Photoactivated luminescent CdSe quantum dots as sensitive cyanide probes in aqueous solutions
Wei Jun
Jin
,
María T.
Fernández-Argüelles
,
José M.
Costa-Fernández
,
Rosario
Pereiro
and
Alfredo
Sanz-Medel
*
Department of Physical and Analytical Chemistry, University of Oviedo, Julián Clavería 8, 33006, Oviedo, Spain. E-mail: asm@uniovi.es; Fax: +34-985 103 125; Tel: +34-985 103 474
First published on 4th January 2005
Abstract
Water-soluble luminescent CdSe quantum dots surface-modified with 2-mercaptoethane sulfonate were synthesized for the selective determination of free cyanide in aqueous solution with high sensitivity (detection limit of 1.1 × 10−6 M), via analyte-induced changes in their photoluminescence after photoactivation.
Low-dimensional semiconductor nanoparticles, more popularly known as quantum dots (QDs), have generated great interest in recent years. Because of their small size (typically from 1 to 10 nanometers) QDs have unique optical properties in comparison with traditional organic dyes, due to quantum-size confinement effects, including large fluorescence quantum yields, narrow and Gaussian emission spectra (full height wide maximum ∼ 30 nm), size- and composition-tunable emission and less susceptibility to photobleaching.1,2 Many studies are focused on the development of new techniques to synthesize high-quality quantum dots with high luminescence quantum yields and to measure their photophysical properties in organic and aqueous media.1–5 Some key advances have shown the interest of QDs in biology and medicine as biomarkers and more recently in analytical chemistry. Two breakthrough papers, by the Nie4 and Alivisatos groups,5 demonstrated that luminescent QDs can be made water-soluble and biocompatible by surface modification and bioconjugation.
Photoluminescent QDs can respond, via changes in their emission, to the presence of different analytes, affording new methodologies for spectrochemical analysis. Considering that the photoluminescence of QDs arises from the recombination of the exciton, it is reasonable to assume that the change of surface charges or components of QDs, caused by the chemical or physical interaction between ions or small molecules present in the nanoparticle environment and the QDs, could affect the efficiency of the core electron–hole recombination, and thus the luminescent emission.6
So far very few reports of inorganic ions determination based on changes in the luminescence of the QDs have been reported.7–9 Expanding applications of QDs to develop inorganic anion sensors in water media is a topic of current interest. To our knowledge, the use of QDs as selective luminophor indicators for anion detection is virtually unexplored. A recent paper has shown the highly sensitive determination of cyanide by using CdSe QDs surface-modified with tert-butyl-N-(2-mercaptoethyl)-carbamate groups;9 unfortunately, organic media were required for the analysis, thus restricting tremendously its analytical potential.
In the present work, a fluorescent probe based on surface-modified CdSe QDs, for the selective determination of trace levels of cyanide in water media, is developed and analytically characterized. Sodium cyanide served as the source of cyanide ions. As a safety precaution, all solutions containing this toxic anion were kept always at a pH higher than 9. Photoluminescent signals were measured with a Perkin-Elmer LS-50B luminescence spectrometer and were obtained at 20 ± 2 °C.
During preparation of the CdSe QDs their inorganic core is capped with an organic layer of a trioctylphosphine/trioctylphosphine oxide mixture (TOP/TOPO). These ligands are hydrophobic and thus nanocrystals doped with these coatings are not compatible with aqueous assay conditions. If analysis of aqueous solutions is desired, hydrophilic capping agents must be introduced after synthesis of QDs. In fact, a further substitution of such an organic cap with polar unidentate 2-mercaptoethane sulfonate10 (MES) or mercaptoacetic groups4 permits tailoring of nanoparticles for efficient dispersal in aqueous solutions with preservation of their photoluminescence.
In our experiments, water-soluble CdSe QDs were prepared using a stepwise procedure consisting of core nanocrystal growth, TOP/TOPO-capping and size selection precipitation, 2-mercaptoethane sulfonic acid cap exchange, and a final wash clean. TOP/TOPO CdSe QDs were synthesized following a procedure described by Peng's group,11 using CdO as precursor, but introducing some slight modifications already described.9 TOP/TOPO capping groups were subsequently exchanged with MES groups.12
Transmission electron microscopy (TEM) was employed in the QDs characterization. TEM experiments were carried out with a JEOL-2000EX II high resolution electron microscope in order to obtain high-resolution images of individual QDs. Fig. 1 shows a TEM image of the aqueous solution containing the synthesized red-CdSe QDs, surface-modified with MES. As can be seen, monodisperse nanocrystals with a narrow size distribution have been synthesized. The diameters of the functionalized CdSe nanoparticles were estimated to be between 2 and 7 nm. However, it is worth noting that only a small percentage of the total particles (below 20%) showed a diameter size greater than 5 nm.
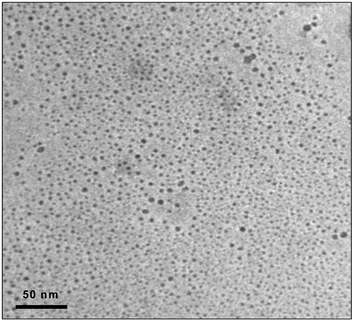 | ||
| Fig. 1 TEM image of an aqueous solution with unaggregated MES-CdSe QDs. | ||
Further, the surface-modified CdSe QDs were spectroscopically characterized. Fig. 2 shows the emission spectra (dotted line) obtained by excitation at 400 nm of an aqueous solution of the surface-modified red QDs after exposing the solution to sunlight for more than 2 days. A maximum at about 580 nm can be observed. The luminescence spectrum from such a photoactivated solution is significantly different from that in the original solution. The QDs in the fresh aqueous solution originally emitted a weak luminescence, with a broader spectrum profile. However, upon exposure to sunlight for about 24 h the luminescence emission is enhanced by over an order-of-magnitude up to a stable level with a narrower profile. Also a blue-shift of the emission maximum spectra was observed. This effect of photo-activation of QDs is a phenomenon reported in recent literature.9,13 Mechanisms such as photoinduced surface reconstruction of the surface atoms of the nanocrystals have been proposed for this activation, but the exact mechanism is not clear yet. Regarding the absorption spectra, a blue-shift of the absorption band was observed after photoactivation, implying a change in size or surface properties of QDs.
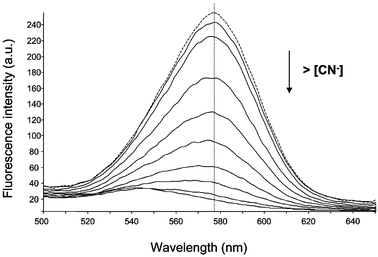 | ||
| Fig. 2 Effect of increasing concentrations of cyanide in the fluorescence emission of the synthesized water-soluble QDs. (Dotted line: fluorescence spectra in absence of CN−). | ||
Luminescence emission of the MES-CdSe QDs turned out to be highly sensitive to the presence of cyanide ions. Fig. 2 shows the effect of increasing concentrations of free cyanide (solid lines) in aqueous solution on the fluorescence emission of the assayed QDs. As can be seen, the QDs fluorescent emission was progressively quenched by the addition of cyanide. Also, a slight blue-shift can be observed. An emission wavelength of 578 nm was used for further analytical studies. The influence of pH on the analytical signal was investigated by the addition of dilute NaOH to achieve pHs higher than 9. It was found that the pH did not affect the analytical signal in the pH interval assayed (from 9 to 11). The effect of trace levels of CN− on the decay time of the QDs fluorescence was also examined. The fluorescence decay observed for MES-CdSe QDs in water diminished with increasing additions of CN−. This result indicates that the observed quenching process seems to be partially dynamic.
The quenching effect of cyanide on the luminescence emission of the MES-CdSe QDs could be used to develop a method for the determination of this anion. A good linear relationship (r > 0.9985) was observed up to cyanide concentrations of at least 2.5 × 10−4 M when using a modified Stern–Volmer plot already proposed for mechanisms where both dynamic and static quenching act together.9,14 The calibration equation obtained in our experiments was:
| ln [(I0/I) − 1] = 1265.7 [Q] + 0.0212, [Q] being expressed in molarity. |
The limit of detection, calculated following the 3σ IUPAC criteria, was 1.1 × 10−6 M (29 µg L−1) of cyanide. It is interesting to note that the sensitivity of the synthesized QDs to CN− detection was unaltered after more than 2 months storage of the luminescent probe in the dark, under ambient conditions.
The quenching of the fluorescence from the MES-CdSe QDs by cyanide anions is rather selective: the influence of other anions (including SO42−, SO32−, NO2−, NO3−, SCN−, Cl−, Br−, I− and acetate) on the luminescence emission from the synthesized QDs was investigated. Results (see Fig. 3) showed that most of these common anions did not exhibit any significant effect on the photoluminescence emission of the QDs, even at relatively high concentration levels. Only, concentrations of I− and SCN− higher than 2 × 10−4 M produced a measurable quenching of the luminescence of the QDs.
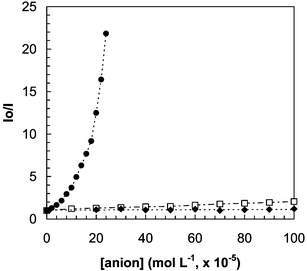 | ||
| Fig. 3 Effect of increasing concentrations of several anions on the luminescence signal from the synthesized MES-CdSe QDs. (●) CN−; (□) I−, SCN−; (◆) SO42−, SO32−, NO3−, NO2−, Cl−, Br− and acetate. | ||
In summary, MES-CdSe quantum dots have been successfully combined to develop a novel and highly sensitive and selective system for optical recognition and determination of cyanide in water. Besides, the long emission wavelength selected and the relatively high separation between the excitation and emission wavelengths (about 180 nm) could allow simplification of the photoluminescent instrumentation for routine measurements. To the best of our knowledge, this is the first use of QDs as selective luminescent probes of an anion in aqueous samples.
This work was supported by the projects MCT-03-BQU-04671 and MAT2003-09074-C02 (Feder Programme and Ministerio de Educación y Ciencia, Spain). Grants to M. T. Fernández-Argüelles (ref. BP04-040) from the Consejería de Educación y Ciencia of the Principado of Asturias and to W. J. Jin (ref. MEC-SABATI-03-521) from the Ministerio de Educación y Cultura, Spain, are acknowledged.
Notes and references
- C. J. Murphy and J. L. Coffer, Appl. Spectrosc., 2002, 56, 16A CrossRef CAS.
- D. M. Willard, Anal. Bioanal. Chem., 2003, 376, 284 CAS.
- C. Landes, C. Burda, M. Braun and M. A. El-Sayed, J. Phys. Chem. B, 2001, 105, 2981 CrossRef CAS.
- W. C. W. Chan and S. Nie, Science, 1998, 281, 2016 CrossRef CAS.
- M. Bruchez, Jr., M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013 CrossRef CAS.
- D. E. Moore and K. Patel, Langmuir, 2001, 17, 2541 CrossRef CAS.
- Y. Chen and Z. Rosenzweig, Anal. Chem., 2002, 74, 5132 CrossRef CAS.
- K. M. Gattás-Asfura and R. M. Leblanc, Chem. Commun., 2003, 2684 RSC.
- W. J. Jin, J. M. Costa-Fernández, R. Pereiro and A. Sanz-Medel, Anal. Chim. Acta, 2004, 522, 1 CrossRef CAS.
- M. Miyake, H. Matsumoto, M. Nishizawa, T. Sakata, H. Mori, S. Kuwabata and H. Yoneyama, Langmuir, 1997, 13, 742 CrossRef CAS.
- L. Qu and X. Peng, J. Am. Chem. Soc., 2002, 124, 2049 CrossRef CAS.
- Purified TOP/TOPO CdSe QDs (20 mg) were suspended in 15 mL of methanol solution containing 300 mg MES and 1.5 mL tetrabutylammonium hydroxide (∼ 25%). The solution was refluxed overnight. The surface-modified QDs were then purified by sonication of the solution in an ultrasonic bath during 2–4 min (until homogenization of the mixture) followed by ultracentrifugation (15000 rpm) during about half an hour in order to separate the QDs and to remove excess of MES and hydrolyzed tetrabutylammonium. The synthesized red-QDs with nanoparticle sizes between 3–5 nm (selected by precipitation) were then dispersed in water. Once the synthesis was finished, the optical properties of the QDs remained unaltered after the purification process (including repeated dissolution and precipitations).
- B. C. Hess, I. G. Okhrimenko, R. C. Davis, B. C. Stevens, Q. A. Schulzke, K. C. Wright, C. D. Bass, C. D. Evans and S. L. Summers, Phys. Rev. Lett., 2001, 86, 3132 CrossRef CAS.
- R. J. Huturbise, A. H. Ackerman and B. W. Smith, Appl. Spectrosc., 2001, 55, 490 CrossRef.
| This journal is © The Royal Society of Chemistry 2005 |
