Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluating the use of chemically modified clinoptilolite zeolite for the simultaneous recovery of ammonium and phosphate from blackwater†
Cynthia J.
Castro
 *,
Hsiang-Yang
Shyu
,
Ben
Hoque
and
Daniel H.
Yeh
*,
Hsiang-Yang
Shyu
,
Ben
Hoque
and
Daniel H.
Yeh
University of South Florida, Civil & Environmental Engineering, 4202 E. Fowler Ave, Tampa, FL 33620, USA. E-mail: cynjcastro@gmail.com
First published on 17th January 2023
Abstract
Natural clinoptilolite zeolite (CZ) was chemically modified with sodium (Na), calcium (Ca), or magnesium (Mg) to recover ammonium and phosphate from simulated blackwater. Chemical modification of CZ with Na, Ca, and Mg was able to increase the mass of each metal by a factor of 2.2, 2.7, and 1.8, respectively. The removal performance of ammonium and phosphate by each chemically modified CZ under concentrations relevant to onsite wastewater treatment systems was evaluated. Initially, single ion isotherm tests were conducted to quantify phosphate and ammonium removal without the presence of the opposite nutrient. Without ammonium present, phosphate removal with Na and Mg modified CZ was marginal, observing sorption capacities of 0.06 ± 0.19 and 0.17 ± 0.12 g P g−1, respectively, at a starting concentration of 95 mg P L−1. At the same starting concentration, Ca modified CZ observed the highest phosphate sorption of 0.45 ± 0.08 g P g−1. Phosphate removal was enhanced by the presence of ammonium in solution for Na, Mg and Ca modified CZ, achieving sorption capacities up to 0.38 ± 0.06, 0.98 ± 0.02 and 2.92 ± 0.01 mg P g−1, respectively, at a starting phosphate concentration of 120 mg P L−1. These removal capacities were associated with phosphate removal rates of 9.5 ± 1.4%, 24.4 ± 0.6% and 72.7 ± 0.4% for Na, Mg and Ca modified CZ. The removal of ammonium remained consistent across a range of phosphate concentrations. The Langmuir isotherm model best predicted the phosphate sorption behavior to Mg and Ca modified CZ when ammonium was not present. When both nutrients were present in solution together, the Freundlich isotherm model best depicted the phosphate sorption behavior to Mg and Ca modified CZ. This change in isotherm behavior was due to a shift in surface reactions from primarily adsorption to primarily complexation. In both cases, phosphorus was recovered onto the zeolite surface as calcium phosphate precipitates. This supports the idea that the Ca content of the CZ, regardless of which metal was used for chemical modification, dictated the phosphate removal performance. X-ray fluorescence revealed that the zeolite granules retained nitrogen, phosphorus, and potassium at a molar ratio of 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.4 which can be used as a complete fertilizer or as an amendment for incomplete fertilizers.
13.4 which can be used as a complete fertilizer or as an amendment for incomplete fertilizers.
Water impactOnsite and decentralized wastewater treatment systems experience high nutrient loading, yet effective and affordable treatment options are still limited to large-scale treatment facilities. Passive and low-oversight treatment options may assist in meeting increasingly stringent effluent standards. This study shows that calcium modified clinoptilolite zeolite is an effective material option for immobilizing phosphate while unaffecting the ammonium sorption capacity of clinoptilolite zeolite, exemplifying the versatility of zeolites as a sustainable dual nutrient recovery option. |
1. Introduction
Nitrogen and phosphorus are necessary nutrients in the production of agricultural crops that maintain global food supplies. However, the discharge of anthropogenic nitrogen and phosphorus into local waterways have detrimental impacts on aquatic ecosystems as well as human health.1 Onsite wastewater treatment systems (OWTS) have only recently been directly recognized as major contributors to nutrient pollution.2 Nitrogen and phosphorus removal is minimal in conventional septic systems, with 10–30% nitrogen and 0–7% phosphorus removal by sludge settling being common performance values.2,3 In the state of Florida, OWTS account for the third highest nitrogen loading to local water bodies, behind only atmospheric deposition and reclaimed water discharge.4,5 Advance secondary OWTS technologies exist that can enable significant reductions in nitrogen by facilitating biological nitrification and denitrification processes and discharging nitrogen as nitrate. Yet, they do not always achieve effluent levels that meet local regulations consistently. In several New England coastal counties considered critical resource areas, studies have shown than advanced secondary OWTS targeted to meet the local standard of 19 mg N L−1 do so 0–78% of the time, with their treatment effectiveness heavily dependent on the treatment train of the applied commercial technology and the daily nitrogen loading rates.6–8 Meanwhile, achieving phosphorus reductions is left to natural attenuation in drainfields or requires an additional secondary or tertiary treatment step.9 Drainfields with high clay content soils do well at removing phosphorus due to their high iron and aluminum content while sandy soils with limited metal oxides do poorly.10 However, phosphate (PO4) can inevitably desorb from soils and reach groundwater sources. Groundwater near OWTS, particularly near aged cesspools and conventional septic systems, has been associated with high phosphorus discharge to nearby lakes which has led to eutrophication.11 When targeted phosphorus removal is desired, chemical dosing of iron (Fe) or aluminum (Al) salts or media adsorption are the primary methods for removal. Treatment units that use Fe-rich media are commercially available, but their adoption and implementation has been slow. Combining nitrogen and phosphorus removal into a single secondary treatment step using sorption media for nutrients management is a promising approach for managing the variable nutrient loading rates inherent to OWTS and to reduce the footprint of secondary and tertiary treatment needed to treat both nutrients separately.A variety of natural, engineered, or industrial waste by-products have been investigated as a sorption media for phosphorus removal.12 Natural materials include mineral apatite, dolomite, bauxite, and calcite, with studies reporting a wide range of sorption capacities, but on average below 5 mg P g−1.12–15 Dolomite, apatite, and calcite are calcium (Ca)-bearing minerals while bauxite is a mineral used as a rich source of aluminum. Industrial by-products tend to yield the highest sorption capacities, with fly ash exhibiting capacities upwards of 260–420 mg P g−1.16,17 For most sorption materials evaluated in the literature, a high CaO content is correlated with improved phosphorus sorption capacities.12 At a pH between 6 and 7, minerals with high Ca content sorb phosphorus through the formation of Ca-PO4 precipitates, such as amorphous calcium phosphate (ACP), dibasic calcium phosphate (DCP), and hydroxyapatite (HAP).18
For nitrogen removal, bentonites, zeolites, biochar, and other clay materials have been widely used for ammonium (NH4) removal in wastewater due to their low cost.19 Zeolites are an attractive material for nitrogen removal because of their high affinity for NH4 uptake and ability to be regenerated and reused over many cycles.20–22 Zeolite is a negatively charged aluminosilicate mineral that can exchange its extra framework cation with NH4. Zeolites have an order or ionic preference of K+ > NH4+ > Na+ > Ca2+ > Mg2+ making them attractive for nitrogen removal and recovery from wastewater.23 While zeolite is used exclusively as a cationic exchange material, it is also capable of phosphorus uptake, particularly orthophosphate.24–27 Orthophosphates can be retained by strong interactions with the loosely bound extra framework cation or with Brønsted acid sites within the zeolite cavities.28,29 There is evidence that phosphates complex with metal framework cations and are retained within the zeolite structure as NaH2PO4, NaHPO4, and followed by H2PO4, with CaHPO4 and MgHPO4 at a much lower presence.29
Recently, using zeolites for the simultaneous removal of nitrogen and phosphorus has gained significant interest. Promising PO4 sorption capacities have been reported for natural (clinoptilolite), chemically altered, thermally altered, and synthesized zeolites but the range of removal is quite variable (0.4–40 mg P g−1) and heavily dependent on the initial concentrations of both PO4 and NH4 and the metal used as the extra framework cation.27,30–32 For clinoptilolite zeolites (CZs) chemically modified with metals such as sodium (Na), Ca, magnesium (Mg), Fe, or aluminum (Al) the concentration of NH4 dictates the level of PO4 removal, however, the maximum capacities are commonly reported but are not achievable in practice. It is not clear how the sorption capacities for both NH4 and PO4 will be influenced by different ratios of NH4 to PO4 that are directly observed in practice and the impact the removal of both ions will have on the effluent quality with regards to dissolved metals. At high nitrogen to phosphorus (N/P) ratios as observed in OWTS (3–40), NH4 can exchange readily with extra framework metals attached to natural zeolites and allow for such metals to complex with PO4, removing both ions from water simultaneously and efficiently.
Therefore, this study aims to identify the sorption performance of chemically modified CZ to remove NH4 and PO4 at concentrations and N/P ratios relevant to onsite wastewater. The objectives are to (1) quantify the sorption performance of chemically modified CZ with three distinct metal cations (Na, Mg, and Ca) for removing both nitrogen and phosphorus in a single reactor; (2) characterize the cation composition of the effluent post sorption; (3) apply sorption isotherm models, including Langmuir and Freundlich, to give insight into the sorption mechanisms; and (4) evaluate the elemental and surface compositional changes of zeolite. We further discuss the implications of using CZ as a treatment method for OWTS and reusing nutrient loaded CZ for agricultural applications.
2. Materials and methods
2.1. Clinoptilolite zeolite pre-treatment
Granular natural CZ composed of hydrous sodium aluminosilicate was used as the primary zeolite source and had a chemical formula of Na6(Al6Si30O72)H2O (KMI Zeolite, Amargoza Valley, NV). This material was chosen due to its low cost and ability to uptake both NH4 and PO4 (see Fig. S1 and Table S1 in ESI† section Material selection). The CZ was sieved and had on average a diameter of 0.85–1 mm. Prior to any testing, the CZ was first rinsed three times with deionized water (DI) and then dried at 105 °C for 24 hours. Three metal cations were selected, Na, Ca, and Mg, to pre-treat CZ for testing. Potassium (K) was initially evaluated as well but did not observe any phosphate removal after contact with 500 or 5000 mg P L−1 in preliminary test and was therefore not used (Table S2†). From here on, each pre-treated CZ is denoted as Na-PT CZ, Mg-PT CZ, or Ca-PT CZ to distinguish between the three metal ions used for pre-treatment. A total of 50 g of washed zeolite was soaked in 500 mL of either 2 M NaCl, CaCl2, or MgCl2 for Na, Ca, or Mg pre-treatment, respectively. The CZ was shaken continuously in an orbital shaker at 150 RPM and allowed to soak in solution for 48 h. Samples of the soaking solution were collected before and after 48 h to verify the mass adsorption of each cation onto the CZ. The granules were then rinsed with ultrapure water and dried at 105 °C for 24 hours and stored in a desiccator.2.2. Single nutrient sorption characterization
The sorption dynamics of NH4 onto zeolite has been heavily documented by previous literature. However, sorption isotherms with only NH4 present were conducted as a control to understand the effects of PO4 presence on the NH4 sorption capacity of the pre-treated CZ used in this study. A total of five NH4 concentrations relevant to the nutrient composition of blackwater were assessed for each pre-treated CZ: 100, 200, 300, 400, and 500 mg NH4–N per liter using NH4Cl in DI water. These concentrations fell within the range of NH4 that has been observed in the permeate of an AnMBR used as an OWTS.20 For each flask, 3 g of Na, Ca, or Mg pre-treated CZ was added into 100 mL of the described NH4 solutions and shaken for 48 h. Liquid samples were taken from the five stock solutions before testing began and from each experiment flask after the 48 h period. All samples were centrifuged and filtered with 0.45 μm filters before processing. The remaining granules were dried for 24 h at 105 °C and stored in an airtight container.A similar procedure was taken for determining the removal capacity of CZ with phosphorus as the main nutrient present. Batch experiments used 0.51 g K2HPO4 and 4.0 g KH2PO4 to yield a phosphate buffer stock solution of 32 mM (1000 mg PO4–P per liter) and was diluted with DI water to yield stock concentrations of 10, 25, 50, 100, and 500 mg PO4–P per liter with an average starting pH of 6.02 ± 0.07. A volume of 100 mL for each concentration was added to a flask containing 3 g of Na, Ca, or Mg pre-treated CZ. All flasks were shaken for 48 h and liquid samples before and after mixing were collected as previously described.
2.3. Ammonium and phosphate sorption characterization
The simultaneous removal of NH4 and PO4 was evaluated under 15 different scenarios. The first ten tests were conducted to develop the sorption models for PO4 and NH4, respectively, while maintaining one nutrient concentration constant and varying the nutrient of interest. The final five tests (11–15) were used to validate whether the sorption capacities were influenced by the presence of the opposite nutrient ion. All tests were conducted similarly to the previously described sorption tests. In five scenarios, the starting NH4 concentration of the solution remained constant near 340 mg N L−1 while the concentration of PO4–P added was varied between 10, 30, 50, 70, and 100 mg P L−1. The next five scenarios varied the NH4 concentration between 100, 200, 300, 400, and 500 mg N L−1 while the concentration of PO4 remained constant near 65 mg P L−1. The constant concentrations of NH4 and PO4 were both selected based on the average concentrations observed previously from a field tested OWTS.33 The last five tests varied both in NH4 and PO4 concentrations, each containing an initial No/Po ratio within the range 3.7–31. Two stocks solutions were prepared where one contained 10.01 g NH4Cl and the other contained 2.98 g (NH4)2HPO4 per liter, thus, eliminating the presence of any competing cation. The pH of the final solutions varied slightly due to the variations of NH4 concentration, but all remained between pH 7–8. The stock solutions were diluted and mixed to reach the desired concentrations of NH4 and PO4. Samples of the soaking solution were collected before and after 48 h to verify the removal of nutrients and the composition of the bulk liquid.2.4. Chemical analysis
Reactive PO4 was measured using the phosphorus molybdovanadate Test ‘N Tube method (HACH method 8114) while NH4 was measured using the nitrogen–ammonia salicylate Test ‘N Tube method (HACH method 10031). All samples were filtered with 0.45 μm filters and processed immediately. During analysis, all procedures were followed as outlined by the manufacturer. The pH of all samples was also measured immediately after sample collection. Relevant cations, such as Na, NH4, K, Ca, and Mg were measured using ion chromatography (Metrohm 930 Compact IC Flex). The ions Na, NH4, and K had a detection limit of 0.2 mg L−1 while the limit was 1.0 mg L−1 for Mg and Ca.2.5. Characterization of material composition
At the end of each 48 hour sorption test, the CZ and liquid mixture were filtered through a 0.45 μm glass fiber filter. The liquid portion was collected for water quality analysis while the solids were rinsed with ultrapure water and dried at 105 °C for 24 hours and stored in a desiccator for downstream microscopy imaging and elemental identification. Eight samples of clinoptilolite zeolite were processed for elemental analysis using X-ray fluorescence (XRF) spectrometry (Bruker S2 PUMA series 2) equipped with a Hisense detector. Six samples were selected from the experimental tests for analyses, including: raw CZ, Na-PT CZ, Ca-PT CZ, Mg-PT CZ, Mg-PT CZ after contact with 0.066 g P L−1 and 0.43 g N L−1, and Ca-PT CZ after contact with 0.067 g P L−1 and 0.32 g N L−1. Additionally, two Ca-PT CZ samples soaked in either 0.5 g P L−1 or 5.0 g P L−1 were also analyzed. Prior to XRF analysis, the granule samples were first ground into fine powders. For six of the eight zeolite samples, the masses of specimen powder processed were 2.135 ± 0.375 g, while 8.0 g and 10.3 g of specimen mass were used for detection of zeolite from the 0.5 g P L−1 and 5.0 g P L−1 experiments, respectively. All detections were performed under helium. Elemental quantification was performed using the SPECTRA.ELEMENTS software.In addition to XRF analysis, microscopic imaging was accomplished using a dual-beam Focused Ion Beam – Scanning Electron Microscope (FIB-SEM) (Quanta 200 3D) with a gallium ion source. All images were captured at a high acceleration voltage of 30 kV and current of 0.35 nA. An IXRF 550i digitizer, along with Iridium Ultra software (version 2.4L) were used for image acquisition, EDS mapping, and spectral analysis.
2.6. Data analysis & calculations
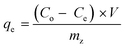 | (1) |
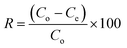 | (2) |
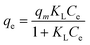 | (3) |
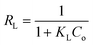 | (4) |
| qe = KFCe1/n | (5) |
3. Results
3.1. Ammonium and phosphorus removal via zeolite sorption
A total of ten different tests containing a variation of NH4 and PO4 concentrations were conducted to determine the equilibrium sorption capacities of CZ pre-treated with either Na, Ca, or Mg. The tests were divided into two groups where tests 1–5 began with PO4 concentrations of 66.5 ± 0.6 P mg L−1 and a range of NH4 concentration spanning 100–550 mg N L−1 (Table 1). The second set of five tests (tests 6–10) began with similar NH4 concentrations of 344 ± 14 mg N L−1 but with variable PO4 concentrations ranging from 10.5 to 121 mg P L−1. Two sets of control tests were conducted with only a single nutrient present, phosphorus or nitrogen, within the ranges previously mentioned.| Test | N o | P o | N o/Po | pHo |
|---|---|---|---|---|
| Constant PO4 and variable NH4 | ||||
| 1 | 103 ± 7.8 | 67.5 ± 0.5 | 1.5 | 7.83 |
| 2 | 206 ± 11 | 66.5 ± 0.0 | 3.1 | 7.65 |
| 3 | 318 ± 4.2 | 66.5 ± 0.0 | 4.8 | 7.55 |
| 4 | 425 ± 8.5 | 66.3 ± 0.4 | 6.4 | 7.46 |
| 5 | 549 ± 24 | 65.8 ± 0.4 | 8.3 | 7.42 |
| Constant NH4 and variable PO4 | ||||
| 6 | 328 ± 4.2 | 10.5 ± 0.7 | 31.2 | 7.14 |
| 7 | 332 ± 1.4 | 36.0 ± 4.2 | 9.2 | 7.59 |
| 8 | 348 ± 4.9 | 56.5 ± 0.0 | 6.2 | 7.65 |
| 9 | 348 ± 0.7 | 81.3 ± 0.4 | 4.3 | 7.90 |
| 10 | 362 ± 0.7 | 121 ± 0.4 | 3.0 | 7.94 |
| Variable NH4 and variable PO4 | ||||
| 11 | 266 ± 2.5 | 8.64 ± 0.0 | 30.9 | 6.96 |
| 12 | 286 ± 15.2 | 33.3 ± 0.2 | 8.59 | 7.42 |
| 13 | 333 ± 6.4 | 60.7 ± 2.1 | 5.46 | 7.57 |
| 14 | 360 ± 4.2 | 79.0 ± 4.7 | 4.56 | 7.64 |
| 15 | 402 ± 5.7 | 109 ± 9.0 | 3.69 | 7.72 |
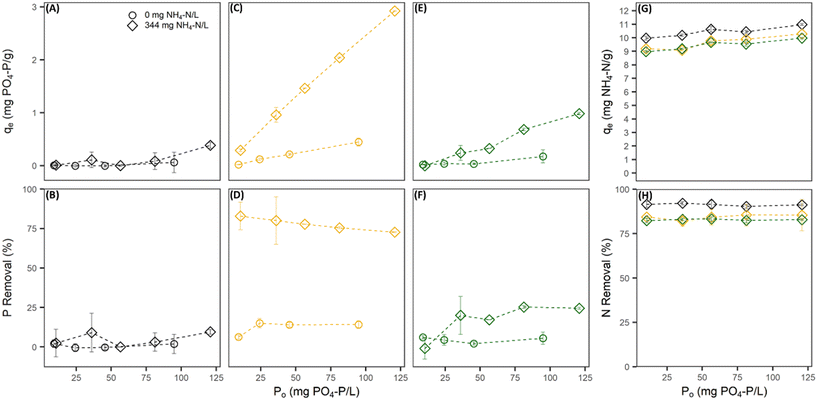
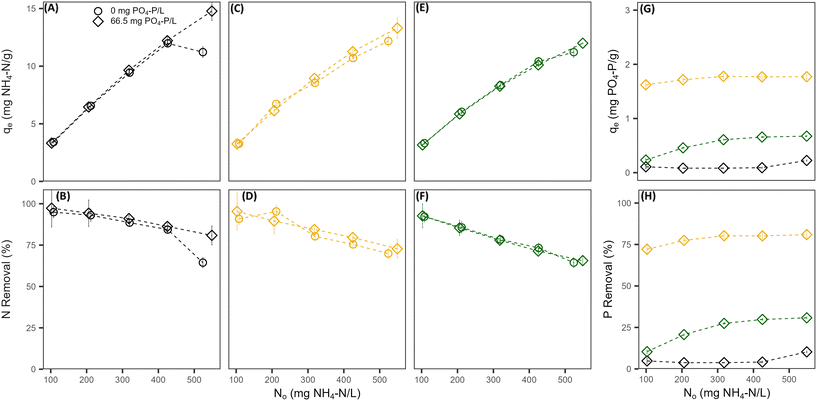
Without the presence of nitrogen, the PO4 sorption capacity of all pre-treated CZ was low and dependent on the pre-treatment cation used. Na-PT CZ showed little to no removal of PO4 (Fig. 1A and B). The PO4 sorption capacity improved slightly for Mg-PT CZ and furthermore with Ca-PT CZ (Fig. 1C–F). Both Mg-PT CZ and Ca-PT CZ PO4 sorption had a positive linear relationship with the initial concentrations, where CZ with higher initial PO4 concentrations observed higher sorption capacities. At a starting concentration of 95 mg P L−1, corresponding to the highest sorption capacities observed when there was no NH4 present, the sorption capacities reached values of 0.06 ± 0.19, 0.17 ± 0.12, and 0.45 ± 0.08 mg P g−1 for Na, Mg, and Ca pre-treated CZ, respectively. The availability of K+ in solution allowed for its exchange with Mg and Ca sorbed onto the CZ, thus, it is possible that higher removal capacities may have been observed had Mg and Ca remained on the CZ. Additional testing confirmed that PO4 sorption capacities of Ca-PT CZ were below 0.10 mg P g−1 for initial concentrations of 9.0, 40.0 and 92.0 mg P L−1 when PO4 was added as H3PO4 (Fig. S2†). Once NH4 was in solution with PO4, sorption capacities improved for Mg-PT CZ and Ca-PT CZ. There was no significant improvement in PO4 sorption using Na-PT CZ with NH4 presence (Fig. 1A and B). At a starting concentration of 120 mg P L−1, the phosphorus sorption capacities were 0.38 ± 0.06, 0.98 ± 0.02, and 2.92 ± 0.01 mg P g−1 for Na, Mg and Ca pre-treatment, which correlated to removal rates of 9.52 ± 1.4%, 24.4 ± 0.6%, and 72.7 ± 0.4%. During these tests, the NH4 removal remained consistently at 91.3 ± 6.3%, 82.3 ± 4.9%, and 84.3 ± 6.8% for Na, Mg, and Ca pre-treated CZ, respectively, and all with sorption capacities above 9 mg N g−1 (Fig. 1G and H).
When phosphorus concentrations in the solution remained constant, the NH4 removal and sorption capacity of all the pre-treated CZ remained consistent with respect to their controls when PO4 was not present (Fig. 2A–F). This was a clear indication that the presence of PO4 in solution had no effect on the sorption capacity of NH4 by ion-exchange regardless of pre-treatment method. However, the presence of NH4 did influence the PO4 sorption capacities of Mg-PT CZ and Ca-PT CZ. As the initial NH4 concentration increased (i.e. higher N/P), PO4 removal percentages and sorption capacities increased at a decreasing rate, stabilizing at N/P greater than 6.4. Mg-PT CZ saw the largest improvement in PO4 removal with increasing NH4 presence, increasing from 10.4% (0.23 mg P g−1) at N/P 1.5 to 30.8% (0.67 mg P g−1) at N/P 8.4. In tests 11–15, where neither NH4 nor PO4 remained constant, NH4 removal was consistent across a range of N/P ratios (Fig. 3). Phosphate removals also remained consistent for Na-PT CZ across a range of N/P ratios, where PO4 sorption decreased with Mg-PT CZ at higher N/P ratios and increased with Ca-PT CZ.
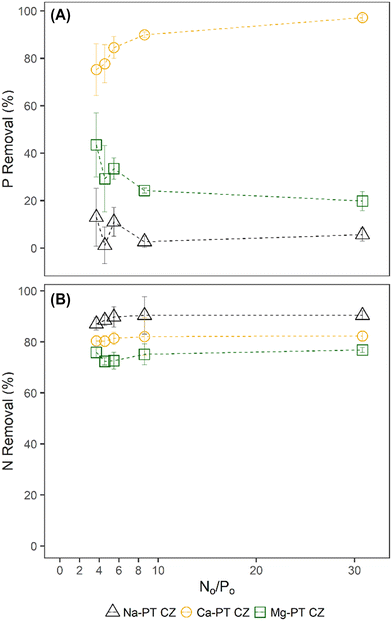 | ||
| Fig. 3 PO4 (A) and NH4 (B) percent removal from tests 11–15 where the initial N/P ratios varied between 3.7–30.8. | ||
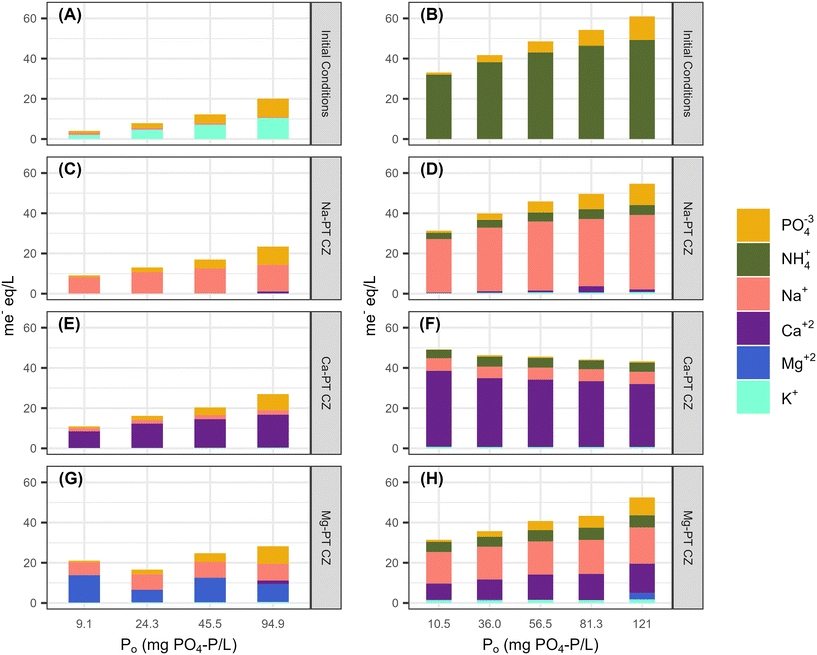
3.2. Ion composition in the bulk liquid
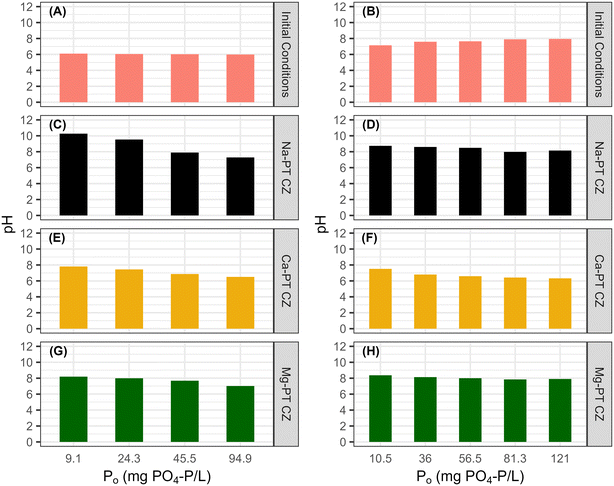
To quantify the ion exchange reactions during PO4 removal, five major cations of interest including Na, NH4, K, Ca, and Mg were measured at equilibrium for tests with PO4 only and with 344 ± 14 mg N L−1 in the background matrix. Phosphorus was the only nutrient present for all the PO4 only tests. Potassium was also present since PO4 was added as potassium phosphate salts (Fig. 4A). The presence of K during the sorption process allowed for the pre-treatment extra framework cations to exchange with K, as K has a stronger affinity for ion-exchange with CZ than Na, Ca, and Mg. After contact with all three pre-treated CZs, there was little to no K present in the bulk liquid at equilibrium (Fig. 4C, E and G). For Na-PT CZ, Na was the only major cation present in the bulk liquid. Similarly, for Ca-PT CZ, Ca was the major cation present in solution at equilibrium with small concentrations of Na present in all samples. For Mg-PT CZ, both Mg and Na were readily found in solution at near equal concentrations, with Ca presence increasing in samples as PO4 concentrations increased. Overall, little PO4 removal was observed while ion exchange occurred between K, Na, Ca, and Mg. However, the starting pH of all solutions began at 6 or less and increased significantly after contact with Na-PT CZ, and increased to a lesser extent when in contact with Ca-PT and Mg-PT CZ, respectively (Fig. 5A, C, E and G). The pH increase was likely to due to ion exchange between Na+ on the CZ surface and H+ in solution after K had exchanged with Na. As phosphorus concentrations increased, and more K+ was present in solution, the pH change was reduced.When PO4 was present with 344 ± 14 mg N L−1 of NH4 in the background matrix, NH4 readily exchange with all the pre-treated clinoptilolite zeolites. With only NH4 available for ion-exchange with the CZ extra framework cations (Fig. 4B), Na-PT CZ was able to readily exchange its Na for NH4, however, small amounts of Ca and K were also present in the bulk liquid (Fig. 4D). Similar results were observed with Ca-PT CZ, where the majority of cation presence was as Ca followed by Na and a small amount of K (Fig. 4F). Interestingly, for Mg-PT CZ, Na was the most abundant cation, followed by Ca and small amounts of K in all the samples (Fig. 4H). Magnesium was only observed when the initial PO4 concentration was 120 mg P L−1.
The starting pH of all the solutions containing both NH4 and PO4 were between 7–8. Minor increases in final pHs were observed when low PO4 concentrations were in contact with Na-PT and Mg-PT CZ due to the loss of NH4 (Fig. 5B, D and H). In this case, PO4 behaved as a buffer, diminishing the pH effects as PO4 concentrations increased. When the solutions were in contact with Ca-PT CZ, the pH dropped below the starting pH for initial PO4 concentrations greater than 10.5 mg P L−1 as Ca–PO4 precipitation is a base consuming reaction (Fig. 5F).
3.3. Sorption isotherms
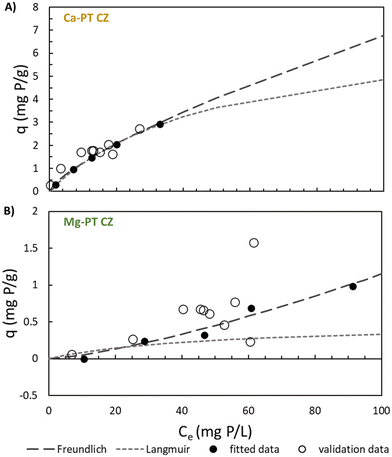
The equilibrium results were fitted to both Freundlich and Langmuir isotherm models for PO4 sorption without NH4 addition, NH4 sorption without PO4 addition, PO4 with 344 ± 14 mg N L−1, and NH4 with 66 ± 0.6 mg P L−1 when each were in contact with Na-PT CZ, Mg-PT CZ, and Ca-PT (Table 2). For PO4 only, the Langmuir model best described both PO4 sorption to Mg-PT CZ and Ca-PT CZ (Fig. S3†), with R2 values of 0.8938 and 0.9995 and exhibiting RSEs less than what was obtained with the Freundlich models in each scenario. Since Na-PT CZ did not readily adsorb PO4, the data did not fit either model, with or without the presence of NH4. When NH4 was present in solution with PO4, the inverse results were observed for PO4 sorption to Mg and Ca pre-treated CZ, with the Freundlich model best describing the sorption behavior. Within the range of PO4 concentrations tested, both Freundlich and Langmuir were able to describe the sorption onto Ca-PT CZ, while statistically Freundlich was a better fit. The PO4 sorption data from tests 1–5 as well as additional sorption data when both NH4 and PO4 were varied (tests 11–15) were also plotted against the Freundlich and Langmuir models for Mg-PT CZ and Ca-PT CZ (Fig. 6). It can be observed that PO4 sorption onto Ca-PT CZ was completely independent of the background NH4 concentration. However, PO4 sorption using Mg-PT CZ was affected by the presence of NH4.| Mg-PT | Na-PT | Ca-PT | Mg-PT | Na-PT | Ca-PT | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | SE | Value | SE | Value | SE | Value | SE | Value | SE | Value | SE | ||
| PO4 with 0 mg N L−1 | PO4 with 344 ± 14 mg N L−1 | ||||||||||||
| Freundlich | K f | 0.0096 | 0.009 | 0.0041 | 0.012 | 0.0095 | 0.002 | 0.0023 | 0.002 | 6.4 × 10−7 | 3.9 × 10−6 | 0.221 | 0.012 |
| n | 1.842 | 0.525 | 3.082 | 5.285 | 1.162 | 0.040 | 0.7411 | 0.125 | 0.3551 | 0.167 | 1.351 | 0.032 | |
| R 2 | 0.8331 | −0.1300 | 0.9988 | 0.9530 | 0.7749 | 0.9990 | |||||||
| p | 0.0196 | 0.5157 | 1.2 × 10−5 | 0.0028 | 0.0311 | 8.4 × 10−6 | |||||||
| RSE | 0.0462 | 0.0287 | 0.0291 | 0.0863 | 0.0744 | 0.0344 | |||||||
| Langmuir | q max | 0.3664 | 0.087 | 0.0365 | 0.037 | 6.098 | 0.683 | 99.52 | 4589 | 1.2 × 103 | 9.6 × 106 | 7.274 | 0.598 |
| K L | 0.0061 | 0.004 | 0.0164 | 0.047 | 0.0009 | 0.0001 | 0.0001 | 0.005 | 1.9 × 10−6 | 0.0148 | 0.0201 | 0.002 | |
| R 2 | 0.8938 | 0.0096 | 0.9995 | 0.9579 | 0.451 | 0.9987 | |||||||
| p | 0.0098 | 0.3832 | 2.8 × 10−6 | 0.0024 | 0.1302 | 1.3 × 10−5 | |||||||
| RSE | 0.0372 | 0.0271 | 0.0184 | 0.1243 | 0.1227 | 0.0425 | |||||||
| NH4 with 0 mg P L−1 | NH4 with 66 ± 0.6 mg P L−1 | ||||||||||||
| Freundlich | K f | 1.706 | 1.311 | 3.397 | 1.311 | 2.334 | 0.881 | 1.490 | 0.069 | 2.549 | 0.233 | 1.847 | 0.156 |
| n | 2.708 | 1.402 | 3.956 | 1.402 | 3.080 | 0.797 | 2.505 | 0.062 | 2.619 | 0.153 | 2.508 | 0.119 | |
| R 2 | 0.9734 | 0.7040 | 0.8317 | 0.9985 | 0.9917 | 0.9945 | |||||||
| p | 0.0012 | 0.0478 | 0.0198 | 1.6 × 10−5 | 0.0002 | 0.0001 | |||||||
| RSE | 0.5357 | 1.942 | 1.433 | 0.1369 | 0.4245 | 0.3003 | |||||||
| Langmuir | q max | 13.04 | 0.784 | 12.96 | 0.937 | 12.39 | 1.705 | 13.79 | 1.271 | 16.70 | 1.305 | 15.61 | 1.466 |
| K L | 0.0307 | 0.006 | 0.0738 | 0.020 | 0.0621 | 0.034 | 0.0253 | 0.007 | 0.0540 | 0.014 | 0.0314 | 0.009 | |
| R 2 | 0.9788 | 0.9385 | 0.7613 | 0.9647 | 0.9761 | 0.9719 | |||||||
| p | 0.0009 | 0.0042 | 0.0341 | 0.0018 | 0.0010 | 0.0013 | |||||||
| RSE | 0.5207 | 0.8876 | 1.713 | 0.7527 | 0.8438 | 0.8286 | |||||||
For NH4 sorption with and without PO4 in the background matrix, both the Freundlich and Langmuir isotherm models fit the data well for the given concentrations tested, all having R2 values well beyond 0.70. The Langmuir models had the best fit for sorption to Mg-PT CZ and Na-PT CZ without PO4 presence, while the Freundlich model fit best for sorption to Ca-PT CZ. The Freundlich models best described NH4 sorption to all three PT-CZs when PO4 was present.
3.4. CZ material characterization
CZ samples prior to any treated and post cation treatment were analyzed for elemental composition using XRF (Table 3). Untreated CZ primarily contained K as the main extra framework cation followed by Ca, Fe, Na, and Mg. For all pre-treated CZ, the iron percent composition did not change, suggesting that while it was present on the CZ it did not play a significant role in ion-exchange with the pre-treatment cations. After CZ was pretreated with Na, the Na composition of CZ increased 2.2-fold, but K remained the main extra framework cation followed by Na and Ca while experiencing no change to the Mg content. After CZ was pretreated with Ca, the Ca composition increased 2.7-fold, increasing from 3.48% to 9.41%. Ca became the main extra framework cation followed closely by K. Both the Na and Mg presence was low within the CZ composition, accounting for less than 0.5% each. For Mg pre-treatment, the metal ion content increased 1.8-fold to 0.9%, however, this percent composition was low compared to other competing cations. K was the main extra framework cation, followed by Ca.| Element | Natural CZ | Na-PT CZ | Mg-PT CZ | Ca-PT CZ | Mg-PT CZ soaked in 0.066 g P L−1 and 0.43 g N L−1 | Ca-PT CZ soaked in 0.067 g P L−1 and 0.32 g N L−1 |
|---|---|---|---|---|---|---|
| BDL = below detection limit. | ||||||
| P | BDL | BDL | BDL | BDL | 0.10 | 0.37 |
| Ca | 3.48 | 2.16 | 4.47 | 9.41 | 3.70 | 6.57 |
| K | 7.78 | 6.43 | 10.7 | 7.24 | 9.62 | 6.26 |
| Na | 1.54 | 3.31 | BDL | 0.38 | BDL | 0.11 |
| Al | 12.4 | 12.5 | 10.2 | 11.4 | 10.6 | 12.6 |
| Si | 69.7 | 70.2 | 67.3 | 65.1 | 69.6 | 68.9 |
| Mg | 0.50 | 0.42 | 0.90 | 0.49 | 1.11 | 0.58 |
| Fe | 3.07 | 2.97 | 2.94 | 3.28 | 3.07 | 3.08 |
For natural CZ (untreated) and all pretreated CZs, there was no phosphorus detected within its elemental composition. The composition was also analyzed for Ca-PT CZ from Test 3 and Mg-PT CZ from Test 4 (Table 1), which each initially in contact with 0.067 g P L−1 and 0.32 g N L−1 and 0.066 g P L−1 and 0.43 g N L−1, respectively. Phosphorus was present on the surface of both CZ samples, with Ca-PT CZ able to retain nearly 4 times more phosphorus than Mg-PT CZ. Since Ca pretreatment observed the best PO4 removal performance, two additional tests were conducted to observe the compositional changes of Ca-PT CZ soaked under 0.5 g P L−1 and 5.0 g P L−1 (Table S2†). Both Ca-PT CZ were able to retain 0.27% and 3.37% phosphorus, respectively. When NH4 was also present in the initial solution with PO4, the percent of phosphorus composition found within the Ca-PT CZ was higher (0.37%) than when PO4 was initially present in solution alone (0.27%) at a much higher initial concentration (Table S3†). These results indicate that the presence of NH4 can significantly enhance phosphorus sorption and recovery when compared to sorption of PO4 alone by CZ.
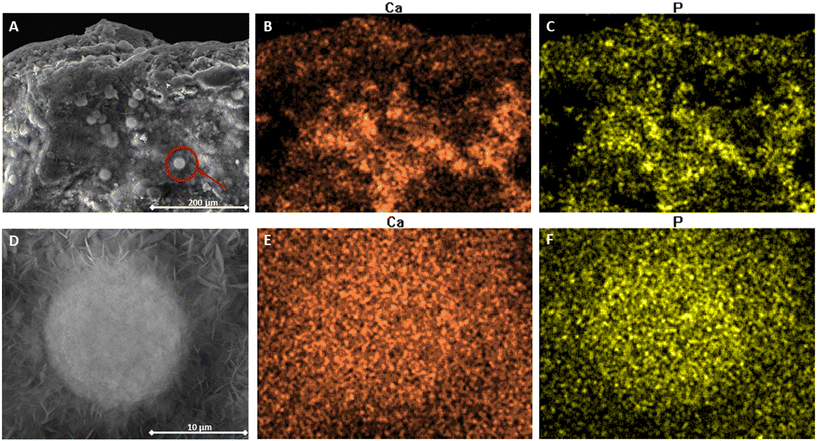
Surface imaging of Ca-PT CZ soaked in 0.067 g P L−1 and 0.32 g N L−1 is shown in Fig. 7. The images reveal small granular aggregates attached to the surface of the CZ (Fig. 7A), with an approximate diameter of 15 μm (Fig. 7D). Small sheath-like crystals can also be observed around the granular aggregates of approximately 1–2.5 μm in length. The elemental distribution of Ca and phosphorus overlapped consistently across the surface of the CZ (Fig. 7B and C). After further magnification onto one of the aggregates on the CZ, Ca and phosphorus are seen to be densely distributed across the same vector space as the aggregate, suggesting that the aggregate is a Ca–PO4 composite. The spectral analysis on the individual aggregate revealed that the concentration by percent weight was primarily oxygen, accounting for 49%, followed by Ca at 27%, and phosphorus at 16%. Elemental nitrogen only comprised 2.5% of the aggregate. The ratio of Ca to phosphorus was 1.69, suggesting that the precipitate was hydroxyapatite (HAP), Ca10(PO4)6(OH)2, which also has a Ca to phosphorus ratio of 1.66.41 Mg-PT CZ was also imaged after soaking in 0.066 g P and 0.32 g N L−1. The surface of Mg-PT CZ also showed, to a much lesser extent, small, localized aggregates of Ca–PO4 precipitates (Fig. S4†).
4. Discussion
4.1. Phosphate removal by clinoptilolite zeolite is improved with the presence of ammonium
Three metal cations frequently found in wastewater sources (Na, Ca, and Mg) were chosen to modify the surface composition of natural CZ to evaluate the simultaneous removal effectiveness and sorption mechanism of NH4 and PO4 at a range of concentrations readily found in blackwater. The preference for NH4 sorption followed the order Na > Mg > Ca while the opposite was true for PO4 sorption, where the preferential order was Ca > Mg > Na. This distinction in cation preference allowed for a lower competition between NH4 and PO4 removal, having no effect on the overall NH4 sorption capacities. The N/P ratio had a significant impact on the removal performance of Ca-PT CZ, increasing PO4 removal rates from 75.2% at 3.7 N/P to 97.2% at 30.8 N/P. Typical values of N/P in blackwater may vary widely, but several studies that have quantified the nutrient concentrations of such wastewaters yield average values near 6–8.33,42,43 This study observed PO4 removal capacities of 1.46 mg P g−1 (Test 8 at 6.2 N/P) and 1.77 mg P g−1 (Test 4 at 6.4 N/P) with NH4 removal capacities of 11.3 mg N g−1 and 9.78 mg N g−1 using Ca-PT CZ. By comparison, Stocker et al. maintained NH4 constant at 780 mg N L−1 and varied the PO4 concentration from 160–820 mg P L−1, which correlated to N/P ratios of 0.95–47.7.31 At a N/P ratio of 4.76 with a starting concentration of 163 mg P L−1 and 777 mg N L−1, PO4 removal was near 2.0 mg P g−1 of Ca modified CZ. The results indicate that under lower concentrations of both nutrients with similar N/P ratios, improved removal capacities can be achieved using Ca-PT CZ.The XRD results revealed that even after modifying the CZ with 2 M of Na and Mg, the dominant extra framework cation remained as K. For Ca-PT CZ, the elemental composition of Ca was greater than K. While K concentration was on par with or higher than the pre-treatment cation, it did not contribute to PO4 removal. This further highlights that the presence of Ca on each of the PT-CZ played a critical role in the direct removal of PO4. The large increase in PO4 removal using Ca-PT CZ when NH4 was also present, and to a lesser extent for Mg-PT CZ, is largely due to several surface reactions occurring simultaneously: (1) ion exchange between NH4 and the extra framework cations; (2) Brønsted–Lowry interactions between dihydrogen phosphate (H2PO4−) and the CZ framework (3) the formation of hydrogen bonds between oxygen atoms on the CZ framework and hydroxyl groups of di- or monohydrogen phosphate ions (HPO42−) sorbed to extra framework divalent cations (Ca or Mg) and (4) Ca–PO4 complexation in the bulk liquid adsorbing to the CZ surface.29,44 Phosphates are also able to complex with the hydroxyl groups of aluminum oxide, however, PO4 removal could only be improved by increasing the aluminum oxide to silicon dioxide ratios. Guaya et al. observed that PO4 removal could be enhanced by more than 10 times from 0.6 mg g−1 using natural zeolite to 7.0 mg g−1 using natural zeolite that was impregnated with aluminum oxides.45 While other theories suggest that surface complexation to aluminum or iron oxides occurs first before the formation of Ca–PO4 precipitates, this is highly dependent in the chemical composition of the CZ. Our study supports that Ca–PO4 complexation is the primary PO4 removal mechanisms using Ca-PT and Mg-PT CZ within the N/P range evaluated.46
The reaction required in removing PO4 affect the ratio of weak acids and conjugate bases in the bulk liquid. To support this, an inverse linear correlation exists between the equilibrium pH and the PO4 sorption capacity in order of Ca, Mg, and Na. As PO4 removal increases, so does the consumption of hydroxides. The Ca-PT bulk liquid pH increased from 6.0 to only 6.5 but observed the highest PO4 removal. The Mg-PT and Na-PT bulk liquid equilibrium pH increased to 7.02 and 7.28, respectively, indicating that the extent of pH increase was due to the removal of NH4 from solution, raising the pH accordingly. The pHs of the effluent were well within the range desired for discharge (6–9), indicating that in practice no pH adjustments may be needed. Other P sorption materials, such as Polonite, has a high sorption capacity for phosphorus but effluent wastewater can have pHs of near 12 as hydroxides are released.12,47
From the EDS and XRD results of NH4 and PO4 loaded Ca-PT CZ and Mg-PT CZ, PO4 is primarily removed by physical sorption and complexation of PO4 and Ca onto the zeolite surface. Karapinar et al. (2009) observed that the addition of zeolite into a solution of Ca, PO4, and NH4, at any given pH between 7.5–10 did not affect the formation of Ca-PO4 precipitates, further supporting that precipitation under this study's experimental conditions happens at the site of Ca.44 While the phase composition of the precipitates on the CZ was not directly investigated, it is speculated that the precipitate is HAP based on the elemental composition of the aggregate, the drop in pH as PO4 uptake occurred, and the EDS images depicting granule and crystal formation. Overall, the presence of NH4 in solution allows for its exchange with Ca, enhancing the PO4 removal by increasing the presence of Ca in the bulk liquid so that CZ can be used as a seed for Ca–PO4 precipitation onto the CZ surface. Increasing the Ca content of CZ composition yields improved PO4 removal and further chemical modification to enhance Ca ion exchange to CZ should be explored. This study did show that significant residual Ca was present within the final effluent wastewater, which could prove to be a challenge in piped systems. Future work should focus on reactor configuration and contact time to optimize both nitrogen and phosphorus removal and minimize Ca losses.
4.2. Phosphate sorption mechanism is altered by ammonium presence
When PO4 was in contact with Mg and Ca pretreated CZ, the PO4 sorption mechanisms was unequivocally altered by the addition of NH4 in solution. Isotherm models for PO4 sorption by Mg-PT CZ and Ca-PT CZ showed that without the presence of NH4, the Langmuir model best fits the resulting sorption. Sorption to both Mg-PT and Ca-PT CZ was favorable, with separation factors ranging between 0.94–0.58 and 0.99–0.90 (Fig. S5†), respectively, within the concentrations of 10–120 mg P L−1 evaluated in this study. Once NH4 was in solution, the sorption mechanism for both Mg-PT CZ and Ca-PT CZ changed due to the competitive nature of PO4 and NH4 for Ca active sites. The adsorption of Ca–PO4 precipitates in the bulk liquid to the CZ surface leads to an increase in the overall size of CZ.44 Therefore, the multilayer binding of Ca–PO4 precipitates onto CZ changes the sorption behavior. How rapidly these complexes form in solution and adsorb to CZ is still to be investigated. The Freundlich model best depicted the change in sorption mechanism. Interestingly, the sorption behavior of PO4 onto zeolite was independent of the concentration of NH4 added (Fig. 5A) which has also been observed by recent studies.31 The presence of NH4 allows for ion-exchange with Ca ions, which then complex with PO4 and bind to CZ. Phosphate sorption will be limited at lower concentrations of initial NH4, beyond the N/P ratios evaluated, as there will be less Ca ions in the bulk liquid due to the limited ion-exchange activity. Future work should also consider the competition between PO4 and other anions, such as sulfide, for complexation with Ca. Similarly, competition between NH4 and other cations, particularly K which has a higher ionic affinity for ion exchange with CZ than NH4, and its effect on PO4 complexation should be explored.4.3. Applications for nutrient recovery at the small-scale
Advance OWTS use biological processes downstream of the septic tank to primarily remove organic contaminants and facilitate partial nitrification and denitrification, but do not readily remove phosphorus. OWTS take advantage of natural attenuation in leach fields to uptake PO4 before reaching discharge points. However, where land usage is limited, and soil types are inadequate, non-point nutrient discharge perpetuates. Natural CZ can be chemically modified more readily with Ca than it is with Na or Mg and while there are other competitive cations on the CZ surface, their presence aids in exchanging with NH4, leaving Ca sites available for PO4 and Ca–PO4 precipitates. Additional tests (data not shown) revealed that with Ca-PT CZ, equilibrium removal of both nutrients was reached within 8 h of contact time. Septic systems typically have 1–3 days of hydraulic retention time before discharging into the drainfields, indicating that Ca-PT CZ would have sufficient time to achieve the results presented in this study. Mixtures of chemically modified CZ with other natural PO4 removal media, including shale, sand, limestone, calcite, dolomite, or pumice, can be used to further enhance PO4 removal by physical sorption.48,49 Used as a single sorption material, CZ can be readily regenerated with alkali brine solutions to recover soluble nitrogen, while Ca–PO4 has been marginally desorbed using bicarbonate as Ca–PO4 bonds are irreversible.20,46 However, rather than recovering soluble ions of NH4 and PO4, CZ loaded with these nutrients can be reutilized as a slow-released fertilizer for agricultural applications. For the Ca-PT CZ soaked in 0.067 g P L−1 and 0.32 g N L−1 in this study, the final sample was able to retain a molar ratio of N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K of 5.3
K of 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.4, making the synthesized material a complete fertilizer. Within the last few years, hydroxyapatite nanoparticle (nHAP) production has been evaluated as a fertilizer for plant nutrition. Typically, fertilizers contain phosphorus in the form of soluble salts such as monoammonium phosphate (MAP), diammonium phosphate (DAP), and triple superphosphate (TSP) as they are readily available for uptake.50,51 There is evidence that HAP, both as a nanoparticle and as a bulk material, can be a source of slow-release phosphorus for P-deficient soils.52,53 Further work is needed on the application of chemically modified CZ as a fertilizer, particularly to quantify the efficiency of phosphorus release to soils or by plant uptake. Beyond the removal of nutrients, the increase in ionic content, particularly sodium and calcium, in the effluent should be considered before discharge to nearby soils. The addition of a desalination and softening step may be required to reduce salinity and hardness.
13.4, making the synthesized material a complete fertilizer. Within the last few years, hydroxyapatite nanoparticle (nHAP) production has been evaluated as a fertilizer for plant nutrition. Typically, fertilizers contain phosphorus in the form of soluble salts such as monoammonium phosphate (MAP), diammonium phosphate (DAP), and triple superphosphate (TSP) as they are readily available for uptake.50,51 There is evidence that HAP, both as a nanoparticle and as a bulk material, can be a source of slow-release phosphorus for P-deficient soils.52,53 Further work is needed on the application of chemically modified CZ as a fertilizer, particularly to quantify the efficiency of phosphorus release to soils or by plant uptake. Beyond the removal of nutrients, the increase in ionic content, particularly sodium and calcium, in the effluent should be considered before discharge to nearby soils. The addition of a desalination and softening step may be required to reduce salinity and hardness.
5. Conclusion
The consistent treatment of both nitrogen and phosphorus by biological means has been a challenge for onsite wastewater treatment which is attributed to high and variable influent loading rates and limited applied technologies for phosphorus removal. One strategy to combat these challenges is to use sorption methods for the simultaneous removal and recovery of nitrogen and phosphorus in a single step. Natural CZ is a low-cost material that can be chemically modified with CaCl2 to increase its Ca content, which readily exchanges with NH4 and precipitates with PO4. Phosphate removal is more complex, and is directly dependent on the extra framework cation composition of the CZ. The results from this study indicated that phosphorus was primarily removed by complexation of Ca–PO4 onto the CZ surface regardless of pre-treatment method and was significantly enhaced when CZ was pre-treated with Ca. The final Ca-PT CZ product was able to retain nitrogen, phosphorus, and potassium, which are important nutrients for agricultural production. When using Ca-based pretreatment methods for nutrient recovery, the effluent wastewater will need further processing to reduce the resulting hardness and salinity. Further exploration is needed on reactor design and operation to minimize the negative impacts to the discharging soils.Author contributions
CJC: conceptualization, investigation, methodology, supervision, data curation, formal analysis, writing – original draft, writing – review & editing. HYS: investigation, methodology, writing – review & editing. BH: investigation, methodology, writing – review & editing. DHY: conceptualization, funding acquisition.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported, in whole or in part, by the Bill & Melinda Gates Foundation [INV-006612]. The authors would like to thank Robert Tufts, Yusuf Emirov, and Alissa Anderson from the Nanotechnology Research & Education Center for their assistance in processing and imaging clinoptilolite zeolite samples for XRF, and FIB-SEM with EDS analysis. We also thank Robert Bair for assisting in reviewing and proofreading the manuscript.References
- D. R. de Figueiredo, U. M. Azeiteiro, S. M. Esteves, F. J. M. Gonçalves and M. J. Pereira, Microcystin-Producing Blooms—a Serious Global Public Health Issue, Ecotoxicol. Environ. Saf., 2004, 59(2), 151–163, DOI:10.1016/j.ecoenv.2004.04.006.
- J. L. Darby and H. Leverenz, Virus, Phosphorus, and Nitrogen Removal in Onsite Wastewater Treatment Processes, CA, Davis, 2004 Search PubMed.
- Washington State Department of Health, Nitrogen Reducing Technologies for Onsite Wastewater Treatment Systems, Report, 2005, No. June, pp. 1–89.
- M. Badruzzaman, J. Pinzon, J. Oppenheimer and J. G. Jacangelo, Sources of Nutrients Impacting Surface Waters in Florida: A Review, J. Environ. Manage., 2012, 80–92, DOI:10.1016/j.jenvman.2012.04.040.
- L. W. Herren, R. A. Brewton, L. E. Wilking, M. E. Tarnowski, M. A. Vogel and B. E. Lapointe, Septic Systems Drive Nutrient Enrichment of Groundwaters and Eutrophication in the Urbanized Indian River Lagoon, Florida, Mar. Pollut. Bull., 2021, 172, 112928, DOI:10.1016/j.marpolbul.2021.112928.
- B. v. Lancellotti, G. W. Loomis, K. P. Hoyt, E. Avizinis and J. A. Amador, Evaluation of Nitrogen Concentration in Final Effluent of Advanced Nitrogen-Removal Onsite Wastewater Treatment Systems (OWTS), Water, Air, Soil Pollut., 2017, 228(10), 383, DOI:10.1007/s11270-017-3558-3.
- B. N. Ross, K. P. Hoyt, G. W. Loomis and J. A. Amador, Effectiveness of Advanced Nitrogen-Removal Onsite Wastewater Treatment Systems in a New England Coastal Community, Water, Air, Soil Pollut., 2020, 231(11), 543, DOI:10.1007/s11270-020-04911-5.
- C. P. Humphrey, M. O'Driscoll and G. Iverson, Comparison of Nitrogen Treatment by Four Onsite Wastewater Systems in Nutrient-Sensitive Watersheds of the North Carolina Coastal Plain, Nitrogen, 2021, 2(2), 268–286, DOI:10.3390/nitrogen2020018.
- N. Diaz-Elsayed, X. Xu, M. Balaguer-Barbosa and Q. Zhang, An Evaluation of the Sustainability of Onsite Wastewater Treatment Systems for Nutrient Management, Water Res., 2017, 121, 186–196, DOI:10.1016/j.watres.2017.05.005.
- C. P. Humphrey, Phosphate Treatment by Five Onsite Wastewater Systems in a Nutrient Sensitive Watershed, Earth, 2022, 3(2), 683–698, DOI:10.3390/earth3020039.
- F. L. Schellenger and F. L. Hellweger, Phosphorus Loading from Onsite Wastewater Systems to a Lake (at Long Time Scales), Lake Reservoir Manage., 2019, 35(1), 90–101, DOI:10.1080/10402381.2018.1541031.
- C. Vohla, M. Kõiv, H. J. Bavor, F. Chazarenc and Ü. Mander, Filter Materials for Phosphorus Removal from Wastewater in Treatment Wetlands-A Review, Ecol. Eng., 2011, 37(1), 70–89, DOI:10.1016/j.ecoleng.2009.08.003.
- N. Bellier, F. Chazarenc and Y. Comeau, Phosphorus Removal from Wastewater by Mineral Apatite, Water Res., 2006, 40(15), 2965–2971, DOI:10.1016/j.watres.2006.05.016.
- X. Yuan, W. Xia, J. An, J. Yin, X. Zhou and W. Yang, Kinetic and Thermodynamic Studies on the Phosphate Adsorption Removal by Dolomite Mineral, J. Chem., 2015, 2015, 853105, DOI:10.1155/2015/853105.
- H. S. Altundoan and F. Tmen, Removal of Phosphates from Aqueous Solutions by Using Bauxite. I: Effect of PH on the Adsorption of Various Phosphates, J. Chem. Technol. Biotechnol., 2002, 77(1), 77–85, DOI:10.1002/jctb.525.
- R. A. Mann and H. J. Bavor, Phosphorus removal in constructed wetlands using gravel and industrial waste substrata, Water Sci. Technol., 1993, 27(1), 107–113 CrossRef CAS.
- Y. Li, C. Liu, Z. Luan, X. Peng, C. Zhu, Z. Chen, Z. Zhang, J. Fan and Z. Jia, Phosphate Removal from Aqueous Solutions Using Raw and Activated Red Mud and Fly Ash, J. Hazard. Mater., 2006, 137(1), 374–383, DOI:10.1016/j.jhazmat.2006.02.011.
- N. Xu, M. Chen, K. Zhou, Y. Wang, H. Yin and Z. Chen, Retention of Phosphorus on Calcite and Dolomite: Speciation and Modeling, RSC Adv., 2014, 4(66), 35205–35214, 10.1039/c4ra05461j.
- B. Han, C. Butterly, W. Zhang, J. He and D. Chen, Adsorbent Materials for Ammonium and Ammonia Removal: A Review, J. Cleaner Prod., 2021, 283, 124611, DOI:10.1016/j.jclepro.2020.124611.
- C. J. Castro, H. Y. Shyu, L. Xaba, R. Bair and D. H. Yeh, Performance and Onsite Regeneration of Natural Zeolite for Ammonium Removal in a Field-Scale Non-Sewered Sanitation System, Sci. Total Environ., 2021, 776, 145938, DOI:10.1016/j.scitotenv.2021.145938.
- W. Zhang, Z. Zhou, Y. An, S. Du, D. Ruan, C. Zhao, N. Ren and X. Tian, Optimization for Zeolite Regeneration and Nitrogen Removal Performance of a Hypochlorite-Chloride Regenerant, Chemosphere, 2017, 178, 565–572, DOI:10.1016/j.chemosphere.2017.03.091.
- E. E. Neag, M. Şenilă, A. I. Török, M. Roman and F. Puskás, Regeneration and Reuse of Natural Zeolite for Ammonium Removal, International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, 2019, vol. 195.2, pp. 651–656, DOI:10.5593/sgem2019/5.2/S20.081.
- L. L. Ames Jr., The Cation Sieve Properties of Clinoptilolite, Am. Mineral., 1960, 45(5–6), 689–700 Search PubMed.
- A. Drizo, C. A. Frost, J. Grace and K. A. Smith, Physico-chemical screening of phosphate-removing substrates for use in constructed wetland systems, Water Res., 1999, 33(17), 3595–3602 CrossRef CAS.
- D. Mitrogiannis, M. Psychoyou, I. Baziotis, V. J. Inglezakis, N. Koukouzas, N. Tsoukalas, D. Palles, E. Kamitsos, G. Oikonomou and G. Markou, Removal of Phosphate from Aqueous Solutions by Adsorption onto Ca(OH)2 Treated Natural Clinoptilolite, Chem. Eng. J., 2017, 320, 510–522, DOI:10.1016/j.cej.2017.03.063.
- B. Zhang, X. Wang, S. Li, Y. Liu, Y. An and X. Zheng, Preferable Adsorption of Nitrogen and Phosphorus from Agricultural Wastewater Using Thermally Modified Zeolite-Diatomite Composite Adsorbent, Water, 2019, 11(10), 2053, DOI:10.3390/w11102053.
- C. Wan, S. Ding, C. Zhang, X. Tan, W. Zou, X. Liu and X. Yang, Simultaneous Recovery of Nitrogen and Phosphorus from Sludge Fermentation Liquid by Zeolite Adsorption: Mechanism and Application, Sep. Purif. Technol., 2017, 180, 1–12, DOI:10.1016/j.seppur.2017.02.031.
- Y. Huang, X. Dong, M. Li, M. Zhang and Y. Yu, Density Functional Theory Study of the Structural and Electronic Properties of H3PO4/ZSM-5, RSC Adv., 2014, 4(28), 14573–14581, 10.1039/c3ra47551d.
- E. L. Uzunova and H. Mikosch, Adsorption of Phosphates and Phosphoric Acid in Zeolite Clinoptilolite: Electronic Structure Study, Microporous Mesoporous Mater., 2016, 232, 119–125, DOI:10.1016/j.micromeso.2016.06.019.
- Q. Cheng, H. Li, Y. Xu, S. Chen, Y. Liao, F. Deng and J. Li, Study on the Adsorption of Nitrogen and Phosphorus from Biogas Slurry by NaCl-Modified Zeolite, PLoS One, 2017, 12(5), e0176109, DOI:10.1371/journal.pone.0176109.
- K. Stocker and M. Ellersdorfer, Phosphate Fixation and P Mineralogy on Natural and Ca-Modified Zeolites During Simultaneous Nutrient Removal, Water, Air, Soil Pollut., 2022, 233(41), 1–13, DOI:10.1007/s11270-022-05509-9.
- X. You, C. Valderrama and J. L. Cortina, Simultaneous Recovery of Ammonium and Phosphate from Simulated Treated Wastewater Effluents by Activated Calcium and Magnesium Zeolites, J. Appl. Chem. Biotechnol., 2017, 92(9), 2400–2409, DOI:10.1002/jctb.5249.
- H.-Y. Shyu, R. A. Bair, C. J. Castro, L. Xaba, M. Delgado-Navarro, R. Sindall, R. Cottingham, A. E. Uman, C. A. Buckley and D. H. Yeh, The NEWgenerator Non-Sewered Sanitation System: Long-Term Field Testing at an Informal Settlement Community in EThekwini Municipality, South Africa, J. Environ. Manage., 2021, 296, 112921, DOI:10.1016/j.jenvman.2021.112921.
- J. Ray, V. Saroyda, Y. S. Ranya, J. C. Cruz, C. Anto-Nio, P. Luis, J. Flestado, R. S. Magalong, K. Zyrell, P. Zagala, C. L. Barba-Cena, J. M. Bumatay, L. F. Bautista, C. C. Deocaris, M. Chester and C. Deocaris, Package “PUPAIM” Type Package Title A Collection of Physical and Chemical Adsorption Isotherm Models Version 0.2.0, 2020, DOI:10.1016/S0001-8686(00)00082.
- H. Swenson and N. P. Stadie, Langmuir's Theory of Adsorption: A Centennial Review, Langmuir, 2019, 35(16), 5409–5426, DOI:10.1021/acs.langmuir.9b00154.
- G. Galamini, G. Ferretti, V. Medoro, N. Tescaro, B. Faccini and M. Coltorti, Isotherms, Kinetics, and Thermodynamics of Nh4 + Adsorption in Raw Liquid Manure by Using Natural Chabazite Zeolite-Rich Tuff, Water, 2020, 12(10), 1–16, DOI:10.3390/w12102944.
- Y. Bulut and Z. Tez, Adsorption Studies on Ground Shells of Hazelnut and Almond, J. Hazard. Mater., 2007, 149(1), 35–41, DOI:10.1016/j.jhazmat.2007.03.044.
- J. Wang and X. Guo, Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method, Chemosphere, 2020, 258, 127279, DOI:10.1016/j.chemosphere.2020.127279.
- B. Meroufel, O. Benali, M. Benyahia, Y. Benmoussa and M. A. Zenasni, Adsorptive Removal of Anionic Dye from Aqueous Solutions by Algerian Kaolin: Characteristics, Isotherm, Kinetic and Thermodynamic Studies, J. Mater. Environ. Sci., 2013, 4(3), 482–491 CAS.
- M. Chiban, A. Soudani, F. Sinan and M. Persin, Single, Binary and Multi-Component Adsorption of Some Anions and Heavy Metals on Environmentally Friendly Carpobrotus Edulis Plant, Colloids Surf., B, 2011, 267–276, DOI:10.1016/j.colsurfb.2010.09.013.
- R. Zhu, R. Yu, J. Yao, D. Wang and J. Ke, Morphology control of hydroxyapatite through hydrothermal process, J. Alloys Compd., 2008, 457, 555–559, DOI:10.1016/j.jallcom.2007.03.081.
- H. Knerr, A. Rechenburg, T. Kistemann and T. G. Schmitt, Performance of a MBR for the Treatment of Blackwater, Water Sci. Technol., 2011, 63(6), 1247–1254, DOI:10.2166/wst.2011.367.
- M. Eshetu Moges, D. Todt and A. Heistad, Treatment of Source-Separated Blackwater: A Decentralized Strategy for Nutrient Recovery towards a Circular Economy, Water, 2018, 10(4), 463, DOI:10.3390/w10040463.
- N. Karapinar, Application of Natural Zeolite for Phosphorus and Ammonium Removal from Aqueous Solutions, J. Hazard. Mater., 2009, 170(2–3), 1186–1191, DOI:10.1016/j.jhazmat.2009.05.094.
- D. Guaya, C. Valderrama, A. Farran, C. Armijos and J. L. Cortina, Simultaneous Phosphate and Ammonium Removal from Aqueous Solution by a Hydrated Aluminum Oxide Modified Natural Zeolite, Chem. Eng. J., 2015, 271, 204–213, DOI:10.1016/j.cej.2015.03.003.
- M. Hermassi, C. Valderrama, N. Moreno, O. Font, X. Querol, N. Batis and J. L. Cortina, Powdered Ca-Activated Zeolite for Phosphate Removal from Treated Waste-Water, J. Appl. Chem. Biotechnol., 2016, 91(7), 1962–1971, DOI:10.1002/jctb.4867.
- S. Gubernat, A. Masłoń, J. Czarnota and P. Koszelnik, Reactive Materials in the Removal of Phosphorus Compounds from Wastewater—A Review, Materials, 2020, 13(15), 3377, DOI:10.3390/ma13153377.
- C. Penn, I. Chagas, A. Klimeski and G. Lyngsie, A Review of Phosphorus Removal Structures: How to Assess and Compare Their Performance, Water, 2017, 9(8), 583, DOI:10.3390/w9080583.
- O. Uzun, Z. Gokalp, H. A. Irik, I. S. Varol and F. O. Kanarya, Zeolite and Pumice-Amended Mixtures to Improve Phosphorus Removal Efficiency of Substrate Materials from Wastewaters, J. Cleaner Prod., 2021, 317, 128444, DOI:10.1016/j.jclepro.2021.128444.
- L. M. Soltys, I. F. Mironyuk, T. R. Tatarchuk and V. I. Tsinurchyn, Zeolite-Based Composites as Slow Release Fertilizers (Review), Phys. Chem. Solid State, 2020, 21(1), 89–104, DOI:10.15330/pcss.21.1.89-104.
- M. R. Maghsoodi, L. Ghodszad and B. Asgari Lajayer, Dilemma of Hydroxyapatite Nanoparticles as Phosphorus Fertilizer: Potentials, Challenges and Effects on Plants, Environ. Technol. Innovation, 2020, 19, 100869, DOI:10.1016/j.eti.2020.100869.
- D. Montalvo, M. J. McLaughlin and F. Degryse, Efficacy of Hydroxyapatite Nanoparticles as Phosphorus Fertilizer in Andisols and Oxisols, Soil Sci. Soc. Am. J., 2015, 79(2), 551–558, DOI:10.2136/sssaj2014.09.0373.
- L. Xiong, P. Wang, M. N. Hunter and P. M. Kopittke, Bioavailability and Movement of Hydroxyapatite Nanoparticles (HA-NPs) Applied as a Phosphorus Fertiliser in Soils, Environ. Sci.: Nano, 2018, 5(12), 2888–2898, 10.1039/C8EN00751A.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ew00753c |
| This journal is © The Royal Society of Chemistry 2023 |