Improvement of tribological and anti-corrosive performance of titanium surfaces coated with dicationic imidazolium-based ionic liquids†
Izabelle M. Gindria,
Danyal A. Siddiquia,
Clarissa P. Frizzob,
Marcos A. P. Martinsb and
Danieli C. Rodrigues*a
aDepartment of Bioengineering, University of Texas at Dallas, Richardson, TX 75080, USA. E-mail: Danieli@utdallas.edu
bDepartment of Chemistry, Universidade Federal de Santa Maria, Santa Maria, Brazil-97105
First published on 15th August 2016
Abstract
In this work, dicationic imidazolium-based ionic liquids (ILs) with amino acid anionic moieties were employed as coatings for commercially pure titanium (Ti) surfaces. Coated and non-coated samples were tested with regard to their anti-corrosive and lubricant properties. Phosphate buffer saline (PBS), artificial saliva, and 50/50 PBS–saliva (v/v) were used as electrolytes during electrochemical testing and as control lubricants for Ti samples during tribological testing. Samples coated with ILs possessing longer alkyl chains in their spacer group and more hydrophobic anionic moieties, such as phenylalanine, demonstrated superior anti-corrosive and lubricant behavior. Protection against corrosion was reflected in lower corrosion current (Icorr) and corrosion rate values as well as independently measured higher polarization resistance values. Immersion medium was also observed to influence the corrosion behavior of samples. Control Ti exhibited increased susceptibility to corrosion in more acidic environments (artificial saliva) while the opposite trend was observed for IL-coated Ti samples. Enhanced lubrication was verified by a significant reduction in the coefficient of friction and total wear volume loss of IL-coated samples in comparison to control Ti.
1. Introduction
Titanium has been the most popular metal for the design of dental implants due to its mechanical strength and naturally forming oxide layer, which confers biocompatibility and corrosion resistance to this material.1 However, in the oral environment, the titanium surface is subject to several potentially damaging factors, such as wear triggered by friction during insertion, stress from mastication, and pH imbalance due to saliva and bacterial biofilm.2 As the implant is inserted, it scrapes against the surface of bone, which can scratch and damage the surface of the metal, thereby compromising the passivity and integrity of the oxide layer.3 During their lifetime in service, dental implants also experience cyclical stresses due to mastication, which have been reported to be in the range of 370 MPa (Hertzian contact pressure) at a testing frequency of 1.2 Hz.4 Also, low pH due to bacterial biofilm adhesion has been associated with disrupting oxide layer formation and exacerbating the corrosion process.5Efforts to develop coatings for commercially pure titanium (cpTi) used in the design of dental implants have focused on increasing titanium bioactivity to improve osseointegration or to provide anti-microbial activity to prevent biofilm formation.6,7 Although corrosion and wear have been considered as etiological factors behind dental implant failures,8,9 coatings designed to improve lubricant and anti-corrosive behavior of these devices have yet to be developed. Furthermore, current surface treatment techniques for implants focus on improving corrosion behavior by either increasing the thickness and uniformity of the oxide layer or by deposition of solid coatings.10 However, these treatments can be considered insufficient as wear mechanisms can continuously damage the metallic surface in vivo, resulting in mechanical failure of coatings such as delamination and formation of crevices between the titanium surface and the solid coating which enhance corrosion processes.11
Ionic liquids (ILs) comprise a class of materials with a wide liquid range (−80 to 100 °C), high conductivity and thermal stability.12,13 The physical and chemical properties of these compounds can be controlled by selecting cationic and anionic moieties with specific structural groups.12,14,15 Due to its versatility, these compounds have been used for a wide range of potential applications such as active pharmaceutical ingredients in drugs,16,17 electrolytes in batteries18 and additives in commercial lubricants.19–21 The anti-corrosive and lubricant properties of imidazolium-based ILs have been investigated on metal surfaces such as titanium, copper and mild steel.22–26 The anionic moiety is observed to play an important role in mitigating corrosion due to the predominant adsorption of these species onto metal surfaces. Studies demonstrate that more hydrophobic anions such as bis(trifluoromethylsulfonyl)imide and sulfates tend to improve the corrosion resistance of metals, whereas small species such as chloride and bromide actually favor corrosion.27 This trend is observed to a greater extent in more acidic media, as reported by Zhang et al.27 In addition to these studies, both monocationic and dicationic imidazolium-based ILs have been used as a lubricant for titanium–steel surfaces to alleviate corrosion and wear caused by sliding contact between the two metals.19,21 The observed improvement in lubrication performance is associated with longer alkyl chains in the side chains of monocationic ILs and in the spacer groups of dicationic ILs, which confer a greater ability of these ILs to form a stable surface layer on titanium.21 Although many studies have been conducted to explore the anti-corrosive and lubricant properties of ILs,19,21,28 there have been no reports about applying these compounds as coatings on biomaterials to improve their surface performance. We propose to investigate the performance of dicationic imidazolium-based ILs (Fig. 1) that have previously shown desirable characteristics such as low toxicity, antimicrobial activity and affinity to titanium surfaces.29–32 These compounds are composed of dicationic imidazolium-based moieties associated with amino acids as the anionic moieties (Fig. 1).29 Previous studies demonstrated that select IL formulations form stable films on titanium surfaces with high adhesion strength and antimicrobial action against bacterial species of relevance in the oral environment, while conditions for integration with soft (supra gingival epithelial cells) and hard (osteoblast-like cells) tissues were maintained.29,31 Based on this previous work, we propose to investigate the performance of ILs as coatings for Ti surfaces in order to functionalize the surface for better corrosion and wear resistance. Combining both tribological and electrochemical testing of non-coated (control) and IL-coated Ti, this study explored the potential reduction in the corrosion rate and wear scar formation by protecting the naturally forming oxide layer with select IL coatings.
2. Experimental
2.1 Materials
1,8-Dibromoctane, L-phenylalanine and L-leucine (MP Biomedicals, Santa Ana, CA, USA); 1,10-dibromodecane, L-methionine, AMBERLITE IRN-78 OH and ethyl ether (Acros Organics, NJ, USA); 1-methylimidazole, acetonitrile, and ethanol (Fisher Scientific, Waltham, MA, USA). 1,8-Bis(3-methylimidazolium-1-yl)octane dihydroxide and 1,10-bis(3-methylimidazolium-1-yl)decane dihydroxide were synthesized from 1,8-bis(3-methylimidazolium-1-yl) octane bromide and 1,10-bis(3-methylimidazolium-1-yl) decane bromide ethanolic solutions, respectively, through reaction employing anion exchange resin. ILs (Fig. 1) were prepared by reacting 10 mM of 1,8-bis(3-methylimidazolium-1-yl)octane dihydroxide or 1,10-bis(3-methylimidazolium-1-yl)decane dihydroxide with 20 mM of respective amino acid. ILs were characterized using nuclear magnetic resonance spectroscopy, mass spectroscopy and differential scanning calorimetry, and the data were in accordance with the literature.12,292.2 Sample preparation
Flat disks (9.5 mm × 4 mm) and rods (9.5 mm × 10 mm) of cold-worked, grade 2 commercially pure titanium (cpTi, McMaster-Carr) were prepared by polishing using silicon carbide paper up to 1200 grit and subsequently fine-polished with polycrystalline diamond (1.0 μm) and nanometer alumina (0.05 μm). All polishing of the samples was performed using a polisher (NANO 1000T, Pace Technologies) with an automated polishing head (FEMTO 1100, Pace Technologies). After polishing, the Ti disks were sequentially cleaned by ultrasonication in acetone, deionized water, and ethanol and left to dry in an oven for 24 hours at 60 °C. Afterward, a thin film of IL was coated onto each Ti disk using a drop casting technique where 5 μL of ethanol solutions containing dissolved IL were pipetted onto a disk and left to dry for 15 minutes in an oven at 60 °C. This step was repeated 10 times with a total of 2 μmol of IL being deposited on each Ti disk. Control and IL-coated Ti disks were left to dry in an oven for 48 hours at 60 °C before tribological testing.2.3 Electrochemical experiments
Prior to electrochemical testing, rod-shaped Ti specimens were prepared by clipping them to an alligator clip and insulating the electrical connection with electrical tape. Afterward, two layers of an insulation coating (Miccrostop, Tolber Chemical Division) were applied over the edges and lateral surface area of the Ti rods and electrical tape in order to only expose the polished specimen surface area to the electrolyte. Ti rods coated with the insulation coating were left to dry in an oven for 24 hours at 60 °C before coating with IL according to the procedure described in Section 2.2.Electrochemical testing of prepared Ti rods were performed using a potentiostat (Interface 1000, Gamry Instruments) and a standard three-electrode cell per general guidelines specified in ASTM F2129 “Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices”33 with the exposed specimen surface area acting as the working electrode. A saturated calomel electrode (SCE) was used as the reference electrode, and a graphite rod was used as the counter electrode. All electrodes were immersed in 5 mL of the electrolyte solution – PBS, artificial saliva, or 50/50 PBS–saliva (v/v) – maintained at 37 °C throughout testing. The open-circuit potential (OCP) of samples was monitored for 1 hour to ensure that the system reached electrochemical equilibrium. Per ASTM G59 “Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements”,34 linear polarization resistance of the specimens was acquired by measuring the current response within ±20 mV of the final OCP value at a scan rate of 1 mV s−1. Finally, Tafel analyses of the specimens were conducted by polarizing the specimen from the recorded OCP value to 250 mV above OCP at a scan rate of 1 mV s−1 in the anodic direction. The corrosion potential (Ecorr), slope of the linear anodic Tafel segment (βa), and corrosion current (Icorr) at equilibrium were then extracted from the anodic Tafel plot. Per ASTM G102 “Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements”,35 the corrosion rate (CR) was calculated from the corrosion current based on Faraday's law using eqn (1):
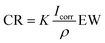 | (1) |
 | (2) |
2.4 Tribological testing
Wear testing was performed in a continuous motion mode using a modified pin-on-disk apparatus mounted on a hybrid rheometer (DHR-3, TA Instruments) based on guidelines specified in ASTM G99 “Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus”36 and ASTM G133 “Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear”.37 Prior to testing, a cold worked, grade 5 titanium alloy (Ti6Al4V) ball (12.7 mm) was held fixed in a ball specimen holder and cleaned with acetone. Specimens were mounted such that the radius of the resulting circular wear scar on each Ti disk was maintained at 1.25 mm. Axial loads of 5 or 10 N were applied onto the Ti disks corresponding to maximum elastic contact stresses of 455 and 573 MPa, respectively. The relative sliding speed of the contact point between Ti6Al4V ball and Ti disk was held constant at 0.05 m s−1 over a sliding distance of 50 m under ambient temperature and air conditions. Axial and frictional forces were measured at a sampling rate of 1 Hz and used to calculate the coefficient of friction. The reported coefficient of friction value was obtained by smoothing the data and averaging the individual measurements taken during testing when it was observed that the friction coefficient value approached some stable value. In situations where “spikes” in the coefficient of friction values were observed or the IL coating was exhausted, these measurements were not incorporated into the calculation of the average coefficient of friction value but were instead documented as specified in ASTM G115 “Standard Guide for Measuring and Reporting Friction Coefficients”.38After wear testing, the Ti disk and Ti6Al4V ball were cleaned with acetone to remove all wear debris and remaining IL on the disk surface in order to characterize the wear scar generated during testing. Linear dimensions of the circular wear scar on each Ti disk were measured using an optical microscope (Digital Microscope VHX-5000, Keyence). The wear track width was measured across six different locations on each wear scar in order to obtain an average wear track width. The total volume loss due to wearing was calculated using eqn (3)–(5) which is applied for spherically shaped pins and disks sliding against each other under elastic deformation conditions:
 | (3) |
 | (4) |
 | (5) |
2.5 Scanning electron microscopy (SEM)
The morphology (secondary electron image) of Ti surfaces for each tribological testing condition was evaluated using a scanning electron microscope (EVO SEM LS 15, Zeis) after tribological testing. The acceleration voltage used was 20 kV.3. Results and discussion
Measurements of key parameters obtained from electrochemical experiments are summarized in Fig. 2. The OCP, linear polarization and anodic Tafel plots of control and IL-coated Ti samples in PBS, PBS–saliva and saliva are shown in Fig. S1–S9, S10–S18 and S19–S27, respectively (ESI†). Both control and IL-coated Ti exhibited monotonic shifts to more noble OCPs over time, which is indicative of the formation of passive stable films on all samples.39 It was observed that IL3 was initially released slower into PBS in comparison to the other IL compositions, as can be seen by the less dramatic increase in OCP. In saliva and PBS–saliva, however, IL1, IL2 and IL3 exhibited smaller changes in OCP values over time relative to the control, demonstrating that these IL compositions have stronger affinity for Ti in saliva. These results are in agreement with our previous work in which it was observed that IL3 strongly interacted with titanium surfaces, which resulted in the maintenance of its antimicrobial activity against bacterial cells for seven days.30Excluding saliva, Ecorr values were similar for control and IL-coated samples in PBS and PBS–saliva. In saliva, control Ti had significantly higher Ecorr values (Fig. 2a) in comparison to PBS and PBS–saliva, and the Ecorr values obtained were similar to those seen in a previous study.40 Generally, more noble corrosion potentials are associated with greater thermodynamic stability and compactness of the outermost film present on the sample surface for a given set of electrochemical conditions including temperature, surface treatment, and solution pH.41 In terms of composition, the main difference between saliva and PBS is the presence of urea in the former media. Although a more stable film forms on control Ti in the presence of this organic compound in saliva (higher observed Ecorr values), it actually increased the corrosion rate as shown in Fig. 2b and in a previous study.42 This observed increase in the corrosion rate of Ti control in artificial saliva relative to PBS can also be attributed to the lower pH (5.5) of saliva in comparison to PBS (7.4) as immersion in more acidic environments is associated with greater dissolution of the TiO2 layer and hence lower corrosion resistance. Among IL-coated Ti samples, IL3-coated samples consistently had the highest Ecorr values across all media tested, demonstrating that it formed the most stable IL film. In PBS and PBS-saliva, IL3-coated samples had Ecorr values even more noble than that of control Ti, suggesting that the IL film formed was more stable than the native oxide layer alone. In saliva, the lower Ecorr value of the IL3-coated samples in comparison to control Ti can be attributed to the presence of a mixed film, possibly due to IL3 molecules competing for adsorption sites with urea molecules.
Anodic Tafel slopes are demonstrated in Fig. 2c. The relatively small variance in these values demonstrated a high level of reproducibility in the results since this parameter is very specific to the sample and solution being tested. The significantly higher slope values of IL3-coated Ti in comparison to control Ti further corroborate IL film stability in all media evaluated (p < 0.05). In a recent study, the interaction between IL1, IL2 and IL3 with TiO2 surfaces has been investigated by our research group.31 Using X-ray photoelectron spectroscopy, it was demonstrated that the interaction between IL3 and Ti was the strongest among the ILs evaluated.31 This result corroborates with the electrochemical parameters obtained in the present work, where IL3 was observed to interact and promote greater stabilization of the oxide layer.31 The stronger interaction is explained based on the structural features of IL3, which has phenylalanine as anionic moiety. The phenyl group of this anion has been shown to improve the IL organization on titanium surface due to the large contact area, which favors close packing of IL molecules on metal surfaces.31 Furthermore, the slightly better performance of IL2 in comparison to IL1 also corroborates with previous XPS results, in which the former was observed to have a higher interaction with titanium.31
Corrosion current density (Fig. 2d), Icorr, and corrosion rate values (Fig. 2b), which are directly proportional, were significantly lower for all IL-coated samples immersed in PBS–saliva and saliva but were only significantly lower (p < 0.05) for IL3-coated Ti immersed in PBS. These results indicate that ILs films worked in protecting the titanium surface and were able to improve its corrosion resistance. Polarization resistance, which is inversely related to corrosion rate, was measured independently through linear polarization resistance experiments and is illustrated in Fig. 2e. The values obtained for IL-coated samples were, in general, superior to those observed for control Ti. IL2-, IL3-, and IL4-coated Ti had significantly higher values in PBS (p < 0.05) while IL2- and IL3-coated Ti also demonstrated significantly improved performance in PBS–saliva and saliva (p < 0.05). Considering the results obtained for control and IL-coated samples in all tested media, the order of increasing corrosion protection among different IL compositions is given as follows: IL1 < IL4 < IL2 < IL3. The lower corrosion resistance of IL1-coated sample, which has a shorter alkyl chain, corroborates with results in the literature where it has been demonstrated that a decrease in the length of the alkyl chain of monocationic imidazolium-based ILs resulted in a decreased corrosion protection of mild steel surfaces.43 The anionic moiety also influences the corrosion protection of Ti. Possessing phenylalanine as its anion, which is the most hydrophobic amino acid among the selected anionic moieties, IL-3 had the best performance as an anti-corrosive coating. This trend has been published in studies employing ILs as corrosion protectors for mild steel.27,43,44 Anionic moieties, such as dicyanamide- and sulfate-based anions, were observed to enhance the corrosion protection of metallic surfaces, while anions containing Cl− resulted in increased corrosion susceptibility.27,44 Therefore, the better performance of IL3-coated samples can be attributed to the molecular structure of this IL. In our previous work, it was demonstrated that this compound forms strongly-bonded and well-ordered films on titanium surfaces.31 As previously stated, these findings are hypothesized to be related to the emergent anti-corrosive properties of IL3-coated Ti. Due to its higher affinity and better organization on the surface, this IL formed a more protective barrier, which avoided the penetration of chemical species that are known to cause corrosion (e.g. Cl−).
The slight better performance of IL-coated samples in more acidic environments (PBS–saliva and saliva) also follows a trend already reported in the literature about the adsorption of amino acids on titanium surfaces.45 It has been shown that more acidic environments favor the adsorption of anionic moieties on titanium surfaces, inhibiting the release of these compounds into the surrounding medium30,45 which contributes to the maintenance of the protective layer formed by IL coatings in solution. This trend is also demonstrated by the higher corrosion inhibition efficiency values (Fig. 2f) observed in PBS–saliva and saliva for all IL-coated samples.
In regards to tribological behavior, IL1-, IL2-, and IL3-coated Ti samples exhibited significantly lower coefficient of friction values (p < 0.05) in comparison to control Ti samples lubricated with PBS, PBS–saliva and saliva as shown in Fig. 3a. However, for IL1-coated samples, the coating of one specimen under 5 N axial loading and another under 10 N axial loading was exhausted during testing, which resulted in coefficient of friction values increasing to values exhibited by control specimens and hence much greater average volume losses as shown in Fig. 3b. In contrast, both IL2 and IL3 exhibited relatively low and constant coefficient of friction values throughout wear testing. On the other hand, IL4 coatings were rapidly exhausted during testing, resulting in coefficient of friction values similar to those exhibited by control specimens (Fig. 3a). In general, lower coefficient of friction values corresponded to smaller wear track widths, and hence smaller wear volume losses on both the Ti samples and Ti6Al4V balls sliding against them. Moreover, significant reductions in the friction coefficient due to the presence of IL1, IL2 and IL3 coatings were maintained and remained stable even after testing separate samples at the higher axial load of 10 N.
 | ||
| Fig. 3 (a) Coefficient of friction (μ) and (b) total volume loss (mm3) of control Ti and IL-coated samples. *Significantly (p < 0.05) different from all other surfaces in the same comparison. | ||
Thin IL films significantly improved the wear performance of Ti6Al4V balls sliding continuously against Ti samples under axial loading. Axial loading conditions (approximately 100 s of MPa) were chosen as worst-case scenarios and were much higher (2 orders of magnitude) than conditions experienced when torque is generated during insertion of a dental implant or when pressure is applied on a dental implant during mastication (approximately 1 s of MPa).46 Despite the extreme loading conditions tested for Ti, most IL-coated Ti samples significantly reduced the friction force under wear testing and maintained low coefficient of friction values, with the exception of IL4. In particular, IL2 and IL3 demonstrated the best performance as they exhibited the most stable friction coefficient measurements throughout testing across all samples at both 5 and 10 N axial loads as shown in Fig. 3 and 4. In contrast, for two samples (one at 5 N and one at 10 N), exhaustion of IL1 coating was observed. The better lubrication performance of IL2 and IL3 (n = 10) in comparison to IL1 (n = 8) is in accordance with trends seen in other studies in which increasing alkyl chain length leads to greater improvement in tribological performance.
 | ||
| Fig. 4 Coefficient of friction of control Ti and IL-coated samples under load of (a) 5 N and (b) 10 N. | ||
In summary, factors influencing coefficient of friction measurements were the relative sliding speed of the contact point between the two surfaces in motion, the applied axial load, and the thickness of the lubricating film. Although all the tribological testing parameters were held constant for a given test condition and the amount of IL-coated on each Ti disk was held constant, IL thin films deposited on Ti samples visibly had variable thickness across the sample surface upon visual inspection and imaging using optical microscopy, with the exception of IL3. As a result, there were local differences in the amount of IL present on a given region of tested Ti samples. Despite this lack of homogeneity, IL2 and IL3 coatings were still able to maintain low coefficient of friction values under extreme loading conditions. This behavior corresponds to the lubrication regime seem in hydrodynamic lubrication where sufficient IL coating must be present on a surface to separate two sliding surfaces from direct contact with each other. In contrast, the heterogeneity of IL1 and IL4 coatings could have resulted in local regions were IL film thickness was too thin. As a result, the lubrication regimes of these wearing samples would rapidly transition to mixed lubrication and eventual boundary lubrication conditions where direct contact between the Ti disk and Ti6Al4V balls occurs.
SEM images reflected the lubrication ability of tested compositions as shown in Fig. 5. While large wear scars were observed for PBS, PBS–saliva, saliva and IL4-coated samples, thin circular and even just half-circular wear scars were observed for IL1-, IL2- and IL3-coated Ti samples. These images also confirmed the results obtained for wear volume losses in which samples with smaller wear scars (IL2-coated Ti and IL3-coated Ti) were observed to have less wear generation (Fig. 3b) than samples with larger wear scars (PBS, PBS–saliva, saliva and IL4-coated Ti).
 | ||
| Fig. 5 SEM images of Ti samples lubricated with PBS, PBS–saliva and saliva, as well as IL-coated samples, after tribological experiments under axial loads of 5 N and 10 N. | ||
4. Conclusion
ILs were employed as coatings on Ti samples and tested for anti-corrosive and lubricant activities. Compounds having longer alkyl chains and more hydrophobic anions emerged as coatings with better anti-corrosive and lubricant properties. The better arrangement of IL molecules at the titanium surface is hypothesized to result in improved performance. After electrochemical testing, IL2 (1,10-bis(3-methylimidazolium-1-yl)decane dimethionine) and IL3 (1,10-bis(3-methylimidazolium-1-yl)decane diphenylalanine) coatings were observed to have the best anti-corrosive properties, in which the corrosion rates were lower than values obtained for control Ti in all media evaluated. Moreover, these compositions also presented lower coefficient of friction and wear volume loss, which proves that even under extreme loading conditions, IL coatings were able to protect the surface against physical damage and wear generation. Overall, our findings support the idea that multi-functional ILs can be designed as novel, anti-corrosive lubricating coatings for titanium surfaces and other metallic biomaterials used in medical devices and implants. Future studies will explore the influence of other structural changes, such as heteroatoms in the side chain and different cationic heads, in the emergent anti-corrosive and lubricant properties of ILs.Acknowledgements
The authors acknowledge the University of Texas at Dallas (UTD) for providing financial support for this study (startup funds DCR), fellowships from Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) (IMG) and Conselho Nacional de Desenvolvimento Científıco e Tecnológico (CNPq) (Universal/Proc. 474895/2013-0 and Universal/Proc. 475556/2012-7 (MAP and CPF)), the Rio Grande do Sul Foundation for Research Support (FAPERGS) (Proc. 2262-2551/14-1 and 2290-2551/14-1 (MAP and CPF)).References
- S. Anil, P. S. Anand, H. Alghamdi and J. A. Jansen, in Implant Dentistry-A Rapidly Evolving Practice, ed. I. Turkyilmaz, Rjeka, 1st edn, 2011, pp. 83–108 Search PubMed
.
- D. C. Rodrigues, P. Valderrama, T. Wilson, K. Palmer, A. Thomas, S. Sridhar, A. Adapalli, M. Burbano and C. Wadhwani, Materials, 2013, 6, 5258–5274 CrossRef
.
- K. W. Frisken, G. W. Dandie, S. Lugowski and G. Jordan, Aust. Dent. J., 2002, 47, 214–217 CrossRef CAS PubMed
.
- M. T. Mathew, S. Abbey, N. J. Hallab, D. J. Hall, C. Sukotjo and M. A. Wimmer, J. Biomed. Mater. Res., Part B, 2012, 100, 1662–1671 CrossRef PubMed
.
- S. Sridhar, T. G. Wilson, K. L. Palmer, P. Valderrama, M. T. Mathew, S. Prasad, M. Jacobs, I. M. Gindri and D. C. Rodrigues, Clin. Implant. Dent. Relat. Res., 2015, 17, e562–e575 CrossRef PubMed
.
- H. S. Alghamdi, R. Bosco, J. J. J. P. van den Beucken, X. F. Walboomers and J. A. Jansen, Biomaterials, 2013, 34, 3747–3757 CrossRef CAS PubMed
.
- T. E. Swanson, X. Cheng and C. Friedrich, J. Biomed. Mater. Res., Part A, 2011, 97, 167–176 CrossRef CAS PubMed
.
- D. G. Olmedo, D. R. Tasat, G. Duffó, M. B. Guglielmotti and R. L. Cabrini, Acta Odontol. Latinoam., 2009, 22, 3–9 Search PubMed
.
- A. C. Vieira, A. R. Ribeiro, L. A. Rocha and J. P. Celis, Wear, 2006, 261, 994–1001 CrossRef CAS
.
- X. Liu, P. Chu and C. Ding, Mater. Sci. Eng., R, 2004, 47, 49–121 CrossRef
.
- A. Zielinski and S. Sobieszczyk, Corros. Rev., 2007, 26, 1–22 CrossRef
.
- H. Shirota, T. Mandai, H. Fukazawa and T. Kato, J. Chem. Eng. Data, 2011, 56, 2453–2459 CrossRef CAS
.
- M. A. P. Martins, C. P. Frizzo, A. Z. Tier, D. N. Moreira, N. Zanatta and H. G. Bonacorso, Chem. Rev., 2008, 108, 2015–2050 CrossRef CAS PubMed
.
- C. P. Frizzo, I. M. Gindri, C. R. Bender, A. Z. Tier, M. A. Villetti, D. C. Rodrigues, G. Machado and M. A. P. Martins, Colloids Surf., A, 2015, 468, 285–294 CrossRef CAS
.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed. Engl., 2000, 39, 3772–3789 CrossRef CAS
.
- C. P. Frizzo, I. M. Gindri, A. Z. Tier, L. Buriol, D. N. Moreira and M. A. P. Martins, in Ionic Liquids-New Aspects for the Future, ed. J. Kadokawa, Intech, Rijeka, Croatia, 1st edn, 2013, pp. 557–579 Search PubMed
.
- H. D. Williams, Y. Sahbaz, L. Ford, T.-H. Nguyen, P. J. Scammells and C. J. H. Porter, Chem. Commun., 2014, 50, 1688 RSC
.
- K. Kwak, S. S. Kumar, K. Pyo and D. Lee, ACS Nano, 2014, 8, 671–679 CrossRef CAS PubMed
.
- M.-D. Bermúdez and A. E. Jiménez, Tribol. Lett., 2009, 33, 111–126 CrossRef
.
- R. Gusain, P. Gupta, S. Saran and O. P. Khatri, ACS Appl. Mater. Interfaces, 2014, 6, 15318–15328 CAS
.
- F. Zhou, Y. Liang and W. Liu, Chem. Soc. Rev., 2009, 38, 2590–2599 RSC
.
- E. Kowsari, M. Payami, R. Amini, B. Ramezanzadeh and M. Javanbakht, Appl. Surf. Sci., 2014, 289, 478–486 CrossRef CAS
.
- M. Scendo and J. Uznanska, Int. J. Corros., 2011, 2011, 718626 Search PubMed
.
- N. V. Likhanova, M. A. Dominguez-Aguilar, O. Olivares-Xometl, N. Nava-Entzana, E. Arce and H. Dorantes, Corros. Sci., 2010, 52, 2088–2097 CrossRef CAS
.
- A. L. Chong, J. I. Mardel, D. R. MacFarlane, M. Forsyth and A. E. Somers, ACS Sustainable Chem. Eng., 2016, 4, 1746–1755 CrossRef CAS
.
- R. Gusain, P. Gupta, S. Saran and O. P. Khatri, ACS Appl. Mater. Interfaces, 2014, 6, 15318–15328 CAS
.
- Q. B. Zhang and Y. X. Hua, Electrochim. Acta, 2009, 54, 1881–1887 CrossRef CAS
.
- M.-D. Bermúdez, A.-E. Jiménez, J. Sanes and F.-J. Carrión, Molecules, 2009, 14, 2888–2908 CrossRef PubMed
.
- I. M. Gindri, D. A. Siddiqui, P. Bhardwaj, L. C. Rodriguez, K. Palmer, C. P. Frizzo, M. A. P. Martins and D. Rodrigues, RSC Adv., 2014, 4, 62594–62602 RSC
.
- I. M. Gindri, K. Palmer, D. A. Siddiqui, S. Aghyarian, C. P. Frizzo, M. A. P. Martins and D. Rodrigues, RSC Adv., 2016, 6, 36475–36483 RSC
.
- I. M. Gindri, D. Siddiqui, C. P. Frizzo, M. A. P. Martins and C. Rodrigues, ACS Appl. Mater. Interfaces, 2015, 7, 27421–27431 CAS
.
- I. M. Gindri, C. P. Frizzo, C. R. Bender, A. Z. Tier, M. A. P. Martins, M. A. Villetti, G. Machado, L. C. Rodriguez and D. C. Rodrigues, ACS Appl. Mater. Interfaces, 2014, 6, 11536–11543 CAS
.
- American Society for Testing and Materials, ASTM F2129 “Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices”, 2008, pp. 1–9 Search PubMed
.
- American Society for Testing and Materials, ASTM G59 “Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements”, 2014, pp. 1–4 Search PubMed
.
- American Society for Testing and Materials, ASTM G102 “Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements”, 1994, pp. 416–422 Search PubMed
.
- American Society for Testing and Materials, ASTM G99 “Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus”, 2011, pp. 1–5 Search PubMed
.
- American Society for Testing and Materials, ASTM G133 “Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear”, 2010, pp. 1–9 Search PubMed
.
- American Society for Testing and Materials, ASTM G115 “Standard Guide for Measuring and Reporting Friction Coefficients”, 2013, pp. 1–13 Search PubMed
.
- R. Bhola, S. M. Bhola, B. Mishra and D. L. Olson, Res. Lett. Phys. Chem., 2009, 2009, 4 Search PubMed
.
- J. C. M. Souza, M. Henriques, R. Oliveira, W. Teughels, J.-P. Celis and L. A. Rocha, Biofouling, 2010, 26, 471–478 CrossRef CAS PubMed
.
- X. Cheng and S. G. Roscoe, Biomaterials, 2005, 26, 7350–7356 CrossRef CAS PubMed
.
- H. Bilhan, T. Bilgin, A. F. Cakir, B. Yuksel and J. A. Von Fraunhofer, J. Biomater. Appl., 2007, 22, 197–221 CrossRef CAS PubMed
.
- Y. Sasikumar, A. S. Adekunle, L. O. Olasunkanmi, I. Bahadur, R. Baskar, M. M. Kabanda, I. B. Obot and E. E. Ebenso, J. Mol. Liq., 2015, 211, 105–118 CrossRef CAS
.
- Y.-C. Wang, T.-C. Lee, J.-Y. Lin, J.-K. Chang and C.-M. Tseng, Corros. Sci., 2014, 78, 81–88 CrossRef CAS
.
- M. Schmidt, Arch. Orthop. Unfall-Chir., 2001, 121, 403–410 CAS
.
- W. Winter, D. Klein and M. Karl, J. Med. Eng., 2013, 2013, 265412 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Open circuit potential, linear polarization resistance and anodic Tafel plots. See DOI: 10.1039/c6ra13961b |
| This journal is © The Royal Society of Chemistry 2016 |


