Conformational modifications induced by internal tandem duplications on the FLT3 kinase and juxtamembrane domains†
Abstract
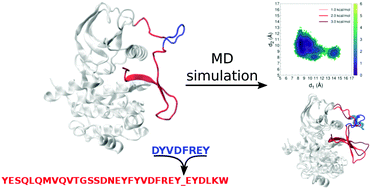
The aberrant expression of FLT3 tyrosine kinase is associated primarily with acute myeloid leukaemia. This blood malignancy is often related to the onset of internal tandem duplications (ITDs) in the native sequence of the protein. The ITDs occur mainly in the juxtamembrane domain of the protein and alter the normal activity of the enzyme. In this work, we have studied the native form of FLT3 and six mutants by molecular dynamics simulations. The catalytic activity of FLT3 is exerted by the tyrosine kinase domain (KD) and regulated by the juxtamembrane (JM) domain. Analysis of the dynamics of these two domains have shown that the introduction of ITDs in the JM domain alters both structural and dynamic parameters. The presence of ITDs allowed the protein to span a larger portion of the conformational space, particularly in the JM domain and the activation loop. The FLT3 mutants were found to adopt more stable configurations than the native enzyme. This was due to the different arrangements assumed by the JM domain. Larger fluctuations of the activation loop were found in four of the six mutants. In the native FLT3, the key residue Tyr572 is involved in a strong and stable interaction with an ion pair. This interaction, which is thought to keep the JM in place hence regulating the activity of the enzyme, was found to break in all FLT3 mutants.


 Please wait while we load your content...
Please wait while we load your content...