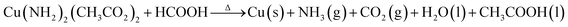Copper inks formed using short carbon chain organic Cu-precursors†
Wen-dong Yang,
Chun-yan Liu*,
Zhi-ying Zhang,
Yun Liu and
Shi-dong Nie
Key Laboratory of Photochemical Conversion and Optoelectronic Materials of Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Zhongguancun, Beijing 100190, PR China. E-mail: cyliu@mail.ipc.ac.cn; Tel: +86-010-82543573
First published on 4th November 2014
Abstract
Cu-based printing inks (20 wt% Cu) and (9.6 wt% Cu) were prepared using the short carbon chain organic Cu-precursors formed during the preparation of the inks, which can easily form a favorable conductive film onto glass slides at 290 °C and 220 °C. The resistance of the film induced by the oxidation of Cu and the remainder of the long carbon chain Cu-precursors markedly decreased, more than usual. The factors influencing the formation and conductive mechanism of the copper ink and film are discussed.
1. Introduction
Printable conductive inks have attracted much attention due to the potential application in low-cost and sophisticated electronic devices, components and integrated smart systems.1–7 Conductive inks that can be patterned by silk-screen printing, inkjet printing, or other printing methods to form conductive tracks are a critical component of printing electronic technology.Up to now, conductive polymers,8 carbon,9,10 graphene,11 and metallic inks12–19 have been used in the formation of conductive tracks. Among these, silver inks are currently favored and rapidly developing. However, high cost, low content, as well as electromigration issues limit the wide industrial application of silver inks.
Copper is a good alternative material to silver due to its low cost, good conductivity, and reduced electromigration effect. Various methods have been developed to synthesize copper nanoparticle (Np) inks.20–26 However, most of them are not economically feasible because of the low throughput and serious agglomeration. Besides, it is very difficult to obtain pure copper Nps, because the reduction of Cu salts tends to stop at the Cu2O stage and copper Nps can easily be oxidized in air. The presence of copper oxides will increase the sintering temperature of the Nps and decrease their conductivity. Moreover, the preparation of Nps inks generally involves a complex process with toxic organic solvents and poor dispersion stability of copper Nps.
To overcome these problems, organic copper decompositon inks are being intensively researched,27–31 where Cu-precursors are generally mono- or di-valent Cu-organic compounds, and there are no problems with oxidation during their preparation and storage. However, at present, three distinct shortcomings of the inks make them hard to apply commercially. First, most of them require a reduction atmosphere (such as hydrogen gas or formic gas), or a special sintering method (intense pulsed light or laser) to make the ink tracks conductive, which results in cost and safety problems and should be avoided for industrial production. Second, the inks generally use nonpolar solvents rather than alcohol species, which could lead to damage to the blanket layer30 and froward compatibility with mass production techniques, especially reverse offset printing. Third, the preparation process is generally complex, and uses long carbon chain Cu-compounds as precursors, which will induce low metallic loading and poor conductivity. To increase the metal load of the ink, Lee et al.29 prepared copper ink by mixing copper(II) neodecanoate and copper hydroxide nanopowder, and annealed the ink trace under an atmosphere of 3% H2 in order to reduce copper hydroxide. The thermal decomposition ink of amine complexes of copper formate27,30,31 was an innovation to organic copper inks, but this is only an example of the ink system, so far. The design and fabrication of well-modulated Cu-inks still present huge challenges. In particular, it is very pressing to economically develop feasible processes to produce alcohol-based copper inks with short carbon chain organic copper compounds.
In the present work, we designed and prepared two alcohol-based organic copper inks of Ink20 (20 wt% Cu) and Ink9.6 (9.6 wt% Cu) with short carbon chain organic copper compounds from the semifinished product during the preparation of the inks. The factors influencing the formation and conductive action of the copper ink and film are discussed.
2. Results and discussion
2.1 Ink20
Ink20 was prepared using copper glycolate as a Cu-precursor, which is derived from the neutralization reaction of hydroxyacetic acid and copper hydroxide. The obtained copper glycolate powder was redispersed in the mixed solvent of terpineol and hydroxyacetic acid (1%), then exposed to ultrasound for an hour to obtain the ink (ESI†).As an important component of the ink, the characteristics of the copper glycolate were investigated by FT-IR spectroscopy, XRD, SEM, EDS (Fig. S1–S4 in the ESI†), DSC and TG (Fig. 1) to confirm the purity, structure and decomposition temperature of the copper glycolate.
The endothermic peak in Fig. 1 appeared at about 287 °C, where the total weight loss was 68%, and implied 32% leftover copper. This was supported by the XRD spectra in Fig. 3. The result is much better than the best one that has been previously reported (14.2%),29 where copper neodecanoate was a metal precursor and a conductive film was produced under a hydrogen atmosphere.
The design and selection of solvent compositions are crucial to ink properties, and should meet various requirements. In the present work, terpineol was used as a high boiling point solvent to suppress the so-called ‘coffee ring effect’18 and solidify the wet film. A given amount of hydroxyacetic acid was used to improve the adhesion of the ink with the substrate and the properties of the copper ink films. As expected, the sintered film, with an average thickness of 1 ± 0.2 μm, had a relatively uniform surface structure, where the Cu Nps accumulated at the centre and the ‘coffee ring effect’ was effectively suppressed (Fig. 2).
 | ||
| Fig. 2 Surface profiles of the copper film deposited on glass after sintering at 290 °C for 60 min under a nitrogen atmosphere. | ||
Based on the observation of the surface profiles and the measured sheet resistance results of the film (sintered at 290 °C), the resistivity was calculated to be about 3.85 × 10−5 Ω cm (Fig. S5†).
The XRD spectra of the sintered copper film in Fig. 3 are in agreement with the values of the face-centered cubic (fcc) crystal structure of copper. No diffraction peaks from any other impurities were detected, indicating that the copper glycolate was directly transformed to copper crystals at 260 °C.
The intensity of the diffraction peaks increased with the sintering temperature, which meant that the crystallization of the Cu Nps improved. As mentioned above, copper is easily oxidized into either Cu2O or CuO. However, the copper in the present sintered copper films showed a stable FCC copper crystal phase, and were significantly oxide-free, which may be attributed to the reduction action of a small amount of hydroxyacetic acid:
The SEM images of the resulting films, sintered at different temperatures, are shown in Fig. 4. When combined with the SEM image of copper glycolate (Fig. S3†) and the study of the thermal decomposition of copper carboxylate,28 it can be deduced that the films simultaneously underwent the following processes during sintering: evaporation of the coated solvent and decomposition of copper glycolate and the neck connecting the produced copper Nps. As shown in Fig. 4, at a lower sintering temperature (260 °C), copper glycolate decomposed slowly and formed relatively large particles with fewer connections among the particles, so the resistivity of the films was greater. With an increased temperature (290 °C), the copper Nps become more uniform and formed connections with each other, and the film was smooth. Meanwhile, pores and voids among the Nps were reduced, and the stacking density was improved greatly, thus, the resistivity gradually decreased. A heat-treatment at higher temperatures (320 °C) could further compact the film, but the resistance of the film decreased much more slowly.
 | ||
| Fig. 4 SEM images of the copper films formed at different sintering temperatures for 60 min: (a) 260, (b) 275, (c) 290, (d) 305, (e) 320 °C. The selected areas were used for the measurements shown in Fig. S6.† | ||
The chemical composition of the copper film sintered at different temperatures was identified by surface energy dispersive spectrometry (Fig. S6†). Three elements (C, O, and Cu) were detected in the films. With increasing temperature from 260 °C to 320 °C, the Cu content increased from 80.47 wt% to 90.88 wt% and the C content decreased from 14.98 wt% to 3.37 wt%, indicated that the copper glycolate almost fully decomposed and volatilized, and the small quantity of O may be from the residual organic molecules. Considering that the copper Nps were easily oxidized at high temperatures, 290 °C was selected as an optimum sintering temperature.
Briefly, the decomposition degree of copper glycolate, the remnant degree of the organic molecules at the interface of the copper particles and the stacking density of the copper Nps are the three dominant factors affecting the conductivity of the sintered copper films.
2.2 Ink9.6
In order to further optimize the prepared system, we designed a new complexation–reduction process of copper acetate and cyclohexylamine together with formic acid to prepare Ink9.6 (ESI†). The as prepared ink had no particles or impurities, and therefore, meets the requirements for dispersion stability and antioxidation.Herein, the lone pair electrons on the nitrogen of cyclohexylamine can coordinate with copper acetate and form copper organic complexes:
Cu(CH3COO)2 + C6H11NH2 → [Cu(NH2)2]+[(CH3COO)2]−
Formic acid was added for the reduction and stabilization of Cu ions. Ethylene glycol was used as a co-solvent with a high boiling point to suppress the ‘coffee ring effect’. Ethanol was used to adjust the surface tension of the ink.
As shown in the DSC-TG results in Fig. 5, the endothermic peaks appeared at around 127 °C and 170 °C, indexed to the evaporation of amine and ethylene glycol. The exothermic peak at about 200 °C might be from the coalescence of the Cu Nps and the partial decomposition of organic species. The total weight loss was 85%, implying that the remaining 15% was copper. 220 °C was the optimum sintering temperature.
The XRD pattern of the sintered copper film of Ink9.6 is in good agreement with the values for the face-centered cubic (fcc) crystal structure of copper. No diffraction peaks from any impurities were detected (ESI Fig. S7†), indicated that copper Nps were produced. In comparison with the previous research results of the thermal decomposition of copper–amine complexes,27,30,31 the following reaction was suggested:
The uniformity, smoothness, and compactness of the sintered films become better when a mixed solvent is used (Fig. S8†). Two elements (C 4.52 wt% and Cu 95.48 wt%) were detected in the films (Fig. S8†), indicating that most of the organic solvent was volatilized, and the little C may be from the residual organic species.
Based on the SEM observations, we chose a film with an average thickness of 1.5 μm to calculate the resistivity (ESI†), which was about 7.5 × 10−5 Ω cm.
Significantly, although the ink contained only 9.6 wt % copper, it still offered good conductivity.
It has been found that formic acid was the most critical factor influencing the conductivity of the film. The experiments showed that a little formic acid could not influence the reduction of the copper ions, but excess acid would react with cyclohexylamine to produce compounds that are hard to decompose at lower temperatures, and lead to poor conductivity of the sintered film. The optimum molar ration of formic acid and copper acetate was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, according to our experiences.
1, according to our experiences.
In the present work, we only used cyclohexylamine in the complexation reaction. However, the types of amine groups are a key factor to the thermal decomposition temperature of the copper film.31 The appropriate amine complexes could significantly decrease the sintering temperature and make inks usable on plastic substrates. This requires further experiments in future efforts.
3. Conclusions
Two kinds of organic copper conductive ink were prepared using the short carbon chain organic Cu-precursors produced by the facile preparation process of the inks. Both of them have good conductivity after sintering, which is comparable to other copper-precursor-based inks.27–31The prepared organic copper decompositon ink, Ink20, used copper glycolate as a precursor, avoiding the usual issue of low metallic loading and bad conductivity from long carbon chain copper precursors, and a favourable conductive film easily formed on glass slides.
During the design and preparation of Ink9.6 a new self-reduction ink system was developed and a pure copper film with the desirable conductivity was obtained after simply sintering at 220 °C in an ambient atmosphere.
Notes and references
- C. N. Hoth, S. A. Choulis, P. Schilinsky and C. Brabec, Adv. Mater., 2007, 19, 3973 CrossRef CAS.
- Y. Chen, J. Au, P. Kazlas, A. Ritenour, H. Gates and M. McCreary, Nature, 2003, 423, 136 CrossRef CAS PubMed.
- S. Magdassi, M. Grouchko and O. Berezin, et al., ACS Nano, 2010, 4, 1943 CrossRef CAS PubMed.
- V. Subramanian, J. M. J. Frechet, P. C. Chang, D. Huang, J. B. Lee, S. E. Molesa, A. R. Murphy, D. R. Redinger and S. K. Volkman, Proc. IEEE, 2005, 93, 1330 CrossRef CAS.
- L. Hu, J. W. Choi, Y. Yang, S. Jeong, F. La Mantia, L. F. Cui and Y. Cui, Proc. Natl. Acad. Sci., 2009, 106, 21490 CrossRef CAS PubMed.
- S. H. Lim, J. W. Kemling, L. Feng and K. S. Suslick, Analyst, 2009, 134, 2453 RSC.
- C. T. Wang, K. Y. Huang, D. T. W. Lin and Y. C. Hu, Sensors, 2010, 10, 5054 CrossRef CAS PubMed.
- H. Sirringhaus, T. Kawase, R. H. Friend, T. Shimoda, M. Inbasekaran, W. Wu and E. P. Woo, Science, 2000, 290, 2123 CrossRef CAS.
- L. Hu, J. Choi, Y. Yang, S. Jeong, F. Mantia, L. Cui and Y. Cui, Proc. Natl. Acad. Sci., 2009, 106, 21490 CrossRef CAS PubMed.
- L. Hu, M. Pasta, F. L. Mantia, L. Cui, S. Jeong, H. D. Deshazer, J. W. Choi, S. M. Han and Y. Cui, Nano Lett., 2010, 10, 708 CrossRef CAS PubMed.
- L. Huang, Y. Huang, J. J. Liang, X. J. Wan and Y. S. Chen, Nano Res., 2011, 4, 675 CrossRef CAS.
- C. N. Chen, C. P. Chen, T. Y. Dong, T. C. Chang, M. C. Chen, H. T. Chen and I. G. Chen, Acta Mater., 2012, 60, 5914 CrossRef CAS PubMed.
- S. Magdassi, M. Grouchko, O. Berezin and A. Kamyshny, ACS Nano, 2010, 4, 1943 CrossRef CAS PubMed.
- S. B. Walker and J. A. Lewis, J. Am. Chem. Soc., 2012, 134, 1419 CrossRef CAS PubMed.
- S. Jeong, H. C. Song, W. W. Lee, Y. Choi, S. S. Lee and B. H. Ryu, J. Phys. Chem. C, 2010, 114, 22277 CAS.
- R. Shankar, L. Groven, A. Amert, K. W. Whites and J. J. Kellar, J. Mater. Chem., 2011, 21, 10871 RSC.
- B. Y. Ahn, D. J. Lorang and J. A. Lewis, Nanoscale, 2011, 3, 2700 RSC.
- W. D. Yang, C. Y. Liu, Z. Y. Zhang, Y. Liu and S. D. Nie, J. Mater. Chem., 2012, 22, 23012 RSC.
- Y. Chang, D. Y. Wang, Y. L. Tai and Z. G. Yang, J. Mater. Chem., 2012, 22, 25296 RSC.
- D. Y. Deng, Y. R. Cheng, Y. X. Jin, T. K. Qi and F. Xiao, J. Mater. Chem., 2012, 22, 23989 RSC.
- D. Y. Deng, Y. R. Cheng, Y. X. Jin, T. K. Qi and F. Xiao, ACS Appl. Mater. Interfaces, 2013, 5, 3839 CAS.
- Y. Lee, J. R. Choi, K. J. Lee, N. E. Stott and D. Kim, Nanotechnology, 2008, 19, 415604 CrossRef PubMed.
- X. F. Tang, Z. G. Yang and W. J. Wang, Colloids Surf., A, 2010, 360, 99 CrossRef CAS PubMed.
- Y. Kim, B. Lee, S. Yang, I. Byun, I. Jeong and S. M. Cho, Curr. Appl. Phys., 2012, 12, 473 CrossRef PubMed.
- N. A. Luechinger, E. K. Athanassiou and W. J. Stark, Nanotechnology, 2008, 19, 445201 CrossRef PubMed.
- E. K. Athanassiou, R. N. Grass and W. J. Stark, Nanotechnology, 2006, 17, 1668 CrossRef CAS.
- A. Yabuki, N. Arrifin and M. Yanase, Thin Solid Films, 2011, 519, 6530 CrossRef CAS PubMed.
- B. Lee, Y. Kim, S. Yang, I. Jeong and J. Moon, Curr. Appl. Phys., 2009, 9, 157 CrossRef PubMed.
- S. J. Kim, J. Lee, Y. H. Choi, D. H. Yeon and Y. Byun, Thin Solid Films, 2012, 520, 2731 CrossRef CAS PubMed.
- D. H. Shin, S. Woo, H. Yem, M. Cha, S. Cho, M. Kang, S. Jeong, Y. Kim, K. Kang and Y. Piao, ACS Appl. Mater. Interfaces, 2014, 6, 3312 CAS.
- A. Yabuki and S. Tanaka, Mater. Res. Bull., 2012, 47, 4107 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra09318f |
| This journal is © The Royal Society of Chemistry 2014 |