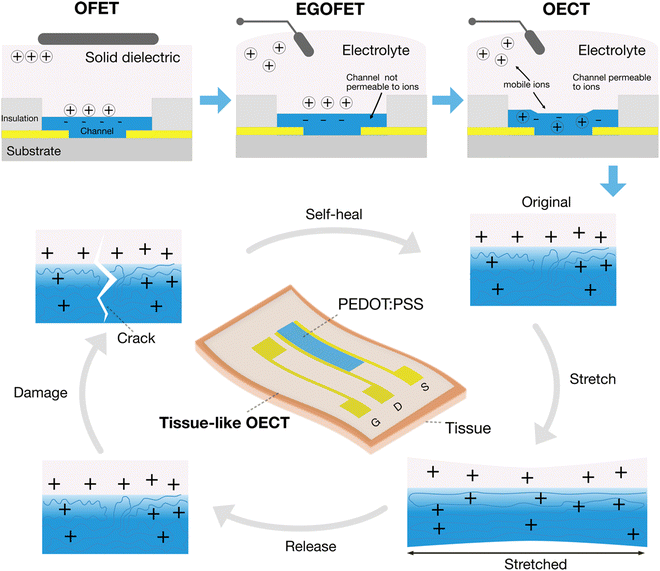
Tissue-like organic electrochemical transistors
Jing
Bai
,
Dingyao
Liu
,
Xinyu
Tian
and
Shiming
Zhang
 *
*
Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China. E-mail: szhang@eee.hku.hk
First published on 18th July 2022
Abstract
Organic bioelectronics has gone wild over the past few decades. As flagship devices, organic electrochemical transistors (OECTs) provide a new choice for next-generation bioelectronic devices because of their excellent water stability and ability to convert small ionic signals to stronger electronic signals (iontronic amplifiers) at extremely low voltage. However, device failure occurs when OECTs are introduced at tissue-electronic interfaces due to the mechanical mismatch between the stretchable tissues and the non-stretchable devices. Tissue-like OECTs aim to make a paradigm shift to unlock the potential of OECTs for applications at soft bioelectronic interfaces by acquiring the mechanical stretchable and healable properties of soft tissues. The stretchability allows OECTs to conform better with soft tissues under motion or deformation, while the self-healing ability permits fast recovery of the electronic functionality after accidental damage. In this article, we highlight the recent trends in developing tissue-like OECTs. Challenges in materials, devices, fabrications, and applications are summarized. Finally, we present our view on how tissue-like OECTs can serve as a new building block to impact exciting new areas, including wearables and implantable medical devices, e-skin, soft robotics, and soft neuroelectronics.
Bioelectronics is a fast-rising discipline involving biology, electronics, chemistry, materials science, and medicine.1,2 Bioelectronic devices are essential medical technologies for the early prediction, screening, diagnosis, and treatment of diseases. Examples include pacemakers, cochlear implants, and neuron probes.
When used for recording health-related signals in vivo, such as deep brain activities, bioelectronic devices are expected to have the following merits to promote their use in practical situations: (i) good biocompatibility to minimize the foreign body reaction. Organic electronics stand out here because they are naturally similar to human tissues.2 This advantage gives rise to the field of organic bioelectronics;2 (ii) high stability in body fluids. While very few organic bioelectronic materials can meet this need, PEDOT:PSS (poly(3,4-ethylenedioxythiophene) polystyrene sulfonate) is an exception;3 and (iii) high signal-to-noise ratio (SNR) to lower the limit of detection. In this regard, transistor transducers are used to amplify the signal in situ.4
The combination of the above three merits points to a fast-rising bioelectronic device, the organic electrochemical transistors (OECTs).5,6 In OECTs, the ionic electrolyte replaces the dielectric layer that is used in common field-effect transistors (FETs) (Fig. 1). The use of an electrolyte as the gating media lowers the operation voltage to less than 1 V due to the large capacitance of the electrical double layer formed at the interface between the electrolyte and a solid conductor. The low operation voltage permits its use inside the human body. It is called an electrochemical transistor, not a FET, because the current modulation is achieved by the electrochemical reaction of the channel. In the case of PEDOT:PSS-based OECTs, the conductivity of the PEDOT:PSS thin film channel can be manipulated through a reversible doping/de-doping process.
OECTs have distinct advantages over other biotransistors.7–9 First, both the oxidized state and reduced state of the PEDOT:PSS-based OECTs are stable in harsh environments, such as in water or at high temperatures. Second, the freely movable ions in the electrolyte can penetrate into the bulk of the channel. This step leads to a higher sensitivity (transconductance) over FETs because the bulk modulation allows the gate to control the conductance state of polymers along the whole channel thickness (volumetric doping), while a FET only allows surface doping.10 Third, the nature of electrophysiological signals of a living creature is an ionic activity, such as action potential, which is analogous to the operation of OECTs.
In addition to the above properties, the fabrication of OECTs is compatible with facile low-temperature printing techniques, making them competitive for large-scale production at a relatively low cost compared to conventional fabrication approaches. Altogether, these merits make OECTs a highly endorsed technology for bioelectronic applications such as biosensing circuits and emerging neuromorphic hardware.
Despite the success of OECTs in the past decade, an additional issue arises when they are introduced to the device/tissue interface: the inherent mechanical mismatch between the device and the tissue causes stability issues.11–13 For example, motion-induced displacement and delamination impair the stability and reliability of signaling and may damage the surrounding tissues.
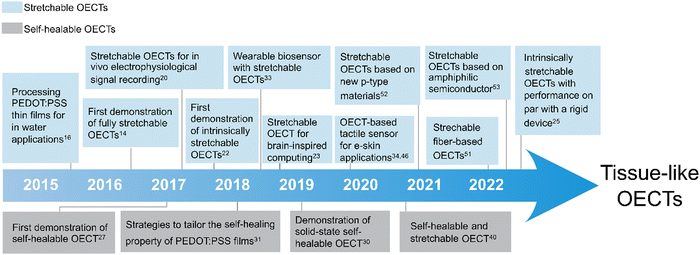
Tissue-like OECTs aim to develop soft, stretchable and healable OECTs to improve their interfacing with tissues (Fig. 1). Stretchable OECTs could minimize the mechanical mismatch toward improved signaling reliability during movement. Self-healing properties could increase the lifetime of the devices when suffering from accidental damages. Because of their potential applications in wearable and implantable medical devices, soft robotics, and soft neuroelectronics, tissue-like OECTs have taken shape in the past few years.
The major difference between tissue-like OECTs and conventional OECTs is the acquisition of additional mechanical properties of soft tissues. It includes the replacement of rigid materials with new low-modulus materials systems for device assembling, the design and fabrication strategies for higher stretchability, and the molecular-level design strategies for stretchability and healability. The ultimate tissue-like OECTs are expected to integrate materials of low modulus, high stretchability and reversible healability in one device, while maintaining high electrical performance and stability comparable to a rigid device. For material selection, in addition to low modulus, high stretchability, and molecular-level designed healability, biocompatibility and water-stability are also critically needed for practical body-centric wearable and implantable applications.
The rise of stretchable OECTs
While the deformation of a bent fiber was used to demonstrate a stretchable device,14 truly stretchable OECTs were missing until 201715 (Fig. 2). In this device architecture, PEDOT:PSS thin films were used as the channel material. PEDOT:PSS is a unique mixed ion-electron conductor where the π-conjugated systems in the PEDOT backbones provide conduction pathways for electrons, while the hydrophilic PSS backbones provide conduction pathways for ions. PEDOT chains and PSS chains entangle together.16 This unique structure leads to efficient ion-electron interaction and conversion once a gate voltage is applied. Before assembling fully stretchable OECTs, the composition of PEDOT:PSS was systematically investigated to ensure the resultant film can maintain stable electrical performance and stability in aqueous environments.17 Besides, a particular fluorinated photoresist, non-reactive with fragile organic electronic materials,18 was used to pattern high-quality PEDOT:PSS films on plastic and elastomers.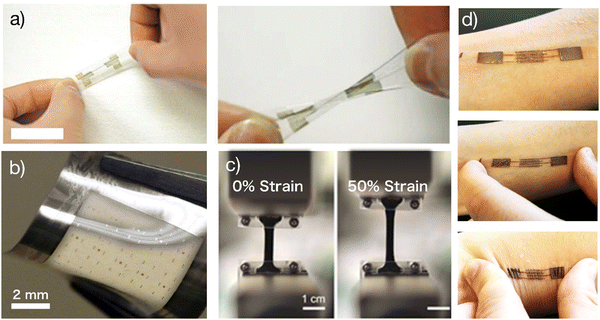 | ||
| Fig. 2 Stretchable OECTs: (a) Optical images of a stretchable OECT. Adapted with permission from ref. 22 copyright 2019 Wiley-VCH; (b) stretchable OECT microelectrodes arrays (10 × 10) with channel length down to 5 μm. Adapted with permission from ref. 14 copyright 2017 American Chemical Society; (c) strain test of the stretchable system. Adapted with permission from ref. 32 copyright 2019 Wiley-VCH; (d) stretchable OECT e-skin. Adapted with permission from ref. 33 copyright 2019 Wiley-VCH. | ||
The first fully stretchable tissue-like OECTs were demonstrated with the buckling method.15 A stretchable solid-state tough hydrogel was brought from other disciplines to endow the device with full stretchability.19 The adhesion between the hydrogel and the substrate was investigated to prevent delamination under strain. Orthogonal photolithography and parylene transfer-patterning manufacturing technologies were combined as the manufacturing strategy to enable the assembling of stretchable OECT microarrays with a channel length less than 10 μm. Afterward, alternative strategies were used to fabricate structurally stretchable OECTs, including the use of laser ablation to pattern metallic interconnections with stretchable serpentine structures20 and the creation of a stretchable honeycomb grid on ultrathin parylene substrates.21 Significantly, in vivo experiments of the latter one proved that, when used for monitoring the electrocardiogram (ECG) signals on the heart surface of rats, stretchable OECTs boosted the SNR to 52 dB because their high conformability helped suppress motion artifacts.21 These results are exciting as they experimentally confirmed that OECT technology does outperform other competitive bioelectronic technologies in obtaining a high SNR, not only under static conditions (52.7 dB)22 but also under moving conditions (52 dB) if a stretchable device can be used.21
While structurally stretchable OECTs are relatively easier to realize, there are potential limitations for practical applications because of the fragility of the artificially constructed stretchable structures. This problem drives the development of more tissue-like intrinsically stretchable OECTs.23,24 For intrinsically stretchable OECTs, the stretchability comes from the inherent mechanical properties of the materials rather than device structures.25 Even though intrinsic stretchability is a natural advantage of organic bioelectronics over other bioelectronic technologies, it remains challenging to assemble an intrinsically stretchable OECT, mainly because of the lack of mature materials systems that can show both suitable electrical properties and intrinsic stretchability, which are considered mutually exclusive.
The first intrinsically stretchable OECTs were demonstrated in 201923 (Fig. 2a). This is made possible by the strategical selection of suitable intrinsically stretchable materials to serve as metallic electrodes, semiconducting channels, hydrogel electrolytes, and insulating layers, respectively. Special efforts were dedicated to processing materials for better intrinsic stretchability. For the PEDOT:PSS channel, it is found that thinner films and lower baking temperature are essential to maximizing intrinsic stretchability on elastomers.23 For the metallic electrodes, reducing the thickness could help gain the intrinsic stretchability on an elastomer through the formation of microcracks.26 The resultant intrinsically stretchable OECTs maintained stable electrical performance under 30% strain (the maximum value the skin can be stretched to).
The demonstration of intrinsically stretchable OECTs further promotes the use of OECTs for soft bioelectronic applications. For example, it was later demonstrated that intrinsically stretchable OECTs can be used to make stretchable neuromorphic transistors to mimic the synaptic function of a neuron.24 Recently, the performance bottleneck of intrinsically stretchable OECTs has been successfully overcome. The first intrinsically stretchable PEDOT:PSS OECT with rigid-device-benchmarkable performance was developed by using low-oxygen permeable elastomers as substrates to improve the de-doping efficiency of PEDOT:PSS thin films.27 Intrinsically stretchable OECTs hold the potential to serve as a fundamental building block for developing e-skins, artificial neurons, and chip-level soft neuroelectronic systems, which may provide artificial intelligence for future soft robotics.
The rise of self-healable OECTs
Self-healing is a unique property of soft tissues. Self-healable electronics aim to repair damage autonomously, which can potentially increase their lifetime.28 The prerequisite to obtaining self-healable OECTs is to develop healability for these materials used for device assembling. The self-healing ability of a given material is usually demonstrated with a 3D shape of greater thickness. However, the OECT is a thin-film semiconductor device where the thickness of each functional layer is typically less than 1 μm, making it difficult to gain self-healing ability.It is observed that PEDOT:PSS thin films (as thin as 1 μm) damaged with a sharp blade can be electrically healed by simply wetting the damaged area with a water droplet29 (Fig. 3). The process is rapid, with a response time of 150 ms. Significantly, after being wetted, the films are transformed into autonomic self-healing materials without the need for external stimulation. This observation reveals the self-healing properties of PEDOT:PSS thin films which was later used to develop various self-healing organic bioelectronic devices.29–33
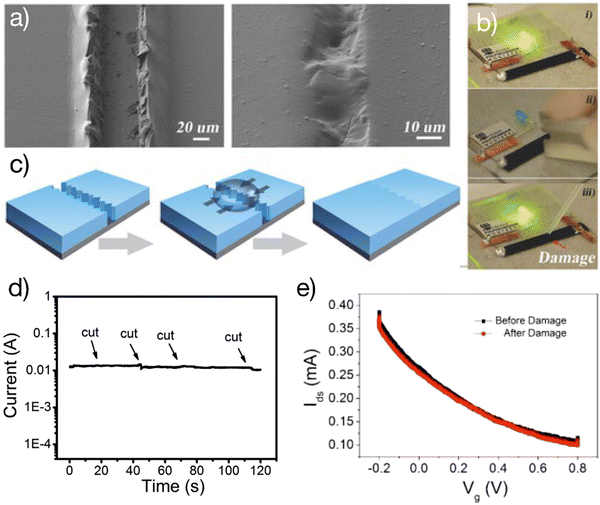 | ||
| Fig. 3 Self-healable OECTs: (a) optical images of the PEDOT: PSS channel before and after water-enabled healing; (b) the healing process restored the conductance of the film and switched on the light-emitting diode; (c) schematic of the water-enabled healing process; (d) real-time current measurement of the wetted PEDOT:PSS thin film upon cutting with razor blades; (e) demonstration of self-healable OECTs where the wetted PEDOT:PSS film was used as the channel. The transfer curve remained identical after damage. Adapted with permission from ref. 27 copyright 2017 Wiley-VCH. | ||
The first self-healing OECTs were demonstrated by using the wet PEDOT:PSS film as the channel material.29 The OECTs autonomically restored their electrical performance after cutting the channel with a razor blade. This work proves that autonomous self-healing ability is achievable in a thin-film semiconductor device (less than 1 μm of thickness). The ability to obtain the self-healing ability in OECTs of smaller film thicknesses has several advantages. For example, there is no need to sacrifice the electrical performance such as the response time and on/off ratio, which happens in devices with greater channel thicknesses.
All-solid-state self-healable OECTs were later demonstrated by processing the PEDOT:PSS channel with a surfactant.32 The device showed a high on/off ratio of above 1000, a high transconductance of 54 mS, and a fast response time of less than 10 ms, promoting their use for practical bioelectronic applications.
The self-healing ability and the stretchability were later combined in an injectable PEDOT:PSS hydrogel fiber.34 These ultrathin fibers were fabricated through a syringe-extrusion method. The fiber is capable of being used as the channel material for OECTs. The freeze-drying method was used to induce a porous microscopic structure in the fiber to facilitate ion penetration into the channel for facile doping/de-doping. The wetted fiber is capable of being stretchable up to 20% of its initial length with healability due to the dynamic hydrogen bonding.
Tissue-like OECTs for soft bioelectronic applications
Because of the potential for soft bioelectronic applications, tissue-like OECTs have become the research interest of more and more research groups in Canada,15,29 the United States,34–37 China,38,39 Japan,21 Korea,40,41 Singapore,32,37,42 Italy,20etc. Emerging research directions include, but not limited to, the design and processing of stretchable materials systems,23,32,42 the development of advanced manufacturing technologies,23,43 interface engineering,27 the optimization of device performance and stability,27,42 and the efforts toward system-level integration (Fig. 4).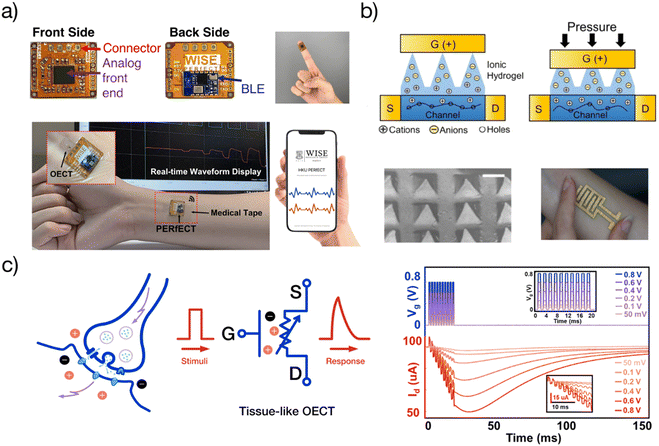 | ||
| Fig. 4 Applications of tissue-like OECTs: (a) a coin-sized wearable analytical unit (PERfECT platform) for wireless characterization of OECTs and similar low-voltage transistors. Adapted with permission from ref. 36 copyright 2022 American Chemical Society; (b) tissue-like stretchable OECTs for pressure sensor and e-skin applications. Adapted with permission from ref. 34 copyright 2021 Institute of Electrical and Electronics Engineers; (c) demonstration of tissue-like OECTs for soft neuroelectronic applications. Adapted with permission from ref. 36 copyright 2022 American Chemical Society. | ||
Wearable and implantable biosensing circuits
Tissue-like OECTs can find several applications in wearable and implantable biosensing. They have already been used as stretchable microelectrode arrays to improve the ECG signaling quality in vivo.44 Another example is to develop soft brain probes for disease monitoring and brain-machine interfaces.45 In addition to obtaining a higher SNR, the stretchable OECT probe has the potential to minimize the motion artifacts and avoid tissue damage under dynamic motion. Tissue-like OECTs can also find applications in developing smart bandages for wound management.46 The improved mechanical properties allow seamless integration with soft bandage. Besides, stretchable OECTs can be used as surgically implanted spinal stimulation devices to help paralyzed people recover.47 For in vivo bioelectronic applications, tissue-like OECTs are supposed to seamlessly and harmlessly interface with the living tissues and organs such as human brain, which favors the use of materials with lower modulus. For specific applications like brain probes, the stretchability of the device should achieve about 10% with modulus < 100 kPa.13,44 Advanced soft fabrication strategies are needed to enable the fabrication of micro OECT arrays for multiplexed signal detection. Last but not least, extreme biocompatibility is required for in vivo uses. Bio-derived elastomers or tissue-derived hydrogels are emerging materials for such applications to facilitate the development of living electrodes or living OECTs.To make a truly wearable system, a compact and wireless OECT readout unit is indispensable. For practical use, the size should be compatible with a smartwatch so that they can find more applications. The challenge in developing such a unit is how to maintain high accuracy during system miniaturization. This conflict was not solved until the recent development of a wireless OECT readout unit, namely “personalized electronic reader for electronic and chemical transistors (PERfECT)”38 (Fig. 4a). The coin-sized PERfECT chip has a high resolution of current reading (1 nA), high sampling rates (200 KSPS), and can characterize the overall performance of low voltage transistors including OECTs. PERfECT can measure 32 OECTs simultaneously and has been commercialized by SESIC Co., Ltd.38 Wireless and truly wearable systems have been demonstrated with stretchable OECTs for the detection of glucose concentrations.
Soft electronic skin
Skin is organic, stretchable, self-healable, and processes signals electrochemically. Besides, its high sensitivity is realized at an ultralow voltage. Tissue-like OECTs possess all these unique properties and thus can serve as a new device paradigm to mimic the function of the skin. Skin-inspired sensitive pressure sensors have been demonstrated based on soft OECTs using a microstructured hydrogel electrolyte36 (Fig. 4b). The pressure sensor can detect a subtle pressure as low as 20 Pa, with a low power consummation of less than 10 μW. There is plenty of room to explore the potential of tissue-like OECTs further to mimic the skin's multi-point and multi-modal functions,48 finally reaching a point where thin-film OECT e-skins can be used for soft robotics and the non-invasive measure of human activities under natural conditions.For e-skin applications, conformability is currently a major concern. The elastic bending energy (EE) can be used to evaluate the conformability of tissue-like OECTs on a soft substrate, which is described by the Elasto-capillarity model shown as following equations:49
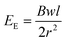 | (1) |
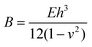 | (2) |
Decreasing the thickness is straightforward, but the decrease of the modulus is limited by the use of the conducting polymer (e.g., PEDOT:PSS) which has a higher modulus. One way to decrease the modulus of the conducting polymer is to blend it with softer stretchability enhancers. The other way is to use low-modulus substrates, while maintaining a high stretchability of PEDOT:PSS OECTs by strain engineering.
Tissue-like OECTs focusing on e-skin applications are expected to obtain a stretchability of at least 30% (maximum value of skin deformation).13,23 Higher stretchability (i.e., >30%) should not be overemphasized as it is likely not required for most practical applications. Another concern is the skin irritation issue, which raises the demand for biocompatibility and breathability. In addition, transparency, water-stability, and self-adhesive properties are also needed in specific application scenarios.
Soft neuroelectronics hardware
Brain-inspired computing is challenging the conventional von Neumann systems because of its energy efficiency and the ability to sense, process, and actuate in a single entity.50 Tissue-like OECTs allow the development of softer neuromorphic computing devices by replacing the current non-stretchable systems. Examples have been shown of using stretchable OECTs to demonstrate a stretchable synapse37,40 (Fig. 4c). Along this line, tissue-like OECTs may facilitate the development of more neuron-like devices because of their similar working mechanism. They own the potential for developing soft neuroelectronic devices and systems for soft artificial neural networks (ANNs), wearable edge computing, and soft robotic applications. For computing applications, the performance of tissue-like OECTs are expected to be comparable with the rigid device. For one thing, they can be better integrated with the rigid-device based circuits. For the other, higher performance (such as higher on/off ratio) may increase the number of the conductance states for advanced in-memory computing applications.Conclusion
In conclusion, we have highlighted tissue-like OECTs for soft bioelectronics applications (Fig. 5). We see several opportunities where stretchable and healable OECTs can facilitate the development of advanced wearable and implantable biosensors, e-skins, and soft robotics. Moreover, we demonstrate that tissue-like OECTs could be a new paradigm for making soft artificial neurons for soft neuroelectronics because of their greater similarities in terms of the material properties, the working mechanism, and the ability to harvest high sensitivity at low voltage.For tissue-like OECT research, it is essential to form a balanced research team with interdisciplinary backgrounds including semiconductor devices, electrochemistry, soft and healable electronic materials, advanced manufacturing, and mechanics (Fig. 6). Besides, electronic engineers and software engineers are indispensable to enabling their integration for translational applications. Communication between disciplines can be challenging at the beginning. However, once the code is decrypted, the high synergy of an interdisciplinary team will be a powerful engine to address specific challenges by decoding it into languages that all can understand.
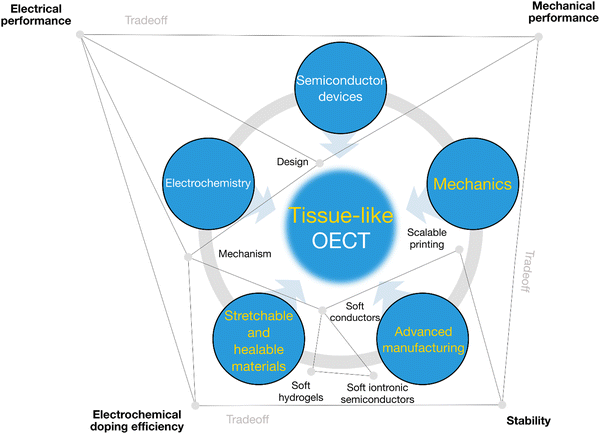 | ||
| Fig. 6 The transdisciplinary approaches toward tissue-like OECTs. Synergetic engineering efforts are required, including semiconductor device physics, electrochemistry, stretchable and healable materials,52,53 advanced manufacturing, and device mechanics. Specific investigations are needed to balance the electrical performance, electrochemical performance, mechanical performance, cyclic stability, water stability and lifetime. | ||
While pushing the paradigm shift from flexible/fragile to stretchable/healable is encouraging, many challenges exist, ranging from materials, interfaces, device performance, manufacturing, and prototyping for practical applications. Some examples are: (i) intrinsic stretchability needs to be obtained for all units without sacrificing too much of electrical properties; (ii) it is challenging to realize scalable manufacturing of tissue-like soft OECTs with conventional microfabrication technologies. This conflict can be mitigated by using printing technologies to process and pattern those soft materials on a case-by-case basis;51 (iii) poor adhesion between layers of soft bioelectronic devices deteriorates the device's performance and stability. Interface engineering is a must to stabilize the system, and customizable design should be available to expand their applications for personalized uses. Besides, non-living electrodes and the limited biomimetic functions of tissue-like OECTs prevent their diverse applications. Therefore, living, biocompatible electrodes and more artificially constructed bionic features still need to be developed.
Despite these challenges, we believe tissue-like OECTs have a bright future because of the rise of soft implantables, soft wearables, and soft robotics. Tissue-like OECTs could be a key supplement to the toolbox of current soft bioelectronic technologies. Beyond these exciting areas, the study of tissue-like OECTs itself is important to extend our understanding and knowledge on this technology, such as their maximum mechanical potential.
Finally, we wish to pass some remarks to newcomers. Tissue-like OECTs is a highly transdisciplinary topic (Fig. 6). While it is easy to demonstrate a device, it is a long journey before one can truly advance the boundary because of those challenges mentioned above. Before starting, it is suggested to conduct a detailed study on the fundamentals of OECTs first, followed by chasing the frontline of soft semiconductors (conducting polymers), soft electrolytes (ion-gels, hydrogels), soft conductors, and design and fabrication strategies for stretchable electronics. Once you are truly engaged in a topic that is cutting the edge, you will gradually enjoy the fulfillment of each day's progress. This should be the same for all scientific research.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. Z. acknowledges the Startup Fund, the Innovation Wing Two Research Fund, the Seed Funding for Strategic Interdisciplinary Research Scheme from the University of Hong Kong (HKU), and The Innovation and Technology Fund (Mainland-Hong Kong Joint Funding Scheme, MHP/053/21) and Shenzhen-Hong Kong-Macau Technology Research Programme (SGDX20210823103537034) for supporting this work.References
- T. Someya, Z. Bao and G. G. Malliaras, The rise of plastic bioelectronics, Nature, 2016, 540, 379–385 CrossRef CAS PubMed.
- J. Rivnay, R. I. M. Owens and G. G. Malliaras, The rise of organic bioelectronics, Chem. Mater., 2014, 26, 679–685 CrossRef CAS.
- G. Dijk, A. L. Rutz and G. G. Malliaras, Stability of PEDOT: PSS-coated gold electrodes in cell culture conditions, Adv. Mater. Technol., 2020, 5, 1900662 CrossRef CAS.
- P. Lin and F. Yan, Organic thin-film transistors for chemical and biological sensing, Adv. Mater., 2012, 24, 34–51 CrossRef CAS PubMed.
- D. Nilsson, et al., Bi-stable and dynamic current modulation in electrochemical organic transistors, Adv. Mater., 2002, 14, 51–54 CrossRef CAS.
- J. Rivnay, et al., Organic electrochemical transistors, Nat. Rev. Mater., 2018, 3, 1–14 CrossRef.
- P. R. Paudel, J. Tropp, V. Kaphle, J. D. Azoulay and B. Lüssem, Organic electrochemical transistors – from device models to a targeted design of materials, J. Mater. Chem. C, 2021, 9, 9761–9790, 10.1039/D1TC01601F.
- H. Sun, J. Gerasimov, M. Berggren and S. Fabiano, n-Type organic electrochemical transistors: materials and challenges, J. Mater. Chem. C, 2018, 6, 11778–11784, 10.1039/C8TC03185A.
- E. R. W. van Doremaele, P. Gkoupidenis and Y. van de Burgt, Towards organic neuromorphic devices for adaptive sensing and novel computing paradigms in bioelectronics, J. Mater. Chem. C, 2019, 7, 12754–12760, 10.1039/C9TC03247A.
- J. Rivnay, et al., Organic electrochemical transistors, Nat. Rev. Mater., 2018, 3, 17086, DOI:10.1038/natrevmats.2017.86.
- J. Kim, R. Ghaffari and D.-H. Kim, The quest for miniaturized soft bioelectronic devices, Nat. Biomed. Eng., 2017, 1, 1–4 CrossRef.
- Y. Jiang, et al., Topological supramolecular network enabled high-conductivity, stretchable organic bioelectronics, Science, 2022, 375, 1411–1417 CrossRef CAS PubMed.
- B. V. Khau, A. D. Scholz and E. Reichmanis, Advances and opportunities in development of deformable organic electrochemical transistors, J. Mater. Chem. C, 2020, 8, 15067–15078, 10.1039/D0TC03118F.
- J. T. Kim, et al., Three-dimensional writing of highly stretchable organic nanowires, ACS Macro Lett., 2012, 1, 375–379 CrossRef CAS PubMed.
- S. Zhang, et al., Patterning of Stretchable Organic Electrochemical Transistors, Chem. Mater., 2017, 29, 3126–3132, DOI:10.1021/acs.chemmater.7b00181.
- J. Rivnay, et al., Structural control of mixed ionic and electronic transport in conducting polymers, Nat. Commun., 2016, 7, 1–9 Search PubMed.
- S. Zhang, et al., Solvent-induced changes in PEDOT: PSS films for organic electrochemical transistors, APL Mater., 2015, 3, 014911 CrossRef.
- S. Zhang, et al., Water stability and orthogonal patterning of flexible micro-electrochemical transistors on plastic, J. Mater. Chem. C, 2016, 4, 1382–1385 RSC.
- J.-Y. Sun, et al., Highly stretchable and tough hydrogels, Nature, 2012, 489, 133–136 CrossRef CAS PubMed.
- B. Marchiori, R. Delattre, S. Hannah, S. Blayac and M. Ramuz, Laser-patterned metallic interconnections for all stretchable organic electrochemical transistors, Sci. Rep., 2018, 8, 1–9 CAS.
- W. Lee, et al., Nonthrombogenic, stretchable, active multielectrode array for electroanatomical mapping, Sci. Adv., 2018, 4, eaau2426 CrossRef CAS PubMed.
- D. Khodagholy, et al., In vivo recordings of brain activity using organic transistors, Nat. Commun., 2013, 4, 1–7 Search PubMed.
- S. Zhang, et al., Tuning the electromechanical properties of PEDOT: PSS films for stretchable transistors and pressure sensors, Adv. Electron. Mater., 2019, 5, 1900191 CrossRef.
- N. Matsuhisa, et al., High-transconductance stretchable transistors achieved by controlled gold microcrack morphology, Adv. Electron. Mater., 2019, 5, 1900347 CrossRef.
- D. Liu, X. Tian, J. Bai, Y. Wang, Y. Cheng, W. Ning, P. K. L. Chan, K. Wu, J. Sun and S. Zhang, Intrinsically Stretchable Organic Electrochemical Transistors with Rigid-Device-Benchmarkable Performance, Adv. Sci., 2022, 2203418, DOI:10.1002/advs.202203418.
- S. P. Lacour, S. Wagner, Z. Huang and Z. Suo, Stretchable gold conductors on elastomeric substrates, Appl. Phys. Lett., 2003, 82, 2404–2406 CrossRef CAS.
- S. Zhang and F. Cicoira, Flexible self-powered biosensors, Nature, 2018, 561, 466–467 CrossRef CAS PubMed.
- J. Kang, J. B.-H. Tok and Z. Bao, Self-healing soft electronics, Nat. Electron., 2019, 2, 144–150 CrossRef.
- S. Zhang and F. Cicoira, Water-Enabled Healing of Conducting Polymer Films, Adv. Mater., 2017, 29, 1703098 CrossRef PubMed.
- S. Kee, M. A. Haque, D. Corzo, H. N. Alshareef and D. Baran, Self-healing and stretchable 3D-printed organic thermoelectrics, Adv. Funct. Mater., 2019, 29, 1905426 CrossRef CAS.
- L. V. Kayser, et al., RAFT polymerization of an intrinsically stretchable water-soluble block copolymer scaffold for PEDOT, Chem. Mater., 2018, 30, 4459–4468 CrossRef CAS PubMed.
- J. Ko, X. Wu, A. Surendran, B. T. Muhammad and W. L. Leong, Self-Healable Organic Electrochemical Transistor with High Transconductance, Fast Response, and Long-Term Stability, ACS Appl. Mater. Interfaces, 2020, 12, 33979–33988 CrossRef CAS PubMed.
- Y. Li, et al., Tailoring the Self-Healing Properties of Conducting Polymer Films, Macromol. Biosci., 2020, 20, 2000146 CrossRef CAS PubMed.
- S. Zhang, et al., Room-temperature-formed PEDOT: PSS hydrogels enable injectable, soft, and healable organic bioelectronics, Adv. Mater., 2020, 32, 1904752 CrossRef CAS PubMed.
- S. Zhang, et al., Hydrogel-enabled transfer-printing of conducting polymer films for soft organic bioelectronics, Adv. Funct. Mater., 2020, 30, 1906016 CrossRef CAS.
- X. Wang, et al., A Sub-1-V, microwatt power-consumption iontronic pressure sensor based on organic electrochemical transistors, IEEE Electron Device Lett., 2020, 42, 46–49 Search PubMed.
- N. Matsuhisa, et al., High-Transconductance Stretchable Transistors Achieved by Controlled Gold Microcrack Morphology, Adv. Electron. Mater., 2019, 5, 1900347, DOI:10.1002/aelm.201900347.
- X. Tian, et al., Pushing OECTs toward Wearable: Development of a Miniaturized Analytical Control Unit for Wireless Device Characterization, Anal. Chem., 2022, 94(16), 6156–6162, DOI:10.1021/acs.analchem.1c05210.
- Y. Li, N. Wang, A. Yang, H. Ling and F. Yan, Biomimicking Stretchable Organic Electrochemical Transistor, Adv. Electron. Mater., 2019, 5, 1900566, DOI:10.1002/aelm.201900566.
- T. D. Nguyen, T. Q. Trung, Y. Lee and N.-E. Lee, Stretchable and Stable Electrolyte-Gated Organic Electrochemical Transistor Synapse with a Nafion Membrane for Enhanced Synaptic Properties, Adv. Eng. Mater., 2021, 2100918 Search PubMed.
- S. Bontapalle, M. Na, H. Park and K. Sim, Fully soft organic electrochemical transistor enabling direct skin-mountable electrophysiological signal amplification, Chem. Commun., 2022, 58, 1298–1301 RSC.
- X. Su, et al., A Highly Conducting Polymer for Self-Healable, Printable and Stretchable Organic Electrochemical Transistor Arrays and Near Hysteresis-Free Soft Tactile Sensors, Adv. Mater., 2022, 2200682 CrossRef CAS PubMed.
- X. Wu, et al., Universal spray-deposition process for scalable, high-performance, and stable organic electrochemical transistors, ACS Appl. Mater. Interfaces, 2020, 12, 20757–20764 CrossRef CAS PubMed.
- W. Lee, et al., Nonthrombogenic, stretchable, active multielectrode array for electroanatomical mapping, Sci. Adv., 2018, 4, eaau2426, DOI:10.1126/sciadv.aau2426.
- I. R. Minev, et al., Electronic dura mater for long-term multimodal neural interfaces, Science, 2015, 347, 159–163, DOI:10.1126/science.1260318.
- A. McLister, J. McHugh, J. Cundell and J. Davis, New Developments in Smart Bandage Technologies for Wound Diagnostics, Adv. Mater., 2016, 28, 5732–5737, DOI:10.1002/adma.201504829.
- B. J. Woodington, et al., Electronics with shape actuation for minimally invasive spinal cord stimulation, Sci. Adv., 2021, 7, eabg7833, DOI:10.1126/sciadv.abg7833.
- S. Chen, A. Surendran, X. Wu and W. L. Leong, Contact Modulated Ionic Transfer Doping in All-Solid-State Organic Electrochemical Transistor for Ultra-High Sensitive Tactile Perception at Low Operating Voltage, Adv. Funct. Mater., 2020, 30, 2006186, DOI:10.1002/adfm.202006186.
- M. Vomero, et al., Conformable polyimide-based μECoGs: Bringing the electrodes closer to the signal source, Biomaterials, 2020, 255, 120178, DOI:10.1016/j.biomaterials.2020.120178.
- Y. van de Burgt, A. Melianas, S. T. Keene, G. Malliaras and A. Salleo, Organic electronics for neuromorphic computing, Nat. Electron., 2018, 1, 386–397, DOI:10.1038/s41928-018-0103-3.
- M. Lerond, W. G. Skene and F. Cicoira, Enhancing the performance of transparent and highly stretchable organic electrochemical transistors by acid treatment and copolymer blending of electrospun PEDOT:PSS fibers, J. Mater. Chem. C, 2022 10.1039/D2TC01134D.
- Y. Dai, et al., Stretchable Redox-Active Semiconducting Polymers for High-Performance Organic Electrochemical Transistors, Adv. Mater., 2022, 34, 2201178, DOI:10.1002/adma.202201178.
- J. Chen, et al., Highly stretchable organic electrochemical transistors with strain-resistant performance, Nat. Mater., 2022, 21, 564–571, DOI:10.1038/s41563-022-01239-9.
| This journal is © The Royal Society of Chemistry 2022 |