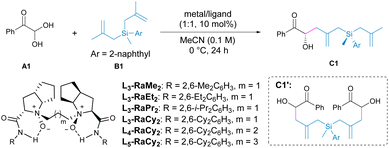Open Access Article
Open Access ArticleCatalytic asymmetric construction of 1,5-remote Si- and C-stereocenters via desymmetrizing ene reaction of bis(methallyl)silanes†
Qiuhui Caoa,
Yuntian Yanga,
Yiwen Meia,
Minghui Jib,
Fei Wangb,
Xiaoming Feng *a and
Weidi Cao
*a and
Weidi Cao *a
*a
aKey Laboratory of Green Chemistry & Technology, Ministry of Education, College of Chemistry, Sichuan University, Chengdu, 610064, P. R. China. E-mail: xmfeng@scu.edu.cn; wdcao@scu.edu.cn
bCenter for Natural Products Research, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu, 610041, China
First published on 1st April 2025
Abstract
The catalytic enantioselective synthesis of chiral silanes has long been a challenging pursuit. Achieving simultaneous construction of remote Si- and C-stereogenic centers in an acyclic molecule via desymmetrization is particularly difficult. Herein, we realized an example of a chiral nickel(II) complex-catalyzed desymmetrizing carbonyl–ene reaction of bis(methallyl)silanes with α-keto aldehyde monohydrates, enabling the highly chemo-, diastereo- and enantioselective synthesis of chiral δ-hydroxy silanes featuring 1,5-remote Si- and C-stereocenters. This protocol demonstrated good functional group tolerance and a broad substrate scope. A bioactivity study revealed its potential applications in the synthesis of bioactive molecules.
Introduction
Enantiomerically enriched silicon-stereogenic silanes hold significant potential in the fields of functional materials,1 medicinal chemistry2 and organic synthesis.3 As natural organosilicon compounds are nonexistent, accessing these compounds relies entirely on chemical synthesis. The desymmetrization of prochiral silanes represents the most prevalent and efficient approach.4 Among these methods, the direct cleavage of Si–X (X = C, H, Cl) bonds or the conversion of functional groups bound to the silicon atom has been well established, yielding chiral silanes with a single silicon stereocenter (Scheme 1a).In contrast, the construction of chiral molecules featuring both silicon- and carbon-stereogenic centers is more challenging due to the need for simultaneous control of diastereo- and enantioselectivity. To date, several intriguing studies have focused on the construction of 1,2-adjacent or 1,3-nonadjacent stereocenters. For examples, asymmetric protoboration5 of divinyl-substituted silanes with B2pin2 was exploited to construct 1,2-Si- and C-stereocenters. Intramolecular asymmetric aryl-transfer6 and the Heck reaction,7 as well as intermolecular Peterson-olefination8 of tetrasubstituted silanes, have generated 1,3-Si- and C-stereocenters. Hydrosilanes-participated catalytic asymmetric hydrosilation9 with alkenes and Si–H insertion10 with α-diazo acetates, have achieved chiral silanes containing 1,2-, 1,3-, or 1,2,3-Si- and C-stereocenters. For the construction of 1,4-remote Si- and C- stereocenters, only two examples have been reported: the homologation11 of silacyclohexanones with CF3CHN2 and the benzoin reaction12 of siladials, both leading to silicon-stereogenic silacycles. However, to our knowledge, protocols for synthesizing chiral silanes with 1,5-remote Si-and C-stereocenters remain unexplored.
Allyl silanes have traditionally served as allylation reagents in organic synthesis through the release of the silyl group. In recent years, bis(methallyl)silanes, a class of symmetrical silanes, have been employed by the List group in carbon–carbon bond formation via silicon–hydrogen exchange13 and cyclization.14 These strategies have emerged as novel and efficient methods for constructing Si-stereogenic centers. Later, they also developed a dynamic kinetic asymmetric transformation of racemic allyl silanes.15
Results and discussion
Inspired by previous success in the carbonyl–ene reaction16 utilizing chiral N,N′-dioxide-metal complexes,17 and driven by our interest in organosilicon chemistry,18 we envisioned that a desymmetrizing carbonyl–ene reaction with prochiral bis(methallyl)silanes could provide an entry to chiral silanes bearing 1,5-remote Si- and C-stereogenic centers. However, this approach faces important challenges, including: (i) Potential competitive reactions, such as allylation and the double carbonyl–ene reaction. (ii) Achieving diastereo- and enantioselective control of 1,5-remote stereogenic centers, especially in acyclic molecules with more flexible conformations. Herein, we describe a chiral N,N′-dioxide/Ni(II) complex-mediated asymmetric carbonyl–ene reaction of glyoxal monohydrates with bis(methallyl)silanes, delivering a wide range of chiral δ-hydroxy silanes with 1,5-remote Si- and C-stereocenters (Scheme 1b).Initially, we chose phenylglyoxal monohydrate A1 and bis(methallyl)silane B1 as the model substrates to optimize the carbonyl–ene reaction conditions. With chiral N,N′-dioxide L3-RaCy2 as the ligand and Ni(OTf)2 as the metal precursor, the desired product C1 was obtained in 54% yield with 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 dr and 99/99% ee (entry 1). Notably, the allylation product was not detected but the byproduct C1′ which underwent a double carbonyl–ene reaction was isolated with 10% yield. The investigation of chiral ligands showed that efficient enantioselectivity could be achieved using different N,N′-dioxides, but the L-ramipril-derived ligand L3-RaCy2 with larger steric hindrance at the 2,6-positions of the amide unit was superior to the ligands generated from other amino acid backbones in terms of diastereoselectivity (entries 2–4, see Table S3† for more details). The chain length linking the two amino amide units had a slight influence on this reaction, affording C1 with 56% yield, 90
11 dr and 99/99% ee (entry 1). Notably, the allylation product was not detected but the byproduct C1′ which underwent a double carbonyl–ene reaction was isolated with 10% yield. The investigation of chiral ligands showed that efficient enantioselectivity could be achieved using different N,N′-dioxides, but the L-ramipril-derived ligand L3-RaCy2 with larger steric hindrance at the 2,6-positions of the amide unit was superior to the ligands generated from other amino acid backbones in terms of diastereoselectivity (entries 2–4, see Table S3† for more details). The chain length linking the two amino amide units had a slight influence on this reaction, affording C1 with 56% yield, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr and 99/99% ee by employment of L4-RaCy2 (entry 5). Switching the counterion of Ni2+ to NTf2− the yield of C1 was improved to 67% (entry 7). Given the generation of byproduct C1′, the amount of B1 was increased, leading to a higher yield (76%) of C1 while maintaining stereoselectivity (entry 8). Upon extending the reaction time to 48 h, the yield of C1 was further enhanced to 81% (entry 9). Screening of other parameters, including metal salt, solvent, temperature, additive and so on, did not yield a better result (see Tables S1–S8† for details). It is worth mentioning that kinetic resolution may be involved in the second carbonyl–ene reaction, which was verified by performing the control experiment that resulted in the formation of C1′ and recovered C1 with an increased dr value (see Fig. S1† for details).
10 dr and 99/99% ee by employment of L4-RaCy2 (entry 5). Switching the counterion of Ni2+ to NTf2− the yield of C1 was improved to 67% (entry 7). Given the generation of byproduct C1′, the amount of B1 was increased, leading to a higher yield (76%) of C1 while maintaining stereoselectivity (entry 8). Upon extending the reaction time to 48 h, the yield of C1 was further enhanced to 81% (entry 9). Screening of other parameters, including metal salt, solvent, temperature, additive and so on, did not yield a better result (see Tables S1–S8† for details). It is worth mentioning that kinetic resolution may be involved in the second carbonyl–ene reaction, which was verified by performing the control experiment that resulted in the formation of C1′ and recovered C1 with an increased dr value (see Fig. S1† for details).
With the optimized reaction conditions in hand (Table 1, entry 9), we proceeded to explore the substrate scope. As depicted in Scheme 2, regardless of the position or electronic property of the substituents on the phenyl group, all the activated aryl aldehydes reacted with B1 smoothly to deliver the corresponding products C2–C26 with 57–90% yield, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15–90
15–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr, 90–99% ee, and exhibited excellent functional group tolerance, including hydroxyl, halogen, nitro, cyano, trifluoromethyl, formyl, ester group, carbonyl, phenyl, vinyl, and ethynyl. Di-, tri-substituted, condensed-ring and heteroaromatic substrates were also well tolerated (C27–C36). Furthermore, this catalytic system was also applicable to aliphatic glyoxal monohydrates. Linear alkyl substituted A37 was converted into C37 with decreased reactivity and stereoselectivity (42% yield, 84
10 dr, 90–99% ee, and exhibited excellent functional group tolerance, including hydroxyl, halogen, nitro, cyano, trifluoromethyl, formyl, ester group, carbonyl, phenyl, vinyl, and ethynyl. Di-, tri-substituted, condensed-ring and heteroaromatic substrates were also well tolerated (C27–C36). Furthermore, this catalytic system was also applicable to aliphatic glyoxal monohydrates. Linear alkyl substituted A37 was converted into C37 with decreased reactivity and stereoselectivity (42% yield, 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 dr, 91%/82% ee), but the cyclopropyl-derived one proved to be a suitable substrate (C38, 89% yield, 84
16 dr, 91%/82% ee), but the cyclopropyl-derived one proved to be a suitable substrate (C38, 89% yield, 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 dr, 99%/99% ee). Additionally, the scope of this reaction was extended successfully to glyoxylate (C39).
16 dr, 99%/99% ee). Additionally, the scope of this reaction was extended successfully to glyoxylate (C39).
| Metal salt | Ligand | Yieldb/% | eec/% | drc | |
|---|---|---|---|---|---|
a Unless otherwise noted, all reactions were carried out with A1 (0.1 mmol), B1 (0.1 mmol), and metal/ligand (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 10 mol%) in MeCN (0.1 M) at 0 °C for 24 h.b Yield of the isolated product.c Determined by SFC analysis on a chiral stationary phase.d B1 (0.2 mmol).e For 48 h. 1, 10 mol%) in MeCN (0.1 M) at 0 °C for 24 h.b Yield of the isolated product.c Determined by SFC analysis on a chiral stationary phase.d B1 (0.2 mmol).e For 48 h. |
|||||
| 1 | Ni(OTf)2 | L3-RaCy2 | 54 | 99/99 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| 2 | Ni(OTf)2 | L3-RaPr2 | 43 | 97/89 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| 3 | Ni(OTf)2 | L3-RaEt2 | 46 | 99/99 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 4 | Ni(OTf)2 | L3-RaMe2 | 33 | 95/92 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 5 | Ni(OTf)2 | L4-RaCy2 | 56 | 99/99 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 6 | Ni(OTf)2 | L5-RaCy2 | 47 | 99/99 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 7 | Ni(NTf2)2 | L4-RaCy2 | 67 | 99/99 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 8d | Ni(NTf2)2 | L4-RaCy2 | 76 | 99/99 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 9d,e | Ni(NTf2)2 | L4-RaCy2 | 81 | 99/99 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
Subsequently, we turned our attention to the scope of bis(methallyl)silanes (Scheme 3). A wide range of aryl methyl substituted bis(methallyl)silanes were successfully transformed into the desired products C40–C59 in 74–90% yield with 92–99% ee and 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25–90
25–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr. Wherein, the silanes (B3–B4) bearing a substituent at the ortho-position of phenyl exhibited lower diastereoselectivity. Benzyl methyl bis(methallyl)silane (B22) was also tested in this reaction, producing C60 with high yield and enantioselectivity, but the diastereoselectivity was poor. A comparative analysis of the results between C40 and C61–C62 revealed that the alkyl substituent on silicon atom also influenced the diastereoselectivity significantly; decreased dr values were obtained with phenyl ethyl bis(methallyl)silane B23 and monohydrosilane B24 as substrates.
10 dr. Wherein, the silanes (B3–B4) bearing a substituent at the ortho-position of phenyl exhibited lower diastereoselectivity. Benzyl methyl bis(methallyl)silane (B22) was also tested in this reaction, producing C60 with high yield and enantioselectivity, but the diastereoselectivity was poor. A comparative analysis of the results between C40 and C61–C62 revealed that the alkyl substituent on silicon atom also influenced the diastereoselectivity significantly; decreased dr values were obtained with phenyl ethyl bis(methallyl)silane B23 and monohydrosilane B24 as substrates.
To evaluate the practicality of the catalytic system, a scale-up experiment was carried out between A9 and B1 under the optimized reaction conditions, delivering the product C9 in 90% yield with 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr and 99/99% ee (Scheme 4a). Further transformations of C9 were also carried out. For instance, C9 underwent a Wittig reaction to yield D while maintaining its stereoselectivity. It was also applied for the late-stage modification of drugs through the introduction of a Si-stereogenic center, as demonstrated by the synthesis of drug derivatives E and F via condensation reaction. Treatment of C20 with 4-bromobenzohydrazide provided the corresponding hydrazone G in 84% yield with 99/99% ee, whose configuration was determined to be (S,S) by X-ray crystallographic analysis19 (Scheme 4b). In light of the potential bioactivity of organosilicon compounds, their in vitro cytotoxicity against human hepatocellular carcinoma was investigated. The outcomes indicated that C35 and C36 had an inhibitory effect on the activity of HCCLM3 (Scheme 4c).
10 dr and 99/99% ee (Scheme 4a). Further transformations of C9 were also carried out. For instance, C9 underwent a Wittig reaction to yield D while maintaining its stereoselectivity. It was also applied for the late-stage modification of drugs through the introduction of a Si-stereogenic center, as demonstrated by the synthesis of drug derivatives E and F via condensation reaction. Treatment of C20 with 4-bromobenzohydrazide provided the corresponding hydrazone G in 84% yield with 99/99% ee, whose configuration was determined to be (S,S) by X-ray crystallographic analysis19 (Scheme 4b). In light of the potential bioactivity of organosilicon compounds, their in vitro cytotoxicity against human hepatocellular carcinoma was investigated. The outcomes indicated that C35 and C36 had an inhibitory effect on the activity of HCCLM3 (Scheme 4c).
 | ||
| Scheme 4 (a) Gram-scale synthesis and further transformations. (b) Determination of the absolute configuration of C20. (c) Bioactivity investigation. (d) Proposed transition state. | ||
Based on the absolute configuration of product C20 and the single-crystal structure of the L4-RaCy2/Ni(II) complex,20 the possible working modes were proposed to understand the stereoselective control of this reaction. As shown in Scheme 4d, both the oxygen atoms of the amide and N-oxide units of the ligand coordinate with the central Ni(NTf2)2 to form the L4-RaCy2/Ni(II) complex in a tetradentate manner; this complex acts as a chiral Lewis acid to activate the glyoxal derivative A20 via bidentate coordination with the dicarbonyl groups. Mechanistically, the carbonyl–ene reaction proceeds via a six-membered cyclic transition state. B1 approaches from the Si face of A20 because the Re face is blocked by the amide unit of the ligand (see the ESI† for details). Meanwhile, due to steric repulsion between the naphthyl of B1 and the chiral ligand (Scheme 4d, bottom), the naphthyl group is directed toward the back side (Scheme 4d, top), producing (S,S)-C20 as the major diastereoisomer.
Conclusions
In conclusion, an efficient catalytic asymmetric desymmetrization of bis(methallyl)silanes with α-keto aldehyde monohydrates was accomplished by employing a chiral N,N′-dioxide/Ni(II) complex catalyst. This protocol provides facile access to acyclic chiral δ-hydroxy silanes bearing 1,5-remote Si- and C-stereocenters in excellent yields with good dr and ee values. The scale-up reaction and product transformations as well as good biological activity illustrate the potential practicality of this methodology. Further endeavor toward the enantioselective synthesis of chiral silanes is underway.Data availability
Further details of the experimental procedure, 1H, 13C{1H} and 19F{1H} NMR, HPLC spectra, SFC spectra, X-ray crystallographic data for G and the L4-RaCy2/Ni(NTf2)2 complex are available in the ESI.†Author contributions
Q. H. C. performed experiments and prepared the manuscript and ESI.† Y. T. Y. participated in the synthesis of substrates. Y. W. M. repeated some experiments. M. H. J. and F. W. conducted bioactivity investigation. W. D. C. helped in modifying the paper and ESI.† W. D. C. and X. M. F. conceived and directed the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We appreciate the National Key Research and Development Program of China (2022YFA1504301) and the National Natural Science Foundation of China (22471179 and 92256302) for financial support. We thank Dr Yuqiao Zhou (Sichuan University) for the X-ray single crystal diffraction analysis.Notes and references
- (a) J. Chen and Y. Cao, Macromol. Rapid Commun., 2007, 28, 1714–1742 CrossRef CAS; (b) Y. Kawakami, Y. Kakihana, O. Ooi, M. Oishi, K. Suzuki, S. Shinke and K. Uenish, Polym. Int., 2009, 58, 279–284 CrossRef CAS; (c) T. Ikeno, T. Nagano and K. Hanaoka, Chem.–Asian J., 2017, 12, 1435–1446 CrossRef CAS PubMed; (d) R. Shintani, N. Misawa, R. Takano and K. Nozaki, Chem.–Eur. J., 2017, 23, 2660–2665 CrossRef CAS PubMed; (e) S. Koga, S. Ueki, M. Shimada, R. Ishii, Y. Kurihara, Y. Yamanoi, J. Yuasa, T. Kawai, T. Uchida, M. Iwamura, K. Nozaki and H. Nishihara, J. Org. Chem., 2017, 82, 6108–6117 CrossRef CAS PubMed.
- (a) R. Tacke and U. Wannagat, Syntheses and Properties of Bioactive Organo-Silicon Compounds, Springer, Heidelberg, 1979 Search PubMed; (b) R. Tacke, D. Reichel, M. Kropfgans, P. G. Jones, E. Mutschler, J. Gross, X. Hou, M. Waelbroeck and G. Lambrecht, Organometallics, 1995, 14, 251–262 CrossRef CAS; (c) M. Mutahi, T. Nittoli, L. Guo and S. M. Sieburth, J. Am. Chem. Soc., 2002, 124, 7363–7375 CrossRef CAS PubMed; (d) A. K. Franz and S. O. Wilson, J. Med. Chem., 2013, 56, 388–405 CrossRef CAS PubMed; (e) E. Remond, C. Martin, J. Martinez and F. Cavelier, Chem. Rev., 2016, 116, 11654–11684 CrossRef CAS PubMed; (f) R. Ramesh and D. S. Reddy, J. Med. Chem., 2018, 61, 3779–3798 CrossRef CAS PubMed.
- (a) C. E. Masse and J. S. Panek, Chem. Rev., 1995, 95, 1293–1316 CrossRef CAS; (b) S. Rendler, G. Auer, M. Keller and M. Oestreicha, Adv. Synth. Catal., 2006, 348, 1171–1182 CrossRef CAS; (c) L.-W. Xu, L. Li, G.-Q. Lai and J.-X. Jiang, Chem. Soc. Rev., 2011, 40, 1777–1790 RSC; (d) L. J. Li, Y. B. Zhang, L. Gao and Z. L. Song, Tetrahedron Lett., 2015, 56, 1466–1473 CrossRef CAS; (e) J. O. Bauer and C. Strohmann, Eur. J. Inorg. Chem., 2016, 18, 2868–2881 CrossRef; (f) Y.-M. Cui, Y. Lin and L.-W. Xu, Coord. Chem. Rev., 2017, 330, 37–52 CrossRef CAS; (g) R. Shintani, Synlett, 2018, 29, 388–396 CrossRef CAS; (h) M. Zhang, S. Gao, J. Tang, L. Chen, A. H. Liu, S. R. Sheng and A. Q. Zhang, Chem. Commun., 2021, 57, 8250–8263 RSC.
- (a) L.-W. Xu, Angew. Chem., Int. Ed., 2012, 51, 12932–12934 CrossRef CAS PubMed; (b) R. Shintani, Asian J. Org. Chem., 2015, 4, 510–514 CrossRef CAS; (c) L. Zheng, X.-X. Nie, Y. C. Wu and P. Wang, Eur. J. Org Chem., 2021, 44, 6006–6014 CrossRef; (d) F. Ye, Z. Xu and L.-W. Xu, Acc. Chem. Res., 2021, 54, 452–470 CrossRef CAS PubMed; (e) Y. C. Ge, X. F. Huang, J. Ke and C. He, Chem Catal., 2022, 2, 2898–2928 CrossRef CAS; (f) Y. C. Wu, L. Zheng, Y. Wang and P. Wang, Chem, 2023, 10, 3461–3514 CrossRef; (g) Y. Zeng and F. Ye, Chin. J. Org. Chem., 2023, 43, 3388–3413 CrossRef CAS; (h) Y. C. Ge, J. Ke and C. He, Acc. Chem. Res., 2025, 58, 375–398 CrossRef CAS PubMed.
- G. Zhang, Y. F. Li, Y. Wang, Q. Zhang, T. Xiong and Q. Zhang, Angew. Chem., Int. Ed., 2020, 59, 11927–11931 Search PubMed.
- R. Kumar, Y. Hoshimoto, H. Yabuki, M. Ohashi and S. Ogoshi, J. Am. Chem. Soc., 2015, 137, 11838–11845 Search PubMed.
- K.-L. Yin, S. Zhao, Y. Qin, S.-H. Chen, B. Li and D. B. Zhao, ACS Catal., 2022, 12, 13999–14005 CrossRef CAS.
- W. G. Guo, Q. Li, Y. Liu and C. Li, Sci. China: Chem., 2023, 66, 2797–2802 CrossRef CAS.
- (a) K. Tamao, K. Nakamura, H. Ishii, S. Yamaguchi and M. Shiro, J. Am. Chem. Soc., 1996, 118, 12469–12470 CrossRef CAS; (b) Z.-Y. Zhao, Y.-X. Nie, R.-H. Tang, G.-W. Yin, J. Cao, Z. Xu, Y.-M. Cui, Z.-J. Zheng and L.-W. Xu, ACS Catal., 2019, 9, 9110–9116 CrossRef CAS; (c) X. Chang, P.-L. Ma, H.-C. Chen, C.-Y. Li and P. Wang, Angew. Chem., Int. Ed., 2020, 59, 8937–8940 CrossRef CAS PubMed; (d) Y.-H. Huang, Y. C. Wu, Z. L. Zhu, S. J. Zheng, Z. H. Ye, Q. Peng and P. Wang, Angew. Chem., Int. Ed., 2022, 61, e202113052 CrossRef CAS PubMed; (e) L. Wang, W. X. Lu, J. W. Zhang, Q. L. Chong and F. K. Meng, Angew. Chem., Int. Ed., 2022, 61, e202205624 CrossRef CAS PubMed; (f) W. X. Lu, Y. M. Zhao and F. K. Meng, J. Am. Chem. Soc., 2022, 144, 5233–5240 CrossRef CAS PubMed.
- (a) Y. Yasutomi, H. Suematsu and T. Katsuki, J. Am. Chem. Soc., 2010, 132, 4510–4511 CrossRef CAS PubMed; (b) Y. Nakagawa, S. Chanthamath, I. Fujisawa, K. Shibatomi and S. Iwasa, Chem. Commun., 2017, 53, 3753–3756 RSC; (c) J. R. Jagannathan, J. C. Fettinger, J. T. Shaw and A. K. Franz, J. Am. Chem. Soc., 2020, 142, 11674–11679 CrossRef CAS PubMed.
- S.-S. Li, S. Sun and J. B. Wang, Angew. Chem., Int. Ed., 2022, 61, e202115098 CrossRef CAS PubMed.
- H. Liu, P. Y. He, X. L. Liao, Y. P. Zhou, X. K. Chen, W. P. Ou, Z. H. Wu, C. Luo, L. M. Yang and J. F. Xu, ACS Catal., 2022, 12, 9864–9871 CrossRef CAS.
- H. Zhou, J. T. Han, N. Nöthling, M. M. Lindner, J. Jenniches, C. Kühn, N. Tsuji, L. Zhang and B. List, J. Am. Chem. Soc., 2022, 144, 10156–10161 CrossRef CAS PubMed.
- J. T. Han, N. Tsuji, H. Zhou, M. Leutzsch and B. List, Nat. Commun., 2024, 15, 5846 CrossRef CAS PubMed.
- H. Zhou, R. Properzi, M. Leutzsch, P. Belanzoni, G. Bistoni, N. Tsuji, J. T. Han, C. D. Zhu and B. List, J. Am. Chem. Soc., 2023, 145, 4994–5000 CrossRef CAS PubMed.
- (a) L. C. Dias, Curr. Org. Chem., 2000, 4, 305–342 CrossRef CAS; (b) X. H. Liu, K. Zheng and X. M. Feng, Synthesis, 2014, 46, 2241–2257 CrossRef CAS; (c) M. Balha, C. Parida and S. C. Pan, Asian J. Org. Chem., 2021, 10, 2440–2453 CrossRef CAS; (d) Y. Yang and C. R. Jones, Synthesis, 2022, 54, 5042–5054 CrossRef CAS; (e) K. Zheng, J. Shi, X. H. Liu and X. M. Feng, J. Am. Chem. Soc., 2008, 130, 15770–15771 CrossRef CAS PubMed; (f) K. Zheng, X. H. Liu, J. N. Zhao, Y. Yang, L. L. Lin and X. M. Feng, Chem. Commun., 2010, 46, 3771–3773 RSC; (g) K. Zheng, Y. Yang, J. N. Zhao, C. K. Yin, L. L. Lin, X. H. Liu and X. M. Feng, Chem.–Eur. J., 2010, 16, 9969–9972 CrossRef CAS PubMed; (h) K. Zheng, C. K. Yin, X. H. Liu, L. L. Lin and X. M. Feng, Angew. Chem., Int. Ed., 2011, 50, 2573–2577 CrossRef CAS PubMed; (i) K. Zheng, X. H. Liu, S. Qin, M. S. Xie, L. L. Lin, C. W. Hu and X. M. Feng, J. Am. Chem. Soc., 2012, 134, 17564–17573 CrossRef CAS PubMed; (j) W. W. Luo, J, N. Zhao, C. K. Yin, X. H. Liu, L. L. Lin and X. M. Feng, Chem. Commun., 2014, 50, 7524–7526 RSC; (k) W. W. Luo, J. N. Zhao, J. Ji, L. L. Lin, X. H. Liu, H. J. Mei and X. M. Feng, Chem. Commun., 2015, 51, 10042–10045 RSC; (l) H. Zhang, Q. Yao, W. D. Cao, S. L. Ge, J. X. Xu, X. H. Liu and X. M. Feng, Chem. Commun., 2018, 54, 12511–12514 RSC; (m) W. Liu, W. D. Cao, H. P. Hu, L. L. Lin and X. M. Feng, Chem. Commun., 2018, 54, 8901–8904 RSC; (n) W. Liu, P. F. Zhou, J. W. Lang, S. X. Dong, X. H. Liu and X. M. Feng, Chem. Commun., 2019, 55, 4479–4482 RSC; (o) L. Z. Hou, T. F. Kang, L. K. Yang, W. D. Cao and X. M. Feng, Org. Lett., 2020, 22, 1390–1395 CrossRef CAS PubMed; (p) X. P. Sang, Y. H. Mo, S. Y. Li, X. H. Liu, W. D. Cao and X. M. Feng, Chem. Sci., 2023, 14, 8315–8320 RSC.
- (a) X. H. Liu, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2011, 44, 574–587 CrossRef CAS PubMed; (b) X. H. Liu, H. F. Zheng, Y. Xia, L. L. Lin and X. M. Feng, Acc. Chem. Res., 2017, 50, 2621–2631 CrossRef CAS PubMed; (c) M.-Y. Wang and W. Li, Chin. J. Chem., 2021, 39, 969–984 CrossRef CAS; (d) S. X. Dong, X. H. Liu and X. M. Feng, Acc. Chem. Res., 2022, 55, 415–428 CrossRef CAS PubMed; (e) D.-F. Chen and L.-Z. Gong, Org. Chem. Front., 2023, 10, 3676–3683 RSC; (f) S. X. Dong, W. D. Cao, M. P. Pu, X. H. Liu and X. M. Feng, CCS Chem., 2023, 5, 2717–2735 CrossRef CAS; (g) Z. J. Xiao, M. P. Pu, Y. Z. Li, W. Yang, F. Wang, X. M. Feng and X. H. Liu, Angew. Chem., Int. Ed., 2025, 64, e202414712 CrossRef CAS PubMed; (h) Q. F. Xu, L. C. Ning, W. T. Xu, L. L. Lin and X. M. Feng, Org. Lett., 2024, 26, 9665–9670 CrossRef CAS PubMed; (i) L. K. Yang, S. Y. Li, L. C. Ning, H. S. Zhao, L. Zhou, W. D. Cao and X. M. Feng, Nat. Commun., 2024, 15, 10866 CrossRef PubMed.
- (a) M. M. Guan, S. Y. Wang, Y. Luo, W. D. Cao, X. H. Liu and X. M. Feng, Chem. Sci., 2021, 12, 7498–7503 RSC; (b) L. Z. Hou, Y. Q. Zhou, H. Yu, T. Y. Zhan, W. D. Cao and X. M. Feng, J. Am. Chem. Soc., 2022, 144, 22140–22149 CrossRef CAS PubMed; (c) L. L. Feng, X. F. Chen, N. Guo, Y. Q. Zhou, L. L. Lin, W. D. Cao and X. M. Feng, Chem. Sci., 2023, 14, 4516–4522 RSC; (d) N. Guo, Y. Luo, L. L. Feng, Z. L. Liu, W. D. Cao and X. M. Feng, Asian J. Org. Chem., 2023, 12, e202300164 CrossRef CAS; (e) L. Z. Hou, W. D. Cao and X. M. Feng, ChemCatChem, 2024, 16, e202400385 CrossRef CAS.
- CCDC 2393874† for G contains the supplentary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- CCDC 2418295† for the L4-RaCy2/Ni(NTf2)2 complex contains the supplentary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C{1H} and 19F{1H} NMR, HPLC and SFC spectra. CCDC 2393874 (G) and 2418295 (L4-RaCy2/Ni(NTf2)2). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc01054c |
| This journal is © The Royal Society of Chemistry 2025 |