Open Access Article
Open Access ArticleAcaricidal activity of geraniol-loaded lignin nanoparticles for the control of Brevipalpus chilensis: an eco-friendly approach to crop protection†
Natalia
Juica
 abe,
Gonzalo
Bustos-Quevedo
cd,
Sindy
Devis
b,
Nicolas
Oneto
a,
Carolina
Klagges
b,
Jeffri
Retamal
abe,
Gonzalo
Bustos-Quevedo
cd,
Sindy
Devis
b,
Nicolas
Oneto
a,
Carolina
Klagges
b,
Jeffri
Retamal
 *a and
Luis
Constandil
*a and
Luis
Constandil
 *ae
*ae
aLaboratorio de Neurobiología, Facultad de Química y Biología, Universidad de Santiago de Chile, Chile. E-mail: jeffri.retamal@usach.cl; luis.constandi@usach.cl
bInstituto de Investigación Interdisciplinar en Ciencias Biomédicas SEK, I3CBSEK, Facultad de Ciencias de la Salud, Universidad SEK, Chile
cInstitute for Infection Prevention and Hospital Epidemiology, Freiburg, Germany
dMedical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany
eCenter for the Development of Nanoscience and Nanotechnology (CEDENNA), Santiago de Chile, Chile
First published on 28th March 2025
Abstract
The environmental pollution and health risks associated with synthetic pesticides have driven increasing interest in plant-derived biopesticides like geraniol. However, their practical application is limited by high volatility and low solubility. In this study, lignin nanoparticles were used as a carrier system to enhance the stability and acaricidal efficacy of geraniol against Brevipalpus chilensis. The nanoprecipitation process enabled the synthesis of spherical geraniol-loaded lignin nanoparticles with an average size of 200 ± 27.2 nm, a surface charge of −29 ± 3.9 mV and an encapsulation efficiency of 46.5%. The release profile of encapsulated geraniol was assessed, and UV exposure assays demonstrated significantly improved stability compared to free geraniol. Bioassays revealed significantly higher mortality rates of Brevipalpus chilensis when treated with geraniol-loaded nanoparticles compared to free geraniol, highlighting the enhanced efficacy of the encapsulated compound. Additionally, nanoparticle formulations exhibited low cytotoxicity in HeLa cells. Overall, this study underscores the potential of lignin nanoparticles as a promising delivery system for optimizing biopesticide formulations in sustainable agriculture.
Environmental significanceInnovative pest control strategies that improve the efficacy of botanical biopesticides are essential for advancing an environmentally friendly agriculture. Nanocarriers have emerged as a promising technology to minimize biopesticide degradation and volatility, thereby increasing the biological activity of these natural compounds. This study underscores the potential of lignin nanoparticles as a delivery nano-system for biopesticides, utilizing the biodegradable, non-toxic and UV-shielding properties of lignin. The nanoparticles developed in this study improved the acaricidal performance of geraniol against a vineyard pest, supporting the development of efficient, safer and sustainable pest control alternatives to reduce the footprint of synthetic pesticides. Furthermore, this work contributes to the understanding of the impact of nanotechnology in agriculture. |
1. Introduction
The widespread environmental pollution, biodiversity loss, and human health risks associated with synthetic pesticides have driven growing interest in plant-derived biopesticides for promoting sustainable agriculture.1 Unlike conventional pesticides, botanical biopesticides offer advantages such as biodegradability, host specificity, lower persistence, and reduced pesticide residue accumulation. Among the most extensively studied biopesticides are the terpenoids, phenolic compounds, and alkaloids.2,3Geraniol (3,7-dimethylocta-trans-2,6-dien-1-ol), an acyclic monoterpene alcohol found in various essential oils, has demonstrated fungicidal, insecticidal, acaricidal, and insect-repellent properties.4,5 Commercially available geraniol-based formulations are currently available for crop protection;6 which are used for controlling mites such as Brevipalpus chilensis Baker (Acari: Tenuipalpidae), a pest known to negatively impact grapevine crop performance and economic yields in countries like Chile.7 However, despite the potential of botanical formulations, their large-scale adoption by farmers remains limited due to challenges such as high volatility, low aqueous solubility, and degradation under environmental conditions. These factors reduce the efficacy of the botanical biopesticides, increasing the need for frequent applications and highlighting the necessity for improved formulations to enhance the performance of compounds like geraniol.8
Nanoscale materials have shown promising potential across several fields including agriculture.9,10 In this regard, polymeric nanoparticles (NPs) have emerged as an innovative tool for addressing the limitations associated with the agricultural application of biopesticides.11,12 Specifically, NPs have been shown to enhance the biological activity of active compounds by improving their solubility, providing sustained release, and protecting them from degradation under environmental conditions. As a result, significant efforts have been made to develop nanostructured systems that optimize biopesticide efficacy.13–15 Among the various biopolymers employed for NP synthesis, lignin stands out due to its biocompatibility, biodegradability, natural ultraviolet (UV)-shielding properties, and high thermal stability.16,17 Moreover, lignin NPs (LNPs) effectively encapsulate hydrophobic molecules, making them attractive carriers for poorly soluble compounds. Additionally, using LNPs is considered an environmentally friendly approach, adding value to lignin, the second most abundant biopolymer on earth.18 Here, this study aimed to develop and characterize an eco-friendly nanosystem based on LNPs loaded with geraniol (LNPs-G) and to assess their acaricidal effects against Brevipalpus chilensis, contributing to sustainable and secure agricultural practices.
2. Experimental
2.1 Materials
Lignin, alkali (5% moisture), geraniol (98% purity), Tween 80°, acetone (for analysis), acetonitrile (ACN, HPLC grade), and methanol (for analysis) were obtained from Sigma-Aldrich (St. Louis, MO, USA).2.2 Nanoprecipitation process
LNPs-G were synthesized using a nanoprecipitation method followed by solvent evaporation, as described previously with certain modifications.19 Briefly, a 2 mg mL−1 of alkali lignin solution was prepared in a mixture of acetone: deionized water (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixture was filtered using a 0.45 μm syringe filter and adjusted to pH 8. Then, geraniol was added in a concentration of 2.5% v/v. A volume of 4.5 mL of the lignin/geraniol solution was added dropwise against ultrapure water using a syringe pump (NE-1000, New Era Pump Systems, USA) at a rate of 1.5 mL min−1, with constant stirring until complete acetone evaporation. The resulting LNPs-G were stored at −80 °C for 48 h and lyophilized for three days (BK-FD-10S, BIOBASE, China). Unloaded LNPs (LNPs-empty) were prepared similarly without the addition of geraniol, to be used as controls. Lyophilized LNPs were resuspended in Milli-Q and maintained for 15 minutes in an ultrasonic bath (Elmasonic S10H, Elma, Germany) for their physicochemical characterization.20
1). The mixture was filtered using a 0.45 μm syringe filter and adjusted to pH 8. Then, geraniol was added in a concentration of 2.5% v/v. A volume of 4.5 mL of the lignin/geraniol solution was added dropwise against ultrapure water using a syringe pump (NE-1000, New Era Pump Systems, USA) at a rate of 1.5 mL min−1, with constant stirring until complete acetone evaporation. The resulting LNPs-G were stored at −80 °C for 48 h and lyophilized for three days (BK-FD-10S, BIOBASE, China). Unloaded LNPs (LNPs-empty) were prepared similarly without the addition of geraniol, to be used as controls. Lyophilized LNPs were resuspended in Milli-Q and maintained for 15 minutes in an ultrasonic bath (Elmasonic S10H, Elma, Germany) for their physicochemical characterization.20
2.3 Physicochemical characterization of LNPs-G
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v), operating at a flow rate of 1 mL min−1 at 25 °C. A 10 μL injection volume was employed, and the detection was performed at a wavelength of 210 nm. The calibration curve was constructed with geraniol concentrations ranging from 0.0005 to 0.5 mg mL−1; a linearity coefficient of 0.999, and a quantification limit of 100 ng mL−1 (Fig. S1†).22
40, v/v), operating at a flow rate of 1 mL min−1 at 25 °C. A 10 μL injection volume was employed, and the detection was performed at a wavelength of 210 nm. The calibration curve was constructed with geraniol concentrations ranging from 0.0005 to 0.5 mg mL−1; a linearity coefficient of 0.999, and a quantification limit of 100 ng mL−1 (Fig. S1†).22
The cumulative release of geraniol from LNPs-G was assessed using the rapid equilibrium dialysis system (RED, Thermo Scientific, USA). LNPs-G were resuspended in an aqueous solution containing 5% (v/v) Tween 80 to enhance geraniol solubility in water. 500 μL of the LNPs-G suspension (0.5 mg mL−1 of geraniol) was loaded into the sample compartment of the dialysis membrane system, while 750 μL of the same aqueous/Tween 80 solution was added to the opposing compartment. The dialysis system was sealed and maintained under constant agitation in an orbital shaker at 25 °C. Samples from the opposing compartment were collected at predeterminate time intervals (3 h, 6 h, 12 h, 18 h, 24 h, 72 h, 96 h, 120 h, 144 h, 168 h and 192), replaced with fresh solutions and quantified by UPLC. Four independent LNPs-G suspensions at the same concentration were tested.24
2.4 Photostability assays
LNPs’ encapsulation capability to protect geraniol from photodegradation was evaluated by exposing 1.5 mL of three independent suspensions of LNPs-G (geraniol concentration of 0.5 mg mL−1) to UV light (365 nm) in a dark chamber at 25 °C under constant agitation in an orbital shaker. The samples were collected at 3 h, 6 h, 9 h, 12 h, 18 h, and 24 h; geraniol extraction and quantification were performed using the method described in section 2.3.3, and the results were compared to free geraniol. Free geraniol, prepared in 5% (v/v) of Tween 80° at a concentration equivalent to that encapsulated in LNPs-G was exposed to the same conditions. LNPs and free geraniol were stored under dark conditions as controls for the assay.2.5 Bioassays to evaluate acaricidal effect of LNPs-G on Brevipalpus chilensis.
The acaricidal effects of LNPs-G, free geraniol and LNPs-empty were evaluated over Brevipalpus chilensis. The specimens were obtained from Ligustrum lucidum plants and transferred to experimental units consisting of two 2 cm leaf disks placed into a moistened cotton in a 10 cm Petri dish. Each leaf disk was infested with 10 female adult mites and sprayed with geraniol encapsulated in concentrations ranging from 0.0005 mg mL−1 to 5 mg mL−1, and for this purpose, LNPs-G were suspended considering the EE% of geraniol. Mortality was evaluated at 24 h, 48 h, 72 h, and 96 h post-treatment under a microscope within 20× magnification, and the results were compared to those of free geraniol and LNPs-empty (in concentrations equivalent to those of encapsulated geraniol and LNPs-G, respectively). The mites were considered dead when movement was not observed upon touching with a brush hair. The mortality rates were calculated and corrected using Abbott's formula.25 The experimental units were retained at 26 °C ± 2 °C under conditions of 65 ± 15% relative humidity and a 16 h/8 h light/dark cycle. The data were expressed as the calculated area under the curve (AUC) from the mortality percentage of preparations over time, and it was expressed as the [Log] mg mL−1 of geraniol to calculate the IC50 value.2.6 Viability of HeLa cells
The viability of HeLa cells was assessed using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay (Biotium, USA), following the manufacturer's instructions. Briefly, 7000 cells were seeded per well into a 96-well plate and incubated at 37 °C in a humidified atmosphere containing 5% CO2 until 90% confluence. The cells were then treated with various concentrations of LNPs-G (0.0002–20 mg mL−1 of geraniol), free geraniol (at an equivalent concentration of encapsulated geraniol), or LNPs-empty and incubated for 24 h. Subsequently, 10 μL of the MTT solution was added to each well and incubated for 4 h. DMSO was then added, and absorbance was measured at 570 and 630 nm using a multiplate reader (EPOCH, BIOTEK, USA). Untreated cells and wells containing the Dulbecco's modified Eagle medium (DMEM, Corning, USA) but lacking cells were employed as controls corresponding to 100% and 0% viability, respectively. Six independent assays were performed, and the data were analyzed using two-way ANOVA and t-tests (GraphPad Software Inc., San Diego, CA).262.7 Data analysis
The data are presented as mean ± standard deviation (SD). Statistical analysis was performed using GraphPad Prism software (version 8.0, GraphPad Software Inc., San Diego, CA). Differences between the groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's post hoc test for multiple comparisons. A p-value less than 0.05 was considered statistically significant.3. Results and discussion
3.1 Preparation and characterization of LNPs-G
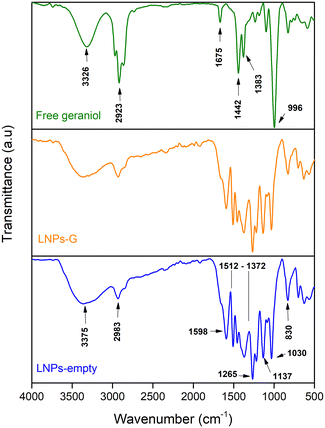
LNP formulations were synthesized by nanoprecipitation without the use of stabilizing agents; compared to other conventional techniques, this methodology is low cost and reproducible, which is crucial for scalability, an ongoing challenge in agricultural nanotechnology.27 After the standardization of the synthesis parameters (Fig. S2 and S3 and Table. S1†), the LNP suspensions were characterized post-synthesis by DLS, ELS, and STEM. Table 1 summarizes the results obtained for the hydrodynamic diameter, PDI, and surface charge determined through cumulant analysis. The LNPs-empty showed an average particle size of 134 ± 17.0 nm, and the incorporation of geraniol resulted in a significant increase in the particle size to 200 ± 27.2 nm, suggesting the incorporation of geraniol into the LNP core with an EE% of 46.5%. In addition, a significant difference in the PDI was not observed between LNPs loaded with geraniol and LNPs-empty. The LNPs are characterized to exhibit a high negative surface charge, attributable to the presence of phenolic and hydroxyl (–OH) groups on lignin monomers.28,29 As we anticipated, the surface charge of the formulations was −29.4 ± 3.9 mV for LNPs-G and −32.4 ± 3.4 mV for LNPs-empty, which suggests an optimal electrostatic repulsion between the particles, which indicates a stable aqueous colloidal suspension formulated by the nanoprecipitation process.30,31 Both (geraniol and empty) LNP formulations exhibited a monomodal size distribution and spherical morphology, observed by STEM. Additionally, the LNPs-G demonstrated a bigger particle size compared to LNPs-empty, consistent with the results of DLS analysis (Table 1 and Fig. 1).The normalized FT-IR spectra of LNPs-G, LNPs-empty, and free geraniol were obtained for comparing their chemical structures (Fig. 2). The FT-IR spectrum of free geraniol exhibited characteristic O–H stretching vibrations at 3326 cm−1, C–H stretching vibrations corresponding to alkanes at 2974, 2923, and 2850 cm−1, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations at 1675 and 1442 cm−1. The deformation of the C–H vibrations from the methyl (CH3) groups were observed at 1383 cm−1, along with the C–O stretching vibrations at 996 cm−1. The spectrum of LNPs-empty showed the expected profile, with O–H vibrations at 375 cm−1, the C–H stretching corresponding to the methyl groups at 2983 cm−1, and the aromatic skeletal vibrations at 1598 cm−1.32,33 Additional bands at 1030 and 830 cm−1 correspond to the in-plane and out-of-plane C–H deformations of the aromatic ring, respectively.
C stretching vibrations at 1675 and 1442 cm−1. The deformation of the C–H vibrations from the methyl (CH3) groups were observed at 1383 cm−1, along with the C–O stretching vibrations at 996 cm−1. The spectrum of LNPs-empty showed the expected profile, with O–H vibrations at 375 cm−1, the C–H stretching corresponding to the methyl groups at 2983 cm−1, and the aromatic skeletal vibrations at 1598 cm−1.32,33 Additional bands at 1030 and 830 cm−1 correspond to the in-plane and out-of-plane C–H deformations of the aromatic ring, respectively.
In contrast, the FT-IR spectrum of LNPs-G did not exhibit any vibrations associated with free geraniol, and no differences were observed when compared with the spectrum of LNPs-empty, suggesting that geraniol is not present on the surface of the NPs. This result is consistent with the freeze-drying process used for NP preparation, which may have resulted in the removal of any unbound geraniol molecules.
3.2 Cumulative release profile of geraniol from LNPs-G
To explore the cumulative release profile of geraniol from LNPs-G, the rapid equilibrium dialysis system was used. Notably, 17 ± 5% of the total geraniol incorporated into the LNPs was released within the first 3 h of dialysis, likely due to the diffusion of molecules near the surface of the LNPs-G (Fig. 3). Additionally, 50 ± 2% and 95 ± 3% of the geraniol was released after 16 and 72 h of dialysis, respectively. de Oliveira et al.15 and Yegin et al.34 reported the release of 50% of geraniol from chitosan/arabic gum-based NPs within 1.7 h and from Pluronic® F127 NPs after 7.25 h of dialysis, respectively; these data suggest that the release of geraniol varies depending on the polymer used for the synthesis of nanoparticles.35 Studies on essential oils encapsulated within LNPs have revealed a sustained release of essential oils from Thymus spp. over a seven-day duration and maximum release of 44.2% (without achieving full release) for the essential oils from Rosa damascena.36 These observations underscore the potential applications of LNPs for pest control, given that sustained release is a critical feature for reducing the required doses of active compounds.3.3 Photostability assays
Botanical compounds are known for their rapid biodegradation, short persistence, and low accumulation of residues associated with UV exposure, which can significantly limit their biological efficacy under field conditions.2,37 Considering the above limitations, a UV exposure assay was conducted to determine whether the incorporation of geraniol into LNPs enhances its photostability.Free geraniol exhibited 50% of degradation after 6.1 h of UV exposure, whereas the LNPs-encapsulated geraniol displayed the same level of degradation only after 11.2 h (Fig. 4). After 24 h of UV exposure, free geraniol was completely degraded, whereas the LNP-encapsulated geraniol retained 40% of its initial content, demonstrating its protective effect. Additionally, neither free geraniol nor LNPs-G exhibited degradation over time when stored in darkness during the assay. The enhanced stability of geraniol upon encapsulation within LNPs-G can be attributed to the UV-shielding properties of lignin, associated with its multiple chromophore and achromatic groups, including conjugated phenols, carbonyl groups, and conjugated double bonds, that allow absorption of UV light at 250–400 nm.38
3.4 Bioassays to evaluate the acaricidal activity of LNPs-G on Brevipalpus chilensis
The acaricidal activity of LNPs-G, free geraniol and LNPs-empty against Brevipalpus chilensis was evaluated based on the IC50 value of geraniol (Fig. 5). The results indicated that LNPs-G exhibited an IC50 of 0.53 mg mL−1, whereas free geraniol and LNPs-empty showed IC50 values of 1.70 mg mL−1 and 24.25 mg mL−1, respectively. These findings suggest that encapsulating geraniol within LNPs enhances its acaricidal effect. Moreover, LNPs-G demonstrated significantly higher acaricidal activity 48 h post-application, implying that the efficacy of geraniol is potentiated overtime (Fig. S4†). Similarly, Campos et al.39 reported the acaricidal effect of nanoencapsulated botanicals against Tetranychus urticae at 12, 24, 48, and 72 h post-application. Additionally, Ebadollahi et al.40 found that essential oils from Thymus eriocalyx were more effective when encapsulated in a nanometric mesoporous material than their non-encapsulated counterparts. The increased efficacy of encapsulated bioactive compounds is generally attributed to their higher bioavailability.41,42 In the case of geraniol, this enhanced bioavailability may result from increased stability, reduced degradation, and sustained release upon encapsulation within LNPs. Notably, LNPs-empty exhibited an acaricidal effect like LNPs-G at low concentrations, suggesting that LNPs themselves contribute to Brevipalpus chilensis’ mortality. This observation aligns with studies that highlights the intrinsic bioactive properties of lignin-based biopolymers.43–453.5 Cell viability assays
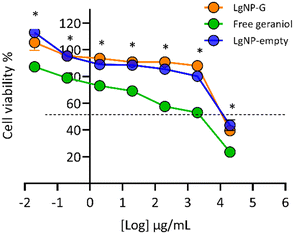
A cytotoxicity assay was conducted to assess whether the synthesized LNPs-G affect the viability of HeLa cells. Free geraniol and LNPs-empty were used as controls for comparison. Cell viability remained above 80% following the treatment with LNPs-G and LNPs-empty at concentrations up to 2000 μg mL−1 (equivalent to 3.3 on the logarithmic scale). In contrast, at this same concentration, free geraniol reduced cell viability to 50%, indicating significantly higher cell toxicity across all the tested concentrations (Fig. 6). These findings suggest that encapsulating geraniol within LNPs mitigates its potential toxicity. Similar results were reported by Zhou et al., 2019.,46 who reported that LNPs had no adverse effects on HeLa cell viability. Furthermore, these results are consistent with the well-documented biocompatibility of LNPs, which is a crucial factor for developing safe and effective formulations for agricultural pest control with minimal toxicity.47,484. Conclusion
Lignin nanoparticles have been widely recognized for their versatility and efficiency as delivery systems for biologically active molecules. In this study, geraniol was successfully encapsulated within lignin nanoparticles using a simple and potentially scalable nanoprecipitation technique. The resulting synthesized nanoparticles improved geraniol stability under UV exposure and facilitated its sustained release, leading to enhanced acaricidal activity against Brevipalpus chilensis compared to free geraniol. Additionally, lignin nanoparticles showed an intrinsic acaricidal effect at low concentrations compared to free geraniol and demonstrated low toxicity toward HeLa cells, supporting the reported biocompatibility of these types of nanoparticles.In summary, this work highlights the potential of lignin nanoparticles as an effective nanocarrier for biopesticides such as geraniol, contributing to the reduction of synthetic agrochemical usage and the promotion of sustainable agriculture. However, despite the potential of lignin nanoparticles, future challenges remain, particularly regarding scalability for large-scale production and in-field evaluations.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors of this work declare no conflicts of interest.Acknowledgements
This work has received funding from the Beca Doctorado Nacional 21202082, ANID and IDeA I + D ID24I10382, Fondecyt regular 1231042, Beca de Apoyo a la Investigación Vridei 021943CC-PAP, CEDENNA grant AFB220001. Jeffri S. Retamal is funded by the POSTDOC_DICYT, Codigo C6d. 022343CC, Vicerrectorıa de Investigacion, Desarrollo e Innovacion (VRIIC). The authors thank Ms. Yaquelin Gonzalez for her technical support and assistance in the laboratory.References
- M. S. Ayilara, B. S. Adelekere, S. A. Akinola, C. A. Fayose, U. T. Adeyemi, L. A. Gbadegesin, R. K. Omole, R. M. Johnson, Q. O. Uthman and O. O. Babalola, Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides and nanobiopesticides, Front. microbiol., 2023, 14, 1040901 CrossRef PubMed.
- F. Acheuk, S. Basiouni, A. A. Shehata, K. Dick, H. Hajri, S. Lasram, M. Yilmaz, M. Emekci, G. Tsiamis, M. Spona-Friedl, H. May-Simera, W. Eisenreich and S. Ntougias, Status and prospects of botanical biopesticides in Europe and Mediterranean countries, Biomolecules, 2022, 12(2), 311 CrossRef CAS PubMed.
- Z. Liu, Q. X. Li and B. Song, Pesticidal activity and mode of action of monoterpenes, J. Agric. Food Chem., 2022, 70(15), 4556–4571 CrossRef CAS PubMed.
- A. Fajdek-Bieda, J. Pawlińska, A. Wróblewska and A. Łuś, Evaluation of the antimicrobial activity of geraniol and selected geraniol transformation products against Gram-positive bacteria, Molecules, 2024, 29(5), 950 CrossRef CAS PubMed.
- W. Chen and A. Viljoen, Geraniol – A review update, S. Afr. J. Bot., 2022, 150, 1205–1219 CrossRef CAS.
- BIOMITE, BIOMITE, acaricida concentrado emulsionable, 2017, available: https://www.sag.gob.cl/sites/default/files/biomite_etiqueta_15-02–2017.pdf Search PubMed.
- N. Penroz and N. Olivares, Parámetros de vida de Brevipalpus chilensis Baker (Acarina:Tenuipalpidae) en tres especies de cítricos, Revista de Citricultura, 2022, 3, 2 Search PubMed.
- A. Khursheed, M. A. Rather, V. Jain, A. R. Wani, S. Rasool, R. Nazir, N. A. Malik and S. A. Majid, Plant-based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects, Microb. Pathog., 2022, 173, 105854 CrossRef CAS PubMed.
- G. S. A. Varshan and S. K. R. Namasivayam, A Green Chemistry Principle for the Biotransformation of Fungal Biomass Derived Chitosan Into Versatile Nano Scale Materials with High Biocompatibility and Potential Biological Activities—A Review, J. Bionanosci., 2024, 14, 4145–4166 CrossRef.
- S. Priyanka, S. K. R. Namasivayam, J. F. Kennedy and M. Meivelu, Starch-chitosan-Taro mucilage nanocomposite active food packaging film doped with zinc oxide nanoparticles - Fabrication, mechanical properties, anti-bacterial activity and eco toxicity assessment, Int. J. Biol. Macromol., 2024, 277(Pt3), 134319 CrossRef CAS PubMed.
- S. Manna, S. Roy, A. Dolai and A. Ravula, Current and future prospects of all-organic nanoinsecticides for agricultural insect pest management, Front. nanotechnol., 2023, 4, 1082018 Search PubMed.
- S. B. Adeyemi, A. M. Akere, J. I. Orege, O. Ejeromeghene, O. B. Orege and J. O. Akolade, Polymeric nanoparticles for enhanced delivery and improved bioactivity of essential oils, Heliyon, 2023, 9(6), e16543 CrossRef CAS PubMed.
- K. Neme, A. Nafady, S. Uddin and Y. B. Tola, Application of nanotechnology in agriculture, postharvest loss reduction and food processing: food security implication and challenges, Heliyon, 2021, 7(12), e08539 CrossRef CAS PubMed.
- S. R. Balusamy, A. S. Joshi, H. Perumalsamy, I. Mijakovic and P. Singh, Advancing sustainable agriculture: a critical review of smart and eco-friendly nanomaterial applications, J. Nanobiotechnol., 2023, 21(1), 372 CrossRef PubMed.
- J. L. de Oliveira, E. V. Ramos, A. E. S. Pereira, L. E. S. Nunes, C. C. L. da Silva, T. Pasquoto, R. Lima, G. Smaniotto, R. A. Polanczyk and L. F. Fraceto, Geraniol encapsulated in chitosan/gum arabic nanoparticles: A promising system for pest management in sustainable agriculture, J. Agric. Food Chem., 2018, 66(21), 5325–5334 CrossRef PubMed.
- L. Dai, R. Liu, L. Hu and Z. Zou, Lignin nanoparticle as a novel green carrier for the efficient delivery of resveratrol, ACS Sustainable Chem. Eng., 2017, 5, 8241–8249 CrossRef CAS.
- M. Gigli, G. Fellet, L. Pilotto, M. Sgarzi, L. Marchiol and C. Crestini, Lignin-based nano-enabled agriculture: A mini-review, Front. Plant Sci., 2022, 13, 976410 CrossRef PubMed.
- C. Vasile and M. L. Baican, Lignins as promising renewable biopolymers and bioactive compounds for high-performance materials, Polymer, 2023, 15(15), 3177 CAS.
- D. Cerro, G. Bustos, C. Villegas, N. Buendia, G. Truffa, M. P. Godoy, F. Rodríguez, A. Rojas, M. J. Galotto, L. Constandil, M. Yáñez-S, J. Romero and A. Torres, Effect of supercritical incorporation of cinnamaldehyde on physical-chemical properties, disintegration, and toxicity studies of PLA/lignin nanocomposites, Int. J. Biol. Macromol., 2021, 167, 255–266 CAS.
- Z. Zhang, C. B. Marín, M. Lefebvre, C. Lefebvre, V. Terrasson and E. Guénin, The preparation of stable spherical alkali lignin nanoparticles with great thermal stability and no cytotoxicity, Int. J. Biol. Macromol., 2022, 222(PtB), 1830–1839 CAS.
- A. V. Malm and J. C. W. Corbett, Improved dynamic light scattering using an adaptive and statistically driven time-resolved treatment of correlation data, Sci. Rep., 2019, 9(1), 13519 CrossRef PubMed.
- B. Pavan, A. Dalpiaz, L. Marani, S. Beggiato, L. Ferraro, D. Canistro, M. Paolini, F. Vivarelli, M. C. Valerii, A. Comparone, L. De Fazio and E. Spisni, Geraniol pharmacokinetics, bioavailability, and its multiple effects on the liver antioxidant and xenobiotic-metabolizing enzymes, Front. Pharmacol., 2018, 9, 18 Search PubMed.
- N. Watcharadulyarat, M. Rattanatayarom, N. Ruangsawasdi and N. Patikarnmonthon, PEG–PLGA nanoparticles for encapsulating ciprofloxacin, Sci. Rep., 2023, 13(1), 266 CAS.
- P. Solar, N. Herrera, D. Cea, S. Devis, F. Gonzalez-Nilo, N. Juica, M. Moreno, M. N. Gai, I. Brescia, S. Henríquez and L. Velasquez, Physicochemical characterization of PHBV nanoparticles functionalized with multiple bioactives designed to be theranostics for lung cancer, J. Cluster Sci., 2021, 32, 1563–1574 CrossRef CAS.
- H. P. Piepho, W. A. Malik, R. Bischof, A. El Hasan, C. Scheer, J. E. Sedlmeier, G. Petschenka and R. T. Voegele, Efficacy assessment in crop protection: A tutorial on the use of Abbott's formula, J. Plant Dis. Prot., 2024, 131, 2139–2160 CrossRef.
- Z. Shakoori, R. Pashaei-Asl, M. Pashaiasl, S. Davaran, H. Ghanbari, E. Ebrahimie and M. Rezayat, Biocompatibility study of P(N-isopropylacrylamide)-based nanocomposite and its cytotoxic effect on HeLa cells as a drug delivery system for cisplatin, J. Drug Delivery Sci. Technol., 2022, 71, 103254 CrossRef CAS.
- M. Gebreyes, K. Mekonnen, P. Thorne, M. Derseh, A. Adie, A. Mulema, S. A. Kemal, L. Tamene, T. Amede, A. Haileslassie, A. Gebrekirstos, W. T. Mupangwa, M. Ebrahim, T. Alene, A. Asfaw, W. Dubale and S. Yasabu, Overcoming constraints of scaling: Critical and empirical perspectives on agricultural innovation scaling, PLoS One, 2021, 16(5), e0251958 CrossRef CAS.
- A. Moreno and M. H. Sipponen, Overcoming challenges of lignin nanoparticles: Expanding opportunities for scalable and multifunctional nanomaterials, Acc. Chem. Res., 2024, 57(14), 1918–1930 CrossRef CAS PubMed.
- I. Pylypchuk, P. Linden, M. Lindstrom and O. Sevastyanova, New insight into the surface structure of lignin nanoparticles revealed by 1H liquid-state NMR spectroscopy, ACS Sustainable Chem. Eng., 2020, 8(36), 13805–13812 CrossRef CAS.
- L. Matsakas, A. Karnaouri, A. Cwirzen, U. Rova and P. Christakopoulos, Formation of lignin nanoparticles by combining organosolv pretreatment of birch biomass and homogenization processes, Molecules, 2018, 23(7), 1822 CrossRef PubMed.
- Z. Zhang, C. Belda Marín, M. Lefebvre, C. Lefebvre, V. Terrasson and E. Guénin, The preparation of stable spherical alkali lignin nanoparticles with great thermal stability and no cytotoxicity, Int. J. Biol. Macromol., 2024, 222(PtB), 1830–1839 Search PubMed.
- Z. Hadiana, M. Malekia, K. Abdib, F. Atyabic, A. Hammadia and R. Khaksar, Preparation and characterization of nanoparticle β-cyclodextrin: Geraniol inclusion complexes, Iran. J. Pharm. Res., 2018, 17(1), 39–51 Search PubMed.
- Z. Zhou, J. Wang, X. Xu, Z. Wang, L. Mao, S. Zhang, H. Zhang, Y. Zhang, Y. Li, Q. Yu and Z. Ning, Lignin-based nanoparticles for combination of tumor oxidative stress amplification and reactive oxygen species responsive drug release, Bioconjugate Chem., 2024, 35(8), 1207–1217 CrossRef CAS PubMed.
- Y. Yegin, K. L. Perez-Lewis, M. Zhang, M. Akbulut and T. M. Taylor, Development and characterization of geraniol-loaded polymeric nanoparticles with antimicrobial activity against foodborne bacterial pathogens, J. Agric. Food Chem., 2016, 170, 64–71 CAS.
- A. Matalanis, O. G. Jones and D. J. McClements, Structured biopolymer-based delivery systems for encapsulation, protection, and release of lipophilic compounds, Food Hydrocolloids, 2011, 25(8), 1865–1880 CrossRef CAS.
- F. Khodadadi, M. Nikzad and S. Hamedi, Lignin nanoparticles as a promising nanomaterial for encapsulation of Rose Damascena essential oil: Physicochemical, structural, antimicrobial, and in-vitro release properties, J. Agric. Food Chem., 2024, 687, 133580 CAS.
- P. M. Ngegb, G. Cui, M. Z. Khalid and G. Zhong, Use of botanical pesticides in agriculture as an alternative to synthetic pesticides, Agriculture, 2022, 12(5), 600 CrossRef.
- X. Wu, H. Lian and X. Li, An ultraviolet shielding material based on lignin nanoparticles engineered with deep eutectic solvents for long-term outdoor application, J. Cleaner Prod., 2023, 430, 139694 CrossRef CAS.
- E. V. R. Campos, P. L. F. Proença and J. L. Oliveira, Carvacrol and linalool co-loaded in β-cyclodextrin-grafted chitosan nanoparticles as sustainable biopesticide aiming pest control, Sci. Rep., 2018, 8, 7623 CrossRef PubMed.
- A. Ebadollahi, J. J. Sendi and A. Aliakbar, Efficacy of nanoencapsulated Thymus eriocalyc and Tymus kotschyanus essential oils by a mesoporus material MCM-41 against Tetranychus urticae (Acari: Tetranychidae), J. Econ. Entomol., 2017, 110(6), 2413–2420 CrossRef CAS PubMed.
- S. B. Adeyemi, A. M. Akere, J. I. Orege, O. Ejeromeghene, O. B. Orege and J. O. Akolade, Polymeric nanoparticles for enhanced delivery and improved bioactivity of essential oils, Heliyon, 2023, 9(6), e16543 CrossRef CAS PubMed.
- A. Romero-Montero, L. J. Melgoza-Ramírez, J. A. Ruíz-Aguirre, A. Chávez-Santoscoy, J. J. Magaña, H. Cortés, G. Leyva-Gómez and M. L. Del Prado-Audelo, Essential-oils-loaded biopolymeric nanoparticles as strategies for microbial and biofilm control: A current status, Int. J. Mol. Sci., 2023, 25(1), 82 CrossRef PubMed.
- K. Li, W. Zhong, P. Li, J. Ren, K. Jiang and W. Wu, Antibacterial mechanism of lignin and lignin-based antimicrobial materials in different fields, Int. J. Biol. Macromol., 2023, 252, 126281 CrossRef CAS PubMed.
- M. M. Hossain, I. M. Scott, B. D. McGarvey, L. Ferrante, F. Berruti and C. Briens, Insecticidal and anti-microbial activity of bio-oil derived from fast pyrolysis of lignin, cellulose, and hemicellulose, J. Pest Sci., 2015, 88, 171–179 Search PubMed.
- E. S. Garcia and P. Azambuja, Lignoids in insects: chemical probes for the study of ecdysis, excretion and Trypanosoma cruzi—triatomine interactions, Toxicon, 2004, 44(4), 431–440 CrossRef CAS PubMed.
- Y. Zhou, Y. Han, G. Li and F. Chu, Effects of Lignin-Based Hollow Nanoparticle Structure on the Loading and Release Behavior of Doxorubicin, Materials, 2019, 12(10), 1694 CrossRef CAS PubMed.
- P. Widsten, T. Tamminen and T. Liitiä, Natural Sunscreens Based on Nanoparticles of Modified Kraft Lignin (CatLignin), ACS Omega, 2020, 5(22), 13438–13446 CrossRef CAS PubMed.
- S. Manna, S. Roy, A. Dolai, A. Reddy, V. Perumal and A. Das, Current and Future Prospects of “All-Organic” Nanoinsecticides for Agricultural Insect Pest Management, Front. Nanotechnol., 2023, 4, 1082128 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5en00155b |
| This journal is © The Royal Society of Chemistry 2025 |