Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Concluding remarks: Carbon dioxide utilization: where are we now?… and where are we going?
Walter
Leitner
 *ab and
Marc
Schmitz
ab
*ab and
Marc
Schmitz
ab
aMax Planck Institute for Chemical Energy Conversion, Stiftstr. 34–36, 45470 Mülheim an der Ruhr, Germany. E-mail: walter.leitner@cec.mpg.de; marc.schmitz@cec.mpg.de
bInstitut für Technische und Makromolekulare Chemie (ITMC), RWTH Aachen University, Worringer Weg 2, 52074 Aachen, Germany
First published on 5th July 2021
Abstract
This publication is reminiscent of the 12 principles of CO2 chemistry as formulated in the first Faraday Discussion on CO2 utilization in 2015. Their visionary significance at the time is brought into context with the current developments in society and industry. “What has changed since then?” and “is our enthusiasm still enough?” are only a few questions that are to be answered in the following from today’s perspective. The synergy of the use of carbon dioxide (CCU) with the concepts of green chemistry as well as the connection to the energy sector is demonstrated using selected examples from industry and research.
Introduction
Setting the scene
A first Faraday Discussion on carbon dioxide utilization was held in 2015. Unfortunately, I was unable to participate in person as I had ruptured my cruciate ligament in a skiing accident a couple of weeks earlier. However, I stayed in touch with Martyn Poliakoff and Emilia Streng during the meeting and we phrased the “12 principles of CO2 chemistry” (Fig. 1), which are modeled according to the 12 principles of green chemistry.1 In a typical Martyn Poliakoff approach, they are associated with a mnemonic where the letters of “CO2 chemistry” are defining some guidelines or goals around challenges and opportunities when using carbon dioxide as raw material. Now, six years later, we have intensively discussed the progress made since then and analyzed the field again. Based on the outcomes we may ask ourselves whether these twelve principles are still valid and if they are reflected in recent developments. I do not want to go through each principle individually, but I will rather pick a few examples from science and application that I consider representative for the concepts to structure the presentation. I will start not with a letter, but with the number “2”. It reads “tomorrow’s world may be different” – which fits very well because we are six years down the road. Let us see what has changed, what remains the same, and what we can learn for the future.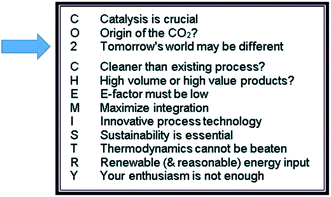 | ||
| Fig. 1 The 12 principles of CO2 chemistry as derived from the Faraday Discussion in 2015.1 | ||
“2”: tomorrow’s world may be different
In his introductory lecture (DOI: 10.1039/D1FD00029B), Volker Sick really pointed out nicely that grasping the full potential of CO2 utilization requires looking into the future, maybe more than in the present, and certainly more than in the past. When the concept of carbon dioxide utilization was envisaged initially under the terminology “carbon capture and utilization, CCU”, it was very much focusing on the question: “how can we get rid of a maximum amount of CO2for a very long period?” This was seen as part of carbon dioxide mitigation strategies for fossil carbon, to dump it somewhere while doing something useful with it. Thus, all the carbon that was targeted at the time was “gray” as removing waste CO2 from the fossil energy system was considered the goal. Incidentally, this fits well with the color of the graphic below taken from a review that we wrote at the time (Fig. 2, left).2 In essence, this strategy is part of global efforts for “de-carbonization”. Wherever fossil resources are still used, we need to get rid of the carbon dioxide afterwards once is generated as waste.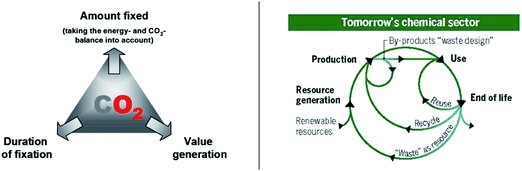 | ||
| Fig. 2 (Left) Carbon capture and utilization as part of CO2 mitigation strategies: “de-carbonization”;2 (right) closed carbon cycles through power-to-X technologies: “de-fossilization”.3 | ||
Today, however, we can look at the potential of CO2 also from a different perspective, because the energy sector is already becoming increasingly decarbonized through the deployment of energy from renewable resources on scale. In various parts of the world, it is already economically preferred to generate electricity from renewable resources. This opens new possibilities for technologies that utilize carbon from non-fossil resources defining a path to “de-fossilization” for those areas, where direct electrification is impossible or not practical. Any carbon required by the chemical sector, including liquid fuels, should come back into the circle at the end of its life (Fig. 2, right).3 So now the question is: “how can we exploit CO2as part of carbon circularity?”.
“R”: renewable (& reasonable) energy input; “O”: origin of the CO2
Fig. 2 is probably the most important slide of the whole presentation reflecting the most important change over the recent years. The closed carbon cycles obviously require the input of additional energy, otherwise we would discuss a perpetuum mobile. We need renewable energy to make the carbon go around and such value circles – rather than value chains – require what we call today “power-to-X” technologies (Fig. 3). In essence, power-to-X technologies harness renewable energy for sectors where we will depend on carbon also in a post-fossil age and CO2 does play an important role as feedstock in this future vision. They are thus directly related to the letters “R” and “O” in the twelve principles of “CO2 CHEMISTRY”.Producing carbon-based liquid fuels can harvest electricity from renewable resources in form of transportable and easily distributed high-density energy carriers. The existing global infrastructure can be used to bring green electrons in form of chemical bonds to the place where they are needed in the mobility and transport sector. We can also make chemical products that are required in an ever-increasing amount to fulfil the needs of a growing global population. There are indispensable existing markets, and we can of course also develop new products with improved functions as Volker Sick has pointed out nicely.
But let us not forget that CCU is not the only option for power-to-X scenarios. We have also other renewable carbon sources such as biomass and plastic waste. So how does CO2 compare to those? This poses new challenges for life cycle analysis as was very nicely reflected in André Bardow’s presentation (DOI: 10.1039/D0FD00134A) for example. It is no longer about getting rid of CO2, we need to identify the frameworks where it is the best carbon source to lower the carbon footprint as compared to the petrochemical value chain. Thus, CO2 utilization provides a trajectory into the area of closed anthropogenic carbon cycles. But if we combine renewable energy with carbon dioxide, should we focus only on high volume products because that takes up a lot of CO2? Or can we also exploit the potential for high value products and make them go in cycles? And what does this mean for other sustainability metrics? These questions lead us directly to the letters “H” and “E”.
“H”: high volume or high value products; “E”: E-factor must be low
One area that we have discussed at this meeting is to capture CO2 by mineralization, e.g., in readily available minerals or inorganic waste. As the formation of inorganic carbonates from CO2 is an exergonic process, the required process energy for grinding, mixing, etc., can even be over-compensated by the significant thermodynamic driving force. In Fig. 4, I have borrowed a graphic from Alissa Park’s presentation (DOI: 10.1039/D1FD00022E) where it is obvious that the feedstocks that are available in terms of minerals and basic waste can bind large amounts of CO2.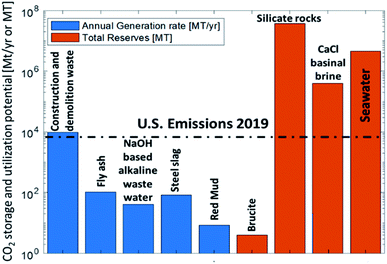 | ||
| Fig. 4 “CO2 capture and utilization in minerals and wastes”, adapted from A. Park, Faraday Discussion Lecture, April 8, 2021, DOI: 10.1039/D1FD00022E. | ||
That is good news. We have a large potential to mitigate CO2 in these materials to fix a lot of CO2 and store it there for a long time, addressing at least two of the corners in the graphic on the left of Fig. 2. There has been impressive scientific progress in the last decade, but many fundamental research questions like the kinetics of these processes on microscopic and macroscopic scale are yet to be unraveled. There is also commercial experience as we heard from the talk of Colin Hills (DOI: 10.1039/D0FD00142B), but some important questions are still open. For the LCA analysis, the systems boundary for the CO2 balance are very important. In the Q&A session it was argued that there is obviously a net CO2 consumption in the process, but that the CO2 balance should in future also be related to conventional minerals that serve the same function. Where will the formed carbonates find their application and what other materials would they replace? How can the concept of circularity improve the sustainability of the building sector? There is clearly a great potential in this area, which may go beyond the amount of waste that can take up CO2 by looking at it also from a product point of view to embrace the idea of a circular economy.
When considering products from the organic chemistry industry, cyclic and polymeric carbonates are species that also remain at the same oxidation level of the carbon +IV and incorporate the entire CO2 molecule (Fig. 5). Like the inorganic carbonates from mineralization, the corresponding reactions have an intrinsic thermodynamic driving force if epoxides are used as reaction partners. For cyclic ethylene and propylene carbonate, these reactions are used industrially already for a long time. More recently, the reaction of propylene oxide and CO2 could also be effectively directed to the production of polymer building blocks commercialized under the trade name Cardyon® by the company Covestro.4,5 A detailed LCA for this product confirms the direct effect of the incorporated CO2 on the “de-fossilization” by partial replacement of propylene oxide from today’s purely petrochemical product.4 In line with Volker Sick’s argument in the introduction (DOI: 10.1039/D1FD00029B), a number of other environmental parameters were improved concomitantly through the reduced dependence on oil-based raw materials. The two components are directly coupled in a fully atom economic reaction under solvent-free conditions resulting in an almost zero E-factor.
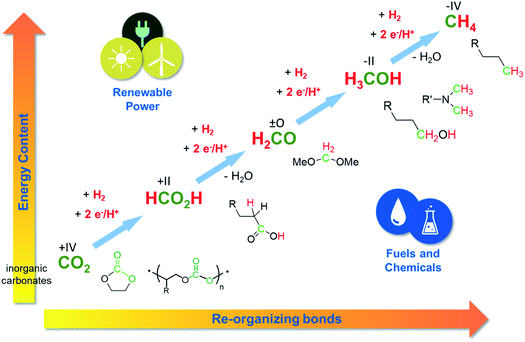 | ||
| Fig. 5 CCU and power-to-X technologies open access to chemical diversity based on CO2. Adapted from ref. 6. | ||
As the chemical conversion of CO2 targets the C1 products formic acid, formaldehyde, methanol, or methane, it changes the oxidation level at carbon to +II, 0, −II and −IV, respectively. The necessary reducing agents can be “green” electrons7 or hydrogen.8 These reagents “pump up” the energy of the system. If they come from a renewable source of power, significant positive effects on the carbon balance and on the CO2-emissions can be achieved.
We have seen many presentations both in the posters as well as in the talks that deal with C1 products including carbon monoxide, which is at the same level as formic acid, or methanol. But mind you, there are many interesting chemical structures out there that could also be formed from CO2 and hydrogen, which are more complex than the single C1 compounds. Fig. 5 exemplifies this molecular playground that we can tap into if we manage to not only reduce the CO2 but also to reorganize chemical bonds at the same time. In this scenario, we can look at CO2 and renewable energy not “only” from the perspective of climate protection, but also as basis for innovative technologies to make fuels and chemicals.
Let us have a look at alcohols as an important class of chemical compounds to exemplify this argument. In the petrochemical value chain, C1 methanol is produced on very large volumes, while longer chain alcohols are produced in lower volumes, but are of high value.
Already today “power to methanol” or CCU-based methanol production is on a very high technology readiness level (TRL) (Fig. 6). A well-known example is the plant of Carbon Recycling International, which operates in Iceland on a kiloton scale, and the company recently announced that they have filed some contracts with China to go up in scale to around 100 kilotons per year. The Carbon2Chem project of ThyssenKrupp Industrial Solutions in Germany utilizes the flue gases from a steel mill as feedstock. The impressive size of fossil-based world scale methanol plants, where mega methanol technologies operate at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year capacity, demonstrate the huge potential for this single product. But with “green” methanol, we could even do a lot of additional things beyond the existing market. It can be used directly as a fuel or to produce hydrocarbon fuels like in the methanol to gasoline process (MtG) and the methanol to kerosene process. Olefins can be produced as chemical feedstocks via the methanol-to-olefin process (MTO). All these technologies are at high technology readiness levels, and many have been demonstrated already on large scale by the petrochemical industry as part of their efforts to use either coal or natural gas instead of crude oil. The big challenge here is economics: but the power-to-X technologies are becoming increasingly competitive with these fossil-based value chains, especially if regulations such as carbon tax or CO2 trading systems are installed in global markets.
000 tons per year capacity, demonstrate the huge potential for this single product. But with “green” methanol, we could even do a lot of additional things beyond the existing market. It can be used directly as a fuel or to produce hydrocarbon fuels like in the methanol to gasoline process (MtG) and the methanol to kerosene process. Olefins can be produced as chemical feedstocks via the methanol-to-olefin process (MTO). All these technologies are at high technology readiness levels, and many have been demonstrated already on large scale by the petrochemical industry as part of their efforts to use either coal or natural gas instead of crude oil. The big challenge here is economics: but the power-to-X technologies are becoming increasingly competitive with these fossil-based value chains, especially if regulations such as carbon tax or CO2 trading systems are installed in global markets.
 | ||
| Fig. 6 Examples for “power-to-methanol” and today’s fossil-based technology on full scale (the pictures are taken from the cited websites). | ||
Longer chain alcohols such as butanol (C4) or hexanol (C6) are produced today over several steps from fossil-based olefins. Interestingly, they can be synthesized directly from CO or syngas (CO/H2) via biotechnology using fermentation processes. As part of the Kopernikus project “P2X”, the syngas fermentation technology developed by EVONIK is coupled to the low-temperature co-electrolysis of Siemens (Fig. 7). At a piloting facility in Marl, Germany, an electrolyzer converts CO2 and water to generate syngas which is fed into the fermenter where the whole cell biocatalysts digest it to form hexanol. The company Beiersdorf, well-known for its brand “Nivea”, is now testing these products as potential replacements for oil-based alcohols in formulations for consumer care applications.
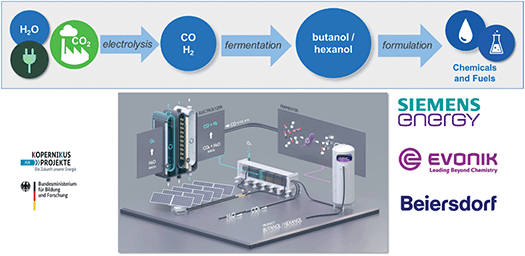 | ||
| Fig. 7 Production of longer chain alcohols via a combination of CO2 electrolysis and fermentation.9,10 | ||
The successful coupling of electrolysis and fermentation leads us directly to the points “maximize integration” and “innovative process technology” corresponding to letters “M” and “I” in our mnemonic.
“M”: maximize integration; “I”: innovative process technology
Carbon dioxide from flue gases as rather concentrated point source will be available also in “post-fossil” times (i.e. from cement industries, chemical plants, or biomass processing). However, taking CO2 from the air can be envisaged for decentralized local productions, where renewable energy is amply available, but no industrial CO2 source is present. Direct air capture (DAC) technologies are researched world-wide. A fully integrated container module comprising the whole production line from DAC to liquid fuels has been developed at the Karlsruhe Institute of Technology (KIT) in collaboration with several start-up companies and SMEs. The container integrates a DAC unit, a solid oxide high temperature co-electrolyzer to make syngas, and a reactor for the Fischer–Tropsch reaction to generate hydrocarbons. Depending on the final downstream processing unit, various “power-to-liquid” products can be obtained (Fig. 8).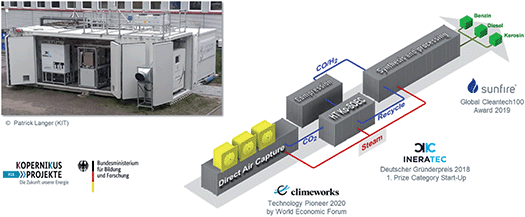 | ||
| Fig. 8 A fully integrated modular system from direct air capture to “power-to-liquid” products.11 | ||
While the technology can also be scaled up, it seems primarily useful for local production using a numbering up approach. Possibilities include isolated areas with heavy-duty machinery, remote airports, or offshore wind platforms. The idea of “crowd oil not crude oil”11 is intriguing, if the robustness of the modules in such realistic environments can be demonstrated.
“C”: cleaner than existing process; “T”: thermodynamics cannot be beaten
While the most obvious prospect of CO2 utilization appears to funnel it into the chemical value chain at the origin, its potential to improve the carbon balance over the existing process may be significantly larger if used as directly as possible for the synthesis of the actual product. This is illustrated here by the production of formic acid, a target molecule that has been studied in this area for a very long time.12,13 The typical petrochemical value chain of formic acid starts from methane to generate carbon monoxide that is first converted to methyl formate which is hydrolyzed to formic acid under recycling of the methanol. Using CO2 as alternative feedstock could be envisaged at each of these process stages, as shown in Fig. 9: to produce methane via the Sabatier reaction, to produce CO via the reverse water gas shift (RWGS) reaction, or in a direct hydrogenation to give formic acid. The analysis of the team of André Bardow (DOI: 10.1039/D0FD00134A) clearly shows that the reduction of the global warming potential is the highest by far in the direct production scenario. As we change the focus from “what is the largest sink for CO2?” to “how to gain the highest reduction in carbon footprint?”, the limiting factor will be the hydrogen and hence the electricity from renewable energy sources.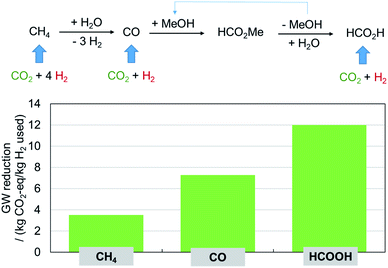 | ||
| Fig. 9 “Power-to-what?”: formic acid as example.14,15 | ||
However, the formic acid reaction suffers from thermodynamic limitations, even though it looks a very simple reaction on paper. The choice of solvent and addition of base are important factors to overcome the thermodynamic barrier for this reaction because it is endergonic under standard conditions. Researchers at BASF came up with a very elegant and clever solution to isolate the free formic acid after it is formed in the presence of a solvent and base.16 The overall energy requirement may be reduced further if CO2 could be applied in scrubbing solutions directly for this reaction. Capturing CO2 from flue gases or other point sources is typically done by absorption in aqueous amine solutions: so, the solvent and base needed for thermodynamic reasons is already there. Rather than absorbing the CO2, setting it free as gas, purifying it and compressing it for the chemical process, would save significant process energy if one could use it directly in these scrubbing solutions. And yes, this works! Recently different groups published about various amine-based solvent systems where the hydrogenation to formic acid can be conducted directly in that scrubbing solution.16–19 All these studies are still done with commercial CO2 as available in research laboratories, and it would be very interesting to see how that works with CO2 taken from real point sources, to see if and how the catalysts would be compatible with that.
This leads us directly to the next two points: catalysts are the key enabler for CO2 conversion to chemical products according to the letter “C”. Their environmental footprint will also impact on the overall sustainability defined in letter “S” as processes move from the lab to large scale applications.
“C”: catalysis is crucial; “S”: sustainability is essential
There is currently a lot of interest to use the 3d earth abundant metals instead of the noble metals of the platinum group in catalysis.20 For CO2 conversion, this may seem like “back to the future”. Many catalytic processes relevant in the context of power-to-X have always been carried out with earth abundant metals: the Fischer–Tropsch process uses iron and cobalt, while the methanol process uses copper and zinc on alumina. Homogeneous catalysis started with the use of cobalt for syngas conversion in industrial hydroformylation. However, the noble metals have just shown extremely good performance in the second half of the 20th century and proved superior in many processes. So, what can we do? We need to learn mechanistically from platinum group metal chemistry and try to transfer this mechanistic knowledge back to the 3d metals while considering their differences, of course. We heard several excellent presentations at this meeting many inspired by nature as she also uses the 3d metals for the interconversion of energy and materials. Therefore, fundamental studies, even without direct application in sight, are crucial to feed that knowledge back to the development of catalysis technology.And almost nowhere is this more important than in electrocatalysis. The electrocatalytic conversion of CO2 is a direct way of using the electrons rather than to make hydrogen as a reducing agent. Ten years ago, it would have been difficult to get a lot of catalyst development in this area covered in a conference, but today it would be easy to fill several days of a conference with only that subject. Specifically, heterogeneous catalysis, supported catalysis as well as molecular catalysis are currently of great scientific interest.7,21–23 Of course, the catalyst is only one component in electrocatalytic conversion and we heard very nice presentations that highlighted that we also need to look at the electrolyte, at the cell, and beyond to the overall system integration. There seems to be a large potential, however, to consider the molecular diversity highlighted in Fig. 5 also for electrocatalytic CO2 conversion and target more complex products than just formate and CO. And surely the catalyst will be the key component to open such reaction pathways by controlling the necessary bond breaking and bond forming events.
“Y”: your enthusiasm is not enough!
Finally, after a couple of exciting days of discussion about the chemistry, the catalysis, the processes, and the systems analysis in this area, we also realize that Martyn Poliakoff was quite right when he assigned the letter “Y” to the statement: “your enthusiasm is not enough!” Let us see why.Volker Sick argued in his presentation (DOI: 10.1039/D1FD00029B) that the rapidly growing number of publications indicates that the field provides an environment to reach technical maturity for applications because there is such a large body of scientific knowledge as reflected in the data shown in Fig. 10. And do not interpret this graph to think there was no research on CO2 conversion before 1980. On the contrary! The book “Carbon Dioxide Activation by Metal Complexes” written by Arno Behr – which I strongly recommend: try to get hold of a copy of this book – was published in 1988 and has over 800 references.24 It provides an excellent background to many of the things that we still discuss today.
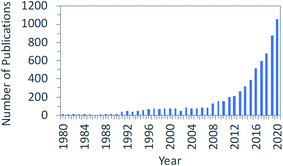 | ||
| Fig. 10 Increasing scientific knowledge and technical maturity, adapted from V. Sick, Faraday Discussion Introductory Lecture, April 7, 2021, DOI: 10.1039/D1FD00029B. | ||
I fully agree with Volker!
I will try to explain why for the example of copolymerization between epoxides and CO2 to give polyols, as mentioned earlier. This reaction was observed for the first time a long time ago by the Japanese chemist Inoue in 1969.25 This was the year when the first man landed on the moon! And there we are, chemists know already about the principle possibility to achieve copolymerization of carbon dioxide with epoxides. Later, in the 90s the field got very, very active.26–28 Many catalysts were synthesized, and the mechanistic understanding was growing rapidly through experimental and theoretical studies. This knowledge allowed for the development of advanced catalysts that enabled an almost perfect alternating co-polymerization to build high molecular weight polymers with a nearly exact 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio of epoxide and CO2.
50 ratio of epoxide and CO2.
So why do I say that this enthusiasm was not enough?
The decisive factor that finally led to commercialization of this reaction was a partnership between academia and industry.29 The knowledge about the required material properties that make the molecules suitable as products in the market was the missing link. The ultimate products for the consumer are polyurethane plastics and the polyols as main components are made today from propylene oxide alone by homo-polymerization. So, the driving force for the industrial development was not to use propylene oxide to capture CO2 but to use CO2 to replace the propylene oxide. This is very important: the benefit of the CO2 technology does not rely on the amount of CO2 that is in the product, it comes from the amount of fossil resources that is avoided. Consequently, the aim is not to incorporate the maximum, but rather the optimum amount of CO2. With incorporation in the range up to ca. 20% of CO2 the polyols turn out to still be very useful for the polyurethanes and the final products. Now the producers of the end products have access to this material at a scale of 5000 tons per year and they will ultimately make the final decision. The technology now is ready, but whether this goes up to a world scale production will depend ultimately on market acceptance. It was very good to see that consumer acceptance was also topic at this conference.
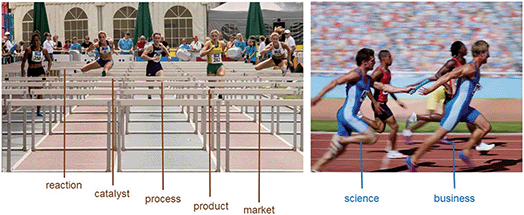
The science and technology around CO2 utilization resembles a hurdle race (Fig. 11). As chemists, we can develop new reactions and effective catalysts, and together with engineers transfer this knowledge into innovative processes. While many of these ideas and developments will drop out early, we can be confident that some of them will make it to the finishing line. But the race isn’t over then: it is then about the product and whether the product comes into the market that makes the difference. At this stage, we must hand over the baton from science and technology to business.
The bad news is: this is very, very difficult and succeeds only in very few cases.
The good news is: science is running fast so we may expect many more opportunities for business to get started!
Conclusions
I will just conclude with a very simple and very positive message: tomorrow will be different and carbon dioxide utilization will be an important pillar of a more sustainable future. It will not just be a bridging technology as part of CO2 mitigation, but it reaches far beyond because it enables closed carbon cycles. That is why it is so important for climate protection: capitalizing on CO2 as feedstock gives us a chance to make carbon-based products by harnessing renewable energy, enabling the de-fossilization of industrial sectors that fulfil crucial requirements of a growing population on this planet.Max Planck said: “insight must proceed application”. Five years ago, we may have analyzed some aspects of CO2 utilization differently, but the 12 principles phrased at the time are still valid. And with every CO2-based technology that will reach the market, we might look back with Nelson R. Mandela and think: “it always seems impossible, only until it’s done”.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to express our sincere thanks to all speakers and chairs at this year’s Faraday Discussion on Carbon Dioxide Utilization for their contributions and discussions. The same goes for all sponsors and funding agencies. Finally, of course, the Royal Society of Chemistry for hosting this event. Further we gratefully acknowledge basic support by the Max Planck Society and the RWTH Aachen University. Parts of the shown work were also supported by the Kopernikus Project P2X: Flexible use of renewable resources—exploration, validation, and implementation of “power-to-X” concepts (FKZ: 03SFK2A0-2) funded by the German Federal Ministry of Education and Research (BMBF). Open Access funding provided by the Max Planck Society.References
- M. Poliakoff, W. Leitner and E. S. Streng, Faraday Discuss., 2015, 183, 9–17 RSC.
- M. Peters, B. Kohler, W. Kuckshinrichs, W. Leitner, P. Markewitz and T. E. Müller, ChemSusChem, 2011, 4, 1216–1240 CrossRef CAS PubMed.
- J. B. Zimmerman, P. T. Anastas, H. C. Erythropel and W. Leitner, Science, 2020, 367, 397–400 CrossRef CAS PubMed.
- R. Meys, A. Katelhon and A. Bardow, Green Chem., 2019, 21, 3334–3342 RSC.
- Cardyon®: A polyol that makes environmental sense, Covestro AG, Leverkusen, 2021, available from https://solutions.covestro.com/en/brands/cardyon?gclid=EAIaIQobChMIrpbtwdSH8QIVDM93Ch0DAAVREAAYASAAEgL3l_D_BwE, accessed 8 Jun 2021 Search PubMed.
- J. Klankermayer and W. Leitner, Philos. Trans. R. Soc., A, 2016, 374, 629–630 CrossRef PubMed.
- N. W. Kinzel, C. Werle and W. Leitner, Angew. Chem., Int. Ed., 2021, 60, 11628–11686 CrossRef CAS PubMed.
- J. Klankermayer, S. Wesselbaum, K. Beydoun and W. Leitner, Angew. Chem., Int. Ed., 2016, 55, 7296–7343 CrossRef CAS PubMed.
- T. Haas, R. Krause, R. Weber, M. Demler and G. Schmid, Nat. Catal., 2018, 1, 32–39 CrossRef CAS.
- Kopernikus Power-to-X, BMBF, Germany, 2021, available from https://www.kopernikus-projekte.de/en/projects/p2x; https://www.kopernikus-projekte.de/en/projects/rheticus, accessed 8 Jun 2021 Search PubMed.
- R. Dittmeyer, M. Klumpp, P. Kant and G. Ozin, Nat. Commun., 2019, 10, 1818 CrossRef PubMed.
- W. Leitner, Angew. Chem., Int. Ed. Engl., 1995, 34, 2207–2221 CrossRef CAS.
- P. G. Jessop, T. Ikariya and R. Noyori, Chem. Rev., 1995, 95, 259–272 CrossRef CAS.
- J. Artz, T. E. Muller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Chem. Rev., 2018, 118, 434–504 CrossRef CAS PubMed.
- A. Sternberg and A. Bardow, Energy Environ. Sci., 2015, 8, 389–400 RSC.
- T. Schaub and R. A. Paciello, Angew. Chem., Int. Ed., 2011, 50, 7278–7282 CrossRef CAS PubMed.
- M. Scott, B. Blas Molinos, C. Westhues, G. Franciò and W. Leitner, ChemSusChem, 2017, 10, 1085–1093 CrossRef CAS PubMed.
- J. Kothandaraman, A. Goeppert, M. Czaun, G. A. Olah and G. S. Prakash, Green Chem., 2016, 18, 5831–5838 RSC.
- D. Wei, H. Junge and M. Beller, Chem. Sci., 2021, 12, 6020–6024 RSC.
- R. M. Bullock, J. G. Chen, L. Gagliardi, P. J. Chirik, O. K. Farha, C. H. Hendon, C. W. Jones, J. A. Keith, J. Klosin, S. D. Minteer, R. H. Morris, A. T. Radosevich, T. B. Rauchfuss, N. A. Strotman, A. Vojvodic, T. R. Ward, J. Y. Yang and Y. Surendranath, Science, 2020, 369, eabc3183 CrossRef CAS PubMed.
- M. N. Jackson and Y. Surendranath, Acc. Chem. Res., 2019, 52, 3432–3441 CrossRef CAS PubMed.
- A. D. Handoko, F. X. Wei, Jenndy, B. S. Yeo and Z. W. Seh, Nat. Catal., 2018, 1, 922–934 CrossRef CAS.
- F. Franco, C. Rettenmaier, H. S. Jeon and B. Roldan Cuenya, Chem. Soc. Rev., 2020, 49, 6884–6946 RSC.
- A. Behr, Carbon Dioxide Activation by Metal Complexes, John Wiley & Sons Incorporated, 1988 Search PubMed.
- S. Inoue, H. Koinuma and T. Tsuruta, Makromol. Chem., 1969, 130, 210 CrossRef CAS.
- M. Cheng, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 1998, 120, 11018–11019 CrossRef CAS.
- M. R. Kember, A. Buchard and C. K. Williams, Chem. Commun., 2011, 47, 141–163 RSC.
- J. Langanke, A. Wolf, J. Hofmann, K. Böhm, M. Subhani, T. Müller, W. Leitner and C. Gürtler, Green Chem., 2014, 16, 1865–1870 RSC.
- Finalists European Inventor Award, Munich, Germany, 2021, available from https://www.epo.org/news-events/events/european-inventor/finalists/2021/gurtler.html, accessed 8 Jun 2021 Search PubMed.
| This journal is © The Royal Society of Chemistry 2021 |