Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Does plasma membrane transbilayer asymmetry coupled to lipid nanodomains drive fast kinetics of FGF2 membrane translocation into the extracellular space?
Fabio
Lolicato
 *ab,
Manpreet
Kaur
*ab,
Manpreet
Kaur
 a,
Ana Marija
Knez
a,
Ana Marija
Knez
 a,
Roberto
Saleppico
a,
Roberto
Saleppico
 a and
Walter
Nickel
a and
Walter
Nickel
 *a
*a
aHeidelberg University Biochemistry Center, Heidelberg, Germany. E-mail: fabio.lolicato@bzh.uni-heidelberg.de; walter.nickel@bzh.uni-heidelberg.de
bDepartment of Physics, University of Helsinki, Helsinki, Finland
First published on 19th February 2025
Abstract
Fibroblast Growth Factor 2 (FGF2) is a potent mitogen secreted from mammalian cells through an unconventional secretory pathway. This process is mediated by direct translocation of FGF2 across the plasma membrane into the extracellular space. It requires several components that are asymmetrically distributed between the two leaflets of the plasma membrane. At the inner plasma membrane leaflet, FGF2 undergoes sequential interactions with the Na,K-ATPase, Tec kinase, and the phosphoinositide PI(4,5)P2. While the Na,K-ATPase, and Tec kinase are auxiliary factors, interactions of FGF2 with PI(4,5)P2 trigger the core mechanism of FGF2 membrane translocation, inducing FGF2-oligomerization-dependent formation of lipidic membrane pores. At the outer plasma membrane leaflet, membrane-inserted FGF2 oligomers are captured and disassembled by Glypican-1 (GPC1), resulting in translocation of FGF2 to the cell surface. In a cellular context, a single FGF2 membrane translocation event occurs within 200 milliseconds. In contrast, in an in vitro system, which uses a fully reconstituted liposomal inside-out system with FGF2 added from the outside and luminal encapsulation of high-affinity heparin molecules, FGF2 membrane translocation takes several minutes. Here, we hypothesize that the observed difference is, at least in part, due to the asymmetrical membrane lipid distribution and the spatial organization of the FGF2 translocation machinery in native plasma membranes. We suggest that the molecular machinery mediating FGF2 membrane translocation assembles in ordered nanodomains, characterized by sphingomyelin (SM), cholesterol and phosphoinositide PI(4,5)P2 coupled together. The transbilayer asymmetry of these lipids likely plays a crucial role in regulating the thermodynamics and kinetics of FGF2-induced membrane pore formation. Therefore, succeeding in reconstituting the FGF2 translocation machinery in artificial membranes with an asymmetric transbilayer distribution of SM, PI(4,5)P2 and other membrane lipids may reveal a direct impact on pore-opening kinetics. Similarly, disrupting lipid asymmetry in cells may significantly impact FGF2 secretion rates, a finding that would underscore the importance of the spatial organization of lipids in membrane dynamics. Testing this hypothesis may advance our understanding of how membrane asymmetry and ordered lipid nanodomains regulate critical biological processes, such as the unconventional secretion of FGF2.
Introduction
The molecular machinery and mechanism driving unconventional secretion of FGF2
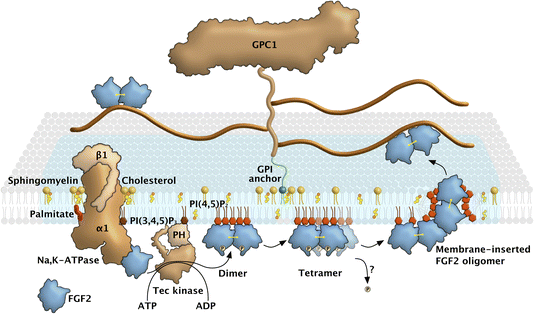
The first pieces of evidence suggesting the existence of ER/Golgi-independent pathways of protein secretion from mammalian cells date back to the late 1980s.1 Along with, for example, Interleukin 1β and members of the galectin family, FGF2 was recognized as one of the first candidates of proteins transported into the extracellular space in a signal peptide-independent manner, suggesting alternative pathways of protein secretion that bypass the classical ER/Golgi route.2 This was experimentally verified by transport of FGF2 into the extracellular space and shown to be insensitive to brefeldin A, an inhibitor of ER/Golgi-dependent protein secretion.3–5 Another early observation was a possible involvement of the plasma membrane resident Na,K-ATPase. This finding was based on the inhibition of unconventional secretion of FGF2 by ouabain, a cardenolide that was known to inhibit the membrane-potential-generating function of this ATP-dependent ion pump.6 However, all of these observations were solely based upon pharmacological evidence and, therefore, not fully conclusive. Due to the lack of advanced techniques at the time, such as genome-wide screening systems based upon RNA interference or CRISPR gene knock-outs, as well as the unavailability of sophisticated in vitro reconstitution systems, mechanistic insights into the molecular machinery mediating unconventional secretion of FGF2 became available only much later starting in the mid-2000s.A key observation in elucidating the FGF2 secretion pathway was the ability of FGF2 to physically traverse the membranes of affinity-purified plasma membrane vesicles with an inside-out topology.7 This suggested unconventional secretion of FGF2 from cells to be mediated by direct translocation across plasma membranes. This conclusion could be confirmed by TIRF imaging of individual FGF2 membrane translocation events at the plasma membrane in real time with single-molecule resolution.8,9 Further key insights that led to the unraveling of this secretory mechanism were its dependence on the phosphoinositide PI(4,5)P2 at the inner leaflet10 and heparan sulfate proteoglycans at the outer leaflet of the plasma membrane.11 The interaction of FGF2 with PI(4,5)P2 was then shown to trigger FGF2 oligomerization, which led to the formation of lipidic membrane pores implicated in FGF2 membrane translocation.12 Using giant unilamellar vesicles with an inside-out topology containing PI(4,5)P2 on their surfaces and luminal encapsulation of heparin molecules with high affinity towards FGF2, the minimal machinery required for directional translocation of FGF2 across membranes could be defined.13 These studies revealed PI(4,5)P2 and heparan sulfates from proteoglycans on opposing sides of the plasma membrane along with the ability of FGF2 to oligomerize and to form lipidic membrane pores to be the minimal machinery driving FGF2 membrane translocation. NMR binding studies of FGF2 towards PI(4,5)P2versus heparin established these interactions to be mutually exclusive, providing a compelling data set that explains the directionality of FGF2 membrane translocation from the cytoplasm into the extracellular space.13 The functional oligomeric state of FGF2 was found to be highly dynamic, with about 4 to 8 FGF2 subunits required to trigger the formation of a membrane pore.13–15 The building blocks of pore-forming FGF2 oligomers were found to be disulfide-bridged dimers that form at the inner plasma membrane leaflet, followed by assembly into higher oligomers with a membrane pore-forming activity.16 It has been hypothesized that FGF2 oligomers cause the local and asymmetric accumulation of the non-bilayer lipid PI(4,5)P2 at the inner plasma membrane leaflet, which triggers the opening of a lipidic membrane pore with a toroidal architecture. The discovery of PI(4,5)P2-induced oligomerization triggering the formation of a membrane pore through which FGF2 reaches the extracellular space also explained earlier findings demonstrating that FGF2 membrane translocation does not involve unfolded intermediates of FGF2.17,18 With all of these aspects in mind, the unusual secretory pathway of FGF2, schematized in Fig. 1, can be considered a protein self-translocation mechanism that, depending on PI(4,5)P2 and heparan sulfates on opposing sides of the plasma membrane, is based upon the ability of FGF2 to trigger a membrane pore through which it is capable of physically traversing the plasma membrane.19
While the early findings on a potential role of the Na,K-ATPase could not be further interpreted in terms of a mechanistic model for quite some time, it gained new attention when the Na,K-ATPase, and Tec kinase were identified as factors whose RNAi-mediated down-regulation limits the efficiency of FGF2 secretion.20 In follow-up studies, a direct interaction of FGF2 with the cytoplasmic domain of the α1 subunit of the Na,K-ATPase could be demonstrated that was found to be sensitive to ouabain.21 It was then shown that the Na,K-ATPase acts upstream of PI(4,5)P2, accumulating FGF2 at the inner plasma membrane leaflet prior to PI(4,5)P2-dependent FGF2 oligomerization and pore formation.22 Based on these findings, it has been proposed that the Na,K-ATPase, like Tec kinase,20,23 functions as an auxiliary factor of the molecular machinery mediating FGF2 membrane translocation into the extracellular space, representing a landing platform for FGF2 at the inner plasma membrane leaflet.2,8,24
The combination of experimental studies in cells and in vitro reconstitution experiments, along with multiscale physics-based computer simulations, proved decisive in elucidating the molecular mechanism by which FGF2 is secreted by direct translocation across the plasma membrane. Even certain discrepancies observed between cell-based data and results from in vitro experiments were informative, spurring new concepts about the spatio-temporal organization of the FGF2 membrane translocation machinery. An example is the kinetics of FGF2 membrane translocation, which is considerably faster in intact cells compared to those observed for artificial membrane systems. While individual events of FGF2 membrane translocation were shown to occur within time intervals of 200 ms,9 this process was found to take place in a time range of minutes when analyzed with giant unilamellar vesicles as model systems,13 as illustrated in Fig. 2. Several parameters may account for this difference, such as the absence of the Na,K-ATPase in the minimal reconstitution system. Similarly, putative redox enzymes, presumably involved in forming disulfide bridges during FGF2 oligomerization in cells, are not included in the in vitro models. Another striking example is the known transbilayer lipid asymmetry of native plasma membranes absent from model membranes used in FGF2 reconstitution experiments. In particular, the exclusive localization of PI(4,5)P2 in the inner leaflet of cellular plasma membranes is strikingly different from the symmetric distribution of PI(4,5)P2 in giant unilamellar vesicles used in in vitro experiments.
Here, we propose the transbilayer asymmetry of membrane lipids in general and PI(4,5)P2, in particular, to play a critical role in lowering the free-energy costs of the conversion of a stable lipid bilayer into a lipidic membrane pore with a toroidal architecture. Furthermore, regarding lateral partitioning, PI(4,5)P2 is known to be enriched in ordered nanodomains of plasma membranes.25 Along with recent findings demonstrating that (i) cholesterol tunes FGF2 binding to PI(4,5)P2,26 (ii) the Na,K-ATPase has multiple contacts to cholesterol regulating its function27 and (iii) the predominant heparan sulfate proteoglycan driving unconventional secretion of FGF2, Glypican-1 (GPC1), is localized in cholesterol-rich domains based upon a GPI anchor,19 we propose ordered nanodomains with an asymmetric transbilayer distribution of PI(4,5)P2 to be the organizational principle of the molecular machinery of FGF2 membrane translocation, contributing to fast FGF2 membrane translocation kinetics of about 200 ms as observed in living cells.9
During apoptosis, the global loss of plasma membrane lipid asymmetry serves as a key mechanism for cell clearance, enabling recognition by phagocytes through “eat me” signals such as PS and phosphatidylinositol phosphates like PI(3,4,5)P3.33,34 This disruption of asymmetry is also observed in cancer cells, which presents both challenges and opportunities for therapeutic strategies.
The loss of transbilayer asymmetry in cancer cells can be problematic, as it has been linked to reduced permeability to chemotherapeutic agents like cisplatin.35 However, it also offers a therapeutic advantage, as the exposure of aminophospholipids such as PE and PS on the outer leaflet creates targets for specific interventions. For instance, small peptides and nanovesicles like OPA and SapC-DOPS exploit this exposure to selectively kill cancer cells, representing a promising strategy for targeted therapy.36,37
Emerging evidence highlights that the temporary loss of plasma membrane lipid asymmetry plays a critical role in maintaining normal physiological functions. For instance, recent studies reveal that plasma membrane SM asymmetry is disrupted during lysosomal damage.38 Calcium release from damaged lysosomes activates SM scramblase, flipping SM to the cytosolic leaflet, which converts it into ceramide. This ceramide facilitates lysosomal repair through an Endosomal Sorting Complex Required for Transport (ESCRT)-independent pathway.
Similarly, the loss of PE asymmetry has been observed during cytokinesis.39 Although the mechanism behind this loss is not yet fully understood, it is crucial for effective cell division.40 During cytokinesis, the two membrane leaflets are coupled, with PE enriched in the outer leaflet of the cleavage furrow, alongside sphingomyelin and cholesterol. In contrast, phosphatidylinositol PI(4,5)P2 is enriched in the inner leaflet. Disrupting the distribution of any of these lipids impairs cell division.41 The strong coupling of lipids, such as SM, cholesterol, and phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2) between the two leaflets during cytokinesis shows intriguing similarities to the mechanism of FGF2 membrane translocation during unconventional secretion. These same lipids have been shown to play a critical role in FGF2 membrane binding and the formation of toroidal pores.2,24
Although it remains unclear why a polyunsaturated lipid-like PI(4,5)P2 coexists in nanodomains with cholesterol and SM—lipids typically are associated with liquid-ordered membrane domains—this arrangement likely creates specialized nanodomains. These nanodomains may involve a complex interplay of membrane curvature and inter-leaflet lipid interactions, increasing membrane tension and thickness.42,43 This unique lipid organization could be key to facilitating processes requiring precise mechanical and biochemical membrane properties.
Key players in ordered nanodomains
Cholesterol is a key component of the liquid-ordered (Lo) phase in lipid bilayers, influencing the membranes’ physical and functional properties, and contributing to the formation and stability of nanodomains. The ordering effect arises from cholesterol’s ability to interact with acyl chains while simultaneously disrupting their tight packing.44 In multi-component lipid mixtures, there is a distinct coexistence between the gel and liquid-disordered (Ld) phases. The addition of cholesterol, which preferentially interacts with long, saturated phospholipids, shifts the gel phase towards a state resembling the Ld phase.45,46 This is because cholesterol enhances membrane thickness by straightening the lipid tails in Lo-phase bilayers, making them thicker than Ld-phase bilayers.47 However, the effect of cholesterol on membrane thickness can vary depending on the lipid composition. For instance, in bilayers with saturated 18-carbon chains, cholesterol’s shorter structure induces deformation or kinking of the longer phospholipid tails, reducing membrane thickness below the phase-transition temperature.48 Within nanodomains, this difference in thickness generates line tension at domain boundaries, dynamically shaping the size and morphology of ordered regions. Because nanodomains exist in non-equilibrium conditions, mechanisms such as the dimpled morphology of lipids and their “pull-and-push” interactions at domain interfaces, along with the presence of amphipathic peptides and proteins at the interface, might play a key role in stabilizing nanodomain boundaries and temporarily preserve distinct domains.49,50Furthermore, due to key structural and interactional differences, cholesterol exhibits a lower affinity for unsaturated lipid chains. The rigid, planar structure of cholesterol’s steroid ring system is better suited to align with the straight, saturated acyl chains, creating a molecular shape mismatch with the kinked, unsaturated chains. However, the two methyl groups at the steroid also play an important role in cholesterol–lipid interactions by reducing sterol tilt in the bilayer, thereby allowing cholesterol to be oriented optimally.51 Additionally, the kinks introduced by double bonds in unsaturated chains hinder tight packing with cholesterol, leading to weaker van der Waals interactions and further reducing affinity.52 Recent studies indicate that cholesterol is predominantly present in the outer leaflet of the plasma membrane, supporting the hypothesis of an asymmetric distribution.53 However, this remains a subject of debate, as experimental methods to directly and conclusively address this question are still lacking. In our previous work, we demonstrated that altering cholesterol levels in the plasma membrane directly influences the unconventional secretory machinery of FGF2 by enhancing FGF2 recruitment at the inner plasma membrane leaflet and facilitating its translocation into the extracellular space, primarily through the modulation of PI(4,5)P2 lipid properties.42
Sphingomyelin is a lipid commonly associated with cholesterol-rich domains.54 This association arises from the complementary structural and chemical properties of SM and cholesterol. SM has a straight, saturated sphingosine chain that aligns well with the planar geometry of cholesterol, promoting tight packing and enhancing the van der Waals interactions. Additionally, the amide group in the sphingosine chain forms hydrogen bonds with the hydroxyl group of cholesterol, further stabilizing cholesterol–SM complexes. This synergy between SM and cholesterol is functionally significant: cholesterol induces an ordering effect on membranes, while SM provides a scaffold that facilitates cholesterol’s interaction with other lipids, creating a mutually stabilizing relationship. SM is asymmetrically distributed in the plasma membrane, with a higher concentration in the outer leaflet. This distribution suggests a coordinated role with cholesterol in membrane organization and function.
PI(4,5)P2 plays an important role in endocytosis, exocytosis, cytokinesis, cytoskeleton activation, vesicle fusion, regulation of other phosphorylated PIs, and many signaling pathways.55 Most PI(4,5)P2-dependent processes happen at the cytosolic leaflet, so it is believed to be localized at the inner leaflet in most of the cells, with a few exceptions, such as HeLa and epithelial cells.56 Few reports exist in the literature focusing on PI(4,5)P2 transbilayer plasma membrane asymmetry. Only recently, a flippase enzyme was reported for PI(4,5)P2, the same as PC flippase.57
The preferential localization of SM and cholesterol within Lo phases is well established.54,58–60 In contrast, little is known about the involvement of phosphatidylinositol (PI), and its phosphorylated derivatives, in Lo and Ld phases. The most abundant PI(4,5)P2 species in brain extracts feature 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 20
0 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 acyl chains, giving this lipid unique properties.61,62 The straight, saturated 18
4 acyl chains, giving this lipid unique properties.61,62 The straight, saturated 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 chain closely aligns with cholesterol’s structure, promoting tight packing and order within membranes. This is further enhanced by forming hydrogen bonds with cholesterol’s hydroxyl group.25 Conversely, the highly unsaturated 20
0 chain closely aligns with cholesterol’s structure, promoting tight packing and order within membranes. This is further enhanced by forming hydrogen bonds with cholesterol’s hydroxyl group.25 Conversely, the highly unsaturated 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 chain introduces flexibility and disorder, favoring disordered regions, and they might promote interdigitation with the outer plasma membrane leaflet. Detergent-resistant membrane (DRM) experiments revealed that PI(4,5)P2 is enriched in ordered regions. Notably, cholesterol depletion using methyl-beta-cyclodextrin causes PI(4,5)P2 to redistribute into disordered regions.63 However, DRM assays are often criticized for potential artifact detergents introduced during the procedure. An alternative study using Giant Plasma Membrane Vesicles (GPMVs) and Lo/Ld-directed peptides has shown faster PI(4,5)P2 metabolism kinetics in Lo regions.64 In this study, they noted that PI(4,5)P2 in Lo regions undergoes rapid turnover, being converted to diacylglycerol (DAG) and inositol trisphosphate (IP3) and replenished at a faster rate compared to PI(4,5)P2 in Ld regions. The reported data suggest that PI(4,5)P2 in Lo regions functions as a dynamic pool, regulating the concentrations of other PI derivatives through the activity of PI kinases and phosphatases. These enzymes are also reported to localize within Lo regions,65,66 further supporting the functional interplay between PI(4,5)P2 and the Lo phase.
4 chain introduces flexibility and disorder, favoring disordered regions, and they might promote interdigitation with the outer plasma membrane leaflet. Detergent-resistant membrane (DRM) experiments revealed that PI(4,5)P2 is enriched in ordered regions. Notably, cholesterol depletion using methyl-beta-cyclodextrin causes PI(4,5)P2 to redistribute into disordered regions.63 However, DRM assays are often criticized for potential artifact detergents introduced during the procedure. An alternative study using Giant Plasma Membrane Vesicles (GPMVs) and Lo/Ld-directed peptides has shown faster PI(4,5)P2 metabolism kinetics in Lo regions.64 In this study, they noted that PI(4,5)P2 in Lo regions undergoes rapid turnover, being converted to diacylglycerol (DAG) and inositol trisphosphate (IP3) and replenished at a faster rate compared to PI(4,5)P2 in Ld regions. The reported data suggest that PI(4,5)P2 in Lo regions functions as a dynamic pool, regulating the concentrations of other PI derivatives through the activity of PI kinases and phosphatases. These enzymes are also reported to localize within Lo regions,65,66 further supporting the functional interplay between PI(4,5)P2 and the Lo phase.
The asymmetric distribution of these lipid types in the plasma membrane likely plays a role in mediating FGF2 membrane translocation. Cholesterol and SM enrichment in the outer leaflet and PI(4,5)P2 primarily located in the inner leaflet highlights a spatial organization that supports membrane-associated processes. This asymmetry may create functional lipid environments, such as ordered nanodomains, that facilitate the recruitment and translocation of FGF2 during unconventional secretion. Thus, developing methods to reproduce this lipid asymmetry in vitro reconstitution experiments or to disrupt it in living cell systems selectively could provide insights into how these lipid distributions regulate FGF2 secretion. Such studies will uncover mechanistic details about the secretory mechanism of FGF2 and reveal broader principles about the interplay between lipid organization and protein function in cellular membranes.
Targeting lipid asymmetry to study its role in the unconventional secretion mechanism of FGF2
The asymmetric distribution of lipids in living cell systems is a significant distinction compared to inside-out reconstituted vesicles, which are challenging to produce. While several reports in the literature on generating asymmetric vesicles exist, only a few studies have yet used them to make physiologically relevant plasma membrane-like asymmetric lipid compositions for functional assays.In our studies on the unconventional secretion mechanism of FGF2, we identified three key lipid species—cholesterol, sphingomyelin (SM), and PI(4,5)P2—that play crucial roles. Thus, developing methods for generating asymmetric vesicles containing these specific lipids in a PC background and investigating their impact on pore formation kinetics could reveal mechanistic insights into this unusual process of protein translocation across a membrane.
Strategies for cellular single-lipid disturbance
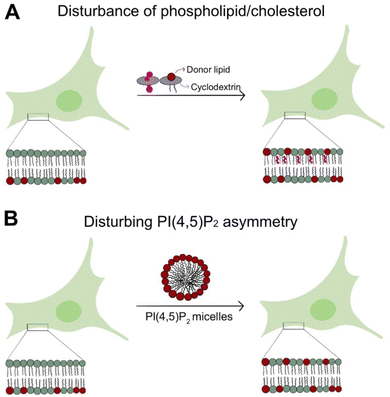
One of the most widely used approaches for manipulating lipid composition at the plasma membrane level is the use of β-cyclodextrin-based methods,67,68 as illustrated in Fig. 3A. β-Cyclodextrins are cyclic oligosaccharides with a hydrophobic cavity that can encapsulate lipid molecules, making them highly effective for lipid delivery or cholesterol removal at the plasma membrane. For example, β-cyclodextrin can be pre-loaded with a specific lipid of interest, such as cholesterol or sphingomyelin, and subsequently incubated with cells. This process facilitates the targeted delivery of these lipids to the outer leaflet of the plasma membrane, potentially disrupting the asymmetry of lipid species such as PI(4,5)P2, which is exclusively located in the inner leaflet. In addition to lipid delivery, (methyl-)β-cyclodextrin can also be employed to extract cholesterol from the plasma membrane outer leaflet. However, while effective, this lipid extraction approach has limitations that can lead to unintended alterations in membrane composition and induce cytotoxic effects. This method has been employed to manipulate cholesterol content in the plasma membrane. It is well established that altering cholesterol levels positively influences the recruitment and translocation of FGF2.26,69 In contrast, little is known about manipulating SM and PI(4,5)P2 levels in cells influencing the mechanisms underlying FGF2 secretion. Sphingomyelin is predominantly located in the outer leaflet of the plasma membrane, where it plays essential roles in maintaining membrane stability, organizing lipid domains, and mediating signaling processes. SM scramblases can translocate SM across the bilayer. Still, their activity is calcium-dependent, which may expose cytosolic leaflet lipids to the outer leaflet and it is coupled with the enzymatic action of sphingomyelinase in the inner leaflet. This enzyme converts SM into ceramide, introducing metabolic changes that complicate the study of SM dynamics. The single-cell injection method is a precise and promising alternative,70 which could deliver SM directly into the inner leaflet. This approach would bypass scramblase activity, offering a controlled environment for investigating lipid redistribution. However, the physical penetration of the cell membrane can cause stress or damage to the cell, potentially affecting its viability or normal function. Furthermore, this approach enables the enrichment of SM in various membrane organelles, which could potentially alter normal cellular functions.On the other hand, phosphatidylinositol 4,5-bisphosphate is predominantly located in the inner leaflet of the plasma membrane, where it functions as a critical signaling lipid and regulator of membrane dynamics.30,71 Its intracellular localization presents opportunities for simple external intervention to disrupt lipid asymmetry. One effective strategy involves the targeted delivery of PI(4,5)P2 micelles directly within the cell medium,70 as illustrated in Fig. 3B. PI(4,5)P2 has a high bilayer partition coefficient and will efficiently incorporate into the outer leaflet of the plasma membrane.
Strategies for asymmetric vesicle generation
There are several methods for creating asymmetric liposomes. Large unilamellar vesicles (LUVs) are usually preferred over giant unilamellar vesicles (GUVs) due to their well-defined size and low susceptibility to high curvature or substrate interactions. In contrast, GUVs, though more fragile, have the advantage of being compatible with confocal microscopy imaging and possess a size range of 5 to 100 μm, closely matching the dimensions of eukaryotic cells.72 This cell-like size makes GUVs valuable as model systems for studying protein–lipid interactions.Asymmetric unilamellar vesicles have been created using five main strategies: (i) The inverted emulsion droplet method uses a lipid-coated oil–water interface to generate GUVs. This approach produces GUVs efficiently; however, the size distribution is broad, and residual organic solvents are often present in the hydrophobic core of the bilayer, which may alter the properties of the bilayer and compromise its integrity.67,73,74 (ii) Changes in pH can manipulate the protonation state of limited anionic lipids, such as phosphatidylglycerol (PG) and phosphatidic acid (PA), to flip between bilayer leaflets in their uncharged state. This creates an uneven lipid distribution, with accumulation on the more alkaline side of the bilayer.75 (iii) Like cellular systems, carrier molecules with hydrophobic cavities, such as methyl-β-cyclodextrin or lipid transfer proteins,76 are commonly employed to facilitate the exchange of lipids in the outer leaflet of vesicles. Catalyzed lipid exchange is compatible with a wide range of phospholipids; however, separating the two vesicle populations is needed afterward to isolate the desired asymmetric liposomes.67 (iv) The hemifusion method is used to mix the lipid composition of GUVs with a supported lipid bilayer (SLB) by inducing membrane fusion using calcium ions. In the hemifusion state, the two leaflets are connected through a hemifusion diaphragm, promoting lipid exchange between the outer leaflets via lateral diffusion. Further fusion events are blocked by removing ions with calcium chelation, and a mechanical shear is applied to detach the GUVs from the SLB.77 (v) The enzymatic method can effectively modify lipid composition in specific bilayer leaflets. For instance, sphingomyelin can be hydrolyzed into ceramide and phosphocholine, while PIP5K1C catalyzes the conversion of PI(4)P to PI(4,5)P2, as illustrated in Fig. 4. This approach enables selective modification of the outer leaflet of synthetic liposomes without affecting the inner leaflet, as the enzymes used are typically not membrane-permeable. However, its application is limited by enzyme availability and cost, substrate specificity, and the need for precise reaction conditions.
Discussion
Several parameters are likely to play key roles in governing the thermodynamics and kinetics of the FGF2 membrane translocation process. The faster kinetics observed in living cells,9 compared to in vitro reconstitution experiments using purified components,13,78 can be attributed to fundamental differences between the two experimental systems. Notably, the absence of auxiliary components in synthetic vesicles such as the Na,K-ATPase and Tec kinase. For example, a variant of FGF2 lacking the two key residues responsible for its interaction with the Na,K-ATPase exhibits a 30–40% reduction in plasma membrane recruitment and translocation efficiency.22 However, these components are not essential for FGF2 membrane translocation in reconstituted systems. In addition, as a surrogate for the authentic GPI-anchored heparan sulfate proteoglycan GPC1, soluble heparin molecules containing high-affinity FGF2 binding sites have been used in in vitro experiments.13,16 Furthermore, we hypothesize that the fast kinetics of this process observed in intact cells is driven by cellular redox systems facilitating the efficient oxidative dimerization of FGF2 disulfide bridges in combination with the asymmetric nature of the plasma membrane. Finally, unlike in living cells where lipid distribution is asymmetric, artificial vesicles exhibit a symmetric lipid arrangement, introducing another layer of difference between the two experimental systems. All of these and maybe additional factors could potentially slow down the kinetics and efficiency of FGF2 membrane translocation observed in vitro compared to living cells.Current views describe the plasma membrane as a composite material, where the exoplasmic leaflet primarily ensures functional barrier integrity, while the cytoplasmic leaflet contributes to membrane fluidity.79 Due to technical limitations, this duality is often lost in in vitro systems, where synthetic liposomes are typically produced with symmetric membranes. For example, a symmetric distribution of SM—which in living cells is predominantly located in the outer leaflet—can lead to excessive membrane rigidity when interacting with cholesterol. This rigidity disrupts the dual nature of the plasma membrane, and it might create an additional barrier that hinders the FGF2 translocation process. Focusing on PI(4,5)P2, its asymmetric distribution within the plasma membrane can play a fundamental role in the membrane translocation mechanism. Our current model proposes that FGF2 undergoes oligomerization at the inner leaflet of the plasma membrane. Notably, a single FGF2 molecule can recruit up to 5 PI(4,5)P2 molecules through high- and low-affinity binding sites.13 Given that FGF2 is known to oligomerize in a membrane-dependent manner into at least tetramers,13–15 this would result in the accumulation of approximately 20 PI(4,5)P2 molecules in a spatially confined area. The localized accumulation of PI(4,5)P2 molecules near the protein is expected to result in a local concentration of ∼20 mol%. PI(4,5)P2 is generally considered as having a positive intrinsic curvature due to its large, charged hydrophilic head group. However, its contribution to local membrane curvature can vary depending on the cellular context and its molecular interactions. Notably, the most abundant PI(4,5)P2 species in brain extracts contain a polyunsaturated acyl chain (arachidonic acid), which is highly disordered and occupies more space, altering the overall shape of the lipid, as suggested by Lin et al.80,81 This characteristic likely shifts PI(4,5)P2’s preference towards negative curvature, which we hypothesize is a critical factor in driving FGF2 pore formation activity. Furthermore, the inner leaflet is already enriched with ∼30 mol% of PE lipids.30 PE are also conical, non-bilayer lipids with intrinsic negative curvature properties. We propose that the enrichment of PI(4,5)P2, driven by FGF2 oligomerization, increases the local concentration of non-bilayer lipids beyond a critical threshold. This accumulation induces negative curvature (Fig. 5B, left panel), destabilizing the membrane and facilitating the pore formation process (Fig. 5C, left panel). Additionally, PI(4,5)P2 carries a highly negative charge under physiological conditions, which would create a charge gradient in the spatially confined region across the membrane, resulting in an electric field that could potentially contribute to the pore formation process.82,83 Contrarily, in a symmetric membrane, the long acyl chains of PI(4,5)P2 (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 20
0 and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) could interdigitate from both sides of the membrane, leading to a symmetrical accumulation in both leaflets.84,85 This would cancel out the charge gradient and curvature-inducing effects, as hypothesized in Fig. 5B, right panel. Furthermore, heparan sulfate chains, which are also negatively charged, are positioned on the outer leaflet of the plasma membrane. A highly negative lipid such as PI(4,5)P2 in the outer leaflet would reduce the proximity of the heparan sulfate chains to the membrane surface due to electrostatic repulsion, as schematized in the right panels of Fig. 5B and C. Notably, FGF2 translocation depends on membrane-proximal heparan sulfate proteoglycans (HSPGs) on the cell surface, which capture and disassemble FGF2 oligomers. Interestingly, among the diverse sub-classes of HSPGs, including syndecans, perlecans, and glypicans, we unexpectedly discovered that Glypican-1 (GPC1) represents the principal and rate-limiting factor driving unconventional secretion of FGF2.19 This is due to its stronger binding affinity for FGF2 and closer proximity to the membrane surface. Therefore, exploring the role of PI(4,5)P2 asymmetry in synthetic and cellular systems is essential to validate the proposed mechanism. Specifically, developing a method to generate PI(4,5)P2-asymmetric vesicles would allow for investigations of FGF2 pore formation and translocation kinetics.
4) could interdigitate from both sides of the membrane, leading to a symmetrical accumulation in both leaflets.84,85 This would cancel out the charge gradient and curvature-inducing effects, as hypothesized in Fig. 5B, right panel. Furthermore, heparan sulfate chains, which are also negatively charged, are positioned on the outer leaflet of the plasma membrane. A highly negative lipid such as PI(4,5)P2 in the outer leaflet would reduce the proximity of the heparan sulfate chains to the membrane surface due to electrostatic repulsion, as schematized in the right panels of Fig. 5B and C. Notably, FGF2 translocation depends on membrane-proximal heparan sulfate proteoglycans (HSPGs) on the cell surface, which capture and disassemble FGF2 oligomers. Interestingly, among the diverse sub-classes of HSPGs, including syndecans, perlecans, and glypicans, we unexpectedly discovered that Glypican-1 (GPC1) represents the principal and rate-limiting factor driving unconventional secretion of FGF2.19 This is due to its stronger binding affinity for FGF2 and closer proximity to the membrane surface. Therefore, exploring the role of PI(4,5)P2 asymmetry in synthetic and cellular systems is essential to validate the proposed mechanism. Specifically, developing a method to generate PI(4,5)P2-asymmetric vesicles would allow for investigations of FGF2 pore formation and translocation kinetics.
In conclusion, by mimicking the natural asymmetry of PI(4,5)P2 in the inner plasma membrane leaflet, we hypothesize that this approach will accelerate the kinetics of FGF2 oligomerization and translocation, as the spatially constrained accumulation of PI(4,5)P2 and the resulting membrane stress and charge gradient would facilitate membrane curvature and pore opening. Conversely, disrupting PI(4,5)P2 asymmetry in living cell systems could offer deeper insights into its critical role in unconventional FGF2 secretion. We predict that the loss of PI(4,5)P2 asymmetry would neutralize the charge gradient, reduce membrane curvature, and increase the distance of heparan sulfate chains from the membrane surface. This, in turn, would slow down the kinetics of FGF2 secretion.
Data availability
All data supporting the findings of this study are included within the manuscript. No additional data or materials were generated or used beyond what is provided in the text and figures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (SFB-1638/1 – 511488495 – Z01; FL, SFB/TRR 186, project A1; WN and FL, DFG LO 2821/1-1; FL, DFG Ni 423/10-1, DFG Ni 423/12-1 and DFG Ni 423/13-1; WN). FL gratefully acknowledges the data storage service SDS@hd supported by the Ministry of Science, Research, and the Arts Baden-Württemberg (MWK), the German Research Foundation (DFG) through grants INST 35/1314-1 FUGG and INST 35/1503-1 FUGG. FL acknowledges the computing resources provided by the CSC – IT Center for Science Ltd (Espoo, Finland) and supported by the state of Baden-Württemberg through bwHPC and the German Research Foundation (DFG) through grant INST 35/1597-1 FUGG.References
- A. Muesch, E. Hartmann, K. Rohde, A. Rubartelli, R. Sitia and T. A. Rapoport, A novel pathway for secretory proteins?, Trends Biochem. Sci., 1990, 15(3), 86–88 CrossRef CAS PubMed.
- M. T. Pallotta and W. Nickel, FGF2 and IL-1β – explorers of unconventional secretory pathways at a glance, J. Cell Sci., 2020, 133(21), jcs250449 CrossRef CAS PubMed.
- R. Z. Florkiewicz, R. A. Majack, R. D. Buechler and E. Florkiewicz, Quantitative export of FGF-2 occurs through an alternative, energy-dependent, non-ER/Golgi pathway, J. Cell. Physiol., 1995, 162(3), 388–399 CrossRef CAS PubMed.
- C. Trudel, V. Faure-Desire, R. Z. Florkiewicz and A. Baird, Translocation of FGF2 to the cell surface without release into conditioned media, J. Cell. Physiol., 2000, 185(2), 260–268 CrossRef CAS PubMed.
- A. Engling, R. Backhaus, C. Stegmayer, C. Zehe, C. Seelenmeyer and A. Kehlenbach, et al., Biosynthetic FGF-2 is targeted to non-lipid raft microdomains following translocation to the extracellular surface of CHO cells, J. Cell Sci., 2002, 115(18), 3619–3631 CrossRef CAS PubMed.
- R. Z. Florkiewicz, J. Anchin and A. Baird, The Inhibition of Fibroblast Growth Factor-2 Export by Cardenolides Implies a Novel Function for the Catalytic Subunit of Na+,K+-ATPase, J. Biol. Chem., 1998, 273(1), 544–551 CrossRef CAS PubMed.
- T. Schäfer, H. Zentgraf, C. Zehe, B. Brügger, J. Bernhagen and W. Nickel, Unconventional Secretion of Fibroblast Growth Factor 2 Is Mediated by Direct Translocation across the Plasma Membrane of Mammalian Cells, J. Biol. Chem., 2004, 279(8), 6244–6251 CrossRef PubMed.
- E. Dimou and W. Nickel, Unconventional mechanisms of eukaryotic protein secretion, Curr. Biol., 2018, 28(8), R406–R410 CrossRef CAS PubMed.
- E. Dimou, K. Cosentino, E. Platonova, U. Ros, M. Sadeghi and P. Kashyap, et al., Single event visualization of unconventional secretion of FGF2, J. Cell Biol., 2019, 218(2), 683–699 CrossRef CAS PubMed.
- K. Temmerman, A. D. Ebert, H. M. Müller, I. Sinning, I. Tews and W. Nickel, A Direct Role for Phosphatidylinositol-4,5-bisphosphate in Unconventional Secretion of Fibroblast Growth Factor 2, Traffic, 2008, 9(7), 1204–1217 CrossRef CAS PubMed.
- C. Zehe, A. Engling, S. Wegehingel, T. Schäfer and W. Nickel, Cell-surface heparan sulfate proteoglycans are essential components of the unconventional export machinery of FGF-2, Proc. Natl. Acad. Sci. U.S.A., 2006, 103(42), 15479–15484 CrossRef CAS PubMed.
- J. P. Steringer, S. Bleicken, H. Andreas, S. Zacherl, M. Laussmann and K. Temmerman, et al., Phosphatidylinositol 4,5-Bisphosphate (PI(4,5)P2)-dependent Oligomerization of Fibroblast Growth Factor 2 (FGF2) Triggers the Formation of a Lipidic Membrane Pore Implicated in Unconventional Secretion, J. Biol. Chem., 2012, 287(33), 27659–27669 CrossRef CAS PubMed.
- J. P. Steringer, S. Lange, S. Čujová, R. Šachl, C. Poojari and F. Lolicato, et al., Key steps in unconventional secretion of fibroblast growth factor 2 reconstituted with purified components, eLife, 2017, 6, e28985 CrossRef PubMed.
- R. Šachl, S. Čujová, V. Singh, P. Riegerová, P. Kapusta and H. M. Müller, et al., Functional Assay to Correlate Protein Oligomerization States with Membrane Pore Formation, Anal. Chem., 2020, 92(22), 14861–14866 CrossRef PubMed.
- V. Singh, S. Macharová, P. Riegerová, J. P. Steringer, H. M. Müller and F. Lolicato, et al., Determining the Functional Oligomeric State of Membrane-Associated Protein Oligomers Forming Membrane Pores on Giant Lipid Vesicles, Anal. Chem., 2023, 95(23), 8807–8815 CrossRef CAS PubMed.
- F. Lolicato, J. P. Steringer, R. Saleppico, D. Beyer, J. Fernandez-Sobaberas and S. Unger, et al., Disulfide bridge-dependent dimerization triggers FGF2 membrane translocation into the extracellular space, eLife, 2024, 12, RP88579 CrossRef PubMed.
- R. Backhaus, C. Zehe, S. Wegehingel, A. Kehlenbach, B. Schwappach and W. Nickel, Unconventional protein secretion: membrane translocation of FGF-2 does not require protein unfolding, J. Cell Sci., 2004, 117(9), 1727–1736 CrossRef CAS PubMed.
- L. C. Torrado, K. Temmerman, H. M. Müller, M. P. Mayer, C. Seelenmeyer and R. Backhaus, et al., An intrinsic quality-control mechanism ensures unconventional secretion of fibroblast growth factor 2 in a folded conformation, J. Cell Sci., 2009, 122(18), 3322–3329 CrossRef CAS PubMed.
- C. Sparn, E. Dimou, A. Meyer, R. Saleppico, S. Wegehingel and M. Gerstner, et al., Glypican-1 drives unconventional secretion of fibroblast growth factor 2, eLife, 2022, 11, e75545 CrossRef CAS PubMed.
- A. D. Ebert, M. Laußmann, S. Wegehingel, L. Kaderali, H. Erfle and J. Reichert, et al., Tec-Kinase-Mediated Phosphorylation of Fibroblast Growth Factor 2 is Essential for Unconventional Secretion, Traffic, 2010, 11(6), 813–826 CrossRef CAS PubMed.
- S. Zacherl, V. G. La, H. M. Müller, S. Wegehingel, E. Dimou and P. Sehr, et al., A Direct Role for ATP1A1 in Unconventional Secretion of Fibroblast Growth Factor 2, J. Biol. Chem., 2015, 290(6), 3654–3665 CrossRef CAS PubMed.
- C. Legrand, R. Saleppico, J. Sticht, F. Lolicato, H. M. Müller and S. Wegehingel, et al., The Na,K-ATPase acts upstream of phosphoinositide PI(4,5)P2 facilitating unconventional secretion of Fibroblast Growth Factor 2, Commun. Biol., 2020, 3(1), 141 CrossRef CAS PubMed.
- G. La Venuta, S. Wegehingel, P. Sehr, H. M. Müller, E. Dimou and J. P. Steringer, et al., Small Molecule Inhibitors Targeting Tec Kinase Block Unconventional Secretion of Fibroblast Growth Factor 2, J. Biol. Chem., 2016, 291(34), 17787–17803 CrossRef CAS PubMed.
- C. Sparn, A. Meyer, R. Saleppico and W. Nickel, Unconventional secretion mediated by direct protein self-translocation across the plasma membranes of mammalian cells, Trends Biochem. Sci., 2022, 47(8), 699–709 CrossRef CAS PubMed.
- Y. Wen, V. M. Vogt and G. W. Feigenson, PI(4,5)P 2 Clustering and Its Impact on Biological Functions, Annu. Rev. Biochem., 2021, 90(1), 681–707 CrossRef CAS PubMed.
- F. Lolicato, R. Saleppico, A. Griffo, A. Meyer, F. Scollo and B. Pokrandt, et al., Cholesterol promotes clustering of PI(4,5)P2 driving unconventional secretion of FGF2, J. Cell Biol., 2022, 221(11), e202106123 CrossRef CAS PubMed.
- R. Kanai, F. Cornelius, B. Vilsen and C. Toyoshima, Cryoelectron microscopy of Na+,K+-ATPase in the two E2P states with and without cardiotonic steroids, Proc. Natl. Acad. Sci. U.S.A., 2022, 119(15), e2123226119 CrossRef CAS PubMed.
- M. S. Bretscher, Asymmetrical Lipid Bilayer Structure for Biological Membranes, Nature New Biol., 1972, 236(61), 11–12 CrossRef CAS PubMed.
- J. Lombard, Once upon a time the cell membranes: 175 years of cell boundary research, Biol. Direct, 2014, 9(1), 32 CrossRef PubMed.
- J. H. Lorent, K. R. Levental, L. Ganesan, G. Rivera-Longsworth, E. Sezgin and M. Doktorova, et al., Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape, Nat. Chem. Biol., 2020, 16(6), 644–652 CrossRef CAS PubMed.
- G. Pabst and S. Keller, Exploring membrane asymmetry and its effects on membrane proteins, Trends Biochem. Sci., 2024, 49(4), 333–345 CrossRef CAS PubMed.
- M. Doktorova, J. L. Symons and I. Levental, Structural and functional consequences of reversible lipid asymmetry in living membranes, Nat. Chem. Biol., 2020, 16(12), 1321–1330 CrossRef CAS PubMed.
- B. Fadeel and D. Xue, The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease, Crit. Rev. Biochem. Mol. Biol., 2009, 44(5), 264–277 CrossRef CAS PubMed.
- O. H. Kim, G. H. Kang, J. Hur, J. Lee, Y. Jung and I. S. Hong, et al., Externalized phosphatidylinositides on apoptotic cells are eat-me signals recognized by CD14, Cell Death Differ., 2022, 29(7), 1423–1432 CrossRef CAS PubMed.
- T. Rivel, C. Ramseyer and S. Yesylevskyy, The asymmetry of plasma membranes and their cholesterol content influence the uptake of cisplatin, Sci. Rep., 2019, 9(1), 5627 CrossRef PubMed.
- D. Patel and S. N. Witt, Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease, Oxid. Med. Cell. Longevity, 2017, 2017(1), 4829180 CrossRef PubMed.
- K. F. N’Guessan, P. H. Patel and X. Qi, SapC-DOPS – a Phosphatidylserine-targeted Nanovesicle for selective Cancer therapy, Cell Commun. Signaling, 2020, 18(1), 6 CrossRef PubMed.
- P. Niekamp, F. Scharte, T. Sokoya, L. Vittadello, Y. Kim and Y. Deng, et al., Ca2+-activated sphingomyelin scrambling and turnover mediate ESCRT-independent lysosomal repair, Nat. Commun., 2022, 13(1), 1875 CrossRef CAS PubMed.
- K. Emoto, T. Kobayashi, A. Yamaji, H. Aizawa, I. Yahara and K. Inoue, et al., Redistribution of phosphatidylethanolamine at the cleavage furrow of dividing cells during cytokinesis, Proc. Natl. Acad. Sci. U.S.A., 1996, 93(23), 12867–12872 CrossRef CAS PubMed.
- K. Emoto and M. Umeda, An Essential Role for a Membrane Lipid in Cytokinesis, J. Cell Biol., 2000, 149(6), 1215–1224 CrossRef CAS PubMed.
- G. Kunduri, U. Acharya and J. K. Acharya, Lipid Polarization during Cytokinesis, Cells, 2022, 11(24), 3977 CrossRef CAS PubMed.
- F. Lolicato, R. Saleppico, A. Griffo, A. Meyer, F. Scollo and B. Pokrandt, et al., Cholesterol promotes clustering of PI(4,5)P2 driving unconventional secretion of FGF2, J. Cell Biol., 2022, 221(11), e202106123 CrossRef CAS PubMed.
- A. Griffo, C. Sparn, F. Lolicato, F. Nolle, N. Khangholi and R. Seemann, et al., Mechanics of biomimetic free-standing lipid membranes: insights into the elasticity of complex lipid compositions, RSC Adv., 2024, 14(19), 13044–13052 RSC.
- T. Róg, M. Pasenkiewicz-Gierula, I. Vattulainen and M. Karttunen, Ordering effects of cholesterol and its analogues, Biochim. Biophys. Acta, Biomembr., 2009, 1788(1), 97–121 CrossRef PubMed.
- H. K. Ijäs, M. Lönnfors and T. K. M. Nyholm, Sterol affinity for phospholipid bilayers is influenced by hydrophobic matching between lipids and transmembrane peptides, Biochim. Biophys. Acta, Biomembr., 2013, 1828(3), 932–937 CrossRef PubMed.
- H. Martinez-Seara, T. Róg, M. Pasenkiewicz-Gierula, I. Vattulainen, M. Karttunen and R. Reigada, Interplay of Unsaturated Phospholipids and Cholesterol in Membranes: Effect of the Double-Bond Position, Biophys. J., 2008, 95(7), 3295–3305 CrossRef CAS PubMed.
- J. V. Bleecker, P. A. Cox, R. N. Foster, J. P. Litz, M. C. Blosser and D. G. Castner, et al., Thickness Mismatch of Coexisting Liquid Phases in Noncanonical Lipid Bilayers, J. Phys. Chem. B, 2016, 120(10), 2761–2770 CrossRef CAS PubMed.
- T. J. McIntosh, The effect of cholesterol on the structure of phosphatidylcholine bilayers, Biochim. Biophys. Acta, Biomembr., 1978, 513(1), 43–58 CrossRef CAS PubMed.
- T. S. Ursell, W. S. Klug and R. Phillips, Morphology and interaction between lipid domains, Proc. Natl. Acad. Sci. U.S.A., 2009, 106(32), 13301–13306 CrossRef CAS PubMed.
- C. Wang, M. R. Krause and S. L. Regen, Push and Pull Forces in Lipid Raft Formation: The Push Can Be as Important as the Pull, J. Am. Chem. Soc., 2015, 137(2), 664–666 CrossRef CAS PubMed.
- T. Róg, M. Pasenkiewicz-Gierula, I. Vattulainen and M. Karttunen, What Happens if Cholesterol Is Made Smoother, Biophys. J., 2007, 92(10), 3346–3357 CrossRef PubMed.
- F. Keller and A. Heuer, Chain ordering of phospholipids in membranes containing cholesterol: what matters?, Soft Matter, 2021, 17(25), 6098–6108 RSC.
- M. Doktorova, J. L. Symons, X. Zhang, H. Y. Wang, J. Schlegel and J. H. Lorent, Cell membranes sustain phospholipid imbalance via cholesterol asymmetry, bioRxiv, 2023, preprint DOI:10.1016/j.cell.2025.02.034.
- M. B. Sankaram and T. E. Thompson, Interaction of cholesterol with various glycerophospholipids and sphingomyelin, Biochemistry, 1990, 29(47), 10670–10675 CrossRef CAS PubMed.
- F. Lolicato, W. Nickel, V. Haucke and M. Ebner, Phosphoinositide switches in cell physiology - From molecular mechanisms to disease, J. Biol. Chem., 2024, 300(3), 105757 CrossRef CAS PubMed.
- A. Yoneda, K. Kanemaru, A. Matsubara, E. Takai, M. Shimozawa and R. Satow, et al., Phosphatidylinositol 4,5-bisphosphate is localized in the plasma membrane outer leaflet and regulates cell adhesion and motility, Biochem. Biophys. Res. Commun., 2020, 527(4), 1050–1056 CrossRef CAS PubMed.
- Y. Muranaka, R. Shigetomi, Y. Iwasaki, A. Hamamoto, K. Nakayama and H. Takatsu, et al., Novel phosphatidylinositol flippases contribute to phosphoinositide homeostasis in the plasma membrane, Biochem. J., 2024, 481(18), 1187–1202 CrossRef CAS PubMed.
- S. N. Ahmed, D. A. Brown and E. London, On the Origin of Sphingolipid/Cholesterol-Rich Detergent-Insoluble Cell Membranes: Physiological Concentrations of Cholesterol and Sphingolipid Induce Formation of a Detergent-Insoluble, Liquid-Ordered Lipid Phase in Model Membranes, Biochemistry, 1997, 36(36), 10944–10953 CrossRef CAS PubMed.
- M. Murata, N. Matsumori, M. Kinoshita and E. London, Molecular substructure of the liquid-ordered phase formed by sphingomyelin and cholesterol: sphingomyelin clusters forming nano-subdomains are a characteristic feature, Biophys. Rev., 2022, 14(3), 655–678 CrossRef CAS PubMed.
- R. F. M. De Almeida, A. Fedorov and M. Prieto, Sphingomyelin/Phosphatidylcholine/Cholesterol Phase Diagram: Boundaries and Composition of Lipid Rafts, Biophys. J., 2003, 85(4), 2406–2416 CrossRef CAS PubMed.
- A. Traynor-Kaplan, M. Kruse, E. J. Dickson, G. Dai, O. Vivas and H. Yu, et al., Fatty-acyl chain profiles of cellular phosphoinositides, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2017, 1862(5), 513–522 CrossRef CAS PubMed.
- S. Ikhlef, N. F. Lipp, V. Delfosse, N. Fuggetta, W. Bourguet and M. Magdeleine, et al., Functional analyses of phosphatidylserine/PI(4)P exchangers with diverse lipid species and membrane contexts reveal unanticipated rules on lipid transfer, BMC Biol., 2021, 19(1), 248 CrossRef CAS PubMed.
- L. J. Pike and J. M. Miller, Cholesterol Depletion Delocalizes Phosphatidylinositol Bisphosphate and Inhibits Hormone-stimulated Phosphatidylinositol Turnover, J. Biol. Chem., 1998, 273(35), 22298–22304 CrossRef CAS PubMed.
- J. Myeong, C. G. Park, B. C. Suh and B. Hille, Compartmentalization of phosphatidylinositol 4,5-bisphosphate metabolism into plasma membrane liquid-ordered/raft domains, Proc. Natl. Acad. Sci. U.S.A., 2021, 118(9), e2025343118 CrossRef CAS PubMed.
- G. Van Den Bogaart, K. Meyenberg, H. J. Risselada, H. Amin, K. I. Willig and B. E. Hubrich, et al., Membrane protein sequestering by ionic protein–lipid interactions, Nature, 2011, 479(7374), 552–555 CrossRef CAS PubMed.
- J. Wang and D. A. Richards, Segregation of PIP2 and PIP3 into distinct nanoscale regions within the plasma membrane, Biol. Open, 2012, 1(9), 857–862 CrossRef CAS PubMed.
- M. Doktorova, F. A. Heberle, B. Eicher, R. F. Standaert, J. Katsaras and E. London, et al., Preparation of asymmetric phospholipid vesicles for use as cell membrane models, Nat. Protoc., 2018, 13(9), 2086–2101 CrossRef CAS PubMed.
- G. Li, S. Kakuda, P. Suresh, D. Canals, S. Salamone and E. London, Replacing plasma membrane outer leaflet lipids with exogenous lipid without damaging membrane integrity, PLoS One, 2019, 14(10), e0223572 CrossRef CAS PubMed.
- F. Lolicato and W. Nickel, A Role for Liquid-Ordered Plasma Membrane Nanodomains Coordinating the Unconventional Secretory Pathway of Fibroblast Growth Factor 2?, Front. Cell Dev. Biol., 2022, 10, 864257 CrossRef PubMed.
- U. Golebiewska, M. Nyako, W. Woturski, I. Zaitseva and S. McLaughlin, Diffusion Coefficient of Fluorescent Phosphatidylinositol 4,5-bisphosphate in the Plasma Membrane of Cells. Martin TU, Mol. Biol. Cell, 2008, 19(4), 1663–1669 CrossRef CAS PubMed.
- J. P. Zewe, A. M. Miller, S. Sangappa, R. C. Wills, B. D. Goulden and G. R. V. Hammond, Probing the subcellular distribution of phosphatidylinositol reveals a surprising lack at the plasma membrane, J. Cell Biol., 2020, 219(3) DOI:10.1083/jcb.201906127.
- R. Dimova, Giant Vesicles and Their Use in Assays for Assessing Membrane Phase State, Curvature, Mechanics, and Electrical Properties, Annu. Rev. Biophys., 2019, 48(1), 93–119 CrossRef CAS PubMed.
- H. Träuble and E. Grell, Carriers and specificity in membranes. IV. Model vesicles and membranes. The formation of asymmetrical spherical lecithin vesicles, Neurosci. Res. Program Bull., 1971, 9(3), 373–380 Search PubMed.
- S. Pautot, B. J. Frisken and D. A. Weitz, Production of Unilamellar Vesicles Using an Inverted Emulsion, Langmuir, 2003, 19(7), 2870–2879 CrossRef CAS.
- M. J. Hope, T. E. Redelmeier, K. F. Wong, W. Rodrigueza and P. R. Cullis, Phospholipid asymmetry in large unilamellar vesicles induced by transmembrane pH gradients, Biochemistry, 1989, 28(10), 4181–4187 CrossRef CAS PubMed.
- B. Bloj and D. B. Zilversmit, Asymmetry and transposition rate of phosphatidylcholine in rat erythrocyte ghosts, Biochemistry, 1976, 15(6), 1277–1283 CrossRef CAS PubMed.
- T. A. Enoki. The use of hemifusion to create asymmetric giant unilamellar vesicles: Insights on induced order domains, in Methods in Enzymology, Elsevier, 2024, pp. 127–159, Available from: https://linkinghub.elsevier.com/retrieve/pii/S0076687924001204 Search PubMed.
- J. P. Steringer, S. Bleicken, H. Andreas, S. Zacherl, M. Laussmann and K. Temmerman, et al., Phosphatidylinositol 4,5-Bisphosphate (PI(4,5)P2)-dependent Oligomerization of Fibroblast Growth Factor 2 (FGF2) Triggers the Formation of a Lipidic Membrane Pore Implicated in Unconventional Secretion, J. Biol. Chem., 2012, 287(33), 27659–27669 CrossRef CAS PubMed.
- G. J. Schütz and G. Pabst, The asymmetric plasma membrane—A composite material combining different functionalities?: Balancing Barrier Function and Fluidity for Effective Signaling, BioEssays, 2023, 45(12), 2300116 CrossRef PubMed.
- X. Lin, H. Wang, Z. Lou, M. Cao, Z. Zhang and N. Gu, Roles of PIP 2 in the membrane binding of MIM I- BAR : insights from molecular dynamics simulations, FEBS Lett., 2018, 592(15), 2533–2542 CrossRef CAS PubMed.
- H. J. Lessen, K. C. Sapp, A. H. Beaven, R. Ashkar and A. J. Sodt, Molecular mechanisms of spontaneous curvature and softening in complex lipid bilayer mixtures, Biophys. J., 2022, 121(17), 3188–3199 CrossRef CAS PubMed.
- R. Dimova, Membrane Electroporation in High Electric Fields, in Advances in Electrochemical Sciences and Engineering, ed. Alkire R. C., Kolb D. M. and Lipkowski J., Wiley, 1st edn, 2011, pp. 335–367, Available from: DOI:10.1002/9783527644117.ch7.
- A. A. Gurtovenko and I. Vattulainen, Pore Formation Coupled to Ion Transport through Lipid Membranes as Induced by Transmembrane Ionic Charge Imbalance: Atomistic Molecular Dynamics Study, J. Am. Chem. Soc., 2005, 127(50), 17570–17571 CrossRef CAS PubMed.
- S. Chiantia and E. London, Acyl Chain Length and Saturation Modulate Interleaflet Coupling in Asymmetric Bilayers: Effects on Dynamics and Structural Order, Biophys. J., 2012, 103(11), 2311–2319 CrossRef CAS PubMed.
- E. H. Chaisson, F. A. Heberle and M. Doktorova, Quantifying Acyl Chain Interdigitation In Simulated Bilayers Via Direct Transbilayer Interactions, bioRxiv, 2024, preprint, DOI:10.1021/acs.jcim.4c02287.
| This journal is © The Royal Society of Chemistry 2025 |