Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Toxicity, arsenic speciation and characteristics of hyphenated techniques used for arsenic determination in vegetables. A review
Bashdar Abuzed Sadee *ab,
Yaseen Galaliab and
Salih M. S. Zebari
*ab,
Yaseen Galaliab and
Salih M. S. Zebari bc
bc
aDepartment of Food Technology, College of Agricultural Engineering Sciences, Salahaddin University-Erbil, KRG, Iraq. E-mail: bashdar.sadee@su.edu.krd
bDepartment of Nutrition and Dietetics, Cihan University-Erbil, Erbil, Iraq
cDepartment of Animal Resource, College of Agricultural Engineering Sciences, Salahaddin University-Erbil, KRG, Iraq
First published on 23rd October 2023
Abstract
Arsenic (As) speciation is an interesting topic because it is well recognized that the toxicity of this metalloid ultimately depends on its chemical form. More than 300 arsenicals exist naturally. However, As can be present in four oxidation states: As−III, As0, AsIII and AsV. Long-term exposure to As from different sources, such as anthropogenic processes, or water, fauna and flora contaminated with As, has put human health at risk for decades. There are many side-effects correlated with exposure to InAs species, such as skin problems, respiratory diseases, kidney problems, cardiovascular diseases and even cancer. There are different levels and types of As in foods, particularly in vegetables. Furthermore, different chemical methods and techniques have been developed. Therefore, this review focuses on the general properties of various approaches used to identify As species in vegetation samples published worldwide. This includes various approaches (different solvents and techniques) used to extract As species from the matrix. Then, versatile chromatographic and non-chromatographic systems to separate different forms of As are reviewed. Finally, the general properties of the most common instruments used to detect As species from samples of interest are listed.
1 Introduction
Vegetables are recognized as crucial parts of the human diet, because they are a rich source of key elements like vitamins, minerals, dietary fiber, and antioxidants. Consuming vegetables on a daily basis, in both raw and cooked states, is good for human growth and development as well as for preventing various diseases,1 particularly when significant global challenges are food instability, malnutrition, and food-related diseases, including diabetes, high blood pressure, cancer, and obesity. These issues have led to an increase in the demand for healthy foods, particularly vegetables. It also encourages the production and consumption of vegetables, which account for 86% of global market share.2The accumulation of heavy metals and metalloids in plants and vegetables has recently received increased attention. Due to the anthropogenic activities , vegetables may be more exposed to environmental contamination than other food systems. When heavy metals are accumulated and taken up by plants in edible and non-edible fractions at a particular level, both animals and people may experience health issues.3 Environmental trace elements are harmful to human health. Numerous studies have demonstrated that these factors can have an impact on the environment and the quality of food. It is commonly recognized that heavy metal contamination can pollute human food supplies, particularly vegetables.4 Plants may become contaminated by metals or microbial development due to a variety of causes, such as the environment, pollution, atmosphere, soil, harvesting, and handling. Determining the quantity of certain metallic elements is crucial, since consuming large amounts of these elements is harmful. The World Health Organization advises adopting appropriate methods and standard measurements to assure the quality of plants and their products.5
As pollution is a recognized human carcinogen that affects hundreds of millions of people worldwide. Inorganic As is a prominent cause of skin, lung, bladder, liver, prostate, and kidney cancer in humans.6 Because it is introduced to the environment both naturally and via human activity, As is a common substance.7 It exists in the pedosphere, hydrosphere, biosphere, atmosphere, and water. The biogeochemical behaviour of As is governed by physical–chemical processes, including oxidation–reduction, precipitation/solubilization, and adsorption/desorption in addition to biological mechanisms, including microbiological processes.8
Heavy metal exposure in vegetables can result from anthropogenic or natural processes. In contrast to anthropocentric metal concentration, naturally occurring metals are found in crusted materials, vapors, and particle matter from volcanoes and continental dust. The most significant and frequent sources of metals in vegetables come from anthropogenic activities, such as extensive long-term pesticide and fertilizer usage, as well as linear, point, and surface metal emissions from industrial activities.9 Additionally, the accumulation of As in environmental samples results from both manmade (using As-based insecticides and herbicides, primarily monomethyl arsenic acid (MMA) and dimethyl arsenic acid (DMA)) and natural (volcanic eruptions) sources. Arsenic trioxides [As2O3], which are used in cosmetics, fireworks, electronics, glass, Cu-based alloys, herbicides, fertilizers, pesticides, and seaweed fertilizers, are examples of As oxides used in industry, mining, agriculture, and other fields.10
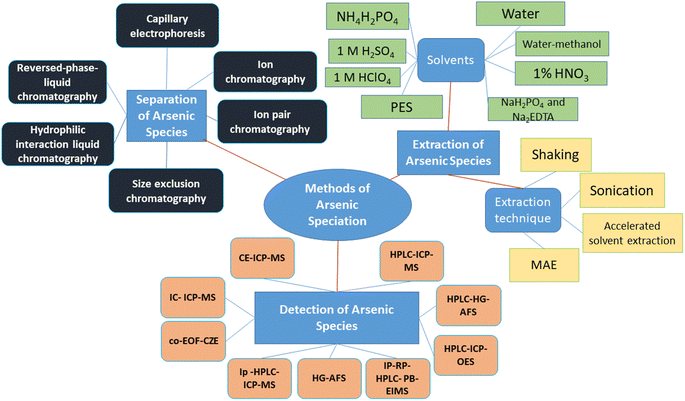
Therefore, this review article summarizes the methods used to extract As species from vegetables, then separating them using chromatographic and non-chromatographic tools, followed by determining the level of As as well as speciating the types using hyphenated techniques (Fig. 1).
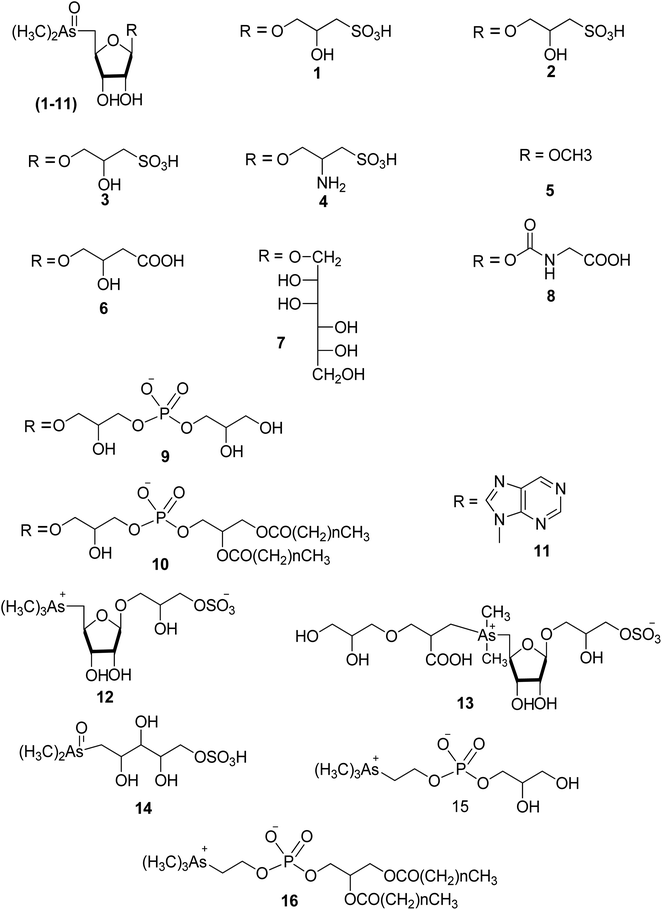 | ||
| Fig. 2 Examples of structures of arsenosugars and arsenolipids, 1–16 of Table 1. | ||
2 Geochemistry of As
As is the 12th most abundant metalloid in the human body and 20th most abundant in nature. As and its derivatives are widely employed in a variety of industries, including agriculture, electronics, metallurgy, and chemical weapons, cattle, insecticides, and fertilizers, in addition to being used as a medicine.11,12 InAs compounds are produced when the element As, which is abundant in the earth's crust at 1.8 ppm by weight, mixes with oxygen, chlorine, and sulfur. The main source of As release and groundwater quality degradation in aquifer systems is rock-water interactions. As typically takes the three allotropic forms of black, yellow, and grey.12 As is an essential component of at least 568 minerals, which are found naturally in the minerals that comprise rocks.10 About 60% of these are arsenates, 20% are sulfides and sulfosalts, 10% are oxides, and the remainder are native elements, metal alloys, arsenites, and arsenides. The principal As-bearing minerals that include As as an anion (arsenide), dianion (diarsenide), or as a sulfarsenide anion or anion(s) are the most significant. These anions are bound to metals such Fe (löllingite, arsenopyrite), Co (cobaltite), and Ni (gersdorffite).13 The most important As-bearing minerals are orpiment (As2S3), realgar (AsS), mispickel (FeAsS), löllingite (FeAs2), niccolite (NiAs), cobaltite (CoAsS), tennantite (Cu12As4S13), and enargite (Cu3AsS4).14 Generally, in groundwater, high levels of naturally occurring As have been reported in aquifers—especially unconsolidated sediment aquifers throughout the world—and have been connected to several adverse health effects.153 As species
A significant part of the human diet is vegetables which can collect As in their edible and non-edible compartments by absorbing it from polluted agricultural soil and water.16 There are numerous inorganic and organic forms of As with different toxicity characteristics. As0 (metalloid arsenic, 0 oxidation state), AsIII (trivalent state, e.g. arsenites), As−III (trivalent state, arsine and arsenide, −3 oxidation state) and AsV (pentavalent state, e.g. arsenates) are the three most common valence states in which it can be found.11,17 A naturally occurring and widely dispersed metalloid, As can be found in soil, water, food, and the environment. As exposure from numerous human activities and sources, such as contaminated groundwater, has grown to be a major global concern. This is due to evidence that As has very hazardous potential to cause detrimental effects on human health. Human exposure to it has been connected to a wide range of diseases, and this poses a serious threat to people's health, economic security, and social standing, particularly in less developed nations.17 Even though As has a reputation for being hazardous, it is generally known that its toxicity relies greatly on the chemical state in which it exists and that the inorganic species AsIII and AsV are considered to be more toxic than organoarsenic compounds (e.g. DMA and MMA).18 Table 1 shows some of the most common As species.| Name | Chemical formula or structure |
|---|---|
| Arsenious acid | H3AsO3 |
| Arsenic acid | H3AsO4 |
| Methyl arsine | CH3AsH2 |
| Dimethylarsine | (CH3)2AsH |
| Trimethylarsine | (CH3)3As |
| Monomethyl arsenic acid | CH3AsO(OH)2 |
| Monomethylarsenous acid | CH3As(OH)2 |
| Dimethyl arsenic acid | (CH3)2AsOH |
| Dimethylarsenous acid | (CH3)2AsOH |
| Trimethylarsinic oxide | TMAO |
| Tetramethylarsonium ion | TMA+ |
| Arsenobetaine | (CH3)3As+CH2COO− |
| Arsenocholine | (CH3)3As+CH2CH2OH |
| Dimethylarsinoylribosides | See structures 1–11 in Fig. 2 |
| Trialklylarsonioribosides | See structures 12, 13 in Fig. 2 |
| Dimethylarsonoulribtol | See structure 14 in Fig. 2 |
| Glecerophosphorarsnocholine | See structure 15 in Fig. 2 |
| Glecerophosphorarsenocholine | See structure 16 in Fig. 2 |
4 Allowable limit of As in vegetation
Many international organizations have established recommended upper limits for the amount of As that should be present in foods. This is due to the heavy enrichment and biotransformation of As and its detrimental effects on human health. Any age or health condition can be negatively impacted by As, which is harmful to humans. Inorganic As (InAs) is the category of As that has the greatest risk of toxicity. As levels are monitored and controlled by the FDA in meals, nutritional supplements, and cosmetics. As cannot be completely prevented or removed from food, although its levels can be brought down. The EU has not yet defined a maximum limit for As in food products. However, in some states, the upper limits are set forth in national legislation. For instance, in the UK, 1 μg g−1 fresh weight of As is the statutory maximum for foods.19 The FDA is recommending a limit or “action level” for InAs in infant rice cereal of 100 μg kg−1. The standard set by the European Commission (EC) for rice used in the manufacturing of food for babies and young children is comparable to this.20 The Scientific Panel on Contaminants in the Food Chain (CONTAM Panel) of the European Food Safety Authority (EFSA) adopted an opinion on As in food on 12 October 2009. In this opinion, the CONTAM Panel stated that the Joint FAO/WHO Expert Committee on Food Additives' (JECFA) provisional tolerable weekly intake (PTWI) of 15 μg kg−1 body weight is no longer appropriate because data have shown that InAs causes cancer of the lung and bladder in addition to skin cancer and that a variety of adverse effects have been reported at exposures lower than those examined by the JECFA. For skin lesions, bladder, lung, and skin cancers, the CONTAM Panel determined a range of benchmark dose lower confidence limits (BMDL01) values between 0.3 and 8 μg kg−1 b.w. per day. The maximum values of InAs for non-parboiled milled rice (polished or white rice); parboiled rice and husked rice; rice waffles, rice wafers, rice crackers and rice cakes; and rice for the manufacturing of foods for infants and children were proposed by the EFSA to be 200, 250, 300, and 100 μg kg−1, respectively,21 China has established a legal cap of 150 μg kg−1 for InAs in rice and rice-based products.22 However, As exists at low concentrations in natural water, and in some locations, the levels of As in drinking water are over the maximum allowable concentration, which is 50 μg L−1, whereas the recommended threshold is 10 μg L−1, according to the Environmental Protection Agency (EPA).235 Toxicity of As
Elemental speciation has been a well-established subject of study in recent years. The mobility, biological availability, and toxicity of an element can be better understood by studying it in its chemical form.24–26 As is typically thought to be metabolized mostly through methylation to lessen its toxicity and this is considered to be a detoxification process. However, by 2020, it was discovered that the synthesis of methylated metabolites containing trivalent As was necessary for at least some of the harmful consequences linked to As exposure. These results were in line with changes in the dynamic behaviour of As brought on by methylation because the trivalent oxidation state of As is linked to increased effectiveness as a cytotoxin and clastogen.27The InAs species (AsIII and AsV) are categorized as species that cause cancer.28 Whereas the organic As (OAs) species (MMA and DMA), despite being less toxic than InAs, are nevertheless categorized as species that provoke cancer,29,30 while AsC and AsB are categorized as non-toxic As species.31 However, intermediate metabolites, such as monomethylarsonous acid (MMAIII) and dimethylarsinous acid (DMAIII), are more toxic than AsIII, AsV, DMAV or MMAV.32
5.1 As and human health
Researchers have identified As as a significant danger factor for both food and water. It exists both naturally and as a result of human activity in the environment in organic and inorganic forms. Exposure to As may have negative health consequences on people as well as other living things. It can also have a variety of side effects, such as skin changes, respiratory issues, cardiovascular problems, digestive system issues, genotoxicity, and mutagenic and carcinogenic effects.16The WHO deemed this situation to be the “largest mass poisoning of a population in history” because Bangladesh as a whole experienced the worst As poisoning public health danger.33 In acute toxicity, As, a toxic metalloid, can cause nausea, vomiting, and severe diarrhea; in chronic toxicity, it can cause cardiovascular disease, diabetes, bladder cancer, and kidney cancer.34 Lung, bladder, kidney, skin, and liver malignancies, neurological disorders, cardiovascular diseases, hypertension, gangrene, diabetes, respiratory diseases, renal diseases, and reproductive diseases are among the prevalent adverse health effects of As exposure.35 The amount of ingested As, dietary status, length of exposure, and immune response of the individual are the key factors influencing the severity of As poisoning. Skin lesions, such as arsenicosis, are the telltale signs of chronic As exposure. Arsenicosis is a worldwide issue, not just a local one in Bangladesh, with Asian nations like Bangladesh, India, and China being the worst impacted.36 More effects of As exposure on the human body are illustrated in Fig. 3.
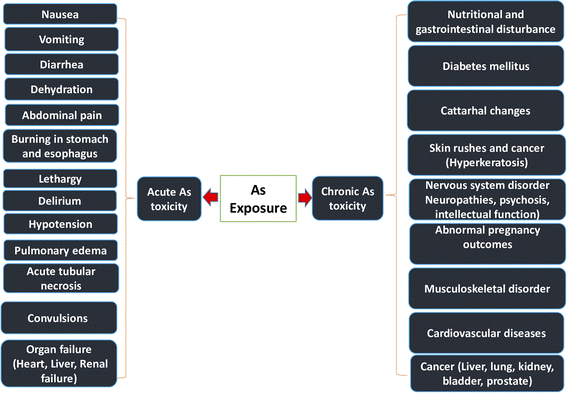 | ||
| Fig. 3 Consequence of As exposure on human organs.37 | ||
6 How As moves into plants and vegetables
In order to understand the mechanism supporting As transport, metabolism, and the detoxification mechanism responsible for scavenging As toxicity in diverse plants including rice, a number of genomes, proteomics, and metabolomic investigations have been conducted. Heavy metals and As can enter the body through specific routes. These routes include inhaling polluted dust, directly ingesting contaminated soil on the surface of foods, and ingesting plant-based foods that have been poisoned at the location of their growth. Vegetables have been reported to have the highest percentage of exposure to As, followed by fruit and fruit juices, rice, and other foods, in locations where there is no water contamination.38As is easily accumulated by plants, which makes it easier for As to go from the soil into the food chain. In the oxic layer of soils, arsenate, which behaves chemically in a similar way to phosphate, is the main InAs species. As a result, AsV can enter plant species via the phosphate transporter system, whereupon the AsV in plant biomass may be changed into AsIII. The amount of phosphate in the soil affects the absorption and phytotoxicity of As. As can displace phosphate in soil particles at low concentrations, increasing absorption and phytotoxicity, whereas high concentrations of phosphate compete with As at root surfaces, decreasing uptake and phytotoxicity.18,39 In addition, As behaviour in the soil environment is influenced by microbial activities and hence As availability in the soil-plant system.32
7 As speciation in vegetables
7.1 Common extraction techniques and solvents
For information on accurate As speciation, it is necessary to maintain the concentration and chemical composition of the original species during the sample preparation and extraction procedures.40 The preferred extraction method for a given application reflects both the matrix and the target species. For the precise measurement of As species in vegetables and plants using HPLC-ICP-MS, a gentle and effective extraction procedure is needed.18 For the detection of As in vegetables, advanced techniques, such as shaking, sonication, accelerated solvent extraction and microwave-assisted extraction (MAE), have also been used (Table 2). However, for many matrices, MAE is an effective substitute for traditional methods because it provides acceptable and repeatable efficiency, shorter extraction duration, use of less solvent, and the ability to do numerous extractions quickly. Numerous investigations on the speciation of As have used this method. Due to the small number of variables involved, such as solvent selection, solvent volume, temperature, extraction time, power, and matrix characteristics, optimization is simple.41,42| Matrix | Technique | Extraction solution | Extraction conditions | As species | Extraction efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| a HPLC: high performance liquid chromatography; ICP-MS: inductively coupled plasma mass spectrometry; HG-AFS: hydride generation atomic fluorescence spectrometry; IC: ion chromatography; LC: liquid chromatography. | ||||||
| Welsh onion, spinach, romaine lettuce, leaf lettuce, leaf mustard, common sowthistle, pakchoi, sweet potato leaves, choy sum, pumpkin leaves | HPLC-ICP-MS | 1% HNO3 | Sonication, 25 °C | AsIII, AsV, DMA | 65.4–94.9 | 54 |
| Garlic | HG-AFS | 1 M H2SO4 | Sonication for 20 min and EDTA, 20 min | AsIII, AsV | 97 | 55 |
| Garlic | HG-AFS | 1 M HClO4 | Sonication for 20 min; and EDTA, 20 min | AsIII, AsV | 80 | 55 |
| Garlic | HG-AFS | Methanol and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Sonication for 20 min; and EDTA, 20 min | AsIII, AsV | 96 | 55 |
| Pteris vittata | HPLC-ICP-MS | 2 mM NaH2PO4 and 0.2 mM | Sonication, 15 min | AsIII, AsV | 98 (fronds) | 50 |
| Na2EDTA (pH 6) | <70 (roots) | |||||
| White mustard | HPLC-ICP-MS | Water | Sonication, 60 min | AsIII, AsV, MMA, DMA | 91 (roots) | 45 |
| 89 (stems) | ||||||
| 50 (leaves) | ||||||
| Leafy vegetables | HPLC-ICP-MS | 1% HNO3 | MAE, 90 °C, 1.5 h | AsIII, AsV, DMA | 77–105 | 52 |
| Different vegetables | HPLC-ICP-MS | 1% HNO3 | MAE, 95 °C for 50 min | AsIII, AsV, MMA, DMA | 95–104 | 56 |
| Spinach | IC-ICP-MS | Methanol and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
MAE, 90 °C, 20 min | AsIII, AsV, MMA, DMA | 45 | 53 |
| Spinach | IC-ICP-MS | PES | MAE, 90 °C for 20 min | AsIII, AsV, MMA, DMA | 100 | 53 |
| Spinach | IC-ICP-MS | 30 mM NH4H2PO4 (pH 5.6) | MAE, 90 °C for 20 min | AsIII, AsV, MMA, DMA | 101 | 53 |
| Different compartments of broad bean | HPLC-ICP-MS | 1% HNO3 | MAE, 95 °C for 50 min | AsIII, AsV, MMA, DMA | 85–108 | 57 |
| Carrots | LC-ICP-MS | Water | Accelerated solvent extraction, 100 °C | AsIII, AsV, MMA, DMA | 80–102 | 58 |
| Pepper plant (fruit part) | HPLC-HG-ICP-MS | Water | Shaking for 14 h | AsIII, AsV, MMA, DMA | 87 | 59 |
| Algae and aquatic plants | LC-ICP-MS | Water | Shaking for 16 h, 25 °C | AsIII, AsV, MMA, DMA, glycerol-arsenosugars | 5–127 | 60 |
| Spinach | IC-ICP-MS | Water | Shaking for 1 h | AsIII, AsV, MMA, DMA | 80 | 53 |
| Spinach | IC-ICP-MS | 30 mM NH4H2PO4 | Shaking for 1 h | AsIII, AsV, MMA, DMA | 70 | 53 |
| Bush leaves | HPLC-HGAFS | Water–methanol | Shaking for 20 min, 60 °C | AsIII, AsV, MMA, DMA | 73 | 48 |
Due to their ability to selectively hydrolyze the important parts of the cell, enzymes may be used as an extraction tool for As species. As a result, the amount of material needed could be greatly reduced, necessitating less sample dilution and enabling the analysis of As species that cannot be extracted using standard methods (water or water/methanol).43 Enzymes have also been employed to treat some food items; freeze-dried apple samples were treated with amylase. Amylase degrades the cellulose in freeze-dried apple samples, increasing the yields of As species during extraction. Acetonitrile–water extraction may then be used to complete the process.43,44 However, the extraction efficiency for As utilizing cellulase as the extraction agent was shown by cellulose to vary significantly for GBW10015 materials. For GBW10015-spinach, cellulase had an extraction efficiency of 119%. Additionally, some As species were retained in the residue because cellulase was unable to completely remove all the As from GBW10015-spinach. Because of this, cellulase is not a suitable extraction agent for extracting As species from plants and vegetables.18 There are many different extraction techniques that have been used for full, total inorganic, and total As speciation. The most commonly used extraction agents are water, methanol, methanol–water solvent systems, and occasionally, though rarely, acetonitrile–water solvent systems. Sequential extractions are also popular. Although water is an inexpensive and affordable solvent that can be used to remove As species in a plant and vegetable matrix, it is incapable of extracting InAs species (AsIII and AsV) that bind protein.45 Low recovery efficiency makes mixtures of methanol and water at various ratios ineffective for removing inorganic As from plant and vegetable tissues.46–48 Trifluoroacetic acid can increase the effectiveness of extraction, but it also has the potential to change the species of As.49 Although phosphate in the plasma could damage the cones of the mass spectrometer due to polymeric deposition and chlorine could interfere with the detection of 75As+ by forming the polyatomic species 40Ar35Cl+ in the subsequent ICP-MS analysis, diluted inorganic acid solutions (such as HNO3, H3PO4, and HCl) work well at extracting As species from plant tissues.50,51 It has been discovered that some mixed salt solutions, such as phosphate buffer solution (PBS) and protein extraction solution (PES), work effectively to remove various As species from plant samples.52,53 Table 2 provides a summary of the main extraction media and extraction techniques utilized to remove As species from vegetables. It should be noted that several of these procedures have fairly low extraction efficiency and are typically time-consuming.
7.2 Techniques used for separation and measuring As species
7.2.1.1 Capillary electrophoresis (CE). Due to its excellent separation efficiency and relatively gentle separation conditions, which would aid in conserving the original As species in samples, CE offers an alternative separation method to HPLC. Because CE is a separation method that operates at the nL min−1 level and because its (electro-osmosis dewatering) EOF is substantially lower than the uptake rate for traditional micro-concentric nebulizers of ICP-MS, coupling of CE and ICP-MS presents a difficult design challenge.62 Since CE has several distinct advantages over GC or HPLC methods, including high resolving power, quick and effective separation, minimal reagent consumption, and the potential for separation with only minor disruptions of the existing equilibrium between different species, CE is an appealing technique for elemental speciation.63 Separation of different organic and inorganic As species has been identified using CE.
7.2.1.2 Chromatographic methods.
7.2.1.2.1 Ion chromatography. Cation-exchange chromatography is utilized with positively charged target molecules. Because the pH for chromatography is lower than the isoelectric point (pI), the molecule is positively charged. The stationary phase in this type of chromatography is negatively charged, and positively charged molecules are loaded to be dragged to it.64 Based on their physicochemical characteristics (pKa values), AsV, MMA, and DMA are deprotonated to create anionic species under neutral pH, whereas AsIII exists as a neutral species. Due to variations in their anionic nature, anion-exchange chromatography is a potential alternative for separating these prevalent As species. Due to its low pKa1 (2.19) and pKa2 (6.98) values, AsV has the strongest negative charge in most mobile phases and elutes slowly. Given that AsIII has high pKa values (pKa1 = 9.23, pKa2 = 12.13, and pKa3 = 13.4), it often occurs as a neutral molecule, which results in relatively minimal retention. Many As compounds, including AsIII, AsV, MMA, DMA, AsB, AsC, oxo-arsenosugars (oxoAsS), thio-arsenosugars (thioAsS), and phenylarsenicals, have been analysed using anion exchange chromatography. Strong anion-exchange columns, such as PRP-X100, are the most popular type of column used for As speciation studies.65
When the stationary phase is positively charged and negatively charged molecules are loaded to be attracted to it (i.e., the pH for chromatography is larger than the pI), this process is known as anion-exchange chromatography. For the speciation investigation of positively charged As molecules, such AsB, AsC, TMAO, or TMA, cation-exchange chromatography is frequently utilized. Similar to anion exchange, cation-exchange chromatography operates by interacting with cationic analytes through the use of a negatively charged stationary phase. Stronger positively-charged analytes retain more information, which is directly correlated with the retention of arsenicals. In 15 minutes, Wolle et al. separated AsIII, MMA, AsV, DMA, AsB, and TMAO using a strong cation-exchange (PRP-X200) column, with AsC and TMA co-eluting.66
7.2.1.2.2 Ion-pair chromatography (IPC). IPC has commonly been used for As speciation studies because it can distinguish between ionic and neutral species. Ion-pair reagents are used in the mobile phase of a typical reversed-phase column (C18) in the IPC process. While the hydrophobic area of the ion-pair reagent interacts with the stationary phase, the charged group of the reagent interacts with the analyte. Tetraalkylammonium, tetrabutylammonium, and tetraethylammonium are frequently utilized as ion-pair reagents for the speciation of anionic and neutral As species. The ion-pair separation of cationic and neutral As species frequently use alkyl sulfonates, such as hexane sulfonic acid and 1-pentane sulfonic acid. The two organic modifiers that are used most frequently are acetonitrile and methanol. Usually included in the mobile phase, they reduce retention duration and alter selectivity. The charged As species must pass through the ion-pair reagents and the hydrophobic stationary phase in order to interact with the conventional stationary phase, whereas the neutral As species can do so directly. In less than 12 minutes, Morita et al. developed a mixed ion-pair method using sodium butanesulfonate and tetramethylammonium hydroxide as the ion-pairing reagents to separate AsV, AsIII, MMA, DMA, AsB, TMAO, TMA, and AsC.67 Eight As species, AsIII, AsV, MMA, DMA, TMAO, tetramethylarsonium, AsC and AsB, have been identified in an extract of tree moss by ion-pair reversed phase HPLC.68
7.2.1.2.3 Reversed-phase-liquid chromatography. Arsenolipids, which comprise fatty acids, phospholipids, phosphatidylcholines, fatty alcohols, and phosphatidylethanolamines, are particularly well analyzed by reversed-phase-liquid chromatography. Arsenolipids can be separated according to the number of carbons, the amount of double bonds, and other functional groups using standard C18 or C8 columns. Reversed-phase HPLC has been used with ICP-MS and ESI-MS to measure and identify several arsenolipids in fish and algae.69,70 AsIII, AsB, DMA, and an arsenosugar (oxo-arsenosugar-glycerol, As 328) in extracts of commercial kelp and bladderwrack have been separated and investigated using ion-pair-reversed-phase high-performance liquid chromatography (IP-RP-HPLC) coupled to particle beam-electron ionization mass spectrometry (PB-EIMS).71 The stability of three arsenolipids—As fatty acids AsFA-362 and AsFA-388, as well as As hydrocarbon AsHC-332—common components of algae—was examined in relation to sample storage and transport as well as their preparation for quantitative analyses.70
7.2.1.2.4 Hydrophilic interaction liquid chromatography (HILIC). Typically, anion-exchange columns and non-volatile solvents, for instance with phosphate buffers as mobile phase, are used to separate highly polar As compounds. Consequently, ESI-MS compatibility is only mild. So, a technique was created using hydrophilic interaction liquid chromatography (HILIC) for the separation of the main transformation products. Thus, compatibility of the used solvents for speciation analysis with both ICP-MS and ESI-MS was accomplished. In order to determine the quantity of the produced products and characterize the primary products developed, quantification of As-containing species was also carried out using high-resolution electrospray (EC)-HILIC-ICP-MS. Thirteen arsenicals were detected and separated using EC-HILIC-ICP-MS in research into roxarsone electrochemical transformation products.72 Xie et al. successfully used a zwitterionic HILIC column and ICP-MS/ESI-MS to separate nine organoarsenicals (i.e., 3-nitro-4-hydroxyphenylarsonic acid (roxarsone, Rox), phenylarsonic acid (PAA), p-arsanilic acid (p-ASA), phenylarsine oxide (PAO), DMA, MMA, AsB, AsC and TMAO) within 45 min.73 Even though HILIC has significant potential for separating more As species in a single run, there are not many instances of it being used for As speciation.
7.2.1.2.5 Size exclusion chromatography (SEC). Size exclusion chromatography is not effective for speciation investigation of tiny As compounds because the size differences between many As species are tiny and they cannot be separated on an SEC column. The investigation of As interactions with big compounds, however, is particularly effective when using SEC. For instance, SEC is frequently used in an As–protein binding study to distinguish protein-bound from free As.74,75 With a focus on maintaining the intact proteins and their As bindings, a novel approach based on size exclusion chromatography linked to electrospray ionization mass spectrometry (SEC-ESI-MS) was developed in a study. Using SEC-ESI-MS, the simultaneous binding of phenylarsine oxide to five distinct peptides and proteins (glutathione, oxytocin, aprotinin, lactalbumin, and thioredoxin) were examined.76 SEC was also utilized to quantify As–biomolecule complexes in Mus musculus liver extracts.77 In order to separate and collect protein-bound As from free As, SEC was utilized; then, the protein-bound As was treated with hydrogen peroxide to liberate it.78 A combination of SEC coupled with ICP-MS, as well as three-dimensional excitation–emission matrix fluorescence spectroscopy combined with parallel factor analysis was used to investigate the roles of dissolved organic matter on As mobilisation and speciation in environmental water.79
7.3 Detection of As species
As can theoretically be found using any spectrometric detector that can identify an element specifically. Atomic absorption spectrometry (AAS), atomic fluorescence spectrometry (AFS), and mass spectrometry (MS) are the methods that are used most frequently.80The AAS with vapour generation assembly (AAS-VGA) method is well known for As trace analysis. The conversion of AsV to AsIII is necessary for appropriate analysis of the total As mixture (AsIII + AsV). The free As atoms that result from the conversion of AsIII to AsH3 vapor and then free As are what provide the AAS absorption signal. This is accomplished using the AAS-attached vapor generation assembly, which contains a reduction channel filled with sodium borohydride and an acid channel filled with 10 M HCl.83
Due to its usefulness, simplicity, and affordability, AAS is an extensively used technique for metal measurement. However, sample pretreatment is frequently performed before the actual detection stage in order to enhance the metrological features of AAS, particularly the sensitivity and the detection limit.84 In reality, optical spectroscopy is a useful tool for identifying AsIII, AsV, DMA, MMA, AsC, AsB and TAMO as well as for detecting considerable hydride arsenosugars and thioarsenate production when used in conjunction with various separation methods and chemical modifiers.85,86
The drawbacks of the conventional HG borohydride/acid system have long been the subject of research into new vapor generation systems, such as electrochemical vapor generation (ECVG) and photochemical vapor generation (photo-CVG). The selective and quantitative conversion of AsIII to AsH3 on the GSH-modified graphite electrode at an applied current of 0.4 A, while both AsIII and AsV on the Cys-modified graphite electrode could generate AsH3 at an applied current of 0.6 A. This allowed for the speciation of As by coupling with AFS. By mixing 15 mg L−1 FeCl3 with acetic acid and formic acid, the ultraviolet vapor generation (UVG) of As has been increased around 10-fold.91 Additionally, under UV light, AsIII and AsV could be transformed to volatile As species using a nano-Au/nano-TiO2 composite.92 AsH3 was produced at 0.6 A of applied current, but MMA and DMA could not form any or only a small amount of hydride under these conditions, achieving the speciation of As by coupling with AFS. HPLC-HG-AFS was employed to measure AsIII, AsV, DMA and MMA in the roots, stems and fruits of strawberry plants.93
7.3.4.1 Interference can be a problem in ICP-MS. When there is an isobaric overlap caused by polyatomic ions generated by the combination of two or more atoms, interference can be a problem in ICP-MS. The most abundant argon isotopes, ambient gases, and the solvents or acids used to prepare the sample all combine to generate the most significant polyatomic ions.11 40As35Cl is a significant polyatomic interference species for As [As is monoisotopic m/z 75]. Refractory oxides may occur as a result of incomplete dissociation or recombination in colder plasma areas, particularly in the boundary layer surrounding the sampler cone.100 When polyatomic interference occurs, a collision/reaction cell can be used to reduce interfering ions by introducing a collision gas (such as helium), a reaction gas (such as oxygen, hydrogen, or CH3F), or a combination of two gases into the ICP-MS. ICP with triple quadrupole tandem mass spectrometry (ICP-QQQ) helps to eliminate isobaric interference, lessen background noise, and enhance selectivity compared to ordinary single quadrupole ICP-MS.98
There are numerous ways to reduce this interference issue in ICP-MS. Polyatomic interference can be reduced by introducing a different gas to the argon plasma, such as nitrogen, oxygen, air, helium, or hydrogen, which can also reduce the inherent polyatomic interference.101 Due to an increase in signal and a decrease in argon and O-based interference, adding nitrogen gas to an argon plasma has been proven to be quite successful.102 However, a more contemporary method that makes use of collision cell technology is currently offered on commercial instruments for the elimination of interference. In the case of As, a collision reaction cell with gases such H2, O2, NH3, CH4, NO, CO2, or C2H4 can reduce the 40Ar35Cl+ interference.103–105 Due to its sensitivity and capacity to resolve isobaric overlap, sector field (SF)-ICP-MS is possibly the most suitable option for elemental speciation research.106 As speciation in cucumber xylem sap is one instance of an As speciation study employing this method.107
CL detection is an optical detection method that provides great selectivity but at a price that is significantly less than that of atomic spectrometric methods. It has frequently been utilized in FI systems to identify both organic and inorganic species. Using luminol-based chemiluminescence detection or chemiluminescence produced by the redox reaction of AsIII with permanganate in the presence of sodium hexametaphosphate, inorganic As species have both been successfully identified in FI systems. The relative detection limits for these techniques were 8 μg L−1, 100 μg L−1, and 0.3 μgL−1, respectively.113
8 As speciation in plants and vegetables
It has long been understood that As is a phytotoxic substance that perturbs the physiological and biochemical processes of plants. The toxicity depends on As speciation, typically, inorganic species are more poisonous to living things, such as plants, people, and other animals, than organic forms.23 Vegetables can absorb As from their surroundings (such as soil, irrigation water, and accumulated dust), leading to their future contamination.114 Therefore, for a population that consumes a lot of vegetables in As-contaminated areas, dietary intake may represent a significant As exposure pathway. As levels in a plant's edible compartments are influenced by the amount of As present in the soil, as well as the plant's capacity for accumulation and translocation.115,116 As is easily accumulated by vegetables, which makes it easier for As to go from the soil to the food chain. In the oxic layer of soils, AsV, which behaves chemically in a similar way to phosphate, is the main InAs species. As a result, AsV can enter plant species via the phosphate transporter system, whereupon the AsV in the plant biomass may be changed into AsIII. The amount of phosphate in the soil affects the absorption and phytotoxicity of As. As can displace phosphate in soil particles at low concentrations, increasing absorption and phytotoxicity, whereas high concentrations of phosphate compete with As at root surfaces, decreasing uptake and phytotoxicity.40 Beside this, a protein transporter supports plants to absorb InAs. Usually, As compartments in roots then transfers to stem and grains.117 Table 3 shows the literature on As species measured in vegetables and plants using different coupling instruments worldwide.| Technique | Sample type | Contents of As species (μg kg−1) | Separation condition | Detection limit ng mL−1 | Ref. |
|---|---|---|---|---|---|
| a NG: not given, co-electro-osmotic flow (co-EOF) capillary zone electrophoresis (CZE), FF: flower and fruits, S: strawberry.b Values are less than limit of quantification; ETV: electrothermal vaporization. | |||||
| HPLC-ICP-MS | Welsh onion | AsIII: 140, AsV: 200, DMA: 0 | Hamilton, PRP-X100 anion exchange column; e mobile phase consisting of 8 mM (NH4)2HPO4 and 2 mM KNO3, pH 8 | NG | 54 |
| Spinach | AsIII: 2460, AsV: 1930, DMA: 0 | ||||
| Romaine lettuce | AsIII: 8970, AsV: 3590, DMA: 0 | ||||
| Leaf lettuce | AsIII: 6010, AsV: 3130, DMA: 0 | ||||
| Leaf mustard | AsIII: 4700, AsV: 5200, DMA: 0 | ||||
| Common sowthistle | AsIII: 340, AsV: 470, DMA: 0.01 | ||||
| Pakchoi | AsIII: 4900, AsV: 2400, DMA: 0 | ||||
| Sweet potato leaves | AsIII: 1210, AsV: 340, DMA: 100 | ||||
| Choy sum | AsIII: 1370, AsV: 650, DMA: 0 | ||||
| Pumpkin leaves | AsIII: 4490, AsV: 3270, DMA: 330 | ||||
| CE-ICP-MS | Solanum lyratum Thunb | AsIII: <LOD, AsV: <LOD, DMA: <LOD | Running buffer solution 50 mmol L−1 H3BO3-12.5 mmol L−1 Na2B4O7, pH = 9.10; sheath liquid 5% (v/v) CH3OH | AsIII: 0.3, AsV: 0.5, DMA: 0.6, AsB: 0.3, AsC: 0.2 | 62 |
| AsB: 420–1300, AsC: <LOD | |||||
| IC-ICP-MS | Spirulina powder samples | AsIII: 28–147, AsV: 170–394 | An anion exchange column (AEC, IonPac AS23), mobile phase 10.0 mmol L−1 (NH4)2HPO4 (pH 5.50); A cation-exchange column (CEC, IonPac CS12), mobile phase 0.10% v/v HCOOH (pH 3.00) | AsIII: 5, AsV: 10, MMA: 5; DMA: 10 | 118 |
| DMA: 32–839; MMA: 67 | |||||
| co-EOF-CZE | Kelp | AsIII, AsV, DMA, MMA | The background solution was 100 mM borax buffer (pH 9.2), the separation voltage was −20 kV. The second stacking step used 60% MeOH | 0.382 to 0.911 | 119 |
| Ion pair HPLC-ICP-MS | Tree moss | AsIII: 48, AsV: 53.7, DMA: 52.0, MMA: 53.7, TMAO: 27.0, tetra: 3.4, AsC: <LOD, AsB: 5.4, arsenosugar X: 42.9 (ng mL−1, as As) | CAPCELL-PAK C18 MG-II (250 mm × 4.6 mm, 5 μm), mobile phase A 10 mmol L−1 sodium butanesulfonate, 4 mmol L−1 TMAH and 4 mmol L−1 malonic acid, methanol/water (0.1/99.9, v/v), pH 3.0: mobile phase B 5 mmol L−1 ammonium acetate, methanol/water (1/99, v/v), pH 7.0: mobile phase C methanol/water (0.1/99.9, v/v), pH 3.0 | AsIII: 0.06, AsV: 0.06, DMA: 0.05, MMA, 0.06 TMAO: 0.07, Tetra: 0.07, AsC: 0.07, AsB: 0.07 | 68 |
| IP-RP-HPLC-PB-EIMS | Kelp bladderwrack extracts | InAs: 6100, DMA: 960 (ng mL−1) | Dionex AS7 column, mobile phase 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4H2O 4H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH w/0.1% trifluoroacetic acid, mobile phase (gradient) (A) 0.5 mmol L−1 HNO3, 2% MeOH (B) 50 mmol L−1 HNO3 MeOH w/0.1% trifluoroacetic acid, mobile phase (gradient) (A) 0.5 mmol L−1 HNO3, 2% MeOH (B) 50 mmol L−1 HNO3 |
AsIII: 0.03, DMA: 0.05, AsB: 0.008 and As 328![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005 0.005 |
71 |
| InAs: 6200, DMA: 620 (ng mL−1) | |||||
| HPLC-ICP-OES | Chinese brake ferns | AsIII: NG, AsV: NG | Hamilton PRP-X100 anion exchange column, mobile phase 30 mM NH4H2PO4 (pH = 6) | AsIII: 32, AsV: 25 | 96 |
| HPLC-ICP-MS | Leaf lettuce | AsIII: 31.8, AsV: 93.1, DMA: 0.5 | NG | NG | 52 |
| Spring onion | AsIII: 95.3, AsV: 142.3, DMA: 2.7 | ||||
| Celery | AsIII: 221.6, AsV: 143.1, DMA: 1 | ||||
| Bok choy | AsIII: 23.4, AsV: 87.9, DMA: <LOD | ||||
| Napa cabbage | AsIII: 30.9, AsV: 80.1, DMA: <LOD | ||||
| Coriander | AsIII: 94.9, AsV: 147.3, DMA: 1.0 | ||||
| Lettuce | AsIII: 79.4, AsV: 114.3, DMA: 1.8 | ||||
| Purple-stem mustard | AsIII: 24.4, AsV: 38.1, DMA: 0.5 | ||||
| Spinach | AsIII: 90.5, AsV: 164.5, DMA: 6.1 | ||||
| Garlic chives | AsIII: 115.3, AsV: 144.3, DMA: 2.0 | ||||
| Rapini | AsIII: 37.9, AsV: 68.3, DMA: 0.3 | ||||
| Turnip green | AsIII: 131.4, AsV: 175.6, DMA: <LOD | ||||
| Choy sum | AsIII: 86.9, AsV: 120.9, DMA: <LOD | ||||
| HPLC-ICP-MS | Carrots | AsIII: ND–230, AsV: 16–96, MMA: ND, DMA: ND, AsB: ND | Column, waters IC-Pak anion HR; mobile phase, 10 mM ammonium carbonate, pH 10 | AsIII: 8, AsV: 6, MMA: 7, DMA: 12, AsB: 7 (ng g−1) | 58 |
| HPLC-HG-AFS | Carrot | AsIII: 90, AsV: ND, DMA: ND, MMA: 22 | Hamilton PRP-X100 column, mobile phase 10 mM K2HPO4/KH2PO4, pH 6.0 (isocratic) | AsIII: 17, AsV: 14, DMA: 15, MMA: 11 (ng g−1) | 120 |
| Kidney beans | AsIII: 42, AsV: ND, DMA: ND, MMA: ND | ||||
| Radish | AsIII: 94, AsV: ND, DMA: ND, MMA: 60 | ||||
| Tomato | AsIII: 54, AsV: ND, DMA: ND, MMA: ND | ||||
| Onion | AsIII: 56, AsV: ND, DMA: ND, MMA: ND | ||||
| Betel nut | AsIII: 26, AsV: ND, DMA: ND, MMA: ND | ||||
| Cauliflower | AsIII: 60, AsV: ND, DMA: ND, MMA: ND | ||||
| Brinja | AsIII: 48, AsV: ND, DMA: ND, MMA: ND | ||||
| Potatoes | AsIII: 47, AsV: ND, DMA: ND, MMA: 34 | ||||
| HPLC-ICPMS | Potatoes | InAs: 8.44, MMA: <0.2, DMA: 0.648, AsB: <2, TMAO: <0.3, TMA: <0.1, AsC: <0.2 (values based on fresh weight) | Column: TSKgel super IC A/C, mobile phase: 0.35 mmol L−1 Na2SO4 (pH 3.1) | AsV: 0.09, AsIII: 0.08, MMA: 0.04, DMA: 0.05, AsB: 0.01, TMAO: 0.07, TMA: 0.04, AsC: 0.05 (ng g−1) | 121 |
| Pulses | InAs: 0.3, MMA: <0.1, DMA: <0.2, AsB: <2, TMAO: <0.2, TMA: <0.1, AsC: <0.1 (values based on fresh weight) | ||||
| Vegetables | InAs: <0.1, MMA: <0.07, DMA: <0.008, AsB: <0.9, TMAO: <0.1, TMA: <0.06, AsC: <0.08 (values based on fresh weight) | ||||
| HPLC-ICP-MS | Broccoli | AsIII: 18.7–35.1, AsV: 25.3–50.4, DMA: ND, MMA: ND | Hamilton PRP-X100 anion-exchange chromatographic column: mobile phase, (NH4)2HPO4 and water | AsIII: 0.068, AsV: 0.073, DMA: 0.086, MMA: 0.065 | 122 |
| Caraway | AsIII: 153–200, AsV: 132–207, DMA: ND–9.24, MMA: ND | ||||
| Lettuce | AsIII: 84–136, AsV: 92.8–142, DMA: ND–8.51, MMA: ND | ||||
| Radish | AsIII: 32.8–53.3, AsV: 58.1–60.9, DMA: ND–4.52, MMA: ND | ||||
| Potato | AsIII: 19.1–23, AsV: 23.9–26.3, DMA: 7.25–10.7, MMA: ND | ||||
| Tomato | AsIII: 13.7–21.5, AsV: 22.7–34.1, DMA: ND, MMA: ND | ||||
| Zucchini | AsIII: 4.59–11.6, AsV: 7.87–17.9, DMA: ND, MMA: ND | ||||
| Green beans | AsIII: 17.9–26, AsV: 28.7–36.6, DMA: ND, MMA: ND | ||||
| HG-AFS | Chards | AsIII: 89.2–90.6, AsV: 14.2–15.3, DMA: 4.1–4.3, MMA: 3.5–3.7 | Sonication at room temperature with H3PO4 1 mol L−1 in the presence of 0.1% (w/v) Triton XT-114 and washing of the solid phase with 0.1% (w/v) EDTA | AsIII: 3.1, AsV: 3.0, MMA: 1.9, DMA: 1.5 (ng g−1) | 123 |
| Aubergine | AsIII: 20.6–20.9, AsV: 61.0–61.9, DMA: 1.1–1.2, MMA: 1.2 | ||||
| HPLC-ICP-MS | Broad bean-root | AsIII: 324, AsV: 1585, DMA: 41, MMA: 68 | Column, Hamilton resin PRP-X100, 10 μm particle size: mobile phase, 20 mM NH4H2PO4, pH 6.0 | NG | 18 |
| Broad bean-stem | AsIII: 35, AsV: 62, DMA: 50, MMA: 44 | ||||
| Broad bean-leaf | AsIII: 91, AsV: 232, DMA: <LOD, MMA: 101 | ||||
| Broad bean-pod | AsIII: 49, AsV: 82, DMA: 70, MMA: 27 | ||||
| Broad bean-bean | AsIII: 9, AsV: 24, DMA: 22, MMA: 55 | ||||
| HG-AFS | Garlic | AsIII: 17.1–22.1, AsV: 54.7–67.6 | Ultrasound-assisted extraction procedure, 1 M H2SO4, 1 M HClO4, and methanol (99.98%) were used. A 0.1% (w/v) solution of the disodium salt of ethylenediaminotetraacetic acid | AsIII: 0.8, AsV: 0.6 (ng g−1) | 55 |
| HPLC-ICP-MS | Herbal tea leaves-FF | AsIII: ND–293, AsV: ND–554, DMA: ND, MMA: ND | Column, hamilton PRP-X100: mobile phase: mmol L−1 (NH4)2HPO4 (pH 6.0) | AsIII: 8.4,AsV: 11.0, MMA: 8.6, DMA: 7.6 (ng g−1) | 124 |
| Herbal tea leaves-S | AsIII: ND–461, AsV: ND–625, DMA: ND, MMA: ND | ||||
| HPLC-ICP-MS | Lotus root | AsIII: <0.4b, AsV: <0.6b, DMA: 3.5–7.9, MMA: <0.3b | Column, diamonsil (2) C18; mobile phase: A: 4 mM TBAH (pH 6.0) | AsIII: 0.078,AsV: 0.15, MMA: 0.078, DMA: 0.081 | 125 |
| B: 4 mM TBAH + 20 mM Cys (pH 6.0) | |||||
9 Certified reference materials (CRM)
There has been an increased demand for various CRM types in chemical analysis during the past few years, as well as new CRM publications regarding their advances and certification. The increase in ISO/IEC 17025 accreditation serves as an example of the rising demand for traceable and trustworthy results in analytical chemistry. The usage of CRMs is highlighted among the several technological requirements in this quality system because of its application in many processes, including method validation, proficiency testing, uncertainty estimation, and quality control.126Environmental control laboratories should utilize CRMs to test or verify the performance of their analytical methods in order to give accurate information and to comply with the data quality objectives of the legislation.127 As-containing CRMs have been produced, but the majority of them have total-element concentration certification. Because of the growing usage of species-specific measurement, species-specific CRM materials are now essential.11 Some certified materials used for As speciation in plants and vegetables are listed in Table 4.
| Name of certified reference material | Extraction media | Certified value (μg kg−1) | Obtained value (μg kg−1) | Extraction efficiency (%) | Ref. |
|---|---|---|---|---|---|
| GBW10048 (GSB-26) | 1% HNO3 under sonication at room temperature (25 °C) | 390 ± 80 | 390 ± 30 | 100 | 54 |
| GBW10015-spinach | 1% HNO3, MAE | 230 ± 30 | 249 ± 8 | 108 | 57 |
| GBW 82301 (peach leaves) | Water–methanol, shaking for 20 min | 340 ± 50 | 248 ± 10 | 73 | 48 |
| BCR 279 sea lettuce (Ulva lactuca) | Water, shaking for 16 h | 3090 ± 200 | 2900 ± 300 | 94 | 60 |
| GBW10049-green onion | Water, MAE, 90 °C, 1.5 h | 520 ± 110 | 489.9 ± 1.2 | 94 | 52 |
| 80 mM NH4H2PO4 (pH = 7), shaking 90 °C, 1.5 h | 520 ± 110 | 324.0 ± 2.3 | 62 | 52 | |
| 80 mM NH4H2PO4 (pH = 7), MAE, 90 °C, 1.5 h | 520 ± 110 | 352.7 ± 9.2 | 68 | 52 | |
| 1% HNO3, shaking, 90 °C, 2 h | 520 ± 110 | 419.3 ± 1.6 | 81 | 52 | |
| 1% HNO3, sonication, 2 h | 520 ± 110 | 224.0 ± 2.8 | 43% | 52 | |
| NCS-ZC-85006 (Tomato leaves) | MAE, HNO3 (69%) | 1050 ± 130 | 940 | 89.5 | 128 |
| CS-PR-2 (parsnip root powder) | MAE, HNO3 (69%) | 30 ± 20 | 30 | 100 | 128 |
| CRM NIST 1573a (Tomato leaves) | Ultrasound-assisted extraction procedure, 1 M H2SO4, 1 M HClO4, and methanol were used. A 0.1% (w/v) solution of the disodium salt of ethylenediaminotetraacetic acid | 112 ± 4 | 110.2 ± 1.7 | 98.4 | 55 |
10 Conclusions
Millions of people worldwide rely heavily on edible plants and vegetables for food, yet when contaminated with toxins like As, they also pose the biggest threat to human health. The outcome of toxic As species present in vegetation on human health is of serious concern because several diseases are linked with toxic forms, especially if they surpass the allowable range. Although a certain limit for As in drinking water has been set, for vegetation this value has not yet been documented. Both can accumulate variable quantities of heavy metals as well as As species from environments such as water and soil, and anthropogenic processes. This review highlights an overview of available methods for measuring As species in edible plants and vegetables. Different chromatographic and non-chromatographic methods, and spectroscopic and coupled techniques were explained to identify and detect As species in edible plants and vegetables. The most promising and realistic methods of determining As species are recently coupled or hyphenated analytical techniques, including ICP-MS, LC-MS, and HPLC/ICP-MS.Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
The authors acknowledge the support of Salahaddin University-Erbil and Cihan University-Erbil.References
- N. Gupta, K. K. Yadav, V. Kumar, S. Krishnan, S. Kumar, Z. D. Nejad, M. M. Khan and J. Alam, Evaluating heavy metals contamination in soil and vegetables in the region of North India: levels, transfer and potential human health risk analysis, Environ. Toxicol. Pharmacol., 2021, 82, 103563 CrossRef CAS PubMed.
- M. Mostafidi, M. R. Sanjabi, F. Shirkhan and M. T. Zahedi, A review of recent trends in the development of the microbial safety of fruits and vegetables, Trends Food Sci. Technol., 2020, 103, 321–332 CrossRef CAS.
- B. A. Sadee, Determination of Elements in Indigenous Vegetables Using ICP-MS, Cihan Univ.-Erbil Sci. J., 2022, 6(1), 26–31 CrossRef.
- B. A. Sadee and R. J. Ali, Determination of heavy metals in edible vegetables and a human health risk assessment, Environ. Nanotechnol., Monit. Manage., 2023, 19, 100761 Search PubMed.
- B. A. Sadee, Determination of trace metals in vegetables using ICP-MS, Zanco J. Pure Appl. Sci., 2022, 34(3), 73–83 Search PubMed.
- M. Costa, Review of arsenic toxicity, speciation and polyadenylation of canonical histones, Toxicol. Appl. Pharmacol., 2019, 375, 1–4 CrossRef CAS PubMed.
- S. W. Al Rmalli, P. I. Haris, C. F. Harrington and M. Ayub, A survey of arsenic in foodstuffs on sale in the United Kingdom and imported from Bangladesh, Sci. Total Environ., 2005, 337(1–3), 23–30 CrossRef CAS PubMed.
- D. Lièvremont, P. N. Bertin and M. C. Lett, Arsenic in contaminated waters: biogeochemical cycle, microbial metabolism and biotreatment processes, Biochimie, 2009, 91(10), 1229–1237 CrossRef PubMed.
- M. M. Haque, N. M. Niloy, M. A. Khirul, M. F. Alam and S. M. Tareq, Appraisal of probabilistic human health risks of heavy metals in vegetables from industrial, non-industrial and arsenic contaminated areas of Bangladesh, Heliyon, 2021, 7(2), e06309 CrossRef CAS PubMed.
- A. Nabi, M. Naeem, T. Aftab, M. M. Khan and P. Ahmad, A comprehensive review of adaptations in plants under arsenic toxicity: physiological, metabolic and molecular interventions, Environ. Pollut., 2021, 290, 118029 CrossRef CAS PubMed.
- B. Sadee, M. E. Foulkes and S. J. Hill, Coupled techniques for arsenic speciation in food and drinking water: a review, J. Anal. At. Spectrom., 2015, 30(1), 102–118 RSC.
- S. Fendorf, H. A. Michael and A. van Geen, Spatial and temporal variations of groundwater arsenic in South and Southeast Asia, Science, 2010, 328(5982), 1123–1127 CrossRef CAS PubMed.
- P. Drahota and M. Filippi, Secondary arsenic minerals in the environment: a review, Environ. Int., 2009, 35(8), 1243–1255 CrossRef CAS PubMed.
- J. Matschullat, Arsenic in the geosphere—a review, Sci. Total Environ., 2000, 249(1–3), 297–312 CrossRef CAS PubMed.
- E. Shaji, M. Santosh, K. V. Sarath, P. Prakash, V. Deepchand and B. V. Divya, Arsenic contamination of groundwater: a global synopsis with focus on the Indian Peninsula, Geosci. Front., 2021, 12(3), 101079 CrossRef CAS.
- M. Ataee, T. Ahmadi-Jouibari, N. Noori and N. Fattahi, The speciation of inorganic arsenic in soil and vegetables irrigated with treated municipal wastewater, RSC Adv., 2020, 10(3), 1514–1521 RSC.
- J. O. Fatoki and J. A. Badmus, Arsenic as an environmental and human health antagonist: a review of its toxicity and disease initiation, J. Hazard. Mater. Adv., 2022, 5, 100052 CrossRef CAS.
- B. A. Sadee, M. E. Foulkes and S. J. Hill, An evaluation of extraction techniques for arsenic in staple diets (fish and rice) utilising both classical and enzymatic extraction methods, Food Addit. Contam.: Part A, 2016, 33(3), 433–441 CrossRef CAS PubMed.
- G. P. Warren, B. J. Alloway, N. W. Lepp, B. Singh, F. J. Bochereau and C. Penny, Field trials to assess the uptake of arsenic by vegetables from contaminated soils and soil remediation with iron oxides, Sci. Total Environ., 2003, 311(1–3), 19–33 CrossRef CAS PubMed.
- FDA, FDA proposes limit for inorganic arsenic in infant rice cereal, https://www.fda.gov/news-events/press-announcements/fda-proposes-limit-inorganic-arsenic-infant-rice-cereal, 2016 Search PubMed.
- The European Commission, Regulation EA. no 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs, Offcial Journal of the European Communities, Brussels, Belgium, 2015 Search PubMed.
- Y. G. Zhu, G. X. Sun, M. Lei, M. Teng, Y. X. Liu, N. C. Chen, L. H. Wang, A. M. Carey, C. Deacon, A. Raab and A. A. Meharg, High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice, Environ. Sci. Technol., 2008, 42(13), 5008–5013 CrossRef CAS PubMed.
- R. K. Mishra, S. Tiwari, A. Patel and S. M. Prasad, Arsenic contamination, speciation, toxicity and defense strategies in plants, Rev. Bras. Bot., 2021, 44(1), 1 CrossRef.
- V. K. Gupta, A. Nayak, S. Agarwal, R. Dobhal, D. P. Uniyal, P. Singh, B. Sharma, S. Tyagi and R. Singh, Arsenic speciation analysis and remediation techniques in drinking water, Desalin. Water Treat., 2012, 40(1–3), 231–243 CrossRef CAS.
- A. M. Ure and C. M. Davidson, Chemical speciation in soils and related materials by selective chemical extraction, Chem. Speciation Environ., 2002, 265–300 Search PubMed.
- M. E. Foulkes, B. A. Sadee and S. J. Hill, Arsenic speciation and its DNA fractionation in the rice plant Oryza sativa, J. Anal. At. Spectrom., 2020, 35(9), 1989–2001 RSC.
- D. J. Thomas, Arsenic methylation–Lessons from three decades of research, Toxicology, 2021, 457, 152800 CrossRef CAS PubMed.
- A. K. Sharma, J. C. Tjell, J. J. Sloth and P. E. Holm, Review of arsenic contamination, exposure through water and food and low cost mitigation options for rural areas, Appl. Geochem., 2014, 41, 11–33 CrossRef CAS.
- D. T. Heitkemper, N. P. Vela, K. R. Stewart and C. S. Westphal, Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry, J. Anal. At. Spectrom., 2001, 16(4), 299–306 RSC.
- E. Sanz, R. Muñoz-Olivas and C. Cámara, A rapid and novel alternative to conventional sample treatment for arsenic speciation in rice using enzymatic ultrasonic probe, Anal. Chim. Acta, 2005, 535(1–2), 227–235 CrossRef CAS.
- Y. Liu, Y. Huang, L. Li, Y. Xiong, L. Tong, F. Wang, B. Fan and J. Gong, Effect of different agricultural conditions, practices, and processing on levels of total arsenic and species in cereals and vegetables: a review, Food Control, 2023, 109876 CrossRef CAS.
- W. Ali, H. Zhang, M. Junaid, K. Mao, N. Xu, C. Chang, A. Rasool, M. Wajahat Aslam, J. Ali and Z. Yang, Insights into the mechanisms of arsenic-selenium interactions and the associated toxicity in plants, animals, and humans: a critical review, Crit. Rev. Environ. Sci. Technol., 2021, 51(7), 704–750 CrossRef CAS.
- A. H. Smith, E. O. Lingas and M. Rahman, Contamination of drinking-water by arsenic in Bangladesh: a public health emergency, Bull. W. H. O., 2000, 78(9), 1093–1103 CAS.
- G. Bjørklund, P. Oliinyk, R. Lysiuk, M. S. Rahaman, H. Antonyak, I. Lozynska, L. Lenchyk and M. Peana, Arsenic intoxication: general aspects and chelating agents, Arch. Toxicol., 2020, 94, 1879–1897 CrossRef PubMed.
- A. P. Cardoso, K. T. Udoh and J. C. States, Arsenic-induced changes in miRNA expression in cancer and other diseases, Toxicol. Appl. Pharmacol., 2020, 409, 115306 CrossRef PubMed.
- M. S. Rahaman, N. Mise and S. Ichihara, Arsenic contamination in food chain in Bangladesh: a review on health hazards, socioeconomic impacts and implications, Int. J. Hyg. Environ. Health, 2022, 2, 100004 CrossRef.
- M. S. Rahaman, M. M. Rahman, N. Mise, M. T. Sikder, G. Ichihara, M. K. Uddin, M. Kurasaki and S. Ichihara, Environmental arsenic exposure and its contribution to human diseases, toxicity mechanism and management, Environ. Pollut., 2021, 289, 117940 CrossRef CAS PubMed.
- A. M. Cooper, D. Felix, F. Alcantara, I. Zaslavsky, A. Work, P. L. Watson, K. Pezzoli, Q. Yu, D. Zhu, A. J. Scavo and Y. Zarabi, Monitoring and mitigation of toxic heavy metals and arsenic accumulation in food crops: a case study of an urban community garden, Plant Direct, 2020, 4(1), e00198 CrossRef CAS PubMed.
- P. Mayorga, A. Moyano, H. M. Anawar and A. Garcia-Sanchez, Uptake and accumulation of arsenic in different organs of carrot irrigated with as-rich water, Clean: Soil, Air, Water, 2013, 41(6), 587–592 CAS.
- C. B’hymer and J. A. Caruso, Arsenic and its speciation analysis using high-performance liquid chromatography and inductively coupled plasma mass spectrometry, J. Chromatogr. A, 2004, 1045(1–2), 1–3 CrossRef PubMed.
- C. S. Eskilsson and E. Björklund, Analytical-scale microwave-assisted extraction, J. Chromatogr. A, 2000, 902(1), 227–250 CrossRef CAS PubMed.
- J. W. McKiernan, J. T. Creed, C. A. Brockhoff, J. A. Caruso and R. M. Lorenzana, A comparison of automated and traditional methods for the extraction of arsenicals from fish, J. Anal. At. Spectrom., 1999, 14(4), 607–613 RSC.
- J. Mattusch, M. Cimpean and R. Wennrich, Enzyme-assisted extraction of arsenic species from plant material, Int. J. Environ. Anal. Chem., 2006, 86(9), 629–640 CrossRef CAS.
- J. A. Caruso, D. T. Heitkemper and C. B'Hymer, An evaluation of extraction techniques for arsenic species from freeze-dried apple samples, Analyst, 2001, 126(2), 136–140 RSC.
- L. Jedynak, J. Kowalska, M. Kossykowska and J. Golimowski, Studies on the uptake of different arsenic forms and the influence of sample pretreatment on arsenic speciation in White mustard (Sinapis alba), Microchem. J., 2010, 94(2), 125–129 CrossRef CAS.
- C. Bergqvist and M. Greger, Arsenic accumulation and speciation in plants from different habitats, Appl. Geochem., 2012, 27(3), 615–622 CrossRef CAS.
- M. J. Ruiz-Chancho, J. F. López-Sánchez, E. Schmeisser, W. Goessler, K. A. Francesconi and R. Rubio, Arsenic speciation in plants growing in arsenic-contaminated sites, Chemosphere, 2008, 71(8), 1522–1530 CrossRef CAS PubMed.
- B. He, Y. Fang, G. Jiang and Z. Ni, Optimization of the extraction for the determination of arsenic species in plant materials by high-performance liquid chromatography coupled with hydride generation atomic fluorescence spectrometry, Spectrochim. Acta, Part B, 2002, 57(11), 1705–1711 CrossRef.
- N. M. Smith, R. Lee, D. T. Heitkemper, K. D. Cafferky, A. Haque and A. K. Henderson, Inorganic arsenic in cooked rice and vegetables from Bangladeshi households, Sci. Total Environ., 2006, 370(2–3), 294–301 CrossRef CAS PubMed.
- D. Zhao, H. B. Li, J. Y. Xu, J. Luo and L. Q. Ma, Arsenic extraction and speciation in plants: Method comparison and development, Sci. Total Environ., 2015, 523, 138–145 CrossRef CAS PubMed.
- K. A. Mir, A. Rutter, I. Koch, P. Smith, K. J. Reimer and J. S. Poland, Extraction and speciation of arsenic in plants grown on arsenic contaminated soils, Talanta, 2007, 72(4), 1507–1518 CrossRef CAS PubMed.
- L. Ma, Z. Yang, Q. Kong and L. Wang, Extraction and determination of arsenic species in leafy vegetables: method development and application, Food Chem., 2017, 217, 524–530 CrossRef CAS PubMed.
- F. Rahman, Z. Chen and R. Naidu, A comparative study of the extractability of arsenic species from silverbeet and amaranth vegetables, Environ. Geochem. Health, 2009, 31, 103–113 CrossRef CAS PubMed.
- X. Zheng, Z. Zhang, J. Chen, H. Liang, X. Chen, Y. Qin, M. J. Shohag, Y. Wei and M. Gu, Comparative evaluation of in vivo relative bioavailability and in vitro bioaccessibility of arsenic in leafy vegetables and its implication in human exposure assessment, J. Hazard. Mater., 2022, 423, 126909 CrossRef CAS PubMed.
- H. Sousa-Ferreira, M. N. Matos-Reyes, M. L. Cervera, S. L. Costa-Ferreira and M. de la Guardia, Screening of toxic inorganic arsenic species in garlic (Allium sativum L.), Food Anal. Methods, 2011, 4, 447–452 CrossRef.
- Z. U. Rehman, S. Khan, K. Qin, M. L. Brusseau, M. T. Shah and I. Din, Quantification of inorganic arsenic exposure and cancer risk via consumption of vegetables in southern selected districts of Pakistan, Sci. Total Environ., 2016, 550, 321–329 CrossRef CAS PubMed.
- B. A. Sadee, M. E. Foulkes and S. J. Hill, A study of arsenic speciation in soil, irrigation water and plant tissue: a case study of the broad bean plant, Vicia faba, Food Chem., 2016, 210, 362–370 CrossRef CAS PubMed.
- N. P. Vela, D. T. Heitkemper and K. R. Stewart, Arsenic extraction and speciation in carrots using accelerated solvent extraction, liquid chromatography and plasma mass spectrometry. This is the work of United States government employees engaged in their official duties. As such it is in the public domain and exempt from copyright© US government, Analyst, 2001, 126(7), 1011–1017 RSC.
- J. Száková, P. Tlustoš, W. Goessler, D. Pavlíková and J. Balík, Comparison of mild extraction procedures for determination of arsenic compounds in different parts of pepper plants (Capsicum annum, L.), Appl. Organomet. Chem., 2005, 19(3), 308–314 CrossRef.
- A. Pell, A. Márquez, J. F. López-Sánchez, R. Rubio, M. Barbero, S. Stegen, F. Queirolo and P. Díaz-Palma, Occurrence of arsenic species in algae and freshwater plants of an extreme arid region in northern Chile, the Loa River Basin, Chemosphere, 2013, 90(2), 556–564 CrossRef CAS PubMed.
- X. M. Xue, C. Xiong, M. Yoshinaga, B. Rosen and Y. G. Zhu, The enigma of environmental organoarsenicals: insights and implications, Crit. Rev. Environ. Sci. Technol., 2022, 52(21), 3835–3862 CrossRef.
- P. Y. Shuai, X. J. Yang, Z. Q. Qiu, X. H. Wu, X. Zhu, G. R. Pokhrel, Y. Y. Fu, H. M. Ye, W. X. Lin and G. D. Yang, Determination of arsenic species in Solanum Lyratum Thunb using capillary electrophoresis with inductively coupled plasma mass spectrometry, J. Sep. Sci., 2016, 39(16), 3239–3245 CrossRef CAS PubMed.
- X. B. Yin, X. P. Yan, Y. Jiang and X. W. He, On-line coupling of capillary electrophoresis to hydride generation atomic fluorescence spectrometry for arsenic speciation analysis, Anal. Chem., 2002, 74(15), 3720–3725 CrossRef CAS PubMed.
- A. J. Ninfa, D. P. Ballou and M. Benore, Fundamental laboratory approaches for biochemistry and biotechnology, John Wiley & Sons, 2009 Search PubMed.
- M. S. Reid, K. S. Hoy, J. R. Schofield, J. S. Uppal, Y. Lin, X. Lu, H. Peng and X. C. Le, Arsenic speciation analysis: a review with an emphasis on chromatographic separations, TrAC, Trends Anal. Chem., 2020, 123, 115770 CrossRef CAS.
- M. M. Wolle, G. M. Rahman and M. Pamuku, Speciation analysis of arsenic in prenatal and children's dietary supplements using microwave-enhanced extraction and ion chromatography–inductively coupled plasma mass spectrometry, Anal. Chim. Acta, 2014, 818, 23–31 CrossRef CAS PubMed.
- Y. Morita, T. Kobayashi, T. Kuroiwa and T. Narukawa, Study on simultaneous speciation of arsenic and antimony by HPLC-ICP-MS, Talanta, 2007, 73(1), 81–86 CrossRef CAS PubMed.
- K. Nan, M. He, B. Chen, Y. Chen and B. Hu, Arsenic speciation in tree moss by mass spectrometry based hyphenated techniques, Talanta, 2018, 183, 48–54 CrossRef CAS PubMed.
- V. Taylor, B. Goodale, A. Raab, T. Schwerdtle, K. Reimer, S. Conklin, M. R. Karagas and K. A. Francesconi, Human exposure to organic arsenic species from seafood, Sci. Total Environ., 2017, 580, 266–282 CrossRef CAS PubMed.
- M. Khan and K. A. Francesconi, Preliminary studies on the stability of arsenolipids: implications for sample handling and analysis, J. Environ. Sci., 2016, 49, 97–103 CrossRef CAS PubMed.
- M. B. Krishna, J. Castro, T. M. Brewer and R. K. Marcus, On-line separation and identification of inorganic and organic arsenic species in ethanolic kelp and bladderwrack extracts through liquid chromatography/particle beam-electron ionization mass spectrometry (LC/PB-EIMS), J. Anal. At. Spectrom., 2009, 24(2), 199–208 RSC.
- L. M. Frensemeier, L. Büter, M. Vogel and U. Karst, Investigation of the oxidative transformation of roxarsone by electrochemistry coupled to hydrophilic interaction liquid chromatography/mass spectrometry, J. Anal. At. Spectrom., 2017, 32(1), 153–161 RSC.
- D. Xie, J. Mattusch and R. Wennrich, Separation of Organoarsenicals by Means of Zwitterionic Hydrophilic Interaction Chromatography (ZIC®-HILIC) and Parallel ICP-MS/ESI-MS Detection, Eng. Life Sci., 2008, 8(6), 582–588 CrossRef CAS.
- S. Shen, X. F. Li, W. R. Cullen, M. Weinfeld and X. C. Le, Arsenic binding to proteins, Chem. Rev., 2013, 113(10), 7769–7792 CrossRef CAS PubMed.
- B. Chen, Q. Liu, A. Popowich, S. Shen, X. Yan, Q. Zhang, X. F. Li, M. Weinfeld, W. R. Cullen and X. C. Le, Therapeutic and analytical applications of arsenic binding to proteins, Metallomics, 2015, 7(1), 39–55 CrossRef CAS PubMed.
- A. C. Schmidt, B. Fahlbusch and M. Otto, Size exclusion chromatography coupled to electrospray ionization mass spectrometry for analysis and quantitative characterization of arsenic interactions with peptides and proteins, J. Mass Spectrom., 2009, 44(6), 898–910 CrossRef CAS PubMed.
- M. Á. García-Sevillano, T. García-Barrera, F. Navarro-Roldán, Z. Montero-Lobato and J. L. Gómez-Ariza, A combination of metallomics and metabolomics studies to evaluate the effects of metal interactions in mammals. Application to Mus musculus mice under arsenic/cadmium exposure, J. Proteomics, 2014, 104, 66–79 CrossRef PubMed.
- B. Chen, X. Lu, S. Shen, L. L. Arnold, S. M. Cohen and X. C. Le, Arsenic speciation in the blood of arsenite-treated F344 rats, Chem. Res. Toxicol., 2013, 26(6), 952–962 Search PubMed.
- X. A. Yang, X. P. Lu, L. Liu, M. B. Chi, H. H. Hu and W. B. Zhang, Selective determination of four arsenic species in rice and water samples by modified graphite electrode-based electrolytic hydride generation coupled with atomic fluorescence spectrometry, Talanta, 2016, 159, 127–136 CrossRef CAS PubMed.
- C. Luvonga, C. A. Rimmer, L. L. Yu and S. B. Lee, Analytical methodologies for the determination of organoarsenicals in edible marine species: a review, J. Agric. Food Chem., 2020, 68(7), 1910–1934 CrossRef CAS PubMed.
- W. Chintakovid, P. Visoottiviseth, S. Khokiattiwong and S. Lauengsuchonkul, Potential of the hybrid marigolds for arsenic phytoremediation and income generation of remediators in Ron Phibun District, Thailand, Chemosphere, 2008, 70(8), 1532–1537 CrossRef CAS PubMed.
- J. Michon, V. Deluchat, R. Al Shukry, C. Dagot and J. C. Bollinger, Optimization of a GFAAS method for determination of total inorganic arsenic in drinking water, Talanta, 2007, 71(1), 479–485 CrossRef CAS PubMed.
- J. R. Behari and R. Prakash, Determination of total arsenic content in water by atomic absorption spectroscopy (AAS) using vapour generation assembly (VGA), Chemosphere, 2006, 63(1), 17–21 CrossRef CAS PubMed.
- V. Andruch, R. Halko, J. Tuček and J. Płotka-Wasylka, Application of deep eutectic solvents in atomic absorption spectrometry, TrAC, Trends Anal. Chem., 2022, 147, 116510 CrossRef CAS.
- M. Z. Kamal and M. Y. Miah, Arsenic Speciation Techniques in Soil Water and Plant: An Overview, in Arsenic Monitoring, Removal and Remediation, 2021 Search PubMed.
- K. F. Akter, Z. Chen, L. Smith, D. Davey and R. Naidu, Speciation of arsenic in ground water samples: a comparative study of CE-UV, HG-AAS and LC-ICP-MS, Talanta, 2005, 68(2), 406–415 CrossRef PubMed.
- D. Sanchez-Rodas, W. T. Corns, B. Chen and P. B. Stockwell, Atomic fluorescence spectrometry: a suitable detection technique in speciation studies for arsenic, selenium, antimony and mercury, J. Anal. At. Spectrom., 2010, 25(7), 933–946 RSC.
- J. F. Heilier, J. P. Buchet, V. Haufroid and D. Lison, Comparison of atomic absorption and fluorescence spectroscopic methods for the routine determination of urinary arsenic, Int. Arch. Occup. Environ. Health, 2005, 78, 51–59 CrossRef CAS PubMed.
- A. L. Lindberg, W. Goessler, M. Grandér, B. Nermell and M. Vahter, Evaluation of the three most commonly used analytical methods for determination of inorganic arsenic and its metabolites in urine, Toxicol. Lett., 2007, 168(3), 310–318 CrossRef CAS PubMed.
- J. L. Gómez-Ariza, D. Sánchez-Rodas, I. Giráldez and E. Morales, A comparison between ICP-MS and AFS detection for arsenic speciation in environmental samples, Talanta, 2000, 51(2), 257–268 CrossRef.
- Y. Wang, L. Lin, J. Liu, X. Mao, J. Wang and D. Qin, Ferric ion induced enhancement of ultraviolet vapour generation coupled with atomic fluorescence spectrometry for the determination of ultratrace inorganic arsenic in surface water, Analyst, 2016, 141(4), 1530–1536 RSC.
- C. H. Lin, Y. Chen, Y. A. Su, Y. T. Luo, T. T. Shih and Y. C. Sun, Nanocomposite-coated microfluidic-based photocatalyst-assisted reduction device to couple high-performance liquid chromatography and inductively coupled plasma-mass spectrometry for online determination of inorganic arsenic species in natural water, Anal. Chem., 2017, 89(11), 5891–5899 CrossRef CAS PubMed.
- A. I. de Las Torres, I. Giráldez, F. Martínez, P. Palencia, W. T. Corns and D. Sánchez-Rodas, Arsenic accumulation and speciation in strawberry plants exposed to inorganic arsenic enriched irrigation, Food Chem., 2020, 315, 126215 CrossRef PubMed.
- H. Yu, C. Li, Y. Tian and X. Jiang, Recent developments in determination and speciation of arsenic in environmental and biological samples by atomic spectrometry, Microchem. J., 2020, 152, 104312 CrossRef CAS.
- M. Chausseau, C. Roussel, N. Gilon and J. M. Mermet, Optimization of HPLC-ICP-AES for the determination of arsenic species, Fresenius' J. Anal. Chem., 2000, 366, 476–480 CrossRef CAS PubMed.
- A. L. Salido and D. Hyatt, Optimization of arsenic speciation conditions in plant material by HPLC-ICPOES, Spectrosc. Lett., 2007, 40(3), 493–499 CrossRef CAS.
- Ž. Fiket, V. Roje, N. Mikac and G. Kniewald, Determination of arsenic and other trace elements in bottled waters by high resolution inductively coupled plasma mass spectrometry, Croat. Chem. Acta, 2007, 80(1), 91–100 Search PubMed.
- B. P. Jackson, A. Liba and J. Nelson, Advantages of reaction cell ICP-MS on doubly charged interferences for arsenic and selenium analysis in foods, J. Anal. At. Spectrom., 2015, 30(5), 1179–1183 RSC.
- E. H. Evans and J. J. Giglio, Interferences in inductively coupled plasma mass spectrometry. A review, J. Anal. At. Spectrom., 1993, 8(1), 1–8 RSC.
- M. A. Vaughan and G. Horlick, Effect of sampler and skimmer orifice size on analyte and analyte oxide signals in inductively coupled plasma-mass spectrometry, Spectrochim. Acta, Part B, 1990, 45(12), 1289–1299 CrossRef.
- N. M. Raut, L. S. Huang, S. K. Aggarwal and K. C. Lin, Mathematical correction for polyatomic isobaric spectral interferences in determination of lanthanides by inductively coupled plasma mass spectrometry, J. Chin. Chem. Soc., 2005, 52(4), 589–597 CrossRef CAS.
- S. J. Hill, M. J. Ford and L. Ebdon, Simplex optimization of nitrogen–argon plasmas in inductively coupled plasma mass spectrometry for the removal of chloride-based interferences, J. Anal. At. Spectrom., 1992, 7(5), 719–725 RSC.
- D. R. Bandura, V. I. Baranov and S. D. Tanner, Reaction chemistry and collisional processes in multipole devices for resolving isobaric interferences in ICP-MS, Fresenius' J. Anal. Chem., 2001, 370, 454–470 CrossRef CAS PubMed.
- D. R. Bandura, V. I. Baranov, A. E. Litherland and S. D. Tanner, Gas-phase ion–molecule reactions for resolution of atomic isobars: AMS and ICP-MS perspectives, Int. J. Mass Spectrom., 2006, 255, 312–327 CrossRef.
- J. Darrouzès, M. Bueno, G. Lespès, M. Holeman and M. Potin-Gautier, Optimisation of ICPMS collision/reaction cell conditions for the simultaneous removal of argon based interferences of arsenic and selenium in water samples, Talanta, 2007, 71(5), 2080–2084 CrossRef PubMed.
- K. L. Linge, Trace element determination by ICP-AES and ICP-MS: developments and applications reported during 2004 and 2005, Geostand. Geoanal. Res., 2006, 30(3), 157–174 CrossRef CAS.
- V. G. Mihucz, E. Tatár, I. Virág, E. Cseh, F. Fodor and G. Záray, Arsenic speciation in xylem sap of cucumber (Cucumis sativus L.), Anal. Bioanal. Chem., 2005, 383, 461–466 CrossRef CAS PubMed.
- S. M. Webb, J. F. Gaillard, L. Q. Ma and C. Tu, XAS speciation of arsenic in a hyper-accumulating fern, Environ. Sci. Technol., 2003, 37(4), 754–760 CrossRef CAS PubMed.
- P. G. Smith, I. Koch and K. J. Reimer, Uptake, transport and transformation of arsenate in radishes (Raphanus sativus), Sci. Total Environ., 2008, 390(1), 188–197 CrossRef CAS PubMed.
- M. Hatayama, T. Sato, K. Shinoda and C. Inoue, Effects of cultivation conditions on the uptake of arsenite and arsenic chemical species accumulated by Pteris vittata in hydroponics, J. Biosci. Bioeng., 2011, 111(3), 326–332 CrossRef CAS PubMed.
- Z. C. Huang, T. B. Chen, M. Lei and T. D. Hu, Direct determination of arsenic species in arsenic hyperaccumulator Pteris vittata by EXAFS, Acta Bot. Sin., 2004, 46(1), 46–50 CAS.
- A. D. Idowu and P. K. Dasgupta, Liquid chromatographic arsenic speciation with gas-phase chemiluminescence detection, Anal. Chem., 2007, 79(23), 9197–9204 CrossRef CAS PubMed.
- C. Lomonte, M. Currell, R. J. Morrison, I. D. McKelvie and S. D. Kolev, Sensitive and ultra-fast determination of arsenic (III) by gas-diffusion flow injection analysis with chemiluminescence detection, Anal. Chim. Acta, 2007, 583(1), 72–77 CrossRef CAS PubMed.
- M. D. Ramirez-Andreotta, M. L. Brusseau, J. F. Artiola and R. M. Maier, A greenhouse and field-based study to determine the accumulation of arsenic in common homegrown vegetables grown in mining-affected soils, Sci. Total Environ., 2013, 443, 299–306 CrossRef CAS PubMed.
- R. Q. Huang, S. F. Gao, W. L. Wang, S. Staunton and G. Wang, Soil arsenic availability and the transfer of soil arsenic to crops in suburban areas in Fujian Province, southeast China, Sci. Total Environ., 2006, 368(2–3), 531–541 CrossRef CAS PubMed.
- N. Karimi and M. Alavi, Arsenic contamination and accumulation in soil, groundwater and wild plant species from Qorveh County, Iran, Biharean Biol., 2016, 10(2), 69–73 Search PubMed.
- H. Neidhardt, U. Kramar, X. Tang, H. Guo and S. Norra, Arsenic accumulation in the roots of Helianthus annuus and Zea mays by irrigation with arsenic-rich groundwater: Insights from synchrotron X-ray fluorescence imaging, Geochemistry, 2015, 75(2), 261–270 CrossRef CAS.
- X. Li, Y. Chen, J. Ye, F. Fu, G. R. Pokhrel, H. Zhang, Y. Zhu and G. Yang, Determination of different arsenic species in food-grade spirulina powder by ion chromatography combined with inductively coupled plasma mass spectrometry, J. Sep. Sci., 2017, 40(18), 3655–3661 CrossRef CAS PubMed.
- Y. L. Yu, S. C. Zhu, M. Z. Shi, F. M. Liu and J. Cao, Two-step micelle-to-solvent stacking of arsenic species from foods in permanently coated tubing for capillary electrophoresis, J. Chromatogr. A, 2022, 1673, 463112 CrossRef CAS PubMed.
- A. J. Signes-Pastor, K. Mitra, S. Sarkhel, M. Hobbes, F. Burló, W. T. De Groot and A. A. Carbonell-Barrachina, Arsenic speciation in food and estimation of the dietary intake of inorganic arsenic in a rural village of West Bengal, India, J. Agric. Food Chem., 2008, 56(20), 9469–9474 CrossRef CAS PubMed.
- T. Oguri, J. Yoshinaga, H. Tao and T. Nakazato, Inorganic arsenic in the Japanese diet: daily intake and source, Arch. Environ. Contam. Toxicol., 2014, 66, 100–112 CrossRef CAS PubMed.
- X. Jia, X. Yang, W. Zhao, Y. Hu and H. Cheng, A method for rapid determination of arsenic species in vegetables using microwave-assisted extraction followed by detection with HPLC hyphenated to inductively coupled plasma-mass spectrometry, J. Sep. Sci., 2019, 42(18), 2957–2967 CrossRef CAS PubMed.
- M. N. Reyes, M. L. Cervera, R. C. Campos and M. de la Guardia, Non-chromatographic speciation of toxic arsenic in vegetables by hydride generation-atomic fluorescence spectrometry after ultrasound-assisted extraction, Talanta, 2008, 75(3), 811–816 CrossRef CAS PubMed.
- R. F. Milani, E. L. de Paiva, L. I. Peron, M. A. Morgano and S. Cadore, Arsenic species in herbal tea leaves and infusions determination by HPLC-ICP-MS, LWT--Food Sci. Technol., 2018, 98, 606–612 CrossRef CAS.
- D. Zhang, S. Yang, H. Cheng, Y. Wang and J. Liu, Speciation of inorganic and organic species of mercury and arsenic in lotus root using high performance liquid chromatography with inductively coupled plasma mass spectrometric detection in one run, Talanta, 2019, 199, 620–627 CrossRef CAS PubMed.
- I. R. Olivares, G. B. Souza, A. R. Nogueira, G. T. Toledo and D. C. Marcki, Trends in developments of certified reference materials for chemical analysis-focus on food, water, soil, and sediment matrices, TrAC, Trends Anal. Chem., 2018, 100, 53–64 CrossRef CAS.
- G. Emma, J. Snell, J. Charoud-Got, A. Held and H. Emons, Feasibility study of a candidate reference material for ions in PM 2.5: does commutability matter also for inorganic matrices?, Anal. Bioanal. Chem., 2018, 410, 6001–6008 CrossRef CAS PubMed.
- J. C. Ng, V. Ciminelli, M. Gasparon and C. Caldeira, Health risk apportionment of arsenic from multiple exposure pathways in Paracatu, a gold mining town in Brazil, Sci. Total Environ., 2019, 673, 36–43 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |