Exploring the origin of the high electro-catalytic activity for nitrate-to-ammonia conversion on electrodeposited Ni/Ru hydroxide hybrids†
Fengling
Zhou
 *a and
Chenghua
Sun
*a and
Chenghua
Sun
 *b
*b
aGuangdong Provincial Key Laboratory of Distributed Energy Systems, Dongguan University of Technology, Dongguan, 523808, China. E-mail: zhoufl@dgut.edu.cn
bDepartment of Chemistry and Biotechnology and Centre for Translational Atomaterials, Swinburne University of Technology, Hawthorn, VIC 3122, Australia. E-mail: chenghuasun@swin.edu.au
First published on 19th April 2023
Abstract
Nitrate electro-reduction reaction (NITRR) offers a promising alternative approach for ammonia synthesis under mild conditions. Our previous studies have shown that electrodeposited Ni/Ru hydroxide hybrid exhibits highly catalytic NITRR activity. Therefore, this study focuses on investigating the origin of the high activity on Ni/Ru hydroxide hybrid by comparing it with drop-cast Ru nanoparticles on nickel foam, which show much lower activity for NITRR. This was attributed to the interaction between Ru and the nickel substrate formed during electrodeposition. To host electrodeposited Ni/Ru hydroxide hybrid nanoparticles, we used a series of nickel foams with varying amounts of surface-oxidized nickel layers prepared by heat treatments. The introduction of moderate oxidized nickel layers in Ni/Ru hydroxide hybrid electrodes increased the interactions between Ru and the Ni species, and the synergistic effect further promotes nitrate-to-ammonia conversion. However, excessive increases in oxidized nickel layers lead to a deterioration in catalytic performance.
Introduction
Ammonia has significant importance beyond being a fertilizer for agriculture and a raw material for the chemical industry. Its advantages in transport have made it a promising energy fuel. However, the current Haber–Bosch production process for ammonia, which is one of the most important chemical reactions in the world, consumes a significant amount of energy and contributes to approximately 1.5% of CO2 emissions annually, causing global environmental problems. Therefore, alternative routes for ammonia synthesis under mild conditions have been sought for decades.1 While electrochemical synthesis of ammonia is promising due to ambient reaction conditions;2 the ultra-strong N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N covalent bond makes it extremely challenging to convert N2 to NH3 with sufficient activity and efficiency.3 Various catalysts have been developed for direct nitrogen-to-ammonia synthesis,4 but the faradaic efficiency and yield rates are still far below the expectations for industrial applications.
N covalent bond makes it extremely challenging to convert N2 to NH3 with sufficient activity and efficiency.3 Various catalysts have been developed for direct nitrogen-to-ammonia synthesis,4 but the faradaic efficiency and yield rates are still far below the expectations for industrial applications.
Another strategy for ammonia synthesis is to electro-catalytic oxidation of N2 to NO3−, followed by further electro-reduction of NO3− to NH3 (NO3− + 8e− + 6H2O → NH3 + 9OH−).5 Unlike N2, nitrate contains single nitrogen and N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N breakage is not required prior to ammonia formation. More importantly, nitrate, as a worldwide industrial waste, has been identified as a major pollutant responsible for gas emissions, acid rain or photochemical smog,6 causing various health-threatening consequences.7 Against this background, effective electro-reduction of NO3− to ammonia not only provides an alternative route for NH3 production, but also converts nitrate waste into valuable chemicals.8 So far, traditional catalysts based on Ti,9 Cu8b,10 or Co,11 have been developed for NO3 electro-reduction, and the energy efficiency for ammonia synthesis is often as high as 90% at low current densities,10a,12 while the challenge is the decrease in reaction selectivity when targeting high production yield at high current.13 To address this issue, Ru with oxygen-doped nanoclusters has been demonstrated to have excellent activity for electrochemical nitrate reduction reactions,14 especially when tensile strains associated with oxygen doping have been introduced, offering ammonia-production rates of 3.25 × 10−7 mol s−1 cm−2 at −0.8 V vs. RHE.14a Such success highlights the outstanding activity of Ru nanoparticles, but such catalysts suffer from the efficiency drop at high potentials (e.g. down to 55.8% at −0.8 V (ref. 14a)). Recently, we have demonstrated a facile and low-cost electrodeposition process for the synthesis of highly active bimetallic Ru–Ni catalysts for nitrate-to-ammonia conversion, exhibiting high nitrate-to-ammonia conversion rate (1.4 ± 0.1 × 10−6 mol s−1 cm−2 at −0.6 V vs. RHE) with almost 100% ammonia selectivity.15 In this study, we conducted further investigations on the catalyst structure to better understand the high efficiency of nitrate-to-ammonia conversion. We compared electrodes prepared by drop-cast Ru nanoparticles onto pristine nickel foam with electrodeposited Ni/Ru hydroxide hybrid. Our results show that the drop-cast electrode exhibited significantly lower nitrate-to-ammonia conversion efficiency than the electrodeposited electrodes. In addition, a series of nickel foams with varying degree of surface oxidation, achieved through heat treatments at room temperature to 500 °C in H2 or Ar gas, serve as 3D substrates to host electrodeposited Ni/Ru hydroxide hybrid nanoparticles. We observed that the Ni/Ru hydroxide hybrid on deeper oxidized nickel layers exhibited improved NITRR performance compared to those with less oxidized layers. However, further increase in the degree of oxidized nickel layers led to a deterioration of the catalytic performance. Comparison of these results revealed that moderate oxidized nickel substrates played a key role in the observed catalytic activity, leading to formation of Ru–O–Ni matrix, which enhanced the NITRR activity with respect to pristine or reduced nickel surface.16
N breakage is not required prior to ammonia formation. More importantly, nitrate, as a worldwide industrial waste, has been identified as a major pollutant responsible for gas emissions, acid rain or photochemical smog,6 causing various health-threatening consequences.7 Against this background, effective electro-reduction of NO3− to ammonia not only provides an alternative route for NH3 production, but also converts nitrate waste into valuable chemicals.8 So far, traditional catalysts based on Ti,9 Cu8b,10 or Co,11 have been developed for NO3 electro-reduction, and the energy efficiency for ammonia synthesis is often as high as 90% at low current densities,10a,12 while the challenge is the decrease in reaction selectivity when targeting high production yield at high current.13 To address this issue, Ru with oxygen-doped nanoclusters has been demonstrated to have excellent activity for electrochemical nitrate reduction reactions,14 especially when tensile strains associated with oxygen doping have been introduced, offering ammonia-production rates of 3.25 × 10−7 mol s−1 cm−2 at −0.8 V vs. RHE.14a Such success highlights the outstanding activity of Ru nanoparticles, but such catalysts suffer from the efficiency drop at high potentials (e.g. down to 55.8% at −0.8 V (ref. 14a)). Recently, we have demonstrated a facile and low-cost electrodeposition process for the synthesis of highly active bimetallic Ru–Ni catalysts for nitrate-to-ammonia conversion, exhibiting high nitrate-to-ammonia conversion rate (1.4 ± 0.1 × 10−6 mol s−1 cm−2 at −0.6 V vs. RHE) with almost 100% ammonia selectivity.15 In this study, we conducted further investigations on the catalyst structure to better understand the high efficiency of nitrate-to-ammonia conversion. We compared electrodes prepared by drop-cast Ru nanoparticles onto pristine nickel foam with electrodeposited Ni/Ru hydroxide hybrid. Our results show that the drop-cast electrode exhibited significantly lower nitrate-to-ammonia conversion efficiency than the electrodeposited electrodes. In addition, a series of nickel foams with varying degree of surface oxidation, achieved through heat treatments at room temperature to 500 °C in H2 or Ar gas, serve as 3D substrates to host electrodeposited Ni/Ru hydroxide hybrid nanoparticles. We observed that the Ni/Ru hydroxide hybrid on deeper oxidized nickel layers exhibited improved NITRR performance compared to those with less oxidized layers. However, further increase in the degree of oxidized nickel layers led to a deterioration of the catalytic performance. Comparison of these results revealed that moderate oxidized nickel substrates played a key role in the observed catalytic activity, leading to formation of Ru–O–Ni matrix, which enhanced the NITRR activity with respect to pristine or reduced nickel surface.16
Results and discussion
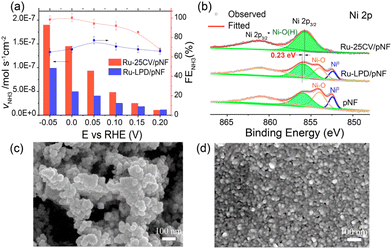
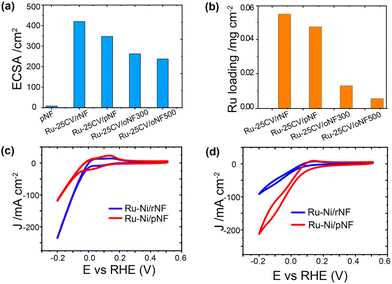
Firstly, a partially oxidized Ru nanoparticle was drop-cast on a pristine nickel foam (pNF) and labelled as Ru-LPD/pNF (as detailed in ESI†). Additionally, the Ru–Ni bimetallic hybrid electrodes were also prepared by a cycle voltammetry (CV) electrodeposition method on pNF by 25 CVs, denoted as Ru-25CV/pNF (as detailed in ESI†).The NITRR activity of the catalysts was evaluated by the cyclic voltammograms (CV) and the liner scan voltammetry (LSV) measured in 1.0 M KOH with and without NaNO3. All the voltages reported in this work were converted by referencing to the reverse hydrogen electrode (RHE) and can calculated by E (V) = E° + 0.059 × pH + 0.2415 with pH = 14. Fig. 1a and b presents the electrochemical performance of the Ru-25CV/pNF and Ru-LPD/pNF. Upon the addition of NO3−, the onset potentials shift from −0.05 V to 0.15 V, indicating a good response to the nitrate reduction reaction. The current density of the Ru–Ni hybrids increases significantly at potentials below 0.2 V (Fig. 1a, red solid line) and exhibits a much higher cathodic current than that of the Ru-LPD/pNF. Since the performance is related to the active surface area and Ru loading amount in the electrodes, the electrochemical active surface area (ECSA) and the Ru loadings were measured to normalize the current as shown in Fig. 1b. Notably, the normalized currents of the Ru-25CV/pNF by the Ru loading (Fig. 1c) or the ECSA (Fig. 1d) are much higher than that of the Ru-LPD/pNF, further confirming the high NITRR activity of the electrodeposited Ru–Ni hybrids.
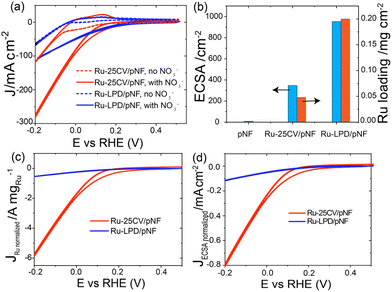 | ||
| Fig. 1 (a) The cyclic voltammetry (CV) of Ru-LPD/pNF and Ru-25CV/pNF measured in Ar saturated-1.0 M KOH with and without 1.0 M NaNO3 at the scan rate of 10 mV s−1. (b) The ECSA calculated from the capacitance data in detailed in ESI and Fig. S1,† and Ru loading amount of Ru-LPD/pNF and Ru-25CV/pNF analysed by ICP-OES. (c) The Ru loading amount normalized and (d) the ECSA normalized CV of Ru-LPD/pNF and Ru-25CV/pNF from a based on the ECSA and Ru loading in b. | ||
Furthermore, the nitrate-to-ammonia conversion efficiency was significantly improved in the Ni–Ru hybrids, as shown in Fig. 2a. For instance, by keeping the FENH3 at almost 100%, the Ru-25CV/pNF (Fig. 2a, red column) produced ammonia at a rate of 1.5 × 10−7 mol s−1 cm−2 at 0 V, which is three times higher than that of the Ru-LPD/pNF (0.5 × 10−7 mol s−1 cm−2 with FE = 68%). In order to investigate the origin of the high catalytic activity of the electrodeposited Ru–Ni hybrid for NITRR, the Ru-25CV/pNF sample was characterized by transmission electron microscopy (TEM), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS) as detailed in Fig. 2b–d and Fig. S2–6.† The Ru-25CV/pNF sample exhibits a layer of nanoclusters with the nanoparticle size at 5–10 nm (Fig. 2d and Fig. S2, 3†), which is smaller than the particle size of the Ru-LPD (∼30 nm, Fig. 2c and Fig. S4†). The XPS results of the Ru 3d and O 1s (Fig. S5 and 6†) indicate the presence of both Ru0 and Ru–O bonds in the Ru-25CV/pNF and the Ru-LPD, which are considered crucial for efficient nitrate-to-ammonia conversion.15
Regarding the Ni states, the XPS spectra of Ni 2p for the Ru-LPD/pNF are similar to those of the pristine NF (Fig. 2b), showing characteristic peaks of Ni0, NiO and Ni-OH. This suggests that the drop casting of Ru-LPD onto pNF does not significantly affect or alter the surface states of the initial nickel foam. However, in the case of electrodeposition, only the Ni-OH peak can be observed, which can be assigned to NiOOH,17 implying that NiO on the pristine NF surface has been converted to NiOOH (Ni2+ → Ni3+), probably due to the Ni/Ru interfacial bonding between Ru and Ni species formed during electrodeposition. This is supported by the shift of the Ni-OH peak to lower energy by 0.23 eV on the electrodeposited Ru-25CV/pNF with respect to the initial nickel foam substrate and the Ru-LPD/pNF.15 The interfacial bonding between Ni and Ru is likely to contribute to the improved NITRR performance for the Ru–Ni hybrids, as evidenced by their higher nitrate reduction current (Fig. 1a and c, d). Whereas, the Ru-LPD/pNF exhibits a lower FENH3 and ammonia yield rate (Fig. 2a).
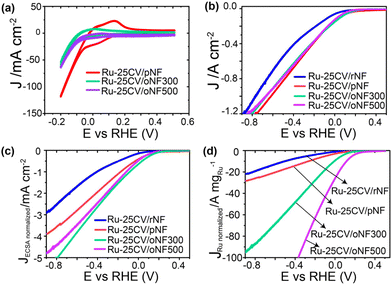
The Ni/Ru interfacial bond is likely formed due to the presence of nickel oxide species, which are typically present on the surface of the pristine nickel foam as indicated in Fig. 2b and in the literature.18 As shown in the synthesis section, the oxidized layer can be reduced by heat treatment in H2 flow (Fig. S7†) and labelled as rNF. The amount of electrodeposited Ru on rNF (0.055 mg cm−2) is slightly more than that on pNF (0.048 mg cm−2), and the former has a higher ECSA (Fig. 3a and b). It is important to investigate whether HER side reaction has been effectively suppressed to achieve high energy efficiency, as a high current response in a reaction does not necessarily lead to a high ammonia production rate. Using the reduced sample for demonstration (Ru-25CV/rNF Fig. 3c, blue line), its HER activity is much higher than that of Ru-25CV/pNF, indicating a good suppression of HER in the Ru-25CV/pNF. Both Ru-25CV/rNF and Ru-25CV/pNF demonstrate excellent response to NO3− addition, but the reaction current of Ru-25CV/rNF is much lower than that of Ru-25CV/pNF as indicated in Fig. 3d. This suggests that nickel oxide, rather than metallic nickel, is favourable for NITRR.
Furthermore, to gain a better understanding of the correlation between the NITRR activity and the nickel oxide interlayer, Ru supported on nickel foam with deeper oxidized surfaces (oxidized nickel foam, oNF) was investigated. The oNFs show nanoparticles merging on the surface, while the rNF and pNF surfaces are smoother (Fig. S8†). As identified by Ni 2p XPS spectra (Fig. S7†), NiO and nickel hydroxide (Ni-OH) species appear on the nickel foam surfaces,19 which can be manipulated by controlled oxidation, as previously reported.10a The Ru film deposited on the oNF, has a much thinner Ru layer than the rNF and pNF supported Ru film (Fig. S9†), which is in consistent with the much lower Ru loading on the oNFs (0.005 mg cm−2 for oNF500 and 0.013 mg cm−2 for oNF300, respectively) as indicated by the ICP-OES results (Fig. 3b).
In terms of the electrochemical performance, both the Ru-25CV/oNF300 and Ru-25CV/oNF500 show lower current density than the Ru-25CV/pNF in KOH (Fig. 4a), indicating better suppressed HER in the deeper oxidized NF supported Ru. In the electrolyte with NO3−, the Ru-25CV/oNF300 has a similar current to the Ru-25CV/pNF, while the Ru-25CV/oNF500 shows a slight decrease in the current density (Fig. 4b). This is probably due to significant decreases of the ECSA and the Ru loading amount on the oNF supports (Fig. 3a and b). To further explore the intrinsic catalytic activity, the electrochemical performances were normalized by the ECSA or the Ru loading mass. It is interesting to note that the normalized LSVs by either the ECSA (Fig. 4c) or the Ru loading mass (Fig. 4d) show significantly enhanced normalized current densities in Ru-25CV/oNF300 and Ru-25CV/oNF500, compared to those with less nickel oxide layers (Ru-25CV/pNF and Ru-25CV/rNF).
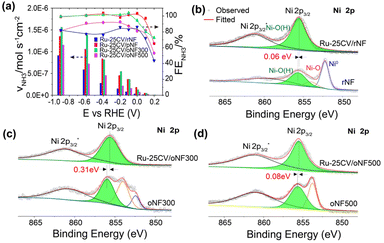
A high current response in a reaction does not necessarily lead to a high ammonia production rate as electrons can be consumed by HER side reaction. The ammonia production performances were examined in Ar saturated-1.0 M KOH with 1.0 M NaNO3 at varied potentials (Fig. 5a). At high cathodic potentials (up to −0.9 V), the FENH3 and VNH3 follow the trend of Ru-25CV/oNF300 ≥ Ru-25CV/pNF > Ru-25CV/oNF500 > Ru-25CV/rNF > Ru-LPD/pNF, indicating the dependence of the oxidized nickel interlayers. By keeping the FENH3 to almost 100%, the Ru-25CV/oNF300 produces ammonia at rate of 3.6 × 10−7 mol s−1 cm−2 at −0.1 V. These results further demonstrate the importance of the oxidized nickel interlayer for achieving high activity in the conversion if nitrate to ammonia. It should be noted that the FENH3 is almost 100% from −0.9 V to −0.1 V on the Ru-25CV/oNF300 and Ru-25CV/pNF, compared to the previously reported NITRR performance with a significant decrease at high overpotentials.10a,14a The ammonia yield rate reaches to as high as 1.4 ± 0.1 × 10−6 mol s−1 cm−2 (or 5.4 ± 0.3 mmol h−1 cm−2) at −0.6 V for Ru-25CV/oNF300, which exceeds most of reported state-of-the-art ammonia synthesis catalysts (Fig. S10†).13,20 The Ru-25CV/oNF300 also exhibits durable and high NITRR performance for up to 100 h with no apparent loss in the selectivity, as shown in Fig. S11.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 The products were also analyzed for the presence of nitrite, hydroxylamine and hydrazine, but no significant levels were found.
15 The products were also analyzed for the presence of nitrite, hydroxylamine and hydrazine, but no significant levels were found.
Zhang and Yu demonstrated that the oxygen doping in Ru can cause stress stains, generating hydrogen radicals and promote the nitrate-to-ammonia conversion.14a Our recent study found that the NF substrate is crucial for efficient nitrate-to-ammonia conversion in Ru catalysts.15 In this study, we investigated the impact of the interaction between the oxidized species of NF substrate and the Ru nanoparticles. While both the electrodeposited Ru films on NF (Ru-25CV/pNF) and the Ru-LPD drop-cast on NF (Ru-LPD/pNF) had reasonable oxygen doping levels, the electrodeposited Ru films on nickel supports with displayed significantly enhanced NITRR activity. The TEM analysis revealed that the Ni/Ru hybrids on NF substrates consist of an amorphous Ru–O–Ni matrix on the nickel foam with a Ni/NiOOH interlayer (Fig. S12†), indicating that the Ni in the Ru layer likely originates from oxidized nickel species on the nickel foam. During Ru electrodeposition, a strong interaction was formed between the Ru and Ni species, In contrast, the drop-casting Ru nanoparticles had a weaker interaction with Ni surfaces (Fig. 1d), resulting in low NITRR activity.14b,21 The difference in activity could be attributed to the strong metal/metal oxide (nanoparticle)–support interactions on the Ru–Ni hybrid electrodes.16,22 The lack of a strong interaction is likely the primary reason for low NITRR activity in Ru-LPD/pNF and electrodeposited Ru without Ni.15
The formation of the Ru–O–Ni metric structure is likely due to the presence of oxidized nickel species on the NF surface. To investigate this, we synthesized Ni/Ru hybrids on NF substrates with varying degrees of oxidized surfaces. The result showed that the Ni-OH peaks in the XPS of Ni 2p were shifted higher in the Ru-25CV/pNF and Ru-25CV/oNF300 samples, compared to the lower peak shifts in the Ru-25CV/rNF and Ru-25CV/oNF500 samples, as shown in Fig. 2b and 5b–d. The XPS results shows a similar presence of Ru0 and Ru–O bonds in the Ru-25CV/pNF and Ru-25CV/rNF (Fig. S6 and S13†), whereas the Ru-25CV/rNF has a less shift of the Ni-OH peak to lower energy by 0.06 eV (Fig. 5b) than the Ru-25CV/pNF (0.23 eV) and Ru-25CV/oNF300 (0.31 eV). These results further demonstrate the essential of the presence of nickel oxides species on formation of the Ni/Ru interfacial bonding. This trend was consistent with the efficiency of the nitrate-to-ammonia conversion on Ni/Ru hybrids (Fig. 5a). It is believed that the increased shifts were due to the synergistic effect induced by oxidized nickel species and oxidized Ru, which enhanced the NITRR activities. The weak H adsorption on the oxidized Ni inhibits the H2 formation, but provides H for the favorable NH3 formation on the nearby Ru site in the Ru–O–Ni matrix with strong NO3− adsorption, thus promoting the activated NO3− to NH3 pathway.23 Furthermore, we found that the oxidized nickel layer did not introduce any significant resistance as indicated by the impedance spectroscopy (Fig. S14†). This suggests that oxidized nickel is crucial for the production of highly active NITRR catalysts. Additionally, the oxygen doping in Ru is also essential for nitrate-to-ammonia conversion, but Ru4+ is likely to be reduced during NITRR, resulting in decreasing performance, as reported in the literature.14a However, the oxidized nickel interlayers and the strong Ru–Ni interactions can probably stabilize Ru4+ and produce sustainable NITRR performance.
Conclusions
This study found that the electrodeposition process produced a more active Ni/Ru hybrid catalyst for electrocatalytic nitrate-to-ammonia conversion than the drop-cast Ru nanoparticles on NF. This was attributed to the interaction between Ru and nickel substrate formed during electrodeposition. In addition, a series of electrodeposited Ni/Ru hybrids with different Ru loading from 0.005 to 0.055 mg cm−2 were synthesized on NF, which has varying oxidized surfaces. The FENH3 and VNH3 follow the trend of Ru-25CV/oNF300 ≥ Ru-25CV/pNF > Ru-25CV/oNF500 > Ru-25CV/rNF > Ru-LPD/pNF. The results showed that the more oxidized NF produced more active Ni/Ru hybrids for nitrate-to-ammonia conversion due to the suppressed hydrogen evolution reaction, with the NF heat treated at 300 °C producing the highest ammonia yield rate with almost 100% faradaic efficiency. This work demonstrates the potential to enhance NITRR performance by tailoring the oxidized nickel interlayer. Further research should focus on tailoring the distribution of bi- or multi-metallic atoms at the near-surface regions to further enhance the catalytic activity and stability.Author contributions
F. Zhou and C. S proposed the research project and analysed the data. F. Zhou conducted the experiments and drafted the manuscript. All the authors reviewed and contributed to this paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
F. Zhou acknowledged the funding support from National Natural Science Foundation of China (21905047) and the Guangdong Provincial Key Laboratory of Distributed Energy Systems, no. 2020B1212060075.References
- S. L. Foster, S. I. P. Bakovic, R. D. Duda, S. Maheshwari, R. D. Milton, S. D. Minteer, M. J. Janik, J. N. Renner and L. F. Greenlee, Catalysts for nitrogen reduction to ammonia, Nat. Catal., 2018, 1, 490–500 CrossRef
.
-
(a) B. H. R. Suryanto, H.-L. Du, D. Wang, J. Chen, A. N. Simonov and D. R. MacFarlane, Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia, Nat. Catal., 2019, 2, 290–296 CrossRef CAS
; (b) X. Zhu Jr., S. Mou, Q. Peng, Q. Liu, Y. Luo, G. Chen, S. Gao and X. Sun, Aqueous electrocatalytic N2 reduction for ambient NH3 synthesis: recent advances in catalyst development and performance improvement, J. Mater. Chem. A, 2020, 8, 1545–1556 RSC
.
-
(a) X. Cui, C. Tang and Q. Zhang, A Review of Electrocatalytic Reduction of Dinitrogen to Ammonia under Ambient Conditions, Adv. Energy Mater., 2018, 8, 1800369 CrossRef
; (b) H. K. Lee, C. S. L. Koh, Y. H. Lee, C. Liu, I. Y. Phang, X. Han, C.-K. Tsung and X. Y. Ling, Favoring the unfavored: Selective electrochemical nitrogen fixation using a reticular chemistry approach, Sci. Adv., 2018, 4, eaar3208 CrossRef PubMed
; (c) W. Xu, G. Fan, J. Chen, J. Li, L. Zhang, S. Zhu, X. Su, F. Cheng and J. Chen, Nanoporous Palladium Hydride for Electrocatalytic N-2 Reduction under Ambient Conditions, Angew. Chem., Int. Ed., 2020, 59, 3511–3516 CrossRef CAS PubMed
; (d) H. Y. F. Sim, J. R. T. Chen, C. S. L. Koh, H. K. Lee, X. Han, G. C. Phan-Quang, J. Y. Pang, C. L. Lay, S. Pedireddy, I. Y. Phang, E. K. L. Yeow and X. Y. Ling, ZIF-Induced d-Band Modification in a Bimetallic Nanocatalyst: Achieving Over 44% Efficiency in the Ambient Nitrogen Reduction Reaction, Angew. Chem., Int. Ed., 2020, 59, 3511–3516 CrossRef PubMed
; (e) C. Liu, Q. Li, C. Wu, J. Zhang, Y. Jin, D. R. MacFarlane and C. Sun, Single-Boron Catalysts for Nitrogen Reduction Reaction, J. Am. Chem. Soc., 2019, 141, 2884–2888 CrossRef CAS PubMed
; (f) Q. Li, S. Qiu, C. Liu, M. Liu, L. He, X. Zhang and C. Sun, Computational Design of Single-Molybdenum Catalysts for the Nitrogen Reduction Reaction, J. Phys. Chem. C, 2019, 123, 2347–2352 CrossRef CAS
.
-
(a) M. Wang, S. Liu, T. Qian, J. Liul, J. Zhou, H. Ji, J. Xiong, J. Zhong and C. Yan, Over 56.55% Faradaic efficiency of ambient ammonia synthesis enabled by positively shifting the reaction potential, Nat. Commun., 2019, 10, 341 CrossRef CAS PubMed
; (b) F. Zhou, L. M. Azofra, M. Ali, M. Kar, A. N. Simonov, C. McDonnell-Worth, C. Sun, X. Zhang and D. R. MacFarlane, Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids, Energy Environ. Sci., 2017, 10, 2516–2520 RSC
; (c) W. Qiu, X.-Y. Xie, J. Qiu, W.-H. Fang, R. Liang, X. Ren, X. Ji, G. Cui, A. M. Asiri, G. Cui, B. Tang and X. Sun, High-performance artificial nitrogen fixation at ambient conditions using a metal-free electrocatalyst, Nat. Commun., 2018, 9, 3485 CrossRef PubMed
; (d) N. Cao, Z. Chen, K. Zang, J. Xu, J. Zhong, J. Luo, X. Xu and G. Zheng, Doping strain induced bi-Ti3+ pairs for efficient N2 activation and electrocatalytic fixation, Nat. Commun., 2019, 10, 2877 CrossRef PubMed
.
-
(a) Y. Liu, J. Mei, C. Shen, M. Huang, M. Yang, Z. Wang, W. Sand and F. Li, Rapid and selective electrochemical transformation of ammonia to N2 by substoichiometric TiO2-based electrochemical system, RSC Adv., 2020, 10, 1219–1225 RSC
; (b) J. Choi, H.-L. Du, C. K. Nguyen, B. H. R. Suryanto, A. N. Simonov and D. R. MacFarlane, Electroreduction of Nitrates, Nitrites, and Gaseous Nitrogen Oxides: A Potential Source of Ammonia in Dinitrogen Reduction Studies, ACS Energy Lett., 2020, 5, 2095–2097 CrossRef CAS
; (c) Y. Wang, Y. Yu, R. Jia, C. Zhang and B. Zhang, Electrochemical synthesis of nitric acid from air and ammonia through waste utilization, Natl. Sci. Rev., 2019, 6, 730–738 CrossRef CAS PubMed
.
- Q. Wang, H. Huang, L. Wang and Y. Chen, Electrochemical removal of nitrate by Cu/Ti electrode coupled with copper-modified activated carbon particles at a low current density, Environ. Sci. Pollut. Res., 2019, 26, 17567–17576 CrossRef CAS PubMed
.
- M. Duca and M. T. M. Koper, Powering denitrification: the perspectives of electrocatalytic nitrate reduction, Energy Environ. Sci., 2012, 5, 9726–9742 RSC
.
-
(a) Y. Yao, S. Zhu, H. Wang, H. Li and M. Shao, A Spectroscopic Study of Electrochemical Nitrogen and Nitrate Reduction on Rhodium Surfaces, Angew. Chem., Int. Ed, 2020, 59, 10479 CrossRef CAS PubMed
; (b) G.-F. Chen, Y. Yuan, H. Jiang, S.-Y. Ren, L.-X. Ding, L. Ma, T. Wu, J. Lu and H. Wang, Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper-molecular solid catalyst, Nat. Energy, 2020, 5, 605–613 CrossRef CAS
; (c) H. Li, C. Yan, H. Guo, K. Shin, S. M. Humphrey, C. J. Werth and G. Henkelman, CuxIr1−x Nanoalloy Catalysts Achieve Near 100% Selectivity for Aqueous Nitrite Reduction to NH3, ACS Catal., 2020, 10, 7915–7921 CrossRef CAS
; (d) Y. Zeng, C. Priest, G. Wang and G. Wu, Restoring the Nitrogen Cycle by Electrochemical Reduction of Nitrate: Progress and Prospects, Small Methods, 2020, 4, 2000672 CrossRef CAS
.
- J. M. McEnaney, S. J. Blair, A. C. Nielander, J. A. Schwalbe, D. M. Koshy, M. Cargnello and T. F. Jaramillo, Electrolyte Engineering for Efficient Electrochemical Nitrate Reduction to Ammonia on a Titanium Electrode, ACS Sustainable Chem. Eng., 2020, 8, 2672–2681 CrossRef CAS
.
-
(a) Y. Wang, A. Xu, Z. Wang, L. Huang, J. Li, F. Li, J. Wicks, M. Luo, D.-H. Nam, C.-S. Tan, Y. Ding, J. Wu, Y. Lum, D. Cao-Thang, D. Sinton, G. Zheng and E. H. Sargent, Enhanced Nitrate-to-Ammonia Activity on Copper-Nickel Alloys via Tuning of Intermediate Adsorption, J. Am. Chem. Soc., 2020, 142, 5702–5708 CrossRef CAS PubMed
; (b) Y.-J. Shih, Z.-L. Wu, C.-Y. Lin, Y.-H. Huang and C.-P. Huang, Manipulating the crystalline morphology and facet orientation of copper and copper-palladium nanocatalysts supported on stainless steel mesh with the aid of cationic surfactant to improve the electrochemical reduction of nitrate and N2 selectivity, Appl. Catal., B, 2020, 273, 119053 CrossRef CAS
; (c) Y. L. Zhao, Y. Liu, Z. J. Zhang, Z. K. Mo, C. Y. Wang and S. Y. Gao, Flower-like open-structured polycrystalline copper with synergistic multi-crystal plane for efficient electrocatalytic reduction of nitrate to ammonia, Nano Energy, 2022, 97, 7 Search PubMed
.
- J. Gao, B. Jiang, C. Ni, Y. Qi, Y. Zhang, N. Oturan and M. A. Oturan, Non-precious Co3O4-TiO2/Ti cathode based electrocatalytic nitrate reduction: Preparation, performance and mechanism, Appl. Catal., B, 2019, 254, 391–402 CrossRef CAS
.
- Y. Wang, W. Zhou, R. Jia, Y. Yu and B. Zhang, Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia, Angew. Chem., Int. Ed., 2020, 59, 5350–5354 CrossRef CAS PubMed
.
- L. Z. Sun and B. Liu, Mesoporous PdN Alloy Nanocubes for Efficient Electrochemical Nitrate Reduction to Ammonia, Adv. Mater., 2023, 35, 8 Search PubMed
.
-
(a) J. Li, G. Zhan, J. Yang, F. Quan, C. Mao, Y. Liu, B. Wang, F. Lei, L. Li, A. W. M. Chan, L. Xu, Y. Shi, Y. Du, W. Hao, P. K. Wong, J. Wang, S.-X. Dou, L. Zhang and J. C. Yu, Efficient Ammonia Electrosynthesis from Nitrate on Strained Ruthenium Nanoclusters, J. Am. Chem. Soc., 2020, 142, 7036–7046 CrossRef CAS PubMed
; (b) K. Liu, X. Zhao, G. Ren, T. Yang, Y. Ren, A. F. Lee, Y. Su, X. Pan, J. Zhang, Z. Chen, J. Yang, X. Liu, T. Zhou, W. Xi, J. Luo, C. Zeng, H. Matsumoto, W. Liu, Q. Jiang, K. Wilson, A. Wang, B. Qiao, W. Li and T. Zhang, Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts, Nat. Commun., 2020, 11, 1263 CrossRef CAS PubMed
.
- F. Zhou and C. Sun, Nitrate-to-Ammonia Conversion on Ru/Ni Hydroxide Hybrid through Zinc-Nitrate Fuel Cell, Small, 2022, 18, 2200436 CrossRef CAS PubMed
.
- S. Hu and W.-X. Li, Theoretical Investigation of Metal-Support Interactions on Ripening Kinetics of Supported Particles, ChemNanoMat, 2018, 4, 510–517 CrossRef CAS
.
- A. M. Hengne, A. K. Samal, L. R. Enakonda, M. Harb, L. E. Gevers, D. H. Anjum, M. N. Hedhili, Y. Saih, K.-W. Huang and J.-M. Basset, Ni–Sn-Supported ZrO2 Catalysts Modified by Indium for Selective CO2 Hydrogenation to Methanol, ACS Omega, 2018, 3, 3688–3701 CrossRef CAS PubMed
.
- M. Grdeń, M. Alsabet and G. Jerkiewicz, Surface Science and Electrochemical Analysis of Nickel Foams, ACS Appl. Mater. Interfaces, 2012, 4, 3012–3021 CrossRef PubMed
.
- M. C. Biesinger, L. W. M. Lau, A. R. Gerson and R. S. C. Smart, The role of the Auger parameter in XPS studies of nickel metal, halides and oxides, Phys. Chem. Chem. Phys., 2012, 14, 2434–2442 RSC
.
-
(a) N. Zhang, J. Shang, X. Deng, L. J. Cai, R. Long, Y. J. Xiong and Y. Chai, Governing Interlayer Strain in Bismuth Nanocrystals for Efficient Ammonia Electrosynthesis from Nitrate Reduction, ACS Nano, 2022, 16, 4795–4804 CrossRef CAS PubMed
; (b) D. Chen, S. C. Zhang, D. Yin, W. P. Li, X. M. Bu, Q. Quan, Z. X. Lai, W. Wang, Y. Meng, C. T. Liu, S. Yip, F. R. Chen, C. Y. Zhi and J. C. Ho, Tailored p-Orbital Delocalization by Diatomic Pt-Ce Induced Interlayer Spacing Engineering for Highly-Efficient Ammonia Electrosynthesis, Adv. Energy Mater., 2023, 13, 11 Search PubMed
; (c) F. Lv, M. Z. Sun, Y. P. Hu, J. Xu, W. Huang, N. Han, B. L. Huang and Y. G. Li, Near-unity electrochemical conversion of nitrate to ammonia on crystalline nickel porphyrin-based covalent organic frameworks, Energy Environ. Sci., 2023, 16, 201–209 RSC
.
- Q. Fu, W.-X. Li, Y. Yao, H. Liu, H.-Y. Su, D. Ma, X.-K. Gu, L. Chen, Z. Wang, H. Zhang, B. Wang and X. Bao, Interface-Confined Ferrous Centers for Catalytic Oxidation, Science, 2010, 328, 1141–1144 CrossRef CAS PubMed
.
-
(a) Q. Fu, F. Yang and X. Bao, Interface-Confined Oxide Nanostructures for Catalytic Oxidation Reactions, Acc. Chem. Res., 2013, 46, 1692–1701 CrossRef CAS PubMed
; (b) J. Xia, M. Volokh, G. Peng, Y. Fu, X. Wang and M. Shalom, Low-Cost Porous Ruthenium Layer Deposited on Nickel Foam as a Highly Active Universal-pH Electrocatalyst for the Hydrogen Evolution Reaction, ChemSusChem, 2019, 12, 2780–2787 CrossRef CAS PubMed
.
-
(a) F.-Y. Chen, Z.-Y. Wu, S. Gupta, D. J. Rivera, S. V. Lambeets, S. Pecaut, J. Y. T. Kim, P. Zhu, Y. Z. Finfrock, D. M. Meira, G. King, G. Gao, W. Xu, D. A. Cullen, H. Zhou, Y. Han, D. E. Perea, C. L. Muhich and H. Wang, Efficient conversion of low-concentration nitrate sources into ammonia on a Ru-dispersed Cu nanowire electrocatalyst, Nat. Nanotechnol., 2022, 17, 759 CrossRef CAS PubMed
; (b) M. Gong, D.-Y. Wang, C.-C. Chen, B.-J. Hwang and H. Dai, A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction, Nano Res., 2016, 9, 28–46 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qi00568b |
| This journal is © the Partner Organisations 2023 |