Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Moving beyond silver in point-of-use drinking water pathogen control
Daniel P.
Huffman
a,
Sarah
Pitell
a,
Paige
Moncure
b,
Janet
Stout
ac,
Jill E.
Millstone
 bd,
Sarah-Jane
Haig
bd,
Sarah-Jane
Haig
 *ae and
Leanne M.
Gilbertson
*ae and
Leanne M.
Gilbertson
 *adf
*adf
aDepartment of Civil and Environmental Engineering, University of Pittsburgh, Benedum Hall, 3700 O'Hara Street, Pittsburgh PA 15261, USA. E-mail: sjhaig@pitt.edu; Tel: (412) 624 9881
bDepartment of Chemistry, University of Pittsburgh, USA
cSpecial Pathogens Laboratory, Pittsburgh PA, USA
dDepartment of Chemical and Petroleum Engineering, University of Pittsburgh, USA
eDepartment of Environmental and Occupational Health, University of Pittsburgh, USA
fDepartment of Civil and Environmental Engineering, Duke University, Wilkinson Building, 534 Research Dr, Durham NC 27710, USA. E-mail: leanne.gilbertson@duke.edu; Tel: (919) 613 6478
First published on 20th February 2024
Abstract
Managing drinking water-associated pathogens that can cause infections in immunocompromised individuals is a persistent challenge, particularly for healthcare facilities where occupant exposures carry a substantial health risk. Incremental advances in point-of-use (POU) devices and modified polymer materials now flood the market, promising to reduce cell concentrations and control biofilm formation. The current leading antimicrobial POU design incorporates silver (Ag), a long standing bacteriostatic used across wide ranging industries. This perspective highlights critical knowledge gaps and fundamental shortcomings associated with existing study designs in the silver-containing POU literature as well as the chemical and microbial processes that underline ongoing critical considerations for pathogen control in drinking water. As a result, we highlight the opportunity to leverage ongoing material discovery and collaboration across disciplines to move us closer towards an affordable, low maintenance approach that addresses the persistent pathogens challenge in drinking water.
Water impactSilver is used in marketed point-of-use devices for the intended purpose of reducing user exposure to drinking water pathogens. Herein, we highlight the underlining chemical principles that influence the antimicrobial action of silver in the drinking water context and biological process considerations that require further attention to elucidate a more comprehensive understanding of performance limitations. Finally, we highlight the opportunity for innovation to address critical and persistent disinfection challenges. |
1. Introduction
Exposure to drinking water-associated pathogens that can cause infections in immunocompromised individuals (DWPIs1), often referred to as opportunistic pathogens, are a leading cause of morbidity and mortality2,3 for susceptible groups (e.g., individuals with AIDS, cancer, cystic fibrosis, undergoing chemotherapy) in the United States.4 Many microorganisms can be considered DWPIs, however following the criteria outlined in Proctor et al.1 we will focus on bacteria that are (1) adapted to grow in drinking water systems, particularly within building plumbing, and (2) frequently cause disease in susceptible populations (e.g., Legionella pneumophila, nontuberculous mycobacteria – NTM, Pseudomonas aeruginosa, Stenotrophomonas maltophilia). Legionella pneumophila, the source of Legionnaires' disease, is the most common reported drinking-water associated outbreak,5 and proven infection cases cost an average of $37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 per admission.2 Hospitalizations from NTM, P. aeruginosa, and L. pneumophila account for 44%, 13%, and 9%, respectively, of waterborne infection hospitalizations in the U.S., and NTM is responsible for the greatest percentage of total deaths, 57% (note: these numbers are underestimated due to underreporting of actual infections emerging from the reporting process).6 Despite a range in existing disinfection regimens,5 there remains a growing number of reports attributing waterborne infectious disease outbreaks to DWPIs7–9 with the incidence of legionellosis7 and NTM8 pulmonary diseases continuing to increase in the USA. As a result, L. pneumophila, Mycobacterium avium, Mycobacterium abscessus, and P. aeruginosa are on the current U.S. EPA's contaminant candidate list.10 There is need to control these persistent organisms at the point of consumer use (e.g., in building fixtures) given the increase in DWPI infection prevalence and incidence, their increasing DWPI abundance found along the drinking water transect, and the highest detection frequency and abundance found at the point of use in buildings.10,11
300 per admission.2 Hospitalizations from NTM, P. aeruginosa, and L. pneumophila account for 44%, 13%, and 9%, respectively, of waterborne infection hospitalizations in the U.S., and NTM is responsible for the greatest percentage of total deaths, 57% (note: these numbers are underestimated due to underreporting of actual infections emerging from the reporting process).6 Despite a range in existing disinfection regimens,5 there remains a growing number of reports attributing waterborne infectious disease outbreaks to DWPIs7–9 with the incidence of legionellosis7 and NTM8 pulmonary diseases continuing to increase in the USA. As a result, L. pneumophila, Mycobacterium avium, Mycobacterium abscessus, and P. aeruginosa are on the current U.S. EPA's contaminant candidate list.10 There is need to control these persistent organisms at the point of consumer use (e.g., in building fixtures) given the increase in DWPI infection prevalence and incidence, their increasing DWPI abundance found along the drinking water transect, and the highest detection frequency and abundance found at the point of use in buildings.10,11
Approaches to reduce the risk posed by DWPIs range from building system-wide (e.g., periodic thermal or chemical shock treatment) to more localized (e.g., point-of-use, POU) approaches. The benefit of POU approaches include the ability to (1) immediately respond to a DWPI detection incident, thus is the current go-to intervention in outbreak situations, and (2) implement proactive, supplemental treatment measures at the fixtures (i.e., showers, faucets) serving vulnerable populations versus treating an entire premise plumbing system. The primary modification to POU strategies in the past 20 years is the incorporation of silver (see Parkinson et al.12 and Table S2 therein of compiled POU device specifications) intending to increase antimicrobial efficacy against DWPIs and extend fixture lifetime. Yet, outbreaks and incidences are on the rise, suggesting the opportunity to innovate POU strategies.
Silver has long been used for its antimicrobial properties beginning with its historical use to preserve food13 to our 21st century advances in precise control of silver nanoparticle (AgNP) size, shape, and surface chemistry to engender specific properties for antimicrobial applications.14,15 Silver can be bacteriostatic (i.e., suppress bacterial growth) and bactericidal (i.e., inactivate bacteria), depending on the system conditions (e.g., how water chemistry affects ion concentrations and contact time).16 The primary mode of action for silver to inactivate bacteria and viruses is via silver ions.17 Nanoparticulate forms of silver introduce physical mechanisms of interaction, and certain features (e.g., reactive sites resulting from certain shapes or crystal facets) induce variable efficacy against a wide range of microbial targets14 and contribute to resistance emergence.18–21 While silver possesses inherent properties that support its widespread use as an antimicrobial agent, characteristics of each use case will influence its efficacy.
Herein, we present our cross-disciplinary viewpoints, based on both our own research and an extensive review of current literature, focused on material-based strategies for point of use pathogen control in water systems (Fig. 1). Our goal is to illuminate commonly overlooked fundamental chemical and biological realities of employing the antimicrobial properties of silver within drinking water matrices, which we see as an opportunity for (1) translation of new material discoveries, and/or (2) a paradigm shift in how we combat DWPIs at the point-of-use.
2. Incorporating silver for DWPI control: findings and limitations of studies, to date
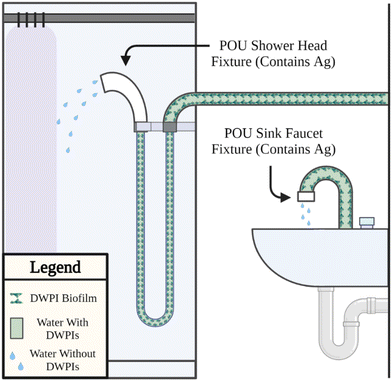
Silver-containing POU devices emerged on the market in 2002 with the Pall AQ-7. The intended function of silver-containing device materials is to lower the bacterial load in drinking water exiting a faucet or shower head. Thus, lowering the user exposure and infection risk. Biofilms are the predominant source of bacteria in a building plumbing system emerging (i) in the plumbing proximal to the POU device, (ii) from a fouled POU filter membrane, referred to as “back contamination”, (iii) from external sources (e.g., splashing from users, liquid disposal), also referred to as “retrograde contamination”, and (iv) from stagnation between use (e.g., in a shower hose). Silver is incorporated into POU devices to slow or eliminate the growth of biofilm. Given the prevalence of this design and lack of robust performance assessment under real or simulated use scenarios, there remains a critical need to uncover the influence of silver on the ability of silver-containing POU devices to mitigate DWPIs. The following are several identified critical limitations to establishing overarching conclusions surrounding the ability for silver to mitigate DWPIs.First, the predominant control in relevant studies assessing the efficacy of silver-containing POU devices against DWPIs22,23 is a faucet or shower head without any installed POU device, rather than a faucet or shower head fitted with a comparable POU device that does not contain silver. Consequently, it is not possible to determine if the observed performance is attributed to the silver component or other device characteristics (e.g., filtration). Second, few studies measure the concentration of silver in the water exiting the fixture and the supposed silver-enabled composite.24 Silver, as silver ion, would indicate the presence of a known inactivation mechanism. (Note: We recognize the potential regulatory implications on quantifiable silver concentration in drinking water depending on the country of implementation.) The absence of silver ion in the exiting drinking water is not conclusive evidence that silver is not present in the system. Silver ion released from the POU device could form strong complexes with chemical components present in drinking water (e.g., chloride ion, vide infra) and would be quantified in a total silver measurement (rather than dissolved silver only). Silver ion could also be consumed by biofilm established on the inside surface of the POU materials, which would render it unquantifiable in the exiting water and underlines the importance of biofilm characterization (vide infra). Third, surveillance case studies12,22,23,25 – commonly used by manufacturers or conducted at a single facility with specific conditions – limit extrapolation of findings to reach broader conclusions about the contributions silver has towards beneficial outcomes. The surveillance approach best mirrors reality but increases experimental variability. Factors such as the total volume of water treated by the device and other external variables introduced by different users are not controlled, confounding the results. An alternative simulated use approach allows more control over system variables yet could miss an unexpected use condition that drives a measured outcome (e.g., retrograde contamination). To maximize the broader applicability of a study's conclusions, the study design should include careful control of system variables while mimicking actual use conditions as closely as possible. Given the tradeoffs of different empirical approaches, future research and comprehensive efficacy testing of existing and novel DWPI mitigation strategies should be pursued in controlled simulated systems and surveillance studies. Finally, variations in sampling procedures can indirectly influence experimental results. For example, several studies include a sequential comparison of DWPI concentrations, which involves comparing water samples collected before and after POU device installation.25,26 While this approach captures fixture-specific use variables, the results are influenced by temporal differences in the plumbing water chemistry. Collecting treatment and non-treatment samples concurrently from different fixtures belonging to the same building plumbing system, and having similar upstream pipe design, helps to preclude confounding factors related to water chemistry fluctuations.12 When employing a concurrent sampling approach, conducting multiple trials with randomized placement of POU devices may be necessary to account for the inherent fixture and use variability.
A consistent limitation across studies is efficacy assessment based on planktonic bacteria, which comprise less than 2% of the total bacteria present in a distribution system (most bacteria are present in biofilms and loose deposits).27 Only a few studies28 include characterization of biofilms present in the POU system of interest and their findings suggest the presence of DWPIs such as Pseudomonas aeruginosa in a sessile biofilm stage within premise plumbing.28 Considering the intended purpose of incorporating silver into POU devices for biofilm prevention, assessment of silver efficacy distinct from the other POU device features (e.g., filtration) necessitates analysis of biofilm on internal device surfaces. Further, characterizing the bacteria present in the biofilm is important for (i) identifying the source of a detected DWPI, (ii) anticipating potential future problematic exposures, (iii) informing mitigation strategies to combat a detected DWPI, and (iv) preventing future infections. In situ biofilm assessment in real systems is challenging due to (1) the biofilm location within the POU device and the intricate pipe network, making non-destructive access difficult, (2) the spatial heterogeneity of biofilms with variations in microbial composition and thickness at different locations within the plumbing system29,30 (3) temporal changes in biofilms influenced by factors such as water flow, temperature, and disinfection practices, and (4) sampling methods for biofilm assessment can be invasive and disruptive to the plumbing system, potentially affecting the biofilm structure and composition.31 These challenges highlight the need to develop non-destructive methods for accurately assessing and monitoring biofilms in situ, possibly by employing localized sensors and machine learning techniques. For example, ATP and electrochemical sensors have been developed to monitor activity in biofilms. Real-time ATP monitoring uses emerging engineering tools, such as microfluidic devices, to quantify the energy product of metabolism as a proxy to the abundance of active cells within the biofilm.32 Electron impedance spectroscopy is another increasingly popular device to monitor real-time biofilm formation onto plumbing surfaces and measures the electron transfer from a microorganism surface to a conductive surface.33 Machine learning techniques, such as naïve Bayesian modeling, can aid in pre-processing data to enhance descriptive models.34 These methods are promising complements to existing destructive sampling approaches (e.g., taking apart the POU device and mechanically removing the biofilm using a swab or into a suspension solution), such as optical coherence tomography, genomics, and transcriptomics. Ultimately, the goal of both in situ and post-operation analysis is to build a comprehensive picture of biofilm dynamics with both the temporal and chemical resolution necessary to make robust progress in the development of effective POU devices.
3. Chemistry considerations of limited silver release from materials used in POU devices
Silver-enabled composites on the market include silver-plastic, silver-ceramic, and silver-textile blends. In these composites, silver may be incorporated in several forms including as elemental silver, AgNPs, or silver salts (such as the Biomaster™ coating). Here, our discussion of the underlying fundamental chemical processes related to silver is centered around AgNPs because their behavior in aqueous media and resulting processes are inclusive of the processes associated with other forms of silver. A comprehensive assessment of the use of silver in POU devices requires an understanding of the chemistry that drives its antimicrobial activity and how this chemistry can change when incorporated into composites.The antimicrobial action of silver, in any form, is typically attributed to the release of silver ions. Therefore, understanding the rate and quantity of silver ion, Ag(I), release from the different silver-containing POU device types is a critical step in assessing efficacy. The relevant chemistry of silver ionization and degradation across length scales is summarized in Johnston et al.,15 including thermodynamic and equilibrium principles underlining silver ion formation from bulk and nanoparticulate silver in the presence of drinking-water relevant ligands (e.g., chloride, sulfur). Herein, we identify key chemical processes that impact performance of silver-enabled composites and what these processes reveal about the feasibility of using Ag in POU materials of construction.
A primary challenge with an ion-driven mechanism in a drinking water system is the stability of the Ag(I). Within the drinking water matrix, silver forms stable complexes and solids with chloride (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ksp = −9.75)35 and sulfide species (log
Ksp = −9.75)35 and sulfide species (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ksp = −53.62),35 and AgNPs experience similar surface complexation chemistries, limiting free Ag(I). In addition, Ag2O products have been found on AgNP surfaces, enabled by the redox potential decreases with NP size,36 which inhibit further surface ionization. Finally, AgNP dissolution has been shown to not reach completion in biological media,37,38 likely due to the system kinetics (e.g., particle aggregation, NP stabilization with ligands).37 Size, shape, and surface chemistry affect AgNP dissolution to Ag(I), however, the effects of each parameter are often difficult to decouple. Generally, a smaller AgNP leads to a larger percentage of surface atoms, thermodynamically driving oxidative processes.39 The surface chemistry of the AgNP also contributes to the stability of the particle as well as the accessibility of oxygen to the AgNP surface. Generally, a smaller ligand with a weak binding affinity will result in more oxidation as there is greater access to undercoordinated surface Ag atoms.40 In a recent study, shape has been found to influence antimicrobial activity through surface reactivity (exposed crystal facets), unique to distinct particle shapes and notable, independent of Ag(I) release.14 It is also useful to note that AgNPs have been found to exhibit particle-specific activity, independent of Ag ions.14 Yet, this mechanism is inherently inhibited when the particles are incorporated into a composite material unless release of the complete AgNP is enabled through system conditions.
Ksp = −53.62),35 and AgNPs experience similar surface complexation chemistries, limiting free Ag(I). In addition, Ag2O products have been found on AgNP surfaces, enabled by the redox potential decreases with NP size,36 which inhibit further surface ionization. Finally, AgNP dissolution has been shown to not reach completion in biological media,37,38 likely due to the system kinetics (e.g., particle aggregation, NP stabilization with ligands).37 Size, shape, and surface chemistry affect AgNP dissolution to Ag(I), however, the effects of each parameter are often difficult to decouple. Generally, a smaller AgNP leads to a larger percentage of surface atoms, thermodynamically driving oxidative processes.39 The surface chemistry of the AgNP also contributes to the stability of the particle as well as the accessibility of oxygen to the AgNP surface. Generally, a smaller ligand with a weak binding affinity will result in more oxidation as there is greater access to undercoordinated surface Ag atoms.40 In a recent study, shape has been found to influence antimicrobial activity through surface reactivity (exposed crystal facets), unique to distinct particle shapes and notable, independent of Ag(I) release.14 It is also useful to note that AgNPs have been found to exhibit particle-specific activity, independent of Ag ions.14 Yet, this mechanism is inherently inhibited when the particles are incorporated into a composite material unless release of the complete AgNP is enabled through system conditions.
In addition to the intrinsic properties of the silver, Ag(I) release from a composite is also dictated by environmental conditions, which are dynamic when incorporated in POU devices (e.g., regular changes in pH, temperature, light, water chemistry, shear forces). The effect of some of these conditions on ion release has been studied, while the impact of others remains unknown. Since Ag(I) release from AgNPs is driven by oxidative dissolution, a decrease in pH will increase ion release.39 Generally, increases in temperature increase Ag(I) release, following Arrhenius behavior.15 Water chemistry and the presence of other ions (e.g., Cl and S) or dissolved organic matter will also influence ion release. For example, small Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag ratios demonstrate lower ion release than AgNPs in pure water whereas large Cl
Ag ratios demonstrate lower ion release than AgNPs in pure water whereas large Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ag ratios demonstrate greater ion release.41 There have been studies on the presence of other dissolved materials, including small molecules and natural organic matter, although there is no consensus on how they influence Ag(I) release.15 Finally, Ag ion release dependence on physical interactions, like shear force, will be dictated by the nature of Ag incorporation (e.g., embedded throughout, surface coated), but additional research is needed to elucidate how these interactions impact the lifetime of material effectiveness.
Ag ratios demonstrate greater ion release.41 There have been studies on the presence of other dissolved materials, including small molecules and natural organic matter, although there is no consensus on how they influence Ag(I) release.15 Finally, Ag ion release dependence on physical interactions, like shear force, will be dictated by the nature of Ag incorporation (e.g., embedded throughout, surface coated), but additional research is needed to elucidate how these interactions impact the lifetime of material effectiveness.
Finally, silver coatings have the advantage of being more adaptable as they can be applied to a variety of different surfaces. Typically, silver coatings are made with metallic silver, which can be deposited on the surface of a substrate with vapor coating,42 sputter coating,43 or ion beam coating.44 When engineering silver composite materials, it is important to understand the longevity of these coatings in complex environments. Some preliminary studies evaluate the efficacy of coatings in hospital settings and found that surface roughness as well as wet versus dry environments affect the antimicrobial activity of silver coatings, where rough and wet conditions promote antimicrobial activity. However, additional research is needed to uncover long term efficacy as a function of polymer type, chemical environment (including exposure to cleaning products), and physical forces.45
4. Microbiology considerations underlining the efficacy and evaluation of silver-enabled POU devices
Bacteria possess many mechanisms for intracellular metal homeostasis, such as metal specific transporters, porin proteins, and efflux pumps.46 While these transport mechanisms are effective at managing essential ion concentrations, non-essential metal ions, like silver, can also be transported into the microorganism. Due to the absence of regulatory processes, silver-induced toxicity can occur leading to microbial dysregulation and death.46 Silver-induced bacterial inactivation results from several possible adverse interactions, including electrostatic interactions between silver and the cell wall causing lysis, disruption of the thiol groups in proteins, and interruption of DNA replication by uncoupling electron transport from oxidative phosphorylation.47 Due to these nonspecific mechanisms of microbial inactivation, it is not surprising that silver is extensively used as an antimicrobial agent in a variety of fields, including on-site and POU drinking water treatment against DWPIs.48 Multiple studies assessing the efficacy of silver-POU devices indicate they are an effective strategy to reduce concentrations of culturable L. pneumophila, P. aeruginosa, Stenotrophomonas spp., Aeromonas spp., Klebsiella spp., nontuberculous Mycobacterium spp., and fungi in drinking water exiting a sink or shower.The focus of most studies to date is on the efficacy of silver-containing POU devices with the underlying assumption that all downstream effects are positive (i.e., prevention of bacterial growth or inactivation of microbes already present) or neutral (i.e., no effect). Given the emerging evidence for silver and AgNPs to induce resistance,18,21,49 it is prudent to also consider potential adverse outcomes of widespread silver use in drinking water building systems. While POU applications of silver are relatively new, copper-silver ionization (CSI) is an established on-site treatment. CSI releases 0.2–0.4 mg L−1 copper and 0.02–0.04 mg L−1 silver into the recirculating hot water50 and has been shown to be more effective at reducing the number of clinical Legionella-induced infections than other on-site strategies (e.g., hyperchlorination, thermal shock and flush regimes).51 However, studies on CSI report an initial decrease followed by an increase in the number of culturable Legionella (over varying time periods) before returning to pre-installation concentrations.52 One explanation for this observation is the emergence of resistance due to consistent, sub-lethal exposure to copper and silver53 (sub-lethal due to poor transport through biofilms,54 poor building plumbing hydraulics,55 or pH influences on ion release). Another is that copper and silver ion concentration fluctuations lead to insufficient inactivation of L. pneumophila allowing for regrowth.54
To date, the mechanism(s) underlying silver ion resistance in many DWPIs is unknown. Possible non-specific heavy metal efflux pumps, similar to that seen for copper resistance,56 could be present within L. pneumophila and other DWPIs. In addition, physical exclusion methods may be deployed by DWPIs, such as intracellular survival within free living amoeba found in building plumbing biofilms as observed when DWPIs are exposed to stress (e.g., low nutrient levels, the presence of biocide53,57). Another protective mechanism for some DWPIs is entry into a viable but nonculturable (VBNC) state, which renders the microbe undetectable using traditional culture techniques, yet they are still alive and capable of causing infection.58 Regardless, these mechanisms result in false-negative results and an underestimation of risk when being evaluated using the gold-standard for detecting and quantifying all life-stages of DWPIs in drinking water: culturing. These limitations highlight the critical need to incorporate culture-independent characterization approaches with standard culturing methods.
Culture-independent techniques overcome many of the disadvantages associated with culture-based methods and provide an exciting opportunity to greatly increase our understanding of DWPI diversity and risk in the building drinking water. Generally, culture-independent methods involve the use of DNA, RNA, or proteins and offer low detection limits, high sensitivity, high specificity, and the ability to detect organisms in the VBNC state,59 bypassing the need for specialized media. Today, real-time PCR or qPCR is the most widely used culture-independent method for the detection and quantification of DWPIs in drinking water60 due to its cost-effectiveness, specificity, and accuracy. Despite the advantages of culture-independent techniques they do have several major drawbacks, specifically their resource intensity and complexity. For example, they require detailed and specific methodology such as optimized nucleic acid extraction procedures, primer design, and quantification assay optimization. Accuracy and detection limits are also crucial components to experimental design, so care needs to be taken for these approaches to be effective.61
Finally, there are several important POU system parameters that affect DWPI behavior and can collectively influence the efficacy evaluation of the silver addition. First, performance of drinking water disinfection interventions involves establishing the concentration and reaction time (together, the CT) that ensures the targeted pathogen removal, yet in POU devices the silver concentration (C) is dynamic (see above for more details) and residence time (T) is likely not adequate to achieve silver-induced inactivation of planktonic microorganisms. Existing research to determine CT values are completed on organisms not native to the drinking water context and/or different growth conditions (i.e., not the drinking water itself). Second, efficacy evaluation of antimicrobial POU devices follows ISO 22196:2011, which involves testing pure cultures of microbes (Escherichia coli and Staphylococcus aureus) after a specific incubation period rather than in a real-use environment. Such testing neglects the effects of the water chemistry and mixed microbiota, the hydraulics and pressure induced in on–off cycling, and the potential for false negatives due to the VBNC state, (note: ASTM 8422:2021, published in January 2022, includes temperature and cycling stress). Finally, studies of POU shower devices to date measure DWPI in the water exiting the fixtures and neglect to measure the most likely route of exposure, inhalation of aerosols, nor comprehensively characterize the biofilm present on internal fixture surfaces which likely seed organisms entering the water phase.
5. Opportunities to advance POU DWPI control
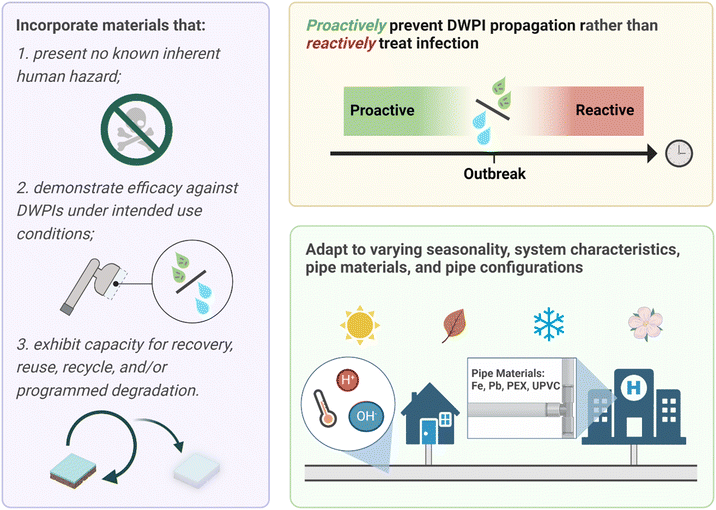
There are critical knowledge gaps limiting conclusions to support enhanced efficacy of silver in POU devices compared with non-silver enabled devices. In fact, there is the potential for silver to have an adverse impact on DWPI presence and abundance (e.g., by initiating resistance mechanism evolution), which merits further attention and study. In addition, characteristics inherent to drinking water chemistry and POU application scenarios implicate fundamental limitations of silver antimicrobial modes of action. Current methodology surrounding the quantification of DWPIs and the assessment of POU device effectiveness can yield biased results due to methodological limitations. For example, DWPIs can evade detection by culture-based methods if present in a VBNC state. Molecular quantification techniques exist, in which DNA and RNA are extracted from water samples and offer a biological footprint. DNA quantification elucidates the microbes that are both alive and dead, while assessing the RNA signatures uncovers only the live microbial populations. Yet, there are technical challenges to methods of RNA extraction and validation, as previously discussed. In the research and industry standards to date, POU device efficacy is not tested under conditions that reflect actual use of the product. Of those POU devices that are studied under conditions reflective of actual use, only the impacts the technology on microbial water quality is explored; impacts on biofilms that develop within the fixture and microbial concentrations in aerosols generated by the product during use (i.e., the most likely exposure route for respiratory infections) have only recently begun to be addressed.62 Thus, the critical research gaps and urgent research need identified herein is (1) POU device efficacy assessments in operating conditions, (2) the use of both culture-independent and culture-dependent strategies, and (3) the assessment of anti-microbial capabilities in matrices beyond the water exiting the fixture (e.g., biofilm within POU, aerosols generated).While the rapid response capabilities and adaptability of POU treatment and mitigation makes it an important strategy for continued DWPI control, the “silver bullet” solution likely does not involve silver after all. There remains immense untapped potential to leverage material science discoveries to advance critically important technologies for DWPI mitigation (Fig. 2). Our ability to design materials with specific properties is driving us ever closer to fully span theory to practice. For example, one can specify a desired nanocomposite glass transition temperature, then use machine learning and artificial intelligence techniques to generate material candidates with varying degrees of synthetic accessibility.63,64 Applying these same approaches to the disinfection context (i.e., enabling computational design for desired disinfection efficacy) would elucidate possibilities beyond the currently available materials, but will rely on mechanistic understanding of current material performance. While the outcome of these approaches can be hypothetical, yet-to-be synthesized materials, there exists a vast array of available synthetic methods enabling precise atomic control for the assembly of materials.
Antimicrobial nanomaterials have been studied for over two decades. Efficient producers of reactive oxygen species (ROS, e.g., nano titanium dioxide, graphene, fullerene, graphitic carbon nitride) are particularly promising in drinking water disinfection applications.65–67 Rather than incorporating inherently antimicrobial materials in which system conditions are known to adversely affect performance, there is an opportunity to design materials that leverage drinking water conditions to activate or enhance their ability to inactivate pathogens. Developing such materials is encouraging given demonstrated advances in switchable polymers, single atom catalysts, and optically enhanced photocatalysis, to name a few.68–73 This opens the door to translating these material capabilities for use in POU devices to advance their design to adapt to the system conditions in which they are applied.
Stimuli-responsive materials for environmental applications are promising “on-demand” antimicrobials, increasing performance longevity and presenting little to minimal risk in their inactivated state. Designing materials to activate and initiate adverse microbial response mechanisms in drinking water contexts is an exciting frontier. The drinking water matrix contains several, mostly predictable, chemical triggers. Opportunities remain to design material ensembles that respond to perturbations intimately connected with a DWPI, or even unique characteristics of a target pathogen, in building plumbing. Stimuli to consider for POU devices include light (e.g., incorporating LEDs), temperature (e.g., of the water or temperature differences), pressure (e.g., from on/off cycling), pH (e.g., change in the water or biological process), chemical presence and threshold concentrations (e.g., chlorine, ion), and EPS from biofilm formation or extra cellular biomolecule from a specific bacteria (e.g., lipopolysaccharides, mycolic acid). The response of the material ensembles could be the release of ROS or other inactivating agent, change in state (e.g., to prevent adherence of early biofilm formation), and detachment or sloughing from a surface.
Translating material discoveries into POU device designs is a known challenge. Tiered, iterative approaches to efficacy and safety testing that involve rapid prototyping and proof of concept that precede comprehensive testing and certification could facilitate the translation. Once demonstrated, future standards for product testing should be expanded to include (1) use conditions, such as variable water flows and on–off cycling, (2) efficacy evaluation using culture and culture-independent methods, and (3) representative DWPIs (i.e., not solely E. coli). Finally, enhanced collaboration of researchers, device manufacturers, and building operators would not only inform product development and testing strategies, but conversations would undoubtedly reveal opportunities to advance capabilities for DWPI control.
The development and translation of demonstrated new materials with high potential for combatting DWPIs into practice is necessary to have a positive impact. The success of these materials in marketed POU devices that are implemented in consumer settings would greatly benefit from early collaboration across disciplines of engineering, microbiology, and chemistry. As demonstrated herein, the details of the application environment of a material will influence the fundamental processes underlining the performance. Further, practical considerations from plumbers, building operators, and standards organizations are critical to the design and suggested use of a given product given that performance is impacted by how a POU device is used.
The field of building drinking water POU treatment is ripe for innovation, for paradigm shifting solutions that control the presence and density of DWPIs. It is time to take a step back and rethink how to protect our increasingly vulnerable populations most effectively and safely.
Author contributions
DPH, SJH and LMG outlined the topic areas to be included. DPH and LMG led discussions pertaining to content and led the revisions. The following authors led writing the respective sections DPH (findings and limitations from current studies), PM and JEM (materials chemistry considerations), SP and SJH (microbiology considerations), LMG (introduction; opportunities). JS provided valuable insights from her professional experiences and feedback on manuscript content. All authors materially participated in the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funding support from NSF CBET Award No. 1935378 and the University of Pittsburgh Summer Undergraduate Research Internship program through the Swanson School of Engineering. We also thank Michael Castro for sharing his valuable insights. Figures created with http://biorender.com/.References
- C. Proctor, E. Garner, K. A. Hamilton, N. J. Ashbolt, L. J. Caverly, J. O. Falkinham, C. N. Haas, M. Prevost, D. R. Prevots, A. Pruden, L. Raskin, J. Stout and S.-J. Haig, Tenets of a Holistic Approach to Drinking Water-Associated Pathogen Research, Management, and Communication, Water Res., 2022, 211, 117997, DOI:10.1016/j.watres.2021.117997.
- S. A. Collier, L. Deng, E. A. Adam, K. M. Benedict, E. M. Beshearse, A. J. Blackstock, B. B. Bruce, G. Derado, C. Edens, K. E. Fullerton, J. W. Gargano, A. L. Geissler, A. J. Hall, A. H. Havelaar, V. R. Hill, R. M. Hoekstra, S. C. Reddy, E. Scallan, E. K. Stokes, J. S. Yoder and M. J. Beach, Estimate of Burden and Direct Healthcare Cost of Infectious Waterborne Disease in the United States, Emerging Infect. Dis., 2021, 27(1), 140–149, DOI:10.3201/eid2701.190676.
- C. Bcheraoui, A. H. Mokdad, L. Dwyer-Lindgren, A. Bertozzi-Villa, R. W. Stubbs, C. Morozoff, S. Shirude, M. Naghavi and C. J. L. Murray, Trends and Patterns of Differences in Infectious Disease Mortality Among US Counties, 1980-2014, JAMA, 2018, 319(12), 1248–1260, DOI:10.1001/jama.2018.2089.
- H. Masur and S. W. Read, Opportunistic Infections and Mortality: Still Room for Improvement, J. Infect. Dis., 2015, 212(9), 1348–1350, DOI:10.1093/infdis/jiv236.
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Division on Earth and Life Studies, Board on Population Health and Public Health Practice, Board on Life Sciences, Water Science and Technology Board and Committee on Management of Legionella in Water Systems, Management of Legionella in Water Systems, National Academies Press(US), Washington, DC, 2019 Search PubMed.
- Findings|Water-related Topics|Healthy Water|CDC, https://www.cdc.gov/healthywater/surveillance/burden/findings.html, (accessed 2023-07-31) Search PubMed.
- Legionnaires Disease History, Burden, and Trends|CDC, https://www.cdc.gov/legionella/about/history.html, (accessed 2022-04-08) Search PubMed.
- J. E. Marshall, R. A. Mercaldo, E. M. Lipner and D. Prevots, Rebecca. Incidence of Nontuberculous Mycobacteria Infections among Persons with Cystic Fibrosis in the United States(2010–2019), BMC Infect. Dis., 2023, 23(1), 489, DOI:10.1186/s12879-023-08468-6.
- J. O. Falkinham, Current Epidemiologic Trends of the Nontuberculous Mycobacteria (NTM), Curr. Environ. Health Rep., 2016, 3, 161–167, DOI:10.1007/s40572-016-0086-z.
- S.-J. Haig, N. Kotlarz, L. M. Kalikin, T. Chen, S. Guikema, J. J. LiPuma and L. Raskin, Emerging Investigator Series: Bacterial Opportunistic Pathogen Gene Markers in Municipal Drinking Water Are Associated with Distribution System and Household Plumbing Characteristics, Environ. Sci.: Water Res. Technol., 2020, 6(11), 3032–3043, 10.1039/D0EW00723D.
- H. Wang, S. Masters, J. O. I. Falkinham, M. A. Edwards and A. Pruden, Distribution System Water Quality Affects Responses of Opportunistic Pathogen Gene Markers in Household Water Heaters, Environ. Sci. Technol., 2015, 49(14), 8416–8424, DOI:10.1021/acs.est.5b01538.
- J. Parkinson, J. L. Baron, B. Hall, H. Bos, P. Racine, M. M. Wagener and J. E. Stout, Point-of-Use Filters for Prevention of Health Care–Acquired Legionnaires' Disease: Field Evaluation of a New Filter Product and Literature Review, Am. J. Infect. Control, 2020, 48(2), 132–138, DOI:10.1016/j.ajic.2019.09.006.
- S. Medici, M. Peana, V. M. Nurchi and M. A. Zoroddu, Medical Uses of Silver: History, Myths, and Scientific Evidence, J. Med. Chem., 2019, 62(13), 5923–5943, DOI:10.1021/acs.jmedchem.8b01439.
- L. M. Stabryla, K. A. Johnston, J. E. Millstone and L. M. Gilbertson, Emerging Investigator Series: It's Not All about the Ion: Support for Particle-Specific Contributions to Silver Nanoparticle Antimicrobial Activity, Environ. Sci.: Nano, 2018, 5(9), 2047–2068, 10.1039/C8EN00429C.
- K. A. Johnston, L. M. Stabryla, L. M. Gilbertson and J. E. Millstone, Emerging Investigator Series: Connecting Concepts of Coinage Metal Stability across Length Scales, Environ. Sci.: Nano, 2019, 6(9), 2674–2696, 10.1039/C9EN00407F.
- R. B.-K. Wakshlak, R. Pedahzur and D. Avnir, Antibacterial Activity of Silver-Killed Bacteria: The “Zombies” Effect, Sci. Rep., 2015, 5(1), 9555, DOI:10.1038/srep09555.
- E. Terzioğlu, M. Arslan, B. G. Balaban and Z. P. Çakar, Microbial Silver Resistance Mechanisms: Recent Developments, World J. Microbiol. Biotechnol., 2022, 38(9), 158, DOI:10.1007/s11274-022-03341-1.
- L. M. Stabryla, K. A. Johnston, N. A. Diemler, V. S. Cooper, J. E. Millstone, S.-J. Haig and L. M. Gilbertson, Role of Bacterial Motility in Differential Resistance Mechanisms of Silver Nanoparticles and Silver Ions, Nat. Nanotechnol., 2021, 16(9), 996–1003, DOI:10.1038/s41565-021-00929-w.
- A. Panáček, L. Kvítek, M. Smékalová, R. Večeřová, M. Kolář, M. Röderová, F. Dyčka, M. Šebela, R. Prucek, O. Tomanec and R. Zbořil, Bacterial Resistance to Silver Nanoparticles and How to Overcome It, Nat. Nanotechnol., 2018, 13(1), 65–71, DOI:10.1038/s41565-017-0013-y.
- E. Valentin, A. L. Bottomley, G. S. Chilambi, E. J. Harry, R. Amal, G. A. Sotiriou, S. A. Rice and C. Gunawan, Heritable Nanosilver Resistance in Priority Pathogen: A Unique Genetic Adaptation and Comparison with Ionic Silver and Antibiotics, Nanoscale, 2020, 12(4), 2384–2392, 10.1039/c9nr08424j.
- C. Gunawan, C. P. Marquis, R. Amal, G. A. Sotiriou, S. A. Rice and E. J. Harry, Widespread and Indiscriminate Nanosilver Use: Genuine Potential for Microbial Resistance, ACS Nano, 2017, 11(4), 3438–3445, DOI:10.1021/acsnano.7b01166.
- J. L. Baron, T. Peters, R. Shafer, B. MacMurray and J. E. Stout, Field Evaluation of a New Point-of-Use Faucet Filter for Preventing Exposure to Legionella and Other Waterborne Pathogens in Health Care Facilities, Am. J. Infect. Control, 2014, 42(11), 1193–1196, DOI:10.1016/j.ajic.2014.08.002.
- T. Sasahara, M. Ogawa, I. Fujimura, R. Ae, K. Kosami and Y. Morisawa, Efficacy and Effectiveness of Showerheads Attached with Point-of-Use(POU) Filter Capsules in Preventing Waterborne Diseases in a Japanese Hospital, Biocontrol Sci., 2020, 25(4), 223–230, DOI:10.4265/bio.25.223.
- M. A. Kessler, F. Osman, J. Marx, A. Pop-Vicas and N. Safdar, Hospital-Acquired Legionella Pneumonia Outbreak at an Academic Medical Center: Lessons Learned, Am. J. Infect. Control, 2021, 49(8), 1014–1020, DOI:10.1016/j.ajic.2021.02.013.
- Z. Barna, K. Antmann, J. Pászti, R. Bánfi, M. Kádár, A. Szax, M. Németh, E. Szegő and M. Vargha, Infection Control by Point-of-Use Water Filtration in an Intensive Care Unit – a Hungarian Case Study, J. Water Health, 2014, 12(4), 858–867, DOI:10.2166/wh.2014.052.
- G. Salvatorelli, S. Medici, G. Finzi, S. D. Lorenzi and C. Quarti, Effectiveness of Installing an Antibacterial Filter at Water Taps to Prevent Legionella Infections, J. Hosp. Infect., 2005, 61(3), 270–271 CrossRef CAS PubMed.
- G. Liu, G. L. Bakker, S. Li, J. H. G. Vreeburg, J. Q. J. C. Verberk, G. J. Medema, W. T. Liu and J. C. Van Dijk, Pyrosequencing Reveals Bacterial Communities in Unchlorinated Drinking Water Distribution System: An Integral Study of Bulk Water, Suspended Solids, Loose Deposits, and Pipe Wall Biofilm, Environ. Sci. Technol., 2014, 48(10), 5467–5476, DOI:10.1021/es5009467.
- M. Totaro, P. Valentini, B. Casini, M. Miccoli, A. L. Costa and A. Baggiani, Experimental Comparison of Point-of-Use Filters for Drinking Water Ultrafiltration, J. Hosp. Infect., 2017, 96(2), 172–176, DOI:10.1016/j.jhin.2016.11.017.
- L. Neu, C. R. Proctor, J.-C. Walser and F. Hammes, Small-Scale Heterogeneity in Drinking Water Biofilms, Front. Microbiol., 2019, 10, 2446, DOI:10.3389/fmicb.2019.02446.
- K. E. Fish, R. Collins, N. H. Green, R. L. Sharpe, I. Douterelo, A. M. Osborn and J. B. Boxall, Characterisation of the Physical Composition and Microbial Community Structure of Biofilms within a Model Full-Scale Drinking Water Distribution System, PLoS One, 2015, 10(2), e0115824, DOI:10.1371/journal.pone.0115824.
- F. C. Pick, K. E. Fish, S. Husband and J. B. Boxall, Non-Invasive Biofouling Monitoring to Assess Drinking Water Distribution System Performance, Front. Microbiol., 2021, 12, 730344, DOI:10.3389/fmicb.2021.730344.
- C. B. Hansen, A. Kerrouche, K. Tatari, A. Rasmussen, T. Ryan, P. Summersgill, M. P. Y. Desmulliez, H. Bridle and H. J. Albrechtsen, Monitoring of Drinking Water Quality Using Automated ATP Quantification, J. Microbiol. Methods, 2019, 165, 105713, DOI:10.1016/j.mimet.2019.105713.
- F. Bimakr, M. P. Ginige, A. H. Kaksonen, D. C. Sutton, G. J. Puzon and K. Y. Cheng, Assessing Graphite and Stainless-Steel for Electrochemical Sensing of Biofilm Growth in Chlorinated Drinking Water Systems, Sens. Actuators, B, 2018, 277, 526–534, DOI:10.1016/j.snb.2018.09.005.
- E. Ramos-Martínez, M. Herrera, J. Izquierdo and R. Pérez-García, Ensemble of Naïve Bayesian Approaches for the Study of Biofilm Development in Drinking Water Distribution Systems, Int. J. Comput. Math., 2014, 91(1), 135–146, DOI:10.1080/00207160.2013.808335.
- M. M. Benjamin, Water Chemistry, Waveland Press, 2nd edn, 2014 Search PubMed.
- V. K. Sharma, J. Filip, R. Zboril and R. S. Varma, Natural Inorganic Nanoparticles – Formation, Fate, and Toxicity in the Environment, Chem. Soc. Rev., 2015, 44(23), 8410–8423, 10.1039/C5CS00236B.
- G. A. Sotiriou, A. Meyer, J. T. N. Knijnenburg, S. Panke and S. E. Pratsinis, Quantifying the Origin of Released Ag+ Ions from Nanosilver, Langmuir, 2012, 28(45), 15929–15936, DOI:10.1021/la303370d.
- K. Loza, J. Diendorf, C. Sengstock, L. Ruiz-Gonzalez, J. M. Gonzalez-Calbet, M. Vallet-Regi, M. Köller and M. Epple, The Dissolution and Biological Effects of Silver Nanoparticles in Biological Media, J. Mater. Chem. B, 2014, 2(12), 1634–1643, 10.1039/C3TB21569E.
- B. Molleman and T. Hiemstra, Time, pH, and Size Dependency of Silver Nanoparticle Dissolution: The Road to Equilibrium, Environ. Sci.: Nano, 2017, 4(6), 1314–1327, 10.1039/C6EN00564K.
- C. Liu, W. Leng and P. J. Vikesland, Controlled Evaluation of the Impacts of Surface Coatings on Silver Nanoparticle Dissolution Rates, Environ. Sci. Technol., 2018, 52(5), 2726–2734, DOI:10.1021/acs.est.7b05622.
- C. Levard, S. Mitra, T. Yang, A. D. Jew, A. R. Badireddy, G. V. Lowry and G. E. Brown, Effect of Chloride on the Dissolution Rate of Silver Nanoparticles and Toxicity to E. Coli, Environ. Sci. Technol., 2013, 47(11), 5738–5745, DOI:10.1021/es400396f.
- M. A. Shevtsov, N. M. Yudintceva, M. I. Blinova, I. V. Voronkina, D. N. Suslov, O. V. Galibin, D. V. Gavrilov, M. Akkaoui, G. Raykhtsaum, A. V. Albul, E. Pitkin and M. Pitkin, Evaluation of the Temporary Effect of Physical Vapor Deposition Silver Coating on Resistance to Infection in Transdermal Skin and Bone Integrated Pylon with Deep Porosity, J. Biomed. Mater. Res., Part B, 2019, 107(1), 169–177, DOI:10.1002/jbm.b.34108.
- C. Balagna, S. Perero, E. Percivalle, E. V. Nepita and M. Ferraris, Virucidal Effect against Coronavirus SARS-CoV-2 of a Silver Nanocluster/Silica Composite Sputtered Coating, Open Ceram., 2020, 1, 100006, DOI:10.1016/j.oceram.2020.100006.
- H. Feng, G. Wang, G. Wu, W. Jin, H. Wu and P. K. Chu, Plasma and Ion-Beam Modification of Metallic Biomaterials for Improved Anti-Bacterial Properties, Surf. Coat. Technol., 2016, 306, 140–146, DOI:10.1016/j.surfcoat.2016.05.059.
- R. M. Ortí-Lucas and J. Muñoz-Miguel, Effectiveness of Surface Coatings Containing Silver Ions in Bacterial Decontamination in a Recovery Unit, Antimicrob. Resist. Infect. Control, 2017, 6(1), 61, DOI:10.1186/s13756-017-0217-9.
- P. Chandrangsu, C. Rensing and J. D. Helmann, Metal Homeostasis and Resistance in Bacteria, Nat. Rev. Microbiol., 2017, 15(6), 338–350, DOI:10.1038/nrmicro.2017.15.
- N. Durán, M. Durán, M. B. de Jesus, A. B. Seabra, W. J. Fávaro and G. Nakazato, Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity, Nanomedicine, 2016, 12(3), 789–799, DOI:10.1016/j.nano.2015.11.016.
- M. E. Vance, T. Kuiken, E. P. Vejerano, S. P. McGinnis, M. F. Hochella, D. Rejeski and M. S. Hull, Nanotechnology in the Real World: Redeveloping the Nanomaterial Consumer Products Inventory, Beilstein J. Nanotechnol., 2015, 6, 1769–1780, DOI:10.3762/bjnano.6.181.
- J. L. Graves, M. Tajkarimi, Q. Cunningham, A. Campbell, H. Nonga, S. H. Harrison and J. E. Barrick, Rapid Evolution of Silver Nanoparticle Resistance in Escherichia Coli, Front. Genet., 2015, 6, 42, DOI:10.3389/fgene.2015.00042.
- S. Triantafyllidou, D. Lytle, C. Muhlen and J. Swertfeger, Copper-Silver Ionization at a US Hospital: Interaction of Treated Drinking Water with Plumbing Materials, Aesthetics and Other Considerations, Water Res., 2016, 102, 1–10, DOI:10.1016/j.watres.2016.06.010.
- J. E. Stout and V. L. Yu, Experiences of the First 16 Hospitals Using Copper-Silver Ionization for Legionella Control: Implications for the Evaluation of Other Disinfection Modalities, Infect. Control Hosp. Epidemiol., 2003, 24(8), 563–568, DOI:10.1086/502251.
- N. Walraven, W. Pool and C. Chapman, Efficacy of Copper-Silver Ionisation in Controlling Legionella in Complex Water Distribution Systems and a Cooling Tower: Over 5 Years of Practical Experience, J. Water Process Eng., 2016, 13, 196–205, DOI:10.1016/j.jwpe.2016.09.005.
- S. G. June and D. M. Dziewulski, Copper and Silver Biocidal Mechanisms, Resistance Strategies, and Efficacy for Legionella Control, J. AWWA, 2018, 110(12), E13–E35, DOI:10.1002/awwa.1144.
- M. LeChevallier, Examining the Efficacy of Copper-Silver Ionization for Management of Legionella: Recommendations for Optimal Use, AWWA Water Sci., 2023, 5(2), e1327, DOI:10.1002/aws2.1327.
- I. Boppe, E. Bédard, C. Taillandier, D. Lecellier, M.-A. Nantel-Gauvin, M. Villion, C. Laferrière and M. Prévost, Investigative Approach to Improve Hot Water System Hydraulics through Temperature Monitoring to Reduce Building Environmental Quality Hazard Associated to Legionella, Build. Environ., 2016, 108, 230–239, DOI:10.1016/j.buildenv.2016.08.038.
- J. A. Delmar, C.-C. Su and E. W. Yu, Heavy Metal Transport by the CusCFBA Efflux System, Protein Sci., 2015, 24(11), 1720–1736, DOI:10.1002/pro.2764.
- S. Giao, S. Wilks, N. Azevedo, M. Vieira and C. Keevil, Incorporation of Natural Uncultivable Legionella Pneumophila into Potable Water Biofilms Provides a Protective Niche against Chlorination Stress, Biofouling, 2009, 25, 335–341, DOI:10.1080/08927010902803305.
- E. Dietersdorfer, A. Kirschner, B. Schrammel, A. Ohradanova-Repic, H. Stockinger, R. Sommer, J. Walochnik and S. Cervero-Aragó, Starved Viable but Non-Culturable(VBNC) Legionella Strains Can Infect and Replicate in Amoebae and Human Macrophages, Water Res., 2018, 141, 428–438, DOI:10.1016/j.watres.2018.01.058.
- F. Y. Ramírez-Castillo, A. Loera-Muro, M. Jacques, P. Garneau, F. J. Avelar-González, J. Harel and A. L. Guerrero-Barrera, Waterborne Pathogens: Detection Methods and Challenges, Pathogens, 2015, 4(2), 307–334, DOI:10.3390/pathogens4020307.
- H. Wang, E. Bédard, M. Prévost, A. K. Camper, V. R. Hill and A. Pruden, Methodological Approaches for Monitoring Opportunistic Pathogens in Premise Plumbing: A Review, Water Res., 2017, 117, 68–86, DOI:10.1016/j.watres.2017.03.046.
- S. Haig, Methods for Detecting and Differentiating Opportunistic Premise Plumbing Pathogens(OPPPs) to Determine Efficacy of Control and Treatment Technologies, 2022, https://www.waterrf.org/research/projects/methods-detecting-and-differentiating-opportunistic-premise-plumbing-pathogens.
- S. Pitell and S.-J. Haig, Assessing the Impact of Anti-Microbial Showerheads on the Prevalence and Abundance of Opportunistic Pathogens in Shower Water and Shower Water-Associated Aerosols, Front. Microbiomes, 2023, 2, 1292571, DOI:10.3389/frmbi.2023.1292571.
- G. X. Gu, C.-T. Chen, D. J. Richmond and M. J. Buehler, Bioinspired Hierarchical Composite Design Using Machine Learning: Simulation, Additive Manufacturing, and Experiment, Mater. Horiz., 2018, 5(5), 939–945, 10.1039/C8MH00653A.
- B. Ma, N. J. Finan, D. Jany, M. E. Deagen, L. S. Schadler and L. C. Brinson, Machine-Learning-Assisted Understanding of Polymer Nanocomposites Composition–Property Relationship: A Case Study of NanoMine Database, Macromolecules, 2023, 56(11), 3945–3953, DOI:10.1021/acs.macromol.2c02249.
- X. Qu, P. J. J. Alvarez and Q. Li, Applications of Nanotechnology in Water and Wastewater Treatment, Water Res., 2013, 47(12), 3931–3946, DOI:10.1016/j.watres.2012.09.058.
- F. Perreault, A. F. Faria and M. Elimelech, Environmental Applications of Graphene-Based Nanomaterials, Chem. Soc. Rev., 2015, 44(16), 5861–5896, 10.1039/C5CS00021A.
- M. S. Mauter, I. Zucker, F. Perreault, J. R. Werber, J.-H. Kim and M. Elimelech, The Role of Nanotechnology in Tackling Global Water Challenges, Nat. Sustain., 2018, 1(4), 166–175, DOI:10.1038/s41893-018-0046-8.
- Z. Zhao, M. Lanzarini-Lopes, E. Westerhoff, X. Long, H. Rho, Y. Bi, L. Ling and P. Westerhoff, Evanescent Wave Interactions with Nanoparticles on Optical Fiber Modulate Side Emission of Germicidal Ultraviolet Light, Environ. Sci.: Nano, 2021, 8(9), 2441–2452, 10.1039/D1EN00199J.
- M. Lanzarini-Lopes, Z. Zhao, F. Perreault, S. Garcia-Segura and P. Westerhoff, Germicidal Glowsticks: Side-Emitting Optical Fibers Inhibit Pseudomonas Aeruginosa and Escherichia Coli on Surfaces, Water Res., 2020, 184, 116191, DOI:10.1016/j.watres.2020.116191.
- H. O'Neal Tugaoen, S. Garcia-Segura, K. Hristovski and P. Westerhoff, Compact Light-Emitting Diode Optical Fiber Immobilized TiO2 Reactor for Photocatalytic Water Treatment, Sci. Total Environ., 2018, 613–614, 1331–1338, DOI:10.1016/j.scitotenv.2017.09.242.
- S. Weon, D. Huang, K. Rigby, C. Chu, X. Wu and J.-H. Kim, Environmental Materials beyond and below the Nanoscale: Single-Atom Catalysts, ACS ES&T Engg, 2021, 1(2), 157–172, DOI:10.1021/acsestengg.0c00136.
- D. Rice, K. Rajwade, K. Zuo, R. Bansal, Q. Li, S. Garcia-Segura and F. Perreault, Electrochemically-Active Carbon Nanotube Coatings for Biofouling Mitigation: Cleaning Kinetics and Energy Consumption for Cathodic and Anodic Regimes, J. Colloid Interface Sci., 2021, 603, 391–397, DOI:10.1016/j.jcis.2021.06.090.
- R. Elashnikov, P. Ulbrich, B. Vokatá, V. S. Pavlíčková, V. Švorčík, O. Lyutakov and S. Rimpelová, Physically Switchable Antimicrobial Surfaces and Coatings: General Concept and Recent Achievements, Nanomaterials, 2021, 11(11), 3083, DOI:10.3390/nano11113083.
| This journal is © The Royal Society of Chemistry 2024 |