Anisotropic Li+ ion conductivity in a large single crystal of a Co(III) coordination complex†
Saet Byeol
Kim‡
a,
Jeung Yoon
Kim‡
b,
Nak Cheon
Jeong
*b and
Kang Min
Ok
 *a
*a
aDepartment of Chemistry, Chung-Ang University, Seoul 06974, Republic of Korea. E-mail: kmok@cau.ac.kr
bDepartment of Emerging Materials Science, DGIST, Daegu 42988, Republic of Korea. E-mail: nc@dgist.ac.kr
First published on 13th October 2016
Abstract
Large single crystals of a novel lithium cobalt coordination compound, LiCo(NC5H3(CO2)2)2(H2O)2.125 [LiCo(PDC)2] were grown via a hydrothermal reaction in high yield. The electrochemical impedance spectroscopy (EIS) data measured on a large single crystal of LiCo(PDC)2 revealed very interesting anisotropic Li+ ion conductivity. The redox potential for Co3+/Co4+ observed at ca. 200 mV in the cyclic voltammogram was consistent with the electric potential where the ionic conductivity occurred. Detailed structural analysis on a series of stoichiometrically equivalent cobalt coordination compounds, ACo(PDC)2 (A = Na+, K+, and H3O+), indicated that the presence of ion channels as well as a suitable cation size is critical for the anisotropic ionic conductivity.
Introduction
Solid state materials containing interstitial cations have attracted enormous attention because the compounds not only provide fundamental electrochemical information1–4 but have promising applications as charge carriers in rechargeable batteries5–7 and fuel cells.8–10 Inorganic compounds exhibiting ionic conductivity, which were first observed from hot PbF2 and Ag2S by Faraday in 1839,11 have been utilized as essential cathode materials in lithium ion batteries12 and solid-state electrolytes.13–15 In addition, ionic polymers such as Nafion and its derivatives have realized their effectiveness as proton-conducting membranes.16 The discovery of superiorly performing and cost-effective alternatives is still an ongoing challenge.Two classes of emerging alternatives include metal–organic frameworks (MOFs), nanoporous crystalline solids assembled by interconnection of inorganic nodes and multitopic organic linkers via coordination bonding,17–28 and organic ionic plastic crystals (OIPCs), crystalline materials consisting of organic ions.29–34 While the representative families of coordination compounds and molecular crystals, MOFs and OIPCs, respectively, are of particular interest attributable to their interesting characteristics, the ionic conductivity phenomena in coordination-molecular crystals consisting of coordination complexes and counter ions have been rarely examined. Only two examples of coordination-molecular crystals revealing the ionic conductivity have been reported thus far.35,36 Although one of the investigations reported an anisotropic ionic conduction using a noncontact microwave method,35 more accurate conductivity measurements require a direct contact method, such as an electrochemical impedance spectroscopy (EIS) technique, for which a sizable crystal containing transition metal cations is required.37–40 To obtain reliable conductivity data, the electrodes should be firmly attached along specific directions in large crystals. Also, the materials should contain channels, through which small cations could be transported. Besides, the overall charge neutrality should be maintained through a reversible redox process on the molecular framework while the interstitial cations are moving. Here, we report a very interesting anisotropic conduction behavior of Li+ ions in a novel molecular crystal of a Co(III) coordination compound. The new molecular crystal, LiCo(NC5H3(CO2)2)2(H2O)2.125, which we denote as LiCo(PDC)2, successfully satisfies all requirements for ionic conduction described above. Large (maximum dimension 8 mm) crystals of LiCo(PDC)2 were easily grown. Also, the coordination geometry formed by the two 2,6-pyridine-dicarboxylate ligands in LiCo(PDC)2 effectively created ion channels in the crystal. In addition, the Co3+ cation can reversibly change its oxidation state to Co4+, as observed in one of the most popular electrochemical materials, LiCoO2. To elucidate the origin of the anisotropic ionic conduction, crystals of similar coordination compounds containing ions with different sizes, ACo(NC5H3(CO2)2)2(H2O)x [ACo(PDC)2; A = Na+, K+, and H3O+; 0 ≤ x ≤ 2], were also grown and comprehensively characterized.
Results and discussion
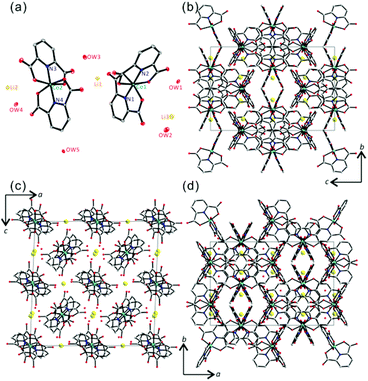
Single crystals of LiCo(PDC)2 were grown in a hydrothermal reaction by combining LiNO3, Co(NO3)2, 2,6-NC5H3(CO2H)2 (2,6-PDC), and water in a Teflon-lined autoclave at 120 °C for 3 days (see the ESI†). Large, violet, rod-shaped crystals were obtained in 93% yield based on the PDC ligand. Single crystal X-ray diffraction suggested that LiCo(PDC)2 crystallizes in the monoclinic space group I2/a (no. 15) and revealed a molecular structure consisting of Li+, Co3+, 2,6-PDC, and water molecules (see Fig. 1a). The starting Co2+ cations were oxidized to Co3+ during the hydrothermal reaction in the presence of nitrate. We successfully synthesized the same product using Co3+ ions, thus confirming the oxidation state of cobalt in LiCo(PDC)2. As shown in Fig. 1a, two unique Co3+ cations exist in a slightly distorted CoN2O4 octahedral environment within the asymmetric unit, with Co–N and Co–O bond lengths of 1.8360(19)–1.8400(19) Å and 1.9023(17)–1.9270(16) Å, respectively. The observed C–N and C–O bond distances in the PDC ligands are 1.326(3)–1.336(3) Å and 1.215(3)–1.316(3) Å, respectively. Three unique Li+ cations are in a LiO4 tetrahedral coordination environment with Li–O contact distances ranging from 1.932(4) to 2.009(5) Å. Occluded water molecules in the crystal reveal hydrogen bonding with carboxylate oxygen atoms ranging from 2.716(4) to 2.859(5) Å. The Li–O contacts and the hydrogen bonds of occluded water molecules may result in a pseudo-three-dimensional topology of LiCo(PDC)2. As shown in Fig. 1, channels were observed along the [010] direction, whereas no open spaces were observed along the [100] and [001] directions.The 1H and 13C NMR spectral data for LiCo(PDC)2 included all H and C atoms in the PDC ligands, for which the assignment of the signals was straightforward. The two hydrogens, Ha and Hb, were observed at 8.79 (t) and 8.53 (d) ppm in the 1H NMR spectrum, and the four carbon atoms, Ca, Cb, Cc, and Cd, were observed at 172.4, 129.5, 152.7, and 143.8 ppm, respectively, in the 13C NMR spectrum (see Fig. 2a). The infrared spectrum of LiCo(PDC)2 included Co–O and Co–N vibrations at ca. 668 and 594 cm−1, respectively (see Fig. 2b). The stretching and bending vibrations of water molecules were also observed at ca. 3360–3490 and 1659 cm−1, respectively. The UV-Vis spectrum of LiCo(PDC)2 revealed broad bands at ca. 225–235, 280–290, and 510 nm, attributed to ligand-to-metal charge transfers, π–π* transitions, and d–d transitions, respectively (see Fig. 2c). The thermogravimetric analysis diagram of LiCo(PDC)2 revealed a weight loss of approximately 8.9% from room temperature to 240 °C attributable to the loss of the occluded water molecule (calculated: 8.8%). Above this temperature, the material lost organic ligands and completely decomposed. The powder X-ray diffraction (PXRD) pattern for the thermally decomposed product was identified as LiCoO2 (see Fig. 2d and ESI†).
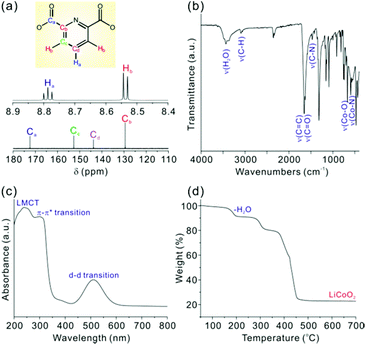 | ||
| Fig. 2 (a) 1H and 13C NMR spectra, (b) IR spectrum, (c) UV-vis spectrum, and (d) TGA diagram of LiCo(PDC)2. | ||
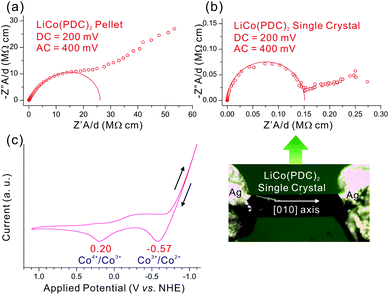
The characteristic structural feature of LiCo(PDC)2 containing Li+ cations inside the channels along a specific direction led us to examine the ionic conductivity of the compound. The electrochemical impedance spectroscopy (EIS) data measured using a pellet prepared by pressing a sample of polycrystalline LiCo(PDC)2 revealed a very low ionic conductivity value of ca. 38 nS cm−1 (see Fig. 3a). Using a large single crystal, however, we observed very interesting anisotropic conductivity along a specific direction. As shown in Fig. 3b and Table 1, a considerably large ionic conductivity of 6.1–6.8 μS cm−1 was obtained when measurements were performed along the [010] direction of the single crystal. However, when the conductivity measurements were performed along the [100] and [001] crystal facets, the impedance spectra were randomly scattered and no ionic conduction was observed (see the ESI†). The conductivity observed along the [010] direction of the single crystal was nearly 170 times greater than that observed in the bulk pellet sample. The poor conductivity obtained from the pellet of LiCo(PDC)2 is attributable to the random orientation of crystallite fragments, which hinders the continuous movement of Li+ ions in a specific direction (see the ESI†). The central transition metal ion, Co3+, should be able to reversibly interchange its oxidation state to maintain charge balance as Li+ cations move through the channels. To determine the Co3+/Co4+ redox potential, electrochemical cyclic voltammetry (CV) was performed for a LiCo(PDC)2/MeOH solution. As shown in Fig. 3c, the redox potential of Co3+/Co4+ is ca. 200 mV, consistent with the ionic conductivity measured at various DC and AC potentials. Reasonable impedance spectra revealing semispherical patterns were only obtained when appropriate potentials ranging from 150 to 400 mV were applied, which strongly indicates that the applied potential is closely associated with the potential of the Co3+/Co4+ redox reactions (see the ESI†). Furthermore, we observed no ionic conduction in the absence of the applied potential. Conductivity measurements at different temperatures on the LiCo(PDC)2 pellet exhibited a characteristic Arrhenius-type activated phenomenon with an activation energy of 0.47 eV, which is comparable to the values observed from other porous materials.41–43 The temperature dependent conductivity data along with the Arrhenius plot for LiCo(PDC)2 can be found in the ESI.†
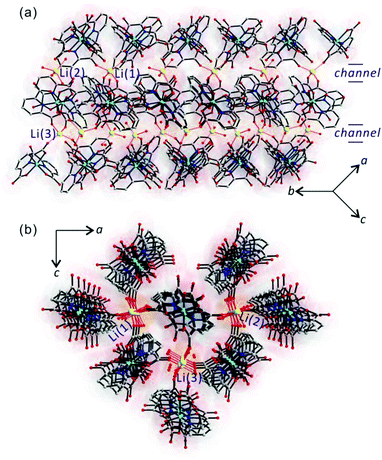
To explain the anisotropic Li+ ion conductivity, the channel structure of LiCo(PDC)2 was examined more closely. As we described earlier, channels running along the [010] direction were observed in LiCo(PDC)2 (see Fig. 4). Within the channels, Li+ cations are arrayed in an ordered fashion. Specifically, Li(1)+ interacts with four carboxylate oxygen atoms, Li(2)+ interacts with four water oxygen atoms, and Li(3)+ comes into contact with two carboxylate oxygen and two water oxygen atoms. However, no possible pathways for Li+ cations exist along the [100] and [001] directions (see Fig. 1 and 4). Thus, Li+ ion conductivity is only observed when the measurement is performed along the [010] direction.
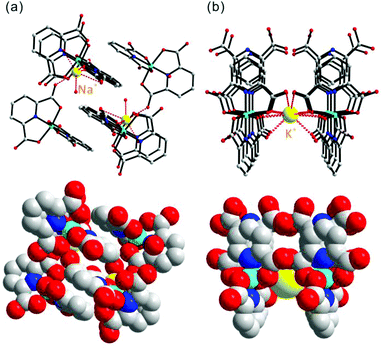
To examine the ionic conduction behaviors of other cations, large single crystals of stoichiometrically equivalent compounds, ACo(PDC)2 (A = Na+, K+, and H3O+), were grown in similar hydrothermal reactions. Detailed syntheses, crystal structures, and full characterization of all molecular compounds are included in the ESI.† The EIS measurement data for the NaCo(PDC)2, KCo(PDC)2, and [H3O]Co(PDC)2 crystals, however, did not indicate any meaningful conductivity, even though the measurements were performed along all three crystallographic directions (see the ESI†). The different conductivity phenomena of these compounds may be attributed to their structural characteristics. Although the stoichiometrically identical cobalt coordination compounds, ACo(PDC)2, have similar molecular structures composed of Co3+ and PDC ligands, their macroscopic crystal packings are dissimilar due to the different sizes of the alkali metal or hydronium cations. In NaCo(PDC)2, strong interactions between the Na+ cations and six oxygen atoms from the carboxylate ligands and water molecules do not create any channel structure (see Fig. 5a). Isostructural KCo(PDC)2 and [H3O]Co(PDC)2 exhibit channels along the [100] and [010] directions (see Fig. 5b). However, as shown in Fig. 5b, the larger cations, K+ or H3O+, are tightly encapsulated by eight oxygen atoms from the four carboxylate groups of the PDC ligands. Because the bulkier cations are locked in place within the channels, the crystals do not exhibit any ionic conduction.
Conclusions
In summary, large single crystals of stoichiometrically equivalent cobalt coordination compounds, ACo(PDC)2 (A = Li+, Na+, K+, and H3O+), were successfully grown via hydrothermal reactions. LiCo(PDC)2 exhibited interesting anisotropic ion conductivity along the [010] direction, whereas crystals containing other larger cations did not exhibit ionic conductivity. Our systematic studies suggest that the presence of ion channels with a suitable size for ion transport is crucial for the directional conductivity. These fundamental studies can be a possible basis for promising applications in useful electrochemical materials, such as rechargeable batteries and solid electrolytes.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (MSIP) (Grant No. 2016R1A2A2A05005298) and the DGIST R&D Program of the MSIP (Grant No. 16-HRMA-01 and 16-BD-0403).Notes and references
- M. S. Whittingham, Chem. Rev., 2004, 104, 4271–4301 CrossRef CAS PubMed.
- M. S. Whittingham, Chem. Rev., 2014, 114, 11414–11443 Search PubMed.
- D. R. Payne and P. V. Wright, Polymer, 1982, 23, 690–693 Search PubMed.
- M. Park, X. C. Zhang, M. D. Chung, G. B. Less and A. M. Sastry, J. Power Sources, 2010, 195, 7904–7929 Search PubMed.
- J. B. Goodenough, Acc. Chem. Res., 2013, 46, 1053–1061 Search PubMed.
- K. Xu, Chem. Rev., 2004, 104, 4303–4417 Search PubMed.
- H. Tukamoto and A. R. West, J. Electrochem. Soc., 1997, 144, 3164–3168 Search PubMed.
- S. M. Haile, D. A. Boysen, C. R. I. Chisholm and R. B. Merle, Nature, 2001, 410, 910–913 CrossRef CAS PubMed.
- G. L. Athens, D. Kim, J. D. Epping, S. Cadars, Y. Ein-Eli and B. F. Chmelka, J. Am. Chem. Soc., 2011, 133, 16023–16036 Search PubMed.
- J. Le Bideau, L. Viau and A. Vioux, Chem. Soc. Rev., 2011, 40, 907–925 Search PubMed.
- A. R. West, Solid State Chemistry and Its Application, Wiley, West Sussex, UK, 2nd edn, 2014 Search PubMed.
- K. Mizushima, P. C. Jones, P. J. Wiseman and J. B. Goodenough, Mater. Res. Bull., 1980, 15, 783–789 Search PubMed.
- N. Kamaya, K. Homma, Y. Yamakawa, M. Hirayama, R. Kanno, M. Yonemura, T. Kamiyama, Y. Kato, S. Hama, K. Kawamoto and A. Mitsui, Nat. Mater., 2011, 10, 682–686 CrossRef CAS PubMed.
- A. Manthiram, Y. Z. Fu, S. H. Chung, C. X. Zu and Y. S. Su, Chem. Rev., 2014, 114, 11751–11787 Search PubMed.
- J. C. Bachman, S. Muy, A. Grimaud, H. H. Chang, N. Pour, S. F. Lux, O. Paschos, F. Maglia, S. Lupart, P. Lamp, L. Giordano and Y. Shao-Horn, Chem. Rev., 2016, 116, 140–162 Search PubMed.
- K. D. Kreuer, S. J. Paddison, E. Spohr and M. Schuster, Chem. Rev., 2004, 104, 4637–4678 Search PubMed.
- O. M. Yaghi, H. Li, C. Davis, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474–484 Search PubMed.
- M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675–702 Search PubMed.
- N. C. Jeong, B. Samanta, C. Y. Lee, O. K. Farha and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 51–54 CrossRef CAS PubMed.
- S. Horike, D. Umeyama, M. Inukai, T. Itakura and S. Kitagawa, J. Am. Chem. Soc., 2012, 134, 7612–7615 Search PubMed.
- S. Horike, D. Umeyama and S. Kitagawa, Acc. Chem. Res., 2013, 46, 2376–2384 Search PubMed.
- T. Yamada, K. Otsubo, R. Makiura and H. Kitagawa, Chem. Soc. Rev., 2013, 42, 6655–6669 Search PubMed.
- M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343–1370 Search PubMed.
- P. Deria, J. E. Mondloch, O. Karagiaridi, W. Bury, J. T. Hupp and O. K. Farha, Chem. Soc. Rev., 2014, 43, 5896–5912 Search PubMed.
- P. Ramaswamy, R. Matsuda, W. Kosaka, G. Akiyama, H. J. Jeon and S. Kitagawa, Chem. Commun., 2014, 50, 1144–1146 Search PubMed.
- M. Sadakiyo, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2014, 136, 13166–13169 CrossRef CAS PubMed.
- J. J. Gassensmith, J. Y. Kim, J. M. Holcroft, O. K. Farha, J. F. Stoddart, J. T. Hupp and N. C. Jeong, J. Am. Chem. Soc., 2014, 136, 8277–8282 Search PubMed.
- H. K. Kim, W. S. Yun, M. B. Kim, J. Y. Kim, Y. S. Bae, J. Lee and N. C. Jeong, J. Am. Chem. Soc., 2015, 137, 10009–10015 Search PubMed.
- D. R. MacFarlane, J. H. Huang and M. Forsyth, Nature, 1999, 402, 792–794 Search PubMed.
- Y. Abu-Lebdeh, P. J. Alarco and M. Armand, Angew. Chem., Int. Ed., 2003, 42, 4499–4501 Search PubMed.
- R. L. Kerr, S. A. Miller, R. K. Shoemaker, B. J. Elliott and D. L. Gin, J. Am. Chem. Soc., 2009, 131, 15972–15973 Search PubMed.
- L. Y. Jin, K. M. Nairn, C. M. Forsyth, A. J. Seeber, D. R. MacFarlane, P. C. Howlett, M. Forsyth and J. M. Pringle, J. Am. Chem. Soc., 2012, 134, 9688–9697 Search PubMed.
- B. E. Kidd, M. D. Lingwood, M. Lee, H. W. Gibson and L. A. Madsen, J. Phys. Chem. B, 2014, 118, 2176–2185 Search PubMed.
- Y. Kiyota, T. Kadoya, K. Yamamoto, K. Iijima, T. Higashino, T. Kawamoto, K. Takimiya and T. Mori, J. Am. Chem. Soc., 2016, 138, 3920–3925 Search PubMed.
- H. Matsui, Y. Ohhta, C. Iida, M. Horii and M. Tadokoro, J. Phys. Soc. Jpn., 2010, 79, 103601 Search PubMed.
- M. Tadokoro, Y. Ohhata, Y. Shimazaki, S. Ishimaru, T. Yamada, Y. Nagao, T. Sugaya, K. Isoda, Y. Suzuki, H. Kitagawa and H. Matsui, Chem. – Eur. J., 2014, 20, 13698–13709 Search PubMed.
- Y. Takahashi, Y. Gotoh, J. Akimoto, S. Mizuta, K. Tokiwa and T. Watanabe, J. Solid State Chem., 2002, 164, 1–4 Search PubMed.
- F. Sauvage, J. M. Tarascon and E. Baudrin, J. Phys. Chem. C, 2007, 111, 9624–9630 Search PubMed.
- J. Y. Li, W. L. Yao, S. Martin and D. Vaknin, Solid State Ionics, 2008, 179, 2016–2019 Search PubMed.
- Y. Chen, Z. Yang, X. Y. Wu, C. Y. Ni, Z. G. Ren and J. P. Lang, Phys. Chem. Chem. Phys., 2011, 13, 10781–10786 Search PubMed.
- T. Yamada, M. Sadakiyo and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 3144–3145 Search PubMed.
- M. Sadakiyo, T. Yamada and H. Kitagawa, J. Am. Chem. Soc., 2009, 131, 9906–9907 Search PubMed.
- J. H. Park, K. Suh, M. R. Rohman, W. Hwang, M. Yoon and K. Kim, Chem. Commun., 2015, 51, 9313–9316 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details for synthesis and characterization of the reported materials. CCDC 1476207–1476210. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qi00314a |
| ‡ S. B. Kim and J. Y. Kim contributed equally to this work and are co-first authors. |
| This journal is © the Partner Organisations 2017 |