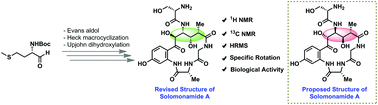
Herein, we report the total synthesis of solomonamide A along with its structural revision for the first time. The natural product possesses very potent anti-inflammatory activity, and it contains a macrocyclic peptide having four consecutive stereocenters on an unnatural amino acid component. The key features in the present synthesis include the application of an Evans aldol reaction, ligand-free Heck macrocyclization and chemoselective oxidations. The challenging task of fixing the stereochemistry of OH at the C5-position was accomplished with the help of DFT calculations, applying a quantum-mechanical (QM)/NMR combined approach. Biological evaluation in a mouse paw edema model revealed that a low dose (0.3 mg kg−1) of the synthesized solomonamide A showed 74% reduction at 6 h, which was comparable to a high dose (10 mg kg−1) standard drug dexamethasone effect (75% at 6 h). Thus, we further confirmed the revised structure of solomonamide A.