Electrical conductivity and conduction mechanisms in (Na0.5Bi0.5TiO3)1−x(BiScO3)x (0.00 ≤ x ≤ 0.25) solid solutions
Abstract
The electrical properties of (Na0.5Bi0.5TiO3)1−x(BiScO3)x (NBT-BS, 0.00 ≤ x ≤ 0.25) solid solutions are established by ac impedance spectroscopy and electromotive force transport number measurements. The bulk conductivity decreases with increasing BS incorporation but the oxide-ion transport number remains high (>0.85) over a wide compositional range 0.00 ≤ x ≤ 0.15 and drops to ∼0.7 for x ≥ 0.20. NBT-BS solid solutions can only present either predominant oxide-ion conduction or mixed ionic-electronic conduction behaviour, indicating that oxide-ion conduction cannot be fully eliminated by incorporation of BS. This is in contrast from our previous study where incorporation of ∼7% BiAlO3 (BA) can fully suppress the oxide-ion conduction in NBT. The conductivity–composition relationships of NBT-BS solid solutions are attributed to a competing effect from lattice expansion, which enlarges the channel for oxygen ion migration, with trapping between B-site acceptor ions, 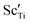 , and oxygen vacancies,
, and oxygen vacancies, 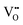 , which decreases oxygen ion migration. Comparisons between NBT-BS, NBT-BA and NBT-BiGaO3 (BG) solid solutions suggest that small acceptor ions on the B-site are more effective in trapping oxygen vacancies and consequently more effective to suppress the oxide-ion conduction and thus reduce dielectric loss at elevated temperatures.
, which decreases oxygen ion migration. Comparisons between NBT-BS, NBT-BA and NBT-BiGaO3 (BG) solid solutions suggest that small acceptor ions on the B-site are more effective in trapping oxygen vacancies and consequently more effective to suppress the oxide-ion conduction and thus reduce dielectric loss at elevated temperatures.

- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...