 Open Access Article
Open Access ArticleLignin-derived bionanocomposites as functional food packaging materials
Muhammad
Zubair
 a,
Zahid
Rauf
b,
Seerat
Fatima
b and
Aman
Ullah
a,
Zahid
Rauf
b,
Seerat
Fatima
b and
Aman
Ullah
 *a
*a
aDepartment of Agricultural, Food and Nutritional Science, South Academic Building University of Alberta, Lab# 540, Edmonton, Alberta T6G 2P5, Canada. E-mail: ullah2@ualberta.ca
bPakistan Forest Institute (PFI), Peshawar 25130, Khyber Pakhtunkhwa, Pakistan
First published on 23rd May 2024
Abstract
Harnessing lignin, which is the second most abundant biopolymer and is cost-effective, biocompatible, and nontoxic, could be a promising alternative to conventional food packaging materials. Each year, millions of tons of lignin are produced, and it is commonly used as a low-value fuel by being burned. However, this inexpensive and abundant bioresource biomass has the potential to be utilized as food packaging materials. It is crucial to explore lignin-based renewable resources to facilitate the shift towards an environmentally friendly materials circular economy. Recent research has shown that lignin-based materials possess excellent anti-oxidant and anti-bacterial properties, in addition to good mechanical and antiviral properties, UV light barrier, and enhanced thermal properties, making them suitable candidates for use as food packaging materials. This study aims to provide current perspectives on the use of lignin based materials for food packaging applications. The article provides a critical analysis of the physicochemical characteristics, processing techniques, and extraction and structural features of lignin from various sources as well as its derived materials. Additionally, it outlines the latest trends in converting lignin into lignin nanoparticles. This comprehensive review concludes with future perspectives on lignin based materials for food packaging applications.
Sustainability spotlightIn today's world, where environmental concerns are at the forefront, lignin, a sustainable biomass, has the potential to revolutionize various industries and alleviate ecological burdens. In the pulp and paper industry, lignin is produced as a byproduct that can be utilized to reduce waste and develop innovative solutions for food packaging materials. By employing lignin's properties, industries can contribute to a more sustainable future by minimizing the waste of petroleum-based materials and developing eco-friendly packaging alternatives. Additionally, lignin-derived materials in food packaging can significantly reduce the food industry's carbon footprint. Through ongoing research and development, lignin has the potential to catalyze transformative change in various industries and pave the way for a greener and more sustainable future. |
Introduction
Lignin, a prevalent aromatic polymer, reinforces plant cell walls alongside cellulose and hemicellulose. Recent data have revealed that the paper sector generates over 50 million metric tons of biomass byproducts yearly.1,2 However, 98% of this organic matter is burned for power generation or ends up in wastewater systems, which negatively affects the ecosystem.3,4 Approximately 2% of the biomass material remains suitable for industrial use, particularly for producing beneficial lignin-based products. Additionally, one-fifth of the planet's total biomass consists of lignin.5 Researchers have discovered that upon combination, these substances form a reinforced structure resembling a supramolecular scaffold, providing cells with chemical and physical resilience to fight against infections and deterioration caused by enzymes and chemicals.6 Plants like jute, cotton, wood pulp, and industrial hemp possess intermediate lamellae that store a significant amount of lignin (up to 35%). The various types of lignin available in the market include kraft lignin (9%), lignosulfonates (88%), and the newly developed sulfur-free organosolv (2%). If current trends continue, it is projected that lignin will be valued at $913.1 million by 2025.7,8 Lignin has numerous industrial applications, such as food packaging materials, drug delivery systems, tissue engineering scaffolds, and sterile biomedical device components.9–11 Its intrinsic UV shielding properties, anti-oxidant capacity, chemical stability, and physical toughness also enhance the value of consumer goods.12Lignin is a polymer composed of three monolignols: syringyl (S) units, guaiacyl (G) units, and p-hydroxyphenyl (H) units. Lignin biomolecules include spatially organized phenolic groups, such as sinapyl, coniferyl, and p-coumaryl, which enable a variety of functional entities and connections.13,14 The heterogeneity of lignin arises from the interaction between its H, G, and S units, resulting in a range of functional groups and connections. The aryl ether-O-4 linkage accounts for approximately 50% of all connections.15
In the past, lignin-containing industrial waste materials and their byproducts were the primary sources of organic components and energy. The structural foundation of lignin is formed by the S, G, and H subunits, which are produced when phenylpropanoid units undergo radical coupling.16 Due to its heterogeneous nature, recovering lignin in its native form is challenging, making it difficult to obtain structural details. However, recent advancements in near-perfect recovery of milled wood lignin (MWL) and cellulolytic enzyme lignin (CEL),17 have partially resolved this issue. Previous research on lignin was limited to the pulp and paper industry, as it was typically discarded as waste in that sector. As a result, studies focused on chemical profiling and structural elucidation.18
Lignins are polysaccharide-bound compounds that exhibit uneven distribution patterns and are intertwined with cellulose and hemicellulose in their natural state.18 The physicochemical characteristics of lignin are influenced by various factors, including the plant source, extraction method, treatment parameters, and other elements.19 Lignin-rich byproducts from industries and agriculture, such as paper and pulp,20 bagasse,21 wood, agricultural waste products, and other organic wastes,22 are abundant in lignin. In contrast, organic wastes like grass, some plant pieces, and other organic wastes produce very little lignin.15
Recently, there has been a growing interest in lignin-derived composites due to their nanoforms, which enhance durability and provide significant value to materials such as fabrics and rubber.23 Native lignin, when considered as a single entity, is unable to provide the expected interaction and performance-boosting properties compared to nano-sized lignins.24 Nanolignins have demonstrated improved water sensing, mechanical, UV shielding, and thermal performance capabilities when impregnated with gluten-containing nanostructures. In contaminated water systems, chitosan and nanolignins have shown methyl orange scavenging ability up to 83%. According to a study, the lignin nanoparticle shape offers improved stability and dispersibility for up to two months. Without using any chemicals, evenly distributed nanolignins can be produced by sonic irradiation.25 Silver ion-loaded nanolignins can be filled with biodegradable and environmentally acceptable cationic poly-electrolyte layers to create silver nanoparticle fillers. When combined with silver ions, the poly-electrolyte surface kills a variety of bacterial species, particularly quaternary-amine-resistant E. coli, Pseudomonas aeruginosa, and Ralstonia sp. It also enables interaction with the bacterial cell membrane.26
In recent patented work, cryogel is produced by embedding lignin nanoparticles in crosslinked gelatin at subfreezing temperatures, resulting in a densely crosslinked microporous composition with pores size between 50–50 μm. This unique combination of collagen-derived natural polymer (gelatin) and natural biowaste polymer (lignin) enhances mechanical performance and shape recovery rate while exhibiting strong free radical scavenging action and inhibiting bacterial growth.27 In another patent, developed a novel method to modify lignocellulosic materials, particularly wood, while preserving their original architectural design. By employing partial delignification and filling of the material's structures, a new composite material is produced that exhibits desirable characteristics. This innovative approach creates possibilities for the development of advanced materials with diverse applications, which could significantly impact various industries.28 Therefore, we believe that it is essential to review the applications of lignin derived environmentally friendly and biodegradable materials with excellent attributes for food packaging. To the best of our current knowledge, a comprehensive review of the lignin structure, sources, extraction and processing techniques, and applications in food packaging materials has not yet been published.
Structure and sources
Lignin, in comparison to cellulose and hemicellulose, possesses a considerably more complex structure.29 It is composed of three fundamental monolignol building blocks, namely phenylpropanoid units (C9-units) p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S), which are polymerized by radicals in plants to form lignin.30 The C9 formula, which represents the average lignin unit composition, is often written as CxHyOz(OCH3)n. These monolignols are interconnected through a variety of carbon–carbon bonds (b-1, b-5, b–b, 5–5, b-6) and inter-unit connections of ether (b-O-4, a-O-4, 4-O-5) to form a three-dimensional macromolecular structure of lignin.31 Like cellulose, lignin lacks a regular structure and is highly chemically heterogeneous due to the irregular mixing of varying quantities of monolignols and various patterns of substitution on their phenylpropanoid units.32 Lignin's polymeric structure has not yet been fully understood. Some researchers propose that lignin should be classified as a random copolymer rather than a biopolymer due to its lack of a repeating monomer in its structure.33,34 Furthermore, nearly all naturally occurring lignin is connected to carbohydrates, primarily hemicelluloses.35 Approximately 40–60% of the inter-unit connections in lignin are composed of bond of b-O-4 of the ether.36 It is important to note that the structure of lignin is significantly influenced by the extraction process, genetic species, culture site, and harvesting season of the parent plant. In terms of the factors that influence the structure of lignin, hardwood lignin is primarily composed of G and S units while a significant portion of G units make up the majority of softwood lignin. The makeup of monolignols and how they are linked together in lignin is determined by the species or source, as demonstrated by the H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) G
G![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S ratios in lignins from radish and carrot roots37 and grass.38 Additionally, lignins from various sections of a plant have distinct fine structures, as seen in Chinese quince lignin.39 Furthermore, the method used to remove lignin affects its fine structure, as shown by the differences in the composition of monolignols and their relationships to cellulose enzymatic lignins, ethanol, alkali, and milled wood.40 The physicochemical differences between lignin and cellulose or hemicellulose are significant. Unlike cellulose, lignin is amorphous in the solid state.
S ratios in lignins from radish and carrot roots37 and grass.38 Additionally, lignins from various sections of a plant have distinct fine structures, as seen in Chinese quince lignin.39 Furthermore, the method used to remove lignin affects its fine structure, as shown by the differences in the composition of monolignols and their relationships to cellulose enzymatic lignins, ethanol, alkali, and milled wood.40 The physicochemical differences between lignin and cellulose or hemicellulose are significant. Unlike cellulose, lignin is amorphous in the solid state.
Lignin is a material that resembles approximately spherical particles that only slightly dissolve in solvents.41,42 Its insolubility in practically all aqueous solutions is widely recognized.43 Measurements of polydispersity (Mw/Mn), number average molecular weight (Mn) and weight average molecular weight (Mw) were frequently made in regards to molecular mass. However, these values varied greatly among the various lignins, sometimes by several orders of magnitude. For example, Hatakeyama44 found that the kraft lignin from beech (Fagus crenata) had Mw, Mn, and Mw/Mn values of 8020 g mol−1, 1840 g mol−1, and 4.35, respectively. Nevertheless, Stark, Yelle, and Agarwal45 reported that the ponderosa pines (Pinus ponderosa) kraft lignin was 4.49 × 107, 3.82 × 107, and 1.18, respectively.
The main issue with lignin solutions during molecular mass measurement is their solubility in organic solvents. According to Brunow and Lundquist,46 a true lignin solution is typically difficult to create and the insoluble portion must coexist. Given that lignin's soluble part is significantly lower than its insoluble portion, there is inevitable ambiguity. Comparably, there is a wide range of documented lignin thermal stabilities concerning the glass transition temperature, with values falling between 89.9 and 174 °C.47 Mainly, the physicochemical characteristics of lignin are dependent on their source.48,49
According to Erdocia et al.,50 the primary functional group found in lignin is the hydroxyl group, which is essential for both its reactivity and how it interacts with surrounding molecules, such as water. Roughly 10% to 13% of all aromatic rings are made up of free phenols.51 The relevance of lignin's safety for use in a variety of foods is particularly significant for the food industry.52
The utilization of agro-processing residues and plants as sources of lignin is of great importance. In India, the production of lignin is facilitated by the large amounts of biowaste generated by the ayurvedic industry. Additionally, other sources of lignin include pine varieties such as red clover, bagasse from sugar cane, lucerne, elephant grass, grass species like Festuca arundinacea, Brazilwood, and several bamboo species like Bambusa vulgaris & Chusquea oxylepis.53
There are numerous advantages to using agricultural byproducts for synthesizing lignin, including environmental, financial, and technological benefits. Lignocellulosic materials from crops such as sugar cane, maize, rice, and wheat supply the majority of biomass for the global agro-industry, while other crops make up a small portion of this biomass. The production of bioethanol from lignin-rich agricultural byproducts using enzymes and genetically modified yeasts can contribute to energy security in an environmentally friendly manner. The disposal of biowastes from the Ayurvedic medicinal industry contributes to the large amounts of industrial biomass that accumulate in landfills. Research supports the use of leftover lignin-containing plant-based products. The team's water-miscible bioactive potential has previously been demonstrated using herbal residues left behind by the Ayurvedic medical sector.22 Traditional solvents, such as water, milk, ghee, oil, etc., listed in Ayurvedic scriptures do not extract all of the phytochemicals from herbal remnants, indicating that there is still potential for further extraction. The research conducted by Vinardell and colleagues focused on the investigation of lignin compounds derived from Acacia nilotica, which is commonly referred to as the babul tree, and their biological significance.54
Preparation methods
Previously, it was commonly understood that plant-based meals contained higher levels of lignin in their non-edible parts, such as seed coverings, cereal husks, and sugarcane bagasse, than in their consumable parts. As a result, the majority of food-grade lignins are derived from these fibrous by-products. To develop a valuable food ingredient and product with added value, an efficient and cost-effective method of separating lignin from these byproducts must be employed. The conventional lignin isolation process involves pulverizing, separating, purifying, and drying. During the pulping process, lignin is released, and the polysaccharide bonds are broken.55 The existing lignin isolation technologies may be broadly classified into two groups (Fig. 1) based on the lignin's physical condition during the pulping process.56 The first group involves extraction techniques that dissolve and completely disperse the lignin in the resultant pulp. In the second group, lignin appears as an insoluble residue in the pulp produced (leaving operations). However, the application scenarios for these two techniques are not well established. Lignin is often measured using a gravimetric technique following isolation due to its low reactivity and inert character.57 Ideally, the optimal isolation process would yield chemically unchanged lignin that is free of non-lignin impurities. However, no existing technique can meet all of these conditions, despite significant efforts to investigate potential isolation approaches to preserve the original structure of lignin.58 In other words, the physicochemical characteristics and structure of lignin are largely determined by the isolation process used. The most common way to refer to lignin is by the method used to separate it, such as milled wood lignin (MWL) or lignin that has been hydrolyzed, alkali, sulfite, or organosolv processed. | ||
| Fig. 1 Procedure of the lignin isolation.59 Adapted and modified with permission of Francis and Taylor publishers. | ||
The process of creating milled wood lignin (MWL) begins with dry biomass being ground in a ball mill until it passes through a 0.50 mm screen, which mechanically breaks the connections between lignin and polysaccharides. Next, the powdered material is dissolved in toluene and extracted using a natural solvent with less than 4% water content, which can be ethanol, acetic acid, or dioxane.
Finally, the lignin found in the natural solvent–water mixture can be recovered as sediments by slowly adding the mixture into deionized water. According to El Hage et al., Holtman et al. and Rencoret et al., this method of preparation falls into the first group.60–62 Although the yield of isolated MWL is often low compared to other techniques, this method has the least impact on lignin structure, as noted by Bjorkman.63 As a result, MWL is frequently used in structural evaluations but is not suitable for commercial use. On the other hand, lignin can also be produced from unprocessed biomass through substantial acid hydrolysis64 or hydrolysis with enzymes40 to break down carbohydrate components and liberate lignin. After the removal of the broken-down carbohydrates, lignin is left as an insoluble residue, which is why this method of preparation falls into the second group.
Cellulase is a commonly utilized enzyme in enzyme-mediated hydrolysis, as reported in a recent study.40 However, other acid hydrolysis agents, such as sulfuric acid,64 nitric acid,65 hydrochloric acid,66 phosphoric acid,67 oxalic acid,68 periodic acid,69 and peracetic acid,68 are also frequently employed in this process. According to Y. Sun and Cheng (2002),70 the acid hydrolysis stage is the most crucial step in this process, as it liberates dissolved polysaccharides and yields pure lignin-containing solid residues. Concentrated or diluted acid solutions can be used for acid hydrolysis at typical pressure and temperature conditions, as indicated by Sluiter and coworkers,71 or at high temperatures (165–195 °C), as reported by Guo and his colleagues.72 Horst et al. mentioned that sulfuric acid and hydrochloric acid are commonly utilized to hydrolyze Klason and Willstätter lignins, which are examples of hydrolytic lignin.66 In the traditional Klason process, the pretreatment step biomass (milled, dewaxed, defatted, deproteinized, etc.) is initially incubated in 72% H2SO4 at 37 °C for two hours. To remove all polysaccharides, the mixture is then reduced to 3% H2SO4 and refluxed for four hours at 800 °C. The Klason process is considered a standard technique for determining the amount of lignin in plant materials due to its high yield.73 For a comprehensive understanding of the Klason process, one may refer to the Carrier et al.64 paper. The Klason process is expected to cause structural changes in lignin due to its strong acidic conditions. Specifically, the formation of phenolic groups and the breakdown of certain aryl and alkyl ethers in units of benzyl alcohol are anticipated.74 Consequently, the Klason procedure is not suitable for adequately preparing lignin for structural characterization. During the acid hydrolysis process, 42% hydrochloric acid was utilized by the Willstatter lignin. The different methods are summarized in Fig. 2.
 | ||
| Fig. 2 Flow diagram of preparation processes of milled wood lignin.59 Adapted and modified with permission of Francis and Taylor publishers. | ||
Regarding the process of enzymatic hydrolysis, lignin's carbohydrates are extracted through the use of enzymes that break down cellulose and hemicelluloses. This results in insoluble leftovers referred to as lignin hydrolyzed enzymatically (EHL), which typically contain approximately 95% of the original lignin and are contaminated with around 10–12% of protein and carbohydrate contaminants, making subsequent analysis more challenging.57,75 According to Yin and his coworkers,76 the enzymatic hydrolysis in this process is often carried out under mild conditions, which preserves the majority of the active functional molecules, particularly phenolic and alcoholic hydroxyl groups, in lignin. An example of the EHL process is provided in the paper by Zhang et al.40
During the pulping stage, lignin is extracted from biomass using a high temperature and a NaOH solution (4–7%).77,78 This results in the formation of a black liquid, which can be separated from the lignin by acidification with HCl or CO2. If a solution of NaOH and Na2S is used as the pulping medium, kraft lignin is generated,79 while if a NaOH solution is used without Na2S, soda lignin is produced.80 These days, softwood and non-wood materials such as peel and pomace are typically processed using the soda process, while hardwood materials are typically processed using the kraft process. During the soda process, lower molecular weight lignin fragments can be produced when alkali hydrolysis partially cleaves the a-ether linkages in lignin.55
The use of strong oxidants, such as anthraquinone (AQ) or H2O2, is frequently employed in the extraction of linked carbohydrates from lignin in alkali solutions.81 In the Kraft method, the Na2S dosage is typically determined to be 16 g per liter of 1 M NaOH. By attaching to the a-carbon atom in the ether linkages, sulfide and bisulfide ions have the potential to break portions of the lignin's ether connections during this process. Unlike soda lignin, kraft lignin contains approximately 1% sulfur in the form of aliphatic groups.82
The level of acidity when precipitating alkali lignin has a significant impact on the molecular weight of the end product; the greater the extent of acidity, the lower the product's molecular weight. The sulfite pulping process, which treats biomass with a water-based solution consisting of sulfur dioxide and a sulfurous acid salt at temperatures ranging from 125 to 1500 °C, results in sulfonated lignin, also known as lignosulfonate.55 Acid hydrolysis may be used in this method to cleave the bonds to polysaccharides,80 and it may also partially destroy the a- and b-ether linkages in lignin.83 The resulting lignin contains carboxylic groups, phenolic/aliphatic hydroxyl groups, and functional groups such as lignosulfonic acid (LA), lignosulfonate, and carboxylic group, depending on the type of sulfurous acid salt used, such as calcium sulfite,84 sodium sulfite,85 or magnesium sulfite.86 Sulfite lignin is water-soluble due to its high sulfonate content (up to 13%).82 Lignin can be extracted from pulping liquors through various methods, such as dialysis, membrane filtration, and alcohol precipitation. According to the study reported by Shimizu et al.,83 these techniques may partially degrade the a- and b-ether linkages in lignin. By using different sulfurous acid salts, like calcium sulphite,87 sodium sulphite,85 or magnesium sulfite,86 the resulting lignin contains functional groups such as carboxylic groups, phenolic/aliphatic hydroxyl groups, and lignosulfonic acid (LA), lignosulfonate, and carboxylic group.
Organosolv lignin is produced by heating biomass in a dilute organic solvent (40–80%, v/v) at temperatures ranging from 1400–2200 °C.88 In this process, the a-ether connections in lignin are broken down using hydrolytic cleavage, and an acid or base catalyst is employed to dissolve the lignin fragments in the solvent.80 A variety of organic solvents, such as acetone, ethanol, methanol, formic acid, or acetic acid, can be used along with different catalysts like HCl and NaOH-AQ combination.89 Some of the commonly used techniques include Alkaline sulfite-AQ-methanol (ASAM),90 Formacell,91 Alcell,89 Organocell,92 and Acetosolv.93 The Alcell process utilizes a sulfuric acid catalyst (approximately 1.0%) and a dilute ethanol solution (about 65%, v/v) to process biomass.94 The Organocell method employs a 30% NaOH catalyst in combination with an aqueous methanol solution (40%, v/v), either with or without AQ.55 The Acetosolv technique involves heating biomass in a 93% (v/v) aqueous acetic acid solution with less than 1% HCl as a catalyst.55 The Formacell approach uses a water-based solution containing peroxyformic acid, which is created when formic acid and H2O2 are combined.95 Finally, the ASAM procedure utilizes an aqueous NaOH solution (approximately 14%) containing methanol (15%), AQ (0.1%), ethylene diamine tetra acetic acid (0.5%), and Na2SO3 (Na2SO3/NaOH ¼ 80/20) at 1500 C.55 Both annual plants and hardwoods can be processed effectively with the organosolv approach. Apart from the ASAM method, organosolv lignin is sulfur-free and has a low molecular weight of roughly 5000.55 Additionally, the organosolv process can be enhanced by using a novel pulping approach of steam explosion before solvent extraction, which has become more and more common as an organosolv technique in recent years.83 Furthermore, pretreating biomass with ion solutions such as 1-ethyl-3-methylimidazolium acetate has been shown to lessen the greenhouse gas emissions of volatile organic solvents, making it a more environmentally friendly option.96
Strategies for lignin incorporation into polymeric film
| Lignin derived materials | Barrier and antibacterial properties | Mechanical properties | Thermal durability | Ref. |
|---|---|---|---|---|
| Lignin—gellan gum-hydroxyethyl cellulose | Enhanced hydrophobicity, UV, antioxidant property | Tensile strength (MPa): 23.0 ± 1.1,39.0 ± 0.8 (respectively, for gellan gum, lignin—gellan gum-hydroxyethyl cellulose) | T g (°C): 149.2 ± 0.5, 156.9 ± 0.3 (for gellan gum, lignin—gellan gum-hydroxyethyl cellulose, respectively) | 111 |
| Exhibited better antibacterial and non-cytotoxic properties | EB (%): 20.3 ± 0.4,32.5 ± 0.4 (respectively, for gellangum, lignin—gellan gum-hydroxyethyl cellulose) | T m (°C): 205.6 ± 0.6, 216.0. ± 0.3 (for gellan gum, lignin—gellan gum-hydroxyethyl cellulose, respectively) | ||
| Cellulose–lignin films | Enhance antibacterial and UV-shielding, durability | Tensile strength (MPa): 75.90 | First weight loss (room temperature to 160 °C) was about 10% | 121 |
| Enhanced antibacterial property, hydrophobicity | T onset of cellulose film is 270 °C, which increased to 275 °C and 290 °C | |||
| Good water vapor and oxygen barrier ability of the phenolated lignin/cellulose | T max (maximum mass loss temperature) values are 291, 308 and 320 °C for the cellulose and cellulose-lignin derived films, respectively | |||
| Carboxymethyl cellulose (CMC) and lignin film | Enhanced physical properties, thickness, solubility, moisture content, and water vapor permeability (WVP) were improved from 0.09 to 0.14 mm, 84.75 to 51.03%, 31.34 to 19.30%, and 4.98 to 1.08 × 10−10 g m−1 s−1 Pa−1, respectively | Tensile strength (TS) increased from 18.29 to 32.61 MPa and elongation at break (EAB) of the CMC-lignin films from and 32.5–45.3% | — | 122 |
| Poly(butylene succinate (PBS) based lignin films | — | PBS tensile modulus (MPa) 636.8 ± 56.9 (machine direction MD), 794.7 ± 50.4 (transverse direction TD) | (PBS melting temperature (Tm) 112.0 °C, crystallization temperature (Tc) 91.0 °C | 123 |
| Tensile strength (MPa) 38.3 ± 3.5 (MD), 35.4 ± 5.3 (TD) | PBS based lignin films Tm 112.3 °C, Tc 90.7 °C | |||
| Elongation at break (%) 279.3 ± 30.9 (MD), (%) 8.8 ± 2.4 (TD) | ||||
| Tensile modulus 652.0 ± 37.5 (MD), 809.7 ± 69.9 (TD) | ||||
| Tensile strength (MPa) 37.3 ± 1.8 (MD), 33.5 ± 2.5 (TD) | ||||
| Elongation at break (%)282.8 ± 15.8 (MD), 6.7 ± 0.5 (TD) | ||||
| Polyvinyl alcohol (PVA)/chitin-lignin nanoparticles (PVA/CI/LNP) films | Enhanced barrier properties | Tensile strength 23.40 ± 1.36 | PVA (degradation temperature) (DT) 75.99 °C, (weight loss) (WL) 95.30% at first peak | 118 |
| PVA moisture (%) 16.33 ± 1.70 | Elongation (%) 121.6 ± 0.12 | PVA/CI/LNP (DT) 96.34 °C, (WL) 97.00% | ||
| PVA/CI/LNP moisture (%) 5.79 ± 1.81 | PVA/Ci/LNP tensile strength 36.82 ± 0.17, elongation (%) 121.2 ± 0.13 | |||
| Alkali lignin lignosulfonate—soy protein isolate | Enhanced thermal, mechanical, and UV barrier qualities decreased penetration of the vapor of water | TS (MPa): 4.74 ± 0.34, 8.01 ± 0.89,10.98 ± 1.02 (respectively, for soy protein, 10% lignosulfonate–soy protein, 10% alkali lignin—soy protein) | First weight loss 50–100 °C. The second weight loss occurred at around 300 °C | 112 |
| EB (%): 126.33 ± 17.9, 79.95 ± 5.32, 7.45 ± 1.24 (respectively, for soy protein, 10% lignosulfonate—soy protein, 10% alkali lignin—soy protein) | ||||
| Lignin—nanocellulose | Improved UV protection and oxygen permeability | Tensile strength (MPa) 22.8 | — | 124 |
| Elastic modulus (GPa) 4.7 | ||||
| Breaking strain (%) 0.7 | ||||
| Lignin—poly (lactic acid) (PLA) | Strong antioxidative action | TS(MPa): ∼40, ∼30 (respectively, for PLA, PLA—40% lignin) | T onset (°C): 323.6, 306.1 for PLA, PLA—40% lignin, respectively) | 125 |
| EB(%): ∼15,∼2 (respectively, for PLA, PLA—40% lignin) | T max (°C): 330.2, 320.7(for PLA, PLA—40% lignin, respectively) |
A recent study has shown that wood-inspired biopolymeric nanocomposite films offer a promising solution to environmental concerns in food packaging. These films are made from sustainable materials, including cellulose nanofibers, lignosulfonates, and beechwood xylans, and possess exceptional qualities such as UV protection, mechanical strength, and fruit preservation (see Fig. 3). This study emphasizes the potential for cost-effective and eco-friendly packaging solutions that prioritize sustainability.113
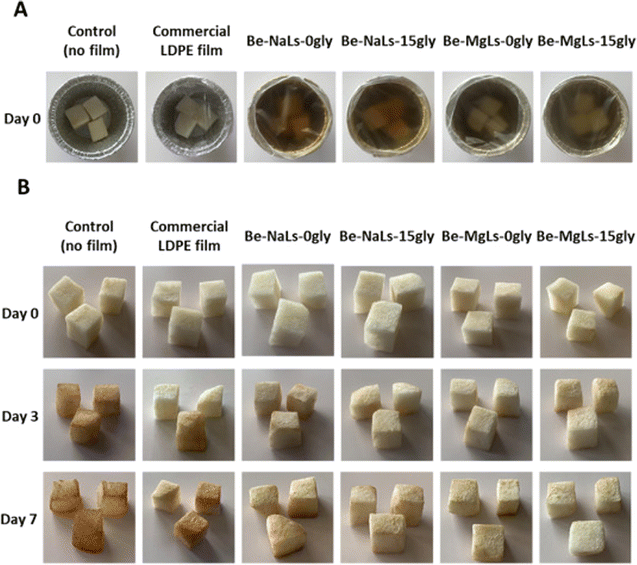 | ||
| Fig. 3 (A) Digital photos of the packed fruits (B) freshly cut pears before and after storage in the refrigerator at 4 °C (3 and 7 days). Reproduced with the permission of Elsevier.113 | ||
A study reported that lignin, an abundant biopolymer presents in agricultural biomass and characterized by its sustainability and biodegradability, can be utilized as a viable alternative for the production of biomaterials, particularly polymer packaging. After extracting and analyzing it from wheat straw, the incorporation of lignin into nanocomposite films showcases its potential to enhance antioxidant, antibacterial, and UV protective properties. The resulting films exhibit strong antimicrobial activity against a variety of pathogens and effective UV protection, highlighting their potential as eco-friendly, cost-effective, and sustainable biomaterials. Moreover, the inherent qualities of lignin-based films—such as low cost, biocompatibility, flexibility, and transparency—further support their applicability in the food packaging industry.114
Recently, researchers have developed lignin nanoparticles (LNPs) using the deep eutectic solvent (DES) anti-solvent approach and combined them with a polyvinyl alcohol (PVA) matrix to create nanocomposite films. These films exhibit improved mechanical, thermal, and hydrophobic qualities, as well as good UV protection, potent antioxidants, and strong antibacterial activity against E. coli and S. aureus as exhibited in Fig. 4. The use of LNPs in PVA-based nanocomposite films holds great promise for their application in active food packaging.115
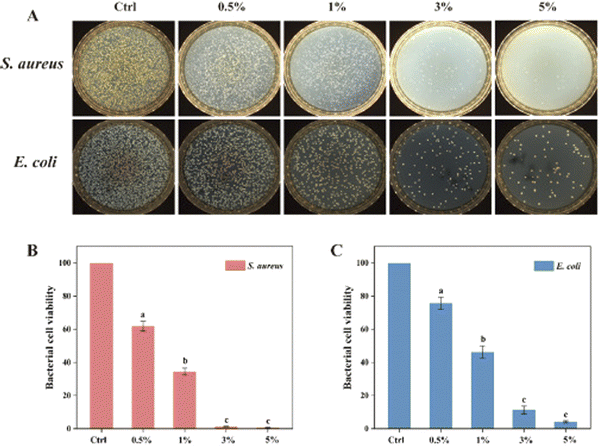 | ||
| Fig. 4 Plate count images of the bactericidal activity of nanocomposite films (A) corresponding to bacterial cell viability of S. aureus (B) and E. coli (C). Reproduced with the permission of Elsevier.115 | ||
Garg and coworkers studied that incorporating lignin's exceptional properties, such as its tensile strength, antioxidant, antibacterial, and UV barrier properties, along with chitosan's film-forming capabilities, could lead to the development of improved food packaging materials. The refined hydrogel formulations show enhanced bioactivity and reduced production costs, suggesting the potential for creating environmentally friendly food packaging solutions. Furthermore, the optimized synthesis method enhances the efficiency of hydrogel film production.116 According to another study, incorporating lignin into biodegradable polylactic acid (PLA) composite films as a reinforcing filler proves effective. Researchers created lignin-grafted polylactic acid co-polymers and mixed them with PLA through in situ polymerization, resulting in films with improved mechanical properties and strong antioxidant capabilities. These findings suggest a promising path for the creation of high-performing, eco-friendly packaging materials.117
An intriguing study investigates the use of oxytenanthera abyssinica derived lignin nanoparticles (LNPs) in enhancing the properties of polyvinyl alcohol/chitosan (PVA/CI) and polyvinyl alcohol/chitin (PVA/CH) films for active food packaging. The addition of LNPs at concentrations of 1% and 3% improved the films' mechanical characteristics, antioxidant capacity, and thermal stability. Furthermore, the inclusion of LNPs enhanced the UV-blocking and antimicrobial properties, and prevented the migration of dietary stimulants, making these films promising candidates for use in active food packaging.118
Researchers have recently uncovered the promising potential of carbon dots (CDs), made from sustainable lignin, for use in intelligent sensing applications in food packaging. By incorporating CDs into a carrageenan biopolymer matrix, a composite film is produced that exhibits increased mechanical characteristics, pH-dependent color change, UV radiation blocking, and enhanced hydrophobicity. Additionally, the film demonstrates good antioxidant and antibacterial qualities, as well as notable decreases in CO2 gas permeability and oxygen transmission rate as presented in Fig. 5. The practical use of this responsive packaging material in tracking milk deterioration through color changes showcases the potential for sustainable and intelligent food packaging solutions.119
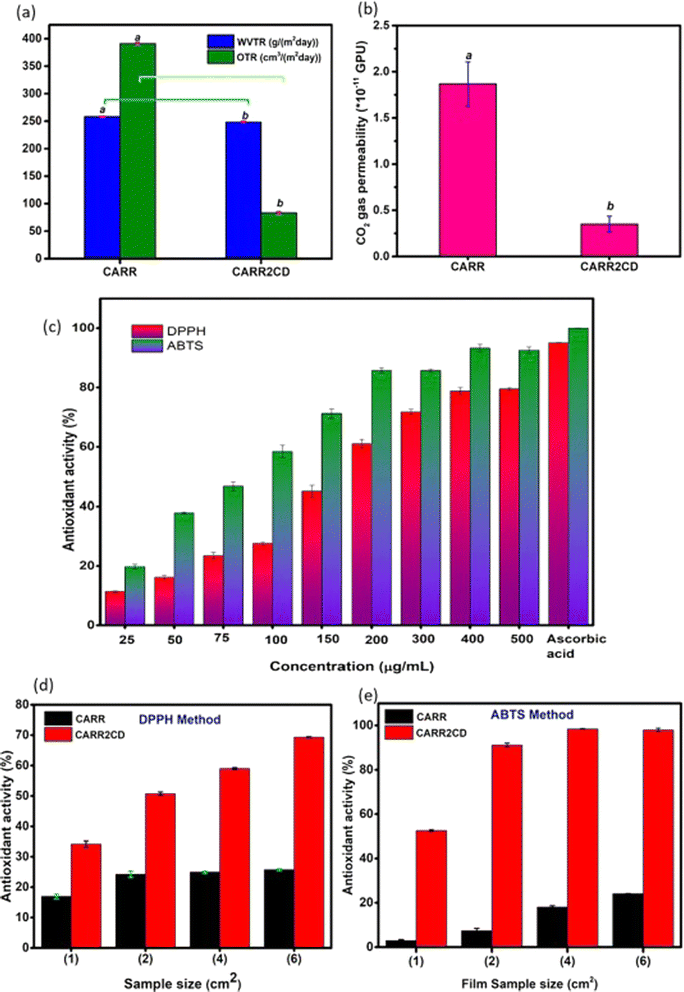 | ||
| Fig. 5 (a) Water and oxygen barrier properties of Carrageenan and Carrageenan-CD films (b) CO2 barrier property of Carrageenan and Carrageenan-CD films (c) antioxidant activities of CD solution against ABTS and DPPH radicals (d) antioxidant activity of films against DPPH free radicals (e) antioxidant activity of films against ABTS free radicals. Reproduced with the permission of Elsevier.119 | ||
A recent study has produced eco-friendly food packaging films by combining lignin with a matrix of potato starch and polyvinyl alcohol (PVA). The addition of lignin significantly improves the tensile strength, UV barrier, antioxidant activity, water vapor barrier, and antibacterial properties of the film. The development of a polymer network structure by hydrogen bonding, as confirmed by structural analysis, increases the interfacial compatibility between polymers. Lignin's high phenolic hydroxyl content facilitates its antioxidant action through a proton-coupled electron transfer pathway. This study presents a promising method for developing multifunctional composite films using elements derived from biomass, which have significant potential for use in food packaging.120
Catignani and Carter139 discovered that adding lignin (l–10%) to the rat diet increased the amount of retinol deposited in the liver by 50–100%, despite the lack of data available at the time. Unfortunately, until recently, no clinical data was available on lignin's antioxidant properties. However, it should be noted that the mechanism underlying lignin's in vivo antioxidant action remains unknown due to the material's poor absorbability from mice's gastrointestinal tracts.140 Despite this, the prebiotic benefits of kraft lignins and Alcell for farm animals suggest a feasible in vivo approach through gut flora regulation.141 Within the food chain, it was found that 2.5% of kraft lignin derived from wood sources was just as effective at preventing maize oil oxidation caused by heating it to 1000 C and aerating it at a rate of 180 mm per minute as 0.03% vitamin E.139 Furthermore, milled wood lignin from green tea leaves may block the autoxidation of linoleic acid by 50%, although it is less efficient than the commercial antioxidants of VE and BHA. Remarkably, lignin has been shown to enhance the antioxidant capacities of a-tocopherol and gallate (EPG).142 Quercetin and lignin have also been found to have a combined antioxidant impact.143 As demonstrated, the specific technique employed and the genetic origins of the starting material have a significant impact on the antioxidant capacity of separated lignin. For instance, Dizhbite and coworkers discovered that alkali lignins from spruce, birch, and aspen—all produced using the same technique—display antiradical capabilities that are, 0.5, 1.0, and 1.1,144 respectively.
The results of the study conducted by Dong et al. indicated that the soda lignins derived from maize stover and prepared under different conditions displayed varying degrees of antioxidant activity. Specifically, the sample extracted at 950 °C for 120 minutes with a solid/solvent ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (w/v) exhibited the highest level of antioxidant activity among the four samples tested.129 This finding is consistent with previous research demonstrating that lignins derived from the same biomass can have distinct antioxidant properties.145 It is widely accepted that the structural features of lignin play a critical role in determining its antioxidant activity. For instance, the presence of unbound phenolic hydroxyl, methoxy, aliphatic hydroxyl, and double bonds between the side chain's outermost carbon atoms have been reported to contribute to lignin's antioxidant activity.144,146 The mechanism by which lignin actsas an antioxidant is depicted in Fig. 6, which includes initiation, propagation, termination, and several advanced oxidized products.
4 (w/v) exhibited the highest level of antioxidant activity among the four samples tested.129 This finding is consistent with previous research demonstrating that lignins derived from the same biomass can have distinct antioxidant properties.145 It is widely accepted that the structural features of lignin play a critical role in determining its antioxidant activity. For instance, the presence of unbound phenolic hydroxyl, methoxy, aliphatic hydroxyl, and double bonds between the side chain's outermost carbon atoms have been reported to contribute to lignin's antioxidant activity.144,146 The mechanism by which lignin actsas an antioxidant is depicted in Fig. 6, which includes initiation, propagation, termination, and several advanced oxidized products.
 | ||
| Fig. 6 Various reactions showing lignin antioxidant mechanism. Reproduced with the permission of Nature Springer Publisher.149 | ||
The concentration of phenolic hydroxyl in lignin is a key structural feature that determines its antioxidant capacity. It is worth noting that studies have shown that low molecular weight lignins tend to have greater antioxidant activity than those with higher molecular weights.147,148 This can be attributed to the fact that lignin fragmentation exposes more phenolic hydroxyls, thereby increasing its antioxidant activity. However, the condensation reaction can harm lignin's antioxidant activity.
| Source of lignin | Filler | Matrix | Microorganisms | Ref. |
|---|---|---|---|---|
| Alkaline lignin | Lignin nanoparticles (LNPs) | Pectin | S. aureus and E.coli | 156 |
| Corn straw | Silver-lignin nanotube | Corn straw | S. aureus and E.coli | 157 |
| Alkaline lignin | Citric acid and acetylated modified LNPs | Polylactic acid | E. coli and M. luteus | 158 |
| Commercial soda lignin (PB1000) | LNPs | Cellulose nanocrystals and cellulose nanofibrils | S. aureus | 159 |
| Alkali lignin (Sigma-Aldrich) | Nanolignin and 0.5 wt% metal oxide nanoparticles (Ag2O, TiO2, WO3, Fe2O3 and ZnFe2O4) | Polylactic acid (PLA) | S. aureus and E. coli | 160 |
| Acacia mangium pulp mill waste | Starch/polylactic acid | Lignin biofilm | E. coli, Salmonella typhi and S. aureus | 161 |
| Lignin (Shandong Longlive co., Ltd.) | Lignin-zinc oxide hybrid particles | (Butyleneadipate-co-terephthalate) (PBAT) | Contact type antibacterial property improved bacterial adhesion decreased | 162 |
| Bamboo/alkaline | Polyethyleneimine-lignin contained cellulose nanofibers | Poly(vinyl alcohol) (PVA) nanocomposite films | S. aureus and E. coli | 163 |
| Lignosulfonate | Tannic acid @ sodium lignosulfonate–Ag nanoparticles | PVA | S. aureus and E. coli | 164 |
 | ||
| Fig. 7 Mechanism of antimicrobial activity of lignin nanoparticles for food packaging applications. Reproduced with the permission of Nature Springer Publisher.149 | ||
With regard to yeast, spruce organ cells contain lignin that inhibits strains of R. rubra, A. pullulans, B. alba, and C. tropicalis, whereas the C. albicans strain is unaffected. However, the oil palm empty fruit bunch alkali lignin is ineffective against Candida albicans, but shows inhibitory effects against Candida lipolytica. Additionally, alkali lignin from maize stover is effective against some strains of fungi. Apple tree pruning leftovers, on the other hand, are observed to hinder the growth of S. cerevisiae. The chemical structure, manufacturing technique, and genetic heritage of lignin all play a role in its antibacterial activity, as do the targeted strain of the microbe and the working concentration. It is clear that lignin's antibacterial activity is not fully established, but its dependence on these factors is evident. In light of the chemical structure of lignin, two primary factors are the side chain structure and its functional moieties. Specifically, the presence of oxygen-containing groups (–OH, –CO, and –COOH) on the side chain is consistently associated with poor inhibitory power. However, isoeugenol, a phenolic component of lignin, displays an excellent inhibitory action due to its double bonds position of the side chain and the methyl group.141 Furthermore, the aromatic compounds linked to the antibacterial activity of lignin are similar to antibiotics like methicillin, carbenicillin, and benzyl penicillin.155
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 155, respectively.168 Furthermore, Matsuhisa and coworkers169 observed a ten-fold increase in the vitality of hepatitis C virus-infected Huh7.5.1 cells when using the low-molecular-weight lignin from Lentinula edodes mycelia solid culture, compared to its hot-water extract, suggesting the lignin's antiviral activity.
000 and 155, respectively.168 Furthermore, Matsuhisa and coworkers169 observed a ten-fold increase in the vitality of hepatitis C virus-infected Huh7.5.1 cells when using the low-molecular-weight lignin from Lentinula edodes mycelia solid culture, compared to its hot-water extract, suggesting the lignin's antiviral activity.
Research has confirmed that lignins possess potential antiviral properties against various viruses, including HIV.167 According to study reported by Srisapoome et al., intramuscular injection of kraft lignin (from Penaeus monodon Linn., black tiger shrimp) and yellow head virus solutions at lignin concentrations of 1–20 mg L−1 before incubation significantly reduced shrimp mortality rates at 14–20 days after injection.170 These findings provide compelling evidence of lignin's antiviral properties. The two main contributing factors for lignin's antiviral activity were found to be lignosulfonic acid (LA) and LCC. As early as 1990, it was discovered that LCC exhibited antiviral properties. Lai et al. provided evidence that LCC from pine cones may prevent HIV replication.171 The LCC from seeds of Pimpinella anisum significantly decreased human cytomegalovirus, measles virus, and herpes simplex virus types 1 and 2 with selectivity index values up to 140, 210, and 3100, respectively.172 In a study, it is reported that the high selectivity index of up to 77 indicates that LCC generated from cocoa mass demonstrates excellent anti-HIV activity.173 LA, a prominent member of the lignin-derived macromolecule family, has a well-established antiviral activity. Qiu et al.174 verified LA's bactericidal potential against HIV-1, with EC50 values versus the R5 and X4 HIV-1 strains being 6.323 mg mL−1 and 1.411 mg mL−1, respectively. Gordts et al.166 reported similar results, demonstrating that LA may significantly inhibit the multiplication of the four HIV strains in MT-4 and peripheral blood mononuclear cells. Furthermore, a clinical trial conducted by Lopez et al.175 found that oral lignin-ascorbic acid supplementation decreased the severity of symptoms and the likelihood of recurrent HSV-1 infection. Vinardell and Mitjans176 analyzed the extensive data about the antiviral in nature functions of LA and LCC. The literature currently available suggests that the possible antiviral efficacy of lignin is dependent on several factors, such as the lignin's source,173 the virus strain,169 and the parameters of treatment (lignin concentration, length of treatment, and stage of virus infection, among others).170,172 The relationship between lignin concentration and dose dependency is commonly established in research studies.169,170 Although the exact mechanism underlying lignin's antiviral effect remains unclear, various research proposals have been put forth. Virus infection occurs when viral apolipoprotein E (apoE) interacts with heparin sulfate on the host cell surface.56 Therefore, lignin's ability to bind to viruses may reduce their adsorption and penetration of host cells. A study suggested that lignin's structural resemblance to heparin sulfate enables it to compete with viruses for cell surface binding, thereby inhibiting their ability to adhere to and infect cells.177 Secondly, lignin has been demonstrated to inhibit the activities of several viral enzymes, including RNA polymerase, reverse transcriptase, protease, and plaque formation,171,178,179 thereby inhibiting viral replication. Lastly, lignin's antioxidant properties may strengthen host cells' defense against viral infection by reducing oxidative stress.175
Lignin's functional groups, such as phenolic units, ketone molecules, and chromophores, enable it to absorb UV light, making it a potent UV blocker. In addition to its antioxidant and radical-scavenging properties, lignin's functional groups contribute to its exceptional UV blocking capabilities.181 Lignin can absorb a broad spectrum of UV radiation with wavelengths ranging from 250 to 400 nm and contains chromophore functional groups.182 Despite its abundance as a byproduct of agriculture, lignin has traditionally had limited utility. However, active packaging, which serves not only as an inert barrier but also as an additional means of preserving food, represents a promising application for this versatile biopolymer.183 Lignin presents a wide range of frameworks, purities, and related properties, which depend on its sort, source, and extraction method. Some of the distinctive qualities of lignin are its capacity to absorb UV light, low polarity (i.e., hydrophobic qualities), and antioxidant properties.184 These characteristics make lignin an appealing component in the production of film materials that have UV blocking and reduced hydrophilicity.185 Lignin has also been studied as a potential additive to polymeric materials to protect them from UV radiation-induced photodegradation, which results in yellowing and a loss of mechanical characteristics.186
Lignin's radiation-protective properties have attracted significant attention, particularly its ability to act as a natural UV blocker. Lignin is the second most abundant renewable biomass on Earth and is rich in aromatic rings, making it an attractive candidate for shielding polymeric materials from UV light.187 Lignin's composition of phenolic units, chromophores, and ketones allows it to naturally block almost the entire UV light spectrum. Additionally, lignin possesses antifungal, antibacterial, and antioxidant qualities.188 The potential use of lignin as a replacement for artificial absorbers in a composite material is due to its strong UV-shielding ability. The sun produces electromagnetic radiation in the form of UV radiation, which can be divided into three wavelength bands: UVA (315–400 nm), UVB (280–315 nm), and UVC (100–280 nm).189 The commercialization of lignin-based UV shield solutions is hampered by their unappealing black appearance, despite their remarkable capacity to absorb UV light.99 Lignin's complex structure, polydispersity in molecular weight, brownish tint, and numerous impurities make it difficult to utilize as a UV blocker. Further research is needed to render lignin a suitable bio-based UV blocker.190
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μg mL−1). Evidence for lignin's ability to lower blood cholesterol dates back to 1968, when research on its ability to bind bile acids in vitro was conducted.199 Recent studies have demonstrated the excellent ability of bamboo shoot shell lignin to bind a range of bile salts, including sodium deoxycholate, sodium chenodeoxycholate, sodium taurocholate, and sodium cholate.150In vivo findings by Thiffault et al.200 revealed that lignin was effective in reducing elevated blood cholesterol levels; however, further research showed that lignin was not successful in sequestering conjugated bile acids.191
μg mL−1). Evidence for lignin's ability to lower blood cholesterol dates back to 1968, when research on its ability to bind bile acids in vitro was conducted.199 Recent studies have demonstrated the excellent ability of bamboo shoot shell lignin to bind a range of bile salts, including sodium deoxycholate, sodium chenodeoxycholate, sodium taurocholate, and sodium cholate.150In vivo findings by Thiffault et al.200 revealed that lignin was effective in reducing elevated blood cholesterol levels; however, further research showed that lignin was not successful in sequestering conjugated bile acids.191
The use of biodegradable materials in food packaging has gained significant attention due to environmental concerns and the depletion of petroleum resources. Cellulose, protein, and starch are commonly used in natural biopolymer packaging sheets, which are more environmentally friendly compared to conventional plastic films. However, these materials suffer from limitations such as poor mechanical and water resistance, which hinder their industrial application. To overcome these limitations, lignin has been incorporated as filler in biodegradable films to enhance their properties. Lignin-incorporated coatings also exhibit antibacterial, antioxidant, and anti-ultraviolet characteristics, making them suitable materials for food packaging. Several studies have investigated the effects of lignin addition on the physical, optical and bioactive properties of various biodegradable materials.
Milling is a widely adopted method for the production of nanolignins from plant sources, and it is a cost-effective approach to generat lignins with nanomolecular-sized particles, as per Sharma and Kumar study.202 For instance, 5 g L−1 of kraft lignin can be homogenized for 4 hours at 15 K rpm to produce 500 nm of kraft lignin-derived nanolignin. Moreover, an acoustic method based on sonication is used to manufacture stable 10–20 nm nanolignin with a uniform distribution of 0.7% lignin solution generated from wheat straw and Sarkanda grass. The optimal parameters for producing such nanoscale materials without the generation of free radicals are 600 W of power inputs for one hour in a uniform stable nanodispersion with a size of 10–20 nm and 20 kHz sound waves.203
The extraction of lignin from biomass sources, such as softwood kraft lignin, has been a challenge due to its complex structure and poor solubility in common solvents. However, by utilizing a well-managed solvent shift approach, mid-sized nanolignins can be produced, despite their relatively low yield. The solvents selected for this method include acetone/water, tetrahydrofuran, dimethyl sulfoxide, and acetone.202 The solvent-shifting method has been shown to yield lignin nanoparticles with a size of 200–500 nm, using kraft lignin and tetrahydrofuran and water, while the particle size of tiny nanolignin was found to be 221 ± 10 nm.192
It has been found that the precipitation of lignin nanoparticles can be influenced by changes in pH, resulting in stable nanostructures with tightly packed lignin moieties. This occurs through two separate processes. In the first process, an ethylene glycol solution containing Indulin AT is added, followed by aqueous HCl, which leads to the formation of biodegradable nanoparticles that are pH stable. In the second procedure, lignin and aqueous sodium hydroxide are mixed, and when coupled with HNO3, they yield the necessary precipitates. This method produces stable nanolignins below pH 5 that are safe for the environment. Furthermore, model microorganisms including Saccharomyces cerevisiae and Chlamydomonas reinhardtii were found to thrive unaffected by lignin-sourced nanostructures, ensuring the viability of cells.202
Caicedo et al.204 synthesized nanomaterials derived from lignin by template synthesis. This process involves a Schiff's base reaction between the aldehyde molecules of thioglycolate lignin and its amino counterparts in the alumina-activated template (APTES). Once hydroxycinnamaldehydes, hydroxycinnamates, or hydroxyzine. Antimicrobial silver nanoparticles with a uniform dispersion of 45–55 nm pseudo spherical, facilitated by reductant-assisted template synthesis of lignin molecules, are produced. Although template synthesis offers numerous benefits, it also causes the toxicity factor associated with surfactants like SDS and CTAB. In addition, the removability of source materials and problems with purification are limiting variables that compromise the overall performance of this technology.
To produce lignin nanofiber scaffolds, a solution containing frozen monomer units is first used. This process results in the formation of ice crystals, which grow and separate the polymer phase. Alkali lignin serves as the source material for homogenous lignin nanofiber scaffolds that are formed under the influence of a rotating drum operating at 300 rpm and 77 K of liquid nitrogen serving as a coolant.
To produce nanolignin via electrospinning, a solution of polymers is required as the base substance. The mixture can be fed through a 100 m wide nozzle that acts as an electrode and/or an auxiliary electrode system once it has been mixed with the appropriate solvent. When employing electrospinning to produce nanolignin, the optimal E-field level ranges from 100 and 500 kV m−1.
Supercritical fluids like carbon dioxide (CO2) are widely utilized in the synthesis of nanolignin due to their non-toxic, non-flammable, and cost-effective nature. Specifically, CO2 is an ideal choice for supercritical fluid-mediated processes owing to its favorable critical pressure and temperature of 7.4 MPa and 304.3 K, respectively. By reacting lignin with CO2 and acetone at 30 MPa and 35 °C, fine lignin nanoparticles with a diameter of 144 nm can be produced.205
The pressurized CO2 antisolvent method can also be employed to create environmentally acceptable lignin nanoparticles (LNPs). In this technique, CO2 is first pumped to a chiller maintained at 258.2 K and then transferred to the precipitator, where it is liquefied. After the precipitating unit has settled, a lignin remedy is added, and the resulting lignin nanoparticles are weighed. The mixture is then exposed to sonication for thirty minutes at room temperature and sprayed through a nozzle controlled by preset flow rates. The particles that pass through the paper filter are collected. It is important to note that this technique does not apply to CO2 immiscible chemicals.202
In this process, an antisolvent such as water is mixed with a lignin solution that has been prepared in an organic solvent. The solvents used are not polar, and water is often employed as an antisolvent because lignin is either immiscible or sparingly soluble.202 In one study, the antisolvent precipitation method was used to produce lignin particles (ALNP and DLNP) measuring 80–104 nm with strong UV shielding and radical scavenging properties. The researchers used acetone and water, constantly stirring at 20 °C and 300 rpm.206 However, this method has some drawbacks, including the instability of the colloid system, the morphology of the generated nanoparticles, and the permanent nature of the solvents.202
Gilca et al.203 used this method to create stable nanoparticles of lignin from a lignin (0.7%) solution. The aqueous solution was then subjected to an hour-long acoustic treatment using an ultrasonic probe that generated a 20 kHz frequency at 600 W. Mild conditions were applied to allow the uniform nanosuspension to dry.203
Another feedstock for LNP synthesis is steam-exploded rice straw lignin (SERSL). To create a homogeneous solution, the SERSL solution mixture and castor oil (20 wt%) were stirred. For four hours, 20 mL of (1 molar) HCl was added in the form of drops at 50 °C with the nitrogen setting in place. The newly generated lignin nanoparticles were thoroughly washed in ethanol and water until the pH reached 7.207
Nanoparticles with a diameter ranging from 45 to 250 nm were manufactured through a new method that utilized precursor materials including kraft lignin and organosolv. The synthesis process began with vortexing a kraft solution in ethylene glycol for 30 minutes and then filtering the mixture using a 0.45 μm pore size syringe filter. Subsequently, 1–3 mL of 0.025 M nitric acid was quickly combined with 5 mL of the purified solution in a scintillation vial, and the mixture was shaken continuously, resulting in the formation of particles.208–210 The second method involved vortexing and filtering the acetone/organosolv solution in stock in the same initial steps. Ultimately, supersaturated lignin was separated into LNPs through the addition of 9.2 mL of water to a 1 mL filtrate solution.211
In another study, Popa et al. investigated the role of hydroxymethylation in the formation of lignin nanoparticles. The process involved stirring a lignin suspension in 47 mm of water for 120 minutes at room temperature using 10 g of lignin derived from Sarkanda grass and wheat straw. The lignin dispersion was then physically agitated for two hours before being sterilized with a 50% solution containing 1.29 g sodium hydroxide and a 25% solution consisting of 3.14 g ammonium hydroxide. The next step involved allowing a 37% solution with 6.7 g formaldehyde to react for four hours at 85 °C. After employing 1 N HCl (pH 2) in a recovery step, a precipitate was created, which was subsequently centrifuged to form a solid phase. The phase was rinsed twice with water and dried to produce LNPs.212
This method transforms lignin-derived non-uniform polymer clusters into uniformly distributed spherical colloidal nanoparticles. In one experiment, alkali lignin was produced by pulping black liquid, which was subsequently purified and acetylated. The acetylated lignin-tetrahydrofuran (ACL-THF) solution (1 mg mL−1) was created by adding tetrahydrofuran to the solution, followed by water. The sudden increase in the ACL-THF solution's scattered light intensity suggests the presence of hydrophobic acetylated lignin molecules in the mixture, which promoted the formation of colloids. The critical water concentration of 44 vol% facilitated the formation of spherical colloids.213,214
Rugose wood components can be transformed into lignin-based nanoparticles using the supercritical antisolvent method, which combines several methods such as centrifugation, dissolution, precipitation, and the use of a CO2 supercritical apparatus. In their patented process for creating industrial-grade xylogen NPs, the researchers chose carbonic acid as their preferred antisolvent.215 The process of creating xylogen nanoparticles, which are particle sizes of less than 0.2 mm that have been alkali treated and freeze-dried to create particles as small as 26 nm. The resulting xylogen NPs have an average particle size of 30 nm.216
These xylogen NPs, when used as fillers in organic rubber, can improve vulcanization and create stable 100 nm lignin nanoparticles. To synthesize the NR/LPCs (natural rubber/lignin-poly (diallyldimethylammonium chloride) (PDADMAC) complexes) composites, a colloidal LPC solution is mixed with a 2% PDADMAC solution at an alkaline pH. The resulting electrostatically assembled solution is then coprecipitated with H2SO4 (pH 2) to create the final product. The necessary steps include filtration, cleaning, and drying under vacuum at 50 °C to produce the NR/LPCs nanocomposites.217
Incorporating lignin into polymer films for food packaging can enhance mechanical strength, gas barrier properties, and provide antioxidant and anti-UV benefits. However, compatibility issues and the risk of phase separation pose challenges when adding lignin to these materials. To effectively address this issue, it is crucial to functionalize lignin or its nanoparticles in order to achieve uniformity in the film. Furthermore, additional research is needed to investigate the relationships between lignin and other compounds, as well as the digestibility of lignin-based films. Assessing the influence of lignin on the compostability and degradability of the final product is also essential, considering its limited degradability in composting environments.
The exact mechanism by which lignin acts as an antibacterial agent is still uncertain and is the subject of ongoing research. In pharmaceutical and biological contexts, lignin's inconsistent molecular weight, impurities, and reactive group composition present both opportunities and challenges, which complicates the evaluation of its efficacy and safety. Despite research demonstrating lignin's antibacterial properties in solution, there is still much to learn about using it as a coating material for antimicrobial surfaces. The development of lignin coatings with the ability to directly inactivate microorganisms appears promising, given the significant role that surface transmission plays in the spread of bacteria. Further studies should focus on refining coating preparation and assessing effectiveness against a diverse range of infections.
In summary, lignin offers a wide range of potential applications, particularly in the food industry, due to its versatility and availability. Its ability to serve as a sustainable material has garnered interest owing to its antioxidant and antibacterial properties. However, further research is necessary to address safety concerns and better understand its interactions with other food components. Moreover, there is a need for more research to demonstrate its effectiveness in real-world food systems and to clarify its in vivo bioactivities. To fully exploit lignin's potential in various sectors such as materials science, pharmaceuticals, and packaging, affordable valorization technologies must be developed. By utilizing lignin's unique properties, we can enhance revenue streams, improve environmental performance, and accelerate the transition toward a more sustainable and eco-friendly economy.
Conflicts of interest
There are no conflicts to declare.References
- R. H. Branco, L. S. Serafim and A. M. Xavier, Fermentation, 2018, 5, 4 CrossRef.
- E. Mnich, N. Bjarnholt, A. Eudes, J. Harholt, C. Holland, B. Jørgensen, F. H. Larsen, M. Liu, R. Manat and A. S. Meyer, Nat. Prod. Rep., 2020, 37, 919–961 RSC.
- A. Rahman, O. Farrok and M. M. Haque, Renewable Sustainable Energy Rev., 2022, 161, 112279 CrossRef.
- R. K. Srivastava, N. P. Shetti, K. R. Reddy and T. M. Aminabhavi, Sci. Total Environ., 2020, 722, 137927 CrossRef CAS.
- A. Boarino and H.-A. Klok, Biomacromolecules, 2023, 24, 1065–1077 CrossRef CAS PubMed.
- M. Xie, W. Muchero, A. C. Bryan, K. Yee, H. B. Guo, J. Zhang, T. J. Tschaplinski, V. R. Singan, E. Lindquist, R. S. Payyavula, J. Barros-Rios, R. Dixon, N. Engle, R. W. Sykes, M. Davis, S. S. Jawdy, L. E. Gunter, O. Thompson, S. P. DiFazio, L. M. Evans, K. Winkeler, C. Collins, J. Schmutz, H. Guo, U. Kalluri, M. Rodriguez, K. Feng, J. G. Chen and G. A. Tuskan, Plant Cell, 2018, 30, 1645–1660 CrossRef CAS PubMed.
- C. D. Pham, M. D. T. Dang, T. B. Ly, K. D. Tran, N. T. Vo, N. H. N. Do, P. T. Mai and P. K. Le, Int. J. Biol. Macromol., 2023, 230, 123175 CrossRef CAS PubMed.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, Ind. Crops Prod., 2019, 139, 111526 CrossRef CAS.
- E. M. Zadeh, S. F. O'Keefe and Y.-T. Kim, ACS Omega, 2018, 3, 7388–7398 CrossRef CAS PubMed.
- D. de Oliveira Begali, L. F. Ferreira, A. C. S. de Oliveira, S. V. Borges, A. R. de Sena Neto, C. R. de Oliveira, M. I. Yoshida and C. I. Sarantopoulos, Int. J. Biol. Macromol., 2021, 180, 262–271 CrossRef CAS PubMed.
- R. Shorey, A. Salaghi, P. Fatehi and T. Mekonnen, RSC Sustainability, 2024, 2(4), 804–831 RSC.
- A. Akbarian, A. Andooz, E. Kowsari, S. Ramakrishna, S. Asgari and Z. A. Cheshmeh, Bioresour. Technol., 2022, 362, 127774 CrossRef CAS PubMed.
- H. Lu, V. Yadav, M. Bilal and H. M. Iqbal, Chemosphere, 2022, 288, 132574 CrossRef CAS PubMed.
- B. Abraham, V. Syamnath, K. Arun, P. F. Zahra, P. Anjusha, A. Kothakotta, Y.-H. Chen, V. K. Ponnusamy and P. Nisha, Sci. Total Environ., 2023, 881, 163316 CrossRef CAS PubMed.
- S. Sethupathy, G. Murillo Morales, L. Gao, H. Wang, B. Yang, J. Jiang, J. Sun and D. Zhu, Bioresour. Technol., 2022, 347, 126696 CrossRef CAS PubMed.
- Z. Ma, J. Wang, H. Zhou, Y. Zhang, Y. Yang, X. Liu, J. Ye, D. Chen and S. Wang, Fuel Process. Technol., 2018, 181, 142–156 CrossRef CAS.
- S. Bertella and J. S. Luterbacher, Trends Chem., 2020, 2, 440–453 CrossRef CAS.
- S. Sharma, A. Sharma, S. I. Mulla, D. Pant, T. Sharma and A. Kumar, Lignin: biosynthesis and transformation for industrial applications, 2020, pp. 1–15 Search PubMed.
- D. Huang, R. Li, P. Xu, T. Li, R. Deng, S. Chen and Q. Zhang, Chem. Eng. J., 2020, 402, 126237 CrossRef CAS.
- M. Tortora, F. Cavalieri, P. Mosesso, F. Ciaffardini, F. Melone and C. Crestini, Biomacromolecules, 2014, 15, 1634–1643 CrossRef CAS PubMed.
- M. Bertolo, L. B. Brenelli de Paiva, V. Nascimento, C. Gandin, M. Neto, C. Driemeier and S. Rabelo, Ind. Crops Prod., 2019, 140, 111591 CrossRef CAS.
- B. Abraham, T. R. Reshmitha, M. M. Navami, L. George, V. V. Venugopalan and P. Nisha, Ind. Crops Prod., 2020, 151, 112451 CrossRef CAS.
- M. P. Ajith, M. Aswathi, E. Priyadarshini and P. Rajamani, Bioresour. Technol., 2021, 342, 126000 CrossRef CAS PubMed.
- P. G, S. As, J. S. Jayan, A. Raman and A. Saritha, Process Saf. Environ. Prot., 2021, 145, 395–410 CrossRef CAS.
- B. Abraham, V. L. Syamnath, K. B. Arun, P. M. Fathima Zahra, P. Anjusha, A. Kothakotta, Y.-H. Chen, V. K. Ponnusamy and P. Nisha, Sci. Total Environ., 2023, 881, 163316 CrossRef CAS PubMed.
- A. P. Richter, J. S. Brown, B. Bharti, A. Wang, S. Gangwal, K. Houck, E. A. Cohen Hubal, V. N. Paunov, S. D. Stoyanov and O. D. Velev, Nat. Nanotechnol., 2015, 10, 817–823 CrossRef CAS PubMed.
- A. Memic and T. Abudula, Antioxidant, antibacterial, injectable lignin-gelatin composite cryogels for wound healing and tissue engineering, US pat., 10881760, King Abdulaziz University, 2021 Search PubMed.
- B. Timotebo and P. A. C. Uduso, Process method for treating structures of lignocellulosic material and method for manufacturing composite structures, JP Pat., 7275335B2, 2015, https://patents.google.com/patent/JP7275335B2/en?q=(Lignin+use+in+Packaging)&oq=Lignin+use+in+Packaging+ Search PubMed.
- J. Deng, T. Xiong, H. Wang, A. Zheng and Y. Wang, ACS Sustain. Chem. Eng., 2016, 4, 3750–3756 CrossRef CAS.
- Q. Zhao, H. Wang, Y. Yin, Y. Xu, F. Chen and R. A. Dixon, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14496–14501 CrossRef CAS.
- R. Vanholme, B. Demedts, K. Morreel, J. Ralph and W. Boerjan, Plant Physiol., 2010, 153, 895–905 CrossRef CAS PubMed.
- M. Baucher, B. Chabbert, G. Pilate, J. Van Doorsselaere, M.-T. Tollier, M. Petit-Conil, D. Cornu, B. Monties, M. Van Montagu and D. Inze, J. Plant Physiol., 1996, 112, 1479–1490 CrossRef CAS PubMed.
- H. Hatakeyama, T. Hatakeyama and J. Advances, Polym. Sci., 2009, 232, 1–63 Search PubMed.
- A. Yanez-McKay, W. Li, R. Mabon and L. Broadbelt, Energy Fuels, 2016, 30, 5835–5845 CrossRef.
- P. Bajpai, Carbon Fibre from Lignin, Springer, 2017 Search PubMed.
- K. Gabov, R. J. Gosselink, A. I. Smeds and P. Fardim, J. Agric. Food Chem., 2014, 62, 10759–10767 CrossRef CAS PubMed.
- M. Bunzel, A. Seiler and H. Steinhart, J. Agric. Food Chem., 2005, 53, 9553–9559 CrossRef CAS PubMed.
- K. Ross and G. Mazza, Int. J. Mol. Sci., 2010, 11, 4035–4050 CrossRef CAS PubMed.
- H.-S. Yin, H.-M. Liu and Y.-L. Liu, Molecules, 2017, 22, 890 CrossRef PubMed.
- Y. Zhang, L. Yang, D. Wang and D. Li, Int. J. Biol. Macromol., 2018, 107, 1193–1202 CrossRef CAS PubMed.
- K. Holtman, H. M. Chang and J. Kadla, J. Wood Chem. Technol., 2007, 27, 179–200 CrossRef CAS.
- N. Terashima, T. Awano, K. Takabe and M. Yoshida, C. R. Biol., 2004, 327, 903–910 CrossRef CAS PubMed.
- H. Ohi, Y. Ju and K. Ken-Ichi, Jpn. Tappi J., 1997,(10), 578–586 Search PubMed.
- H. Hatakeyama and T. J. B. l. Hatakeyama, Proteins, Bioactive Nanocomposites, 2010, pp. 1–63 Search PubMed.
- N. M. Stark, D. J. Yelle and U. P. Agarwal, in Lignin in Polymer Composites, ed. O. Faruk and M. Sain, William Andrew Publishing, 2016, pp. 46–66, DOI:10.1016/B978-0-323-35565-0.00004-7.
- G. Brunow and K. Lundquist, Lignin Lignans: Advances in Chemistry, 2010, vol. 1 Search PubMed.
- R. J. Sammons, D. P. Harper, N. J. Labbe, J. J. Bozell, T. J. Elder and T. Rials, J. Bioresour., 2013, 8, 2752–2767 Search PubMed.
- Y.-C. Lu, Y. Lu and X. Fan, Lignin: Biosynthesis and Transformation for Industrial Applications, 2020, pp. 17–75 Search PubMed.
- O. Y. Abdelaziz and C. P. Hulteberg, Waste Biomass Valorization, 2017, 8, 859–869 CrossRef CAS.
- X. Erdocia, R. Prado, M. Corcuera and J. Labidi, J. Ind. Eng. Chem., 2013, 20, 1103–1108 CrossRef.
- M. Ek, G. Gellerstedt and G. Henriksson, Ljungberg Textbook: Pulp and Paper Chemistry and Technology, De Gruyter Open, 2007 Search PubMed.
- P. Guiraud, R. Steiman, F. Seigle-Murandi and J. L. Benoit-Guyod, Ecotoxicol. Environ. Saf., 1995, 32, 29–33 CrossRef CAS PubMed.
- S. Haghdan, S. Renneckar and G. D. Smith, Lignin in Polymer Composites, 2016, vol. 1, pp. 1–11 Search PubMed.
- M. P. Vinardell and M. Mitjans, Int. J. Mol. Sci., 2017, 18, 1219 CrossRef PubMed.
- H. Chung and N. R. Washburn, in Lignin in Polymer Composites, ed. O. Faruk and M. Sain, William Andrew Publishing, 2016, pp. 13–25, DOI:10.1016/B978-0-323-35565-0.00002-3.
- J. Jiang, W. Cun, X. Wu, Q. Shi, H. Tang and G. Luo, J. Virol., 2012, 86, 7256–7267 CrossRef CAS PubMed.
- E. P. Feofilova and I. S. Mysyakina, Prikl. Biokhim. Mikrobiol., 2016, 52, 559–569 CAS.
- S. Y. Lin and C. W. Dence, Methods in Lignin Chemistry, Springer Science & Business Media, 2012 Search PubMed.
- J. Tao, S. Li, F. Ye, Y. Zhou, L. Lei and G. Zhao, Crit. Rev. Food Sci. Nutr., 2020, 60, 2011–2033 CrossRef PubMed.
- K. Holtman, H.-M. Chang, H. Jameel and J. Kadla, J. Wood Chem. Technol., 2006, 26, 21–34 CrossRef CAS.
- R. El Hage, N. Brosse, L. Chrusciel, C. Sanchez, P. Sannigrahi and A. Ragauskas, Polym. Degrad. Stab., 2009, 94, 1632–1638 CrossRef CAS.
- J. Rencoret, G. Marques, A. Gutiérrez, L. Nieto, J. Jiménez-Barbero, A. T. Martinez and J. del Río, Ind. Crops Prod., 2009, 30, 137–143 CrossRef CAS.
- A. J. Bjorkman, Ind. Eng. Chem., 1957, 49, 1395–1398 CrossRef CAS.
- M. Carrier, A. Loppinet-Serani, D. Denux, J.-M. Lasnier, F. Ham-Pichavant, F. Cansell and C. Aymonier, Biomass Bioenergy, 2011, 35, 298–307 CrossRef CAS.
- D. L. Brink, Method of Treating Biomass Material, US Pat., 5366558, 1993 Search PubMed.
- D. J. Horst, J. J. R. Behainne, P. P. de Andrade Júnior and J. L. Kovaleski, J. Energy Sustain. Dev., 2014, 23, 78–84 CrossRef CAS.
- X. Ma and G. Zhao, J. Appl. Polym. Sci., 2011, 121, 3525–3530 CrossRef CAS.
- V. Chaturvedi and P. Verma, 3 Biotech, 2013, 3, 415–431 CrossRef PubMed.
- I. M. Cabott, Experiments on the pulping of periodate lignin by the sulfite process, PhD thesis, 1951, https://escholarship.mcgill.ca/concern/theses/v979v615m.
- Y. Sun and J. Cheng, Bioresour. Technol., 2002, 83, 1–11 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter, D. Templeton and D. Crocker, Determination of structural carbohydrates and lignin in biomass, in Laboratory Analytical Procedure (LAP), National Renewable Energy Laboratory, 2008, 1617, pp. 1–16 Search PubMed.
- G.-L. Guo, W.-H. Chen, W.-H. Chen, L.-C. Men and W.-S. Hwang, Bioresour. Technol., 2008, 99, 6046–6053 CrossRef CAS.
- M. Bunzel, A. Schu€ßler and G. H. Saha, J. Agric. Food Chem., 2011,(23), 12506–12513 CrossRef CAS PubMed.
- L. E. Beramendi-Orosco, M. Castro-Díaz, C. E. Snape, C. H. Vane and D. J. Large, Org. Geochem., 2004, 35, 61–72 CrossRef CAS.
- Z. Hu, T.-F. Yeh, H.-M. Chang, Y. Matsumoto and J. Kadla, Holzforschung, 2006, 60, 389–397 CAS.
- Q. Yin, W. Yang, C. Sun and M. Di, Bioresources, 2012, 7, 5737–5748 CrossRef.
- J. Domínguez-Robles, R. Sánchez, E. Espinosa, D. Savy, P. Mazzei, A. Piccolo and A. Rodríguez, Int. J. Mol. Sci., 2017, 18, 327 CrossRef PubMed.
- J. Gierer, Wood Sci. Technol., 1980, 14, 241–266 CrossRef CAS.
- A. Maček, Prog. Energy Combust. Sci., 1999, 25, 275–304 CrossRef.
- G. Jiang, D. Nowakowski and T. Bridgwater, Thermochim. Acta, 2010, 498, 61–66 CrossRef CAS.
- K. Lundquist, R. Simonson and K. Tingsvik, Pulp Pap. J., 1981, 63 Search PubMed.
- N.-E. Mansouri and J. Salvadó, Ind. Crop. Prod., 2007, 26, 116–124 CrossRef.
- K. Shimizu, K. Sudo, H. Ono, M. Ishihara, T. Fujii and S. Hishiyama, Biomass Bioenergy, 1998, 14, 195–203 CrossRef CAS.
- A. Corey, K. Wamsley, T. Winowiski and J. Moritz, J. Appl. Poultry Res., 2014, 23, 418–428 CrossRef CAS.
- L. Bosong, L. Wei, Z. Qi, W. Tiejun and M. Longlong, J. Anal. Appl. Pyrolysis, 2014, 108, 106355 Search PubMed.
- S. Kang, B. Li, J. Chang and J. Fan, J. Bioresour., 2011, 6, 243–252 CAS.
- X. Ouyang, X. Qiu and P. Chen, Colloids Surf., A, 2006, 282, 489–497 CrossRef.
- F. Ferdosian and C. Xu, Conversion of Lignin into Bio-Based Chemicals and Materials, Springer, 2017 Search PubMed.
- E. Muurinen, Organosolv pulping: A review and distillation study related to peroxyacid pulping, 2000, https://urn.fi/URN:ISBN:9514256611 Search PubMed.
- H. Kirçi, S. Bostanci and M. K. Yalinkilic, Technology, 1994, 28, 89–99 Search PubMed.
- S. Hidayati, A. Zuidar, W. Satyajaya, M. Murhadi and D. Retnowati, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 344, 012006 CrossRef.
- A. Lindner and G. Wegener, J. Wood Chem. Technol., 1988, 8, 323–340 CrossRef CAS.
- G. Vázquez, G. Antorrena, J. González, S. Freire and S. López, J. Bioresour. Technol., 1997, 59, 121–127 CrossRef.
- X. Pan, D. Xie, R. Yu, D. Lam and J. Saddler, Ind. Eng. Chem. Res., 2007, 46, 2609–2617 CrossRef CAS.
- M. Suchy and D. S. Argyropoulos, in Oxidative Delignification Chemistry, ACS Symposium Series, American Chemical Society, 2001, vol. 785, ch. 1, pp. 2–43 Search PubMed.
- K. Saha, J. Dasgupta, S. Chakraborty, F. Antunes, J. Sikder, S. Curcio, J. Santos, H. Arafat and S. da Silva, Cellulose, 2017, 24, 1–17 CrossRef.
- E. A. Agustiany, M. Rasyidur Ridho, M. Rahmi DN, E. W. Madyaratri, F. Falah, M. A. R. Lubis, N. N. Solihat, F. A. Syamani, P. Karungamye and A. Sohail, J. Polym. Compos., 2022, 43, 4848–4865 CrossRef CAS.
- V. P. Saraf and W. G. Glasser, J. Appl. Polym. Sci., 1984, 29, 1831–1841 CrossRef CAS.
- Y. Zhang and M. Naebe, ACS Sustain. Chem. Eng., 2021, 9, 1427–1442 CrossRef CAS.
- T. Wang, M. Wang, L. Fu, Z. Duan, Y. Chen, X. Hou, Y. Wu, S. Li, L. Guo and R. Kang, Sci. Rep., 2018, 8, 1557 CrossRef PubMed.
- S. Li, J. Zhou, G. Chen, S. Qin, H. Li and Y. Chen, Trans. Chin. Soc. Agric. Eng., 2021, 37, 279–286 Search PubMed.
- Y. Wang and G. W. Padua, Macromol. Mater. Eng., 2003, 288, 886–893 CrossRef CAS.
- L. R. Chiappero, S. S. Bartolomei, D. A. Estenoz, E. A. Moura and V. V. Nicolau, J. Polym. Environ., 2021, 29, 450–459 CrossRef CAS.
- S. Edebali, Iran. J. Chem. Chem. Eng., 2020, 39, 245–251 CAS.
- D. Dixit, R. Pal, G. Kapoor and M. Stabenau, Lightweight Ballistic Composites, Elsevier, 2016, pp. 157–216 Search PubMed.
- Y. Kang, Z. Chen, B. Wang and Y. Yang, Ind. Crops Prod., 2014, 56, 105–112 CrossRef CAS.
- A. Gregorova, M. Hrabalova, R. Kovalcik and R. Wimmer, J. Polym. Eng., 2011, 51, 143–150 CrossRef CAS.
- R. Wulandari, J. Phys.: Conf. Ser., 2019, 1295, 012058 CrossRef CAS.
- F. S. Mostafavi and D. Zaeim, Int. J. Biol. Macromol., 2020, 159, 1165–1176 CrossRef CAS PubMed.
- N. M. Sá, A. L. Mattos, L. M. Silva, E. S. Brito, M. F. Rosa and H. M. Azeredo, Int. J. Biol. Macromol., 2020, 161, 1337–1345 CrossRef PubMed.
- B. Rukmanikrishnan, S. Ramalingam, S. K. Rajasekharan, J. Lee and J. Lee, Int. J. Biol. Macromol., 2020, 153, 55–62 CrossRef CAS PubMed.
- E. M. Zadeh, S. F. O'Keefe and Y.-T. Kim, ACS Omega, 2018, 3, 7388–7398 CrossRef CAS PubMed.
- J. M. Silva, C. Vilela, A. V. Girão, P. C. Branco, J. Martins, M. G. Freire, A. J. Silvestre and C. S. Freire, Carbohydrate Polym., 2024, 337, 122112 CrossRef CAS PubMed.
- S. Kirar, D. Mohne, M. Singh, V. Sagar, A. Bhise, S. Goswami and J. Bhaumik, Sustain. Mater. Technol., 2024, e00864 CAS.
- Q. Zheng, P. O. Osei, S. Shi, S. Yang and X. Wu, Food Biosci., 2024, 59, 104022 CrossRef CAS.
- S. Garg and A. Avanthi, bioRxiv, 2024, preprint, DOI:10.1101/2024.05.03.592363.
- P. Beniwal, D. Guliani and A. P. Toor, J. Polym. Res., 2024, 31, 68 CrossRef CAS.
- L. A. Worku, M. G. Tadesse, A. Bachheti, D. Pandey, A. K. Chandel, A. W. Ewuntu and R. K. Bachheti, Int. J. Biol. Macromol., 2024, 254, 127644 CrossRef CAS PubMed.
- U. Sangeetha, N. Sudhakaran, P. Parvathy, M. Abraham, S. Das, S. De and S. K. Sahoo, Int. J. Biol. Macromol., 2024, 266, 131005 CrossRef CAS PubMed.
- S. Wang, Y. Hao, Q. He and Q. Gao, J. Thermoplast. Compos. Mater., 2024, 08927057241233566 CrossRef.
- Y. He, H.-C. Ye, T.-T. You and F. Xu, Food Hydrocolloids, 2023, 137, 108355 CrossRef CAS.
- A. Riaz, H. Mostafa, K. G. Lawal, N. Sivapragasam, T. Ramachandran, F. Hamed, I. Manikas, B. Sundarakani, C. Stathopoulos and S. Maqsood, Food Biophys., 2024, 1–13 Search PubMed.
- P. Nuamduang, R. Auras, C. Winotapun, B. Hararak, W. Wanmolee and P. Leelaphiwat, Int. J. Biol. Macromol., 2024, 131185 CrossRef CAS PubMed.
- J. Dou, T. Vuorinen, H. Koivula, N. Forsman, M. Sipponen and S. Hietala, ACS Appl. Nano Mater., 2021, 4, 2921–2929 CrossRef CAS.
- E. S. Esakkimuthu, D. DeVallance, I. Pylypchuk, A. Moreno, M. H. Sipponen and J. Frontiers, Biotechnol. Bioeng., 2022, 10, 1025076 Search PubMed.
- M. Vinardell, V. Ugartondo and M. Mitjans, Ind. Crops Prod., 2008, 27, 220–223 CrossRef CAS.
- N. H. A. Latif, A. A. Rahim, N. Brosse and M. H. Hussin, Int. J. Biol. Macromol., 2019, 130, 947–957 CrossRef CAS PubMed.
- M. F. Li, S. N. Sun, F. Xu and R. C. Sun, Food Chem., 2012, 134, 1392–1398 CrossRef CAS PubMed.
- X. Dong, M. Dong, Y. Lu, A. Turley, T. Jin and C. Wu, Ind. Crops Prod., 2011, 34, 1629–1634 CrossRef CAS.
- W. Gong, Z. Xiang, F. Ye and G. Zhao, Ind. Crops Prod., 2016, 91, 340–349 CrossRef CAS.
- A. Salanti, L. Zoia, M. Orlandi, F. Zanini and G. Elegir, J. Agric. Food Chem., 2010, 58, 10049–10055 CrossRef CAS PubMed.
- W. Gong, Z. Ran, F. Ye and G. Zhao, Food Chem., 2017, 228, 455–462 CrossRef CAS PubMed.
- S. S. Qazi, D. Li, C. Briens, F. Berruti and M. M. Abou-Zaid, Molecules, 2017, 22, 372 CrossRef PubMed.
- A. Arshanitsa, J. Ponomarenko, T. Dizhbite, A. Andersone, R. Gosselink, J. Putten, M. Lauberts and G. Telysheva, J. Anal. Appl. Pyrolysis, 2013, 103, 78–85 CrossRef CAS.
- V. Ugartondo, M. Mitjans and M. P. Vinardell, Bioresour. Technol., 2008, 99, 6683–6687 CrossRef CAS PubMed.
- B. KoŠíková, J. Lábaj, A. Kovalcik and D. Slameňová, Holzforschung, 2006, 60, 166–170 Search PubMed.
- E. P. o. F. Additives and N. S. a. t. Food, EFSA J., 2011, 9, 2319 Search PubMed.
- J. Wang, L. Tian, B. Luo, S. Ramakrishna, D. Kai, X. J. Loh, I. H. Yang, G. R. Deen and X. Mo, Colloids Surf. B Biointerfaces, 2018, 169, 356–365 CrossRef CAS PubMed.
- G. L. Catignani and M. E. Carter, J. Food Sci., 1982, 47, 1745 CrossRef CAS.
- Joint FAO and WHO Expert Committee on Food Additives, Safety evaluation of certain food additives/prepared by the sixty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA), in Safety evaluation of certain food additives/prepared by the sixty-ninth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JEFCA), 2009, https://kohahq.searo.who.int/cgi-bin/koha/opac-detail.pl?biblionumber=31771&shelfbrowse_itemnumber=56006 Search PubMed.
- B. Baurhoo, C. Ruiz-Feria and X. Zhao, Anim. Feed Sci. Technol., 2008, 144, 175–184 CrossRef CAS.
- K. Toh, H. Yokoyama, H. Noda and Y. Yuguchi, J. Food Biochem., 2010, 34, 192–206 CrossRef.
- D. Liu, Y. Li, Y. Qian, Y. Xiao, S. Du and X. Qiu, ACS Sustain. Chem. Eng., 2017, 5, 8424–8428 CrossRef CAS.
- T. Dizhbite, G. Telysheva, V. Jurkjane and U. Viesturs, Bioresour. Technol., 2004, 95, 309–317 CrossRef CAS PubMed.
- A. García, G. Spigno and J. Labidi, Ind. Crops Prod., 2017, 104, 242–252 CrossRef.
- R. Y. Nsimba, N. West and A. A. Boateng, J. Agric. Food Chem., 2012, 60, 12525–12530 CrossRef CAS PubMed.
- L. An, G. Wang, H. Jia, C. Liu, W. Sui and C. Si, Int. J. Biol. Macromol., 2017, 99, 674–681 CrossRef CAS PubMed.
- X. Pan, J. F. Kadla, K. Ehara, N. Gilkes and J. N. Saddler, J. Agric. Food Chem., 2006, 54, 5806–5813 CrossRef CAS PubMed.
- Anushikha and K. K. Gaikwad, Biomass Convers. Biorefin., 2023, 1–13 Search PubMed.
- E. Gogo, A. Opiyo, K. Hassenberg, C. Ulrichs and S. Huyskens-Keil, Postharvest Biol. Technol., 2017, 129, 107–117 CrossRef CAS.
- J. Sunthornvarabhas, S. Liengprayoon, T. Lerksamran, C. Buratcharin, T. Suwonsichon, W. Vanichsriratana and K. Sriroth, Sugar Technol., 2018, 21, 355–363 CrossRef.
- A. M. A. Nada, A. I. ElDiwany and A. M. Elshafei, Acta Biotechnol., 1989, 9, 295–298 CrossRef CAS.
- J. D. C. Medina, A. L. Woiciechowski, A. Zandona Filho, L. Bissoqui, M. D. Noseda, L. P. de Souza Vandenberghe, S. F. Zawadzki and C. R. Soccol, Ind. Crop. Prod., 2016, 94, 630–637 CrossRef.
- B. Baurhoo, A. Letellier, X. Zhao and C. A. Ruiz-Feria, Poultry Sci., 2007, 86, 2509–2516 CrossRef CAS PubMed.
- M. T. Madigan, J. M. Martinko and J. Parker, Brock Biology of Microorganisms, Prentice hall, Upper Saddle River, NJ, 2015 Search PubMed.
- S. Zhang, X. Cheng, Q. Fu, Y. Li, P. Wu, Y. Qiao, J. Yan, L. Si, G. I. Waterhouse and H. Li, Food Hydrocolloids, 2023, 143, 108783 CrossRef CAS.
- Y. Lu, M. Jiang, Y. Pan, F. Wang, W. Xu, Y. Zhou and X. Du, Int. J. Biol. Macromol., 2024, 254, 127630 CrossRef CAS PubMed.
- E. Cavallo, X. He, F. Luzi, F. Dominici, P. Cerrutti, C. Bernal, M. L. Foresti, L. Torre and D. Puglia, Molecules, 2020, 26, 126 CrossRef PubMed.
- E. Gerbin, G. Rivière, L. Foulon, Y.-M. Frapart, B. Cottyn, M. Pernes, C. Marcuello, B. Godon, A. Gainvors-Claisse and D. Crônier, Int. J. Biol. Macromol., 2021, 181, 136–149 CrossRef CAS PubMed.
- E. Lizundia, I. Armentano, F. Luzi, F. Bertoglio, E. Restivo, L. Visai, L. Torre and D. Puglia, ACS Appl. Bio Mater., 2020, 3, 5263–5274 CrossRef CAS PubMed.
- F. Falah, D. Zulfiana, M. Septiano, D. S. Nawawi, F. P. Sari, W. Fatriasari and N. N. Solihat, Antimicrobial activity of food packaging biofilms derived from lignin-starch-poly (lactic acid), in AIP Conference Proceedings, AIP Publishing, 2024, vol. 2973, p. 1 Search PubMed.
- L. Xiao, Z. Yao, Y. He, Z. Han, X. Zhang, C. Li, P. Xu, W. Yang and P. Ma, Ind. Crops Prod., 2022, 187, 115515 CrossRef CAS.
- Y. Li, Y. Chen, Q. Wu, J. Huang, Y. Zhao, Q. Li and S. Wang, Polymers, 2022, 14, 1705 CrossRef CAS PubMed.
- X. Zhang, W. Liu, D. Sun, J. Huang, X. Qiu, Z. Li and X. Wu, ChemSusChem, 2020, 13, 4974–4984 CrossRef CAS PubMed.
- H. Sakagami, T. Kushida, T. Oizumi, H. Nakashima and T. Makino, Pharmacol. Therapeut., 2010, 128, 91–105 CrossRef CAS PubMed.
- S. C. Gordts, G. Férir, T. D’huys, M. I. Petrova, S. Lebeer, R. Snoeck, G. Andrei and D. Schols, PLoS One, 2015, 10, e0131219 CrossRef PubMed.
- H. Sakagami, K. Asano, K. Satoh, K. Takahashi, M. Kobayashi, N. Koga, H. Takahashi, R. Tachikawa, T. Tashiro, A. Hasegawa, K. Kurihara, T. Ikarashi, T. Kanamoto, S. Terakubo, H. Nakashima, S. Watanabe and W. Nakamura, In Vivo, 2007, 21, 499–505 CAS.
- H. Sakagami, K. Satoh, H. Fukamachi, T. Ikarashi, A. Shimizu, K. Yano, T. Kanamoto, S. Terakubo, H. Nakashima, H. Hasegawa, A. Nomura, K. Utsumi, M. Yamamoto, Y. Maeda and K. Osawa, In Vivo, 2008, 22, 327–332 Search PubMed.
- K. Matsuhisa, S. Yamane, T. Okamoto, A. Watari, M. Kondoh, Y. Matsuura and K. Yagi, Biochem. Biophys. Res. Commun., 2015, 462, 52–57 CrossRef CAS PubMed.
- P. Srisapoome, K. Hamano, I. Tsutsui and K. Iiyama, Fish Shellfish Immunol., 2018, 72, 494–501 CrossRef CAS PubMed.
- P. K. Lai, J. Donovan, H. Takayama, H. Sakagami, A. Tanaka, K. Konno and M. Nonoyama, AIDS Res. Hum. Retrovir., 1990, 6, 205–217 CrossRef CAS PubMed.
- J.-B. Lee, C. Yamagishi, K. Hayashi and T. Hayashi, Biosci. Biotechnol. Biochem., 2011, 75, 459–465 CrossRef CAS PubMed.
- H. Sakagami, M. Kawano, M. M. Thet, K. Hashimoto, K. Satoh, T. Kanamoto, S. Terakubo, H. Nakashima, Y. Haishima, Y. Maeda and K. Sakurai, In Vivo, 2011, 25, 229–236 CAS.
- M. Qiu, Q. Wang, Y. Chu, Z. Yuan, H. Song, Z. Chen and Z. Wu, PLoS One, 2012, 7, e35906 CrossRef CAS PubMed.
- B. S. Lopez, M. Yamamoto, K. Utsumi, C. Aratsu and H. Sakagami, In Vivo, 2009, 23, 1011–1016 CAS.
- M. P. Vinardell and M. Mitjans, Int. J. Mol. Sci., 2017, 18, 1219 CrossRef PubMed.
- A. Raghuraman, V. Tiwari, Q. Zhao, D. Shukla, A. K. Debnath and U. R. Desai, Biomacromolecules, 2007, 8, 1759–1763 CrossRef CAS PubMed.
- T. Ichimura, T. Otake, H. Mori and S. Maruyama, Biosc. Biotech. Biochem., 1999, 63, 2202–2204 CrossRef CAS PubMed.
- K. Nagata, H. Sakagami, H. Harada, M. Nonoyama, A. Ishihama and K. Konno, Antivir. Res., 1990, 13, 11–21 CrossRef CAS PubMed.
- W. Yang, Y. Weng, D. Puglia, G. Qi, W. Dong, J. M. Kenny and P. Ma, Int. J. Biol. Macromol., 2020, 144, 102–110 CrossRef CAS PubMed.
- R. Kaur, S. Bhardwaj, S. Chandna, K.-H. Kim and J. Bhaumik, J. Clean. Prod., 2021, 317, 128300 CrossRef CAS.
- H. Sadeghifar, R. Venditti, J. Jur, R. Gorga and J. Pawlak, ACS Sustain. Chem. Eng., 2016, 5, 625–631 CrossRef.
- Q. Xing, P. Buono, D. Ruch, P. Dubois, L. Wu and W. Wang, ACS Sustain. Chem. Eng., 2019, 7, 4147–4157 CrossRef CAS.
- Y. Kim, J. Suhr, H.-W. Seo, H. Sun, S. Kim, I.-K. Park, S.-H. Kim, Y. Lee, K.-J. Kim and J.-D. Nam, Sci. Rep., 2017, 7, 43596 CrossRef.
- P. Posoknistakul, C. Tangkrakul, P. Chaosuanphae, S. Deepentham, W. Techasawong, N. Phonphirunrot, S. Bairak, C. Sakdaronnarong and N. Laosiripojana, ACS Omega, 2020, 5, 20976–20982 CrossRef CAS PubMed.
- R. Kaur, N. S. Thakur, S. Chandna and J. Bhaumik, J. Mater. Chem. B, 2020, 8, 260–269 RSC.
- A. Raman, A. Sankar, A. SD, A. Anilkumar and A. Saritha, Macromol. Mater. Eng., 2022, 307, 2200114 CrossRef CAS.
- Q. Xing, D. Ruch, P. Dubois, L. Wu and W.-J. Wang, ACS Sustain. Chem. Eng., 2017, 5, 10342–10351 CrossRef CAS.
- F. Avelino, D. R. de Oliveira, S. E. Mazzetto and D. Lomonaco, Int. J. Biol. Macromol., 2019, 125, 171–180 CrossRef CAS PubMed.
- Y. Chai, Y. Wang, B. Li, W. Qi, R. Su and Z. He, Langmuir, 2021, 37, 7219–7226 CrossRef CAS PubMed.
- D. Barnard and K. Heaton, Gut, 1973, 14, 316–318 CrossRef CAS PubMed.
- P. Figueiredo, K. Lintinen, A. Kiriazis, V. Hynninen, Z. Liu, T. Bauleth-Ramos, A. Rahikkala, A. Correia, T. Kohout, B. Sarmento, J. Yli-Kauhaluoma, J. Hirvonen, O. Ikkala, M. A. Kostiainen and H. A. Santos, Biomaterials, 2017, 121, 97–108 CrossRef CAS PubMed.
- H. Sakagami, J. Pharmacol. Sci., 2014, 126, 92–106 CrossRef CAS PubMed.
- A. Y. Mehta, B. M. Mohammed, E. J. Martin, D. F. Brophy, D. Gailani and U. R. Desai, J. Thromb. Haemostasis, 2016, 14, 828–838 CrossRef CAS PubMed.
- B. Saluja, J. N. Thakkar, H. Li, U. R. Desai and M. Sakagami, Pulm. Pharmacol. Therapeut., 2013, 26, 296–304 CrossRef CAS PubMed.
- I. L. Cameron, W. E. Hardman and D. W. Heitman, Nutr. Cancer, 1997, 28, 170–176 CrossRef CAS PubMed.
- L. Andrijevic, K. Radotic, J. Bogdanovic, D. Mutavdzic and G. Bogdanovic, Journal of B.U.ON. : official journal of the Balkan Union of Oncology, 2008, 13, 241–244 Search PubMed.
- R. G. Saratale, G. D. Saratale, G. Ghodake, S. K. Cho, A. Kadam, G. Kumar, B. H. Jeon, D. Pant, A. Bhatnagar and H. S. Shin, Int. J. Biol. Macromol., 2019, 128, 391–400 CrossRef CAS PubMed.
- M. A. Eastwood and R. H. Girdwood, Lancet, 1968, 2, 1170–1172 CrossRef CAS PubMed.
- C. Thiffault, M. Bélanger and M. Pouliot, Can. Med. Assoc. J., 1970, 103, 165–166 CAS.
- N. Dey, G. Kumar, A. S. Vickram, M. Mohan, R. R. Singhania, A. K. Patel, C. D. Dong, K. Anbarasu, S. Thanigaivel and V. K. Ponnusamy, Bioresour. Technol., 2022, 344, 126171 CrossRef CAS PubMed.
- S. Sharma and A. Kumar, Lignin, Springer, 2020 Search PubMed.
- I. A. Gilca, V. I. Popa and C. Crestini, Ultrason. Sonochem., 2015, 23, 369–375 CrossRef CAS PubMed.
- H. M. Caicedo, L. A. Dempere and W. Vermerris, Nanotechnology, 2012, 23, 105605 CrossRef PubMed.
- A. A. Myint, H. Lee, B. Seo, W.-S. Son, J. Yoon, T. J. Yoon, H. J. Park, J. Yu, J. Yoon and Y.-W. Lee, Green Chem., 2015, 18, 2129–2146 RSC.
- S. R. Yearla and K. Padmasree, J. Exp. Nanosci., 2016, 11, 289–302 CrossRef CAS.
- O. ur Rahman, S. Shi, J. Ding, D. Wang, S. Ahmad and H. Yu, New J. Chem., 2018, 42, 3415–3425 RSC.
- W. Yang, J. Owczarek, E. Fortunati, M. Kozanecki, A. Mazzaglia, G. M. Balestra, J. M. Kenny, L. Torre and D. Puglia, Ind. Crops Prod., 2016, 94, 800–811 CrossRef CAS.
- W. Yang, E. Fortunati, F. Bertoglio, J. Owczarek, G. Bruni, M. Kozanecki, J. Kenny, L. Torre, L. Visai and D. Puglia, Carbohydrate Polym., 2018, 181, 275–284 CrossRef CAS PubMed.
- M. Yang, W. Zhao, S. Singh, B. Simmons and G. Cheng, Nanoscale Adv., 2019, 1, 299–304 RSC.
- A. P. Richter, B. Bharti, H. B. Armstrong, J. S. Brown, D. Plemmons, V. N. Paunov, S. D. Stoyanov and O. D. Velev, Langmuir, 2016, 32, 6468–6477 CrossRef CAS PubMed.
- V. I. Popa, A.-M. Capraru, S. Grama and T. Malutan, Cellul. Chem. Technol., 2011, 45, 221 CAS.
- Y. Qian, Y. Deng, X. Qiu, H. Li and D. Yang, J. Green Chem., 2014, 16, 2156–2163 RSC.
- Y. Qian, Q. Zhang, X. Qiu and S. Zhu, J. Green Chem., 2014, 16, 4963–4968 RSC.
- S. Zhiqiang, S. Wenfeng, Z. Jun, Z. Baoyou, Z. Bin, Y. Lei, Z. Baishi, Z. Xiuhua, Z. Jian and H. Luming, Chinese Pat., 102002165, 2011 Search PubMed.
- L. Zhiming, L. Guochao and W. Haiying, Chinese Pat., CN 103145999, 2013 Search PubMed.
- C. Jiang, H. He, H. Jiang, L. Ma and D. Jia, eXPRESS Polym. Lett., 2013, 7, 480–493 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |
