Ulrich Arnold and Ronald T. Raines
Org. Biomol. Chem., 2016,14, 6780-6785
DOI:
10.1039/C6OB00980H,
Paper
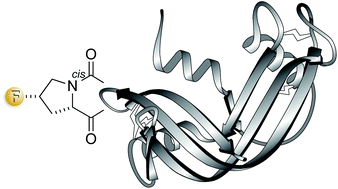
The conformational attributes of proline can have a substantial effect on the folding of polypeptide chains into a native structure and on the stability of that structure. Replacing the 4S hydrogen of a proline residue with fluorine is known to elicit stereoelectronic effects that favor a cis peptide bond. Here, semisynthesis is used to replace a cis-proline residue in ribonuclease A with (2S,4S)-4-fluoroproline. This subtle substitution accelerates the folding of the polypeptide chain into its three-dimensional structure and increases the thermostability of that structure without compromising its catalytic activity. Thus, an appropriately situated fluorine can serve as a prosthetic atom in the context of a protein.