Yuuki Maekawa, Zachary T. Ariki, Masakazu Nambo and Cathleen M. Crudden
Org. Biomol. Chem., 2019,17, 7300-7303
DOI:
10.1039/C9OB01099H,
Communication
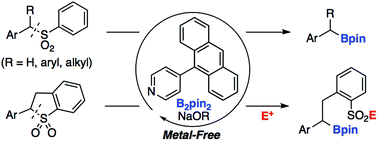
Herein, we report the transition-metal free, pyridine-catalyzed desulfonative borylation of benzyl sulfones with bis(pinacolato)diboron (B2pin2). A variety of benzhydryl- and benzyl boronic esters could be synthesized from readily prepared sulfone derivatives. The borylation of cyclic sulfones accompanied by ring opening also proceeded to afford the corresponding sulfonate, which could be converted into functionalized sulfones and sulfonamides.