Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Amino acids as highly efficient modulators for single crystals of zirconium and hafnium metal–organic frameworks†
Ross J.
Marshall
a,
Claire L.
Hobday
b,
Colin F.
Murphie
a,
Sarah L.
Griffin
a,
Carole A.
Morrison
b,
Stephen A.
Moggach
*b and
Ross S.
Forgan
*a
aWestCHEM School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow, G12 8QQ, UK. E-mail: Ross.Forgan@glasgow.ac.uk; Web: http://www.forganlab.com
bEaSTCHEM School of Chemistry, University of Edinburgh, Joseph Black Building, David Brewster Road, Edinburgh, EH9 3FJ, UK. E-mail: s.moggach@ed.ac.uk; Web: http://www.moggach.chem.ed.ac.uk
First published on 4th February 2016
Abstract
The synthesis of zirconium and hafnium metal–organic frameworks (MOFs) often relies on coordination modulation – the addition of competing monotopic modulators to reaction mixtures – to reproducibly generate highly crystalline material. Typically, large excesses of monocarboxylic acids such as formic, acetic and benzoic acid are applied, but access to diffraction quality single crystals, particularly of UiO-66 topology MOFs, remains troublesome. Herein, we show that amino acids, in particular L-proline, are highly efficient modulators of Zr and Hf MOFs of the UiO-66 series, with as little as four equivalents affording access to large, diffraction quality single crystals that are free of defects. Five crystal structures are reported, including MOFs which previously could not be characterised in this manner, with molecular dynamics simulations utilised to understand dynamic disorder. Additionally, a series of MOFs are characterised in depth, allowing a comparison of the thermal stabilities and porosities for Zr and Hf analogues. We also show that the protocol can be extended to microwave synthesis, and that modulating ability varies dramatically across a series of amino acids. Access to single crystals has facilitated our own in depth study of the mechanical properties of these MOFs, and we expect that our protocols will enable the discovery of new Zr and Hf MOFs as well as offer new insights into their materials properties.
Introduction
Metal–organic frameworks (MOFs) are 3D hybrid materials that have received appreciable amounts of interest in recent years1 as the almost infinite combination of inorganic metal clusters and organic spacing ligands achievable allows highly specialised materials to be investigated for use in a wide range of applications including selective gas capture and sequestration,2 drug delivery3 and catalysis.4 Some MOFs have been known to exhibit relatively low chemical and mechanical stabilities, but the use of zirconium, a strong Lewis acid that demonstrates a high affinity for oxygen, results in resilient MOFs as a result of the robust Zr–O bonds that tether the organic and inorganic moieties.5Many zirconium MOFs adopt the well-documented UiO-66 framework topology containing Zr6O4(OH)4 secondary building units (SBUs) connected by 12 bridging dicarboxylate ligands, thereby expanding to form cubic framework structures containing large octahedral cages and smaller tetrahedral cages.5b Incorporation of organic ligands that present different coordination environments, including tri- and tetra-topic ligands, into Zr MOFs leads to materials with alternative structures and topologies.6 Functionalised ligands presenting greater chemical complexity, or bearing a functional group required for specific post-synthetic modification, have been incorporated in the anticipation of producing highly specialised materials.6b,d,e,7 Hafnium displays similar chemical properties to zirconium; hafnium MOFs are isostructural with their zirconium analogues, where direct substitution of the Zr6 SBUs by Hf6 ones is observed.8 There are fewer reports of hafnium MOFs in the literature,6i,9 but they have shown great promise in medicinal applications,10 as well as in catalysis11 and CO2 fixation.12
Coordination modulation13 results in materials with enhanced crystallinity and has recently enabled the isolation of single crystals of zirconium and hafnium MOFs with the UiO-66 topology.6b,d,e,7c,d,9b,14 This technique usually requires the addition of large excesses of small foreign materials directly to the MOF synthesis, with small monodentate organic compounds, such as acetic acid and benzoic acid, routinely employed. The precise role of the modulator during synthesis is not known, although it is believed that the crystallisation kinetics are altered, introducing reversibility to the system and ultimately resulting in products with improved crystallinity.15 Preformation of Zr6O4(OH)4(RCO2)12 building blocks containing the monodentate modulators may also be responsible for the enhanced crystallinity of the resulting MOFs.14b Under certain conditions, modulation can also promote defect formation, which is prevalent9d,14f,16 in UiO-66 type MOFs of both Zr and Hf, and these defects can be exploited to confer the MOFs with enhanced gas uptake,17 proton conduction,18 mechanical5f or catalytic19 properties.
Recently the scope of modulators has been broadened and the role of hydrochloric acid in zirconium MOF synthesis has been investigated. HCl modulation was observed to increase the rate of product formation whilst providing access to otherwise inaccessible materials, therefore it has been postulated that HCl could perform numerous roles during synthesis, including conditioning the DMF solvent or assisting in the pre-formation of Zr6 clusters.16b,20 Despite these advances, reliable isolation of X-ray quality single crystals of UiO-66 topology Zr and Hf MOFs remains troublesome, and so we seek to extend the range of potential modulators for both bulk and single crystal syntheses of Zr and Hf MOFs.
Results and discussion
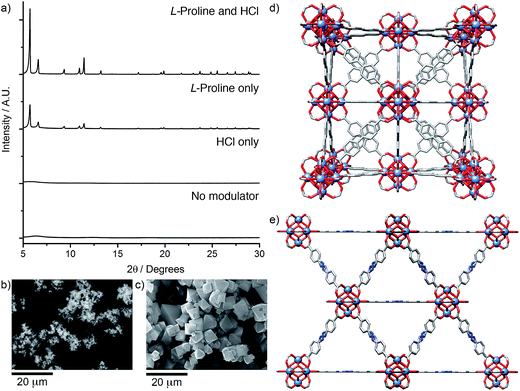
Herein, we fully investigate for the first time the ability of amino acids to act as modulating agents in the directed synthesis of both zirconium and hafnium MOFs. Amino acids can be viewed as attractive modulating agents as they are readily available small biomolecules and the large diversity of side-chains within these compounds may potentially result in unprecedented modulating properties. We have investigated21 a comprehensive range of ligands (L1–L8, Scheme 1) and report the influence of seven amino acids on the crystallinity of the products obtained, which have the general formula [M6O4(OH)4L6]n, where M = Zr4+ or Hf4+, and are referred to as M–L for simplicity. MOF syntheses were carried out to a general synthetic protocol (see ESI, Section S2†). One equivalent each of MCl4 and the chosen ligand were dissolved separately in reagent grade N,N-dimethylformamide (DMF), with five equivalents of the amino acid and one equivalent of HCl added to the metal solution. The solutions were combined (the final concentrations of metal and ligand being 45 mM), sonicated and heated for 24 h at 120 °C. The resultant precipitates were thoroughly washed by DMF and methanol, and vacuum dried.22 Powder X-ray diffraction (PXRD) analysis was used to determine the extent of formation and crystallinity of the MOFs in comparison to identical control samples with no amino acid added.Our examination of this broad experimental parameter space showed dramatic improvements in crystallinity of the Zr MOFs of the longer linkers, L5–L8, when certain amino acids were used as modulators; the results for Zr-L5 (Fig. 1) are representative of the longer linkers. The single crystal structure of Zr-L5 has been reported9b previously, using 47 equivalents of acetic acid as modulator, and comparison of the PXRD patterns of the amino acid modulated syntheses with that predicted from the crystal structure confirm that Zr-L5 is formed cleanly, and that the smaller, more hydrophobic amino acids, in concert with HCl, significantly enhance the crystallinity of the product in comparison to syntheses using HCl alone.23
 | ||
| Fig. 1 Stacked PXRD patterns of Zr-L5 synthesised with different amino acid modulators (five equivalents), compared with Zr-L5 synthesised with one equivalent of HCl only and the pattern predicted from the previously reported crystal structure.9b An unknown crystalline impurity, marked by an asterisk, was occasionally found in β-alanine modulated samples. | ||
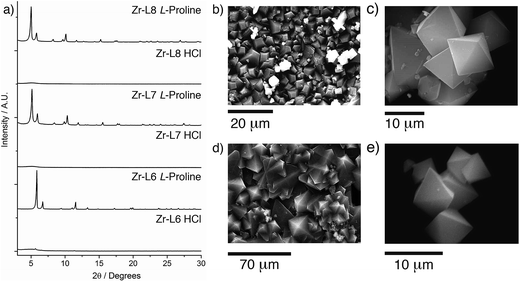
Across the Zr MOFs of L5–L8, L-proline stands out as the most effective modulator, with considerable improvements in the crystallinity of the MOFs (Fig. 2a). SEM images of materials synthesised according to the general synthetic procedure using five equivalents of L-proline (Fig. 2b and d) show Zr-L5 and Zr-L7 to form sheets of polycrystalline material, which has been observed previously24 for Zr-L7, with intergrown crystals of around 5–10 μm for Zr-L5 and up to 50 μm for Zr-L7. In contrast, Zr-L6 and Zr-L8 tend to form discrete octahedral crystals (Fig. 2c and e), around 10–20 μm in size for Zr-L6 (but with occasional larger crystals up to 60 μm) and 5–10 μm size for Zr-L8, which also forms some spherical polycrystalline assemblies (see ESI, Section S3†).
In contrast, the synthesis of Zr MOFs of L1–L4, all terephthalate-based linkers, did not show any appreciable benefits when amino acid modulation was employed in comparison with samples prepared using HCl alone. The binding of monocarboxylic acids to the zirconium cluster in situ has been proposed to decelerate the rate of crystallisation, through establishing exchange equilibrium with the bridging organic ligands,15 and so we are unable to account for this unusual, seemingly size-selective phenomenon.
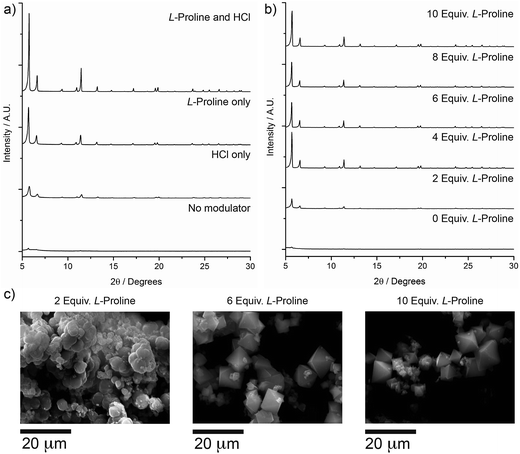
The overwhelming performance exhibited by L-proline directed future modulation studies of zirconium MOFs. Considering our synthetic technique combines HCl and L-proline it was vital to investigate the modulation ability of L-proline in the presence/absence of HCl, considering the recent work detailing the advantages of addition of HCl to zirconium MOF syntheses.16b,20Zr-L6 (UiO-67) was used to demonstrate the effect of L-proline modulation (see ESI, Section S4†). PXRD patterns (Fig. 3a) showed that while addition of one equivalent of HCl alone to solvothermal syntheses was capable of intermittently producing relatively crystalline material,23 addition of five equivalents of L-proline consistently produced better quality materials, and a synergistic effect appears to be present when both are incorporated into syntheses. Proline equivalency studies on Zr-L6 (see ESI Section S4†) revealed that the addition of between 4 and 10 equivalents of L-proline to syntheses alongside HCl resulted in significantly improved PXRD patterns (Fig. 3b). SEM images of the synthesised materials (Fig. 3c) showed that when two equivalents of L-proline are added to the synthesis, the Zr-L6 that forms is ill-defined with a globular morphology, while samples with between four and ten equivalents of L-proline added to syntheses resulted in well-defined octahedral crystals around 10–20 μm in size.
Examination of both PXRD patterns and SEM images suggest that four to five equivalents of L-proline and one equivalent of HCl represent the most efficient modulating conditions. Syntheses can also be carried out under microwave heating,25 with reduced synthesis times (see ESI, Section S5†). Zr-L6 prepared in 1 h in a single mode microwave cavity, using five equivalents of L-proline, showed comparable particle size, morphology and porosity to samples prepared by heating for 24 h in the oven.
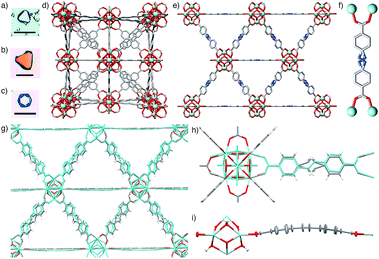
Further optimisation of the L-proline/HCl modulated conditions allowed the synthesis (see ESI, Section S6†) of single crystals of many of the MOFs (Fig. 4a–c). High quality single crystals of Zr-L6, approximately 60 μm in size, were synthesised solvothermally, at 120 °C in DMF for 24 h, in the presence of 5 equivalents of L-proline and one equivalent of HCl, which is minimal when compared to the large excess of modulators commonly required for effective modulation. Until recently, Zr-L6 had only been isolated as a microcrystalline powder however, during the course of this study single crystals were reported by a number of different groups. Kaskel et al. synthesised the MOF solvothermally, using 117 equivalents of acetic acid modulator,26 Lillerud et al. used 30 equivalents of benzoic acid and one equivalent of HCl as modulators in solvothermal syntheses using glassware first pre-treated with basic aqueous solution,27 Kim et al. reported a synthesis modulated by 30 equivalents of benzoic acid,28 while Shafir and Gutov reported a similar benzoic acid (30 equivalents) modulated route which included a subsequent “healing” step using additional L6. Our own determination of the crystal structure of Zr-L6 showed it to be very similar to those reported recently (Fig. 4d), with no evidence of defects or diffuse scattering observed.29
Similarly, L-proline modulation yielded 50–100 μm single crystals of both Zr-L7 and Zr-L8, which have “bent” linkers. Zr-L7 was reported previously in 2012 by Behrens et al., with the use of 30 equivalents of benzoic acid as the modulator resulting in crystalline sheets rather than discrete single crystals, and weak diffraction combined with disorder meaning no crystal structure could be elucidated.24 A recent catalytic study reported an acetic acid (525 equivalents) modulated synthesis of Zr-L7 that resulted in micron-sized particles.30 Isolation of discrete octahedral single crystals of Zr-L7 and Zr-L8 was possible using four equivalents of L-proline and one equivalent of HCl in a solvothermal synthesis at 100 °C for 48 h in DMF. During repeated syntheses, it was noted that order of addition was key to gaining large single crystals; addition of HCl to a pre-sonicated mixture of the other reagents provided optimal results.
Determination of the crystal structures of the closely related Zr-L7 and Zr-L8 MOFs revealed them to be isostructural (Fig. 4e). Despite the non-linearity of the “bent” azobenzene and stilbene linkers, L7 and L8, the resultant MOFs crystallise in the cubic Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group, with the ligands disordered at their interiors and exhibiting geometric frustration (Fig. 4f). The longer linkers result in larger unit cells; for Zr-L7, a = 29.3248(8) Å and for Zr-L8, the larger value of a = 29.8884(3) Å may be due to the longer length of the C
m space group, with the ligands disordered at their interiors and exhibiting geometric frustration (Fig. 4f). The longer linkers result in larger unit cells; for Zr-L7, a = 29.3248(8) Å and for Zr-L8, the larger value of a = 29.8884(3) Å may be due to the longer length of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond compared to the N
C bond compared to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond.31 Indeed, the separation between the Zr6 metal clusters, measured from analogous carboxylate oxygen atoms on an individual linker molecule, increases from 13.3104(2) Å in Zr-L7 to 13.5713(1) Å in Zr-L8, illustrating the increase in linker length.
N bond.31 Indeed, the separation between the Zr6 metal clusters, measured from analogous carboxylate oxygen atoms on an individual linker molecule, increases from 13.3104(2) Å in Zr-L7 to 13.5713(1) Å in Zr-L8, illustrating the increase in linker length.
Molecular dynamics (MD) simulations were utilised to understand the nature of the disorder in the MOFs and also to elucidate the slight differences in unit cell values (see ESI, Section S7†). We have previously used MD simulations to characterise the structural properties of Zr-L7 in a communication discussing its mechanical properties,21 and so analogous calculations were carried out on Zr-L8. The simulations confirm that the increase in unit cell length from Zr-L7 to Zr-L8 is a consequence of moving from an azobenzene to a stilbene linker, and reveal that the geometrically frustrated ligands, L7 and L8, are accommodated in the structures by bowing out of the mirror plane, sitting above and below the plane with bowing angles as high as 10° (Fig. 4g, average value 5(3)°, see ESI, Section S7†). The average effect of this dynamic disorder process, wherein the linkers bend and flip between opposite sides of the mirror plane, is the frustrated linear arrangement as observed in the crystal structure.
The time-averaged structures generated by these simulations show a close correlation to the spatially-averaged disorder in the experimental crystal structures (Fig. 4h), suggesting that the flexing of the ligand is responsible for the disorder, and atomic pair-distribution functions derived from the MD trajectory show that the ligand is indeed the most dynamic component of the structure (Fig. 4i). Finally, key bond distances within the Zr6 clusters of Zr-L8 match closely to those of its solid-state structure and those calculated for UiO-66 (Zr-L1) by Lamberti et al.,16d validating the simulations. The disorder model applied to the structures of Zr-L7 and Zr-L8 is very representative of the bulk material; PXRD patterns predicted from the single crystal structures match the experimental patterns from bulk-synthesised samples closely (see ESI, Section S7†).
Following effective amino acid modulation of the zirconium MOFs, we investigated the suitability of this technique for the synthesis of their hafnium analogues (see ESI, Section S8†). We initially sought to investigate the effect of both HCl and L-proline inclusion during the synthesis of Hf-L6, analogous to the control experiments performed for Zr-L6 (Fig. 5a). Surprisingly, HCl alone proved to be an ineffective modulator for synthesis of Hf-L6, even in the quantities found to modulate the synthesis of Zr-L6 by Farha et al.,16b however L-proline is observed to greatly enhance crystallinity. As with Zr-L6, it was observed that a combination of one equivalent of HCl and five equivalents of L-proline in the synthetic mixture leads to the greatest improvement in crystallinity of Hf-L6, yielding 10–20 μm octahedral crystals instead of amorphous material (Fig. 5b and c). Subsequently, syntheses of Hf-MOFs of L1–L8 were attempted using HfCl4 as a replacement for ZrCl4 in the reaction mixture, and modulated by six different amino acids.32 As with the zirconium analogues, the greatest enhancement in crystallinity from amino acid modulation is observed for the Hf MOFs of the longer linkers, L5–L8, although para-aminobenzoic acid seems to slightly improve the PXRD patterns of the Hf MOFs of the terephthalate ligands, L1–L4, when it is used as the modulator. For the longer linkers, the incorporation of smaller hydrophobic amino acids into MOF synthesis results in significantly improved crystallinity. L-Proline is again found to be the most effective modulator, although the synthesis of Hf-L8 seems to benefit most from inclusion of L-tryptophan.
Single crystals of Hf-L6 suitable for X-ray diffraction were isolated from solvothermal syntheses in DMF at 120 °C for 24 h with five equivalents of L-proline and one equivalent of HCl as modulators (see ESI, Section S9†). With small modifications in the synthesis procedure, it was possible to isolate single crystals of Hf-L7, using three equivalents of L-proline and one of HCl as modulators in a solvothermal synthesis in DMF at 100 °C for 48 h. Both MOFs are, as expected, isostructural to their zirconium analogues, with a comparison of some pertinent crystallographic details from the five structures (Table 1) illustrating the similarities between the Zr and Hf analogues. We have as yet been unable to isolate single crystals of Hf-L8, likely due to the tendency to form spherical microcrystalline architectures when modulated by L-proline in a manner similar to Zr-L8 (see ESI, Section S8†).
| MOF | Unit cell edge/Å | Unit cell Vol/Å3 | M–OOC bond/Å | M6 separationa/Å |
|---|---|---|---|---|
| a Measured as the separation between opposite carboxylate oxygen atoms of one linker molecule. | ||||
| Zr-L6 | 26.8564(5) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370.6(11) 370.6(11) |
2.227(3) | 11.209(4) |
| Hf-L6 | 26.7451(1) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130.9(2) 130.9(2) |
2.214(2) | 11.171(4) |
| Zr-L7 | 29.3248(8) | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 217.7(12) 217.7(12) |
2.233(6) | 12.960(8) |
| Hf-L7 | 29.2116(9) | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926.7(20) 926.7(20) |
2.194(12) | 12.959(17) |
| Zr-L8 | 29.8884(3) | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 699.8(8) 699.8(8) |
2.199(6) | 13.419(8) |
With a range of analogous Zr and Hf MOFs in hand, it was possible to examine their physical properties and similarities. Previous reports of the monocarboxylic acid modulation of Zr-L6 have described6d,16a,19,28 incorporation of acid modulators into the structure, postulated to be at defect or surface inclusion sites, and so 1H NMR spectra of acid-digested samples of Zr and Hf MOFs of L5–L8 were examined for evidence of L-proline incorporation (see ESI, Section S10†). Only Zr-L5 and Hf-L5 appeared to contain any appreciable quantities of L-proline or its breakdown products, which may be due to their smaller pore size than the other MOFs, although their incorporation at defects or as surface ligands cannot be ruled out. Thermogravimetric analysis (TGA) of the MOFs confirms that the Hf MOFs retain the thermal stability of their Zr analogues (see ESI, Section S11†), while an additional mass loss event at 350–450 °C for Zr-L5 and Hf-L5 is observed that can be ascribed to removal of incorporated L-proline, with a modulator content of 10% w/w calculated for the two analogous MOFs. Subtle changes in TGA profiles are evident as the ligand is changed for both the Zr and Hf MOFs, indicating that the organic linker has a role to play in the thermal stability of the MOFs.
N2 adsorption isotherms (see ESI, Section S12†) carried out at 77 K on the L-proline modulated materials show the high surface areas expected for highly crystalline MOFs (Fig. 6). In the case where the Zr MOFs have been reported previously,9b,16b,24,30 the BET surface areas closely correlate to those previously measured; 1300 m2 g−1 (Zr-L5), 2465 m2 g−1 (Zr-L6), 2830 m2 g−1 (Zr-L7) and 2950 m2 g−1 (Zr-L8). The value for Zr-L5 is slightly lower than reported previously by Kaskel et al. (1400 m2 g−1)9b which may be a consequence of the presence of L-proline, as indicated by 1H NMR spectroscopy and TGA. Pore size distributions calculated using QSDFT show an increase in major pore diameter in concert with the increase in linker length. The Hf analogues show decreased gravimetric surface areas in line with the increased mass of Hf compared to Zr, however, the surface areas of Hf-L5 (810 m2 g−1) and Hf-L8 (2020 m2 g−1) are slightly lower than expected based on this mass increase. Interestingly, both of these MOFs display spherical microcrystalline aggregates under SEM imaging, whereas all other MOFs formed either discrete single crystals or films, but the effect of morphology on accessible surface area has not been further investigated. The BET surface areas of Hf-L6 (1930 m2 g−1) and Hf-L7 (2270 m2 g−1) correlate closely to their corresponding Zr analogues.
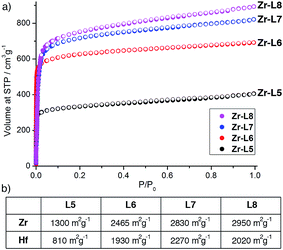 | ||
| Fig. 6 (a) N2 adsorption isotherms (77 K) for Zr-L5, Zr-L6, Zr-L7 and Zr-L8. (b) BET surface areas for all Zr and Hf MOFs, as derived from their N2 adsorption isotherms. | ||
Conclusions
To conclude, we report that amino acids, in concert with HCl, can effectively modulate the syntheses of zirconium and hafnium MOFs, enhancing crystallinity and ultimately resulting in the isolation of previously inaccessible MOFs, many as single crystals suitable for X-ray diffraction. Whilst a range of amino acids have been investigated, the greatest success has been achieved using L-proline, which is required in as little as four equivalents – a great reduction when compared with the large excess of modulator routinely required for single crystal synthesis (see ESI, Section S13†) – to offer unprecedented access to defect free single crystals of a number of UiO-66 topology MOFs of both Zr and Hf.33 The ability to reliably synthesise Zr and Hf MOFs simply and efficiently has allowed us to compare the properties of the analogous materials. We have found that the change in metal has little effect on thermal stability, and N2 adsorption isotherms show the MOFs have the high surface areas expected from materials of excellent crystallinity. Access to single crystals of Zr MOFs has already facilitated detailed investigations of their mechanical properties by high pressure single crystal X-ray diffraction and nanoindentation,21 and also single-crystal to single-crystal post-synthetic modification.14e We expect that adoption of amino acid modulated synthetic protocols, either solvothermally or microwave heated, will result in the improved synthesis of other Zr and Hf MOFs, and we are currently attempting to elucidate the mechanism of modulation while extending the protocol to MOFs linked by other metals.Acknowledgements
RSF thanks the Royal Society for receipt of a University Research Fellowship and the University of Glasgow for funding. The research was supported at the University of Glasgow by EPSRC (EP/L004461/1). CLH thanks the EPSRC and the University of Edinburgh for a studentship. CAM acknowledges the UK Car-Parrinello consortium (EP/K01465X/1) for access to the high performance computing resource ARCHER, as managed by the Edinburgh Parallel Computing Service. The authors gratefully acknowledge pump-priming funding from the EPSRC Directed Assembly Network (PP 14 05 03). We thank Mr Jim Gallagher at the University of Glasgow for assistance with SEM. The data which underpin this work are available at http://dx.doi.org/10.5525/gla.research-data.251. CCDC 1442841–1442842† contain the supplementary crystallographic data for this paper.References
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 6149 CrossRef PubMed
.
-
(a) D. Britt, D. Tranchemontagne and O. M. Yaghi, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11623–11627 CrossRef CAS PubMed
; (b) S. Ma and H.-C. Zhou, Chem. Commun., 2010, 46, 44–53 RSC
; (c) L. J. Murray, M. Dinca and J. R. Long, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC
; (d) K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T.-H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed
.
-
(a) A. C. McKinlay, R. E. Morris, P. Horcajada, G. Férey, R. Gref, P. Couvreur and C. Serre, Angew. Chem., Int. Ed., 2010, 49, 6260–6266 CrossRef CAS PubMed
; (b) P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie, T. Baati, J. F. Eubank, D. Heurtaux, P. Clayette, C. Kreuz, J.-S. Chang, Y. K. Hwang, V. Marsaud, P.-N. Bories, L. Cynober, S. Gil, G. Férey, P. Couvreur and R. Gref, Nat. Mater., 2010, 9, 172–178 CrossRef CAS PubMed
; (c) P. Horcajada, C. Serre, G. Maurin, N. A. Ramsahye, F. Balas, M. Vallet-Regí, M. Sebban, F. Taulelle and G. Férey, J. Am. Chem. Soc., 2008, 130, 6774–6780 CrossRef CAS PubMed
.
-
(a) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450–1459 RSC
; (b) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS PubMed
; (c) A. Corma, H. García and F. X. Llabrés i Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS PubMed
.
-
(a) R. J. Marshall, T. Richards, C. Hobday, C. F. Murphie, C. Wilson, S. A. Moggach, T. D. Bennett and R. S. Forgan, Dalton Trans., 2016 10.1039/C1035DT03178H
; (b) J. H. Cavka, S. Jakobsen, U. Olsbye, N. Guillou, C. Lamberti, S. Bordiga and K. P. Lillerud, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed
; (c) J. B. DeCoste, G. W. Peterson, H. Jasuja, T. G. Glover, Y.-G. Huang and K. S. Walton, J. Mater. Chem. A, 2013, 1, 5642–5650 RSC
; (d) M. Kandiah, M. H. Nilsen, S. Usseglio, S. Jakobsen, U. Olsbye, M. Tilset, C. Larabi, E. A. Quadrelli, F. Bonino and K. P. Lillerud, Chem. Mater., 2010, 22, 6632–6640 CrossRef CAS
; (e) J. E. Mondloch, M. J. Katz, N. Planas, D. Semrouni, L. Gagliardi, J. T. Hupp and O. K. Farha, Chem. Commun., 2014, 50, 8944–8946 RSC
; (f) B. van de Voorde, I. Stassen, B. Bueken, F. Vermoortele, D. De Vos, R. Ameloot, J.-C. Tan and T. D. Bennett, J. Mater. Chem. A, 2015, 3, 1737–1742 RSC
; (g) H. Wu, T. Yildirim and W. Zhou, J. Phys. Chem. Lett., 2013, 4, 925–930 CrossRef CAS PubMed
; (h) L.-M. Yang, E. Ganz, S. Svelle and M. Tilset, J. Mater. Chem. C, 2014, 2, 7111–7125 RSC
; (i) C. Hobday, R. J. Marshall, C. F. Murphie, T. Richards, D. Allan, T. Duren, F.-X. Coudert, R. S. Forgan, C. A. Morrison, S. A. Moggach and T. D. Bennett, Angew. Chem., Int. Ed., 2016, 55, 2401–2405 CrossRef CAS PubMed
.
-
(a) R. Wang, Z. Wang, Y. Xu, F. Dai, L. Zhang and D. Sun, Inorg. Chem., 2014, 53, 7086–7088 CrossRef CAS PubMed
; (b) H. Furukawa, F. Gándara, Y.-B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369–4381 CrossRef CAS PubMed
; (c) V. Guillerm, F. Ragon, M. Dan-Hardi, T. Devic, M. Vishnuvarthan, B. Campo, A. Vimont, G. Clet, Q. Yang, G. Maurin, G. Férey, A. Vittadini, S. Gross and C. Serre, Angew. Chem., Int. Ed., 2012, 51, 9267–9271 CrossRef CAS PubMed
; (d) A. Schaate, P. Roy, A. Godt, J. Lippke, F. Waltz, M. Wiebcke and P. Behrens, Chem.–Eur. J., 2011, 17, 6643–6651 CrossRef CAS PubMed
; (e) A. Schaate, P. Roy, T. Preuße, S. J. Lohmeier, A. Godt and P. Behrens, Chem.–Eur. J., 2011, 17, 9320–9325 CrossRef CAS PubMed
; (f) W. Morris, B. Volosskiy, S. Demir, F. Gándara, P. L. McGrier, H. Furukawa, D. Cascio, J. F. Stoddart and O. M. Yaghi, Inorg. Chem., 2012, 51, 6443–6445 CrossRef CAS PubMed
; (g) T. C. Wang, W. Bury, D. A. Gómez-Gualdrón, N. A. Vermeulen, J. E. Mondloch, P. Deria, K. Zhang, P. Z. Moghadam, A. A. Sarjeant, R. Q. Snurr, J. F. Stoddart, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2015, 137, 3585–3591 CrossRef CAS PubMed
; (h) D. Feng, Z.-Y. Gu, J.-R. Li, H.-L. Jiang, Z. Wei and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 10307–10310 CrossRef CAS PubMed
; (i) D. Feng, H.-L. Jiang, Y.-P. Chen, Z.-Y. Gu, Z. Wei and H.-C. Zhou, Inorg. Chem., 2013, 52, 12661–12667 CrossRef CAS PubMed
; (j) D. Feng, K. Wang, J. Su, T.-F. Liu, J. Park, Z. Wei, M. Bosch, A. Yakovenko, X. Zou and H.-C. Zhou, Angew. Chem., Int. Ed., 2015, 54, 149–154 CrossRef CAS PubMed
; (k) J. E. Mondloch, W. Bury, D. Fairen-Jimenez, S. Kwon, E. J. DeMarco, M. H. Weston, A. A. Sarjeant, S. T. Nguyen, P. C. Stair, R. Q. Snurr, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2013, 135, 10294–10297 CrossRef CAS PubMed
; (l) W. Liang, H. Chevreau, F. Ragon, P. D. Southon, V. K. Peterson and D. M. D'Alessandro, CrystEngComm, 2014, 16, 6530–6533 RSC
.
-
(a) P. W. Siu, Z. J. Brown, O. K. Farha, J. T. Hupp and K. A. Scheidt, Chem. Commun., 2013, 49, 10920–10922 RSC
; (b) S. J. Garibay and S. M. Cohen, Chem. Commun., 2010, 46, 7700–7702 RSC
; (c) H.-L. Jiang, D. Feng, T.-F. Liu, J.-R. Li and H.-C. Zhou, J. Am. Chem. Soc., 2012, 134, 14690–14693 CrossRef CAS PubMed
; (d) B. Wang, H. Huang, X.-L. Lv, Y. Xie, M. Li and J.-R. Li, Inorg. Chem., 2014, 53, 9254–9259 CrossRef CAS PubMed
; (e) K. Manna, T. Zhang and W. Lin, J. Am. Chem. Soc., 2014, 136, 6566–6569 CrossRef CAS PubMed
; (f) H. Fei and S. M. Cohen, Chem. Commun., 2014, 50, 4810–4812 RSC
.
- S. Jakobsen, D. Gianolio, D. S. Wragg, M. H. Nilsen, H. Emerich, S. Bordiga, C. Lamberti, U. Olsbye, M. Tilset and K. P. Lillerud, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 125429 CrossRef
.
-
(a) V. Bon, I. Senkovska, I. A. Baburin and S. Kaskel, Cryst. Growth Des., 2013, 13, 1231–1237 CrossRef CAS
; (b) V. Bon, I. Senkovska, M. S. Weiss and S. Kaskel, CrystEngComm, 2013, 15, 9572–9577 RSC
; (c) V. Bon, V. Senkovskyy, I. Senkovska and S. Kaskel, Chem. Commun., 2012, 48, 8407–8409 RSC
; (d) M. J. Cliffe, W. Wan, X. Zou, P. A. Chater, A. K. Kleppe, M. G. Tucker, H. Wilhelm, N. P. Funnell, F.-X. Coudert and A. L. Goodwin, Nat. Commun., 2014, 5, 4176 CAS
; (e) M. Zhang, Y.-P. Chen, M. Bosch, T. Gentle, K. Wang, D. Feng, Z. U. Wang and H.-C. Zhou, Angew. Chem., Int. Ed., 2014, 53, 815–818 CrossRef CAS PubMed
; (f) P. Xydias, I. Spanopoulos, E. Klontzas, G. E. Froudakis and P. N. Trikalitis, Inorg. Chem., 2014, 53, 679–681 CrossRef CAS PubMed
.
-
(a) K. Lu, C. He and W. Lin, J. Am. Chem. Soc., 2014, 136, 16712–16715 CrossRef CAS PubMed
; (b) K. E. deKrafft, W. S. Boyle, L. M. Burk, O. Z. Zhou and W. Lin, J. Mater. Chem., 2012, 22, 18139–18144 RSC
.
- J. Zheng, M. Wu, F. Jiang, W. Su and M. Hong, Chem. Sci., 2015, 6, 3466–3470 RSC
.
- M. H. Beyzavi, R. C. Klet, S. Tussupbayev, J. Borycz, N. A. Vermeulen, C. J. Cramer, J. F. Stoddart, J. T. Hupp and O. K. Farha, J. Am. Chem. Soc., 2014, 136, 15861–15864 CrossRef CAS PubMed
.
- C. V. McGuire and R. S. Forgan, Chem. Commun., 2015, 51, 5199–5217 RSC
.
-
(a) M. I. Gonzalez, E. D. Bloch, J. A. Mason, S. J. Teat and J. R. Long, Inorg. Chem., 2015, 54, 2995–3005 CrossRef CAS PubMed
; (b) V. Guillerm, S. Gross, C. Serre, T. Devic, M. Bauer and G. Férey, Chem. Commun., 2010, 46, 767–769 RSC
; (c) B. Li, B. Gui, G. Hu, D. Yuan and C. Wang, Inorg. Chem., 2015, 54, 5139–5141 CrossRef CAS PubMed
; (d) L. Li, S. Tang, C. Wang, X. Lv, M. Jiang, H. Wu and X. Zhao, Chem. Commun., 2014, 50, 2304–2307 RSC
; (e) R. J. Marshall, S. L. Griffin, C. Wilson and R. S. Forgan, J. Am. Chem. Soc., 2015, 137, 9527–9530 CrossRef CAS PubMed
; (f) C. A. Trickett, K. J. Gagnon, S. Lee, F. Gándara, H.-B. Bürgi and O. M. Yaghi, Angew. Chem., Int. Ed., 2015, 54, 11162–11167 CrossRef CAS PubMed
; (g) G. Wißmann, A. Schaate, S. Lilienthal, I. Bremer, A. M. Schneider and P. Behrens, Microporous Mesoporous Mater., 2012, 152, 64–70 CrossRef
.
- G. Zahn, P. Zerner, J. Lippke, F. L. Kempf, S. Lilienthal, C. A. Schroder, A. M. Schneider and P. Behrens, CrystEngComm, 2014, 16, 9198–9207 RSC
.
-
(a) O. V. Gutov, M. G. Hevia, E. C. Escudero-Adán and A. Shafir, Inorg. Chem., 2015, 54, 8396–8400 CrossRef CAS PubMed
; (b) M. J. Katz, Z. J. Brown, Y. J. Colon, P. W. Siu, K. A. Scheidt, R. Q. Snurr, J. T. Hupp and O. K. Farha, Chem. Commun., 2013, 49, 9449–9451 RSC
; (c) G. C. Shearer, S. Chavan, J. Ethiraj, J. G. Vitillo, S. Svelle, U. Olsbye, C. Lamberti, S. Bordiga and K. P. Lillerud, Chem. Mater., 2014, 26, 4068–4071 CrossRef CAS
; (d) L. Valenzano, B. Civalleri, S. Chavan, S. Bordiga, M. H. Nilsen, S. Jakobsen, K. P. Lillerud and C. Lamberti, Chem. Mater., 2011, 23, 1700–1718 CrossRef CAS
.
- H. Wu, Y. S. Chua, V. Krungleviciute, M. Tyagi, P. Chen, T. Yildirim and W. Zhou, J. Am. Chem. Soc., 2013, 135, 10525–10532 CrossRef CAS PubMed
.
- J. M. Taylor, S. Dekura, R. Ikeda and H. Kitagawa, Chem. Mater., 2015, 27, 2286–2289 CrossRef CAS
.
- F. Vermoortele, B. Bueken, G. Le Bars, B. van de Voorde, M. Vandichel, K. Houthoofd, A. Vimont, M. Daturi, M. Waroquier, V. van Speybroeck, C. Kirschhock and D. E. De Vos, J. Am. Chem. Soc., 2013, 135, 11465–11468 CrossRef CAS PubMed
.
- F. Ragon, P. Horcajada, H. Chevreau, Y. K. Hwang, U. H. Lee, S. R. Miller, T. Devic, J.-S. Chang and C. Serre, Inorg. Chem., 2014, 53, 2491–2500 CrossRef CAS PubMed
.
- The synthesis and crystallographic characterisation of Zr-L6 and Zr-L7 were recently reported by us in a preliminary communication concerning their mechanical properties (see ref. 5i). The synthesis and crystallographic characterisation of Zr-L8 were recently reported by us in a communication concerning the effect of post-synthetic modification on mechanical properties (see ref. 5a).
- During the course of our study, it became apparent that Zr-L8 was unstable in the presence of methanol, which has previously been observed for certain MOFs of the UiO-66 topology (see ref. 5c). To avoid sample degradation, acetone replaced methanol in the work up of all MOFs containing L8 (see ESI†).
- HCl has previously been reported to enhance the synthesis of members of the UiO-66 series when incorporated in large quantities into solvothermal syntheses (see ref. 16b and 20). In our hands, syntheses of Zr-MOFs of ligands L5–L8 with the addition of only one equivalent of HCl did not give reproducible results, particularly in the synthesis of Zr-L6 (UiO-67) with some products showing good crystallinity and others being completely amorphous. This observation correlates with previous work by Behrens, et al., who reported that addition of HCl did not improve their syntheses of UiO-67 (see ref. 6d).
- A. Schaate, S. Dühnen, G. Platz, S. Lilienthal, A. M. Schneider and P. Behrens, Eur. J. Inorg. Chem., 2012, 2012, 790–796 CrossRef CAS
.
-
(a) Y. Li, Y. Liu, W. Gao, L. Zhang, W. Liu, J. Lu, Z. Wang and Y.-J. Deng, CrystEngComm, 2014, 16, 7037–7042 RSC
; (b) M. Taddei, P. V. Dau, S. M. Cohen, M. Ranocchiari, J. A. van Bokhoven, F. Costantino, S. Sabatini and R. Vivani, Dalton Trans., 2015, 44, 14019–14026 RSC
.
- G. Nickerl, M. Leistner, S. Helten, V. Bon, I. Senkovska and S. Kaskel, Inorg. Chem. Front., 2014, 1, 325–330 RSC
.
- S. Øien, D. Wragg, H. Reinsch, S. Svelle, S. Bordiga, C. Lamberti and K. P. Lillerud, Cryst. Growth Des., 2014, 14, 5370–5372 Search PubMed
.
- N. Ko, J. Hong, S. Sung, K. E. Cordova, H. J. Park, J. K. Yang and J. Kim, Dalton Trans., 2015, 44, 2047–2051 RSC
.
-
Zr-L6 crystallises in the Fm
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m cubic space group, with a unit cell edge a = 26.8564(5) Å for our DMF-solvated sample, a value very similar to the reported parameters for (i) a fully DMF solvated sample, where a = 26.896(3) Å (see ref. 26), a second DMF solvated sample, where a = 26.783(3) Å (see ref. 28), a third DMF solvated sample, where a = 26.812(2) Å (see ref. 16a), (iv) a partially desolvated crystal, where a = 26.8809(3) Å (see ref. 27), and (v) a powdered sample, where a = 26.8499(15) Å (see ref. 6d).
m cubic space group, with a unit cell edge a = 26.8564(5) Å for our DMF-solvated sample, a value very similar to the reported parameters for (i) a fully DMF solvated sample, where a = 26.896(3) Å (see ref. 26), a second DMF solvated sample, where a = 26.783(3) Å (see ref. 28), a third DMF solvated sample, where a = 26.812(2) Å (see ref. 16a), (iv) a partially desolvated crystal, where a = 26.8809(3) Å (see ref. 27), and (v) a powdered sample, where a = 26.8499(15) Å (see ref. 6d). - L. T. M. Hoang, L. H. Ngo, H. L. Nguyen, H. T. H. Nguyen, C. K. Nguyen, B. T. Nguyen, Q. T. Ton, H. K. D. Nguyen, K. E. Cordova and T. Truong, Chem. Commun., 2015, 51, 17132–17135 RSC
.
- Our experimental values for Zr-L7 compare well to previously reported values of a = 29.4227(4) Å and a = 29.4433(4) Å for powder and single crystal examples, respectively (see ref. 24) and a = 29.3994(2) Å in the space group Fm
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m; for a powder sample (see ref. 30).
m; for a powder sample (see ref. 30). - L-Arginine was not used in these evaluations as it showed no modulating effect for the Zr-MOFs and generally produced tar-like materials rather than powder.
- While some modulators that are usually utilised in large amounts during synthesis of Zr MOFs are available at very low cost (e.g. formic and acetic acid) our highly efficient amino acid modulated syntheses dramatically cut down on waste and do not routinely induce defects in the MOFs.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1442841–1442842. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ta10401g |
| This journal is © The Royal Society of Chemistry 2016 |