Open Access Article
Open Access ArticleThe genus Capsicum: a phytochemical review of bioactive secondary metabolites†
A. S. Antonioa,
L. S. M. Wiedemanna and
V. F. Veiga Junior *ab
*ab
aChemistry Department, Institute of Exact Sciences, Amazonas Federal University, Avenida Rodrigo Octávio, 6200, Coroado, CEP: 69.077-000, Manaus, AM, Brazil
bChemistry Section, Military Institute of Engineering, Praça General Tibúrcio, 80, Praia Vermelha, Urca, CEP: 22.290-270, Rio de Janeiro, RJ, Brazil. E-mail: valdir.veiga@gmail.com
First published on 19th July 2018
Abstract
The Capsicum genus is one of the most popular plants consumed and cultivated worldwide, containing approximately 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 varieties of pepper. Due to its wide biodiversity, the chemical composition within the genus also presents a great variability. Its major applications are in food and pharmacological industry, as pepper presents a chemical composition rich in capsaicinoids, carotenoids, flavonoids and volatile compounds which is attributed to the ability of the fruit to remove insipidity, produce aromas and act against oxidative diseases. Due the existence of several cultivars there is a huge intraspecific chemical variability within each species, which can be considered as an obstacle when selecting and cultivating a species to be applied as a natural product source for a specific objective. The usage of pepper-based products in different industrial areas requires pre-established ranges of chemical compounds, such as capsaicinoids, which in high concentration are toxic when consumed by humans. Applying a pepper with a chemical profile closely related to the concentration that is required after industrial processing can improve efficacy and effectiveness of the process. An insight into the chemical characteristics of major secondary bioactive compounds within Capsicum, the factors that affect their concentration and their chemosystematic implication are reported and discussed.
000 varieties of pepper. Due to its wide biodiversity, the chemical composition within the genus also presents a great variability. Its major applications are in food and pharmacological industry, as pepper presents a chemical composition rich in capsaicinoids, carotenoids, flavonoids and volatile compounds which is attributed to the ability of the fruit to remove insipidity, produce aromas and act against oxidative diseases. Due the existence of several cultivars there is a huge intraspecific chemical variability within each species, which can be considered as an obstacle when selecting and cultivating a species to be applied as a natural product source for a specific objective. The usage of pepper-based products in different industrial areas requires pre-established ranges of chemical compounds, such as capsaicinoids, which in high concentration are toxic when consumed by humans. Applying a pepper with a chemical profile closely related to the concentration that is required after industrial processing can improve efficacy and effectiveness of the process. An insight into the chemical characteristics of major secondary bioactive compounds within Capsicum, the factors that affect their concentration and their chemosystematic implication are reported and discussed.
Introduction
Capsicum is the most economically important genus in the Solanaceae family, composed of nearly 30 species.1 Their fruits, popularly known as peppers or chili, present a bell-shape and a wide range of colors, such as yellow, green, red and orange. They have been largely used by human society as a herb and spice, presenting the first records of usage dating back to 7000 B.C., as part of the Mexican Indians' diet.2The production and usage of Capsicum fruits has spread worldwide since the marine exploration period,2,3 when several civilizations started to consume and cultivate them, which were able to adapt and be domesticated in different environmental conditions due to the hybridization process.2,4,5 The fast increase of their popularity was due to their outstanding properties, for instance, it is known that before 1600 B.C. the fruits had been used as pigments, food spices and in the treatment of stomach disorders and headaches.2,6,7 Pepper usage in food and as a medicine has attracted several researchers to study each of the chemical properties attributed to the characteristics of peppers. Meanwhile, the consumption of Capsicum fruits spread and acquired more applications, mainly as medicines.2,6,8–12
Later on, with the development of research within the genus species, their properties were attributed to the abundant presence of carotenoids, flavonoids and capsaicinoids in the peppers.13–22 In addition, the three chemical classes had already been reported as bioactive compounds,18,23–25 the first two metabolites are also responsible for the attractive colors of peppers, while capsaicinoids attribute a taste characteristic known as pungency, which is responsible for the worldwide popularity of these fruits.26 Pungency consists of an irritation in the terminal nerves that produce a sensation of pain and heat, which enables its application in culinary uses as an efficient form to remove insipidity and attribute new tastes to meal.18 Recently, Capsicum fruits have been applied in various industries as spices, food colorants, painkillers, cardiovascular protectors, anti-inflammatories, antioxidants, anticancer and antitumor agents.15,18,19
The application of peppers as a raw material for industry depends on the content of the bioactive compounds within the fruits. These fruits are usually applied in industrial processes as a dry powder (paprika) or an oleoresin (concentrated extract of essential oils, waxes, carotenoids, flavonoids and capsaicinoids).27–29 These pepper-based products are usually bought from a producer and later applied in several industrial purposes. Thus, the same oleoresin or paprika used for pharmacological formulations may be applied in the food or military industry.27–32
An implication of this is the possible loss of effectiveness and efficiency of the product, as each objective requires a specific chemical profile.33–37 For instance, the military usage of Capsicum oleoresin requires a product with less than 1.6% of capsaicinoids content,37 the colorant paprika requires the presence of red fraction carotenoids38,39 and for pharmacological usage the capsaicin concentration cannot be greater than 2.64 mg.40,41
As the chemical profile of pepper varies in accordance to the species, seasonality, environmental conditions and even the life cycle of the plant,5,22–24,42–45 it can be difficult to choose the Capsicum species and cultivars that are economically profitable to a specific objective.29,41,46–49 Therefore, to comprehension of the properties, composition and factors affecting the chemical profile of Capsicum fruits may assist the selection of cultivar/cultivation method; advancing the industrial usage of pepper-based products, increasing the productivity and the development of post-harvest technology.18,25,50,51
Additionally, as the hybridization processes promote the existence of several cultivars with similar morphologic characteristics, it is difficult to distinguish each cultivar44,52,53 and identify them unambiguously. Increasing the productivity and effectiveness of a pepper-based product will also require an unequivocal identification of the cultivar which is being used. A tool that can be used to enhance the systematic identification of the genus is the application of the chemical profile to characterize each cultivar.4,54,55
Despite the large quantity of reviewed papers covering Capsicum bioactivity,14,15,18,19,22,51 few have reported on the phytochemistry perspective and applications.56 In this paper we propose to present a review of the major secondary metabolites within the Capsicum genus that may subsidize the development of its industrial/commercial usage and chemosystematics approaches, focused on cultivars identification.
Capsaicinoids
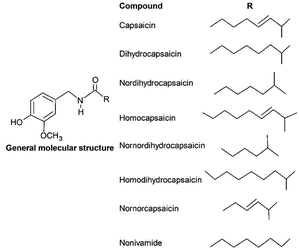
Capsaicinoids (CAPS) consists of a group of substances closely related with an alkaloid typical molecular structure, produced only within the Capsicum genus, and capable of producing pungency. This characteristic, as much as their bioactivity, is a result of the molecular structure which allows them to interact differently with the nervous receptors and cellular membrane.26,57,58The general chemical structure of capsaicinoids (Fig. 1) is composed of a vanillyl group bonded with an amide and an alkyl chain.17 The molecular diversity within capsaicinoids occurs due to the carbon chain, which may present itself as: (i) unsaturated and ramified, as in capsaicin; (ii) saturated and ramified, as in dihydrocapsaicin; or (iii) saturated and linear, as in nonivamide.59,60 This molecular structure provides the CAPS with an amphiphilic and no basicity behavior, atypically of what is observed in alkaloids in general.17,59,60
Among the compounds that constitute the capsaicinoids class, only four of them are usually studied due to their higher concentration in situ,60 these are the major compounds capsaicin and dihydrocapsaicin, and the minor ones homocapsaicin and nordihydrocapsaicin, whose structure can be observed in Fig. 1.
The molecular structure of CAPS is resistant to ionizing radiation, although it readily decomposes over 50% of its original concentration when exposed to temperatures over 80 °C. This thermal degradation is initially attributed to the cleavage of the carbon–nitrogen bond, to produce the vanillyl group and an acyl chain.59–61 It demonstrates that the temperature within any process for natural products based on capsaicinoids must be carefully considered to avoid thermal decomposition. The vanillyl group within CAPS is reported to be a proton donor, which provides these molecules with the ability to stabilize radical species and interact with cellular membranes, enzymes and nervous receptors.46,62 Therefore, CAPS bioactivity is mainly attributed to the vanillyl group present in the structure.18,19
The most important biological interaction of CAPS is with the transient receptor potential vanilloid-1 (VR1), a sensorial receptor responsible for the neural response to temperature variations, acidosis, pain and osmolarity, which generates the sensation of heat and/or pain as a warning to the body of possible dangerous situations. This receptor consists of a nonselective cation channel mainly activated by proton sites within different molecules and vanilloids functional groups.19 The importance of VR1 is mainly due to the regulation of this receptor by CAPS, which produces not only the pungent sensation but also provides pain relief, gastrointestinal protection, and antioxidant properties, such as antitumoral, anticancer and anti-inflammatory effects.17,19,22,63
The mechanism of interaction of CAPS and VR1 was described by Hayman and Kam.18 Briefly, the bioactivity of CAPS is closely related to the down regulation or desensitization of VR1 for relatively long periods of time, as pungent CAPS can remain attached to VR1 without suffering cleavage due to its amphiphilic behavior.18,19
Regarding its solubility, capsaicinoids are soluble in medium to low polarity solvents, such as alcohol, methanol, ethanol, and acetonitrile, this last one being the most applied in CAPS extraction, as it is recognized as the solvent in which CAPS presents the highest solubility.18,51 In addition, the phenolic functional group within vanillyl enables CAPS to suffer a phase transfer when exposed to basic conditions, in which it can form a phenolate ion which may form a salt in the presence of metals, increasing its solubility in aqueous solution.58
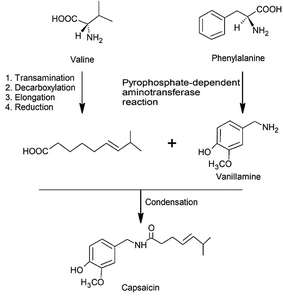
Biosynthesis and catabolism
Capsaicinoids are mainly biosynthesized in the placenta of the fruit, where there is the largest concentration of vanillylamine.3,44,64–66 The formation occurs by the condensation of vanillylamine with an acyl acid precursor, as can be observed in Fig. 2, by the phenylpropanoid pathway.17The acyl precursor, which originates the C9–C11 ranched chain, is synthesized by a series of enzymatic based reactions starting from an amino acid, and presenting as fundamental steps the transamination, decarboxylation, elongation and reduction. The elongation process, promoted by the malonylCoA, can occur from two to four cycles, depending on which CAPS is being synthesized.17,65 In the meantime, the final step consists of a ketone to alcohol reduction and dehydration to provide the double bond to the fatty acid chain or the saturation of the carbon chain.17 Each CAPS has a unique acyl precursor, such as 8-methyl-trans-6-nonenoic acid (capsaicin), 8-methylnonanoic acid (dihydrocapsaicin), 7-methylnonanoic acid (nordihydrocapsaicin), 9-methyldecanoic acid (homodihydrocapsaicin) and 9-methyldec-trans-7-enoic acid (homocapsaicin).67–69
Among the naturally synthesized CAPS is the nonivamide or N-(4-hydroxy-3-methoxybenzyl)-nonanoic acid amide. This alkaloid has a pungency level very similar to capsaicin, although it is biosynthesized in smaller quantities, corresponding to approximately 3% of the total CAPS content.9,70 The similarity in pungency allows nonivamide to be used as an adulterant in pepper-based products which have low levels of capsaicin. Adulteration with nonivamide is a several problem, as this alkaloid does not present the same biological activity and nutritional levels as capsaicin, generating products with low efficiency for the pharmaceutical and food industry.27,38,70,71 The usage of nonivamide as an adulterant was only reduced at the beginning of the 20th century with the development of analytical methods capable of distinguishing and separating the similar molecular structures.27,72,73
Vanillylamine share with CAPS the same exclusivity, that is produced only within the Capsicum genus, and acts as a precursor for the vanillyl group present in the CAPS structure.17 Its precursor is L-phenylalanine, which reacts by the cinnamic acid pathway in a pyrophosphate-dependent reaction to generate trans-cinnamic acid, trans-p-coumaric acid, trans-caffeic acid, trans-ferulic acid, vanillin and finally vanillylamine.17,74–76 The synthesis of CAPS can be limited by either the concentration of vanillylamine, when considering the plant compartment, or the concentration of the acyl precursor, when considering the biosynthesis within the placenta tissue in which vanillylamine is abundant.3,17,77
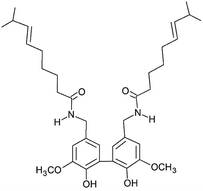
CAPS biosynthesis produces a peak of concentration within 30–50 days after flowering, the period in which the pungency is usually higher in the whole fruit, and after that period the CAPS concentration start to naturally decrease due to plant metabolism.14,17 This reaction is mediated by the peroxidase enzyme over the vanillyl group, which is the main oxidative site within capsaicinoids. Peroxidase promotes the oxidative phenol coupling of CAPS to form a dimer (5,5′-dicapsaicin in Fig. 3) and 4′-O-dicapsaicinether,42,78 which are closed related to the lignin metabolites in a mechanism described by Díaz et al.14
In human and mammals, CAPS are readily metabolized within the liver in the presence of the P450 enzyme14,15,17,79 by a complex mechanism, which was described by Reilly and Yost.15 Briefly, P450 interacts mainly with the vanillyl group through several different reactions of aromatic hydroxylation, O-demethylation, benzylic oxidation, ω-hydroxylation and dehydrogenation. Additionally, Kawada et al. also reported an in vivo and in vitro experiment using the liver cells of rats, and detected, as metabolites of CAPS (through enzymatic hydrolyzation), vanillylamine, vanillin, vanillyl alcohol, vanillic acid and 8-methylnonanoic acid.80
Capsaicinoids content
The economic importance of the Capsicum species is most related to its biological activities and flavoring properties.18,42,81 In this context, the capsaicinoid content is an important characteristic that must be evaluated and controlled during cultivation and processing of the fruit. Over 20 alkaloids have been reported in this class, distributed in 4 main groups based in the similarity of their molecular structure with the major CAPS. The distribution of capsaicinoids within each group is approximately: capsaicin group (69%), dihydrocapsaicin group (22%), nordihydrocapsaicin group (7%), homocapsaicin and homodihydrocapsaicin analogues groups (1%).16,82 It is obvious that the major CAPS are within the capsaicin and dihydrocapsaicinoids groups, more specifically capsaicin and dihydrocapsaicin, and can correspond to 89–98% of the total capsaicinoids content in the fresh fruits.16,21,23,82 Among the minor CAPS already reported in natural products nonivamide, nornordihydrocapsaicin, homodihydrocapsaicin, and nordihydrocapsaicin, nornorcapsaicin have been reported.61,73 Additionally, the concentration of minor CAPS can be used as an indicator for adulteration, such as nonivamide, which is naturally present at a concentration lower than 3% of the total content.17CAPS are mainly found within the fruit organs where they are biosynthesized, such as the placenta tissue where most of it is produced and in the interlocular septa, a tissue that produces smaller quantities of CAPS.77 After production and accumulation in these tissues, CAPS are induced to diffuse to other compartments within the fruit66,77,83 and plant organs.17,42,84 Even with the diffusion processes, it is estimated that over 85% of the capsaicinoids total content can be located within the placenta, reaching a concentration that can be 10 times higher than the CAPS content in other organs during the ripening stages.17,77,85 The organ with the second greatest accumulation of CAPS is the seed, as these metabolic processes have protective and proliferation actions, allowing the reproduction of the plant.66,77,83,86
Even though metabolomics in plants is a very complex and dynamic science, some exceptions to this approach have been reported. Nugroho et al. reported varieties of Capsicum annuum L. whose fruit presents higher CAPS content in the last stages of fruit development in the septum, which may indicate their accumulation in this organ after production in the placenta tissue, as postulated before.42
In general, the CAPS profile is more often studied during the period of maximum production of these alkaloids, which usually occurs close to the end of the fruit immature stage, at the moment when CAPS are most required by the plant, as these metabolites are an agent protective against herbivores and also responsible for the scarification of seeds.17,26,44,87 In the period of maximum CAPS production most species presents more capsaicin than dihydrocapsaicin, typically in a proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.23,24,43,82
1.23,24,43,82
Although this general proportion has been used in several papers, there is no solid evidence that this approach can be considered true for a certain species or cultivar. The capsaicinoids profile does not present a homogeneous behavior in cultivars collected in different locations, and even in cases in which they are subject to the same conditions a wide range of concentrations can be observed for each capsaicinoid.88 Some exception to these general approximations can be found in a closer observation of different cultivar and varieties within a species,44 as described by Duelund and Mouritsen88 and Bae et al.48 in their studies on the Espelette and Tuxtlas (C. annuum) cultivars.
Table 1 shows a brief overview of the major CAPS content in several varieties, in which we can emphasize the wide range of values that each of the major CAPS present. For instance, their content varies from 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 to 5.87 μg of capsaicin g−1 of fruit; 68
760 to 5.87 μg of capsaicin g−1 of fruit; 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 to 0.1 μg of dihydrocapsaicin g−1 of fruit; 5350 to 0.1 μg of nor-dihydrocapsaicin g−1 of fruit; and 5410 to 0.31 μg of homo-dihydrocapsaicin g−1 of fruit. As may be observed in Table 1, the C. chinense and C. annuum are the most targeted species when researching the Capsicum genus. This fact can be attributed to their moderate to high pungency and cultivation pattern.
470 to 0.1 μg of dihydrocapsaicin g−1 of fruit; 5350 to 0.1 μg of nor-dihydrocapsaicin g−1 of fruit; and 5410 to 0.31 μg of homo-dihydrocapsaicin g−1 of fruit. As may be observed in Table 1, the C. chinense and C. annuum are the most targeted species when researching the Capsicum genus. This fact can be attributed to their moderate to high pungency and cultivation pattern.
| Species | Cultivar | C | DHC | n-DHC | h-DHC | Ref | Species | Cultivar | C | DHC | n-DHC | h-DHC | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a C = capsaicin; DHC = dihydrocapsaicin; n-DHC = nordihydrocapsaicin; h-DHC = homodihydrocapsaicin; n.d = not described; *values are presented as μg g−1 of fresh fruit. | |||||||||||||
| Chinense | Carolina Reaper | 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 760 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 470 |
1140.0 | 1312 | 88 | Annuum | Huai Si Thon Kanlapaphruek | 1644 | 1089 | n.d | n.d | 24 |
| Chinense | Trinidad Scorpion | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 120 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
3770 | 5410 | 88 | Annuum | Jariza* | 1539.1–60.08 | 1048.8–52.64 | 238.49–13.34 | 82.76–3.52 | 120 |
| Chinense | IH Jolokia | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 640 640 |
35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420 |
5050 | 3670 | 88 | Chinense | n.d* | 1369.51 | 229.35 | 8.25 | 1.65 | 121 |
| Chinense | Buth Orange Copenhagen | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 890 890 |
5050 | 3150 | 88 | Chinense | Cumari | 1302.7 | 196.2 | 8.7 | 7.1 | 84 |
| Chinense | Habalolokia, Yellow | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 350 |
5350 | 4140 | 88 | Pubescens | n.d | 1280–130 | 2070–250 | 1220–90 | n.d | 122 |
| Chinense | Habanero red type 2 | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 871 871 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 132 132 |
1102 | n.d | 23 | Chinense | Habanero | 1128.3 | 321.6 | 11.4 | 8.6 | 84 |
| Chinense | Naga morich | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 510 510 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 592 592 |
1041 | n.d | 23 | Annuum | Jalapeño | 1101 | 843 | 180 | n.d | 23 |
| Chinense | Red Savina | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 790 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 410 |
2060 | 1680 | 88 | Frutescens | Malagueta | 969.1 | 490.6 | 111.6 | 21.9 | 84 |
| Chinense | Habanero | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 010 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 410 |
3000 | 2710 | 88 | Frutescens | Tabasco | 917 | 351 | 66 | n.d | 23 |
| Chinense | Fatali | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
6770 | 1880 | 2150 | 88 | Chinense | Baiana | 832.9 | 192.7 | 7.2 | 11.3 | 84 |
| Chinense | Bhut Jolokia | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900–10 900–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
7910–3330 | n.d | n.d | 86 | Annuum | Cayenne* | 717.8–300.3 | 779.5–223.0 | 198.1–47.3 | 47.9–20.5 | 78 |
| Chinense | Habanero chocolate | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 454 454 |
3092 | 608 | n.d | 23 | Annuum | Sinpezon | 716 | 308 | 78 | n.d | 23 |
| Frutescens | Tabasco | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 610 |
6630 | 1460 | 1030 | 88 | Annuum | Serrano | 707 | 527 | 112 | n.d | 23 |
| Chinense | Fatali | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800–13 800–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
6450–5310 | n.d | n.d | 86 | Annuum | Espalette | 690 | 660 | 410 | 470 | 88 |
| Chinense | Caribbean red | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
4410 | n.d | n.d | 86 | Annuum | Mokho 2 | 614 | 609 | n.d | n.d | 24 |
| Chinense | Habanero chocolate | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400–4120 400–4120 |
3720–1070 | n.d | n.d | 86 | Annuum | Bola* | 593.03–579.03 | 528.65–56.17 | 150.61–141.19 | 43.43–7.80 | 120 |
| Chinense | Habanero orange | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
6020 | n.d | n.d | 86 | Annuum | Jeromin* | 554.38–190.3 | 440.74–40.75 | 114.10–34.91 | 37.39–14.98 | 120 |
| Chinense | Habanero orange | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 042 |
9332 | n.d | n.d | 24 | Annuum | Serrano | 361 | 23,00 | 630 | 1160 | 88 |
| Chinense | Habanero yellow | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2280 | n.d | n.d | 86 | Chinense | Fatali | 312 | 512.1 | 72.1 | n.d | 84 |
| Chinense | Habanero Orange | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 156 156 |
4273 | 433 | n.d | 23 | Annuum | 730 F1 | 307.7 | 208 | 40 | 112.9 | 123 |
| Chinense | Habanero Antillas red | 8720 | 3350 | n.d | n.d | 86 | Annuum | Jalapeño | 296–132 | 233–61.3 | n.d | n.d | 86 |
| Chinense | Habanero white | 8248 | 1859 | 340 | n.d | 23 | Annuum | Ancho | 290 | 770 | 250 | 580 | 97 |
| Chinense | Habanero Golden | 8175 | 1389 | 144 | n.d | 23 | Annuum | 1245 F1 | 271 | 123.4 | 24.4 | 32.6 | 123 |
| Chinense | Devils tongue | 7530 | 2210 | n.d | n.d | 86 | Baccatum | Dedo-de-moça | 247.5 | 120.2 | 17.1 | n.d | 84 |
| Chinense | Scotch bonnet | 5961 | 2331 | 68 | n.d | 23 | Annuum | Jalapeño* | 217.5–115.8 | 121.3–54 | n.d | n.d | 48 |
| Chinense | Habanero red type 1 | 4904 | 1359 | 100 | n.d | 23 | Annuum | Cayenne* | 211.07–20.8 | 114.6–16.4 | n.d | n.d | 48 |
| Baccatum | Omnicolor | 4710 | 6660 | 720 | 1790 | 88 | Annuum | Guajillo | 170 | 610 | 120 | 560 | 97 |
| Baccatum | Toftegaard lemon chili | 4630 | 4870 | 690 | 920 | 88 | Baccatum | Bishop Crown* | 158.88–198.64 | 196.23–95.39 | 54.00–24.36 | 20.61–0.94 | 120 |
| Chinense | Habanero white | 4523 | 811 | n.d | n.d | 24 | Annuum | Serademre 8 | 149.2 | 72.5 | 15.8 | 63.9 | 123 |
| Chinense | Naga Jolokia | 4469.4 | 2319.9 | 416.3 | 363.2 | 84 | Annuum | Toftegaard Hot Banana | 125 | 102 | 790 | 830 | 88 |
| Chinense | Habanero peach | 4280 | 1600 | n.d | n.d | 86 | Pubescens | n.d* | 118.27–73.93 | 152.41–45.47 | 42.94–36.72 | 1.05–0.31 | 121 |
| Frutescens | Ratchaburi | 3238 | 1414 | n.d | n.d | 24 | Baccatum | n.d* | 81.6–5.87 | 39.23–3.39 | 7.98–3.36 | 1.33 | 121 |
| Baccatum | Aji Verde | 3100 | 1610 | 540 | 660 | 88 | Annuum | Serrano* | 81.3–39.1 | 87.7–47.8 | n.d | n.d | 48 |
| Frutescens | Malagueta* | 1956.81–706.09 | 1404–779.58 | 451.46–198.16 | 156.06–50.99 | 120 | Annuum | Amazon F1 | 16 | 0.1 | 10.2 | 65.8 | 123 |
| Chinense | Murupi | 1776.7 | 226.4 | 10.8 | 14.3 | 84 | Annuum | Kusak 295 F1 | 11 | 1.7 | 0.1 | 15.4 | 123 |
Pungency levels are directly related to capsaicinoids content, mainly capsaicin and dihydrocapsaicin, which are considered the most pungent CAPS.23 In addition, its remarkable taste and bioactivity,22,56,89 as previously cited in this article and discussed by Wahyuni et al., CAPS presents a high toxicity degree to humans.56 In mammalian organism, CAPS act as an inflammatory and carcinogenic agent18,41,90 causing irritation in mucous membranes in the digestive and respiratory systems and cellular rupture, when orally ingested.28,35,36 It is estimated that the 50% of the CAPS lethal dose by oral ingestion in humans is 5.0 g kg−1,40,41 with a safe daily intake dose of 2.64 mg of CAPS.41
Depending on the commercial application of the pepper (or pepper-based product) a lower or higher concentration of CAPS may be required. For medicinal proposes, cultivars with moderate to low CAPS content are preferable due to the alkaloid toxicity, that can make it difficult to formulate medicines that are safe to be used by humans.18,90,91 In the food industry and for military usage, it is desirable that a product contains a large concentration of CAPS. Thus, cultivars with moderate to high CAPS content are usually applied.27,28,92,93
The C. chinense cultivars are mainly known for their elevated levels of pungency, usually presenting higher concentrations of capsaicin then other species. Therefore, C. chinense cultivars are associated with a high toxicity to human usage, which made their use as a medicine more difficult when compared to other species, such as C. annuum, which is popularly applied as a spice or even as a chemical weapon.15,41,94,95
C. annuum is the species with the most natural hybridization ability within the genus, which made it possible for it to be easily domesticated and cultivated worldwide. It is estimated that this species correspond to one of the most produced vegetables, representing approximately 9.8% of the total planted area on Earth.2,49,96
Additionally, it should be pointed out that C. annuum cultivars are more suitable to present species with dihydrocapisaicin as a major capsaicinoid during the maturity stage, although its inversion or even the presence of low levels of capsaicin cannot be directly correlated to low pungency levels, as cultivars such as Toftegaard Hot Banana and Guajillo are scaled as have moderate pungency in the Scoville heat scale, with a 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 5300 and 13
000 ± 5300 and 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 704.8 ± 2275.7 Scoville heat unit, respectively.88,97 It must be pointed out that all capsaicinoids influence the Scoville heat, although it is not common for researchers to describe this minor CAPS constitution, a fact that could help to elucidate behavior such as that observed in the Toftegaard Hot Banana and Guajillo.
704.8 ± 2275.7 Scoville heat unit, respectively.88,97 It must be pointed out that all capsaicinoids influence the Scoville heat, although it is not common for researchers to describe this minor CAPS constitution, a fact that could help to elucidate behavior such as that observed in the Toftegaard Hot Banana and Guajillo.
The variability of CAPS content is one of the factors that causes researchers to avoid the use of their profile, exclusively, as a reliable chemotaxonomic marker. This was discussed by Zewdie and Bosland, whose research demonstrated that there is no statistical relationship between capsaicinoids content within a specific cultivar or species, in means of the presence/absence, proportion and concentration of each of the principal CAPS.44 The absence of studies focused on the chemosystematics of the Capsicum genus is mainly related to the large chemical variability induced by the intraspecific and interspecific hybridization which occurs, not only by natural means as a consequence of the evolution process, but also by the human intervention that has been carried out since the marine exploration periods.4,44,98–100
In addition to the process of hybridization, the variability in CAPS profile can be attributed not only to the hybridization process but also to other factors, which can be either endogenous (species genetic characters and ripening stage) and exogenous (such as seasoning, climatic conditions and soil fertility).42,48,77,85 In addition, the mechanisms of CAPS regulation is not consolidated, it had been reported that their biosynthesis and catabolism undergoes very sensitive mechanisms.88
Factors affecting CAPS concentration
As the Capsicum species has a great biodiversity, with the existence of hundreds of different cultivars to each single species, a comprehensive study of how and which internal or external factors influence CAPS biosynthesis is extremely hard to accomplish or confirm. Despite this difficulty, it is known that the genotype, environmental conditions and fruit maturity stage have a great influence over the CAPS profile.88,97Production and accumulation of CAPSs is generally related to the expression of specific alleles in DNA, mainly the one denominated Pun1, which had been reported even in non-pungent species with unfunctional behavior. In addition, there is no solid information about the transcription mechanism of these genetic markers on the complex metabolic pathway of CAPS.48,101–103
Pun1 regulation has been associated with the quantity of CAPS produced by a certain cultivar and their susceptibility to undergo modifications in their CAPS profile when exposed to environmental stress conditions. A slight modification in its transcription among the different cultivars can cause the heterogeneous behavior in the CAPS profile observed within each species, a fact that determined that the genotype should be considered as the most important factor (among the three previously cited) affecting the CAPS content.52,97,102–107
When considering the external factors that can influence the CAPS content those that are emphasized are the temperature, amount of sunlight, soil fertility and water availability.64,108,109 Temperature and sunlight have been reported to present negative and positive correlations in accordance with the type of cultivar, although there is no homogeneity in the behavior of species.97,104,105,110
The correlation between CAPS content, temperature and sunlight can be postulated either as a compensatory mechanism of protection or a competition process. A positive correlation can indicate that CAPS can act as protective metabolites against oxidative stress from UV radiation. While, a negative correlation could appear as plants exposed to high temperatures use photosynthates in the growth process, for the production of lignin-like compounds, in a competitive metabolic pathway, which would reduce the CAPS content as a consequence.97,104,105,110,111 The heterogeneous behavior observed in these factors and the existence of cultivars that maintain a uniform CAPS content when they are varied,105 indicates that the influence of these factors is only of minor importance in the CAPS profile for economical and phylogenetic approaches.
The effect of soil fertility shows less concordance within the literature. Published work can be found discussing that either potassium and nitrogen have a positive or negative relationship with the CAPS content104,112–115 and also discussing whether the water holding capacity could decrease the CAPS content or present a heterogeneous correlation, similar to that described for temperature effects.116,117 In these studies the research was conducted without consideration of several other factors that affect soil fertility, such as granulometry, residual microbial biomass, micronutrients, organic carbon and relief type.118 Thereby, there is still a lack of information about how soil fertility affect CAPS content.
As previously cited, the CAPS content changes in accordance with the ripening stage to provide protective and pollinating properties.17,78,119 Although a slight variation occurs within cultivars, the fruit maturity is usually reached after 40 to 50 days,42,78,119 which is the moment where the major CAPS produced by each cultivar will be in a larger amount due the decrease in the peroxidase isoenzymes responsible for CAPS catabolism.76,85 Capsaicin and dihydrocapsaicin generally undergo the largest changes during ripening as they are the capsaicinoids in the greatest quantities within the Capsicum species.17,45,78 Depending on the days after anthesis, the CAPS profile will present several different proportions that can also include a moment of shift in each of the CAPS present in larger quantities.42,78,119
Capsinoids
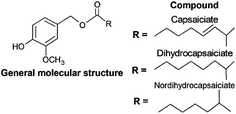
For decades, CAPS have being extensively explored by industry due to their bioactivity and pungency properties, although their application is limited due to the pungency and toxicology effects that small quantities produce in humans.19,124,125 A promising substitute to CAPS for industrial proposes is their analogue structure substances known as capsinoids, for which the main differential characteristic is the lower pungency.19Capsinoids can be defined as a group of substances within the CAPS class that are also synthesized exclusively within the Capsicum genus, in non-pungent and pungent cultivars.126 Capsinoids generally present a relatively low concentration in fruit (approximately 300 μg g−1).126–128 As can be observed in Fig. 4, their molecular structure differs from CAPS as there is an ester functional group in the connection of the vanillyl group with the carbon chain.19,124,126
In addition, the relatively few studies on the pharmaceutical application of capsinoids are mainly focused on capsiate. It has been reported that capsiate presents similar properties to capsaicin in terms of pain relief, anticarcinogenic agent and in the prevention of cardiovascular and gastrointestinal diseases, highlighting their possible application as a less toxic substitute for capsaicin.19,124,129
Carotenoids
Carotenoids are a group of nearly 750 tetraterpenoid compounds,130,131 widely distributed in plants and known by their chromogenic properties which attribute vivid orange, yellow and red colors to organisms.132 This class of pigments can be synthesized in all photosynthetic organisms, and in some non-photosynthetic organisms playing an important role in photosynthesis, photoprotection and stabilization of free radicals.130,131 They are not produced by animals, but carotenoids play an important role in their nutrition and health as they present antioxidant and anticarcinogenic activities and act as precursors to other essential compounds, such as vitamin A and retinoic acid.23,130–132The variety of colors within the Capsicum genus are partially promoted by the variety of carotenoids present within it, mainly the oxygenated ones, whose profile can change as a function of the maturity stage, species and cultivars, proving the complexity of the biosynthesis of carotenoids.16,23,39
Chemical structure, biosynthesis and classification
Carotenoids present a low polar molecular structure based on a tetraterpenoid skeleton, in which the most common structures presents 40 carbon atoms derived from isoprene units (Fig. 5).Their biosynthesis begins with the mevalonic acid which is submitted to several reactions to produce the building block C5 precursors isopentenyl diphosphate and dimethylallyl pyrophosphate.39,133,134 Furthermore, these precursors are submitted to condensation reactions to produce geranylgeranyl phosphate, which is the precursor for the first compound with 40 carbons in the metabolic pathway, the phytoene that presents no color.133–136
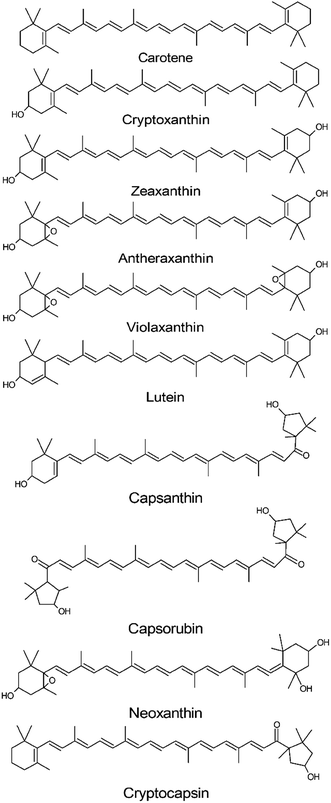
Among the sequential reactions that phytoene can undergo are a series of desaturation and isomerization reactions that will produce the red colored lycopene, which can undergo cyclization to originate α-carotene and β-carotene (orange colored carotenoids). In addition, carotene can produce the yellow colored xanthophylls lutein and zeaxanthin by the hydroxylation of their α or β structure, respectively.132,133 All these reactions are accompanied by the formation of conjugated double bonds, which can vary from 7–15 in colored carotenoids, while the colorless carotenoids present a maximum of five conjugated carbon double bonds.136 On the other hand, carotenoids in the Capsicum species usually contain nine conjugated double bonds.
Carotenoids can be divided into two main groups in accordance with their chemical structure: carotenes and xanthophylls. Carotenes groups include linear or cyclized hydrocarbons, as α-carotene and β-carotene, while xanthophylls include carotenoids with oxygenated functions such as hydroxy-, keto-, methoxy-, epoxy and carbonyl, such as those that occur in lutein, violaxanthin, neoxanthin and zeaxanthin.23,133,134,136 Another classification is based on the chromogenic properties of carotenoids.137 In this concept, carotenoids are divided into two fractions, yellow and red.136,138 Each fraction is related to the specific chemical structure characteristic that allows the compound to interact differently with visible radiation.138 Therefore, their conjugated double bond, length and oxygenated functions allow them to present a wide variety of absorption values in visible light.136,139
The yellow fraction is composed of carotenoids that present yellow or orange colors when observed with the human eye.139,140 The carotenoids of this fraction present different degrees of oxidation and include the precursors to the substances that compose the red fraction.137,139 Examples of the yellow fraction members are hydrocarbon based carotenoids (β and α-carotene), hydroxylated carotenoids (β-cryptoxanthin and zeaxanthin) and epoxidized carotenoids (curcubitaxanthin A and violaxanthin).138,139,141
The red fraction differs structurally from the yellow by the substitution of the 3-hydroxy β rings for a 3-hydroxyacylcyclopentane ring, which confers them with a greater stability.138 Among this fraction the ketocarotenoids can be found, that are almost exclusive to the Capsicum genus,137,139,141 the capsanthin, capsanthin 5,6-epoxide and capsorubin.
Carotenoids content within Capsicum
Capsicum fruits can be considered carotenogenic fruits due to the richness of the carotenoids profile within them, which contains over 50 different carotenoids.23,39,142,143 In addition, most of the carotenoids described within Capsicum are commonly found in other vascular plants,131,134–136 there is a restricted conjunction of the compounds from this class that are considered almost exclusive to the genus, as previously cited.The different colors observed in Capsicum fruits from different species, cultivars and maturity stages are partially associated with the profile of the carotenoids and their chromogenic activity.23,122,138 It had been reported by several authors that the carotenoid profile drastically changes during ripening.139–141,143 The colors of the fruits have a direct correlation with the major carotenoids, a fact that can be used to divide the study of carotenoids in Capsicum fruits in accordance to the color of the mature fruit.23,144,145
Rodriguez-Burruezo et al. compared the differences between the carotenoids profiles in accessions of C. baccatum, C. pubescens and C. annuum with genotype to produce yellow/orange mature fruits and those that produce red mature fruits.140 In this research, the accessions with yellow fruits presented as violaxanthin as the major carotenoid, which comprised 40 to 70% of the total carotenoids content. Other characteristics of yellow/orange accessions were the presence of cis-violaxanthin, antheraxanthin, and lutein as part of the major carotenoids and the absence of compounds from the red isochromic fraction. On the other hand, the red-fruited accessions presented capsanthin as the major carotenoid (composing 35 to 50% of the total carotenoid content) and low quantities of the yellow isochromic fraction, it was this fact that made these accessions richer in carotenoids than the yellow-fruited accessions. These results have being confirmed by other authors, which in a general manner have also described the ketocarotenoids of capsanthin, capsanthin 5,6-epoxide and capsorubin as being the major carotenoids found in different varieties of red pepper fruits in the maturity stage.137–139,141,143
The absence of the red isochromic fraction in the Capsicum species with yellow fruits is usually associated with the inactivation of the enzyme capsanthin–capsorubin synthase,140,144 which is basically responsible for the transformation of violaxanthin and antheraxanthin on capsorubin and capsanthin.131,141,144 This inactivation also explains the accumulation of violaxanthin as the major carotenoid in yellow-fruited species. Among these species the presence of lutein as a major carotenoid has also been reported.23,39,144 In Table 2 the main carotenoids described for Capsicum can be observed, emphasizing that carotenoids from the yellow and red fraction are inversely proportional, as previously cited.
| Species | Cultivar | Atrx | Nxt | Vxt | Lutein | Zxt | α-Ctxt | α-Crtn | β-Crtn | Capsb | Capst | Crypt | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Atrx = antheraxanthin; Nxt = neoxanthin; Vxt = violaxanthin; Zxt = zeaxanthin; α-Ctxt = α-cryptoxanthin; α-Crtn = α-carotene; β-Crtn = β-carotene; Capsb = capsorubin; Capst = capsanthin; Crypt = cryptocapsin; n.d = not detected.b mg 100 g−1 of dry fruit.c μg 100 g−1 of fresh fruit.d % of total carotenoid content. | |||||||||||||
| Annuum | Ancho | n.d | n.d | n.d | n.d | 4.29d | 5.28d | 2.1d | 20.9d | n.d | 9.69d | 0.24d | 160 |
| Annuum | Guajillo | 7.07d | n.d | n.d | n.d | 1.88d | 2.24d | 1.27d | 17.9 d | n.d | 12.6d | 10d | 160 |
| Annuum | 730 f1 | n.d | n.d | n.d | n.d | 17.15d | n.d | n.d | 5.03d | n.d | 53.13d | n.d | 123 |
| Annuum | 1245 f1 | n.d | n.d | n.d | n.d | 14.16d | n.d | n.d | 5.38d | n.d | 54.97d | n.d | 123 |
| Annuum | Amazon F1 | n.d | n.d | n.d | n.d | 25.54d | n.d | n.d | 4.63d | n.d | 46.96d | n.d | 123 |
| Annuum | Serademre 8 | n.d | n.d | n.d | n.d | 16.66d | n.d | n.d | 4.13d | n.d | 53.03d | n.d | 123 |
| Annuum | Kusak 295 F1 | n.d | n.d | n.d | n.d | 14.77d | n.d | n.d | 5.06d | n.d | 53.40d | n.d | 123 |
| Annuum | Jalapeño | n.d | n.d | 1.2b | n.d | 6.1b | 3.1b | n.d | 7.7b | 0.1b | 0.1b | n.d | 161 |
| Annuum | Vergasa | 1100c | n.d | 2.3c | n.d | 4.0c | 1.5c | n.d | 4.3c | 3.0c | 26.2c | n.d | 147 |
| Annuum | Ferrari F1 | 7.0d | 2.2d | 6.5d | n.d | n.d | n.d | n.d | 1.7d | 3.1d | 48.3d | n.d | 140 |
| Annuum | Vélez F1 | 4.8d | n.d | 67.5d | 13.4d | 1.7d | 0.7d | 2.4d | 0.9d | n.d | n.d | n.d | 140 |
| Annuum | Serrano | 0.2d | n.d | n.d | n.d | 0.2d | n.d | n.d | n.d | n.d | 3.3d | n.d | 23 |
| Annuum | Sinpezon | 0.5d | n.d | n.d | 0.4d | 0.1d | 0.1d | n.d | 10.8d | n.d | 2.9d | n.d | 23 |
| Annuum | Nagano | n.d | n.d | n.d | n.d | n.d | n.d | 0.60b | 2.01b | 4.01b | 22.88b | n.d | 39 |
| Annuum | Adami red | n.d | n.d | 2.59b | n.d | n.d | n.d | 0.53b | 1.88b | n.d | n.d | n.d | 39 |
| Annuum | Mazzora | 6.19b | 0.43b | 0.29b | 28.39b | 151.39b | 0.40b | 1.00b | 1.23b | n.d | n.d | n.d | 39 |
| Annuum | Raon orange | 0.89b | 0.25b | n.d | 22.24b | 88.80b | 0.71b | 0.81b | 0.76b | n.d | n.d | n.d | 39 |
| Annuum | Jorrit | 0.81b | 0.70b | 0.14 b | 8.75b | n.d | 0.04b | 0.09b | 0.15b | n.d | n.d | n.d | 39 |
| Annuum | Raon yellow | 1.35b | 1.00b | 2.85b | 21.08b | n.d | 0.09b | 0.04b | 0.30b | n.d | n.d | n.d | 39 |
| Annuum | Szentesi kosszarvú | n.d | n.d | 1.33d | n.d | 16.88d | 0.31d | 0.19d | 9.19d | 1.97d | 29.25d | 0.53d | 142 |
| Annuum | Ancho | n.d | n.d | 14.5d | n.d | 4.29d | 5.28d | 2.1d | 20.9d | 2.17d | 9.69d | 0.24d | 160 |
| Annuum | Guajillo | 7.07d | 0.28d | 13.2d | n.d | 1.88d | 2.24d | 1.27d | 17.9d | 0.35d | 12.6d | 10.0d | 160 |
| Annuum | Mulato | n.d | 2.20d | 22.0d | 0.88d | 3.56d | 0.72d | 2.98d | 14.9d | 4.20d | 11.2d | 0.62d | 160 |
| Annuum | Yellow cayenne | 20.1c | 34.6c | 151c | 226c | 40.4c | n.d | n.d | 24.0c | n.d | 0.0c | n.d | 151 |
| Annuum | Taballo | 249c | 54.4c | 1119c | 614c | 279c | n.d | n.d | 548c | n.d | 0.0c | n.d | 151 |
| Annuum | Hierro | 186c | 34.2c | 249c | 17.8c | 56.4c | n.d | n.d | 1524c | n.d | 786c | n.d | 151 |
| Baccatum | 7.8d | 9.9d | 9.2d | n.d | 2.5d | n.d | 0.6 | 3.1d | 7d | 27.6d | n.d | 140 | |
| Baccatum | Campana | 116c | 30.7c | 172c | 20.5c | 70.7c | n.d | n.d | 1258c | n.d | 230c | n.d | 151 |
| Baccatum | Aji angelo | 283c | 35.1c | 214c | 59.2c | 95.6c | n.d | n.d | 1150c | n.d | 592c | n.d | 151 |
| Chacoense | Chaco | 225c | 32.1c | 194c | 31.0c | 58.8c | n.d | n.d | 682c | n.d | 848c | n.d | 151 |
Carotenoids modifications during ripening
One of the factors that most influences the carotenoid content is the ripening process, which causes qualitative and quantitative modifications.23,122,139–141,144 The first modification that should be emphasized is in the form of the presentation of this class of compounds, which can appear in plants as free carotenoids or esterified,23,139 whereas the first cited form occurs mainly in leaves, although they appear in significant quantities in immature fruits.143 During ripening, carotenoids are synthesized and esterified with saturated or unsaturated fatty acids.139,144,146The esterification process occurs to give carotenoids more stability against thermal, photo and enzymatic oxidative reactions and increase their liposulibility.143,146 This allows carotenoids to raise their accumulation in fruit and in the maturity stage as these compounds are responsible for photo-protection, assisting photosynthesis, promoting the biosynthesis of other essential compounds (e.g. abscisic acid) and attracting insects to promote pollination and seed dispersal.130,139,144
Esterification occurs mainly in the red isochromic fraction, present in the chromoplast,139,146 which are esterified by short chain fatty acids, such as lauric, myristic, palmitic and linoleic acids.139,146,147 Meanwhile, the yellow isochromic fractions are mainly esterified by longer chain fatty acids such as mystiric, palmitic and the unsaturated form of linoleic acid, which is the major fatty acid reported in this fraction, comprising approximately 50% of the total fatty acids used in esterification.139,146 Moreover, in the ripe fruit only 21.3% of the total carotenoids remains in their free form.146
The carotenoid profile changes during ripening differently in accordance with the genotype, although its generally occurs with an increase in the total carotenoid content, which can be from approximately 10 to 90 fold.141,144,148 In the species with red fruits, the ripening process is associated with a greater increase in the total carotenoids content when compared to those with yellow fruits.39,144 In contrast to this affirmation, Agostini-Costa et al. found no significant difference between the increase in the carotenoid total content in yellow-fruited and red-fruited cultivars, which could raise the possibility that the genotype could control the increase of carotenoids.152
In the unripe red-fruit the main carotenoids are lutein and neoxanthin, which during ripening, almost completely vanish giving way to capsanthin, capsorubin and cryptocapsin.139,143,144,149 Capsanthin is usually responsible for the enlargement of the content of carotenoids in red-fruited species, as they comprise approximately 35–70% of the total carotenoid content in the ripe stage.143,144 Additionally, the intensity of the red color in the mature fruit has a direct correlation with the amount of capsanthin present.143,144
Chloroplast pigments, lutein, neoxanthin and β-carotene, mainly present in the vegetative stage gradually decrease in concentration during ripening, for two main reason: due to their use in the biosynthesis of chromoplast pigments (e.g. antheraxanthin, violaxanthin and capsanthin) and the inhibition of their synthesis to increase the production of the chromoplast pigments. The chloroplast pigments are usually associated with the process of photosynthesis, which is unfunctionalized during ripening, a fact that explains the decrease in their concentration, as long as they are not required during fruit development.141,143,144
The ripening in yellow-fruited species is extremely different to the red ones. During the immature stage both colors of fruit present lutein and β-carotene as major carotenoids. As ripening goes on, there is two possible scenarios in the yellow fruits, the accumulation of lutein and maintenance of β-carotene or the lowering of the levels of both previously cited carotenoids concomitantly with the increase in violaxanthin and α-carotene.139,143,144,150,151 Furthermore, violaxanthin and lutein are described as the main carotenoids in ripe yellow-fruited species.152 In species in which violaxanthin is the major carotenoid in ripe fruit, it comprises approximately 30–50% of the total content,139 while lutein can comprises 41–67%.144
The carotenoid profile is one of the characteristics associated with the quality of the Capsicum fruit and other natural products derived from it.143,147,153 This fact is associated either with the bioactivity that carotenoids give to the natural product, enriching their value as a healthy product, and the attractive color they promote in the natural product, which make them more attractive.81,143,153–156 Furthermore, acquiring proper knowledge about how each factor affects the carotenoid profile has become crucial for industrial applications of the Capsicum species.
The content of the carotenoids can vary during the cultivation, processing and storage of the natural product.117 In the cultivation context, establishing the behavior of the carotenoids content in different environmental conditions is important to determine the best place to cultivate them and strategies for the cultivation method.117,157 As carotenoids are part of the secondary metabolic system, environmental stress can modify their profile in order to provide metabolics that can maintain the plant health even in stressful conditions.117,158,159 Lee et al. reported that high temperatures induce the accumulation of xanthophyll, such as lutein, which can increase the resistance to thermal oxidative stress.152,158 In addition, xanthophylls are the precursors of abscisic acid, which regulate stomatal closure to avoid loss of water during drought scenarios.158
The accumulation of xanthophylls is indicated as the main factor that increases the total carotenoid content in species growth in temperature stress conditions.117,162,163 Okunlola et al.117 reported the effect of different degrees of drought stress on Capsicum species, which are known as sensitive species to excessive and restrained water availability conditions.157,164,165 In this paper was reported that the vegetative stages of Capsicum plants are more sensitive to the lack of water, presenting as one of the consequences a severe decrease in the total content of carotenoids, while the flowering and fruiting stages presented minor modifications in carotenoids content when exposed to drought stress. Moreover, the species tested responded to stress differently emphasizing the request for further studies approaching different genotypes.
In addition there have been several studies published concerning the stability of carotenoids during storage or when exposed to different temperatures, relatively few of these are focused on Capsicum products, which, as previously cited, present carotenoids as being almost exclusively to the genus; an exclusive class of substance that had been report to interact with carotenoids (capsaicinoids); and have a great biodiversity.23,143,156,166–168 In a study of Giuffrida et al., it had been demonstrated that the structure of the carotenoid was the controlling factor for their degradation during storage at different temperatures.156 The stability observed followed the following trend di-ester carotenoid > mono-ester carotenoid > free carotenoid.
Natural products, such as paprika, usually require the fruit to be submitted to a drying process, which can be performed using several techniques, as the step can cause the degradation of substances which are desirable in the final product the choice of the method must be made carefully.134,156,159,168,169
Cao et al. demonstrated in this context that the drying methods in which the temperature is raised slowly, such as sun drying and hot air drying, are preferable to those using an ultraviolet irradiation source as abrupt temperature changes and longer exposition time can increase the deterioration of the molecular structure.168 Additionally, Chuyen et al. demonstrated that the length of the time of exposure to heat, during the drying processes, may be the key factor to avoid degradation instead of the temperature itself, which would allow the use of higher temperatures.169
In terms of the processing the extraction procedure should be emphasized, Nath et al. demonstrated that the use of an enzyme-assisted extraction method can extract approximately 80% of the total carotenoid content, as enzymes are able to dissolve molecular membranes in which carotenoids are associated in plant tissue, thereby stabilizing the carotenoids structure in the process.153 Although the use of enzymes in extraction can present some difficulties in storage, to prevent enzyme degradation it still is a more environmentally friendly method when compared to the use of organic solvents, such as methanol, and time-consuming techniques, such as Soxhlet extraction.1,170,171
Flavonoids
Flavonoids are a class of chemical compounds that consist of more than 7000 secondary plant metabolites and are based on a structure with fifteen carbon atoms.172–174 This class of compound became famous due to their bioactive properties in mammals, who are not able to synthesize them,173 mainly due to their antioxidant activity.25 Other properties related to flavonoids are protective against inflammatory processes, hypertension, arthritis and AIDS.25,173,175 Due to these characteristics they have become very attractive to several branches of industry, such as food, pharmaceutical and clothing.173,175–177Flavonoids are known by their chromogenic properties as well as carotenoids, which are also partially responsible for the vivid colors observed by human eye in fruits and plants.172 Their accumulation in plants is associated with several other functions including UV protection, growth regulation, antimicrobial agents and attraction to pollinators.175,178,179
Flavonoids content
Among the pigments found in the Capsicum species, the presence of flavonoids, as well as the other previously cited class, should be emphasized.48 They are mainly accumulated in the peel, and although most published papers represent their content as aglycones flavonoids, they can occur in pepper as conjugated O-glycosides and C-glycosides derivates.180 Within Capsicum the quercetin and luteolin are described as the major flavonoids, representing approximately 41% of the total flavonoid content,46,48,122,158,180 generally in their hydrolysed form. Usually the content of flavonoids in pepper are expressed as the sum of these cited compounds, due to the difficulty of performing an acid hydrolysis in several of the other conjugate flavonoids.122,127 In Table 3 the content of flavonoids detected in different varieties of Capsicum can be observed.| Species | Variety | Quercetin | Luteolin | Ref. |
|---|---|---|---|---|
| C. annuum | CA408 | 2.0 | 3.1 | 48 |
| C. annuum | Mesilla | 1.0 | 5.3 | 48 |
| C. annuum | Ixtapa | 2.7 | 7.0 | 48 |
| C. annuum | TMJ | n.d | 3.2 | 48 |
| C. annuum | Pa137 | 3.7 | 1.2 | 48 |
| C. annuum | B58 | 2.7 | 3.9 | 48 |
| C. annuum | Tuxtlas | 1.2 | 2.3 | 48 |
| C. annuum | B22 | 1.65 | 5.88 | 158 |
| C. annuum | Banana Supreme | 49.89 | 7.18 | 158 |
| C. annuum | Sweet Jalapeño | 1.48 | 4.74 | 158 |
| C. annuum | Rio Grande Gold | 10.31 | 10.30 | 158 |
| C. chinense | TMH | Not described | 2.3 | 48 |
| C. chinense | Orange Habanero | 3.71 | 5.72 | 158 |
| C. chinense | Habanero | 10.21 | 4.96 | 176 |
| C. chinense | Habanero | 15.11 | n.d | 48 |
| C. spp. | PI 357509 | 48.33 | 8.51 | 158 |
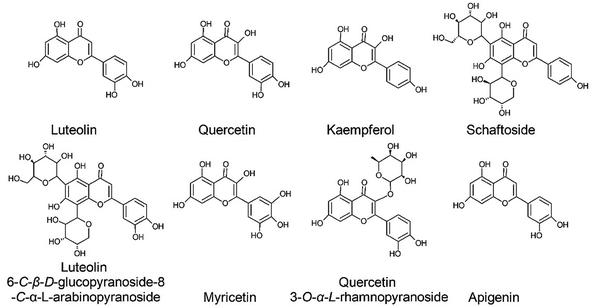
The following have been reported as part of the flavonoid profile within cultivars: quercetin, luteolin, kaempferol, apigenin, myricetin, quercetin 3-O-α-L-rhamnopyranoside, luteolin 6-C-β-D-glucopyranoside-8-C-α-L-arabinopyranoside, luteolin 7-O-(2-apiosyl-6-acetyl)-glucoside, luteolin-7-O-(2-apiosyl-diacetyl)-glucoside, apigenin 6 C-β-D-glucopyranoside-8-C-α-L-arabinopyranoside known as schaftoside, luteolin 7-O-[2-(β-D-apiofuranosyl)-β-D-glucopyranoside], chrysoeriol-7-O-(2-apiosyl-6-acetyl)glucoside, luteolin 6-C-hexosyl-8-C-rhamnosyl, apigenin-7-O-glucopyranoside, luteolin 6-C-rhamnosyl-8-C-hexosyl, luteolin-8-C-hexoside, luteolin-C-6-malonyl-C-pentoside, chrysoeriol 6,8-di-C-hexoside, luteolin 6-C-(6-malonyl)hexosyl-8-C-hexoside and luteolin 6 C-(6-malonyl)hexosyl-8-C-pentoside, as demonstrated in Fig. 6.48,158,180–182 As can be observed in Table 3, there is no pattern in the flavonoids content, which is reported to present a wide variability in the function of the genotype.46,158,176,182
Ripening and environmental effects in flavonoids content
Flavonoids content presents a contradictory pattern when compared to that discussed for CAPS and carotenoids during ripening. It has been reported that during ripening the flavonoid content decreases up until 85% of the original content in the vegetative stages.48,180,182This pattern can be associated with the bioactivity of quercetin, which is responsible for the photoprotection of the plant cells during photosynthesis.163,183 The intensity of this modification is correlated to the genotype factor. For example, while the C. chinense quercetin levels were described as 156.96 μg g−1 of fresh fruit during the immature stage and 10.21 μg g−1 of fresh fruit during mature stage,176 a C. annuum cultivar presented values of 3.3 μg g−1 of fresh fruit in the immature stage and 2.7 μg g−1 of fresh fruit in the mature stage.48 This contradictory behavior in relation to carotenoids and CAPS brings up the question of which stage of development the fruit can be used most efficiently as a rich source of antioxidant compounds, as flavonoids have been reported to influence the antioxidant activity.46
As with the previously cited class of substances, flavonoids are also reported to be influenced by environmental factors.163,176,184 As an example, in a paper published by Lee et al. it was demonstrated that the environmental effect on the flavonoids content presents a different pattern as a function of the genotype and that this variation is not as significant in flavonoids as it is for carotenoids and CAPS.163 In this research it was reported that the flavonoids content presented an increase when exposed to an area with higher temperature, which stimulates the production of these compounds as photoprotective agents for the plant organism.
Volatile composition
As one of the most consumed natural products worldwide, pepper must present attractive properties such as a pleasant flavor and aroma to induce the consumer to associate that specific product to a delicious aliment with high nutritional benefits.54,185–187The volatile fraction within Capsicum fruits is responsible for their aroma and can also provide the compound with bioactivity. This fraction had been reported as being rich and diverse, with over 200 substances described,54 including compounds classified as terpenes, hydrocarbons, alcohols, aldehydes, ketones, acids, esters, lactones and phenolics.
Among the substances identified in the volatile profile are thiol methane, dimethyl sulfide, dimethyl amine, acetaldehyde, propanal, acetone, 2-nonanone, hexane, acetic acid, 1-pentanol, limonene, pentadecane, ethyl ester propanoic acid, ethanol, 2-methyl-1-tetradecene, hexyl n-valerate, β-cubene, β-caryophyllene, butyrate, hexanal, α-pinene, linalool, limonene, hexyl isobutanoate, α-terpineol, methyl salicylate, heptyl isopentanoate, decanoic acid, β-cubebene, germacrene D, 6-methyl-4-heptenyl 2-methylpropanoate, pentyl 4-methyl-2-pentanoate, ethyl 3-methylpentanoate, 2-butyl acetate, α-copaene, byperene, α-humulene, γ-cadinene, and α-calacorene.54,60,121,188,189
Pino et al. characterized several C. chinense subjects of Habanero and found a specific profile in accordance to fruit colors.188 Cultivars with red fruits are usually associated with high amounts of hydrocarbons, which are correlated to the biosynthesis of capsaicin and degradation of carotenoids54,188 while orange-fruits correlate to high quantities of esters.188
Additionally, Kollmannsberger et al. characterized accessions of C. chinense and C. baccatum volatile profile by the presence of a high concentration and diversity of esters, while C. pubescens were almost absent in this class of substances. Moreover C. baccatum and C. chinense differentiate between themselves in the concentration of terpenoids, in which C. baccatum presented lower concentrations.121 Regarding the terpenoids profile, C. chinense and C. pubescens were identified by a different major terpenoid, which was cubenenes for C. chinense and ylangenes for C. pubescens.
In the volatile fraction there are a group of substances that are usually well associated to the Capsicum species as they are not very common in other tall plants, these are the alkyl-methoxy-pyrazines, which are usually associated with a very distinct aroma.188,190–193 The principal reported components of pyrazines are tetramethyl-pyrazine (oleoresin and paprika),190,191 3-isobutyl-2-methoxypyrazine in low concentrations in fruit,192,194 2-isobutyl-3-methoxypyrazine involved in the ripening processes of C. annuum cv. Kulai,193 and 2-isobutyl-3-methoxypyrazine in the mature fruits of C. chinense.188
Chemosystematic perspective
The chemosystematic study of botanic species is a promising tool in the discovery of biodiversity, although few researches focus on this topic even when dealing with botanical families with great economical interest, such as the Solanaceae family.99,100,106,195 From the 19th century to the year of 2008the systematics for Solanaceae were rewritten eight times, considering only those with good acceptance among the scientific community. Among these systematic proposals only 2 of them included chemical characters, the first in 1987 and the second in 2001.99Pigatto et al.99 (2015) performed a chemosystematic study of the Solanaceae family, to determine whether each species can synthesize two distinct classes of metabolites, tropane alkaloids and calysteliges. In this study it was highlighted that the Capsicum genus, four of the genera among the 29 studied, were unable to synthesize tropane alkaloids, presenting only two specific structure types of calysteliges (tetrahydroxynortropanes and pentahydroxynortropanes) and nicotinoids. This behavior was proposed as a unique pattern found in the New World species.
In addition to the genus level characterization, other researchers proved that it was possible to distinguish the Capsicum species through their chemical profile of flavonoids, carotenoids, and volatile compounds.4,52,54,127,196 According to Patel et al., peppers can be systematically studied by their aroma differences, being classified in accordance to the volatile profile within these classes of compounds.54 Classification into seven distinct groups in accordance to the concentration of the class of substances was proposed, as observed in Table 4.
| Group | Low concentration (peak area below 20%)a | High concentration (peak area above 30%)a |
|---|---|---|
| a Concentration are given as percentages of total mean gas chromatography peak areas. | ||
| 1 | Ketones and aldehydes | Hydrocarbons |
| 2 | — | Hydrocarbons, ketones and aldehydes |
| 3 | Hydrocarbons | Ketones and aldehydes |
| 4 | — | Terpenes |
| 5 | Terpenes | — |
| 6 | — | Ester |
| 7 | Mean values of all class | |
A relevant problem regarding the chemosystematic study of the Capsicum species is the great intraspecific and interspecific variability derived from the diversity of varieties and cultivars generated by the endogenous process of hybridization and exogenous genetic manipulation.4,52,197 In addition, the absence of a standard method to prepare and analyze samples of peppers, and a lack of definition as to which chemical characters are of greater relevance also makes it difficult to obtain a reliable database for the chemosystematic analysis of the genus.52,106,195,197
One strategy to overcome the absence of predefined chemical markers for the chemosystematic study is the application of the untargeted metabolomic, in which all detectable metabolites in the sample are analyzed simultaneously.198
Wahyuni et al. used this strategy to classify 32 pepper accessions from four distinct species into five groups according to their chemical profile of semi-polar metabolites, which included flavonoids, phenyl-propanoids, terpenoids, amino acids and fatty acid derivatives.197 Briefly, C. annuum accessions were separated into two groups by the different accumulation of acyclic diterpenoid phytatetraene; C. baccatum group was composed of all the analyzed accessions of this species, which were differentiated from the others by the presence of phenolic compounds with a sulfate conjugation; and the C. chinense and C. frutescens groups were separated and defined by their abundance of flavonoid glycosides.
Although the untargeted metabolomic offers the advantage of obtaining a sufficient number of characters to perform a high resolution systematic analysis and allows the discovery of new metabolites along the sample. It also presents certain disadvantages such as obtaining a huge amount of data, making it difficult to process data, and the appearance of undesired variations in the analytical response due to the presence of contaminating compounds (substances that can produce side reactions during analysis) in the sample or even the need for technical adjustments to perform the analysis.198–200 For example, Wahyuni et al. obtained 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 372 ions during analysis by mass spectrometry, of which 297 showed significant differences between the analyzed samples and only 88 were effectively identified.197 Thus, the target metabolomic should not necessarily be excluded as a possibility.
372 ions during analysis by mass spectrometry, of which 297 showed significant differences between the analyzed samples and only 88 were effectively identified.197 Thus, the target metabolomic should not necessarily be excluded as a possibility.
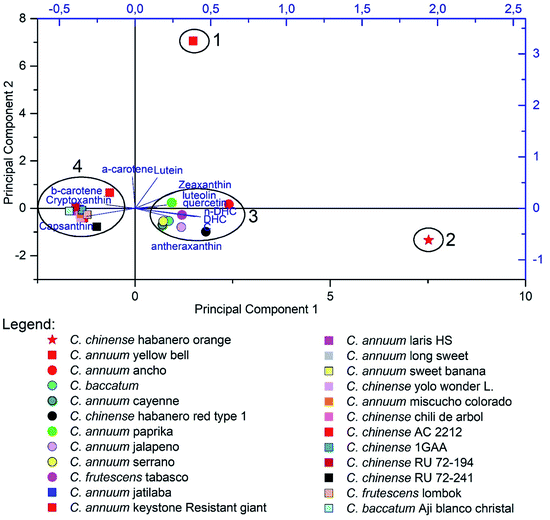
As previously discussed, the capsaicinoids and carotenoids profile in the Capsicum species are not classified as good chemotaxonomic markers due to their variability, despite the existence of unique compound from these classes within Capsicum.4,44,157 In this paper we tested a targeted metabolomic approach, in which the concentration or presence of defined compounds are used to establish similarity between different samples. The major compounds from capsaicinoids, carotenoids and flavonoids classes described during this review were used as variables to perform a Principal Component Analysis (PCA) of samples analyzed by other researchers.64,78,127,150,156,158,160,162,176,201–205 Before the PCA analysis, data were normalized to reduce the influence of different analytical methods applied by each researcher (see ESI material†).
PCA results are summarized in Fig. 7, in which the first two main components extracted represented a variation of 56.44% of the data, and the Principal Component 1 (36.09%) presented a positive correlation with the evaluated capsaicinoids, while the Principal Component 2 (20.35%) was correlated with carotenoids and flavonoids. In Fig. 7, it is possible to observe the formation of four groups. Group 1 corresponds to a non-pungent species with yellow fruit, which differs from the others due to the high levels of lutein and α-carotene, which do not participate in the biosynthetic route of the carotenoids capsathin and capsorubin, which were present at low levels in the constituent species of Group 1. Both of these mentioned substances belong to the red fraction of the carotenoids and are considered practically exclusive to the Capsicum genus, being synthesized mainly in pungent fruits of red, orange or brown color.144,156
Group 2 contains the sample with the highest level of pungency among those used,176 differing from the others by their high levels of capsaicinoids. When comparing groups 3 and 4 it can be noted that they are separated by the quantitative contents of their metabolites. While Group 3 presents high levels of carotenoids, Group 4 has a high concentration of flavonoids.
Although the observed groups do not present homogeneity in relation to the species within them, it can be noted that their formation is related to the biosynthetic routes, which may indicate pepper varieties that facilitate the prospection of individuals with economic interest without the need for extensive exploratory studies.196,206 The high variability of the data, responsible for the formation of Group 4, can be attributed to the intraspecific and interspecific variation within Capsicum varieties, the exogenous factors such as the cultivation conditions could cause the grouping of distinct species, such as in Group 4.158,176 It is noteworthy that the data used were not obtained by the same analytical methods, which can also generate an increase in the variation of the response obtained for each variable, thus highlighting the importance of the use of standardized methods in chemometric studies, in order to avoid undesirable patterns of variation.198,199
Another strategy to reduce intra and interspecific variation is through the use of a high number of individuals to represent a given species. This was suggested by Ballard et al., despite the logistic difficulty of his proposal, a sufficient number of specimens to represent a species would be 100 individuals.4
The best strategy for the systematic analysis of a botanical genus known for a great difficulty in the identification/differentiation of their species would be by the usage of the 3 basic foundations that describe a botanical individual, their morphology, chemical composition and genetic code. Singh et al.52 and Wahyuni et al.197 used a similar approach to evaluate the chemical and genetic data of the Capsicum varieties classification. However, in both cases, it highlighted the necessity for a definition of which genes and chemical compounds are the most significant for the systematic analysis. Part of the scarcity of data that could help in the chemosystematics study of the Capsicum genus occurs due to the wide economic interest, which means that most researchers focus on obtaining data that favors the use of these fruits by the industry, mainly the pharmaceutical sector.
Final considerations
The chemical composition of peppers enable them to be applied for different purposes. Among these uses are: a natural source of bioactive metabolites, a food additive for color and aroma, a pungent spice or even as a chemical weapon. For each of those products, the pepper need to possess a precise chemical profile, each of which can be specifically designed due to the cultivation processes, if the phytochemical behavior is well-know. A proper selection of cultivar, cultivation methods and harvest period, based on the chemical profile that is requested, can enhance the economic viability of pepper crops, increasing the reliability of post-harvest processes and production by the potential use of a lower fruit weight to obtain a product with the same quality.One of the greatest difficulties in the metabolomic study of the Capsicum genus is the large biodiversity that exists due to the natural hybridization, domestication and genetic manipulation processes. This diversity generates a wide range of metabolomic profiles within each species of Capsicum, raising the intraspecific and interspecific variability. Among the major classes of compounds found within the genus it has become a difficult task to understand the biochemical behavior of the Capsicum species when exposed to stress conditions.
A systematic study of the genus could be performed with the application of chemometric tools which are usually not applied in most of the papers published on this topic, whose focus is mainly to report a chemical pattern. It also may be necessary to divide cultivars by the main compound accumulating in their mature stage, such as lutein, violaxanthin, capsanthin, β-carotene, capsaicin, dihydrocapsaicin, sulfur volatile compound and terpenoids. This systematic study may provide a reliable form to identify cultivars, understand and predict their biochemical alteration and adaptation skills, and enable the management of cultivation areas.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support of the Coordination for the Improvement of Higher Education Personnel (CAPES) and the National Council for Scientific and Technological Development (CNPq).References
- X. Dong, X. Li, L. Ding, F. Cui, Z. Tang and Z. Liu, LWT--Food Sci. Technol., 2014, 59, 396–402 CrossRef.
- V. S. Govindarajan, Crit. Rev. Food Sci. Nutr., 1985, 22, 109–176 CrossRef PubMed.
- A. Garcés-Claver, M. S. Arnedo-Andrés, J. Abadía, R. Gil-Ortega and A. Álvarez-Fernández, J. Agric. Food Chem., 2006, 54, 9303–9311 CrossRef PubMed.
- R. E. Ballard, J. W. McClure, W. H. Eshbaugh and K. G. Wilson, Am. J. Bot., 1970, 57, 225 CrossRef.
- S. Das, K. C. Teja, B. Duary, P. K. Agrawal and S. S. Bhattacharya, Sci. Hortic., 2016, 213, 354–366 CrossRef.
- V. S. Govindarajan and M. N. Sathyanarayana, Crit. Rev. Food Sci. Nutr., 1991, 29, 435–474 CrossRef PubMed.
- M. Sganzerla, J. P. Coutinho, A. M. T. de Melo and H. T. Godoy, Food Res. Int., 2014, 64, 718–725 CrossRef PubMed.
- A. Berzal-Herranz, A. de la Cruz, F. Tenllado, J. R. Díaz-Ruíz, L. López, A. I. Sanz, C. Vaquero, M. T. Serra and I. García-Luque, Virology, 1995, 209, 498–505 CrossRef PubMed.
- G. A. Cordell and O. E. Araujo, Ann. Pharmacother., 1996, 27, 770–776 Search PubMed.
- K. Iwai, T. Suzuki and H. Fujiwake, Agric. Biol. Chem., 1979, 43, 2493–2498 Search PubMed.
- K. L. Bajaj and G. Kaur, Mikrochim. Acta, 1979, 71, 81–86 CrossRef.
- P. H. Todd, M. G. Bensinger and T. Biftu, J. Food Sci., 1977, 42, 660–665 CrossRef.
- I. Tolan, D. Ragoobirsingh and E. Y. S. A. Morrison, Phytother. Res., 2001, 15, 391–394 CrossRef PubMed.
- J. Díaz, F. Pomar, Á. Bernal and F. Merino, Phytochem. Rev., 2004, 3, 141–157 CrossRef.
- C. a. Reilly and G. S. Yost, Drug Metab. Rev., 2006, 38, 685–706 CrossRef PubMed.
- F. Conforti, G. A. Statti and F. Menichini, Food Chem., 2007, 102, 1096–1104 CrossRef.
- E. Fattourusso and O. Taglialatela-Scafati, Modern Alkaloids: Structure, Isolation, Synthesis and Biology, Wiley-VCH, Germany, 2nd edn, 2007 Search PubMed.
- M. Hayman and P. C. A. Kam, Curr. Anaesth. Crit. Care, 2008, 19, 338–343 CrossRef.
- X. J. Luo, J. Peng and Y. J. Li, Eur. J. Pharmacol., 2011, 650, 1–7 CrossRef PubMed.
- J. Roy, P. Saha, S. Sultana and A. S. Kenyon, Bull. W. H. O., 1997, 75, 19–22 Search PubMed.
- D. Nica-badea, Rev. Chim., 2016, 67, 1–4 Search PubMed.
- M. Lu, C.-T. Ho and Q. Huang, J. Food Drug Anal., 2017, 25, 27–36 CrossRef PubMed.
- D. Giuffrida, P. Dugo, G. Torre, C. Bignardi, A. Cavazza, C. Corradini and G. Dugo, Food Chem., 2013, 140, 794–802 CrossRef PubMed.
- P. Sricharoen, N. Lamaiphan, P. Patthawaro, N. Limchoowong, S. Techawongstien and S. Chanthai, Ultrason. Sonochem., 2017, 38, 629–639 CrossRef PubMed.
- H. M. Merken and G. R. Beecher, J. Agric. Food Chem., 2000, 48, 577–598 CrossRef PubMed.
- K. C. Baby and T. V. Ranganathan, Biocatal. Agric. Biotechnol., 2016, 7, 95–101 Search PubMed.
- C. A. Reilly, D. J. Crouch, G. S. Yost and A. A. Fatah, J. Chromatogr. A, 2001, 912, 259–267 CrossRef PubMed.
- M. F. Yeung and W. Y. M. Tang, Hong Kong Med. J., 2015, 21, 542–552 Search PubMed.
- A. C. de Aguiar, J. F. Osorio-Tobón, L. P. S. Silva, G. F. Barbero and J. Martínez, J. Supercrit. Fluids, 2018, 133, 86–93 CrossRef.
- O. Monago-Maraña, M. Guzmán-Becerra, A. Muñoz de la Peña and T. Galeano-Díaz, J. Food Compos. Anal., 2018, 67, 10–18 CrossRef.
- D. Dussault, K. D. Vu and M. Lacroix, Meat Sci., 2014, 96, 514–520 CrossRef PubMed.
- J. Shaikh, R. Bhosale and R. Singhal, Food Chem., 2006, 94, 105–110 CrossRef.
- J. E. Mendelson, B. K. Tolliver, K. L. Delucchi, M. J. Baggott, K. Flower, C. W. Harris, G. P. Galloway and P. Berger, Forensic Toxicol., 2010, 28, 33–37 CrossRef.
- M. D. L. Reyes-escogido, E. G. Gonzalez-mondragon and E. Vazquez-tzompantzi, Molecules, 2011, 16, 1253–1270 CrossRef PubMed.
- O. M. J. Adang and J. Mensink, Policing: An International Journal of Police Strategies & Management, 2004, 27, 206–219 Search PubMed.
- R. J. Haar, V. Iacopino, N. Ranadive, S. D. Weiser and M. Dandu, BMC Public Health, 2017, 17, 1–14 CrossRef PubMed.
- J. Patocka and K. Kuca, Military Medical Science Letters, 2011, 80, 72–79 CrossRef.
- C. Zhou, D. Ma, W. Cao, H. Shi and Y. Jiang, Food Addit. Contam., Part A, 2018 DOI:10.1080/19440049.2018.1457801.
- J. S. Kim, C. G. An, J. S. Park, Y. P. Lim and S. Kim, Food Chem., 2016, 201, 64–71 CrossRef PubMed.
- Y. J. Kim, A. R. Payal and M. K. Daly, Surv. Ophthalmol., 2016, 61, 434–442 CrossRef PubMed.
- M. K. Meghvansi, S. Siddiqui, M. H. Khan, V. K. Gupta, M. G. Vairale, H. K. Gogoi and L. Singh, J. Ethnopharmacol., 2010, 132, 1–14 CrossRef PubMed.
- L. H. Nugroho, AIP Conf. Proc., 2016, 1744, 020034 CrossRef.
- K. G. Sweat, J. Broatch, C. Borror, K. Hagan and T. M. Cahill, Food Chem., 2016, 210, 606–612 CrossRef PubMed.
- Y. Zewdie and P. W. Bosland, Biochem. Syst. Ecol., 2001, 29, 161–169 CrossRef PubMed.
- G. F. Barbero, A. Liazid, L. Azaroual, M. Palma and C. G. Barroso, Int. J. Food Prop., 2016, 19, 485–493 CrossRef.
- M. R. Loizzo, A. Pugliese, M. Bonesi, F. Menichini and R. Tundis, LWT--Food Sci. Technol., 2015, 64, 623–631 CrossRef.
- H. A. A. Gibbs and L. W. O'Garro, HortScience, 2004, 39, 132–135 Search PubMed.
- H. Bae, G. K. Jayaprakasha, K. Crosby, K. S. Yoo, D. I. Leskovar, J. Jifon and B. S. Patil, J. Food Compos. Anal., 2014, 33, 195–202 CrossRef.
- X. Wang, C. Zou, Y. Zhang, X. Shi, J. Liu, S. Fan, Y. Liu, Y. Du, Q. Zhao, Y. Tan, C. Wu and X. Chen, J. Cleaner Prod., 2018, 171, 934–943 CrossRef.
- F. Ma, Q. Yang, B. Matthäus, P. Li, Q. Zhang and L. Zhang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2016, 1021, 137–144 CrossRef PubMed.
- R.-M. Stoica, M. Moscovici and N. B. Tomulescu, Scientific Bulletin Series F. Biotechnologies, 2016, 20, 93–98 Search PubMed.
- S. Singh, D. R. Singh, R. Balaji, D. Nayak, A. K. Singh and N. Kumar, Afr. J. Biotechnol., 2013, 12, 5729–5737 Search PubMed.
- I. Domínguez-Martínez, O. G. Meza-Márquez, G. Osorio-Revilla, J. Proal-Nájera and T. Gallardo-Velázquez, J. Korean Soc. Appl. Biol. Chem., 2014, 57, 133–142 CrossRef.
- K. Patel, C. Ruiz, R. Calderon, M. Marcelo and R. Rojas, Food Res. Int., 2016, 89, 471–475 CrossRef PubMed.
- M. Ritota, F. Marini, P. Sequi and M. Valentini, J. Agric. Food Chem., 2010, 58, 9675–9684 CrossRef PubMed.
- Y. Wahyuni, A. R. Ballester, E. Sudarmonowati, R. J. Bino and A. G. Bovy, J. Nat. Prod., 2013, 76, 783–793 CrossRef PubMed.
- P. Dawan, T. Satarpai, P. Tuchinda, J. Shiowatana and A. Siripinyanond, Talanta, 2017, 162, 460–465 CrossRef PubMed.
- W.-K. Ryu, H.-W. Kim, G.-D. Kim and H.-I. Rhee, J. Food Drug Anal., 2017, 25, 798–803 CrossRef PubMed.
- A. Alberti, V. Galasso and B. Kovac, J. Phys. Chem. A, 2008, 112, 5700–5711 CrossRef PubMed.
- C. Y. Cheok, B. Sobhi, N. Mohd Adzahan, J. Bakar, R. Abdul Rahman, M. S. Ab Karim and Z. Ghazali, Food Chem., 2017, 216, 10–18 CrossRef PubMed.
- U. Schweiggert, R. Carle and A. Schieber, Anal. Chim. Acta, 2006, 557, 236–244 CrossRef.
- R. Mohammad, M. Ahmad and L. Y. Heng, Sensor, 2013, 13, 10014–10026 CrossRef PubMed.
- G. S. Subramanian, A. Karthik, S. B. Kamath, K. Prabahar, A. Ranjithkumar, S. Pathak and N. Udupa, J. Planar Chromatogr.--Mod. TLC, 2008, 21, 6007–6010 CrossRef.
- A. González-Zamora, E. Sierra-Campos, R. Pérez-Morales, C. Vázquez-Vázquez, M. A. Gallegos-Robles, J. D. López-Martínez and J. L. García-Hernández, J. Chem., 2015, 2015, 1–10 CrossRef.
- M. Monforte-González, F. Medina-Lara, G. Gutiérrez-Carbajal and F. Vázquez-Flota, J. Liq. Chromatogr. Relat. Technol., 2007, 30, 1697–1704 CrossRef.
- R. Ananthan, K. Subhash and T. Longvah, Food Chem., 2016, 238, 51–57 CrossRef PubMed.
- S. Liu, W. Li, Y. Wu, C. Chen and J. Lei, PLoS One, 2013, 8, e48156 CrossRef PubMed.
- C. Aza-González, H. G. Núñez-Palenius and N. Ochoa-Alejo, Plant Cell Rep., 2011, 30, 695–706 CrossRef PubMed.
- H. Fujiwake, T. Suzuki and K. Iwai, Agric. Biol. Chem., 1982, 46, 2519–2592 Search PubMed.
- H. L. Constant, G. A. Cordell and D. P. West, J. Nat. Prod., 1996, 59, 425–426 CrossRef.
- J. Walker, J. P. Ley, J. Schwerzler, B. Lieder, L. Beltran, P. M. Ziemba, H. Hatt, J. Hans, S. Widder, G. E. Krammer and V. Somoza, Mol. Nutr. Food Res., 2017, 61, 5–6 CrossRef PubMed.
- A. Peña-Alvarez, E. Ramírez-Maya and L. Á. Alvarado-Suárez, J. Chromatogr. A, 2009, 1216, 2843–2847 CrossRef PubMed.
- S. Saha, S. Walia, A. Kundu, C. Kaur, J. Singh and R. Sisodia, Int. J. Food Prop., 2014, 18, 1535–1545 CrossRef.
- J. Curry, M. Aluru, M. Mendoza, J. Nevarez, M. Melendrez and M. A. O'Connell, Plant Sci., 1999, 148, 47–57 CrossRef.
- P. Kirschbaum-Titze, E. Mueller-Seitz and M. Petz, J. Agric. Food Chem., 2002, 50, 1264–1266 CrossRef PubMed.
- P. Kirschbaum-Titze, C. Hiepler, E. Mueller-Seitz and M. Petz, J. Agric. Food Chem., 2002, 50, 1260–1263 CrossRef PubMed.
- H. Buczkowska, R. Nurzynska-Wierdak, H. Labuda and A. Salaya, Acta. Sci. Pol. Hortorum Cultus, 2016, 15, 185–198 Search PubMed.
- G. F. Barbero, A. G. Ruiz, A. Liazid, M. Palma, J. C. Vera and C. G. Barroso, Food Chem., 2014, 153, 200–206 CrossRef PubMed.
- M. Kuzma, K. Fodor, G. Maász, P. Avar, G. Mózsik, T. Past, E. Fischer and P. Perjési, J. Pharm. Biomed. Anal., 2015, 103, 59–66 CrossRef PubMed.
- T. Kawada, T. Suzuki, M. Takahashi and K. Iwai, Toxicol. Appl. Pharmacol., 1984, 72, 49–56 CrossRef.
- H. G. Daood, G. Palotás, G. Palotás, G. Somogyi, Z. Pék and L. Helyes, Food Res. Int., 2014, 65, 231–237 CrossRef.
- L. Koleva-Gudeva, S. Mitrev, V. Maksimova and D. Spasov, Hem. Ind., 2013, 67, 671–675 CrossRef.
- N. A. Golubkina, E. A. Dzhos, O. N. Pishnaya, M. I. Mamedov and S. M. Nadezhkin, Russian Agricultural Sciences, 2014, 40, 27–31 CrossRef.
- T. Stipcovich, G. F. Barbero, M. Ferreiro-González, M. Palma and C. G. Barroso, Food Chem., 2018, 239, 217–224 CrossRef PubMed.
- B. Estrada, M. A. Bernal, J. Díaz, F. Pomar and F. Merino, J. Agric. Food Chem., 2000, 48, 6234–6239 CrossRef PubMed.
- A. Schmidt, G. Fiechter, E. M. Fritz and H. K. Mayer, J. Food Compos. Anal., 2017, 60, 32–37 CrossRef.
- D. W. Barchenger and P. W. Bosland, Sci. Hortic., 2016, 203, 29–31 CrossRef.
- L. Duelund and O. G. Mouritsen, Food Chem., 2017, 221, 913–918 CrossRef PubMed.
- W. D. Rollyson, C. A. Stover, K. C. Brown, H. E. Perry, C. D. Stevenson, C. A. McNees, J. G. Ball, M. A. Valentovic and P. Dasgupta, J. Controlled Release, 2014, 196, 96–105 CrossRef PubMed.
- M. Archuleta, Oleoresin Capsicum: toxicology evaluation and hazard review, California, 1995 Search PubMed.
- M. d. L. Reyes-Escogido, E. G. Gonzalez-Mondragon and E. V. Tzompantzi, Molecules, 2011, 16(2), 1253–1270 CrossRef PubMed.
- K. G. Liljana, M. Viktorija, S. D. Marija, R. Gulabovski and I. J. Emilija, J. Food Sci. Technol., 2013, 917–922 Search PubMed.
- G. Melgar-Lalanne, A. J. Hernández-Álvarez, M. Jiménez-Fernández and E. Azuara, Food Bioprocess Technol., 2017, 10, 51–76 CrossRef.
- Y. J. Surh and S. Sup Lee, Life Sci., 1995, 56, 1845–1855 CrossRef PubMed.
- H. Krishnatreyya, H. Hazarika, A. Saha and P. Chattopadhyay, Eur. J. Pharmacol., 2018, 819, 114–121 CrossRef PubMed.
- C. Hayano-Kanashiro, N. Gámez-Meza and L. Á. Medina-Juárez, Crop Sci., 2016, 56, 1 CrossRef.
- A. González-Zamora, E. Sierra-Campos, J. G. Luna-Ortega, R. Pérez-Morales, J. C. R. Ortiz and J. L. García-Hernández, Molecules, 2013, 18, 13471–13486 CrossRef PubMed.
- C.-L. Lin, Y.-F. Kang, W.-J. Li, H.-T. Li, C.-T. Li and C.-Y. Chen, Chem. Nat. Compd., 2016, 52, 1145–1146 CrossRef.
- A. G. S. Pigatto, C. C. Blanco, L. A. Mentz and G. L. G. Soares, An. Acad. Bras. Cienc., 2015, 87, 2139–2149 CrossRef PubMed.
- A. G. S. Pigatto, L. A. Mentz and G. L. G. Soares, Cloning & Transgenesis, 2015, 04, 1–8 Search PubMed.
- N. Keyhaninejad, J. Curry, J. Romero and M. A. O'Connell, Plant Sci., 2014, 215–216, 59–68 CrossRef PubMed.
- Y. Tanaka, H. Yoneda, M. Hosokawa, T. Miwa and S. Yazawa, Sci. Hortic., 2014, 165, 242–245 CrossRef.
- M. J. Rodríguez-Maza, A. Garcés-Claver, S. W. Park, B. C. Kang and M. S. Arnedo-Andrés, Mol. Breed., 2012, 30, 889–898 CrossRef.
- T. Gurung, S. Techawongstien, B. Suriharn and S. Techawongstien, HortScience, 2011, 46, 1576–1581 Search PubMed.
- T. Gurung, S. Techawongstien, B. Suriharn and S. Techawongstien, Euphytica, 2012, 187, 11–18 CrossRef.
- B. M. Walsh and S. B. Hoot, Int. J. Plant Sci., 2012, 162, 1409–1418 CrossRef.
- E. A. Moscone, M. A. Scaldaferro, M. Grabiele, N. M. Cecchini, Y. S. García, R. Jarret, J. R. Daviña, D. A. Ducasse, G. E. Barboza and F. Ehrendorfer, Acta Hortic., 2007, 745, 137–169 CrossRef.
- K. P. Harvell and P. W. Bosland, HortScience, 1997, 32, 1292 Search PubMed.
- D. R. Wilson, R. C. Muchow and C. J. Murgatroyd, Field Crops Res., 1995, 43, 1–18 CrossRef.
- N. Jeeatid, S. Techawongstien, B. Suriharn, P. W. Bosland and S. Techawongstien, Hortic., Environ. Biotechnol., 2017, 58, 103–110 CrossRef.
- G. R. Cramer, K. Urano, S. Delrot, M. Pezzotti and K. Shinozaki, BMC Plant Biol., 2011, 11, 163 CrossRef PubMed.
- F. Medina-Lara, I. Echevarría-Machado, R. Pacheco-Arjona, N. Ruiz-Lau, A. Guzmán-Antonio and M. Martinez-Estevez, HortScience, 2008, 43, 1549–1554 Search PubMed.
- C. D. Johnson and D. R. Decoteau, HortScience, 1996, 31, 1119–1123 Search PubMed.
- M. Monforte-González, A. Guzmán-Antonio, F. Uuh-Chim and F. Vázquez-Flota, J. Sci. Food Agric., 2010, 90, 764–768 Search PubMed.
- M. A. Islam, S. S. Sharma, P. Sinha, M. S. Negi, B. Neog and S. B. Tripathi, Sci. Hortic., 2015, 183, 66–71 CrossRef.
- N. Ruiz-Lau, F. Medina-Lara, Y. Minero-García, E. Zamudio-Moreno, A. Guzmán-Antonio, I. Echevarría-Machado and M. Martínez-Estévez, HortScience, 2011, 46, 487–492 Search PubMed.
- G. O. Okunlola, O. A. Olatunji, R. O. Akinwale, A. Tariq and A. A. Adelusi, Sci. Hortic., 2017, 224, 198–205 CrossRef.
- M. E. C. Claessen, W. D. O. Barreto, J. L. De Paula and M. N. Duarte, Embrapa, 2009, 2, 7–9 Search PubMed.
- C. I. Victoria-Campos, J. D. J. Ornelas-Paz, O. P. Ramos-Aguilar, M. L. Failla, C. Chitchumroonchokchai, V. Ibarra-Junquera and J. D. Pérez-Martínez, Food Chem., 2015, 181, 325–332 CrossRef PubMed.
- G. F. Barbero, A. Liazid, M. Ferreiro-González, M. Palma and C. G. Barroso, Int. J. Food Prop., 2015, 19, 984–992 CrossRef.
- H. Kollmannsberger, A. Rodríguez-Burruezo, S. Nitz and F. Nuez, J. Sci. Food Agric., 2011, 91, 1598–1611 CrossRef PubMed.
- S. W. Meckelmann, C. Jansen, D. W. Riegel, M. van Zonneveld, L. Ríos, K. Peña, E. Mueller-Seitz and M. Petz, Eur. Food Res. Technol., 2015, 241, 817–825 CrossRef.
- A. Topuz and F. Ozdemir, J. Food Compos. Anal., 2007, 20, 596–602 CrossRef.
- D. Y. Kwon, Y. S. Kim, S. Y. Ryu, M. R. Cha, G. H. Yon, H. J. Yang, M. J. Kim, S. Kang and S. Park, J. Nutr. Biochem., 2013, 24, 1078–1085 CrossRef PubMed.
- A. Macho, C. Lucena, R. Sancho, N. Daddario, A. Minassi, E. Muñoz and G. Appendino, Eur. J. Nutr., 2003, 42, 2–9 CrossRef PubMed.
- S. Singh, R. Jarret, V. Russo, G. Majetich, J. Shimkus, R. Bushway and B. Perkins, J. Agric. Food Chem., 2009, 57, 3452–3457 CrossRef PubMed.
- Y. Wahyuni, A. R. Ballester, E. Sudarmonowati, R. J. Bino and A. G. Bovy, Phytochemistry, 2011, 72, 1358–1370 CrossRef PubMed.
- R. L. Jarret, J. Bolton and B. Perkins, HortScience, 2014, 49, 107–108 Search PubMed.
- A. Rosa, M. Deiana, V. Casu, S. Paccagnini, G. Appendino, M. Ballero and M. A. Dessí, J. Agric. Food Chem., 2002, 50, 7396–7401 CrossRef PubMed.
- N. Nisar, L. Li, S. Lu, N. C. Khin and B. J. Pogson, Mol. Plant Pathol., 2015, 8, 68–82 Search PubMed.
- J. J. Huang, S. Lin, W. Xu and P. C. K. Cheung, Biotechnol. Adv., 2017, 35, 597–618 CrossRef PubMed.
- T. Sun, H. Yuan, H. Cao, M. Yazdani, Y. Tadmor and L. Li, Mol. Plant Pathol., 2018, 11, 58–74 Search PubMed.
- E. Valduga, P. O. Tatsch, L. Tiggemann, H. Treichel, G. Toniazzo, J. Zeni, M. Di Luccio and A. Fúrigo, Quim. Nova, 2009, 23, 2429–2436 Search PubMed.
- P. M. Dewick, Medicinal Natural Products: A Biosynthetic Approach, Wiley-VCH, New York, 2009 Search PubMed.
- J. Hirschberg, Curr. Opin. Plant Biol., 2001, 4, 210–218 CrossRef PubMed.
- G. Britton, FASEB J., 1995, 9, 1551–1558 CrossRef PubMed.
- D. Hornero-Méndez and M. I. Minguez-Mosquera, J. Agric. Food Chem., 2001, 49, 3584–3588 CrossRef.
- A. Pérez-Gálvez and M. I. Mínguez-Mosquera, J. Agric. Food Chem., 2001, 49, 4864–4869 CrossRef.
- M. del R. Gómez-García and N. Ochoa-Alejo, Int. J. Mol. Sci., 2013, 14, 19025–19053 CrossRef PubMed.
- A. Rodriguez-Burruezo, C. Gonzalez-Mas Mdel and F. Nuez, J. Food Sci., 2010, 75, S446–S453 CrossRef PubMed.
- D. Hornero-Méndez, R. Gómez-Ladrón De Guevara and M. I. Mínguez-Mosquera, J. Agric. Food Chem., 2000, 48, 3857–3864 CrossRef.
- J. Deli, Z. Matus and G. Tóth, J. Agric. Food Chem., 1996, 44, 711–716 CrossRef.
- R. Arimboor, R. B. Natarajan, K. R. Menon, L. P. Chandrasekhar and V. Moorkoth, J. Food Sci. Technol., 2015, 52, 1258–1271 CrossRef PubMed.
- S. H. Ha, J. B. Kim, J. S. Park, S. W. Lee and K. J. Cho, J. Exp. Bot., 2007, 58, 3135–3144 CrossRef PubMed.
- A. Vera, J. Chávez, J. Carrillo and M. López, Chil. J. Agric. Res., 2011, 71, 578–585 CrossRef.
- M. I. Mínguez-Mosquera and D. Hornero-Méndez, J. Agric. Food Chem., 1994, 42, 640–644 CrossRef.
- A. Marín, F. Ferreres, F. A. Tomás-Barberán and M. I. Gil, J. Agric. Food Chem., 2004 Search PubMed.
- B. H. Davies, S. Matthews and J. T. O. Kirk, Phytochemistry, 1970, 9, 797–805 CrossRef.
- U. Schweiggert, D. R. Kammerer, R. Carle and A. Schieber, Rapid Commun. Mass Spectrom., 2005, 19, 2617–2628 CrossRef PubMed.
- I. Bonaccorsi, F. Cacciola, M. Utczas, V. Inferrera, D. Giuffrida, P. Donato, P. Dugo and L. Mondello, J. Sep. Sci., 2017, 7, 1–80 Search PubMed.
- A. Pugliese, M. R. Loizzo, R. Tundis, Y. O'Callaghan, K. Galvin, F. Menichini and N. O'Brien, Food Chem., 2013, 141, 2606–2613 CrossRef PubMed.
- T. da Silveira Agostini-Costa, I. da Silva Gomes, L. A. M. P. de Melo, F. J. B. Reifschneider and C. S. da Costa Ribeiro, J. Food Compos. Anal., 2017, 57, 73–79 CrossRef.
- P. Nath, C. Kaur, S. G. Rudra and E. Varghese, Agric. Res., 2016, 5, 193–204 CrossRef.
- A. J. Meléndez-Martínez, G. Britton, I. M. Vicario and F. J. Heredia, Food Res. Int., 2005, 38, 931–936 CrossRef.
- J. Robertson and K. Stevens, Nat. Prod. Rep., 2017, 31, 1721 RSC.
- D. Giuffrida, P. Dugo, G. Torre, C. Bignardi, A. Cavazza, C. Corradini and G. Dugo, Food Res. Int., 2014, 65, 163–170 CrossRef.
- D. A. Kopsell and D. E. Kopsell, Trends Plant Sci., 2006, 11, 499–507 CrossRef PubMed.
- J. J. Lee, K. M. Crosby, L. M. Pike, K. S. Yoo and D. I. Leskovar, Sci. Hortic., 2005, 106, 341–352 CrossRef.
- J. Cui, M. Yang, S. Park, D. Son, E. S. Jeong and S. I. Cho, Indian J. Agric. Res., 2017, 51, 149–154 Search PubMed.
- O. Collera-Zúñiga, F. García Jiménez and R. Meléndez Gordillo, Food Chem., 2005, 90, 109–114 CrossRef.
- B. Cervantes-Paz, E. M. Yahia, J. De Jesús Ornelas-Paz, C. I. Victoria-Campos, V. Ibarra-Junquera, J. D. Pérez-Martínez and P. Escalante-Minakata, Food Chem., 2014, 146, 188–196 CrossRef PubMed.
- V. M. Russo and L. R. Howard, J. Sci. Food Agric., 2002, 82, 615–624 CrossRef.
- C.-J. Lee, E. Yoo, J. Shin, J. Lee, H. Hwang and B. Kim, Mol. Cells, 2005, 19, 262–267 Search PubMed.
- H. M. Taylor, W. R. Jordan and T. R. Sinclair, Limitations to efficient water use in crop production, American Society of Agronomy, Madison, 1983 Search PubMed.
- M. M. Moreno, F. Ribas, A. Moreno and M. J. Cabello, Span. J. Agric. Res., 2003, 1, 65–74 CrossRef.
- D. B. R. Amaya, Food Carotenoids: Chemistry, Biology and Technology, IFT Press, Oxford, 2015 Search PubMed.
- H. G. Daood, J. Kapitány, P. Biacs and K. Albrecht, J. Sci. Food Agric., 2006, 86, 2450–2457 CrossRef.
- Z. Cao, L. Zhou, J. Bi, J. Yi, Q. Chen, X. Wu, J. Zheng and S. Li, J. Sci. Food Agric., 2016, 96, 3596–3603 CrossRef PubMed.
- H. V. Chuyen, P. D. Roach, J. B. Golding, S. E. Parks and M. H. Nguyen, J. Food Process. Preserv., 2017, 41, 1–9 Search PubMed.
- M. Mokhtar, M. Russo, F. Cacciola, P. Donato, D. Giuffrida, A. Riazi, S. Farnetti, P. Dugo and L. Mondello, J. Food Process. Preserv., 2016, 9, 1381–1390 Search PubMed.
- R. K. Saini and Y.-S. Keum, Food Chem., 2018, 240, 90–103 CrossRef PubMed.
- T. Iwashina, J. Plant Res., 2000, 113, 287–299 CrossRef.
- M. Waksmundzka-Hajnos, J. Sherma and T. Kowalska, Thin layer chromatography in phytochemistry, CRC Press, Boca Raton, 2008 Search PubMed.
- A. De Villiers, P. Venter and H. Pasch, J. Chromatogr. A, 2015, 1430, 16–78 CrossRef PubMed.
- A. Rodriguez-Mateos, D. Vauzour, C. G. Krueger, D. Shanmuganayagam, J. Reed, L. Calani, P. Mena, D. Del Rio and A. Crozier, Arch. Toxicol., 2014, 88, 1803–1853 CrossRef PubMed.
- J. D. Butcher, K. M. Crosby, K. S. Yoo, B. S. Patil, A. M. H. Ibrahim, D. I. Leskovar and J. L. Jifon, HortScience, 2012, 47, 574–579 Search PubMed.
- M. L. Falcone Ferreyra, S. P. Rius and P. Casati, Front. Plant Sci., 2012, 3, 1–15 Search PubMed.
- R. A. Dixon and G. M. Pasinetti, Plant Physiol., 2010, 154, 453–457 CrossRef PubMed.
- B. H. Havsteen, Pharmacol. Ther., 2002, 96, 67–202 CrossRef PubMed.
- A. Marín and F. A. Tomás-barberán, J. Agric. Food Chem., 2004, 52, 3861–3869 CrossRef PubMed.
- M. Materska, S. Piacente, A. Stochmal, C. Pizza, W. Oleszekc and I. Perucka, Phytochemistry, 2003, 63, 893–898 CrossRef PubMed.
- Y. K. Jang, E. S. Jung, H. A. Lee, D. Choi and C. H. Lee, J. Agric. Food Chem., 2015, 63, 9452–9460 CrossRef PubMed.
- M. Ghasemnezhad, M. Sherafati and G. A. Payvast, J. Funct. Foods, 2011, 3, 44–49 CrossRef.
- S. W. Meckelmann, D. W. Riegel, M. van Zonneveld, L. Ríos, K. Peña, E. Mueller-Seitz and M. Petz, Eur. Food Res. Technol., 2014, 240, 273–283 CrossRef.
- C. Qin, C. Yu, Y. Shen, X. Fang, L. Chen, J. Min, J. Cheng, S. Zhao, M. Xu, Y. Luo, Y. Yang, Z. Wu, L. Mao, H. Wu, C. Ling-Hu, H. Zhou, H. Lin, S. Gonzalez-Morales, D. L. Trejo-Saavedra, H. Tian, X. Tang, M. Zhao, Z. Huang, A. Zhou, X. Yao, J. Cui, W. Li, Z. Chen, Y. Feng, Y. Niu, S. Bi, X. Yang, W. Li, H. Cai, X. Luo, S. Montes-Hernandez, M. A. Leyva-Gonzalez, Z. Xiong, X. He, L. Bai, S. Tan, X. Tang, D. Liu, J. Liu, S. Zhang, M. Chen, L. Zhang, L. Zhang, Y. Zhang, W. Liao, Y. Zhang, M. Wang, X. Lv, B. Wen, H. Liu, H. Luan, Y. Zhang, S. Yang, X. Wang, J. Xu, X. Li, S. Li, J. Wang, A. Palloix, P. W. Bosland, Y. Li, A. Krogh, R. F. Rivera-Bustamante, L. Herrera-Estrella, Y. Yin, J. Yu, K. Hu and Z. Zhang, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 5135–5140 CrossRef PubMed.
- M. N. Mashabela, K. M. Selahle, P. Soundy, K. M. Crosby and D. Sivakumar, J. Food Sci., 2015, 80, H2612–H2618 CrossRef PubMed.
- F. Korel, N. Baǧdatlioǧlu, M. Ö. Balaban and Y. Hişil, J. Agric. Food Chem., 2002, 50, 3257–3261 CrossRef PubMed.
- J. Pino, M. González, L. Ceballos, A. R. Centurión-Yah, J. Trujillo-Aguirre, L. Latournerie-Moreno and E. Sauri-Duch, Food Chem., 2007, 104, 1682–1686 CrossRef.
- J. Pino, E. Sauri-Duch and R. Marbot, Food Chem., 2006, 94, 394–398 CrossRef.
- J. M. Guadayol, J. Caixach, J. Ribe and J. Caban, J. Agric. Food Chem., 1997, 45, 1868–1872 CrossRef.
- N. Kocsis, M. Amtmann, Z. Mednyánszky and K. Korány, J. Food Compos. Anal., 2002, 15, 195–203 CrossRef.
- H. Simian, F. Robert and I. Blank, J. Agric. Food Chem., 2004, 52, 306–310 CrossRef PubMed.
- M. M. Mazida, M. M. Salleh and H. Osman, J. Food Compos. Anal., 2005, 18, 427–437 CrossRef.
- R. Naef, A. Velluz and A. Jaquier, J. Agric. Food Chem., 2008, 56, 517–527 CrossRef PubMed.
- O. R. Gottliebt and M. R. de M. B. Borin, Quim. Nova, 2012, 35, 2105–2114 CrossRef.
- Y. Wahyuni, A. R. Ballester, Y. Tikunov, R. C. H. de Vos, K. T. B. Pelgrom, A. Maharijaya, E. Sudarmonowati, R. J. Bino and A. G. Bovy, Metabolomics, 2013, 9, 130–144 CrossRef PubMed.
- Y. Wahyuni, V. Stahl-Hermes, A. R. Ballester, R. C. H. de Vos, R. E. Voorrips, A. Maharijaya, J. Molthoff, M. V. Zamora, E. Sudarmonowati, A. C. M. Arisi, R. J. Bino and A. G. Bovy, Mol. Breed., 2014, 33, 503–518 CrossRef PubMed.
- E. Gorrochategui, J. Jaumot, S. Lacorte and R. Tauler, TrAC, Trends Anal. Chem., 2016, 82, 425–442 CrossRef.
- T.-L. Han, Y. Yang, H. Zhang and K. P. Law, F1000Research, 2017, 6, 967 Search PubMed.
- I. Aretz and D. Meierhofer, Int. J. Mol. Sci., 2016, 17 Search PubMed.
- L. R. Howard, S. T. Talcott, C. H. Brenes and B. Villalon, J. Agric. Food Chem., 2000, 48, 1713–1720 CrossRef PubMed.
- D. J. Simpson, M. R. Baqar and T. H. Lee, Z. Pflanzenphysiol., 1977, 83, 293–308 CrossRef.
- J. de J. Ornelas-Paz, J. M. Martínez-Burrola, S. Ruiz-Cruz, V. Santana-Rodríguez, V. Ibarra-Junquera, G. I. Olivas and J. D. Pérez-Martínez, Food Chem., 2010, 119, 1619–1625 CrossRef.
- H. Bae, G. K. Jayaprakasha, J. Jifon and B. S. Patil, Food Chem., 2012, 134, 1912–1918 CrossRef PubMed.
- Y. Lee, L. R. Howard and B. Villalón, J. Food Sci., 1995, 60, 473–476 CrossRef.
- K. Ortmayr, T. J. Causon, S. Hann and G. Koellensperger, TrAC, Trends Anal. Chem., 2016, 82, 358–366 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra02067a |
| This journal is © The Royal Society of Chemistry 2018 |