Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Visible light promoted thiol-ene reactions using titanium dioxide†
Venugopal T.
Bhat
,
Petar A.
Duspara
,
Sangwon
Seo
,
Nor Syazwani Binti
Abu Bakar
and
Michael F.
Greaney
*
School of Chemistry, University of Manchester, Oxford Rd, Manchester, M13 9PL, UK. E-mail: michaelgreaney@manchester.ac.uk
First published on 3rd February 2015
Abstract
The radical addition of thiols to alkenes is reported under photoredox conditions, using visible light and TiO2 as a cheap and readily available photocatalyst.
Recent developments in visible light photoredox catalysis (PRC) have substantially expanded the scope and application of organic photochemistry.1 Using a domestic lightbulb as the light source, rather than specialist UV equipment, the process uses a photo-active catalyst to mediate electron transfer between reactants, resulting in novel transformations that frequently proceed under mild conditions. The vast majority of PRC in organic synthesis to date is based on two classes of catalyst; transition metal complexes, such as the archetypal Ru(bpy)3Cl2 and Ir(ppy)3 systems, or organic dyes such as methylene blue or eosin-Y. The Noble metals of the first class are expensive and a limited resource, but are highly optimisable in terms of ligand-metal tuning. Organic dyes lack this particular quality, but may be tuned via conventional synthesis and are usually less expensive. We were interested in exploiting a third class of photoredox catalyst; semi-conducting metal oxides such as titania (TiO2). With 4 million tons of titania being produced per annum, the material is cheap, readily accessible, and non-toxic (commonly ingested as food additive E 171), as well as offering the process benefits of heterogeneous catalysis such as simple removal by filtration.2 The UV photoredox properties of TiO2 have been extensively investigated and applied on an industrial scale to areas such as water treatment and photovoltaics. Applications to organic synthesis, however, remain under-developed, particularly in the area of visible light photochemistry.3,4 We were interested in developing a titania-mediated PRC reaction using visible light, and chose to investigate the thiol-ene reaction for this purpose.
The addition of thiols to alkenes is a radical reaction that is typically initiated with UV light.5 Although an old reaction,6 its reliability, substrate scope, and compatibility has led to renewed interest in the context of click chemistry, where the facile C–S bond formation can be used as a powerful ligation strategy in biological and materials science.7 Indeed, the largest field of application for thiol-ene chemistry is currently in polymer science, where thiols are cross-linked with alkenes under UV curing conditions, producing polymers with very uniform step-growth properties.8 Recent literature reports have established PRC for thiol-ene addition using both Ru(II) and eosin-Y photocatalysis, providing an important frame of reference for our inchoate titania process.9–11
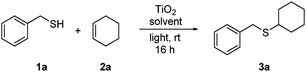
We were aware at the outset that titania visible light PRC would likely require a nanoparticulate form of the catalyst. Unmodified titania absorbs very weakly in the visible region, but there is evidence that substrate binding to the surface can shift the absorption and enable electron transfer.4a,c–e Rueping and co-workers, for example, have recently demonstrated visible light photoredox activity in dehydrogenative coupling reactions using commercially available titania P25 nanoparticles,4d,e and we chose to begin our studies using this material (aeroxide® from Sigma-Aldrich). Using the addition of benzyl mercaptan (1a) to cyclohexene (2a) as a test reaction, we were pleased to observe very high conversions into adduct 3a using one equivalent of TiO2 in MeCN under irradiation with a domestic light bulb (Table 1). The reaction was also viable in DCM (entry 2), under neat conditions (entry 3), and using blue LEDs as an alternative light source (entry 4).
| Entry | TiO2 (equiv.) | Solvent | Light source | Yieldb (%) |
|---|---|---|---|---|
| a Cyclohexene (1.0 mmol), benzyl mercaptan (4.0 mmol), titania (TiO2 P25) and solvent (1.0 mL) at room temperature for 16 h; light source placed 10–15 cm away from reaction vials. b Yields determined by 1H NMR using nitromethane as the internal standard. c Isolated yields. d Foil-wrapped flask for 16 h. e Reaction for 40 h. f Using degassed MeCN under N2 atmosphere. g Using 2.5 mmol of benzyl mercaptan. | ||||
| 1 | 1.0 | MeCN | 20 W light | 98 (96c) |
| 2 | 1.0 | DCM | 20 W light | 96 |
| 3 | 1.0 | — | 20 W light | 97 |
| 4 | 1.0 | MeCN | Blue LED | 80 |
| 5d | 1.0 | MeCN | No light | 0 |
| 6e | 0 | MeCN | 20 W light | 20 |
| 7 | 0 | MeCN | Blue LED | 0 |
| 8 | 0.5 | MeCN | 20 W light | 88 |
| 9 | 0.1 | MeCN | 20 W light | 82 |
| 10e | 0.1 | MeCN | 20 W light | 92 |
| 11f | 1.0 | MeCN | 20 W light | 50 |
| 12g | 1.0 | MeCN | 20 W light | 84 |
Control experiments established that titania in the dark gave no reaction (entry 5), but there was a small background reaction with ambient light in the absence of titania (20% conversion). We were pleased to see that the titania loading could be reduced to catalytic quantities with little drop in overall yield (entries 8–10), and we established that oxygen was beneficial for the reaction. Use of an inert atmosphere and de-gassed solvent halved the efficiency of the process (entry 11). This was an expected requirement, as an oxidative quencher is needed to balance charge and prevent hole-electron recombination in the titania photocatalyst (vide infra).
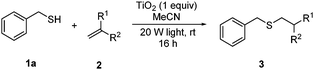
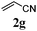
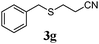
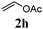
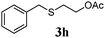
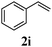
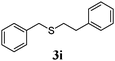
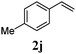
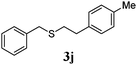
With a titania PRC system in place, we moved on to investigate substrate scope, beginning with the alkene partner. We were pleased to find that a variety of primary and 1,1-disubstituted substrates underwent successful thiol-ene reaction with benzyl mercaptan (Table 2). The reaction was tolerant of a variety of functional groups (ester, alcohol, silane, nitrile, halogen) giving the thiol ene adducts 3 in generally high yields. Substitution was tolerated at the 2- and 3-positions of the alkene (entries 3 and 4), but 1,2-disubstitution was restricted to cyclic alkenes. Vinyl acetate and acrylonitrile were both productive, interestingly undergoing no reaction at all in the absence of PRC (entries 6 and 7). More reactive alkenes such as styrenes gave very high yields of the thiol ene products, but background reaction was high for 2i and 2j.
| Entry | Alkene | Product | Yieldb (%) |
|---|---|---|---|
| a Alkene (1.0 mmol), benzyl mercaptan (4.0 mmol), titania (TiO2 P25, 1.0 mmol) and MeCN (1.0 mL) at room temperature for 16 h; light source placed 10–15 cm away from reaction vials. b Isolated yields; numbers in parentheses are the yield obtained under standard reaction conditions where titanium dioxide is absent. | |||
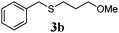
| 1 |

|
|
85 (9) |
| 2 |
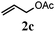
|
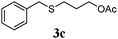
|
75 (61) |
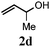
| 3 |
|
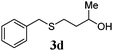
|
78 (12) |
| 4 |
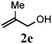
|

|
89 (68) |
| 5 |
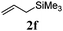
|
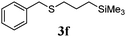
|
97 (31) |
| 6 |
|
|
43 (0) |
| 7 |
|
|
80 (0) |
| 8 |
|
|
98 (90) |
| 9 |
|
|
98 (93) |
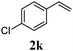
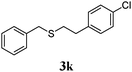
| 10 |
|
|
95 (30) |
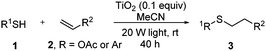
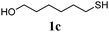
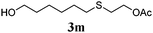
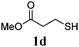
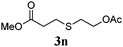
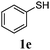
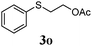
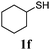
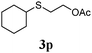
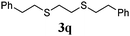
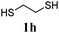
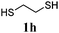
Thiol scope was initially trialled (Table 3) using catalytic amounts of titania (0.1 equiv.) and vinyl acetate (2h), an alkene with zero background reaction for the addition of benzyl mercaptan (Table 1, entry 7). We were pleased to find that the catalytic protocol was successful, with primary alkyl thiols (entries 1–3) and thiophenols (entry 4) undergoing thiol-ene reaction under the longer reaction time of 40 h. Increasing steric hindrance around the thiol was deleterious, with cyclohexyl thiol reacting in moderate yield (entry 5) and tert-butyl thiol undergoing no reaction, even with stoichiometric titania (entry 6). We could extend the reaction to a double thiol-ene reaction, with ethane and propane dithiol undergoing addition to styrenes in good yield (entries 7–9). p-Methoxystyrene proved less reactive, and required stoichiometric quantities of titania to yield 3t in an acceptable timeframe.
| Entry | Thiol | Product | Yieldc (%) |
|---|---|---|---|
| a Thiol (4.0 mmol), vinyl acetate (1.0 mmol), titania (TiO2 P25, 0.1 mmol) and MeCN (1.0 mL) at room temperature for 16–40 h; light source placed 10–15 cm away from reaction vials. b Dithiol (1 mmol), styrene (4 mmol) and conditions as previous. c Isolated yields; numbers in parentheses are the yield obtained under standard reaction conditions where titanium dioxide is absent. d 1 equiv. of titania used. | |||
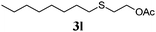
| 1 |
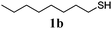
|
|
86 (0) |
| 2 |
|
|
79 (8) |
| 3 |
|
|
87 (30) |
| 4 |
|
|
61 (14) |
| 5 |
|
|
30 (5) |
| 6d |
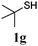
|
— | 0 |
| 7 |
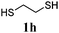
|
|
68 (0) |
| 8 |
|
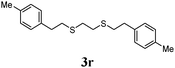
|
73 (0) |
| 9 |
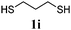
|
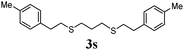
|
70 (0) |
| 10d |
|

|
57 (0) |
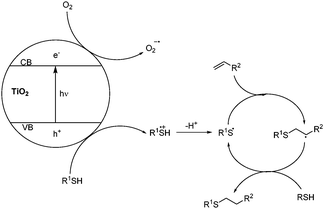
A mechanism for the reaction is outlined in Scheme 1, based upon the photo-excitation of electrons to the conduction band of the titania catalyst. The resultant holes are reductively quenched by the thiol, generating a thiyl radical cation, which can lose a proton to give a thiyl radical. Oxygen acts as a sacrificial electron acceptor, enhancing reaction efficiency by reducing hole-electron recombination in the titania. The thiyl radical can then initiate the thiol-ene cycle through addition to an alkene and generation of an alkyl radical, which propagates the reaction by abstracting a hydrogen atom from the thiol starting material.
In conclusion, we have developed a titania photoredox system for the thiol-ene reaction that uses visible light. The photocatalyst is cheap, robust, readily available, and easily processed out of the reaction for re-use. Applications of this method to the broad remit of thiol-ene chemistry are ongoing in our laboratory.
We thank the University of Manchester and the EPSRC for funding.
Notes and references
- Recent reviews: (a) J. M. R. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (c) S. Fukuzumi and K. Ohkubo, Org. Biomol. Chem., 2014, 12, 6059–6071 RSC; (d) D. P. Hari and B. König, Chem. Commun., 2014, 50, 6688–6699 RSC; (e) M. N. Hopkinson, B. Sahoo, J. L. Li and F. Glorius, Chem. – Eur. J., 2014, 20, 3874–3886 CrossRef CAS PubMed; (f) D. A. Nicewicz and T. M. Nguyen, ACS Catal., 2014, 4, 355–360 CrossRef CAS.
- (a) C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC; (b) P. Roy, S. Berger and P. Schmuki, Angew. Chem., Int. Ed., 2011, 50, 2904–2939 CrossRef CAS PubMed; (c) B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- Selected examples of UV titania photoredox catalysis: (a) C. M. Wang and T. E. Mallouk, J. Am. Chem. Soc., 1990, 112, 2016–2018 CrossRef CAS; (b) C. W. Lai and T. E. Mallouk, Chem. Commun., 1993, 1359–1361 RSC; (c) S. Higashida, A. Harada, R. Kawakatsu, N. Fujiwara and M. Matsumura, Chem. Commun., 2006, 2804–2806 RSC; (d) X. J. Lang, H. W. Ji, C. C. Chen, W. H. Ma and J. C. Zhao, Angew. Chem., Int. Ed., 2011, 50, 3934–3937 CrossRef CAS PubMed; (e) D. W. Manley, R. T. McBurney, P. Miller, R. F. Howe, S. Rhydderch and J. C. Walton, J. Am. Chem. Soc., 2012, 134, 13580–13583 CrossRef CAS PubMed; (f) D. W. Manley, R. T. McBurney, P. Miller and J. C. Walton, J. Org. Chem., 2014, 79, 1386–1398 CrossRef CAS PubMed; (g) D. W. Manley and J. C. Walton, Org. Lett., 2014, 16, 5394–5397 CrossRef CAS PubMed.
- With visible light: (a) F. Parrino, A. Ramakrishnan and H. Kisch, Angew. Chem., Int. Ed., 2008, 47, 7107–7109 CrossRef CAS PubMed; (b) M. Cherevatskaya, M. Neumann, S. Fuldner, C. Harlander, S. Kummel, S. Dankesreiter, A. Pfitzner, K. Zeitler and B. König, Angew. Chem., Int. Ed., 2012, 51, 4062–4066 CrossRef CAS PubMed; (c) X. Lang, W. Ma, Y. Zhao, C. Chen, H. Ji and J. Zhao, Chem. – Eur. J., 2012, 18, 2624–2631 CrossRef CAS PubMed; (d) M. Rueping, J. Zoller, D. C. Fabry, K. Poscharny, R. M. Koenigs, T. E. Weirich and J. Mayer, Chem. – Eur. J., 2012, 18, 3478–3481 CrossRef CAS PubMed; (e) C. Vila and M. Rueping, Green Chem., 2013, 15, 2056–2059 RSC; (f) C. D. McTiernan, S. P. Pitre, H. Ismaili and J. C. Scaiano, Adv. Synth. Catal., 2014, 356, 2819–2824 CrossRef CAS.
- F. Dénès, M. Pichowicz, G. Povie and P. Renaud, Chem. Rev., 2014, 114, 2587–2693 CrossRef PubMed.
- T. Posner, Ber. Dtsch. Chem. Ges., 1905, 38, 646–657 CrossRef.
- C. E. Hoyle and C. N. Bowman, Angew. Chem., Int. Ed., 2010, 49, 1540–1573 CrossRef CAS PubMed.
- (a) A. B. Lowe, Polym. Chem., 2014, 5, 4820–4870 RSC; (b) N. Brummelhuis, C. Diehl and H. Schlaad, Macromolecules, 2008, 41, 9946–9947 CrossRef ; and references therein.
- (a) E. L. Tyson, M. S. Ament and T. P. Yoon, J. Org. Chem., 2013, 78, 2046–2050 CrossRef CAS PubMed; (b) E. L. Tyson, Z. L. Niemeyer and T. P. Yoon, J. Org. Chem., 2014, 79, 1427–1436 CrossRef CAS PubMed.
- M. H. Keylor, J. E. Park, C.-J. Wallentin and C. R. J. Stephenson, Tetrahedron, 2014, 70, 4264–4269 CrossRef CAS PubMed.
- (a) C. A. DeForest and K. S. Anseth, Nat. Chem., 2011, 3, 925–931 CrossRef CAS PubMed; (b) C. A. DeForest and K. S. Anseth, Angew. Chem., Int. Ed., 2012, 51, 1816–1819 CrossRef CAS PubMed; (c) H. Shih and C. C. Lin, Macromol. Rapid Commun., 2013, 34, 269–273 CrossRef CAS PubMed; (d) H. Shih, A. K. Fraser and C. C. Lin, ACS Appl. Mater. Interfaces, 2013, 5, 1673–1680 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation data for all new compounds. See DOI: 10.1039/c4cc09987g |
| This journal is © The Royal Society of Chemistry 2015 |