Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solar H2 evolution in water with modified diketopyrrolopyrrole dyes immobilised on molecular Co and Ni catalyst–TiO2 hybrids†
Julien
Warnan‡
a,
Janina
Willkomm‡
a,
Jamues N.
Ng
a,
Robert
Godin
 b,
Sebastian
Prantl
b,
James R.
Durrant
b and
Erwin
Reisner
b,
Sebastian
Prantl
b,
James R.
Durrant
b and
Erwin
Reisner
 *a
*a
aChristian Doppler Laboratory for Sustainable SynGas Chemistry, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, CB2 1EW, UK. E-mail: reisner@ch.cam.ac.uk; Web: http://www-reisner.ch.cam.ac.uk
bDepartment of Chemistry, Imperial College London, Exhibition Road, London, SW7 2AZ, UK
First published on 3rd February 2017
Abstract
A series of diketopyrrolopyrrole (DPP) dyes with a terminal phosphonic acid group for attachment to metal oxide surfaces were synthesised and the effect of side chain modification on their properties investigated. The organic photosensitisers feature strong visible light absorption (λ = 400 to 575 nm) and electrochemical and fluorescence studies revealed that the excited state of all dyes provides sufficient driving force for electron injection into the TiO2 conduction band. The performance of the DPP chromophores attached to TiO2 nanoparticles for photocatalytic H2 evolution with co-immobilised molecular Co and Ni catalysts was subsequently studied, resulting in solar fuel generation with a dye-sensitised semiconductor nanoparticle system suspended in water without precious metal components. The performance of the DPP dyes in photocatalysis did not only depend on electronic parameters, but also on properties of the side chain such as polarity, steric hinderance and hydrophobicity as well as the specific experimental conditions and the nature of the sacrificial electron donor. In an aqueous pH 4.5 ascorbic acid solution with a phosphonated DuBois-type Ni catalyst, a DPP-based turnover number (TONDPP) of up to 205 was obtained during UV-free simulated solar light irradiation (100 mW cm−2, AM 1.5G, λ > 420 nm) after 1 day. DPP-sensitised TiO2 nanoparticles were also successfully used in combination with a hydrogenase or platinum instead of the synthetic H2 evolution catalysts and the platinum-based system achieved a TONDPP of up to 2660, which significantly outperforms an analogous system using a phosphonated Ru tris(bipyridine) dye (TONRu = 431). Finally, transient absorption spectroscopy was performed to study interfacial recombination and dye regeneration kinetics revealing that the different performances of the DPP dyes are most likely dictated by the different regeneration efficiencies of the oxidised chromophores.
Introduction
Utilising solar energy to split water for the production of renewable hydrogen (H2) is a promising strategy to satisfy our demand for sustainable and storable energy.1–3 Dye-sensitised photocatalysis (DSP) has emerged as a functional bio-inspired approach for sunlight-driven H2 evolution in water by means of co-immobilising a dye and a catalyst on a semiconductor in suspension (Fig. 1a–c),4 and this approach can also be adopted in dye-sensitised photoelectrosynthesis cells.4–13 DSP systems can be readily assembled through simultaneous attachment of an anchor-bearing molecular photosensitiser and H2 evolution catalyst to the surface of an inorganic wide-band gap semiconductor such as TiO2.4,6 The semiconductor displays dual functionality as it acts as a scaffold for co-immobilisation of the dye and catalyst and, importantly, enables efficient charge separation and accumulation of multiple long-lived, low-potential electrons for catalytic fuel generation.4,14 Thus, DSP systems can be regarded as a self-assembled triadic architecture that demonstrates a greater functionality than previously reported homogeneous molecular structures with the added benefit of straightforward assembly from readily available molecular and semiconductor components.15–19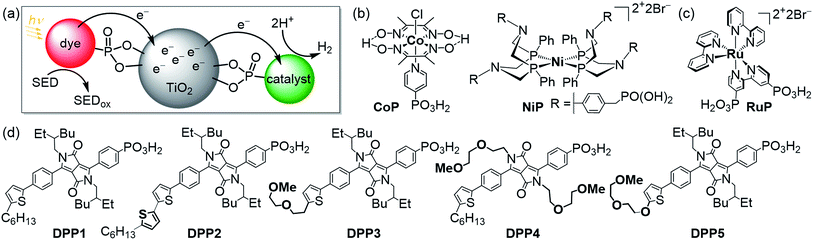 | ||
| Fig. 1 (a) Schematic representation of dye-sensitised photocatalysis (DSP) with a dye and H2 evolution catalyst co-immobilised onto TiO2 nanoparticles via a phosphonate anchoring group (i.e., dye|TiO2|catalyst assemblies).4 (b) Chemical structures of the molecular H2 evolution catalysts NiP and CoP (a hydrogenase and Pt were also employed as catalysts; see text),20,21 (c) the dye RuP,22 and (d) DPP dyes developed in this study (see Scheme 1 for synthetic route). | ||
To date, DSP has often employed precious metal-containing dyes such as the phosphonated ruthenium tris(bipyridine)-based dye RuP (Fig. 1c) anchored to a semiconductor.20–23 Despite having beneficial features such as a broad absorption band, a metal-to-ligand charge transfer transition and long-lived charge separated state, ruthenium-dyes challenge future scale-up and low cost applications due to their scarcity, modest molar absorption and the relative lack of simple fine-tuning. Such limitations were also experienced in the past with dye-sensitised solar cell technology, where ruthenium dyes provided benchmark performances for more than a decade.24 Recently, organic chromophores (π-conjugated systems) have reached photovoltaic efficiencies of approximately 13% and thereby surpassed Ru-containing photosensitisers.25 This is notably due to several advantages of the metal-free chromophores in terms of tunability and strong π–π* transitions. These dyes have been carefully optimised in terms of electronic properties, side chains and engineering of anchoring groups to control the charge transfer processes at the interface with the semiconductor and the redox mediator in an organic electrolyte solution.26 Organic dyes are promising candidates for H2 evolution via DSP if they can demonstrate efficient operation in aqueous solution. Light-driven H2 evolution with organic dyes in combination with a metal oxide semiconductor has been previously reported, but these systems required either a Pt co-catalyst, a p-type semiconductor electrode, organic solvents or an anchor-free diffusional dye.8,27–30 Only few studies are available with organic chromophores under DSP conditions and even less with commonly used aqueous electron donors, such as triethanolamine (TEOA) or ascorbic acid (AA), or with a molecular catalyst in a semi-heterogeneous photocatalytic scheme.11,31–34
Herein, we report the preparation of five phosphonic acid-containing diketopyrrolopyrrole (DPP) photosensitisers bearing different side-chains and electronically active substituents (Fig. 1d). DPP was chosen as chromophore because of its numerous advantages such as well-established synthesis, adjustable photophysical properties and high performances as already reported in optoelectronic devices such as organic transistors and organic and hybrid solar cells.35–37
Rational modification of the chromophore architecture provides decisive information for the future preparation of new organic dyes capable of competing with and surpassing the efficiency of Ru dye-based systems. Phosphonic acid was chosen as the anchoring functionality because of its strong attachment to metal oxide surfaces under acidic and pH neutral conditions, whilst allowing electron injection from the excited state of the photosensitiser into the conduction band of TiO2.38 To the best of our knowledge, these are the first examples of phosphonic acid bearing DPP chromophores, highlighting the chemical compatibility of these two key chemical moieties. The DPP dyes were evaluated using a variety of techniques such as UV-Vis and fluorescence spectroscopy as well as electrochemistry. The photocatalytic activity of the DPP chromophores on TiO2 nanoparticles was studied in the presence of the molecular complexes CoP or NiP (Fig. 1b) as well as a hydrogenase and Pt as H2 evolution catalysts in an aqueous sacrificial reaction medium under UV-filtered simulated solar light irradiation.20,21 Finally, charge separation and dye regeneration kinetics were investigated by transient absorption spectroscopy. The dye RuP (Fig. 1c) was also evaluated under the same conditions to assess the performance of the DPP dyes in comparison to previously established DSP systems.4
Results and discussion
Synthesis of DPP dyes
The synthesis of the DPP dyes is summarised in Scheme 1. The diphenyl DPP core was prepared following a previously described method and consists of a pseudo-Stobbe condensation of 1-bromo-4-cyanobenzene with diethyl succinate.39 The N-alkylation of the lactams was subsequently achieved in the presence of potassium carbonate and the branched alkyl 1-bromo-2-ethylhexane (Br-2EH) or the linear alkyl 1-bromo-2-(2-methoxyethoxy)ethane (Br-ME), affording compounds 1 or 2, respectively.40,41 These side chains were chosen to increase the general solubility of the dye in organic solvents. Yet, they differ in nature and polarity with the 2EH chain being expected to provide more hydrophobicity and bulkiness to the chromophore. Compounds 1 and 2 were desymmetrised to give the thiophenyl compounds 7 to 11 upon Suzuki–Miyaura or Stille cross-coupling reactions with 1 equiv. of the thiophene derivatives 3 to 6 (see ESI† for chemical structures) in the presence of [Pd(PPh3)4]. The thiophenyl derivatives 7 to 11 were isolated in moderate yield after purification (28–38%) due to a statistical distribution in cross-coupling products (formation of mono- and bis-coupled adducts). | ||
| Scheme 1 Synthetic route to DPP dyes: (i) [Pd(PPh3)4], Na2CO3, THF/H2O, 16 h, 70 °C; (ii) [Pd(PPh3)4], toluene, 16 h, 80 °C; (iii) HPO(OEt)2, [Pd(PPh3)4], Et3N, THF, microwave, 120 °C, 0.5 h; (iv) (a) bromotrimethylsilane, DCM, 12 h, r.t. and (b) MeOH/DCM, 2 h, r.t. See ESI† for experimental details and chemical structures of compounds 3–6. | ||
The phosphonic acid anchoring group was added in two steps. A micro-wave assisted Hirao cross-coupling reaction was performed in the presence of diethyl phosphite to give compounds 12 to 16, followed by hydrolysis of the corresponding phosphonic esters using bromotrimethylsilane and methanol (MeOH) to give DPP1 to DPP5 in good yields. All dyes were soluble in common organic solvents such as MeOH, dichloromethane and tetrahydrofuran and sufficiently soluble in aqueous buffer solutions to allow for their immobilisation on the metal oxide surfaces. The detailed synthetic procedures and characterisation of all compounds (high resolution mass spectrometry, elemental analysis, FT-IR, 1H, 13C & 31P NMR spectroscopy) are available in the ESI.†
Electronic absorption spectroscopy
To assess their electronic properties and the impact of the chemical modifications, electronic absorption spectra of the novel DPP dyes were recorded in solution and after chemisorption on transparent mesoporous TiO2 films on glass slides (Table 1, Fig. 2 and S1–S3†). N,N-Dimethylformamide (DMF) was first used as a strong solubilising solvent for solution spectra. In this polar and aprotic solvent, all diphenyl-based DPP chromophores display a strong characteristic absorption centred around 490 nm (εDPP > 1.5 × 104 M−1 cm−1 at λ = 490 nm, Fig. 2a and S1†), matching the solar spectrum maximum intensity wavelength, with all dyes absorbing strongly between 400 and 550 nm.| Dye | λ max (ε)/nm (M−1 cm−1) | E 00 /eV | E(S+/S)b/V vs. NHE | E(S+/S*)b,c/V vs. NHE | ΔGinjd/eV | ΔGrege/eV | ||
|---|---|---|---|---|---|---|---|---|
| pH 4.5 | pH 7.0 | AA | TEOA | |||||
| a E 00 = (1240/λabs–fluo) with λabs–fluo available in ESI (Table S1†). b S = ground state of the sensitiser, S* = excited state of the sensitiser, S+ = oxidised sensitiser. c E(S+/S*) = E(S+/S) − E00. d ΔGinj calculated from the equation: ΔGinj = E(S+/S*) − ECB(TiO2) with ECB(TiO2) = −0.70 V vs. NHE at pH = 7 and ECB(TiO2) = −0.55 eV vs. NHE at pH = 4.5.43,44 e ΔGreg calculated from the equation: ΔGreg = −(E(S+/S) − E(SED+/SED)) with E(SED+/SED)AA = 0.20 V vs. NHE45 and E(SED+/SED)TEOA = 0.82 V vs. NHE.46 | ||||||||
| DPP1 | 489 (2.0 × 104) | 2.32 | 1.15 | −1.17 | −0.62 | −0.47 | −0.95 | −0.33 |
| DPP2 | 496 (2.6 × 104) | 2.27 | 1.10 | −1.17 | −0.62 | −0.47 | −0.90 | −0.28 |
| DPP3 | 490 (2.3 × 104) | 2.32 | 1.19 | −1.13 | −0.58 | −0.43 | −0.99 | −0.37 |
| DPP4 | 489 (1.7 × 104) | 2.33 | 1.17 | −1.16 | −0.61 | −0.46 | −0.97 | −0.35 |
| DPP5 | 494 (1.7 × 104) | 2.30 | 1.01 | −1.29 | −0.74 | −0.59 | −0.81 | −0.19 |
| RuP | 457 (1.1 × 104) | 1.90 (ref. 42) | 1.37 | −0.78 (ref. 42) | −0.23 | −0.08 | −1.17 | −0.55 |
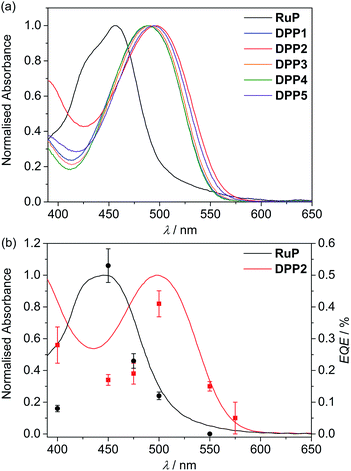 | ||
| Fig. 2 UV-Vis absorption spectra of (a) DPP and RuP in DMF solution (see Fig. S1†) and (b) DPP2 (red trace) and RuP (black trace) adsorbed on a thin mesoporous TiO2 film at room temperature. The wavelength-dependent EQE values obtained for RuP|TiO2|NiP (black circles) and DPP2|TiO2|NiP (red squares) are also shown. EQE conditions: 2.5 mg TiO2, 0.025 μmol of NiP, 0.05 μmol of DPP2 or RuP in aqueous AA solution (3 mL, 0.1 M, pH 4.5), 25 °C, 3.03 or 3.15 mW cm−2 (see text). | ||
In a polar protic solvent such as MeOH, no significant spectral differences were observed between DPP1, DPP3 and DPP4 as their structures only differ in electronically inactive side-chains (Fig. S2a†). However, slight bathochromic shifts were observed for the DPP5 and DPP2 absorption maxima as a result of the strong electron-donating O-substituted side-chain and increased conjugation length from the second thiophene unit, respectively. More substantial spectral differences were observed between the different DPP dyes in non-polar aprotic solvents such as toluene (Fig. S2b†). Due to enhanced molecular solubility, DPP2 features a sharper absorption peak in toluene than in MeOH, whereas the more hydrophilic DPP4 exhibits a broader absorption most likely due to dye aggregation/organisation. Such behaviour indicates that the side-chains' nature/polarity directly affects its interaction with the media, thereby potentially modulating electronic communication with the electrolyte components (e.g. proton source or electron donating reagent).
Immobilisation of the DPP photosensitisers on TiO2 films allowed for better insight into the absorption ability of the light-harvesting system in a state closer to the photocatalytic DSP conditions. As a result of a slight dye aggregation, absorption peaks are marginally broadened towards higher wavelengths, reaching 575 nm in the case of DPP2 (Fig. S3†). Among all dyes, DPP2 displays the broadest light-harvesting window, which potentially allows more photons to be collected, and consequently gives rise to a higher electron injection probability. As a comparison, the phosphonated Ru-dye RuP exhibits strong absorption close to the UV region with a sharp onset around 515 nm (Fig. 2a and S3†). The maximum intensity of the metal-to-ligand charge transfer (MLCT) transition in RuP was recorded at 457 nm, with a weaker molar absorption intensity (εRuP ≈ 1.1 × 104 M−1 cm−1) compared to the DPP dyes.
Emission spectroscopy
In order to gain information about the dyes' singlet excited state (S1), the steady-state photoluminescence of the DPP dyes was recorded in DMF solution at room temperature. The zero–zero excitation energies (E00) were estimated at the intersection between the normalised absorption and luminescence spectra40 (Fig. S4†) and the results are summarised in Tables 1 and S1.†Photoexcitation at 460 nm results in a strong emission for all dyes with a maximum centred at approximately 570 nm (±10 nm). In the case of DPP1, DPP3 and DPP4, similar E00 values were obtained (E00 ≈ 2.32 eV). Slightly smaller values were determined for DPP2 and DPP5 (E00 ≈ 2.30 eV), in line with their red-shifted absorption characteristics. Medium Stokes shifts were recorded for all dyes (Δ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 2500 to 3000 cm−1), indicating notable reorganisation of the dyes' dipole moment in the excited state. The reorganisation could originate from the diminution of the thiophene-phenyl angle as previously reported.40 Among all the dyes, DPP2 and DPP5 featured the largest shifts, which could also suggest a stronger ability for efficient charge transfer as the result of a major change between the excited and ground state dipoles.
= 2500 to 3000 cm−1), indicating notable reorganisation of the dyes' dipole moment in the excited state. The reorganisation could originate from the diminution of the thiophene-phenyl angle as previously reported.40 Among all the dyes, DPP2 and DPP5 featured the largest shifts, which could also suggest a stronger ability for efficient charge transfer as the result of a major change between the excited and ground state dipoles.
Electrochemical properties
The redox potentials of the DPP dyes were recorded by cyclic voltammetry after immobilisation on mesoporous indium tin oxide electrodes47 in an acetonitrile solution containing tetrabutylammonium tetrafluoroborate (0.1 M) as electrolyte (Table 1). Cyclic voltammetry shows that all DPP dyes exhibit an irreversible oxidation wave, located at approximately Eonset ≈ 1.17 V vs. NHE (onset potential)48 for DPP1, DPP3 and DPP4 (Fig. S5†). The marginal differences between the oxidation potential of these three photosensitisers confirm the minor impact of side chain modification on the electronic properties with the main electrochemical processes being localised on the DPP core. The onset of the oxidation wave is shifted to Eonset = 1.01 V vs. NHE in the case of DPP5, where the electron donating, O-substituted thiophene facilitates the chromophore oxidation. DPP2 also exhibits a less positive oxidation potential at Eonset = 1.10 V vs. NHE, most likely due to the additional thiophene unit. This difference could have a significant influence on the regeneration ability of an oxidised dye by a chemical reductant such as a sacrificial electron donor (SED) in photocatalytic schemes (see transient absorption spectroscopy study below).TEOA (pH 7) and AA (pH 4.5) have been used as SEDs in this study. The driving force for dye regeneration (ΔGreg) following oxidative quenching of each dye's excited state by TiO2 was estimated. The low redox potential of AA (E(SED+/SED)AA < 0.20 V vs. NHE, pH 4.5)45,49–51 allows it to thermodynamically act as a strong electron-donating reagent for all oxidised dyes, generating a highly favourable regeneration reaction (ΔGreg < −0.80 eV). However, TEOA's more positive redox potential for oxidation (E(SED+/SED)TEOA = 0.82 V vs. NHE, pH 7)46 provides considerably less driving force, implying a potentially sluggish regeneration of DPP dyes (ΔGreg ≈ −0.19 to −0.37 eV). This driving force differs from RuP (Eonset = 1.37 V vs. NHE, Table 1), where a sufficiently exergonic situation (ΔGreg ≈ −0.55 eV) is expected to allow an efficient reaction with TEOA.
The addition of E00 to the dye's E(S+/S) provides an estimate for the excited state oxidation potential E(S+/S*) of the DPP dyes relevant for oxidative quenching. Apart from DPP5, comparable values were obtained for all DPP dyes (E(S+/S*) ≈ −1.15 V vs. NHE), indicating that the different substituents have little influence on the excited state energy levels. In the case of the alkyloxy-functionalised DPP5, a more negative E(S+/S*) of approximately −1.29 V vs. NHE was obtained, which shows that the electron donating group affected both the HOMO and LUMO energy levels. The driving force for electron injection, ΔGinj, from the dye's excited state to the TiO2 conduction band (CB; ECB(TiO2) = (−0.29 V – 0.059 V × pH) vs. NHE)43,44 was calculated for both pH values and proved to be sufficient for all DPP photosensitisers (ΔGinj < −0.4 eV).
Photocatalysis of DPP|TiO2 with molecular catalysts
The DPP dyes were co-immobilised with the molecular H2 evolution catalysts CoP or NiP (Fig. 1b) on TiO2via their phosphonic acid functionalities.4,20,21,52 The DSP systems were self-assembled by dispersing Evonik P25 TiO2 nanoparticles in a buffered and SED-containing aqueous solution, followed by addition of the molecular catalyst and dye to give the dye|TiO2|catalyst assemblies (see ESI† for details). In a standard experiment, 2.5 mg of TiO2 and 0.05 μmol of dye were used in 3 mL of aqueous SED solution with different amounts of either CoP or NiP in a sealed photoreactor (headspace = 4.84 mL). The photocatalytic activity of the DPP|TiO2|catalyst assemblies was studied in the presence of the SED TEOA (pH 7.0 with CoP) or AA (pH 4.5 with NiP) under previously identified optimal conditions for the applied molecular catalysts.20,21 The deaerated DPP|TiO2|CoP and DPP|TiO2|NiP suspensions were kept at 25 °C and irradiated with UV-filtered simulated solar light (AM 1.5G, 100 mW cm−2, λ > 420 nm). The UV cut-off filter prevented band gap excitation of TiO2 and ensured that light is only harvested by the molecular dye. The corresponding RuP-based system, RuP|TiO2|catalyst, was also studied for comparison.Binding of CoP, NiP and RuP to TiO2 has been studied previously and a maximum loading capacity of approximately 0.15 μmol of molecular components on 2.5 mg of TiO2 was determined.20,21 Attachment of the DPP dyes to TiO2 particles under experimental conditions was confirmed for DPP1 and DPP4. 0.05 μmol of dye were stirred with 2.5 mg of TiO2 in aqueous AA or TEOA solution. The modified TiO2 particles were separated via centrifugation and UV-Vis spectroscopy of the supernatant showed that more than 80% of DPP dye was adsorbed onto the particles (Table S2†), ensuring a strong light-harvesting ability for both the most hydrophilic (DPP4) and the corresponding hydrophobic (DPP1) dye on TiO2.
Control experiments established that no H2 or only trace amounts were produced in the absence of either DPP dye, TiO2, molecular catalyst, light, or SED. No H2 was also detected, in presence of ZrO2 nanoparticles, instead of TiO2 (Table S3†), due to the high-energy level of the CB of ZrO2 (ECB(ZrO2) = (−1.0 V – 0.059 V × pH) vs. NHE) preventing the electron injection from the excited state of DPP1 and DPP4.53 Upon addition of potassium phosphate (0.1 M, pH adjusted to corresponding SED solution) to the aqueous SED solution, no H2 was produced by the DSP systems due to displacement of the molecular components from the TiO2 surface by excess phosphate ions,45,54 demonstrating that a homogeneous reductive quenching pathway is not contributing to H2 evolution. Experiments in the presence of the corresponding Co and Ni metal salts instead of the molecular catalysts showed no H2 evolution (Table S3†). Thus, all components of the DSP are vital for light-driven H2 evolution. It further demonstrates that the system proceeds via oxidative quenching of DPP and a ‘through particle’ electron transfer mechanism requiring TiO2 as an electron mediator,21 and only immobilised molecular components on a semiconductor with suitable band energies (e.g., TiO2) can contribute to the photoactivity.
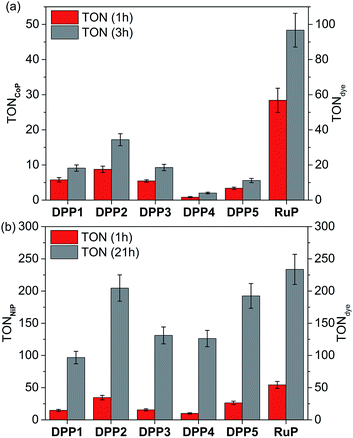
The photocatalytic activity of DPP|TiO2|CoP was studied in TEOA solution (0.1 M) at pH 7 and the results are summarised in Tables 2, S4 and S5,† and Fig. 3a, S6 and S7.† The TiO2 nanoparticles were loaded with CoP and the corresponding DPP dye (0.05 μmol each) and the amount of CoP optimised for a maximum CoP-based turnover number (TONCoP). Among the DPP|TiO2|CoP assemblies, a maximum turnover frequency, TOFCoP of 8.8 ± 0.9 h−1 and TOFDPP = 17.5 ± 1.8, was obtained with DPP2. Only trace amounts of H2 were observed with DPP4, whereas a TOFDPP of 6 to 12 h−1 was achieved with DPP1, DPP3 and DPP5.
| System | TOFcatf/h−1 | TOFdyeg/h−1 | n(H2)/μmol (1 h) | TONcatf | TONdyeg |
|---|---|---|---|---|---|
| a General conditions: samples contained dye and catalyst loaded onto P25 TiO2 nanoparticles (2.5 mg) in a total volume of 3 mL of SED solution and were irradiated with UV-filtered simulated solar light (100 mW cm−2, AM 1.5G, λ > 420 nm) at 25 °C. b CoP (0.05 μmol) and dye (0.05 μmol) on TiO2 in aqueous TEOA solution (3 mL, pH 7, 0.1 M), see Table S5 for results for DPP1, DPP3, DPP4 and DPP5. c NiP (0.025 μmol) and dye (0.05 μmol) on TiO2 in aqueous AA solution (3 mL, pH 4.5, 0.1 M). d [NiFeSe]-H2ase (50 pmol) and dye (0.05 μmol) on TiO2 in AA-MES solution (3 mL, pH 6, 0.1 M each). e Pre-platinised TiO2 (2.5 mg) and dye (0.05 μmol) in aqueous AA solution (3 mL, pH 4.5, 0.1 M). f TOFcat and TONcat were calculated as follows: TOFcat = n(H2) after 1 h/n(catalyst) and TONcat = n(H2) after x h/n(catalyst). g TOFdye and TONdye were calculated as follows: TOFdye = 2n(H2) after 1 h/n(dye) and TONdye = 2n(H2) after x h/n(dye). h Not determined due to the unknown amount of catalytically active sites; control experiments and optimisations of DSP systems are listed in Tables S3 to S7. | |||||
| Dye|TiO 2 | CoP | |||||
| DPP2 | 8.8 ± 0.9 | 17.5 ± 1.8 | 0.43 ± 0.04 | 17.2 ± 1.7 (3 h) | 34.4 ± 3.4 (3 h) |
| RuP | 28.4 ± 3.4 | 56.8 ± 6.9 | 1.42 ± 0.17 | 48.4 ± 4.8 (3 h) | 96.7 ± 10.0 (3 h) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Dye|TiO 2 | NiP | |||||
| DPP1 | 14.7 ± 1.5 | 14.7 ± 1.5 | 0.38 ± 0.04 | 96.8 ± 9.7 (21 h) | 96.8 ± 9.7 (21 h) |
| DPP2 | 34.6 ± 3.5 | 34.6 ± 3.5 | 0.86 ± 0.09 | 204.6 ± 20.5 (21 h) | 204.6 ± 20.5 (21 h) |
| DPP3 | 15.5 ± 1.6 | 15.5 ± 1.6 | 0.39 ± 0.04 | 131.1 ± 13.1 (21 h) | 131.1 ± 13.1 (21 h) |
| DPP4 | 10.0 ± 1.0 | 10.0 ± 1.0 | 0.25 ± 0.03 | 126.3 ± 12.6 (21 h) | 126.3 ± 12.6 (21 h) |
| DPP5 | 26.4 ± 2.6 | 26.4 ± 2.6 | 0.66 ± 0.07 | 192.4 ± 19.2 (21 h) | 192.4 ± 19.2 (21 h) |
| RuP | 54.3 ± 5.4 | 54.3 ± 5.4 | 1.35 ± 0.14 | 233.6 ± 23.4 (21 h) | 233.6 ± 23.4 (21 h) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Dye|TiO 2 |H 2 ase | |||||
| DPP2 | 8650 ± 1100 | 17.3 ± 2.2 | 0.43 ± 0.06 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 ± 11 600 ± 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (21 h) 100 (21 h) |
175 ± 22 (21 h) |
| RuP | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 1246 500 ± 1246 |
25.0 ± 2.5 | 0.62 ± 0.06 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ± 22 100 ± 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 (21 h) 300 (21 h) |
182 ± 45 (21 h) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Dye|TiO 2 |Pt | |||||
| DPP2 | n.d.h | 337 ± 33.7 | 8.4 ± 0.8 | n.d.h | 2660 ± 265 (24 h) |
| RuP | n.d.h | 71.3 ± 7.1 | 1.8 ± 0.2 | n.d.h | 431 ± 95 (24 h) |
The results for the DPP dyes are in approximate accordance with trends expected from the electronic properties. The slightly better performance of DPP2 may be due to its broader light absorption window and the poor performance of DPP5 due to the smallest ΔGreg. For DPP4, the linear hydrophilic core side chain appears to have a negative impact on the performance of the dye at pH neutral conditions. This effect could be explained by the formation of a dense packed layer of dye that induced a steric effect possibly preventing the dye regeneration from the SED.31 This is confirmed to some extent by the higher loading of DPP4 than DPP1 (Table S2†). Furthermore, the similar performances observed for DPP1 and DPP3 (≈11 h−1) indicate a minimal impact of the thiophene's “tailing” side-chain hydrophilicity under the employed conditions.
In general, the lower performance of the DPP-based DSP systems compared to RuP|TiO2|CoP (TOFCoP = 28.4 ± 3.4 h−1, TOFRuP = 56.8 ± 6.9 h−1) can be attributed to the small driving force for regeneration of the oxidised dye (ΔGreg > −0.35 eV) by TEOA after electron transfer from the excited dye to the TiO2-CB. In agreement, we observe a significant bleaching of the orange colouration of the DPP-sensitised TiO2 nanoparticles as a result of dye degradation in the absence of an efficient electron regeneration process after one hour of light exposure. This correlates well with the observed cessation of photo-H2 generation of DPP|TiO2|CoP within the first hours of irradiation (Fig. 3a and S7,†Tables 2 and S5†).
The DPP dyes were subsequently studied with the molecular H2 evolution catalyst NiP co-adsorbed on TiO2 nanoparticles in an aqueous pH 4.5 AA solution (0.1 M). The amount of NiP (0.025 μmol) was optimised for a maximum TONNiP (Table S6 and Fig. S8†). The following trend based on TOFNiP and TOFDPP was observed for DPP|TiO2|NiP: DPP2 > DPP5 > DPP3 ≈ DPP1 > DPP4 (Fig. 3b and S9†, Tables 2 and S7†), with DPP2 achieving the highest TOFNiP/DPP of 34.6 ± 3.5 h−1. With DPP2 and DPP5, TONNiP/DPP of 204.6 ± 20.5 and 192.4 ± 19.2 were obtained after 21 h of visible light irradiation, comparing well with the corresponding RuP-based DSP (TONNiP/DPP of 233.6 ± 23.4). The DPP dyes therefore exhibit a good stability, allowing for prolonged H2 generation with high performance in DSP with NiP.
The large driving force available for regeneration of DPP+ (ΔGreg < −0.80 eV) when using AA as SED is likely a key reason for the better performance of the DPP dyes in aqueous AA compared to TEOA solution.
The DSP systems with DPP1, DPP3 and DPP4 feature a similar TOFDPP (10 to 15 h−1), which agrees with their almost identical electronic properties. However, long-term irradiation (21 h) of the DPP3- and DPP4-based systems results in a higher TONDPP than with DPP1 (Tables 2 and S7,†Fig. 3b and S9†). This observation suggests that the side chains' polarity has a secondary but not negligible impact on the dye stability/efficiency with a synergistic relationship between the nature of the SED and/or pH variation. While bulky lipophilic chains positioned on the core (the oxidation centre) appear advantageous to the system under pH neutral conditions (TONDPP1 ≈ TONDPP3 > TONDPP4, see above), the presence of hydrophilic chains appeared to be beneficial (TONDPP3 ≈ TONDPP4 > TONDPP1) at pH 4.5. The better performance of DPP4 at pH 4.5 compared to pH 7 is presumably due to the concomitant binding of AA to the TiO2 surface, thereby preventing strong deleterious aggregation of the DPP dye (see above).
The additional O-donor functionality in DPP5 presumably accounts for the better performance compared to DPP3 due to improved charge separation properties as observed in fluorescence and UV-Vis experiments. This is in contrast to the performance at pH 7, where DPP3 performed better than DPP5. Under pH neutral conditions, the small ΔGreg is presumably the limiting factor (−0.37 vs. −0.19 eV), whereas ΔGreg is sufficiently large at pH 4.5 (<−0.8 eV) that other parameters like the push–pull architecture dominate the performance. Similarly, in the case of DPP2, the extended absorption window allows for better light absorption resulting in the highest performance amongst all DPP|TiO2|NiP assemblies.
In contrast to the DPP-based DSP systems studied herein, there are two mechanistic H2 evolution pathways possible for RuP|TiO2|NiP in AA (Fig. S10†).21,45 In addition to the ‘through particle’ pathway, where RuP* is oxidatively quenched by the semiconductor CB, reductive quenching of RuP* by AA is also possible. In the latter case, a strongly reducing dye species (RuP−) is formed, which can directly reduce NiP to initiate H2 evolution through an ‘on particle’ pathway.21 This might account for the higher TOFNiP of RuP|TiO2|NiP as two pathways contribute toward H2 production as opposed to a pure ‘through particle’ pathway in DSP with the DPP dyes (see above).
External quantum efficiency
The external quantum efficiency (EQE) was determined for DPP2|TiO2|NiP at different wavelengths and compared to RuP|TiO2|NiP. The obtained EQE values match well with the absorption profiles of the dyes on TiO2 giving the highest value at their corresponding absorption maxima (Fig. 2b and Table S8†). Notably, RuP|TiO2|NiP did not show any photo-H2 activity at λ = 550 nm, whereas DPP2|TiO2|NiP was still active at this wavelength (EQE ≈ 0.15%). This highlights the good solar light absorption properties of DPP2 and confirms that light from a wide range of the visible spectrum can successfully be used for H2 evolution in DPP2|TiO2|NiP.An EQE of approximately 0.3 and 0.4% was achieved with DPP2|TiO2|NiP at λ = 400 and 500 nm, respectively, which is in the same range as previously reported EQE values of molecular DSP systems with RuP.45,54 All EQE values were recorded using the standard conditions from the photocatalysis experiments and no optimisation was performed. EQEs represent a lower limit for an internal quantum yield, which would assume that all incident light was absorbed by the sample.
Photocatalysis of DPP|TiO2 with hydrogenase and Pt
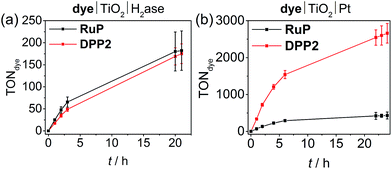
We also studied the best performing DPP chromophore, DPP2, in combination with previously established benchmark catalysts, which have been applied in DSP systems, i.e. the reversible H2 cycling catalysts hydrogenase (H2ase)55,56 and Pt.22,32 Using hydrogenase allows establishing the biocompatibility of DPP dyes and Pt as a catalyst eliminates or at least substantially reduces kinetic limitations from catalyst turnover and allows for evaluation of the true potential of the organic dyes.For experiments with hydrogenase, P25-TiO2 (2.5 mg) was loaded with DPP2 or RuP (0.05 μmol) in an aqueous AA-MES solution (3 mL, 0.1 M each, pH 6, MES = 2-(N-morpholino)ethanesulfonic acid) and a [NiFeSe]-H2ase from Desulfomicrobium baculatum (50 pmol) was added to the deaerated suspension.57 This hydrogenase was selected for its well-established properties as highly active H2 evolution catalyst that displays O2-resistance paired with little inhibition by H2 and its excellent attachment to metal oxide surfaces.58,59 Pt was pre-deposited on P25 TiO2 nanoparticles22 and the modified particles (2.5 mg) were sensitised with either DPP2 or RuP (0.05 μmol) after suspending the particles in aqueous AA solution (3 mL, 0.1 M, pH 4.5). As for experiments performed with CoP and NiP, all samples were stirred at 25 °C and irradiated with UV-filtered simulated solar light (100 mW cm−2, AM 1.5G, λ > 420 nm).
Similar to the NiP-based DSP systems, RuP|TiO2|H2ase and DPP2|TiO2|H2ase displayed similar photoactivity (TONDPP2 = 175 ± 22 and TONRuP = 182 ± 45, Fig. 4a, Tables 2 and S9†). This result may originate from the low amount of H2ase available at the TiO2 surface, generating a catalysis-limited system. However, the activity of the DPP2-based system (TOFenzyme ≈ 8.7 × 103) compares well with a previously reported carbon nitride|TiO2|H2ase hybrid,56 confirming a good compatibility of the DPP chromophore with the biocatalyst.
When using Pt as H2 evolution catalyst, the DPP2-containing assembly significantly outperforms RuP|TiO2|Pt, achieving a TOFdye of 337 ± 33.7 and 71.3 ± 7.1, respectively (Fig. 4b, Tables 2 and S10†). Notably, the DPP2|TiO2|Pt was also found to be considerably more efficient with a TONdye of 2660 ± 265 after 24 h of irradiation, whereas a TONRuP of only 431 ± 95 was observed for RuP. The higher efficiency of the DPP-based system could stem from altered kinetic pathways. With Pt being a fast H2 evolution catalyst, the systems are less limited by charge recombination kinetics (see transient absorption spectroscopy), but more likely by the number of available CB electrons in TiO2 – this is a direct consequence of the DPP photosensitisers' enhanced light-harvesting and electron injection abilities.
Transient absorption spectroscopy
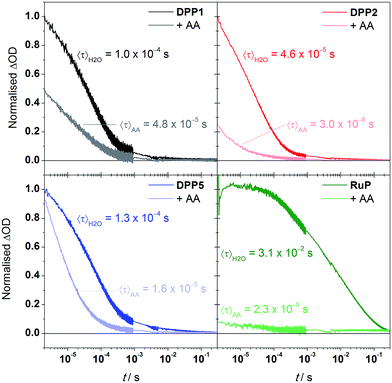
We performed transient absorption spectroscopy (TAS) measurements to evaluate both the charge recombination and dye regeneration processes. To reach high efficiencies, the productive charge transfer steps must compete favourably with the undesired energy loss pathways. For example, electron injection should occur faster than excited state relaxation, and oxidised dye regeneration should be faster than charge recombination.60We monitored the charge-separated state produced upon the photoexcitation at 500 nm of DPP-sensitised TiO2 films by following the transient change in absorption at 700 nm, assigned to photogenerated dye cation absorption. Normalised results for DPP1, DPP2, and DPP5 are shown in Fig. 5 (see Fig. S11† for non-normalised traces). Measurements were attempted for DPP3 and DPP4, but these dyes proved to be highly unstable under the TAS conditions in the absence of a SED (it is likely that chemical transformations following photo-oxidation of the dyes causes the instability). We expect the extinction coefficients of the oxidised DPP dyes to be similar on the basis of the similar ground state optical properties. We may thus compare the initial signal amplitude, proportional to the concentration of oxidised DPP produced, observed for the different DPP dyes. The initial amplitudes at 2 μs will be related to the charge injection yield and is the highest for DPP5, consistent with its larger ΔGinj compared to DPP1 and DPP2. A decrease of 20% is seen for DPP1 compared to DPP2. As the two dyes possess the same ΔGinj, the change potentially reflects differential dye orientation or polarity of the side chains. DPP1 shows the lowest initial amplitude, which might explain its lower photoactivity compared to DPP2 and DPP5.
The decays presented in Fig. 5 could be well-described by a stretched exponential expression (fits shown in Fig. S11†), in line with the dispersive recombination kinetics observed in TiO2 caused by charge trapping/detrapping.61,62 We characterised the lifetime of the charge separated state from the mean lifetime 〈τ〉 obtained from fitting (see ESI†). All three DPP dyes show decays comparable to previous reports of DPP-sensitised TiO2,63 and have similar charge-separated lifetimes near 100 μs, suggesting that the observed differences in activity between dyes are not due to changes in this recombination lifetime. The excited state dynamics of the DPP photosensitisers on TiO2 were also compared to RuP (excitation at 450 nm, monitoring at 700 nm). In line with previous investigations,21 the transient signal decays on the millisecond timescale. The mean lifetime for RuP was 31 ms, roughly 300-fold longer than observed for the DPP-based dyes. The increased charge separation lifetime is possibly due to decreased electronic coupling or an increased spatial charge separation between the photosensitiser cation and the TiO2 surface in the case of RuP.64
We next performed TAS measurements in the presence of AA (10 mM) to investigate dye regeneration (Fig. 5). Quenching of the oxidised dyes was confirmed by observation of a reduced signal amplitude and shortening of 〈τ〉, both for the DPP dyes and RuP. The reduction in initial signal amplitude showed large variations between DPP dyes, ranging from less than 10% for DPP5 to 75% for DPP2, suggesting faster and efficient (>90%) regeneration for the latter. The shape of the decays indicates that for DPP1 and DPP2 dye regeneration mainly takes place on the sub-μs timescale while the same process takes place in approximately 10 μs for DPP5. We calculated the regeneration efficiencies from the competitive kinetics of regeneration and charge recombination (see ESI† for details): the regeneration is most efficient (94% yield) for DPP2, which may partly be the reason for its best performance in DSP. Although the regeneration kinetics are significantly slower in the case of DPP5, regeneration is relatively efficient (87% yield) as competition with charge recombination is still favourable, and is in line with the comparable photoactivity to DPP2. DPP1 showed the lowest regeneration yield, 52%, another factor that may limit its photoactivity. The regeneration yields do not correlate directly with ΔGreg and appear to rely primarily on other factors such as the dyes' hydrophobicity, orientation or push–pull architecture.65,66
Comparative experiments with RuP showed a more significant sub-μs quenching of the oxidised dye, with the initial amplitude decreasing by over 90% in the presence of AA, and overall shows quantitative regeneration. The more efficient regeneration with RuP is consistent with its larger ΔGreg, and consistent with the slightly higher photoactivity obtained for this dye in the systems without Pt.
Despite the high regeneration yields, overall quantum efficiencies of the hybrid systems RuP|TiO2|NiP and DPP2|TiO2|NiP are below 1%. This discrepancy can be explained by the increased electron density in the CB of TiO2 under continuous irradiation, which will lead to faster charge recombination kinetics that reduces the regeneration yield in bulk photocatalysis experiments.67 We have previously determined that the first reduction of molecular catalyst on RuP-sensitised TiO2 occurs on the μs to ms timescales.14,21,54 However, the second electron transfer required for catalytic turnover to produce H2 was several orders of magnitude slower than the first reduction step.14 The multi-electron nature of proton reduction therefore gives photo-generated TiO2-CB electrons time to undergo charge recombination and additional competing side reactions such as reduction of oxidised donor (AA) or oxidation products of the SED thereby limiting the overall efficiency of the system.
Conclusions
In summary, we report the use of DPP-sensitised TiO2 for the assembly of a molecule-based DSP system for light-driven H2 generation in water without the need for a precious metal-containing component. Five novel DPP dyes bearing different side chains and a phosphonic acid-anchoring group, for robust immobilisation on metal oxide semiconductors, have been synthesised and are reported. The dyes exhibit strong light absorption over a wide range of the visible light spectrum (λ = 400 to 575 nm) and operate as efficient photosensitisers when adsorbed on TiO2. We demonstrate preliminary structure–activity relationships between the DPP chromophore modifications and the solar-driven H2 evolution performances of the dye|TiO2|catalyst systems. Changing energetic parameters such as broader light-harvesting range and push–pull design architecture by adding of a conjugated thiophene or an electron rich unit, as in DPP2 or DPP5, was revealed to be beneficial for the H2 evolution performances (i.e. TOF and TON) as long as they allow for efficient electron injection and dye regeneration. In parallel, we confirmed that tuning non-energetic parameters (e.g. steric hindrance, position and nature of the solubilising side chains) plays a decisive role on the dye organisation at the TiO2 surface and the electronic communication with the media's components (DPP4). It is also evident that kinetic parameters (e.g. the lifetime of the charge-separated state) need to be considered and should be adapted in line with the catalyst kinetics to allow for sufficient time to perform the two-electron H2 evolution reaction. The performance of the dye in DSP systems does ultimately also depend on the pH, SED, chemical catalyst and mechanistic details, which implies that the comparison between two dyes' activity should be taken with caution. Nevertheless, the present study provides the basis for further studies to more fully rationalise dye design and structure–activity relationships in the future.Compared to previous systems with the phosphonated Ru dye RuP, the DPP-systems can absorb light at higher wavelengths (up to 575 nm) and match the performance of the Ru dye in terms of stability and turnover numbers.21,22,45,52 It is promising that despite faster recombination kinetics of the DPP cations, reasonably efficient dye regeneration by AA is still observed. The compatibility of DPP with a hydrogenase demonstrates its biocompatibility and replacing the molecular catalysts by Pt demonstrates that DPP-based dyes outperform RuP in this system, which shows much scope for further development. We have therefore established phosphonated DPP dyes as an excellent alternative to precious metal-containing dyes in aqueous DSP schemes. The five DPP dyes studied herein are first-generation dyes and not yet fully optimised, leaving room for further tuning through core and side chain engineering to improve light absorption, charge separation and regeneration yields. DPP chromophores have therefore great potential in DSP and, more widely, in aqueous photocatalysis.
Acknowledgements
Support by the Christian Doppler Research Association (Austrian Federal Ministry of Science, Research and Economy and National Foundation for Research, Technology and Development), the OMV Group and the Ministry of Education (Singapore) is gratefully acknowledged. RG is grateful to FRQNT for a Postdoctoral Fellowship and JRD thanks the European Science Foundation project Intersolar (291482) for support. We also thank Dr Juan C. Fontecilla-Camps and Dr Christine Cavazza (CNRS Grenoble, France) for providing us with the hydrogenase, Dr Manuela A. Gross for providing the molecular complexes NiP and RuP, Dr Timothy Rosser for his help recording the emission spectra of the dyes and Dr Benjamin C. M. Martindale and Charles E. Creissen for helpful discussions and comments on the manuscript.Notes and references
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z.-X. Guo and J. Tang, Energy Environ. Sci., 2015, 8, 731–759 CAS.
- N. S. Lewis, Science, 2016, 351, aad1920 CrossRef PubMed.
- Y.-H. Lai, D. W. Palm and E. Reisner, Adv. Energy Mater., 2015, 5, 1501668 CrossRef.
- J. Willkomm, K. L. Orchard, A. Reynal, E. Pastor, J. R. Durrant and E. Reisner, Chem. Soc. Rev., 2016, 45, 9–23 RSC.
- L. Alibabaei, H. Luo, R. L. House, P. G. Hoertz, R. Lopez and T. J. Meyer, J. Mater. Chem. A, 2013, 1, 4133–4145 CAS.
- Y. Ma, X. Wang, Y. Jia, X. Chen, H. Han and C. Li, Chem. Rev., 2014, 114, 9987–10043 CrossRef CAS PubMed.
- H. Tian, ChemSusChem, 2015, 8, 3746–3759 CrossRef CAS PubMed.
- F. Li, K. Fan, B. Xu, E. Gabrielsson, Q. Daniel, L. Li and L. Sun, J. Am. Chem. Soc., 2015, 137, 9153–9159 CrossRef CAS PubMed.
- Z. Yu, F. Li and L. Sun, Energy Environ. Sci., 2015, 8, 760–775 CAS.
- M. Wang, K. Han, S. Zhang and L. Sun, Coord. Chem. Rev., 2015, 287, 1–14 CrossRef CAS.
- D.-I. Won, J.-S. Lee, J.-M. Ji, W.-J. Jung, H.-J. Son, C. Pac and S. O. Kang, J. Am. Chem. Soc., 2015, 137, 13679–13690 CrossRef CAS PubMed.
- X. Zhang, T. Peng and S. Song, J. Mater. Chem. A, 2016, 4, 2365–2402 CAS.
- M. A. Gross, C. E. Creissen, K. L. Orchard and E. Reisner, Chem. Sci., 2016, 7, 5537–5546 RSC.
- A. Reynal, F. Lakadamyali, M. A. Gross, E. Reisner and J. R. Durrant, Energy Environ. Sci., 2013, 6, 3291–3300 CAS.
- T. A. Moore, D. Gust, P. Mathis, J.-C. Mialocq, C. Chachaty, R. V. Bensasson, E. J. Land, D. Doizi, P. A. Liddell, W. R. Lehman, G. A. Nemeth and A. L. Moore, Nature, 1984, 307, 630–632 CrossRef CAS.
- P. A. Liddell, D. Kuciauskas, J. P. Sumida, B. Nash, D. Nguyen, A. L. Moore, T. A. Moore and D. Gust, J. Am. Chem. Soc., 1997, 119, 1400–1405 CrossRef CAS.
- A. Magnuson, Y. Frapart, M. Abrahamsson, O. Horner, B. Åkermark, L. Sun, J.-J. Girerd, L. Hammarström and S. Styring, J. Am. Chem. Soc., 1999, 121, 89–96 CrossRef CAS.
- J. Warnan, J. Gardner, L. Le Pleux, J. Petersson, Y. Pellegrin, E. Blart, L. Hammarström and F. Odobel, J. Phys. Chem. C, 2014, 118, 103–113 CAS.
- B. H. Farnum, K.-R. Wee and T. J. Meyer, Nat. Chem., 2016, 8, 845–852 CrossRef CAS PubMed.
- F. Lakadamyali and E. Reisner, Chem. Commun., 2011, 47, 1695–1697 RSC.
- M. A. Gross, A. Reynal, J. R. Durrant and E. Reisner, J. Am. Chem. Soc., 2014, 136, 356–366 CrossRef CAS PubMed.
- E. Bae and W. Choi, J. Phys. Chem. B, 2006, 110, 14792–14799 CrossRef CAS PubMed.
- J. Zhang, P. Du, J. Schneider, P. Jarosz and R. Eisenberg, J. Am. Chem. Soc., 2007, 129, 7726–7727 CrossRef CAS PubMed.
- A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- Y. Ooyama and Y. Harima, ChemPhysChem, 2012, 13, 4032–4080 CrossRef CAS PubMed.
- L. J. Antila, P. Ghamgosar, S. Maji, H. Tian, S. Ott and L. Hammarström, ACS Energy Lett., 2016, 1, 1106–1111 CrossRef CAS.
- B. van den Bosch, J. A. Rombouts, R. V. A. Orru, J. N. H. Reek and R. J. Detz, ChemCatChem, 2016, 8, 1392–1398 CrossRef CAS.
- J.-S. Lee, D.-I. Won, W.-J. Jung, H.-J. Son, C. Pac and S. O. Kang, Angew. Chem., Int. Ed., 2017, 56, 976–980 CrossRef CAS PubMed.
- K. A. Click, D. R. Beauchamp, Z. Huang, W. Chen and Y. Wu, J. Am. Chem. Soc., 2016, 138, 1174–1179 CrossRef CAS PubMed.
- S.-H. Lee, Y. Park, K.-R. Wee, H.-J. Son, D. W. Cho, C. Pac, W. Choi and S. O. Kang, Org. Lett., 2010, 12, 460–463 CrossRef CAS PubMed.
- R. P. Sabatini, W. T. Eckenhoff, A. Orchard, K. R. Liwosz, M. R. Detty, D. F. Watson, D. W. McCamant and R. Eisenberg, J. Am. Chem. Soc., 2014, 136, 7740–7750 CrossRef CAS PubMed.
- K. Narayanaswamy, A. Tiwari, I. Mondal, U. Pal, S. Niveditha, K. Bhanuprakash and S. P. Singh, Phys. Chem. Chem. Phys., 2015, 17, 13710–13718 RSC.
- M. Yin, S. Ma, C. Wu and Y. Fan, RSC Adv., 2015, 5, 1852–1858 RSC.
- Z. Hao and A. Iqbal, Chem. Soc. Rev., 1997, 26, 203–213 RSC.
- S. Qu and H. Tian, Chem. Commun., 2012, 48, 3039–3051 RSC.
- Y. Li, P. Sonar, L. Murphy and W. Hong, Energy Environ. Sci., 2013, 6, 1684–1710 CAS.
- C. Queffélec, M. Petit, P. Janvier, D. A. Knight and B. Bujoli, Chem. Rev., 2012, 112, 3777–3807 CrossRef PubMed.
- D. G. Farnum, G. Mehta, G. G. I. Moore and F. P. Siegal, Tetrahedron Lett., 1974, 15, 2549–2552 CrossRef.
- J. Warnan, L. Favereau, Y. Pellegrin, E. Blart, D. Jacquemin and F. Odobel, J. Photochem. Photobiol., A, 2011, 226, 9–15 CrossRef CAS.
- H. Ftouni, F. Bolze and J.-F. Nicoud, Dyes Pigm., 2013, 97, 77–83 CrossRef CAS.
- D. F. Zigler, Z. A. Morseth, L. Wang, D. L. Ashford, M. K. Brennaman, E. M. Grumstrup, E. C. Brigham, M. K. Gish, R. J. Dillon, L. Alibabaei, G. J. Meyer, T. J. Meyer and J. M. Papanikolas, J. Am. Chem. Soc., 2016, 138, 4426–4438 CrossRef CAS PubMed.
- J. M. Bolts and M. S. Wrighton, J. Phys. Chem., 1976, 80, 2641–2645 CrossRef CAS.
- Y. Xu and M. A. A. Schoonen, Am. Mineral., 2000, 85, 543–556 CrossRef CAS.
- J. Willkomm, N. M. Muresan and E. Reisner, Chem. Sci., 2015, 6, 2727–2736 RSC.
- M. Kirch, J.-M. Lehn and J.-P. Sauvage, Helv. Chim. Acta, 1979, 62, 1345–1384 CrossRef CAS.
- N. M. Muresan, J. Willkomm, D. Mersch, Y. Vaynzof and E. Reisner, Angew. Chem., Int. Ed., 2012, 51, 12749–12753 CrossRef CAS PubMed.
- The onset potential for oxidation of the dyes E(S+/S) was determined as shown in Fig. S5 in the ESI† and is solely a rough estimate for the thermodynamic redox potential E1/2(S+/S).
- P. Schluga, C. G. Hartinger, A. Egger, E. Reisner, M. Galanski, M. A. Jakupec and B. K. Keppler, Dalton Trans., 2006, 1796–1802 RSC.
- J. J. Ruiz, A. Aldaz and M. Dominguez, Can. J. Chem., 1977, 55, 2799–2806 CrossRef CAS.
- Redox potentials ranging from 0.0 to 0.2 V vs. NHE have been reported for ascorbic acid. A redox potential E(SED+/SED) = 0.2 V vs. NHE was used to calculate the minimum available driving force for dye generation (ΔGreg) when using AA as SED.
- F. Lakadamyali, M. Kato and E. Reisner, Faraday Discuss., 2012, 155, 191–205 RSC.
- K. Sayama and H. Arakawa, J. Phys. Chem., 1993, 97, 531–533 CrossRef CAS.
- F. Lakadamyali, A. Reynal, M. Kato, J. R. Durrant and E. Reisner, Chem.–Eur. J., 2012, 18, 15464–15475 CrossRef CAS PubMed.
- E. Reisner, D. J. Powell, C. Cavazza, J. C. Fontecilla-Camps and F. A. Armstrong, J. Am. Chem. Soc., 2009, 131, 18457–18466 CrossRef CAS PubMed.
- C. A. Caputo, L. Wang, R. Beranek and E. Reisner, Chem. Sci., 2015, 6, 5690–5694 RSC.
- E. Garcin, X. Vernede, E. C. Hatchikian, A. Volbeda, M. Frey and J. C. Fontecilla-Camps, Structure, 1999, 7, 557–566 CrossRef CAS PubMed.
- C. Wombwell, C. A. Caputo and E. Reisner, Acc. Chem. Res., 2015, 48, 2858–2865 CrossRef CAS PubMed.
- D. Mersch, C.-Y. Lee, J. Z. Zhang, K. Brinkert, J. C. Fontecilla-Camps, A. W. Rutherford and E. Reisner, J. Am. Chem. Soc., 2015, 137, 8541–8549 CrossRef CAS PubMed.
- A. Listorti, B. O'Regan and J. R. Durrant, Chem. Mater., 2011, 23, 3381–3399 CrossRef CAS.
- A. N. M. Green, E. Palomares, S. A. Haque, J. M. Kroon and J. R. Durrant, J. Phys. Chem. B, 2005, 109, 12525–12533 CrossRef CAS PubMed.
- Y. Zhao, J. R. Swierk, J. D. Megiatto, B. Sherman, W. J. Youngblood, D. Qin, D. M. Lentz, A. L. Moore, T. A. Moore, D. Gust and T. E. Mallouk, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 15612–15616 CrossRef CAS PubMed.
- F. Guo, X. Liu, Y. Ding, F. Kong, W. Chen, L. Zhou and S. Dai, RSC Adv., 2016, 6, 13433–13441 RSC.
- J. N. Clifford, E. Palomares, M. K. Nazeeruddin, M. Grätzel, J. Nelson, X. Li, N. J. Long and J. R. Durrant, J. Am. Chem. Soc., 2004, 126, 5225–5233 CrossRef CAS PubMed.
- J. N. Clifford, E. Palomares, M. K. Nazeeruddin, M. Grätzel and J. R. Durrant, J. Phys. Chem. C, 2007, 111, 6561–6567 CAS.
- K. C. D. Robson, K. Hu, G. J. Meyer and C. P. Berlinguette, J. Am. Chem. Soc., 2013, 135, 1961–1971 CrossRef CAS PubMed.
- S. A. Haque, Y. Tachibana, R. L. Willis, J. E. Moser, M. Grätzel, D. R. Klug and J. R. Durrant, J. Phys. Chem. B, 2000, 104, 538–547 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, synthetic procedures, additional tables and figures. See DOI: 10.1039/c6sc05219c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |