
Yu-Kui Tian
a and
Yu-Kui Tian *b
*b
aCAS Key Laboratory of Soft Matter Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei 230026, P. R. China
bDepartment of Chemistry, Tianjin University, Tianjin 300354, P. R. China. E-mail: yukui.tian@tju.edu.cn; Tel: +86 22 27403475
cClinic Medical College of Anhui Medical University, Hefei 230012, P. R. China
First published on 5th December 2018
Abstract
Metallo-supramolecular polymers with the incorporation of benzothiadiazole-substituted organoplatinum moiety have been successfully constructed. The designed monomer displays intense fluorescence signals, which are severely quenched upon the supramolecular polymerization process. On–off switching of fluorescence can be further exploited for data security materials in response to the chemical stimuli. Accordingly, the resulting supramolecular polymers can be regarded as a novel and efficient candidate toward information processing applications.
Metallo-supramolecular polymers (MSPs), which represent polymeric assemblies constructed by reversible metal–ligand coordination, are considered as an important class of organic/inorganic hybrid supramolecular materials. Owing to the incorporation of metallic complexes in the polymer chain, these macromolecular metal-containing systems not only possess the properties of traditional organic polymers (viscosity, processability, etc.), but also exhibit redox, optical, electrochromic, catalytic and magnetic properties.1–5 In addition, due to the incorporation of reversible and weak recognition moieties on the supramolecular polymeric backbones, MSPs show unique stimuli-responsive characters, which is essential to develop environment-adaptable materials.6–9
The structure of MSPs and their physical properties can be elaborately regulated by the coordination ligand motifs.10,11 Terpyridines and their structural analogs are the most popular chelating end-groups to form MSPs, ascribed to their capability to complex with a variety of transition metals.10,12–16 Furthermore, the linkage on the polytopic ligand is also of crucial importance, since it dictates the structure arrangement and physicochemical properties of the targeted MSPs assemblies.10 Up to now, a variety of π-conjugated organic chromophores have been incorporated into the backbone of MSPs.17–22 In stark contrast, the employment of π-conjugated organometallic units as the linkages has been far-less exploited.23
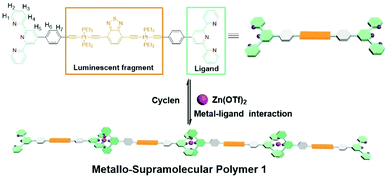
In this work, we sought to attain this objective, and construct a new type of MSPs with the involvement of platinum acetylide linkage. The monomeric structure is showed in Scheme 1: terpyridine moieties were incorporated on both sides of the monomer, in which the rigid benzothiadiazole-functionalized dinuclear platinum(II) acetylide moiety serves as the linkage unit. Herein, the platinum(II) acetylide derivatives were chosen as the linkages based on the following two considerations. First, platinum(II) acetylide unit features the intriguing photo-physical properties such as larger stokes shifts and higher photoluminescence quantum yields, which are primarily arised from the overlapping of d-orbitals of the transition metal with p-orbitals of the alkyne ligands.24–26 As a result, it endows the resulting supramolecular polymers with the fascinating optical and electrochromic properties.27–31 Second, the rigid linkage incorporated in the monomer structure restrains the tendency for cyclization. The reduced critical polymerization concentration (CPC) value promotes the linear supramolecular polymerization process.32–35 In the meantime, due to the dynamic properties of metal–ligand interactions, the resulting supramolecular polymer was anticipated to possess the stimuli-responsive properties, which display the potential applications as the smart materials.36–39
The synthetic route for monomer is quite straightforward. As shown in Scheme S1,† Sonogashira coupling reaction between terpyridine and benzothiadiazole-functionalized dinuclear platinum(II) acetylide moieties was employed as the key step to construct the designed monomer. All of the synthetic compounds were fully characterized with NMR and ESI-MS spectra (Fig. S1–S4, ESI†). The introduction of dinuclear platinum(II) acetylide unit is expected to achieve low critical polymerization concentration (CPC) value for the supramolecular polymerization process. As a consequence, it facilitates to fabricate fully rigid supramolecular polymers with intriguing optical and electrochromic properties.
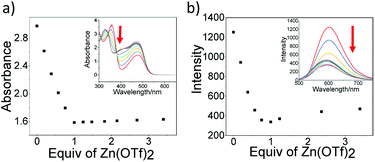
Non-covalent complexation between the terpyridine-contained monomer and metal ion Zn2+ was first investigated via the spectroscopic measurements. For the monomer itself, it exhibits two absorption bands with the maximum wavelength located at 358 nm and 477 nm, respectively (Fig. 1a). The high-energy absorption at 358 nm is mainly derived from the π–π* intraligand transitions. For the low-energy absorptions at 477 nm, it could be ascribed to the interplay between the π-conjugated benzothiadiazole acetylene ligand and the transition metal Pt2+.28 With the gradual addition of Zn2+ to the monomer in CHCl3/CH3OH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), an obvious decrease absorbance at 370 nm was observed (Fig. 1a), indicating the transformation from free terpyridine species to the metal–terpyridine complex.38 Upon excitation of monomer at 420 nm, the relative quantum yield is determined to be 3.5%, while the emission lifetime is 1.3 ns (Fig. S7†). As shown in Fig. 1b, with the stepwise addition of the Zn2+, the fluorescence of monomer gradually decreases, and reaches the minimum at 602 nm when the ratio of monomer/Zn2+ achieves to 1.0. Also, the 1
1, v/v), an obvious decrease absorbance at 370 nm was observed (Fig. 1a), indicating the transformation from free terpyridine species to the metal–terpyridine complex.38 Upon excitation of monomer at 420 nm, the relative quantum yield is determined to be 3.5%, while the emission lifetime is 1.3 ns (Fig. S7†). As shown in Fig. 1b, with the stepwise addition of the Zn2+, the fluorescence of monomer gradually decreases, and reaches the minimum at 602 nm when the ratio of monomer/Zn2+ achieves to 1.0. Also, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation was validated by the ITC experiment (Fig. S5†).
1 complexation was validated by the ITC experiment (Fig. S5†).
As widely documented, Zn2+ could complex with terpyridine to form two kinds of metallic complex species, Zn(tpy)22+ and Zn(tpy)2+.22 However, UV/Vis and fluorescence measurements could not distinctly distinguish such types of complex. In this regard, 1H NMR titration measurements were employed to get further insights into the exchange kinetics of Zn2+–terpyridine complexes. As shown in Fig. 2, when the feed-ratio of Zn2+/monomer is below 1.0 (Fig. 2a–d), the terpyridine proton H1 exhibits the remarkable upfield shift from 8.74 to 7.80 ppm due to the shielding effect, while both H3 and H5 exhibit obviously downfield shifts (H3: from 7.92 to 8.17 ppm; H5: from 8.60 to 8.95 ppm). Upon adding 1.0 equivalent Zn2+, the original uncomplexed terpyridine signals totally disappeared, suggesting the formation of dimeric Zn(tpy)22+. However, once Zn2+/monomer ratio is further increased, the newly signals evolved and gradually strengthen, suggesting the formation of Zn(tpy)2+.38
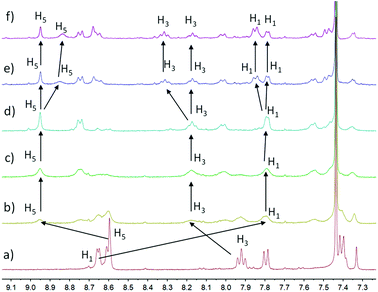 | ||
| Fig. 2 Partial 1H NMR spectra (300 MHz, CDCl3: CD3OD (2/1, v/v)): (a) monomer, (b–f) gradual titration of Zn2+ into monomer. | ||
After confirming the non-covalent complexation behavior between Zn2+and monomer in relatively low concentration as the monomeric state, the supramolecular polymerization process was further studied via concentration-dependent 1H NMR measurement. According to Fig. S8,† for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of monomer and Zn(OTf)2 at 2.0 mM, the aromatic protons on terpyridine moiety show one set of well-defined sharp signals, implying the dominance of oligomers (Fig. S8a†). In sharp contrast, the broadening of all signals at high concentration of monomer suggests the formation of supramolecular polymer (Fig. S8e†). Such conclusion could be also validated by two-dimensional diffusion order spectrum experiment (DOSY), which is a convenient and efficient technique to monitor the size variation of the dynamic aggregations. When the monomer concentration increased from 0.5 to 30.0 mM, the measured diffusion coefficients of metallo-supramolecular polymer 1 decreased remarkably from 2.81 × 10−11 to 8.91 × 10−12 m2 s−1 (Fig. S9†), implying the formation of large-sized supramolecular polymeric assemblies at high concentration.
1 mixture of monomer and Zn(OTf)2 at 2.0 mM, the aromatic protons on terpyridine moiety show one set of well-defined sharp signals, implying the dominance of oligomers (Fig. S8a†). In sharp contrast, the broadening of all signals at high concentration of monomer suggests the formation of supramolecular polymer (Fig. S8e†). Such conclusion could be also validated by two-dimensional diffusion order spectrum experiment (DOSY), which is a convenient and efficient technique to monitor the size variation of the dynamic aggregations. When the monomer concentration increased from 0.5 to 30.0 mM, the measured diffusion coefficients of metallo-supramolecular polymer 1 decreased remarkably from 2.81 × 10−11 to 8.91 × 10−12 m2 s−1 (Fig. S9†), implying the formation of large-sized supramolecular polymeric assemblies at high concentration.
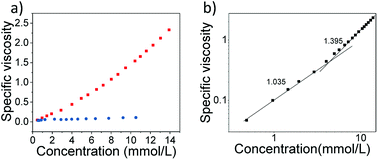
Next, capillary viscosity measurements were performed to study the macroscopic properties of the resulting supramolecular assemblies. All of the viscosity studies were performed in DMSO containing 0.05 M tetrabutylammonium hexafluorophosphate to exclude the polyelectrolyte effect.39 As shown in Fig. 3a, the monomer shows the comparably shallow curve for the specific viscosities. In sharp contrast, the specific viscosity of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of Zn2+ and monomer changes exponentially as a function of monomer concentration, revealing the formation of high-molecular-weight supramolecular polymer.
1 mixture of Zn2+ and monomer changes exponentially as a function of monomer concentration, revealing the formation of high-molecular-weight supramolecular polymer.
The double logarithmic plots of versus specific viscosity concentration were further obtained for 1 (Fig. 3b), which displays a clear slope change. In the low concentration range, the slope value was determined to be 1.03, which indicates the presence of oligomers with the constant size. Remarkably, a sharp rise in the viscosity was observed when the concentration exceeds the critical polymerization concentration value (around 4.0 mM), and the slope value was estimated up to 1.40. Such phenomenon is in highly consistent with the aformentioned DOSY and 1H NMR results, suggesting the formation of large supramolecular polymeric assemblies at high concentration.
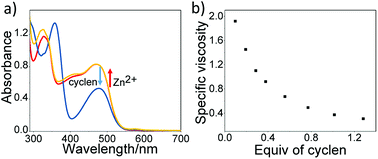
Stimuli-responsive properties of the resulting supramolecular polymer were further exploited, by manipulating the dynamic property of Zn2+–terpyridine recognition motif. It is well known that 1,4,7,10-tetraazacyclododecane (cyclen) exhibits higher affinity toward Zn2+ than terpyridine unit. With the addition of cyclen, the decrease of Zn2+/tpy absorption bands (λ = 477 nm), together with the increase of fluorescence intensity (λ = 602 nm), suggest that cyclen competitively complexes with terpyridine to destroy the Zn2+/terpyridine interactions (Fig. 4a, and S10†). Such process could also be validated by capillary viscosity experiments, which is shown in Fig. 4b. With the stepwise addition of cyclen, the specific viscosity of the metallo-supramolecular polymer decreases and gradually levels off, suggesting the disassembly of metallo-supramolecular polymer. However, with the further addition of Zn2+, metallo-supramolecular polymer could be recovered. The conclusion could be manifested by the reappearance of Zn2+/tpy absorption band, the decrease of fluorescence intensity (Fig. S10†), and the restore of the original 1H NMR signals (Fig. S11†). Hence, the successive addition of the competitive ligand cyclen and Zn2+ provide a possible method to manipulate the reversible assembly/disassembly of the metallo-supramolecular polymer.
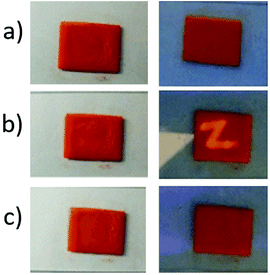
As mentioned above, the fluorescence intensity of resulting supramolecular polymers could be regulated by stepwise addition of cyclen and Zn2+. By taking advantage of the “on/off” fluorescent switching properties, we sought to explore their potential applications for data security. In detail, the solution of metallo-supramolecular polymer 1 was doped into polylactic acid (PLA) and formed a bright orange film (1.2 cm × 1.0 cm). The fluorescence intensity of resulting film is relatively lower, owing to the existence of Zn2+/terpyridine metal–ligand interactions. The resulting film could be used as a drawing board for the applications in document security, by employing the solution of cyclen as a magic ink (Fig. 5). In detail, when a letter “Z” was written on the surface of the drawing board, there have almost no changes for the film under daylight. However, the patterned security feature of “Z” encrypted with the solution of cyclen could be readily visualized under UV light, which could be ascribed to the disassembly of Zn2+–terpyridine complexes. Moreover, the letter “Z” could also be wiped by immerging the film into the Zn2+ solution and the drawing board could be recovered.
In summary, we have constructed the rigid metallo-supramolecular polymer, by using metal–ligand interactions as the non-covalent connecting bonds. Benzothiadiazole-functionalized dinuclear platinum(II) acetylide moiety was incorporated, rendering fascinating photo-physical properties to the resulting monomer and supramolecular polymeric assemblies. 1H NMR, UV/Vis, ITC, DOSY, and viscosity measurements were employed to investigate the supramolecular polymerization process. Additionally, the metallo-supramolecular polymer 1 could be reversibly assembled and disassembled upon adding the competitive ligands. By taking advantage of the fluorescence “on/off” switch process, the resulting supramolecular polymer could be used as fluorescent security materials. Therefore, the current work provides a convenient and efficient approach to fabricate supramolecular polymer toward smart information processing materials.40,41
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21704075, 21871245).Notes and references
- G. R. Whittell, M. D. Hager, U. S. Schubert and I. Manners, Nat. Mater., 2011, 10, 176 CrossRef CAS.
- A. Winter and U. S. Schubert, Chem. Soc. Rev., 2016, 45, 5311 RSC.
- M. Higuchi, J. Mater. Chem. C, 2014, 2, 9331 RSC.
- A. Gasnier, G. Royal and P. Terech, Langmuir, 2009, 25, 8751 CrossRef CAS PubMed.
- L. Yu, Z. Wang, J. Wu, S. Tu and K. Ding, Angew. Chem., Int. Ed., 2010, 49, 3627 CrossRef CAS PubMed.
- R. J. Wojtecki, M. A. Meador and S. J. Rowan, Nat. Mater., 2011, 10, 14 CrossRef CAS PubMed.
- Y. Han, Y. Tian, Z. Li and F. Wang, Chem. Soc. Rev., 2018, 47, 5165 RSC.
- M. Chiper, R. Hoogenboom and U. S. Schubert, Macromol. Rapid Commun., 2009, 30, 565 CrossRef CAS PubMed.
- J. B. Beck and S. J. Rowan, J. Am. Chem. Soc., 2003, 125, 13922 CrossRef CAS PubMed.
- A. Wild, A. Winter, F. Schluetter and U. S. Schubert, Chem. Soc. Rev., 2011, 40, 1459 RSC.
- A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729 CrossRef CAS PubMed.
- M. Burnworth, L. Tang, J. R. Kumpfer, A. J. Duncan, F. L. Beyer, G. L. Fiore, S. J. Rowan and C. Weder, Nature, 2011, 472, 334 CrossRef CAS PubMed.
- D. W. R. Balkenende, S. Coulibaly, S. Balog, Y. C. Simon, G. L. Fiore and C. Weder, J. Am. Chem. Soc., 2014, 136, 10493 CrossRef CAS.
- H. Wang, X. Qian, K. Wang, M. Su, W.-W. Haoyang, X. Jiang, R. Brzozowski, M. Wang, X. Gao, Y. Li, B. Xu, P. Eswara, X.-Q. Hao, W. Gong, J.-L. Hou, J. Cai and X. Li, Nat. Commun., 2018, 9, 1815 CrossRef PubMed.
- L. Gao, Z. Zhang, B. Zheng and F. Huang, Polym. Chem., 2014, 5, 5734 RSC.
- Y. K. Tian, L. Chen, Y. J. Tian, X. Y. Wang and F. Wang, Polym. Chem., 2013, 4, 453 RSC.
- Y. Zhou, H. Y. Zhang, Z. Y. Zhang and Y. Liu, J. Am. Chem. Soc., 2017, 139, 7168 CrossRef CAS.
- S. Pai, M. Schott, L. Niklaus, U. Posset and D. G. Kurth, J. Mater. Chem. C, 2018, 6, 3310 RSC.
- S. Pai, M. Moos, M. H. Schreck, C. Lambert and D. G. Kurth, Inorg. Chem., 2017, 56, 1418 CrossRef CAS PubMed.
- P. Stenclova, K. Sichova, I. Sloufova, J. Zednik, J. Vohlidal and J. Svoboda, Dalton Trans., 2016, 45, 1208 RSC.
- T. Vitvarova, J. Svoboda, M. Hissler and J. Vohlidal, Organometallics, 2017, 36, 777 CrossRef CAS.
- R. Dobrawa, M. Lysetska, P. Ballester, M. Gruene and F. Wuerthner, Macromolecules, 2005, 38, 1315 CrossRef CAS.
- C. Chakraborty, R. K. Pandey, U. Rana, M. Kanao, S. Moriyama and M. Higuchi, J. Mater. Chem. C, 2016, 4, 9428 RSC.
- S. Archer and J. A. Weinstein, Coord. Chem. Rev., 2012, 256, 2530 CrossRef CAS.
- V. Prusakova, C. E. McCusker and F. N. Castellano, Inorg. Chem., 2012, 51, 8589 CrossRef CAS PubMed.
- E. Glimsdal, M. Carlsson, T. Kindahl, M. Lindgren, C. Lopes and B. Eliasson, J. Phys. Chem. A, 2010, 114, 3431 CrossRef CAS PubMed.
- H. Masai, J. Terao, S. Makuta, Y. Tachibana, T. Fujihara and Y. Tsuji, J. Am. Chem. Soc., 2014, 136, 14714 CrossRef CAS PubMed.
- X. Wang, Y. F. Han, Y. Y. Liu, G. Zou, Z. Gao and F. Wang, Angew. Chem., Int. Ed., 2017, 56, 12466 CrossRef CAS PubMed.
- E. T. Shi, Z. Gao, M. Yuan, X. Y. Wang and F. Wang, Polym. Chem., 2015, 6, 5575 RSC.
- B. A. D. Neto, P. H. P. R. Carvalho and J. R. Correa, Acc. Chem. Res., 2015, 48, 1560 CrossRef CAS PubMed.
- B. A. D. Neto, J. R. Correa and R. G. Silva, RSC Adv., 2013, 3, 5291 RSC.
- Y. Liu, Y. Yu, J. Gao, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2010, 49, 6576 CrossRef CAS PubMed.
- Y. Liu, R. Fang, X. Tan, Z. Wang and X. Zhang, Chem. Eur. J., 2012, 18, 15650 CrossRef CAS PubMed.
- Y. Liu, Z. Huang, X. Tan, Z. Wang and X. Zhang, Chem. Commun., 2013, 49, 5766 RSC.
- R. Joseph, A. Nkrumah, R. J. Clark and E. Masson, J. Am. Chem. Soc., 2014, 136, 6602 CrossRef CAS PubMed.
- H. Hofmeier, R. Hoogenboom, M. E. L. Wouters and U. S. Schubert, J. Am. Chem. Soc., 2005, 127, 2913 CrossRef CAS PubMed.
- G. Groeger, W. Meyer-Zaika, C. Boettcher, F. Groehn, C. Ruthard and C. Schmuck, J. Am. Chem. Soc., 2011, 133, 8961 CrossRef CAS.
- Y. Ding, P. Wang, Y. K. Tian, Y. J. Tian and F. Wang, Chem. Commun., 2013, 49, 5951 RSC.
- Y. K. Tian and F. Wang, Macromol. Rapid Commun., 2014, 35, 337 CrossRef CAS PubMed.
- Z. Gao, Y. Han and F. Wang, Nat. Commun., 2018, 9, 3977 CrossRef PubMed.
- Z. Gao, Y. Han, Z. Gao and F. Wang, Acc. Chem. Res., 2018, 51, 2719 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra08615j |
| This journal is © The Royal Society of Chemistry 2018 |