 Open Access Article
Open Access ArticleRecent advances in 2D MXene-based heterostructures for gas sensing: mechanisms and applications in environmental and biomedical fields
Lanting
Qian
 *ab,
Farnood
Rahmati
*c,
Fengchao
Li
d,
Tianzhu
Zhang
a,
Tao
Wang
a,
Haoze
Zhang
b,
Shuo
Yan
e and
Yun
Zheng
*a
*ab,
Farnood
Rahmati
*c,
Fengchao
Li
d,
Tianzhu
Zhang
a,
Tao
Wang
a,
Haoze
Zhang
b,
Shuo
Yan
e and
Yun
Zheng
*a
aInstitute of New Energy Materials and Engineering, College of Materials Science and Engineering, Fujian Engineering Research Center of High Energy Batteries and New Energy Equipment & Systems, Fuzhou University, Fuzhou 350108, P. R. China. E-mail: yunzheng@fzu.edu.cn
bDepartment of Chemical Engineering, University of Waterloo, Waterloo, ON N2L 3G1, Canada. E-mail: ltqian@uwaterloo.ca
cDepartment of Chemistry, University of Guelph, Guelph, ON N1G 2W1, Canada. E-mail: fpakrava@uguelph.ca
dShandong Weifang Ecological Environment Monitoring Center, Weifang 261000, P. R. China
eDepartment of Materials and Engineering, University of Ottawa, ON K1N 6N5, Canada
First published on 20th March 2025
Abstract
MXenes, a unique class of 2D transition metal carbides, have gained attention for gas sensing applications due to their distinctive properties. Since the synthesis of Ti3C2Tx MXene in 2011, significant progress has been made in using MXenes as chemiresistive sensors. Their layered structure, abundant surface groups, hydrophilicity, tunable conductivity, and excellent thermal properties make MXenes ideal for low-power, flexible, room temperature gas sensors, fostering scalable and reproducible applications in portable devices. This review evaluates the latest advancements in MXene-based gas sensors, beginning with an overview of the elemental compositions, structures, and typical fabrication process of MXenes. We subsequently examine their applications in gas sensing domains, evaluating the proposed mechanisms for detecting common volatile organic compounds such as acetone, formaldehyde, ethanol, ammonia, and nitrogen oxides. To set this apart from similar reviews, our focus centered on the mechanistic interactions between MXene sensing materials and analytes (particularly for chemiresistive gas sensors), leveraging the distinct functionalities of MXene chemistries, which can be finely tuned for specific applications. Ultimately, we examine the current limitations and prospective research avenues concerning the utilization of MXenes in environmental and biomedical applications.
1. Introduction
Gas sensors are critical in various applications, including disease diagnosis, environmental monitoring, public safety, and food quality evaluation.1–3 Over the past five decades, numerous technologies have been developed for gas detection, encompassing conductometric, optical, electrochemical, thermoelectric, and acoustic sensors.4–12 Among these, chemiresistive sensors are of particular interest due to their scalable production methods, low-cost, small footprint, and seamless integration with standard integrated circuit technologies.13–15 Since the 1960s, metal oxide semiconductors (MOS) have been extensively studied for their application in chemiresistive gas sensors. Their simple structure and high sensitivity to stimuli have positioned MOS-based materials in the ideal space for sensor integration. The gas-sensing properties of pristine MOS, corresponding heterostructures, surface modifications, and micro- and nanostructures have been thoroughly documented in various reviews and book chapters.16–25 Consequently, MOS-based materials have been successfully commercialized and deployed in many fields ranging from industrial and agriculture to pharmaceutical applications.26–30 Despite the advantages of MOS-based gas sensors, several practical constraints and unresolved challenges impede their further advancement. A primary concern is the requirement for high operating temperatures, typically 200–500 °C, to activate the adsorption of ionized chemical species necessary for detection reactions. Typically, this requirement translates to incorporating a heating element in the sensor device, limiting their potential application in many fields and low-power devices.31–34 The high operating temperature of MOS-based sensors necessitates increased sensor design and fabrication complexity and diminished sensitivity due to thermally induced Ostwald ripening of nanoparticles.35,36 Furthermore, elevated temperature significantly limits these sensors’ wide adoption and application, particularly in hazardous environments where flammable gases may ignite. In addition, MOS-based gas sensors face additional technical challenges, including baseline resistance drift, insufficient selectivity, and sensitivity to humidity, further limiting their use-case in many areas.37–39To overcome these limitations described above, researchers have focused on exploring a wide range of material combinations and architectures, modifying sensing material surfaces, and scalable production methodologies. As a result, the critical metrics on assessment of a sensor device are often categorized into four key elements: sensitivity, selectivity, recovery time, and stability. Concurrently, there is a growing emphasis on detecting gas molecules at or near room temperature to usher in a new wave of sensing devices that are compatible with the current surge in consumer electronics.40–43 The rapid growth of the Internet of Things (IoT) and flexible electronics has driven a heightened demand for gas sensors that are flexible, wearable, energy-efficient, and compact.26 Further, advancements in nanotechnology have stimulated interest in gas-sensing technologies that focus on device miniaturization and enhanced sensing performance through the application of innovative low-dimensional materials.44–46 Recently, emerging 2D materials have attracted significant interest for the development of room temperature chemiresistive gas sensors, thanks to their high surface-to-volume ratio and exceptional physicochemical properties. The introduction of graphene-based gas sensors spurred exploration into a broader range of 2D materials for room temperature gas sensing applications.47–52 Since 2011, MXenes—a subset of 2D materials including transition metal carbides, nitrides, carbonitrides, and oxycarbides—have gained considerable attention due to their unique surface chemistry, tunable interlayer spacing,53,54 and diverse physicochemical properties.55–57
The MXene family consists of a wide variety of materials based on composition and structure, such as single transition metal MXenes, ordered phases, and high-entropy (h) MXenes.58–67 Recent research58,68–71 has shown that MXene-based materials offer excellent gas sensing performance at room temperature, capable of detecting ultra-low gas concentrations due to their high signal-to-noise ratio. However, pristine MXene-based gas sensors face challenges, including low sensitivity, baseline drift, susceptibility to cross-interference, and a narrow band gap, which limits surface reactions and sensor responsiveness. Additionally, MXenes’ tendency to self-stack hinders gas diffusion and access to active sites, further restricting their ability to detect low-concentration gases.72 While pristine MXenes offer a promising foundation for developing selective and sensitive room temperature gas sensors, further research is needed to better understand the gas sensing mechanisms and to scale up production of MXene-based sensors. Overcoming these challenges, such as improving response, selectivity, detection limits, and reversibility, is essential for unlocking the full potential of MXene-based materials in advanced gas sensing technologies.
In this review, we explored recent advancements in 2D MXene-based heterostructures, focusing specifically on gas sensing mechanisms (particularly for chemiresistive gas sensors) within environmental and biomedical applications. Unlike other studies, we emphasized the unique mechanistic interactions between MXenes and analytes, enabled by the tunable chemistries of MXenes, which facilitate enhanced sensitivity and selectivity for various gases. Our comprehensive analysis includes the distinct functionalities and room temperature capabilities of MXenes, making them viable for low-power, scalable gas-sensing applications. By addressing existing limitations and proposing future research avenues, this review serves as a valuable resource for researchers, academics, and industry professionals aiming to push the boundaries of MXene-based sensing technologies. We believe this work will contribute to advancing the integration of MXenes into next generation sensor technologies, aligning with the growing demands of environmental and health monitoring.
2. 2D materials for gas sensing
In 2011, the landscape of 2D materials was predominantly composed of semiconductors, semimetals, and insulators characterized by low electronic conductivities and carrier concentrations. At that time, few 2D materials could be produced in quantities sufficient for applications beyond microelectronics. Solution-processed 2D materials, with the exceptions of graphene and hexagonal boron nitrides, exhibited limited flake sizes due to low mechanical strength, which resulted in fracture during delamination. Many of these materials also demonstrated hydrophobic properties and instability in air.73 The discovery of a family of 2D carbides and nitrides possessing metallic conductivity, hydrophilicity, processing ease, high yields, and large flakes subsequently transformed the materials science field.74 Early transition metal carbides and nitrides had established themselves as an important material class, distinguished by their high metallic electrical conductivity, exceptional hardness, and superior chemical stability. These materials had been extensively studied for decades as bulk ceramic materials, primarily for high-temperature applications and cutting tools. Their applications extended to functional composites, catalysts, and electrochemical energy storage.The strong bonding between transition metal and carbon/nitrogen atoms, predominantly through covalent and metallic bonds,75 had previously made dimensionality reduction—from bulk 3D solids to nanomaterials including 2D sheets and 1D nanoribbons or nanotubes—particularly challenging. This necessitated unconventional approaches to achieve dimensionality reduction, and as a result, Gogotsi and coworkers developed an approach that utilized the difference in strength between metal–metal bonds and metal–carbon/nitrogen bonds to selectively etch monoatomic metal layers from the MAX phases. These MAX phases were layered ternary carbides and nitrides with a composition of Mn+1AXn, where M represented an early transition metal, A primarily comprised group 13 and 14 elements, X denoted carbon and/or nitrogen, and n ranged from 1 to 4. In 1996, Barsoum and El-Raghy achieved a breakthrough by producing single-phase pure samples of Ti3SiC2, demonstrating its machinability and excellent electrical and thermal conductivity.76 By 1997, they had established that Ti3SiC2 was one of 50 phases, most of 2011 which had been discovered by Nowotny and colleagues in the 1960s.77,78 The discovery of Ti4AlN3 in 1999 led to the designation of these materials as Mn+1AXn or MAX phases.79
Structurally, the MAX phases exhibited layered hexagonal characteristics (P63/mmc space group) and could be described as transition metal carbide/nitride sheets of octahedral blocks, with X atoms positioned in the octahedron centers, connected by pure A layers.80 In 2011, Naguib and coworkers demonstrated that immersing Ti3AlC2 in hydrofluoric acid at room temperature enabled selective etching of the Al layers, resulting in the first 2D titanium carbide—Ti3C2.81 By 2012,82 this selective etching method had been shown to apply to numerous other MAX phases containing aluminum A-layers, one of the more common A-elements in MAX phases. This development led to the designation of this new family as MXenes, emphasizing their relationship to MAX phases and their dimensionality. While approximately 70 MAX phases existed in 2011,83 by the time of documentation, the number had grown to 150, with new discoveries occurring regularly, providing an expanding array of precursors for MXenes. The critical performance metrics of gas sensor devices (sensitivity, selectivity, response and recovery time, detection limit, etc.) fundamentally depend on the characteristics of the sensing material utilized. Specifically, the large surface area of the sensing material facilitates the interaction between the material's active surface and the target gas molecules, while the availability of active surface sites enables efficient and selective adsorption of gas molecules.84 In this context, two-dimensional nanostructures, which possess unique material properties that inherently differ from bulk structures, have garnered extensive research interest for development of high-performance gas sensors. In addition to providing an extensive surface area and thus increased active sites, 2D materials demonstrate several notable advantages, including facile surface functionalization, adjustable electronic structure, potential for 3D architectural assembly via stacking or sandwich-style approaches, superior flexibility enabling wearable use-cases enabling effective device integration compatibility, and exceptional mechanical durability.85 As a result of implementation of 2D structures in this field, substantial improvements in the “4S” sensor performance parameters (sensitivity, selectivity, stability, and speed—response/recovery time) have been made in the past two decades.86 For gas sensing applications, three primary categories of two-dimensional materials have emerged as the most extensively studied: (i) metal oxides,87–90 (ii) graphene-based structures,91–93 and (iii) dichalcogenides.94–96 Metal oxide-based structures demonstrate exceptional sensitivity to gas molecules while maintaining robust stability.97,98 These materials offer significant advantages in terms of cost-effective production and versatile manufacturing capabilities, enabling the creation of diverse nanostructured morphologies. The operational principle of metal oxide sensors relies on conductivity changes within the sensing surface layer, which vary according to environmental gas presence. The sensing mechanism progresses through distinct stages: initial adsorption of oxygen species on the semiconductor surface, electron transfer between semiconductor and oxygen, adsorption of the target gas, chemical reaction occurrence, electron transfer to the semiconductor, and finally, product desorption. The material's nanostructure and morphology play crucial roles in sensor performance. Specifically, porous structures facilitate enhanced surface-to-volume ratios, while large specific surface areas provide abundant active sites for gas molecule adsorption.97,99 Graphene-based materials present compelling opportunities for gaseous molecule detection, primarily due to their exceptional electrical conductivity, remarkably high specific surface area, and superior charge carrier mobility.99–101 These materials operate through a direct charge transfer mechanism, whereby the adsorption and desorption of gas molecules induce changes in local charge carrier concentration. The electrical conductivity undergoes either an increase or decrease, depending on whether the gas acts as an electron donor or acceptor.102 Two-dimensional layered structures of transition metal dichalcogenides, notably MoS2, MoSe2, and WS2 as frequently reported in literature, exhibit favorable semiconducting properties combined with high surface area and exceptional surface sensitivity. These characteristics have led to their widespread adoption in gas detection applications.103,104 Their sensing mechanism closely parallels that of graphene-based materials, relying on charge transfer processes between the surface and adsorbed molecules.105
Beyond their general sensing capabilities, 2D materials provide an excellent foundation for developing gas sensors that operate effectively at low or room temperatures.106 For example, Zhang and Yin successfully demonstrated high ethanol-sensing properties using SnO2 nanosheets at a relatively low operating temperature of 165 °C. The researchers attributed this exceptional sensor performance to the mesoporous texture of the nanosheets, combined with small grain sizes and surface defects, resulting in high response rates, rapid response/recovery times, and excellent selectivity.107 While metal oxide-based gas sensors are able to function at low temperatures, graphene-based materials and transition metal dichalcogenides enable the development of room-temperature sensors.108 The creation of graphene/CNT hybrid films and Pd-decorated graphene structures facilitates high-performance NO2 detection at room temperature, presenting opportunities for low-power sensor device development.109 Similarly, sensors utilizing WS2 nanoflakes or WS2 and MoS2 thin films demonstrate significant potential for room-temperature ammonia detection, offering both high sensitivity and selectivity.104,110 MXenes represent the next evolution of 2D materials particularly suited for high-performance sensor development, further enabling low- and room-temperature applications with excellent sensitivity and response time. These materials possess exceptional electronic, physical, chemical, and mechanical properties, including large specific surface area, narrow and tunable bandgap, rapid electron transfer capability, and adjustable surface chemistry. While MXenes exhibit a 2D layered graphene-like morphology, they distinguish themselves from other 2D materials through superior response characteristics and enhanced signal-to-noise ratios, attributed to strong functional group binding with analytes. Their near-free electron states, positioned around the Fermi level, enable rapid charge-carrier transport through electron transport channels.111–113 As a result, the superior sensing properties of MXenes stem primarily from their ability to effectively host a wide range of surface functional groups, which form strong bonds with analyte gases, and their metallic conductivity, facilitating rapid electron transfer and mobility.114,115 The selectivity of MXene-based sensors depends significantly on several factors, including surface-gas molecule interactions, MXene compositions and charge states, and flake orientation. Additionally, their controllable surface terminations offer substantial opportunities for structural modification, leading to enhanced properties and improved sensing performance.116
3. 2D MXenes and MXene heterostructures
3.1 MAX and MXene structure
Since their discovery by Gogotsi et al. in 2011,81 two-dimensional (2D) MXenes with nanosheet (NS) morphology have garnered significant interest due to their unique features, including their 2D structure, high conductance, tunable bandgap, excellent mechanical flexibility, and hydrophilicity. MXenes are derived from their precursor materials known as MAX phases, which have the formula Mn+1AXn. In MAX phases, n ranges from 1 to 3, M is a transition metal, and A represents an element from groups 13–16 of the periodic table. Fig. 1 illustrates the elements involved in MAX phases and the corresponding MXenes, depicting a visual categorization of the precursor elements on the periodic table.117 MXenes are characterized by the general formula Mn+1XnTx, where M represents a transition metal (e.g., Mo, Ti, Zr, Cr), X denotes either carbon (C) or nitrogen (N), n ranges from 1 to 4, and Tx indicates surface termination groups (e.g., –H, –O, –OH, –F). The atomic structure of MXenes consists of layers of M atoms arranged in a honeycomb-like 2D lattice, interspersed with X ions occupying the octahedral sites between adjacent metal layers, yielding highly ordered two-dimensional surfaces enabling a wide range of potential surface modifications.118–120 Typically, the MXene structure is derived from separation of the MAX-phase precursor via selective etching and delamination in stages.121,122Fig. 1b and c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123,124 depicts a visual of the transition stages of Ti-based MAX phase, illustrating the inception of 2D layered MXene via typical HF etching and delamination synthesis route.
123,124 depicts a visual of the transition stages of Ti-based MAX phase, illustrating the inception of 2D layered MXene via typical HF etching and delamination synthesis route.
 | ||
| Fig. 1 (a) Periodic table displaying elements in MAX phases and MXenes, including surface terminations and intercalant cations derived from experimental investigations.74 (b) left to right: SEM images of Ti3AlC2 MAX powder, multilayered Ti3C2Tx MXene powder synthesized using 30 wt% HF, single MXene sheet post delamination.80 (c) Schematic demonstrating Mo2Ga2C MAX and MXene structures.81 | ||
3.2 Synthesis of MXenes
| Mn+1AlX1 + 3HF = Mn+1Xn + AlF3 + 1.5H2 | (1) |
| Mn+1Xn + 2HF = Mn+1XnF2 + H2 | (2) |
| Mn+1Xn + 2H2O = Mn+1(OH)2 + H2 | (3) |
The M–X (X = C, N) bonds in Mn+1Xn layers are strong covalent-metal interactions, while the M–Al bonds are relatively weak metallic bonds, enabling selective bond cleaving via exposure to selective etchants. For MAX-phases containing more electronegative elements like Si instead of Al as the binding element, an additional oxidizing agent (e.g., H2O2, FeCl3, HNO3, NH4S2O8, KMnO4) is often used alongside HF.125,126
However, the high toxicity and reactivity of hydrofluoric acid used in the etching process often result in defective MXenes and dramatic heating of the reaction system, leading to potential oxidation of the resulting MXenes.127,128 To address these issues, a milder approach was developed using in situ generated HF through the interaction of HC acid with various metal fluorides (Li, Na, K, Fe). This method allows for better control of the synthesis process, producing less defective MXenes with fewer Mn+1Xn layers.68,129,130 The etchant composition significantly influences the surface functional groups (e.g., –F,–OH, –O, –Cl) that form, which in turn affect the electrochemical properties of MXenes.
While these delamination strategies demonstrate notable improvements in yield from the original synthesis methodology, a scalable method to synthesize large defect-free MXenes is highly sought after to enable large-volume production. In this effort, to obtain large, non-defective Ti3C2Tx particles, the Minimally Intensive Layer Delamination (MILD) technique was developed, optimizing the interaction between the Ti3AlC2 MAX-phase and the HCl-LiF system.121,123 This method employs higher n(LiF)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(Mn+1AlXn) and n(HCl)
n(Mn+1AlXn) and n(HCl)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(LiF) ratios to facilitate aluminum layer etching and separate accordion-like MXene aggregates into individual plates without ultrasound, requiring only shaking. MXenes synthesized by the MILD method exhibit distinct morphology, aggregation patterns after drying, and mechanical and electrophysical properties compared to those produced by conventional etching methods using lithium fluoride solution in hydrochloric acid.
n(LiF) ratios to facilitate aluminum layer etching and separate accordion-like MXene aggregates into individual plates without ultrasound, requiring only shaking. MXenes synthesized by the MILD method exhibit distinct morphology, aggregation patterns after drying, and mechanical and electrophysical properties compared to those produced by conventional etching methods using lithium fluoride solution in hydrochloric acid.
Reproducible and scalable MXene synthesis methodologies are still being developed to enable application in sensing devices. Given the similarities between MXenes and other 2D materials, many studies on MXene-based sensor device fabrication employ established methods like dip-coating or drop- coating to apply modified MXene structures for investigation of sensing properties [Fig. 2].133–135 While the sensor device fabrication is crucial in demonstration of its commercial and scaling viability, device fabrication and integration will not be covered in the scope of this review.
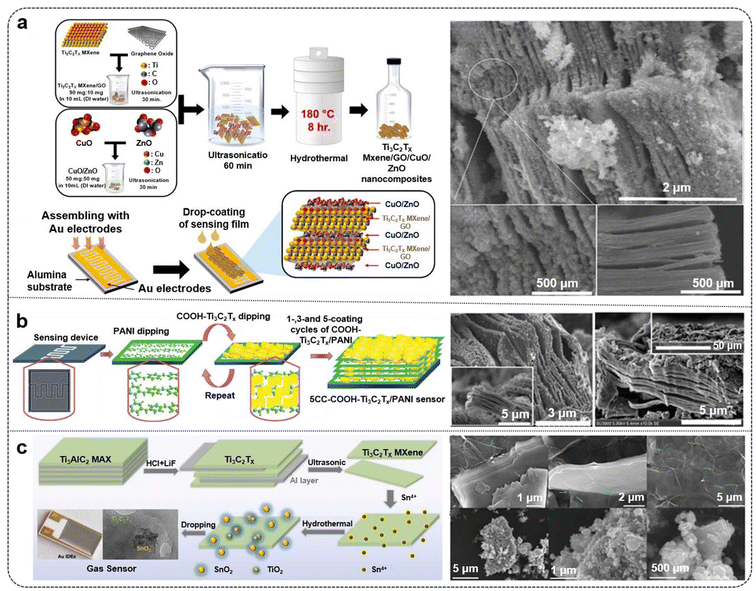 | ||
| Fig. 2 (a) Synthesis schematic diagram of Ti3C2Tx MXene/GO/CuO/ZnO nanocomposites.90 (b) Schematic of sensor fabrication process using dip-method and coating cycles of COOH-Ti3C2Tx /PANI.91 (c) Illustration of preparation process of Ti3C2Tx-SnO2 nanocomposite. The right side includes SEM images of the as-prepared MXene composites.92 | ||
The properties of synthesized MXenes exhibit significant variations depending on the chosen synthetic route, as these characteristics are fundamentally influenced by their surface functional groups, structural defects, and interlayer architectures. These two-dimensional materials demonstrate remarkable versatility, fulfilling the essential requirements for functional gas-sensing devices. The following discussion examines the latest reports on key intrinsic properties of MXenes that contribute to their gas-sensing capabilities, with particular emphasis on their structure–property relationships and sensing mechanisms.
4. Gas sensing principles
The sensing mechanism exhibited by MXene structures represents a distinct paradigm from that of metal oxides, demonstrating complexity that differs from the conventional surface adsorption or charge transfer mechanisms observed in traditional two-dimensional (2D) materials. While MOS-based sensors operate through well-established mechanisms involving interactions between gas molecules and pre-adsorbed oxygen species at the surface, MXenes exhibit fundamentally different sensing behavior.The intrinsic sensing mechanisms of both 2D MXenes and their heterostructures are central to their gas- sensing capabilities. Computational simulations have provided valuable insights into the gas-sensing mechanisms of pristine 2D MXenes. However, MXene heterostructures introduce additional complexity through their unique attributes, particularly in terms of their electronic structures and adsorption models, resulting in distinct sensing mechanisms.4.1 Sensing mechanism of 2D MXenes
Since the initial discovery of MXenes, numerous MXene permutations have been reported,57,67,69 with Ti3C2Tx has emerged as the most extensively investigated member for gas sensing applications. Ti3C2Tx sensors exhibit behavior characteristic of p-type semiconductors, a phenomenon attributed to the presence of surface functional groups including –O, –OH, –F and etc. In gas sensing applications, charge carrier transfer is predominantly driven by physisorption and chemisorption. This attribute is influenced by the operating temperature, where at ambient temperatures, physisorption predominates such that a target gas molecule is absorbed onto the MXene surface without the mediation of adsorbed oxygen species. As a result, the measured electrical response of the system is changed via the process of adsorption and desorption, giving rise to gas detection mechanism.136 This contrasts with MOS-based sensing mechanism, where adsorption of Oxygen on the surface is enabled at high temperature by capturing free electrons of the MOS, such that upon presence of a reducing gas, the surface density of adsorbed oxygen is changed thus allowing current flow, in accordance with its semiconductor properties. This fundamental difference in sensing mechanisms between MOS- and MXene-based gas sensors plays a crucial role in enabling ambient-temperature gas sensors, which are in high demand.The electrical response is fundamentally determined by the chemical nature of the gas analytes, where reducing gases often induce an increase in electrical resistance, while the opposite is observed for oxidizing gases. This phenomenon is well-illustrated in Ti3C2 MXene interfaces with ammonia, where the generated electrons combine with holes in the MXene structure, resulting in a reduction in surface charge- carrier concentration and a corresponding increase in the resistance of Ti3C2Tx sensors.137,138 The p-type Ti3C2Tx sensing reaction is demonstrated in eqn (4) and (5):
| 2NH3 + 3O− → N2 + 3H2O + 3e− | (4) |
| NH3 + OH− → NH2 + H2O + e− | (5) |
MXene-based gas sensors generally operate at ambient temperature to avoid surface oxidation; therefore, the dominant adsorbed oxygen ions are typically of the O2− type. Upon introduction of an external gas, a reaction occurs between the adsorbed O2− ions and the gas, resulting in a change of MXene electrical conductivity. Further, in the case of a reducing gas, the interaction yields electrons, resulting in both hole formation and electron recombination, leading to an increase in MXene resistivity via hole depletion. Alternatively, when an oxidizing gas is encountered, electrons are consumed, leading to hole accumulation, decreasing resistivity.
4.2 Sensing mechanism of MXenes heterostructures
The gas sensing mechanism in MXene heterostructures is notably more complex due to the interplay between MXene and various materials, including metal oxides, polymers, and metal nanoparticles. These combinations result in diverse energy-band diagram configurations as these components interact with target molecules of either reducing or oxidizing gases. For instance, MXenes with p-type conductivity can be integrated with n-type or p-type semiconductors, forming p–p139 or n–p140 junctions based on the sensor device arrangement. Further additions of active materials to the MXene composites often adds another layer of complexity into the gas sensing process, where elucidation of fundamental sensing mechanisms aims to optimize the composition of the heterostructures. We chose Sn-based MXene composites as an example to discuss MXene heterostructure sensing mechanisms due to the consensus across many reports on their enhancing effect for MXenes.Sn-based MXene composites stand out among MXene heterostructures owing to their low cost, non- toxicity, and availability.140,141 A first report to the best of our knowledge, on SnO2/MXene heterostructures capable of sensing NO2 gas at ppb-level proposed the following mechanistic process. Given the priori on SnO2 as a n-type semiconductor property with high electronegativity and intrinsic oxygen vacancies, a Schottky barrier is formed by addition of MXene, where due to a mismatch in Fermi level, electrons are injected from MXene to SnO2 until system is equilibrated, leading to the band bending [Fig. 3a].142 Further, introduction of target gases results affects the electron depletion layer (EDL), thus modulating electrical conductivity of the system.
 | ||
| Fig. 3 (a) Sensing mechanism of SnO2-MXene under air and NO2 conditions.99 (b) NH3 sensing mechanism of Ti3C2Tx-SnO2 composite sensor.100 (c) Schematic of NH3 sensing mechanism of Ti3C2Tx MXene and SnO.101 | ||
Yu and coworkers used Ti3C2Tx-SnO2, which was shown to have excellent NH3 sensing response at room temperature due to the hybridization with SnO2via facile hydrothermal synthesis route.143 Similarly, this work concluded the enhanced sensing property of the Sn-based MXene composite is due to the modulation of the depletion layer because of reactions between NH3 molecule and the adsorbed oxygen ions [Fig. 3b]. Additionally, Ti3C2Tx MXene functional groups provide favorable sites for gas adsorption, although an excessive amount of MXene can reduce response due to the blocking of active sites by numerous –OH termination groups.143 In another study focused on SnO/MXene sensors for NH3 detection, it was observed that when the Ti3C2Tx MXene/SnO nanocomposite was exposed to ambient air at room temperature, adsorbed oxygen ions (O2−) contributed to the development of a thicker electron depletion layer, resulting in increased resistance. When the sensor was then exposed to ammonia vapor, NH3 reacted with water molecules to produce NH4+, which subsequently underwent an oxidation reaction with the adsorbed oxygen O2−. This process released electrons back into the Ti3C2Tx MXene/SnO sensing material. Electron transfer occurred from Ti3C2Tx to SnO due to Ti3C2Tx having a lower work function compared to SnO, causing band bending [Fig. 3c]. Essentially, the formation of a p–n junction enabled a greater return of electrons to the composite, thereby reducing the thickness of the electron depletion layer and resulting in lower resistance for the Ti3C2Tx MXene/SnO nanocomposite in the presence of ammonia vapor. The authors remarked that the synergy between thin layer of SnO, high conductivity and specific area of Ti3C2Tx MXene, along with the p–n junction forming between the two materials was the enabler for the high response and high selectivity of the sensor towards ammonia.144
Across the screened reports, the mono- and bi-metallic additions to MXene follows similar trends in terms of mechanistic processes of gas sensing, with many reported optimizations in specific surface area and active site accessibly to enable maximum utilization of MXene framework as an ideal chemiresistive gas sensing device. We will dive further into MXene-based heterostructures modified for selective gas sensing towards common gases such as ammonia, and NO2.
5. MXene-nanocomposites for gas sensing
5.1 Volatile organic compounds (VOC) gas sensors
The rapid pace of industrialization has led to higher emissions of toxic, flammable, and explosive gases, posing significant risks to both ecological systems and human health. Of special concern is acetone (CH3COCH3), a vital organic chemical extensively used in various industrial applications. However, despite its widespread utility, the flammable, explosive, and toxic nature of acetone imposes considerable threats to public safety and occupational health.145–148 Prolonged exposure to acetone can result in severe health issues such as central nervous system depression, irritation of mucous membranes, ocular inflammation, and dizziness. At elevated concentrations, exposure could lead to unconsciousness, coma, or even death. Consequently, developing efficient methods for the rapid detection of acetone has become an urgent research priority. In this subsection, we briefly review a compilation of recent reports on modified MXenes designed for selectivity towards VOCs, including acetone, ethanol, and formaldehyde. A recent report by Sun et al. demonstrated the utility of ZnSnO3/ZnO nanofibers on a Ti3C2Tx MXene sheet, whereby inclusion of the MXene component in the composite increased the sensor response value by a factor of 3.5, establishing a significant enhancement over the pristine nanofibers.149 From an applied point of view, this enhancement in sensor sensitivity was observed in the optimum working temperature of the sensor where the highest response value is achieved, which was reported to be reduced from 200 °C to 120 °C. The synthesis of ZnSnO3/ZnO/Ti3C2Tx MXene composites was realized through a two-step process, initially creating ZnSnO3/ZnO nanofibers via electrospinning, followed by their attachment to MXene sheets using a self-assembly method, which takes advantage of electrostatic interaction between the MXene and the nanofibers. In this composite architecture, ZnSnO3/ZnO composite nanofibers acted as the active core material, while Ti3C2Tx MXene nanosheets served as the conductive substrate. Furthermore, the composite structure established a p–n heterojunction between the n-type semiconductors (ZnO and ZnSnO3) and the p-type Ti3C2Tx MXene, facilitating enhanced electron transfer within the assembly, demonstrating the typical characteristic of n-type oxide-semiconductors. As a result, the three- dimensional composite structure, composed of ZnSnO3/ZnO nanofibers distributed on 2D Ti3C2Tx MXene nanosheets, was shown to synergistically accelerate electron transfer rates within gas-sensitive materials.The synergies and heterostructures of Ti3C2Tx MXene nanosheets with ZnSnO3/ZnO in ZnSnO3/ZnO/Ti3C2Tx MXene composites were examined using a surface charge model, revealing intricate interactions between the components. With a band gap of 3.48 eV for ZnSnO3/ZnO and the metallic nature of MXene—characterized by conduction and valence bands crossing the Fermi level—the composite displayed unique electronic properties. The difference in work functions between the MXene nanosheets (3.9 eV) and ZnSnO3/ZnO (5.17 eV) triggered a charge transfer mechanism, where free electrons migrated from the MXene nanosheets to the ZnSnO3/ZnO material. Fig. 4a visualizes this charge redistribution, which results in the formation of an electron depletion layer in the MXene nanosheets and an accumulation layer in the ZnSnO3/ZnO material, consequently generating an internal electric field region akin to a barrier. This barrier ultimately impeded further electron transfer, facilitating the establishment of an equilibrium Fermi level within the ZnSnO3/ZnO/Ti3C2Tx MXene composite. Sun et al. further concluded that the enhancement of the ZnSnO3 MOS perovskite sensing capability by incorporating MXene was proven to be a direct result of formation of heterojunctions with the MXene framework, thereby enabling a new paradigm of improvements to MOS-based sensors.
 | ||
| Fig. 4 (a) Illustration of gas sensing mechanism of ZnSnO3/ZnO/Ti3C2Tx,106 and (b) In O2/ZnO3/Ti3C2Tx MXene composites.111. | ||
Considering the widespread use of VOCs in various industrial and consumer applications, researchers recognize their significant contribution to atmospheric pollution and potential for causing irreversible damage to human health.150,151 Ethanol, a prominent VOC and important chemical raw material extensively utilized in food processing, medical treatment, and industrial production, garnered particular attention due to its pervasive presence and associated health risks. Prolonged exposure to ethanol gas was found to induce a range of adverse effects, including headaches, liver and kidney dysfunction, and paralysis of the central nervous system.152,153 Consequently, the scientific community emphasized the critical importance of developing high-sensitivity monitoring systems for ethanol detection, aiming to mitigate its detrimental impact on environmental and human health.
In a study by Zhang et al., metal–organic framework (MOF) derived In2O3/ZnO was synthesized with Ti3C2Tx MXene to make a composite for detecting ethanol at room temperature, presenting a new strategy for room temperature gas sensing via utility of MOF and MXenes. They explored the mechanism of the MOF-MXene composite, finding that In2O3 and ZnO acted as n-type semiconductors, with In2O3/ZnO/Ti3C2Tx MXene nanocomposites demonstrating n-type semiconductor characteristics.154 Moreover, band structure analysis revealed that both the conduction band and valence band of Ti3C2Tx MXene crossed the Fermi level, verifying its metallic properties and superior electrical conductivity [Fig. 4b]. Electrons were the primary charge carriers in the sensing process, thus, electron transfer occurred from In2O3 to ZnO, creating an n–n heterojunction at their interface. Similarly, electrons from Ti3C2Tx MXene moved to In2O3, leading to band bending until reaching Fermi level equilibrium. In an air environment, O2 captured electrons from ZnO, In2O3, and Ti3C2Tx MXene to form oxygen ions (O2−). This process led to the formation of electron accumulation layers (EAL) and electron depletion layers (EDL) at the ZnO/In2O3 interface, while a hole accumulation layer (HAL) emerged at the In2O3/Ti3C2Tx MXene junction. When ethanol gas was introduced, the molecules diffused and reacted with O2− ions on the In2O3/ZnO/Ti3C2Tx MXene surface. As the reaction progressed, electrons released from the O2− ions combined with the holes in the HAL, reducing its thickness and increasing the Schottky barrier. Additionally, the formation of an n-n heterojunction reduced the thickness of the EDL and EAL, increasing electron concentration and decreasing the material's resistance.
Several factors were reported as critical contributors to the superior ethanol sensing capabilities of the In2O3/ZnO/Ti3C2Tx MXene nanocomposites. First, the substantial specific surface area (48.9 m2g−1) improved the adsorption of oxygen molecules and target gases, significantly enhancing sensor response. Secondly, Ti3C2Tx MXene's rich functional groups (–O, –F, –OH) provided ample active sites for gas reactions. The inherent conductivity Ti3C2Tx raised the carrier concentration in the material, thereby boosting ethanol sensing performance. Additionally, constructing the ternary In2O3/ZnO/Ti3C2Tx MXene nanocomposite system markedly increased oxygen content (39.13%), further enhancing sensing efficiency. This report by Zhang and colleagues provides another excellent example of MXene derivatives that enable a significant boost to traditional sensing active materials that are hindered by low conductivity, selectivity, and poor stability. It is evident that incorporating MXene into traditional structures that excel at target gas adsorption can yield multiplicative and synergistic improvements without a significant synthesis or fabrication barrier, paving the way for applied and scaled-up solutions.
Detection of Formaldehyde (HCHO), a harmful indoor pollutant known to cause severe health issues, including respiratory system damage and cancer risk, has been a focus of investigation in the sensor research community.155–157 Among various materials proposed for sub-ppm level formaldehyde detection in air, MOS-based materials, particularly SnO2, had been extensively investigated158–161 due to their fast response and low cost. While pristine Ti3C2Tx MXene exhibited gas-sensing capability for NH3, NO2, and H2, its performance is known to further improve when combined with metal oxide semiconductors like ZnO and CuO. However, premature oxidation of Ti3C2Tx MXenes is a known failure mode wherein affecting the sensor's long-term stability when exposed to high humidity or high temperatures. It is well-documented that pristine MXene can exhibit instability due to the chemical oxidation of Ti3C2Tx to TiO2 in ambient environment, modulating sensor properties which lead to baseline shifts.129–131 Given the importance of surface chemical states on gas sensing behaviour, the sensing performance of MXenes can be regulated through surface oxidations.
In a study conducted by Niu et al.,162 the authors reported that TiO2 generated through the synthesis process may form an n–n junction with SnO2 nanosheets, which prior research indicated could improve the response to formaldehyde sensing. To tackle the stability issues associated with nanocomposites, they developed a hierarchical SnO2/MXene nanocomposite using a simple one-step hydrothermal method. Additionally, to prevent uncontrolled oxidation of Ti3C2Tx MXene, they implemented a strategy involving pre-oxidation of the nanocomposites through an annealing process at 200 °C in air, which resulted in remarkable sensing stability over a four-week duration. The experimental findings showed that the synthesized SnO2-based MXene nanocomposite demonstrated improved sensing performance for formaldehyde compared to pure SnO2 samples, while the pre-oxidized MXene exhibited minimal response to formaldehyde at 160 °C. The authors attributed the enhancements in sensing and selectivity to the synergistic interaction between SnO2 nanosheets and TiO2/Ti3C2Tx MXene within the nanocomposites.162 The sensing mechanism of SnO2, an n-type metal oxide semiconductor, was explained as the interaction between formaldehyde gas and absorbed oxygen species, resulting in decreased resistance of SnO2 materials [Fig. 5]. In air, oxygen molecules adsorbed onto the surface of SnO2 nanosheets captured electrons from the conduction band, leading to the formation of various oxygen species (O2−, O−, and O22−). The predominant species in the temperature range of 120–200 °C were O2− and O−. This process created an electron depletion region on the SnO2 surface, which increased the initial resistance. When formaldehyde gas was introduced, the molecules reacted with the O2− and O− species. In the SnO2/MXene nanocomposites, pre-oxidizing the Ti3C2Tx MXene increased the concentration of oxygen vacancies, providing additional active sites for the adsorption of both oxygen and formaldehyde molecules. As a result, the sensor exhibited more significant changes in resistance in response to formaldehyde exposure.
 | ||
| Fig. 5 Schematic of formaldehyde sensing mechanism of pure SnO2 and SnO2/TiO2/Ti3C2Tx MXene composite.119 | ||
It is worth noting however, that excessive Ti3C2Tx MXene addition resulted in extremely low carrier concentration in the composite and correspondingly high resistance (∼700 Mohm for SnO2/MXene-5 and SnO2/MXene-10), whereby electrons released from the sensing reaction had to traverse numerous interfaces/boundaries between TiO2/Ti3C2Tx MXene and SnO2 in the composites. As such, it is understood that careful control of synthesis procedures and composition control is critical in application and scale-up of durable MXene composites, with one proven strategy proven to be through pre-oxidation of MXene framework.
5.2 NH3 gas sensors
Ammonia gas was identified as one of the most prevalent gases found in household and industrial cleaners, chemical production, refrigeration systems, agricultural products, and fertilizers.163–168 Furthermore, it is well known that NH3 detection could serve as a significant biomarker for disease diagnosis through exhaled breath analysis, particularly for conditions such as chronic liver disease, chronic kidney disease, and peptic ulcer symptoms. Additionally, NH3 has been established as a quality indicator for various food products, including fish, meat, and marine produce freshness in the produce industry.169,170 Consequently, room temperature detection of NH3 became crucial for numerous industries and applications. Although NH3 is also considered a VOC, due to a wider range of applications and subsequent demand for sensing devices, we selected to dedicate a subsection specifically in recent developments of MXene sensors for detection of ammonia.In gas sensing applications, pristine Ti3C2Tx MXene exhibits high selectivity to NH3 at room temperature, thanks to its surface terminal functionalities, which include O, F, and OH groups. These functionalities enhance active sites for NH3 molecule adsorption through hydrogen bonding, making Ti3C2Tx MXene a promising material for room temperature NH3 sensing. However, several challenges remain, such as low sensitivity, prolonged recovery time for pristine Ti3C2Tx MXene, and resistance drift following NH3 exposure.171,172
To address these limitations, researchers incorporated additional carbonaceous nanomaterials such as graphene, carbon nanotubes (CNTs), reduced graphene oxide (rGO), and graphene oxide (GO) to enhance NH3 gas molecule reactions. For instance, MXene/rGO nanocomposite fibers demonstrated improved NH3 sensing response at room temperature due to increased concentration in the conduction band and activated adsorption sites. Nevertheless, the sensor response remained relatively low (6.8% at 50 ppm of NH3) with prolonged recovery times exceeding 20 minutes.173–175 In efforts to further enhance NH3 sensing properties of MXene-based gas sensors, metal oxides were selected as effective doping materials due to their ability to generate abundant charge carriers. Copper oxide (CuO) and zinc oxide (ZnO) emerged as prominent metal oxides in gas sensing applications, offering advantages such as good electrical conductivity, non- toxicity, high surface activity sites and long-term stability.176–178
Seekaew et al.133 reported the synthesis of Ti3C2Tx MXene/GO/CuO/ZnO nanocomposites using the hydrothermal method, exploring various weight ratios to determine the optimal combination for enhanced NH3 sensing at room temperature. The authors demonstrated that the Ti3C2Tx MXene/GO/CuO/ZnO gas sensors exhibited superior response and exceptional selectivity toward NH3 gas under ambient conditions, highlighting the potential of these nanocomposites for room temperature ammonia detection applications. To obtain the composites, the authors added appropriate amounts of Ti3C2Tx MXene and graphene oxide (GO) to deionized water and sonication to obtain a homogenous mixture. Separately, CuO and ZnO powders were also mixed in deionized water and sonicated, followed by the two mixture solutions being combined and sonicated again to reach homogeneity. The mixture then underwent a hydrothermal process at 180 °C for 8 h, and dried to obtain the active sensing material [Fig. 2a]. The authors further investigated the selectivity of six different gas sensors based on varying weight ratios of Ti3C2Tx MXene/GO/CuO/ZnO nanocomposites. Their study examined the responses of gas sensors to various gases and volatile organic compounds (VOCs) at room temperature, including ammonia (NH3), formaldehyde (CH2O), ethanol (C2H5OH), methanol (CH3OH), isopropanol (C2H8O), toluene (C7H8), and acetone (C2H6O), each at a concentration of 200 ppm. As illustrated in Fig. 6a, the results indicated that all gas sensors exhibited decreased resistance when exposed to NH2, while showing negligible changes in response to the other VOCs.
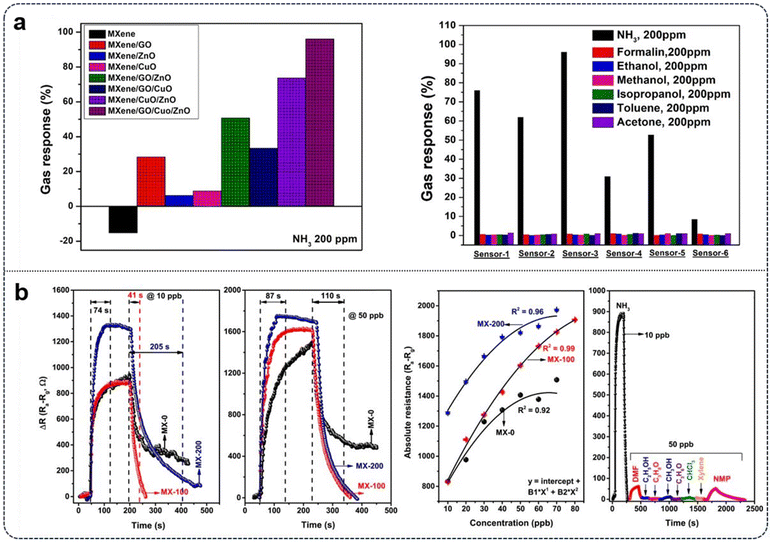 | ||
| Fig. 6 (a) left: Gas response profile of different compositions of GO, ZnO, and CuO on MXene, right: selectivity histograms of different gases based on different sensor composition rations at room temperature.90 (b) left: Absolute response profiles at various gas concentrations, including gas selectivity behaviour of MX-100 towards 10 ppb of NH3, right: comparison of sensor response recovery after exposure to NH3 on various sensors at 10 ppb and 50 ppb.136 | ||
Notably, all sensors, particularly Sensor-3, displayed high selectivity for NH3 compared to the other gases and VOCs, with the Ti3C2Tx MXene/GO/CuO/ZnO sensor showing no response to the common oxidizing gas NO2 at room temperature. In evaluating the Ti3C2Tx MXene/GO/CuO/ZnO gas sensors with different weight ratios, researchers found that all configurations exhibited high selectivity and responsiveness to NH3, with the optimal composition determined to be a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 weight ratio of Ti3C2Tx MXene/GO/CuO/ZnO. Under these optimized conditions, the sensor demonstrated impressive performance characteristics at room temperature, including a 59.9% response to 100 ppm of NH2, along with rapid response and recovery times of 26 and 25 seconds, respectively. Furthermore, the optimized Ti3C2Tx MXene/GO/CuO/ZnO gas sensor exhibited a combination of desirable traits, including high selectivity, significant response magnitude, excellent repeatability, long-term stability, and quick response and recovery times, while maintaining consistent performance across a relative humidity range of 30–70% RH.
5 weight ratio of Ti3C2Tx MXene/GO/CuO/ZnO. Under these optimized conditions, the sensor demonstrated impressive performance characteristics at room temperature, including a 59.9% response to 100 ppm of NH2, along with rapid response and recovery times of 26 and 25 seconds, respectively. Furthermore, the optimized Ti3C2Tx MXene/GO/CuO/ZnO gas sensor exhibited a combination of desirable traits, including high selectivity, significant response magnitude, excellent repeatability, long-term stability, and quick response and recovery times, while maintaining consistent performance across a relative humidity range of 30–70% RH.
The room temperature NH3-sensing mechanism of the Ti3C2Tx MXene/GO/CuO/ZnO nanocomposite was elucidated based on the formation of multiple p–n heterojunctions at the interfaces of its constituent materials. While Ti3C2Tx MXene typically exhibited metallic electronic properties, it demonstrated p-type semiconductor behavior in this work, as evidenced by increased resistance upon exposure to electron- donating NH3. Conversely, GO, CuO, and ZnO displayed n-type conductivity, although their semiconductor properties were noted to be dependent on various factors such as surface functional groups and thermal treatment. The proposed NH3-sensing mechanism was predicated on forming multiple p–n heterojunctions, facilitated by the varying work functions of the composite materials. As illustrated in Fig. 7, upon exposure to NH3 gas, the interaction between NH3 molecules and oxygen species (O2−) resulted in electron release to the conduction band of the nanocomposite, leading to a reduction in the electron depletion layer width and the upward band bending. This process decreased the potential barrier at the p–n heterojunction. It promoted the formation of a narrow electron accumulation layer in the n-type components, ultimately causing a decrease in the sensor's resistance at room temperature. The high selectivity of the Ti3C2Tx MXene/GO/CuO/ZnO gas sensor towards NH3 was attributed to the polar nature of NH3 molecules and the presence of numerous functional groups on the nanocomposite surface, which facilitated stronger hydrogen bonding and enhanced sensing interactions.
 | ||
| Fig. 7 (a) Schematic of work function and Fermi level positions of Ti3C2Tx and p-type WS2 MXene composite at different gas exposures, illustration of charge transfer process, and work functions of different pristine materials. (b) Synthesis procedure of ME + To3C2Tx/WS2 sensor. (c) Sensing value of Ti3C2Tx/WS2 sensors on various electrodes.139 | ||
In another study, Dogra et al.179 diverged from the common approach of synthesizing metal oxide semiconductor (MOS) and MXene composites separately before combination, instead employing a novel bottom-up methodology to grow MOS directly on the MXene surface during calcination. This technique, which improved the junction contact between TiO2 and Ti3C2Tx MXene, emphasized the critical role of an optimum MOS/MXene ratio in maximizing sensor response. The researchers developed a facile approach for synthesizing Ti3C2Tx MXene and Ti3C2Tx/TiO2 composites, wherein TiO2 was grown through a kinetically controlled calcination process. Their findings revealed that calcination temperature was crucial in determining the overall morphology and sensing performance of Ti3C2Tx/TiO2 composites. They also demonstrated that both extremely low and high device resistances were unsuitable for effective conductivity modulation during the sensing process. The enhanced sensing performance was attributed to the formation of heterointerfaces between Ti3C2Tx and TiO2, which facilitated conductivity modulation upon interaction with gas molecules and improved overall sensing capabilities.
Moreover, the response transients revealed varying recovery behaviors among the devices, with MX-0 failing to fully recover its base resistance after gas removal, while MX-100 demonstrated nearly identical resistance changes and recovery to its initial state, with a response time comparable to MX-0. MX-200 exhibited the largest resistance change, albeit with a slightly delayed recovery [Fig. 6b]. At lower concentrations (50 ppb), MX-100 and MX-200 displayed similar recovery times, while MX-0 continued to show delayed recovery, suggesting an interplay between increased resistance due to calcination and various sensing parameters. Among the devices tested, MX-200 demonstrated the largest relative response across the investigated range of ammonia concentrations.
The authors concluded that MXene-based sensing devices exhibited real-time sensitivity to NH3 under ambient temperature and relative humidity conditions, showcasing their effectiveness in ammonia detection. The devices were exposed to various interfering gases to assess selectivity, a critical factor in sensor performance. The selectivity, influenced by factors such as adsorption and desorption energies of gas molecules, was favourable towards ammonia. This preferential detection was attributed to the composite nature of the sensor (Ti3C2Tx/TiO2), with certain crystal planes in TiO2 being more sensitive to NH3 adsorption. To validate this selectivity, response transients were acquired for other volatile compounds, including dimethyl formamide, ethanol, isopropyl alcohol, methanol, acetone, chloroform, xylene, and N-methyl pyrrolidone, at a concentration of 50 ppb each. The results demonstrated a significantly larger response to ammonia than these interfering gases, confirming the MXene-based devices’ selectivity towards reducing ammonia molecules [Fig. 6b].
The reported sensing mechanism agrees with previous studies, wherein TiO2, an n-type semiconductor with higher electronegativity, exhibited intrinsic oxygen vacancies and, similar to MXene, demonstrated a tendency to absorb atmospheric oxygen on its surface. The adsorbed oxygen is transformed into O2 after electrons are abstracted from the oxide surface. In the composite system, oxygen adsorption became competitive between the semiconducting oxide and MXene, though in both cases, the adsorbed oxygen converted to O2−. TiO2, with a band gap of 3.2 eV, possessed a work function of 5.1 eV, while the typical work function for MXene ranged from 3.9 eV to 4.8 eV, depending on surface group termination. Assuming a work function of 3.9 eV for MXene, electron flow occurred from MXene to TiO2 (ϕ = 5.1 eV), forming a depletion layer at the TiO2/Ti3C2Tx composite interface.
The interaction of target gases, whether oxidizing or reducing, modulated the electrical conductivity of the composite. Reducing gases, such as ammonia, donate electrons upon interaction with the sensing surface through two possible mechanisms: interaction with adsorbed O− ions or OH− ions associated with processed MXene, both processes resulting in electron release. The released electrons compensated for the depletion layer charges after recombination, enhancing device resistance due to reduced p-type character, which was reflected as an increase in overall device resistance.
5.3 NOx gas sensors
Among toxic gases, nitrogen dioxide (NO2) monitoring became particularly crucial due to its association with elevated incidences of respiratory diseases and mortality. Studies demonstrated that prenatal and postnatal exposures to elevated NO2 levels increased the risk of asthma, hay fever, rhinitis, pneumonia, and eczema in children.180,181A study by Quan et al.182 explored the additional enhancements to the WS2-based MXene composite by optimization of a flexible electrode material, where three different electrode materials including MXene, graphene, and carbon nanotube, were tested. In a comparative study of flexible printed films, the conductivity of three distinct compositions was evaluated, revealing that the ME composition exhibited superior conductivity, achieving a remarkable 7230 S cm−1. To assess the adhesion properties between the paper substrate, MXene, and Ti3C2Tx/WS2 material, they further conducted a simple tape stripping test, concurrently measuring changes in baseline resistance and NO2 response of the ME + Ti3C2Tx/WS2 sensor. Although delamination was observed, the baseline resistance and the response to 200 ppb NO2 of the flexible Ti3C2Tx/WS2 gas sensor remained stable before and after tape stripping, thus demonstrating the excellent adhesion performance of ME and Ti3C2Tx/WS2 gas sensing materials when printed on qualitative filter paper. Electrodes were fabricated using Au interdigital electrode (AuE) type gas sensors using Ti3C2Tx, WS2, and Ti3C2Tx/WS2, while flexible electrode type gas sensors were fabricated using ME + Ti3C2Tx and ME + Ti3C2Tx/WS2. As depicted in Fig. 8c, the AuE + Ti3C2Tx device exhibited higher conductivity compared to other sensors, however, its NO2 gas sensing performance was poor, primarily due to the high stacking density of synthetic Ti3C2Tx. The Schottky-type connection between AuE and WS2, resulted in incomplete recovery response to 1 ppm NO2, indicating hampered charge transfer due to mismatched work functions at the AuE-WS2 interface. The AuE + Ti3C2Tx/WS2 sensor exhibited p-type sensing behavior, suggesting NO2 molecule adsorption inhibited charge carrier transport. Notably, the measured response values showed minimal standard deviations (maximum 0.5%) across various NO2 concentrations, indicating robust electrode manufacturing stability.
 | ||
| Fig. 8 (a) Fabrication procedure of 3D MoS2/MXene heterostructure aerogel. (b) Response and recovery profile of MoS2/MXene towards NO2, including selectivity profile towards NO2. (c) Illustration of pristine MXene and MoS2/MXene, including Bader charge analysis between NO2 molecules and sensor.140 | ||
Analysis of the calculated work functions, Fermi level values, and electronic band structures revealed the energy level dynamics between metallic Ti3C2Tx and semiconductor WS2. Upon contact, electron transfer occurred from WS2 to Ti3C2Tx due to work function differences until Fermi level equilibrium was achieved. As illustrated in Fig. 8a, under ambient conditions, oxygen molecules captured electrons from the material, forming chemically adsorbed O2− ions on the surface and creating hole accumulation layers (HALs). Upon NO2 introduction, its higher electron affinity (2.30 eV versus 0.44 eV for O2) facilitated electron transfer, forming NO3− and expanding the HALs, thereby reducing resistance. The enhanced NO2 sensing performance of AuE + Ti3C2Tx/WS2 was attributed to both the formation of the Ti3C2Tx/WS2 heterojunction layer and the extensive contact areas provided by the 2D/2D heterostructures, which effectively improved carrier transportation.
Continuing the topic of MXene sensing enhancement via optimization of electrode morphology and composition, Kim and coworkers183 presented a synthesis strategy for a three-dimensional (3D) MoS2/MXene heterostructure aerogel, which was achieved through physical mixing of MoS2 and MXene nanosheets in deionized water, followed by freeze-drying to form a highly porous hierarchical network, as illustrated in Fig. 8a. Spectroscopic characterization demonstrated that this approach substantially minimized the oxidation degree of the MXene layer while successfully forming van der Waals heterojunctions between MoS2 and MXene, as confirmed by charge density difference analysis. The fabrication process of the MoS2/MXene composite aerogel (MMA) began with the synthesis of Ti3C2Tx sheets through selective etching of Ti3AlC2 in a mixture of LiF and HCl, followed by sonication-induced delamination. MoS2 nanosheets were prepared using a hydrazine-assisted ball-milling process on bulk MoS2, whereby the synthesized sheets were then directly assembled into a MoS2/MXene gel in an aqueous solution. Due to their similar hydrophilicities and negative charges, a stable aqueous MoS2/Ti3C2Tx suspension was readily achieved. To prevent significant oxidation and structural damage, authors employed mild sonication conditions. During freeze-casting, Ti3C2Tx sheets interconnected to form a 3D framework, with MoS2 nanosheets assembling on the MXene framework surfaces. The final MMA composite aerogel was obtained after removing ice crystal templates through freeze-drying, with MMA-X samples where X represented the mass ratio of Ti3C2Tx to MoS2.
The incorporation of the MoS2 catalytic layer significantly enhanced the resistance variation response toward NO2 gas. To elucidate the enhancement mechanisms, the researchers investigated charge transfer in two systems: (1) pristine MXene + NO2 and (2) MXene/MoS2 + NO2. Bader charge analysis and charge density calculations revealed decreasing charge transfer from sensing layers to NO2 in the order of MXene surface (−0.669 eV), MoS2 edge on MMA (−0.437 eV), and MoS2 basal plane on MMA (−0.176 eV). Although pristine MXene demonstrated the highest charge transfer, the interaction between NO2 and MXene surface resulted in hydrogen terminating group withdrawal, leading to charge compensation and reduced overall charge density alteration (0.04 eV). The presence of MoS2 effectively suppressed HNO2 formation at both basal plane and edge, resulting in higher charge density alterations in the gas-sensing layer compared to the MXene basal plane. Further analysis showed charge transfer occurred primarily from MoS2 (−0.447 eV), while MXene's charge density remained relatively stable (0.011 electron gain). The researchers observed that beyond improved response and accelerated NO2 desorption through transition from chemisorption to physisorption, the MXene's highly selective response toward NO2 was maintained after MoS2 decoration. This suggested that the enhancement mechanism was specific to NO2 gas molecules, as no significant improvements were observed in responses to other interfering gas species (H2, acetone, NH3, ethanol, and propanol). This work further demonstrates the multiple avenues of utilization and optimization of MXenes for selective and responsive gas-sensing applications. In addition, it is evident that many previously reported composite sensing materials and frameworks are easily implementable with MXene, raising significant opportunities for rapid development toward a scalable, cost-effective, room temperature gas sensor.
6. Designing MXene heterostructures for gas sensing
It has become evident that a single type of MXene or metal oxide (MO) material proves insufficient for achieving optimal gas sensing response levels. Furthermore, it is also well known the sensing performance of metal oxide materials can be enhanced through integration with conductive materials. When developing MXene hybrid materials for superior sensing performance, several critical factors warrant consideration. The primary requirement involves ensuring the metal oxide material possesses a high specific surface area, as this characteristic facilitates an increased number of available contact points. Additionally, the integration methodology between the metal oxide material and MXene must maintain simplicity. Furthermore, careful regulation of the metal oxide material's work function is essential, as this parameter significantly influences the charge transfer processes occurring at the MO-MXene interface. These requirements make metal oxide nanoparticles, with their inherent high specific surface area and straightforward fabrication characteristics, particularly suitable candidates for MXene hybrid development.184The resulting composite materials, formed through the combination of MXene and metal oxides, demonstrate distinctive morphological features and properties that result in enhanced sensing capabilities. Moreover, the integration of these heterogeneous structures results in enhanced sensing performance across various volatile organic compounds (VOC) and gaseous molecular species.
Extensive research has been conducted on MXene/metal oxide (hydroxide) materials for gas sensing applications, encompassing investigations of TiO2, SnO2, ZnO, WO3, Fe2O3, CuO, In2O3, Co3O4, Ni(OH)2, W18O49, and V2O5, among various other compounds as shown in Table 1. Beyond traditional semiconducting metal oxides, researchers have explored a diverse range of materials in developing MXene-metal oxide hybrids for gas sensing applications. Notable examples include Ti3C2Tx/1D-K2W7O22 for acetone detection,185 Ti3C2Tx/Co3O4 for formaldehyde sensing, Ti3C2Tx/Ni(OH)2 and Ti3C2Tx/V2O5/CuWO4 for NH3 detection, and Ti3C2Tx/Fe2(MoO4)3 for n-butane detection. Nanomaterials of varying dimensionality (0D, 1D, 2D, and 3D) demonstrate unique structural characteristics, resulting in substantially different gas-sensitive layers even when utilizing identical metal oxide compositions. Metal oxide nanomaterials, particularly those with 1D structures (such as nanotubes, nanobelts, and nanowires) and 2D configurations (including nanoflakes, nanoplates, and nanosheets), have gained increasing prominence in the development of chemiresistive gas sensors. The selection of nanomaterials with appropriate structural properties has emerged as a crucial factor in optimizing gas sensor parameters, warranting detailed investigation of gas sensors based on these nanomaterials.
| Analyte | MXene composite | Operating temperature (°C) | Analyte concentration (ppm) | Response (%) | Response/recovery time (s s−1) | LOD (ppb) | Ref. |
|---|---|---|---|---|---|---|---|
| Acetone | Ti3C2Tx/TiO2 | 350 | 2 | 180 | — | 20 | 186 |
| Ti3C2Tx/SnO-SnO2 | 25 | 100 | 12.1 | 18/9 | — | 185,187 | |
| Ti3C2Tx/W18O49 | 300 | 20 | 11.6 | 5.6/6 | 170 | 185 | |
| Ti3C2Tx/(α-/γ-Fe2O3) | 255 | 100 | 215.2 | 13/8 | — | 188 | |
| Ti3C2Tx/α-Fe2O3 | 25 | 5 | 16.6 | 5/5 | — | 189 | |
| Ti3C2Tx/K2W7O22 | 25 | 2.86 | 250 | ± | — | 190 | |
| Ti3C2Tx/ZnO | 320 | 100 | 14.4 | 8/12 | — | 191 | |
| Toluene | Ti3C2Tx/CuO | 250 | 50 | 11.4 | 270/10 | 320 | 184 |
| Methane | Ti3C2Tx/In2O3 | 25 | 5 | 29.6 | 6.5/3.5 | — | 192 |
| Ethanol | Ti3C2Tx/TiO2 | 25 | 100 | 22.47 | — | — | 193 |
| Ti3C2Tx /TiO2 | 25 | 100 | 5.90 | 226/79 | 194 | ||
| Ti3C2Tx/SnO2 | 230 | 10 | 500 | 14/26 | — | 195 | |
| Ti3C2Tx/MOF-derived Co3O4 | 200 | 50 | 190 | 50/45 | 1000 | 196 | |
| Ti3C2Tx/MoO2/MoO3 | 25 | 5 | 3 | 46/276 | — | 197 | |
| n-Butane | Ti3C2Tx/Fe2(MoO4)3 | 120 | 100 | 43.1 | 18/24 | — | 198 |
| Formaldehyde | Ti3C2Tx/Co3O4 | 25 | 10 | 9.2 | 83/5 | 10 | 199 |
| Ti3C2Tx/SnO2 | 160 | 20 | 10.7 | — | — | 200 | |
| Ti3C2Tx/ZnSnO3 | 25 | 100 | 194.7 | 6.2/5.1 | — | 201 | |
| Hexanal | Ti3C2Tx/TiO2 | 25 | 10 | 3.4 | 293/461 | 217 | 202 |
| Triethylamine | Ti3C2Tx/SnO2 | 140 | 50 | 33.9 | 1/1 | — | 203 |
| Ti3C2Tx/WO3 | 25 | 10 | 277.78 | 2/3 | — | 204 | |
| Isopropanol | Ti3C2Tx/TiO2/MoO3 | 25 | 50 | 245 | 100/40 | 50.45 | 205 |
| NH3 | Ti3C2Tx/SnO2 | 25 | 50 | 40 | 36/44 | 4.29 | 206 |
| Ti3C2Tx/SnO | 23 | 10 | 67 | 61/119 | 1000 | 207 | |
| Ti2CTx/TiO2 | 25 | 10 | 3.1 | — | 100 | 208 | |
| Ti3C2Tx/TiO2 | 25 | 10 | 3.1 | 33/277 | 500 | 209 | |
| Ti3C2Tx /(001)TiO2 | 25 | 30 | 40.6 | 10/5 | 5 | 209 | |
| Ti3C2Tx/WO3 | 25 | 1 | 22.3 | 119/228 | 210 | ||
| Ti3C2Tx/α-Fe2O3 | 25 | 5 | 18.3 | 2.5/2 | — | 211 | |
| Ti3C2Tx/CuO | 25 | 100 | 24.8 | 43/26 | — | 212 | |
| Ti3C2Tx/In2O3 | 25 | 30 | 63.8 | 42/209 | 213 | ||
| Ti3C2Tx/In2O3 | 18 | 20 | 100.7 | 60/300 | — | 214 | |
| Ti3C2Tx/MOF-derived In2O3 | 25 | 5 | 60.6 | 3/2 | — | 215 | |
| Ti3C2Tx/Ni(OH)2 | 25 | 50 | 11.6 | 78/- | 216 | ||
| Ti3C2Tx/V2O5/CuWO4 | 25 | 51 | 53.5 | 1.6/4 | 300 | 217 | |
| NO2 | Ti3C2/TiO2 | 25 | 5 | 16.02 | — | 125 | 218 |
| Ti3C2/TiO2 | 175 | 100 | 19.76 | 150/72 | — | 219 | |
| Ti3C2Tx/ZnO | 25 | 100 | 41.93 | 34/105 | 220 | ||
| Ti3C2Tx/ZnO | 25 | 20 | 367.63 | 22/10 | 221 | ||
| Ti3C2Tx/ZnO | 25 | 0.05 | 81 | 17/24 | 0.2 | 222 | |
| Ti3C2Tx/ZnO | 160 | 8 | 3.6 | 191/254 | — | 223 | |
| Ti3C2Tx/ZnO | 25 | 10 | 25 | — | — | 224 | |
| Ti3C2Tx/WO3 | 25 | 0.06 | 27 | 162/19.8 | — | 225 | |
| Ti3C2Tx/WO3 | 25 | 20 | 12 | 96/129 | — | 226 | |
| Ti3C2Tx/SnO2 | 25 | 0.03 | 231 | 146/102 | — | 227 | |
| Ti3C2Tx/SnO2 | 150 | 10 | 24.8 | — | 100 | 228 | |
| Ti3C2Tx-SnO2-TiO2 | 25 | 10 | 32.4 | — | 100 | 229 | |
| Ti3C2Tx/CuO | 25 | 50 | 56.99 | 16.6/31.3 | — | 230 | |
| Ti3C2Tx/Co3O4/Al2O3 | 25 | 100 | 40.3 | 1.3/13.3 | 10 | 231 | |
| H2 | Ti3C2Tx/TiO2 | 150 | 500 | 70 | — | — | 232 |
SnO2 represents a highly effective material for gas sensing applications due to its distinctive properties as an inorganic n-type semiconductor exhibiting a wide band gap (3.6 eV to 3.8 eV). The exceptional responsiveness of SnO2 to various harmful gases and VOCs establishes it as a remarkably promising material for gas sensing applications.185,195,203 Furthermore, the advancement of SnO2-MXene gas sensors has garnered significant attention from the research community owing to its potential to enhance both the selectivity and sensitivity of gas sensing devices. In their research, He et al.206 investigated a gas-sensing platform through the integration of 2D MXene heterojunctions with SnO2 nanoparticles, which were synthesized utilizing a hydrothermal method. The resultant MXene/SnO2 heterojunctions powder was applied to interdigitated electrodes, and the sensor demonstrated a 40% higher sensitivity to 50 ppm NH3 compared to a pure MXene sensor when operating at room temperature. ZnO, an n-type semiconductor with a band gap of 3.37 eV is often investigated in for use in gas sensing applications owing to its high response signal to various reducing or oxidizing gases, affordability, and abundance. As a result, reports of ZnO or combinations of, have recently become widespread in selective sensing of NO2 at room temperature.220–224 Similar properties have been reported in Tungsten oxide (WO3) where reports demonstrate high sensitivity and selectivity for NH3, yielding nearly 16 times higher gain than pristine Ti3C2Tx.210 Oxides of iron, copper, and indium have been steadily reported to significantly boost MXene-based sensors’ performance with sensitivity towards acetone,189 toluene,184 and ammonia,213,214 respectively. Although significant efforts have been made to enable room temperature sensing via incorporation of metal oxide heterojunctions with MXenes as the framework, high operating temperatures, baseline drift, and junction active site degradation or fouling hinder widespread adoption and application of MXene-based sensors.
7. Challenges and future directions
MXene-based heterostructures have shown significant potential in gas sensing applications, offering high sensitivity, selectivity, and operability at room temperature. However, several challenges must be addressed to enable their widespread adoption and practical implementation. This section outlines the key limitations of current MXene-based gas sensors and proposes future research directions to overcome these challenges.7.1 Stability and oxidation sensitivity
One of the primary limitations of MXenes is their susceptibility to oxidation under ambient conditions, which can degrade sensor performance over time. For example, the oxidation of Ti3C2Tx MXene to TiO2 alters its electrical conductivity and sensing properties, leading to baseline drift and inconsistent responses. Future research should focus on developing passivation strategies—such as protective coatings, controlled surface functionalization, or encapsulation techniques—to enhance long-term stability.7.2 Selectivity and cross-sensitivity
While MXene-based sensors exhibit excellent sensitivity, achieving high selectivity remains a significant challenge. Many target gases exhibit similar adsorption behaviors on MXene surfaces, leading to cross-sensitivity issues. To improve specificity, the incorporation of selective receptors—such as functionalized polymers or molecularly imprinted layers—could be explored. Additionally, machine learning-based signal processing and pattern recognition techniques may help distinguish between different gas analytes.7.3 Scalability and integration with flexible electronics
The fabrication of MXene-based sensors at a large scale, while maintaining high reproducibility, remains a challenge. Conventional methods like drop-casting and vacuum filtration often result in inhomogeneous film formation, which affects sensor performance. Future research should focus on scalable synthesis and deposition techniques, such as inkjet printing and roll-to-roll manufacturing, to enable cost-effective, large-area sensor fabrication. Additionally, the integration of MXene-based sensors with flexible and wearable electronics represents an exciting opportunity for real-world applications in biomedical and environmental monitoring.7.4 Understanding sensing mechanisms and gas adsorption behavior
Although experimental and computational studies have provided valuable insights into the gas-sensing mechanisms of MXenes, a comprehensive understanding of charge transfer dynamics, surface interactions, and gas adsorption kinetics is still lacking. In situ characterization techniques—such as operando X-ray spectroscopy, Raman spectroscopy, and atomic-scale imaging—are essential to elucidate fundamental sensing mechanisms and optimize sensor design.7.5 Balancing sensitivity and response time
While some sensors (e.g., Ti3C2Tx/WO3 for NH3) demonstrate fast response/recovery times (∼1–3 s), others with higher sensitivity (e.g., Ti3C2Tx/In2O3 for NH3) tend to have longer recovery times (∼300 s). This highlights an inherent trade-off between rapid gas adsorption/desorption dynamics and strong gas interactions. Understanding and balancing these trade-offs—depending on the specific application—will be critical for optimizing sensor performance.8 Summary and outlook
8.1 Summary
In conclusion, MXene-based heterostructures have emerged as highly promising materials for advanced gas sensing applications due to their unique two-dimensional structure, tunable surface chemistry, and excellent conductivity. Since the first synthesis of Ti3C2Tx MXene in 2011, substantial progress has been made in enhancing the performance of these materials, particularly for room temperature gas detection. The versatility of MXenes, which allows for various surface functionalities such as –F, –O, and –OH groups, provides a means of tailoring their sensing properties. This has enabled MXenes to detect VOCs like acetone, ethanol, formaldehyde, and gases such as ammonia and nitrogen oxides at extremely low concentrations.Despite their potential, pristine MXenes face challenges such as baseline drift, low sensitivity at sub- ppm levels, and self-stacking tendencies that hinder gas molecule diffusion. Researchers have developed MXene-based heterostructures to overcome these issues by integrating metal oxides (e.g., SnO2, ZnO, CuO), polymers, and other 2D materials. These heterostructures introduce new p–n and n–n junctions that modulate charge transfer, leading to improved response times, enhanced stability, and increased gas selectivity. For example, MXene-SnO2 composites have shown exceptional sensitivity to ammonia at room temperature, benefiting from the formation of depletion layers and an abundance of surface-active sites. Similarly, ZnO/MXene composites have successfully lowered operating temperatures for VOC detection, making them well-suited for low-power, portable devices.
Significant advancements have also been made in MXene synthesis techniques, such as the minimally intensive layer delamination method, which improves control over surface defects and functionalization. This has led to the production of higher-quality MXenes with enhanced gas-sensing properties. Additionally, pre-oxidizing MXenes to improve stability in humid and high-temperature environments has addressed a key challenge, enabling more durable and reliable sensor performance.
8.2 Outlook
However, several challenges remain before MXene-based gas sensors can be fully realized in commercial applications. Scalability in synthesis, long-term operational stability, and performance under varying environmental conditions, such as humidity and temperature, require further optimization. Addressing the self-stacking nature of MXenes, which limits gas diffusion, will also be critical for maximizing sensor performance. Several key challenges in sensing chemistries of MXenes are summarized below:Nonetheless, the versatility and room temperature operability of MXene-based heterostructures offer significant potential for applications ranging from environmental monitoring and industrial safety to medical diagnostics and IoT. In-depth studies of the fundamental gas-sensing mechanisms, aided by computational modeling and experimental verification, will be essential for the development of next-generation MXene sensors. Continued progress in this field will pave the way for the large-scale commercialization of MXene-based gas sensors, establishing them as a key technology for future advanced sensing systems.
Data availability
Figures used for this article are available corresponding to the cited articles in the paper. No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Y. Z. thank the funding National Natural Science Foundation of China (22250710676), the Natural Science Foundation of Fujian Province (2024J01261), the Educational Research Projects for Young and Middle-aged Teachers in Fujian Province (JZ230002), Fujian Province Super 100 Talents Program, Fujian Province 100 Talents Program, Fujian Province Minjiang Scholar Program. Y. Z. thank Fuzhou University Testing Fund of precious apparatus (2024T010). Y. Z., W. T., and T. Z. would like to acknowledge Fujian province, and the Institute of New Energy Materials and Engineering, College of Materials Science and Engineering, Fuzhou University. L. Q. thank Natural Sciences and Engineering Research Council of Canada (NSERC) for financial support. L. Q. gratefully thank the Nanoscale journal for this invitation article.References
- R. Zhang, J. Jiang and W. Wu, Nanoscale, 2023, 15, 3079–3105 RSC
.
- S. Dhall, B. R. Mehta, A. K. Tyagi and K. Sood, Sens. Int., 2021, 2, 100116 Search PubMed
.
- M. V. Nikolic, V. Milovanovic, Z. Z. Vasiljevic and Z. Stamenkovic, Sensors, 2020, 20, 6694 Search PubMed
.
- G. Hagen, J. Herrmann, X. Zhang, H. Kohler, I. Hartmann and R. Moos, Sensors, 2023, 23, 2930 CAS
.
- Y. Yu, Z. Hu, S.-Y. Lien, Y. Yu and P. Gao, ACS Appl. Mater. Interfaces, 2022, 14, 47696–47705 Search PubMed
.
- Y. Pan, M. Qin, P. Wang, L. Yang, L. Zhang, C. Yan, C. Zhang and W. Wang, ACS Sens., 2022, 7, 612–621 Search PubMed
.
- X. Li, W. Sun, W. Fu, H. Lv, X. Zu, Y. Guo, D. Gibson and Y.-Q. Fu, J. Mater. Chem. A, 2023, 11, 9216–9238 Search PubMed
.
- M. Massetti, F. Jiao, A. J. Ferguson, D. Zhao, K. Wijeratne, A. Würger, J. L. Blackburn, X. Crispin and S. Fabiano, Chem. Rev., 2021, 121, 12465–12547 CAS
.
- J. Wu, G. Yue, W. Chen, Z. Xing, J. Wang, W. R. Wong, Z. Cheng, S. Y. Set, G. Murugan, X. Wang and T. Liu, ACS Photonics, 2020, 7, 2923–2940 CAS
.
- C. Love, H. Nazemi, E. El-Masri, K. Ambrose, M. S. Freund and A. Emadi, Sensors, 2021, 21, 3423 Search PubMed
.
- M. A. Franco, P. P. Conti, R. S. Andre and D. S. Correa, Sens. Actuators Rep., 2022, 4, 100100 Search PubMed
.
- M. I. A. Asri, M. N. Hasan, M. R. A. Fuaad, Y. M. Yunos and M. S. M. Ali, IEEE Sens. J., 2021, 21, 18381–18397 Search PubMed
.
- S. M. Majhi, A. Mirzaei, S. Navale, H. W. Kim and S. S. Kim, Nanoscale, 2021, 13, 4728–4757 Search PubMed
.
- J.-Y. Jeon, B.-C. Kang, Y. T. Byun and T.-J. Ha, Nanoscale, 2019, 11, 1587–1594 RSC
.
- Z. U. Abideen, W. U. Arifeen and Y. M. N. D. Y. Bandara, Nanoscale, 2024, 16, 9259–9283 Search PubMed
.
- W. Zhang, X. Chen, Y. Chen, H.-Y. Li and H. Liu, Nanoscale, 2024, 16, 12883–12908 Search PubMed
.
- J. Li, X. Chen, X. Zhu, Y. Jiang, X. Chang and S. Sun, Chin. Chem. Lett., 2024, 35, 108286 Search PubMed
.
- S. Hong, M. Wu, Y. Hong, Y. Jeong, G. Jung, W. Shin, J. Park, D. Kim, D. Jang and J.-H. Lee, Sens. Actuators, B, 2021, 330, 129240 Search PubMed
.
- Y. Jo, Y. K. Jo, J. Lee, H. W. Jang, I. Hwang and D. J. Yoo, Adv. Mater., 2023, 35, 2206842 Search PubMed
.
- H. Zhang, Y. Guo and F. Meng, Chemosensors, 2022, 10, 231 Search PubMed
.
- C. Schultealbert, T. Baur, A. Schütze, S. Böttcher and T. Sauerwald, Sens. Actuators, B, 2017, 239, 390–396 Search PubMed
.
- J. Zhang, Z. Qin, D. Zeng and C. Xie, Phys. Chem. Chem. Phys., 2017, 19, 6313–6329 Search PubMed
.
- P. Rai, R. Khan, S. Raj, S. M. Majhi, K.-K. Park, Y.-T. Yu, I.-H. Lee and P. K. Sekhar, Nanoscale, 2014, 6, 581–588 RSC
.
- S. Sun, Nanoscale, 2015, 7, 10850–10882 Search PubMed
.
- N. A. Isaac, I. Pikaar and G. Biskos, Microchim. Acta, 2022, 189, 196 Search PubMed
.
- J. B. A. Gomes, J. J. P. C. Rodrigues, R. A. L. Rabêlo, N. Kumar and S. Kozlov, J. Sens. Actuator Netw., 2019, 8, 57 Search PubMed
.
-
M. Fleischer and M. Lehmann, Solid State Gas Sensors-Industrial Application, Springer Science & Business Media, 2012, vol. 11 Search PubMed
.
- A. Tricoli, M. Righettoni and A. Teleki, Angew. Chem., Int. Ed., 2010, 49, 7632–7659 CrossRef CAS PubMed
.
- D. Kohl, J. Phys. D: Appl. Phys., 2001, 34, R125 Search PubMed
.
- S. C. Anjankar and R. Dhavse, Arabian J. Sci. Eng., 2024, 49, 7013–7028 CrossRef CAS
.
- S. Xue, S. Cao, Z. Huang, D. Yang and G. Zhang, Materials, 2021, 14, 4263 Search PubMed
.
- A. Caron, N. Redon, F. Thevenet, B. Hanoune and P. Coddeville, Build. Environ., 2016, 107, 19–28 CrossRef
.
- G. Korotcenkov , Conventional approaches.
-
M. Grassi, P. Malcovati and A. Baschirotto, Sensors and Microsystems: AISEM 2009 Proceedings, Springer, 2010, pp. 25–40 Search PubMed
.
- H. J. Lee, U. J. Yang, K. N. Kim, S. Park, K. H. Kil, J. S. Kim, A. M. Wodtke, W. J. Choi, M. H. Kim and J. M. Baik, Nano Lett., 2019, 19, 4306–4313 Search PubMed
.
- D. K. Pattadar and F. P. Zamborini, Langmuir, 2019, 35, 16416–16426 CrossRef CAS PubMed
.
- Y. Shimizu and M. Egashira, MRS Bull., 1999, 24, 18–24 Search PubMed
.
- Y. Tang, Y. Zhao and H. Liu, ACS Sens., 2022, 7, 3582–3597 CrossRef CAS PubMed
.
- A. Singh, S. Sikarwar, A. Verma and B. C. Yadav, Sens. Actuators, A, 2021, 332, 113127 Search PubMed
.
- S. C. Dhanabalan, B. Dhanabalan, X. Chen, J. S. Ponraj and H. Zhang, Nanoscale, 2019, 11, 3046–3101 RSC
.
- J. Zhang, X. Liu, G. Neri and N. Pinna, Adv. Mater., 2016, 28, 795–831 Search PubMed
.
- L. Zhu and W. Zeng, Sens. Actuators, A, 2017, 267, 242–261 Search PubMed
.
- Y. Tang, Y. Zhao and H. Liu, ACS Sens., 2022, 7, 3582–3597 CrossRef CAS PubMed
.
- S. G. Chatterjee, S. Chatterjee, A. K. Ray and A. K. Chakraborty, Sens. Actuators, B, 2015, 221, 1170–1181 CrossRef
.
- M. Mittal and A. Kumar, Sens. Actuators, B, 2014, 203, 349–362 CrossRef CAS
.
- S. Basu and P. Bhattacharyya, Sens. Actuators, B, 2012, 173, 1–21 CrossRef CAS
.
- S.-J. Choi and I.-D. Kim, Electron. Mater. Lett., 2018, 14, 221–260 CrossRef CAS
.
- D. J. Buckley, N. C. G. Black, E. G. Castanon, C. Melios, M. Hardman and O. Kazakova, 2D Mater., 2020, 7, 032002 CrossRef CAS
.
- V. Shukla, J. Warna, N. K. Jena, A. Grigoriev and R. Ahuja, J. Phys. Chem. C, 2017, 121, 26869–26876 CrossRef CAS
.
- Y. Kim, S. Lee, J. Song, K. Y. Ko, W. J. Woo, S. W. Lee, M. Park, H. Lee, Z. Lee and H. Choi, Adv. Funct. Mater., 2020, 30, 2003360 CrossRef CAS
.
- Y. Kim, S.-K. Kang, N.-C. Oh, H.-D. Lee, S.-M. Lee, J. Park and H. Kim, ACS Appl. Mater. Interfaces, 2019, 11, 38902–38909 CrossRef CAS PubMed
.
- S.-J. Choi and I.-D. Kim, Electron. Mater. Lett., 2018, 14, 221–260 CrossRef CAS
.
- Y. Sun, S. Li, Y. Zhuang, G. Liu, W. Xing and W. Jing, J. Membr. Sci., 2019, 591, 117350 CrossRef CAS
.
- Y. Du, Z. Yan, W. You, Q. Men, G. Chen, X. Lv, Y. Wu, K. Luo, B. Zhao and J. Zhang, Adv. Funct. Mater., 2023, 33, 2301449 CrossRef CAS
.
- W. Zeng, X. Ye, Y. Dong, Y. Zhang, C. Sun, T. Zhang, X. Guan and L. Guo, Coord. Chem. Rev., 2024, 508, 215753 CrossRef CAS
.
- S. Zhang, X. Li, Y. Gao, L. Li, L. Bao and X. Li, J. Mater. Sci. Technol., 2025, 211, 89–97 CrossRef CAS
.
- S. A. Kazemi, S. A. Ogunkunle, O. Allen, W. Wen, A. W.-C. Liew, S. Yin and Y. Wang, J. Phys.: Mater., 2023, 6, 035004 CAS
.
- J. Li, X. Chen, X. Zhu, Y. Jiang, X. Chang and S. Sun, Chin. Chem. Lett., 2024, 35, 108286 CrossRef CAS
.
- F. Shahzad, S. A. Zaidi and R. A. Naqvi, Crit. Rev. Anal. Chem., 2022, 52, 848–864 CrossRef CAS PubMed
.
- J. Yang, M. Naguib, M. Ghidiu, L. Pan, J. Gu, J. Nanda, J. Halim, Y. Gogotsi and M. W. Barsoum, J. Am. Ceram. Soc., 2016, 99, 660–666 CrossRef CAS
.
- M. Han, K. Maleski, C. E. Shuck, Y. Yang, J. T. Glazar, A. C. Foucher, K. Hantanasirisakul, A. Sarycheva, N. C. Frey and S. J. May, J. Am. Chem. Soc., 2020, 142, 19110–19118 CAS
.
- L. Wang, M. Han, C. E. Shuck, X. Wang and Y. Gogotsi, Nano Energy, 2021, 88, 106308 CAS
.
- T. L. Tan, H. M. Jin, M. B. Sullivan, B. Anasori and Y. Gogotsi, ACS Nano, 2017, 11, 4407–4418 CAS
.
- R. Meshkian, Q. Tao, M. Dahlqvist, J. Lu, L. Hultman and J. Rosen, Acta Mater., 2017, 125, 476–480 CAS
.
- S. K. Nemani, B. Zhang, B. C. Wyatt, Z. D. Hood, S. Manna, R. Khaledialidusti, W. Hong, M. G. Sternberg, S. K. R. S. Sankaranarayanan and B. Anasori, ACS Nano, 2021, 15, 12815–12825 CAS
.
- Z. Du, C. Wu, Y. Chen, Z. Cao, R. Hu, Y. Zhang, J. Gu, Y. Cui, H. Chen and Y. Shi, Adv. Mater., 2021, 33, 2101473 CAS
.
- J. Zhou, Q. Tao, B. Ahmed, J. Palisaitis, I. Persson, J. Halim, M. W. Barsoum, P.OÅ. Persson and J. Rosen, Chem. Mater., 2022, 34, 2098–2106 CAS
.
- Q. Li, Y. Li and W. Zeng, Chemosensors, 2021, 9, 225 CAS
.
- S. J. Kim, H.-J. Koh, C. E. Ren, O. Kwon, K. Maleski, S.-Y. Cho, B. Anasori, C.-K. Kim, Y.-K. Choi and J. Kim, ACS Nano, 2018, 12, 986–993 CAS
.
- E. Lee, A. VahidMohammadi, B. C. Prorok, Y. S. Yoon, M. Beidaghi and D.-J. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 37184–37190 CAS
.
- Q. Xia, Y. Fan, S. Li, A. Zhou, N. Shinde and R. S. Mane, Diamond Relat. Mater., 2023, 131, 109557 CAS
.
- H. Zhang, H. He, Y. Huang, S. Pu, Y. Xie, J. Huang, X. Jiang, Y. Wang, S. Wang and H. Peng, Appl. Surf. Sci., 2023, 639, 158183 CrossRef CAS
.
- M. Naguib, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2021, 33, 2103393 CrossRef CAS PubMed
.
- Y. Gogotsi and Q. Huang, ACS Nano, 2021, 15, 5775–5780 CrossRef CAS PubMed
.
- K. Schwarz, Crit. Rev. Solid State Mater. Sci., 1987, 13, 211–257 CrossRef CAS
.
- M. W. Barsoum and T. El-Raghy, J. Am. Ceram. Soc., 1996, 79, 1953–1956 CrossRef CAS
.
- M. W. Barsoum, D. Brodkin and T. El-Raghy, Scr. Mater., 1997, 36, 535–541 CrossRef CAS
.
- W. Jeitschko, H. Nowotny and F. Benesovsky, Monatsh. Chem. Verw. Teile Anderer Wiss., 1963, 94, 672–676 CrossRef CAS
.
- M. W. Barsoum, L. Farber, I. Levin, A. Procopio, T. El-Raghy and A. Berner, J. Am. Ceram. Soc., 1999, 82, 2545–2547 CrossRef CAS
.
- M. W. Barsoum, Prog. Solid State Chem., 2000, 28, 201–281 CAS
.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CAS
.
- M. Naguib, O. Mashtalir, J. Carle, V. Presser, J. Lu, L. Hultman, Y. Gogotsi and M. W. Barsoum, ACS Nano, 2012, 6, 1322–1331 CAS
.
- M. Sokol, V. Natu, S. Kota and M. W. Barsoum, Trends Chem., 2019, 1, 210–223 CAS
.
- E. Lee and D.-J. Kim, J. Electrochem. Soc., 2020, 167, 037515 CAS
.
- X. Liu, T. Ma, N. Pinna and J. Zhang, Adv. Funct. Mater., 2017, 27, 1702168 Search PubMed
.
- Y. Pei, X. Zhang, Z. Hui, J. Zhou, X. Huang, G. Sun and W. Huang, ACS Nano, 2021, 15, 3996–4017 CAS
.
- D. Ju, H. Xu, J. Zhang, J. Guo and B. Cao, Sens. Actuators, B, 2014, 201, 444–451 CAS
.
- T. Li, W. Zeng, H. Long and Z. Wang, Sens. Actuators, B, 2016, 231, 120–128 CAS
.
- Z. Li, N. Wang, Z. Lin, J. Wang, W. Liu, K. Sun, Y. Q. Fu and Z. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 20962–20968 CAS
.
- Q. Li, Y. Du, X. Li, G. Lu, W. Wang, Y. Geng, Z. Liang and X. Tian, Sens. Actuators, B, 2016, 235, 39–45 CAS
.
- K. Z. Milowska and J. A. Majewski, J. Phys. Chem. C, 2014, 118, 17395–17401 CAS
.
- N. M. Nurazzi, N. Abdullah, S. Z. N. Demon, N. A. Halim, A. F. M. Azmi, V. F. Knight and I. S. Mohamad, Nanotechnol. Rev., 2021, 10, 330–369 CAS
.
- V. Kumar, K. Vikrant and K.-H. Kim, TrAC, Trends Anal. Chem., 2019, 121, 115694 CAS
.
- E. Lee, Y. S. Yoon and D.-J. Kim, ACS Sens., 2018, 3, 2045–2060 CAS
.
- R. Kumar, W. Zheng, X. Liu, J. Zhang and M. Kumar, Adv. Mater. Technol., 2020, 5, 1901062 CrossRef CAS
.
- R. Kumar, N. Goel, M. Hojamberdiev and M. Kumar, Sens. Actuators, A, 2020, 303, 111875 CrossRef CAS
.
- Y.-F. Sun, S.-B. Liu, F.-L. Meng, J.-Y. Liu, Z. Jin, L.-T. Kong and J.-H. Liu, Sensors, 2012, 12, 2610–2631 CrossRef CAS PubMed
.
- C. Wang, L. Yin, L. Zhang, D. Xiang and R. Gao, Sensors, 2010, 10, 2088–2106 CrossRef CAS PubMed
.
- X. Liu, T. Ma, N. Pinna and J. Zhang, Adv. Funct. Mater., 2017, 27, 1702168 CrossRef
.
- J. H. Choi, J. Lee, M. Byeon, T. E. Hong, H. Park and C. Y. Lee, ACS Appl. Nano Mater., 2020, 3, 2257–2265 CrossRef CAS
.
-
T. Yang, X. Zhao, Y. He and H. Zhu, Graphene, Elsevier, 2018, pp. 157–174 Search PubMed
.
- S. S. Varghese, S. Lonkar, K. K. Singh, S. Swaminathan and A. Abdala, Sens. Actuators, B, 2015, 218, 160–183 CrossRef CAS
.
- R. K. Jha, J. V. D'Costa, N. Sakhuja and N. Bhat, Sens. Actuators, B, 2019, 297, 126687 CrossRef CAS
.
- T. Järvinen, G. S. Lorite, J. Peräntie, G. Toth, S. Saarakkala, V. K. Virtanen and K. Kordas, Nanotechnology, 2019, 30, 405501 CrossRef PubMed
.
- B. Cho, M. G. Hahm, M. Choi, J. Yoon, A. R. Kim, Y.-J. Lee, S.-G. Park, J.-D. Kwon, C. S. Kim and M. Song, Sci. Rep., 2015, 5, 8052 CrossRef CAS PubMed
.
- E. Lee, A. VahidMohammadi, B. C. Prorok, Y. S. Yoon, M. Beidaghi and D.-J. Kim, ACS Appl. Mater. Interfaces, 2017, 9, 37184–37190 CrossRef CAS PubMed
.
- L. Zhang and Y. Yin, Sens. Actuators, B, 2013, 185, 594–601 CAS
.
- H. Y. Jeong, D.-S. Lee, H. K. Choi, D. H. Lee, J.-E. Kim, J. Y. Lee, W. J. Lee, S. O. Kim and S.-Y. Choi, Appl. Phys. Lett., 2010, 96, 213105 Search PubMed
.
- M. Aleixandre, M. C. Horrillo, A. Benito and W. K. Maser, in 2018 Spanish Conference on Electron Devices (CDE), IEEE, 2018, pp. 1–4.
- S.-H. Li, F.-F. Meng, Z. Chu, T. Luo, F.-M. Peng and Z. Jin, Adv. Condens. Matter Phys., 2017, 2017, 9720973 Search PubMed
.
- R. A. Soomro, S. Jawaid, Q. Zhu, Z. Abbas and B. Xu, Chin. Chem. Lett., 2020, 31, 922–930 CAS
.
- C. Verma and K. K. Thakur, Eur. J. Mol. Clin. Med., 2020, 7, 4429–4450 Search PubMed
.
- A. Hermawan, B. Zhang, A. Taufik, Y. Asakura, T. Hasegawa, J. Zhu, P. Shi and S. Yin, ACS Appl. Nano Mater., 2020, 3, 4755–4766 CAS
.
- E. Lee, A. VahidMohammadi, Y. S. Yoon, M. Beidaghi and D.-J. Kim, ACS Sens., 2019, 4, 1603–1611 CAS
.
- K. Deshmukh, T. Kovářík and S. K. K. Pasha, Coord. Chem. Rev., 2020, 424, 213514 CAS
.
- S. J. Kim, H.-J. Koh, C. E. Ren, O. Kwon, K. Maleski, S.-Y. Cho, B. Anasori, C.-K. Kim, Y.-K. Choi and J. Kim, ACS Nano, 2018, 12, 986–993 CAS
.
-
B. Anasori and Y. Gogotsi, 2D Metal Carbides and Nitrides (MXenes) Structure, Properties and Applications, 2019, pp. 3–12 Search PubMed
.
- H. Huang, R. Jiang, Y. Feng, H. Ouyang, N. Zhou, X. Zhang and Y. Wei, Nanoscale, 2020, 12, 1325–1338 CAS
.
- J. Zou, J. Wu, Y. Wang, F. Deng, J. Jiang, Y. Zhang, S. Liu, N. Li, H. Zhang and J. Yu, Chem. Soc. Rev., 2022, 51, 2972–2990 RSC
.
- D. Wang, F. Li, R. Lian, J. Xu, D. Kan, Y. Liu, G. Chen, Y. Gogotsi and Y. Wei, ACS Nano, 2019, 13, 11078–11086 CrossRef CAS PubMed
.
- Y. Wei, P. Zhang, R. A. Soomro, Q. Zhu and B. Xu, Adv. Mater., 2021, 33, 2103148 CrossRef CAS PubMed
.
- M. Murugesan, K. R. Nagavenkatesh, P. Devendran, N. Nallamuthu, C. Sambathkumar and M. K. Kumar, Mater. Sci. Eng., B, 2024, 309, 117607 CrossRef CAS
.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Chem. Mater., 2017, 29, 7633–7644 CrossRef CAS
.
- J. Halim, S. Kota, M. R. Lukatskaya, M. Naguib, M.-Q. Zhao, E. J. Moon, J. Pitock, J. Nanda, S. J. May, Y. Gogotsi and M. W. Barsoum, Adv. Funct. Mater., 2016, 26, 3118–3127 CAS
.
-
M. Alhabeb, K. Maleski, T. S. Mathis, A. Sarycheva, C. B. Hatter, S. Uzun, A. Levitt and Y. Gogotsi, MXenes, Jenny Stanford Publishing, 2023, pp. 451–462 Search PubMed
.
- M. A. El Saeed, F. A. Deorsola and R. M. Rashad, Int. J. Refract. Met. Hard Mater., 2012, 35, 127–131 CrossRef CAS
.
- F. Xia, J. Lao, R. Yu, X. Sang, J. Luo, Y. Li and J. Wu, Nanoscale, 2019, 11, 23330–23337 RSC
.
- R. A. Soomro, P. Zhang, B. Fan, Y. Wei and B. Xu, Nano-Micro Lett., 2023, 15, 108 CrossRef CAS PubMed
.
- F. Xia, J. Lao, R. Yu, X. Sang, J. Luo, Y. Li and J. Wu, Nanoscale, 2019, 11, 23330–23337 RSC
.
- R. A. Soomro, P. Zhang, B. Fan, Y. Wei and B. Xu, Nano-Micro Lett., 2023, 15, 108 CrossRef CAS PubMed
.
- F. Cao, Y. Zhang, H. Wang, K. Khan, A. K. Tareen, W. Qian, H. Zhang and H. Ågren, Adv. Mater., 2022, 34, 2107554 CAS
.
- M. A. Hope, A. C. Forse, K. J. Griffith, M. R. Lukatskaya, M. Ghidiu, Y. Gogotsi and C. P. Grey, Phys. Chem. Chem. Phys., 2016, 18, 5099–5102 RSC
.
- Y. Seekaew, S. Kamlue and C. Wongchoosuk, ACS Appl. Nano Mater., 2023, 6, 9008–9020 CrossRef CAS
.
- L. C. T. Cao, M.-H. Zhou, P. Opaprakasit, P. Sreearunothai, Y. Nagao, S. Boonruang, H. Fallah, S.-F. Tseng and S.-H. Hsu, Adv. Mater. Interfaces, 2023, 10, 2300166 CrossRef CAS
.
- H. Yu, L. Dai, Y. Liu, Y. Zhou, P. Fan, J. Luo and A. Zhong, J. Alloys Compd., 2023, 962, 171170 CAS
.
- S. M. Aghaei, A. Aasi and B. Panchapakesan, ACS Omega, 2021, 6, 2450–2461 Search PubMed
.
- S. J. Kim, H.-J. Koh, C. E. Ren, O. Kwon, K. Maleski, S.-Y. Cho, B. Anasori, C.-K. Kim, Y.-K. Choi, J. Kim, Y. Gogotsi and H.-T. Jung, ACS Nano, 2018, 12, 986–993 CAS
.
- L. Qian, S. Durairaj, S. Prins and A. Chen, Biosens. Bioelectron., 2021, 175, 112836 CAS
.
- F. Guo, C. Feng, Z. Zhang, L. Zhang, C. Xu, C. Zhang, S. Lin, H. Wu, B. Zhang, A. Tabusi and Y. Huang, Sens. Actuators, B, 2023, 375, 132885 CAS
.
- Z. Wang, F. Wang, A. Hermawan, Y. Asakura, T. Hasegawa, H. Kumagai, H. Kato, M. Kakihana, J. Zhu and S. Yin, J. Mater. Sci. Technol., 2021, 73, 128–138 CAS
.
- P. Song, D. Xiong, W. Jiang, H. Zhang, K. Wu, Y. Xie, Z. Feng, T. Wang, Q. Wang and M. He, Ionics, 2023, 29, 3991–4000 CAS
.
- S. Gasso, M. K. Sohal and A. Mahajan, Sens. Actuators, B, 2022, 357, 131427 CAS
.
- H. Yu, L. Dai, Y. Liu, Y. Zhou, P. Fan, J. Luo and A. Zhong, J. Alloys Compd., 2023, 962, 171170 CAS
.
- L. Yao, X. Tian, X. Cui, R. Zhao, M. Xiao, B. Wang, X. Xiao and Y. Wang, Sens. Actuators, B, 2022, 358, 131501 CAS
.
- A. Marchand, J. Ménard, P. Brochu and S. Haddad, Environ. Toxicol. Pharmacol., 2021, 88, 103737 CAS
.
- T. Fowler, B. Thompson and J. Mueller, Rem. J., 2011, 22, 9–28 Search PubMed
.
- H. Guo, Z. H. Ling, K. Cheung, D. W. Wang, I. J. Simpson and D. R. Blake, Atmos. Environ., 2013, 65, 80–88 CAS
.
- K. Karimi, M. Tabatabaei, I. S. Horváth and R. Kumar, Biofuel Res. J., 2015, 2, 301–308 CAS
.
- B. Sun, Y. Ding, Q. Wang and P. Song, Sens. Actuators, B, 2024, 409, 135541 CrossRef CAS
.
- D. D. Hsu, D. Inman, G. A. Heath, E. J. Wolfrum, M. K. Mann and A. Aden, Environ. Sci. Technol., 2010, 44, 5289–5297 CrossRef CAS PubMed
.
- D. Pimentel, Nat. Resour. Res., 2003, 12, 127–134 CrossRef
.
- S. K. Lin, Molecules, 2001, 6, 1019–1020 CrossRef
.
- P. C. Badger and J. D. Broder, HortScience, 1989, 24, 227–232 Search PubMed
.
- S. Zhang, Y. Ding, Q. Wang and P. Song, Sens. Actuators, B, 2023, 393, 134122 CrossRef CAS
.
- T. Salthammer, S. Mentese and R. Marutzky, Chem. Rev., 2010, 110, 2536–2572 CAS
.
- C. W. F. Yu and J. T. Kim, Indoor Built Environ., 2012, 21, 137–149 CAS
.
- H. Reingruber and L. B. Pontel, Curr. Opin. Toxicol., 2018, 9, 28–34 Search PubMed
.
- H. Reingruber and L. B. Pontel, Curr. Opin. Toxicol., 2018, 9, 28–34 Search PubMed
.
- Z. Li, Q. Zhao, W. Fan and J. Zhan, Nanoscale, 2011, 3, 1646–1652 CAS
.
- Z. Deng, Y. Zhang, D. Xu, B. Zi, J. Zeng, Q. Lu, K. Xiong, J. Zhang, J. Zhao and Q. Liu, ACS Sens., 2022, 7, 2577–2588 CAS
.
- S. R. Mishra and M. Ahmaruzzaman, Nanoscale, 2022, 14, 1566–1605 CAS
.
- G. Niu, M. Zhang, B. Wu, Y. Zhuang, R. Ramachandran, C. Zhao and F. Wang, Ceram. Int., 2023, 49, 2583–2590 CAS
.
- P. Berwal, S. Kumar and B. Khandelwal, J. Energy Inst., 2021, 99, 273–298 CAS
.
- J. I. van der Vlugt, Chem. Soc. Rev., 2010, 39, 2302–2322 CAS
.
- X. Meng and K. N. Han, Miner. Process. Extr. Metall. Rev., 1996, 16, 23–61 CAS
.
- S. M. McGinn and H. H. Janzen, Can. J. Soil Sci., 1998, 78, 139–148 CAS
.
- D. A. Khudhur, T. A. Abdullah and N. Norazahar, ACS Chem. Health Saf., 2022, 29, 394–404 CrossRef CAS
.
- Y. Huang, S. C. Lee, K. F. Ho, S. S. H. Ho, N. Cao, Y. Cheng and Y. Gao, Atmos. Environ., 2012, 59, 224–231 CAS
.
- M. P. Diana, W. S. Roekmijati and W. U. Suyud, in E3S Web of Conferences, EDP Sciences, 2018, vol. 73, p. 06003.
- C. Liu, Y. Qiao, Y. Guo and K. Guo, Matter, 2024, 7, 2673–2675 CAS
.
- K. Cheng, X. Tian, S. Yuan, Q. Feng and Y. Wang, Sensors, 2024, 24, 4465 CAS
.
- S. Atkare, S. D. Kaushik, S. Jagtap and C. S. Rout, Dalton Trans., 2023, 52, 13831–13851 CAS
.
- Y. Peng and J. Li, Front. Environ. Sci. Eng., 2013, 7, 403–411 CAS
.
- W. Chen, F. Deng, M. Xu, J. Wang, Z. Wei and Y. Wang, Sens. Actuators, B, 2018, 273, 498–504 CrossRef CAS
.
- S. Zhu, H. Sun, X. Liu, J. Zhuang and L. Zhao, Sci. Rep., 2017, 7, 14773 CrossRef PubMed
.
- D. K. Chaudhary, Y. S. Maharjan, S. Shrestha, S. Maharjan, S. P. Shrestha and L. P. Joshi, J. Phys. Sci., 2022, 33, 97–108 CrossRef CAS
.
- R. K. Bedi and I. Singh, ACS Appl. Mater. Interfaces, 2010, 2, 1361–1368 CrossRef CAS PubMed
.
- S. Bhuvaneshwari and N. Gopalakrishnan, J. Colloid Interface Sci., 2016, 480, 76–84 CrossRef CAS PubMed
.
- N. Dogra, S. Gasso, A. Sharma, K. K. Sharma and S. Sharma, Surf. Interfaces, 2024, 48, 104290 CrossRef CAS
.
- A. Faustini, R. Rapp and F. Forastiere, Eur. Respir. J., 2014, 44, 744–753 CAS
.
- R. W. Atkinson, B. K. Butland, H. R. Anderson and R. L. Maynard, Epidemiology, 2018, 29, 460–472 Search PubMed
.
- W. Quan, J. Shi, H. Luo, C. Fan, W. Lv, X. Chen, M. Zeng, J. Yang, N. Hu, Y. Su, H. Wei and Z. Yang, ACS Sens., 2023, 8, 103–113 CAS
.
- S. Kim, H. Shin, J. Lee, C. Park, Y. Ahn, H.-J. Cho, S. Yuk, J. Kim, D. Lee and I.-D. Kim, ACS Nano, 2023, 17, 19387–19397 CAS
.
- A. Hermawan, B. Zhang, A. Taufik, Y. Asakura, T. Hasegawa, J. Zhu, P. Shi and S. Yin, ACS Appl. Nano Mater., 2020, 3, 4755–4766 CAS
.
- S. Sun, M. Wang, X. Chang, Y. Jiang, D. Zhang, D. Wang, Y. Zhang and Y. Lei, Sens. Actuators, B, 2020, 304, 127274 CAS
.
- H. Pazniak, I. A. Plugin, M. J. Loes, T. M. Inerbaev, I. N. Burmistrov, M. Gorshenkov, J. Polcak, A. S. Varezhnikov, M. Sommer and D. V. Kuznetsov, ACS Appl. Nano Mater., 2020, 3, 3195–3204 CAS
.
- Z. Wang, F. Wang, A. Hermawan, Y. Asakura, T. Hasegawa, H. Kumagai, H. Kato, M. Kakihana, J. Zhu and S. Yin, J. Mater. Sci. Technol., 2021, 73, 128–138 CAS
.
- D. Huang, H. Li, Y. Wang, X. Wang, L. Cai, W. Fan, Y. Chen, W. Wang, Y. Song and G. Han, Chem. Eng. J., 2022, 428, 131377 CAS
.
- M. Liu, J. Ji, P. Song, M. Liu and Q. Wang, Sens. Actuators, B, 2021, 349, 130782 CAS
.
- O. Ama, M. Sadiq, M. Johnson, Q. Zhang and D. Wang, Chemosensors, 2020, 8, 102 CAS
.
- Y. Zhu, Y. Ma, D. Wu and G. Jiang, Sens. Actuators, A, 2022, 344, 113740 CAS
.
- M. Liu, Z. Wang, P. Song, Z. Yang and Q. Wang, Ceram. Int., 2021, 47, 23028–23037 CAS
.
- M. Hou, S. Guo, L. Yang, J. Gao, T. Hu, X. Wang and Y. Li, Ceram. Int., 2021, 47, 7728–7737 CAS
.
- Z. Wang, F. Wang, A. Hermawan, J. Zhu and S. Yin, Funct. Mater. Lett., 2022, 15, 2251007 CAS
.
- Z. Shao, Z. Zhao, P. Chen, J. Chen, W. Liu, X. Shen and X. Liu, Inorg. Nano-Met. Chem., 2024, 54, 898–906 CAS
.
- X. Bu, F. Ma, Q. Wu, H. Wu, Y. Yuan, L. Hu, C. Han, X. Wang, W. Liu and X. Li, Sens. Actuators, B, 2022, 369, 132232 CAS
.
- S. Zhang, P. Song, Y. Zheng, Y. Ding and Q. Wang, J. Alloys Compd., 2022, 925, 166663 CAS
.
- S. Zou, J. Gao, L. Liu, Z. Lin, P. Fu, S. Wang and Z. Chen, J. Alloys Compd., 2020, 817, 152785 CrossRef CAS
.
- D. Zhang, Q. Mi, D. Wang and T. Li, Sens. Actuators, B, 2021, 339, 129923 CrossRef CAS
.
- G. Niu, R. Ramachandran, C. Zhao and F. Wang, in 2021 21st International Conference on Solid-State Sensors, Actuators and Microsystems (Transducers), 2021, pp. 831–834.
- Z. Sima, P. Song, Y. Ding, Z. Lu and Q. Wang, Appl. Surf. Sci., 2022, 598, 153861 CrossRef CAS
.
- D. Kuang, L. Wang, X. Guo, Y. She, B. Du, C. Liang, W. Qu, X. Sun, Z. Wu, W. Hu and Y. He, J. Hazard. Mater., 2021, 416, 126171 CrossRef CAS PubMed
.
- D. Liang, P. Song, M. Liu and Q. Wang, Ceram. Int., 2022, 48, 9059–9066 CrossRef CAS
.
- Z. Li, D. Zhang, X. Wang, X. Liu, Y. Yang, C. Du, J. Guo and Y. Zhang, J. Alloys Compd., 2023, 939, 168730 CrossRef CAS
.
- Y. Yao, Y. Han, M. Zhou, L. Xie, X. Zhao, Z. Wang, N. Barsan and Z. Zhu, J. Mater. Chem. A, 2022, 10, 8283–8292 RSC
.
- T. He, W. Liu, T. Lv, M. Ma, Z. Liu, A. Vasiliev and X. Li, Sens. Actuators, B, 2021, 329, 129275 CrossRef CAS
.
- L. Yao, X. Tian, X. Cui, R. Zhao, M. Xiao, B. Wang, X. Xiao and Y. Wang, Sens. Actuators, B, 2022, 358, 131501 CrossRef CAS
.
- H. Tai, Z. Duan, Z. He, X. Li, J. Xu, B. Liu and Y. Jiang, Sens. Actuators, B, 2019, 298, 126874 CrossRef CAS
.
- D. Zhang, S. Yu, X. Wang, J. Huang, W. Pan, J. Zhang, B. E. Meteku and J. Zeng, J. Hazard. Mater., 2022, 423, 127160 CrossRef CAS PubMed
.
- X. Guo, Y. Ding, D. Kuang, Z. Wu, X. Sun, B. Du, C. Liang, Y. Wu, W. Qu, L. Xiong and Y. He, J. Colloid Interface Sci., 2021, 595, 6–14 CrossRef CAS PubMed
.
- M. Liu, J. Ji, P. Song, J. Wang and Q. Wang, J. Alloys Compd., 2022, 898, 162812 CAS
.
- D. Wang, D. Zhang, Y. Yang, Q. Mi, J. Zhang and L. Yu, ACS Nano, 2021, 15, 2911–2919 CrossRef CAS PubMed
.
- M. Zhou, Y. Han, Y. Yao, L. Xie, X. Zhao, J. Wang and Z. Zhu, Ceram. Int., 2022, 48, 6600–6607 CrossRef CAS
.
- Z. Liu, T. He, H. Sun, B. Huang and X. Li, Sens. Actuators, B, 2022, 365, 131918 CrossRef CAS
.
- M. Liu, J. Wang, P. Song, J. Ji and Q. Wang, Sens. Actuators, B, 2022, 361, 131755 CrossRef CAS
.
- D. Kuang, X. Guo, Z. Zhu, Y. Ding, X. Sun, Z. Wu, L. Zhang, Y. Zhou and Y. He, Ceram. Int., 2021, 47, 19471–19480 CrossRef CAS
.
- F. Ranjbar, S. Hajati, M. Ghaedi, K. Dashtian, H. Naderi and J. Toth, J. Hazard. Mater., 2021, 416, 126196 CrossRef CAS PubMed
.
- T. Yang, L. Gao, W. Wang, J. Kang, G. Zhao, D. Li, W. Chen and H. Zhang, Nano-Micro Lett., 2021, 13, 63 CrossRef PubMed
.
- S. Liu, M. Wang, G. Liu, N. Wan, C. Ge, S. Hussain, H. Meng, M. Wang and G. Qiao, Appl. Surf. Sci., 2021, 567, 150747 CrossRef CAS
.
- Z. Yang, L. Jiang, J. Wang, F. Liu, J. He, A. Liu, S. Lv, R. You, X. Yan, P. Sun, C. Wang, Y. Duan and G. Lu, Sens. Actuators, B, 2021, 326, 128828 CAS
.
- C. Fan, J. Shi, Y. Zhang, W. Quan, X. Chen, J. Yang, M. Zeng, Z. Zhou, Y. Su, H. Wei and Z. Yang, Nanoscale, 2022, 14, 3441–3451 RSC
.
- J. Wang, Y. Yang and Y. Xia, Sens. Actuators, B, 2022, 353, 131087 CAS
.
- X. Liu, H. Zhang, Y. Song, T. Shen and J. Sun, Sens. Actuators, B, 2022, 367, 132025 CrossRef CAS
.
- S. Gasso, M. K. Sohal and A. Mahajan, Sens. Actuators, B, 2022, 357, 131427 CAS
.
- D. Wang, D. Zhang, J. Guo, Y. Hu, Y. Yang, T. Sun, H. Zhang and X. Liu, Nano Energy, 2021, 89, 106410 CAS
.
- S. Gasso and A. Mahajan, ACS Sens., 2022, 7, 2454–2464 CAS
.
- S. Gasso, M. K. Sohal and A. Mahajan, Sens. Actuators, B, 2022, 357, 131427 CAS
.
- S. Kang, A. Mirzaei, K. Y. Shin, W. Oum, D. J. Yu, S. S. Kim and H. W. Kim, Sens. Actuators, B, 2023, 375, 132882 CAS
.
- X. Wu, Y. Gong, B. Yang, Z. Mao, Z. Yan, C. Su, S. Xiong, X. Long and X. Wang, Appl. Surf. Sci., 2022, 581, 152364 CAS
.
- F. Guo, C. Feng, Z. Zhang, L. Zhang, C. Xu, C. Zhang, S. Lin, H. Wu, B. Zhang, A. Tabusi and Y. Huang, Sens. Actuators, B, 2023, 375, 132885 CAS
.
- B. Sun, F. Qin, L. Jiang, J. Gao, Z. Liu, J. Wang, Y. Zhang, J. Fan, K. Kan and K. Shi, Sens. Actuators, B, 2022, 368, 132206 CAS
.
- S. A. M. Chachuli, M. N. Hamidon, M. Ertugrul, M. S. Mamat, O. Coban, F. N. Tuzluca, Y. O. Yesilbag and N. H. Shamsudin, J. Alloys Compd., 2021, 882, 160671 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |
