Controllable synthesis of spherical cerium oxide particles
Hepeng Zhang*,
Wenbin Wang,
Baoliang Zhang,
Huan Li and
Qiuyu Zhang
Key Laboratory of Applied Physics and Chemistry in Space of Ministry of Education, School of Science, Northwestern Polytechnical University, Xi'an 710129, China. E-mail: zhanghepeng@nwpu.edu.cn
First published on 21st March 2016
Abstract
Spherical CeO2 submicron particles were synthesized by a facile PVP-assisted hydrothermal method. By the analysis of XRD, FESEM, TEM and selected area electron diffraction (SAED), we confirmed that the as-obtained spherical CeO2 particles were formed by the oriented aggregation of fluorite CeO2 nanocrystals. The formation mechanism was discussed by varying synthetic parameters such as reaction temperature, and the concentration of cerium salt. In our synthetic process, Ce(NO3)3·6H2O was first hydrolyzed to rod-like organic cerium salt by the assistance of PVP, then the organic cerium salt was decomposed into CeO2 nanocrystals. Finally, spherical CeO2 particles were formed by the oriented aggregation of these CeO2 nanocrystals through the interaction between the same crystal planes of different nanocrystals and the assistance of PVP.
1. Introduction
Cerium oxide (CeO2) has brilliant properties such as large oxygen storage capacity and quick mutation between the oxidation state of cerium between Ce(III) and Ce(IV). The unique properties of nanoscale ceria, such as higher specific surface area and more exposed atoms, make it an ideal candidate in the field of catalysts,1–5 water gas shift,6–8 oxygen sensing materials9 and photovoltaic materials.10In the past decades, a number of methods have been applied to synthesize CeO2 particles, such as hydrothermal methods11–14 spray pyrolysis methods15,16 and microemulsion methods.17,18 Various morphologies of CeO2 particles have also been obtained, including nanorods, nanotubes, nanowires, microplates and other morphological structures.19–29 In particularly, spherical CeO2, due to its excellent size dependence effects, has been widely employed in solid oxide fuel cell (SOFC) and ultra-smooth surface polishing liquid. However, the size-controllable hydrothermal synthesis of spherical CeO2 particles has rarely been reported. Zhou et al.30 have successfully fabricated size-controllable spherical CeO2 particles in the diameter range of 100–800 nm through a PVP-assisted hydrothermal method, and have studied their electrochemical properties. Wei et al.31 have prepared spherical CeO2 particles, range from 200 nm to 300 nm, utilizing PVP, urea and hydrogen peroxide as additives through hydrothermal method. Although spherical CeO2 particles have been successfully prepared, the role of PVP and the formation mechanism are not very clear.
In this paper, we further studied the synthesis of spherical CeO2 particles through a facile PVP-assisted hydrothermal method. After varying synthetic parameters, the formation process of rod-like organic cerium salt decomposing into CeO2 nanocrystals was proposed. And based on this, we deduced the role of PVP and the formation mechanism of spherical CeO2 particles.
2. Experiment
Cerium nitrate (Ce(NO3)3·6H2O), polyvinylpyrrolidone were purchased from Sinopharm Chemical Reagent Co., Ltd. All the chemical reagents were analytically pure. Ultrapure water was produced by an apparatus for pharmaceutical purified water (Aquapro Co., Ltd.).A typical synthesis of spherical CeO2 particles is introduced as below: 2 mmol Ce(NO3)3·6H2O and 4 mmol (repeating units) PVP were dissolved into 35 mL of distilled water and stirred for 15 min with a magnetic stirrer to get good homogeneity. The mixed solution was next transformed into a 50 mL Teflon-lined stainless steel autoclave and treated at 200 °C for 150 min under the autogenous pressure. After cooling to room temperature naturally, the light-yellow products were harvested by centrifuging at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min, washing with distilled water for 3 times. Some of the powders were dispersed in water for further characterization, and the rest were treated by freezing vacuum drying for 12 h.
000 rpm for 10 min, washing with distilled water for 3 times. Some of the powders were dispersed in water for further characterization, and the rest were treated by freezing vacuum drying for 12 h.
X-ray diffraction patterns were recorded on shimadzu XRD-600 X-ray diffractometer, and then the size of the CeO2 crystals was calculated by Scherer formula (D = Kγ/B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ). An X-ray photoelectron spectroscopy (XPS) experiment was carried on a Kratos Axis Ultra DLD system at room temperature. The binding energy were normalized to signal for adventitious carbon at 284.8 eV. The particle size was determined with a laser diffraction particle sizer analyzer of LS13320 (Beckman Coulter, Inc.). The morphology of CeO2 was examined by field emission scanning electron microscope (FESEM) using JSM-6700 and transmission electron microscopy (TEM) using Tecnai G2 F20 S-TWIN. The percentage of atom were obtained from Energy Dispersive Spectrometer using Oxford INCA.
θ). An X-ray photoelectron spectroscopy (XPS) experiment was carried on a Kratos Axis Ultra DLD system at room temperature. The binding energy were normalized to signal for adventitious carbon at 284.8 eV. The particle size was determined with a laser diffraction particle sizer analyzer of LS13320 (Beckman Coulter, Inc.). The morphology of CeO2 was examined by field emission scanning electron microscope (FESEM) using JSM-6700 and transmission electron microscopy (TEM) using Tecnai G2 F20 S-TWIN. The percentage of atom were obtained from Energy Dispersive Spectrometer using Oxford INCA.
3. Results and discussion
3.1 Sample characterization
Fig. 1 shows the FESEM images of the as-prepared spherical CeO2, Fig. 1A reveals that the morphology of as-prepared CeO2 is mostly spherical structure with a uniform magnification FESEM image of the sample is shown in Fig. 1B. It can be seen that the particles sizes are quite uniform. The average diameter is about 442 nm and the standard deviation is 92 nm. High spherical structure, and the surface is not smooth. In light of the TEM image (Fig. 1C), it can be concluded that the as-prepared spherical CeO2 is constituted by the aggregation of some small CeO2 crystals. Selected area electron diffraction (SAED) patterns are showed in the inset 1 of Fig. 1C, indicating that the aggregation of small CeO2 crystals is not irregular but oriented and the crystal plane belongs to (200) with a space of 2.72 Å, which agrees well with the inset 2 of Fig. 1C.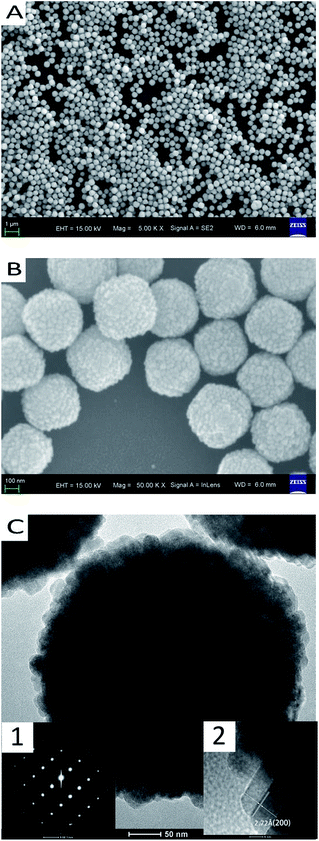 | ||
| Fig. 1 FESEM and TEM images of spherical CeO2 particles: (A) 5k magnification FESEM image; (B) 50k magnification FESEM image; (C) TEM image: 1 SAED pattern, 2 high resolution TEM image. | ||
Fig. 2 shows the X-ray diffraction (XRD) pattern of the as-prepared spherical CeO2 particles. From the figure, the XRD pattern can be indexed to pure face-centered cubic (fcc) fluorite CeO2 with a lattice constant a = 5.418 Å (0.5411 nm, JCPDS Card no. 34-0394). The diffraction peaks are strong and narrow, indicating that the degree of crystallinity is pretty high. The crystal size of the (111) surface in the vertical direction, calculated by Debye–Scherrer formula, is 19.8 nm.
To further investigate the chemical status of as-prepared spherical CeO2, analyses of elements and chemical valence analysis were carried out by XPS, as shown in Fig. 3. The elements Ce, O, C can be detected from Fig. 3A, and they are assigned to Ce 3d, O 1s and C 1s binding energies, respectively. Six peaks at BEs 881.8, 888.9, 897.8, 900.5, 907.7, and 916.3 (marked with u1, u2, u3, v1, v2, v3) can found in Fig. 3B, where u and v come from Ce 3d5/2 and Ce 3d3/2 states, respectively.32 The Ce 3d XPS spectrum of Ce4+ has been fitted with six peaks, and if some Ce3+ species also present, four more peaks would be detected. Thus, Ce4+ is the only oxidation state of the as-prepared spherical CeO2. This result will be in great agreement with the O 1s XPS spectra showed in Fig. 3C. The peak at BE = 528.9 eV, which is attributed to oxide species, is considered as being fingerprints characterizing of CeO2 while peak at 530.3 eV is assigned to Ce2O3.33 Whereas the peak at 531 eV (BE) is attributed to surface adsorbed oxygen such as O− or OH−. Consequently, it can be confirmed that Ce4+ is the only oxidation state of as-prepared spherical CeO2.
The TG curve of the as-prepared spherical CeO2 particles is shown in the Fig. 4. It can be seen from the TG curve that the weight loss can be mainly divided into 2 steps. The first weight loss between 50 and 200 °C is 2%, which can be ascribed to partly volatilization of the water in CeO2 and the bond water of CeO2 crystal surface. The second step between 200 and 550 °C is attributed to the decomposition of residual PVP in the CeO2 crystals, which confirmed that final products contains 8% of PVP.
3.2 Controllable synthesis of spherical CeO2
To study the formation mechanism of spherical CeO2 particles synthesized through PVP-assisted hydrothermal method and control the size and morphology of spherical CeO2, we investigated some influence factors related to the synthesis of CeO2.No product was obtained at 140 °C for 24 h and the reaction solution kept clear, which was different from what Zhou et al. reported. Thus, the reaction temperature was increased to 180 and 200 °C at the reaction time of 24 h. The FESEM images of the products at different temperature are shown in Fig. 5A and B. The product of 180 °C contains not only spherical CeO2 particles but also rod-like substance, as shown in Fig. 5A. Furthermore, under the excitation of high-energy electron beam, the rod-like substance was decomposed, indicating that these substances were not stable, and little residue was observed after excitation. By the analysis of Energy Dispersive Spectrometer (EDS), which is shown in Table 1, the atom percentage of Ce decreased from 19.07% in spherical CeO2 to 7.73% in rod-like substances, which indicating that these rod-like substances would be mainly composed of organic matter. The XRD pattern of the product, prepared in 180 °C, is shown in Fig. 6. All the typical diffraction peaks of cubic fluorite CeO2 could be found, and at the same time, some impure peaks could be observed. These impure peaks were close to the diffraction peaks of some organic ceria salt, so it could be preliminarily ascertained that the rod-like substance might be some organic ceria formed by PVP and cerium ions. The rod-like substance would disappear gradually with a longer reaction time or higher reaction temperature, so we believe that the rod-like substance was intermediate products. Pure spherical CeO2 particles was obtained at the reaction temperature of 200 °C, as shown in Fig. 5B. The particle size distributed unevenly. The average diameter is about 987 nm and its standard deviation is 316 nm.
| Elements | C | O | Ce |
|---|---|---|---|
| Atom percentage of rod-like substances/% | 49.10 | 43.16 | 7.73 |
| Atom percentage of spherical ceria/% | 38.05 | 42.88 | 19.07 |
The FESEM images of as-prepared CeO2 particles at different reaction time in 200 °C are shown in Fig. 7. With the reaction time increasing, the mean particle size increased and the monodispersity became weak, which might be caused by Ostwald mechanism.13,34 Fig. 8 shows the SEM images of CeO2 particles at different molar ratios of Ce(NO3)3·6H2O to PVP (repeating units). The particle size increased with the amount of PVP increasing from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 while other synthetic parameters kept unchanged. When the molar ratios of Ce(NO3)3·6H2O to PVP (repeating units) was 1
4 while other synthetic parameters kept unchanged. When the molar ratios of Ce(NO3)3·6H2O to PVP (repeating units) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, interparticle bonding was formed. When the amount of PVP was low, the oriented aggregation force of nanocrystals which was formed by the interaction of the same crystal planes was not effectively prevented. As the amount of PVP increasing, the size of final CeO2 particles increased owing to the increase of the intermediate products (PVP cerium salt) which further caused an acceleration in the precipitation and the growth rate of the CeO2 nanocrystals. SEM images and size distribution of the CeO2 particles with different amounts of Ce(NO3)3·6H2O (molar ratio of Ce(NO3)3·6H2O to PVP kept unchanged) are shown in Fig. 9.
1, interparticle bonding was formed. When the amount of PVP was low, the oriented aggregation force of nanocrystals which was formed by the interaction of the same crystal planes was not effectively prevented. As the amount of PVP increasing, the size of final CeO2 particles increased owing to the increase of the intermediate products (PVP cerium salt) which further caused an acceleration in the precipitation and the growth rate of the CeO2 nanocrystals. SEM images and size distribution of the CeO2 particles with different amounts of Ce(NO3)3·6H2O (molar ratio of Ce(NO3)3·6H2O to PVP kept unchanged) are shown in Fig. 9.
 | ||
| Fig. 7 SEM images of CeO2 samples synthesized at 200 °C with different reaction time: (A) 2.5 h; (B) 3 h; (C) 6 h; (D) 12 h; (E) 24 h. | ||
 | ||
Fig. 8 SEM images and size distribution of CeO2 samples synthesized at different molar ratios of Ce(NO3)3·6H2O to PVP (repeating units): (A) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (B) 1 1; (B) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; (C) 1 2; (C) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; (D) 1 3; (D) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. 4. | ||
 | ||
| Fig. 9 SEM images and size distribution of CeO2 samples synthesized with different amounts of Ce(NO3)3·6H2O: (A) 2 mmol; (B) 4 mmol; (C) 6 mmol; (D) 8 mmol. | ||
It can be seen from Fig. 9A–D that the as-prepared spherical CeO2 particles at different amounts of Ce(NO3)3·6H2O are of good monodispersity. With the increase of the cerium salt concentration, the particle size changed complicatedly, it was an increase–decrease–increase process. We think that the formation process of spherical CeO2 can be divided into 4 steps and describe as follows: (1) cerium salt was hydrolyzed into organic cerium salt with the assistance of PVP; (2) ceria crystal nucleus were formed by the decomposition of organic cerium at high temperature and high pressure; (3) nucleus grew; (4) CeO2 nanocrystals aggregated into spherical CeO2 particles with the assistance of PVP. The main factors effect on the size of as-prepared spherical CeO2 particles are believed to be: (1) the amounts and concentration of the nucleus formed initially; (2) the growth rate and size of the nucleus. Nucleus grew faster when the amount of the nucleus was less, thus the crystals size became larger, which led to an increase in particle size of the as-prepared spherical CeO2 particles. With crystal nucleus number increasing, nanocrystals which can be aggregated into spherical CeO2 increased. This means that the number of the spherical CeO2 in per unit volume of liquid increased, and therefore the particle size decreased. Initial cerium salt concentration had a complex effect on the particle size of the final spherical CeO2, because the initial formation amount of organic cerium salt, which was directly affected by initial cerium salt concentration, had an direct effect on the formation amount of ceria crystal nucleus, and at the same time would influence the Ostwald ripening process of the nucleus.
The change curve of crystal nucleation and growth rate with concentration are shown in Fig. 10.34 Nucleation takes place when the concentration is more than C*min. As nucleation proceed, nucleation ceases and nucleus begin to grow up when the concentration decreases to between C*min and Cs. The corresponding relationship between the nucleation rate and concentration in different systems are different and can be divided into curve a and b. We do believe that cerium salt system agrees well with curve b: the nucleation rate changes little when the concentration is a little more than C*min and nucleation changes obviously when the concentration is close to C*max. In the systems containing 2 mmol and 4 mmol cerium salt, the amount of nucleation had little difference, because the concentration of cerium salt was a little more than C*min and the concentration had little effect on the nucleation rate. Individual crystal was larger in the system containing 4 mmol cerium salt owing to their high nucleus growth rate, which caused a larger size of final product. When the amount of cerium salt was 6 mmol, the nucleation rate increased greatly, leading to a large number and high concentration of crystals. The spherical CeO2 particles were formed by the inter-collision and oriented aggregation of crystal nucleus after a transient nucleus growth, resulting in an increase of spherical CeO2 number, thus the size of final product decreased. As the amount of cerium salt increasing to 8 mmol, the amount of nucleation had little change compared to 6 mmol cerium salt, but the nucleus growth rate became faster in later stage, which eventually caused an increase of the particle size.
3.3 Formation mechanism of spherical CeO2
In this paper, CeO2 particles were synthesized by hydrothermal method and we studied their influence factors in the morphology of CeO2 particles. Based on the experimental data obtained above, we proposed the formation mechanism of spherical CeO2 particles through a hydrothermal method with the assistance of PVP. The formation process can be expressed by the Fig. 11. When PVP was dissolved in water, the solution became acidic owing to the complex adsorption between PVP and OH− (pH = 4.21, showed by step 1). After the addition of Ce(NO3)3, the pH of the solution became lower (pH = 3.56), caused by the complexing and hydrolyzing of the Ce3+ and carbonyl oxygen in PVP (showed by step 2). With temperature increasing, nitrate ion connected with Ce3+ could be hydrolyzed and replaced by OH− (showed by step 4). The rod-like organic cerium salt crystalline was formed by the hydrolytic crosslinking reaction of organic cerium precursor at high temperature (showed by step 5). Then the organic crystals nucleated gradually and formed CeO2 crystals with some PVP adsorption on the surface. Adsorption between PVP and different planes was not same, because atoms on different CeO2 crystal surfaces were different. It has been reported that PVP had a strong binding action with {100} crystal planes,35 therefore, the growth of {100} crystal planes is inhibited, resulting in the formation of cubic CeO2 crystals (showed by step 6). These formed crystals had large surface energy owing to their small size (below 100 nm). Thus, these small crystals tended to aggregate into big particles by the interaction of the hydroxy or PVP absorbed on the surface. The surface energy was lower when the same crystal plane contacted each other, therefore these aggregation must be oriented, which could be confirmed by SEAD pattern in Fig. 1C.4. Conclusions
In this paper, spherical CeO2 submicron particles were successfully synthesized by a hydrothermal method with the assistance of PVP. We demonstrated that the as-prepared spherical CeO2 particles were formed by the oriented aggregation of fluorite ceria crystals with the aid of XRD and SAED. Our study revealed that the Ce(NO3)3·6H2O was firstly hydrolyzed to rod-like organic cerium salt by the assistance of PVP, then the organic cerium salt was decomposed into CeO2 nanocrystals. Finally, spherical CeO2 particles were formed by the oriented aggregation of these CeO2 nanocrystals through the interaction between crystal planes and the assistance of PVP.Acknowledgements
The authors are grateful for the financial support provided by the National Natural Science Foundation of China (No. 51173146) and the financial support provided by the National Natural Science Foundation of Shaanxi (No. 2015JM2050, 2015JQ2055).Notes and references
- J. Graciani, K. Mudiyanselage, F. Xu, A. E. Baber, J. Evans, S. D. Senanayake, D. J. Stacchiola, P. Liu, J. Hrbek and J. F. Sanz, Science, 2014, 345, 546–550 CrossRef CAS PubMed.
- S. Luo, T.-D. Nguyen-Phan, A. C. Johnston-Peck, L. Barrio, S. Sallis, D. A. Arena, S. Kundu, W. Xu, L. F. Piper and E. A. Stach, J. Phys. Chem. C, 2015, 119, 2669–2679 CAS.
- W. Lei, T. Zhang, L. Gu, P. Liu, J. A. Rodriguez, G. Liu and M. Liu, ACS Catal., 2015, 5, 4385–4393 CrossRef CAS.
- Y. Su, Z. Tang, W. Han, P. Zhang, Y. Song and G. Lu, CrystEngComm, 2014, 16, 5189–5197 RSC.
- J. B. Park, J. Graciani, J. Evans, D. Stacchiola, S. D. Senanayake, L. Barrio, P. Liu, J. F. Sanz, J. Hrbek and J. A. Rodriguez, J. Am. Chem. Soc., 2009, 132, 356–363 CrossRef PubMed.
- I. Gonzalez, R. Navarro, W. Wen, N. Marinkovic, J. Rodriguez, F. Rosa and J. Fierro, Catal. Today, 2010, 149, 372–379 CrossRef CAS.
- K. Ueno and H. Misawa, NPG Asia Mater., 2013, 5, e61 CrossRef CAS.
- P. Zhang, T. Wang and J. Gong, Adv. Mater., 2015, 27, 5328–5342 CrossRef CAS PubMed.
- P. Jasinski, T. Suzuki and H. U. Anderson, Sens. Actuators, B, 2003, 95, 73–77 CrossRef CAS.
- T. Ichimura, K. Fujiwara and H. Tanaka, Sci. Rep., 2014, 4, 5818 CAS.
- H. R. Tan, J. P. Y. Tan, C. Boothroyd, T. W. Hansen, Y. L. Foo and M. Lin, J. Phys. Chem. C, 2011, 116, 242–247 Search PubMed.
- N. C. Wu, E. W. Shi, Y. Q. Zheng and W. J. Li, J. Am. Ceram. Soc., 2002, 85, 2462–2468 CrossRef CAS.
- E. Y. H. Teo, M. Lin, Z. Y. Fu, S. C. Ng, J. P. Y. Tan and H. R. Tan, ECS Trans., 2013, 50, 63–74 CrossRef.
- J. P. Y. Tan, H. R. Tan, C. Boothroyd, Y. L. Foo, C. B. He and M. Lin, J. Phys. Chem. C, 2011, 115, 3544–3551 CAS.
- H. Wang, J.-J. Zhu, J.-M. Zhu, X.-H. Liao, S. Xu, T. Ding and H.-Y. Chen, Phys. Chem. Chem. Phys., 2002, 4, 3794–3799 RSC.
- X. Feng, D. C. Sayle, Z. L. Wang, M. S. Paras, B. Santora, A. C. Sutorik, T. X. Sayle, Y. Yang, Y. Ding and X. Wang, Science, 2006, 312, 1504–1508 CrossRef CAS PubMed.
- J. Zhang, X. Ju, Z. Wu, T. Liu, T. Hu, Y. Xie and Z. Zhang, Chem. Mater., 2001, 13, 4192–4197 CrossRef CAS.
- F. Gu, Z. Wang, D. Han, C. Shi and G. Guo, Mater. Sci. Eng., B, 2007, 139, 62–68 CrossRef CAS.
- R.-J. La, Z.-A. Hu, H.-L. Li, X.-L. Shang and Y.-Y. Yang, Mater. Sci. Eng., A, 2004, 368, 145–148 CrossRef.
- C. Sun, H. Li, H. Zhang, Z. Wang and L. Chen, Nanotechnology, 2005, 16, 1454 CrossRef CAS.
- R. Yu, L. Yan, P. Zheng, J. Chen and X. Xing, J. Phys. Chem. C, 2008, 112, 19896–19900 CAS.
- L. Yan, R. Yu, J. Chen and X. Xing, Cryst. Growth Des., 2008, 8, 1474–1477 CAS.
- F. Zhang, Q. Jin and S.-W. Chan, J. Appl. Phys., 2004, 95, 4319–4326 CrossRef CAS.
- F. Zhang, S.-W. Chan, J. E. Spanier, E. Apak, Q. Jin, R. D. Robinson and I. P. Herman, Appl. Phys. Lett., 2002, 80, 127–129 CrossRef CAS.
- Z. Yang, D. Han, D. Ma, H. Liang, L. Liu and Y. Yang, Cryst. Growth Des., 2009, 10, 291–295 Search PubMed.
- X.-H. Lu, X. Huang, S.-L. Xie, D.-Z. Zheng, Z.-Q. Liu, C.-L. Liang and Y.-X. Tong, Langmuir, 2010, 26, 7569–7573 CrossRef CAS PubMed.
- Z. L. Wang and X. Feng, J. Phys. Chem. B, 2003, 107, 13563–13566 CrossRef CAS.
- C. Pan, D. Zhang, L. Shi and J. Fang, Eur. J. Inorg. Chem., 2008, 2429–2436 CrossRef CAS.
- X. Han, L. Li and C. Wang, CrystEngComm, 2012, 14, 1939–1941 RSC.
- F. Zhou, X. Zhao, H. Xu and C. Yuan, J. Phys. Chem. C, 2007, 111, 1651–1657 CAS.
- J. Wei, Z. Yang, Y. Yang and H. Wei, Cryst. Res. Technol., 2011, 46, 201–204 CrossRef CAS.
- F. ç. Larachi, J. Pierre, A. Adnot and A. Bernis, Appl. Surf. Sci., 2002, 195, 236–250 CrossRef CAS.
- S. Fang, Y. Xin, L. Ge, C. Han, P. Qiu and L. Wu, Appl. Catal., B, 2015, 179, 458–467 CrossRef CAS.
- T. Sugimoto, Adv. Colloid Interface Sci., 1987, 28, 65–108 CrossRef CAS.
- R. Si, Y.-W. Zhang, L.-P. You and C.-H. Yan, J. Phys. Chem. B, 2006, 110, 5994–6000 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |







