Metal oxides and polysaccharides: an efficient hybrid association for materials chemistry†
B.
Boury
* and
S.
Plumejeau
CMOS-Institut Charles Gerhardt, Université Montpellier 2 CC1701, Pl E. Bataillon, 34090 Montpellier, France. E-mail: bruno.boury@univ-montp2.fr
First published on 4th September 2014
Abstract
Metal oxides and polysaccharides in nature and in laboratories: limits and aims of the review. Part 1: Different ways to associate metal oxides and polysaccharides. Part 2: Controlled growth of metal oxide nanoparticles throughout polysaccharide fibers. Part 3: Biotemplating and bio-replication on the micro- to nanoscale. Part 4: Chemical transformation of polysaccharide fibers by mineralisation. Perspectives and concluding remarks. Biopolymers and inorganic minerals are often associated in nature, and living organisms benefit from these materials with a sophisticated and hierarchical architecture. Inspired by nature, chemists have tried to extend these combinations by associating natural polymers with inorganic materials that do not occur naturally in living organisms. In this review, we propose to focus only on research conducted on the association between polysaccharides and metal oxides. Over the last 10–15 years, substantial research has been focused on finding ways to combine these two types of materials, with the goal of mastering the morphology, porosity, composition and structure of the hybrid materials (metal oxide@polysaccharide) or pure metal oxides obtained after polysaccharide elimination. There are many possibilities for interactions between metal cations and the chemical functionality of the carbohydrate, thus allowing different approaches, as the structure and functionality of the polysaccharide are of major importance. Because of the sophisticated architecture that can be achieved on the one hand, and the potential sustainable use of these biopolymers (a green approach) on the other hand, these material elaboration processes offer a unique way for chemists to prepare functional hybrid materials and metal oxides (e.g. luminescent materials, catalysts, absorbent materials, magnetic composites, anode and photocatalyst materials). To be as comprehensive as possible, this review is limited to some natural polysaccharides. After contextualisation, we successively considered metal oxide growth control by biotemplating, the replication of raw and refined polysaccharide templates, ending with a discussion of the most recent approaches like mineralisation.
Introduction
Biopolymers and metal oxides
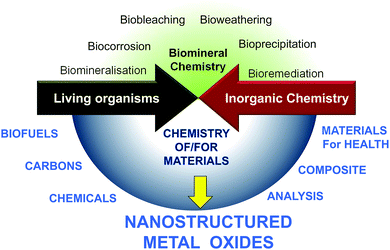
Living organisms depend on abiotic factors such as the composition of inorganic solids with which they are in contact, and in turn they contribute to transformation.7 At the same time, very early in evolution, inorganic materials were incorporated into structures of living beings such as exoskeletons of diatomaceous organisms, shells or corals,8 endoskeletons of sponges, fishes and animals,9 macro- or nanoparticles (otoliths of fish and mammals, magnetosomes of magnetotactic bacteria).4,6,10 However, besides what we learn from nature, chemists can now consider new types of mineral/biomaterial associations with objectives that differ completely from those found in living organisms.A limited number of minerals are used by living organisms (mainly silicon oxide, carbonates, and some phosphates, oxalates and sulfides), whereas the chemist takes advantage of every opportunity offered by the elements of the periodic table. Contemporary chemists also strive to use all sources of renewable materials and the ultimate goals of chemists when using materials may differ completely from the natural functions of the same materials. Therefore, besides the substantial amount of research focused on the natural interaction between mineral and living processes (upper part of Fig. 2),8,9,11–14 the field of research with the aim of producing new materials required for energy, health and environmental applications is growing (Fig. 1). In nature, metal oxides are generally not closely associated with polysaccharides but more with proteins, with the latter currently being studied for their involvement in the nucleation and growth of inorganic materials in living organisms.11,15–18 MO@PS therefore represents a new field of research.
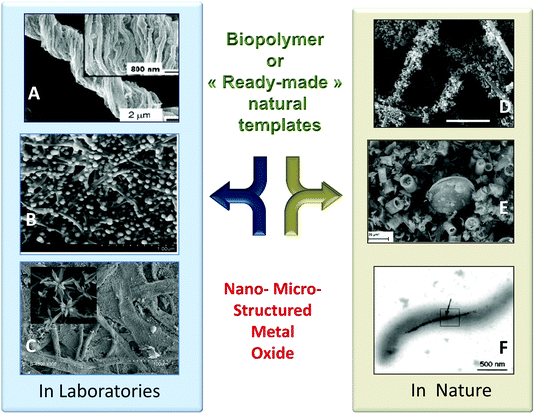 | ||
| Fig. 1 Example of PS@MO synthesised in laboratories (A, B, C) and found in nature (D, E, F). (A) Titania replica of natural cotton fibers by sol-gel impregnation (Reprinted with permission from ref. 1, Copyright (2003) American Chemical Society). (B) Dispersion of ZnO particles along bacterial cellulose fibers (Reprinted from ref. 2, Copyright (1959), with permission from Elsevier). (C) TiO2 replica by mineralisation of filter paper with TiCl4 under anhydrous conditions (Reprinted from ref. 3, Copyright (2013), with permission from Wiley and Sons). (D) Uranium oxide nanoparticles on fungal hyphae (Reprinted from ref. 3, Copyright (2007), with permission from Wiley and Sons). (E) Natural diatomite from a Borownica deposit in Poland (Reprinted from ref. 5, Copyright (2010), with permission from Elsevier). (F) Magnetite nanoparticles in Magnetospirillum magnetotacticum (From ref. 6, Reprinted with permission from AAAS (Copyright 1998)). | ||
Context and objectives of the review
Plant fossilisation studies and the characterisation of cellulose structures were probably the first investigations related to this field, followed quickly after by the use of soluble polysaccharides in the sol–gel process and the templating of natural fibers and crystals. The interest in MO@PS is the result of knowledge and processes concerning polysaccharides and also the demand for metal oxides, mixed- or doped-metal oxides and even MO@C composites.MO is high in demand for industrial and high-tech applications, their properties being substantially altered by the porosity, crystallinity, morphology, doping, particle size and, last but not least, their arrangement in hierarchical structures from the macro- to the nano- and micro-scale (for example in the case of TiO2,19,20 and other oxides in electrochemical devices21 or sensors22).
On the other hand, polysaccharides are hugely diversified in terms of structure and functionality, and those not requiring purification are among the “greenest” possible materials. Their shaping at the nanoscale has also definitively opened a new area with the production of nanocrystals, nanorods, nanoplatelets, nanofibers or aerogels of various biopolymers.23–28 Recently, a new type of polysaccharide was introduced in this field, i.e. exopolysaccharides (EPS) produced by microalgae and cyanobacteria for their phototaxis, has been used for photo-patterning and mineral replica.29,30
Therefore, at the crossroads between these two materials science domains (MO and PS) there are different fields of research: enhancing the conversion of PS into renewable fuels and chemicals, transforming PS into functional carbons, using them as modifiers of the MO surface, combining them into nanocomposites, etc.
Here we assess the usefulness of PS in the MO preparation process to control MO synthesis, in terms of size, morphology, porosity, composition, etc. The interplay between MO and PS is at a different level: in controlling the chemistry leading to MO formation, in MO molding (morphology, size, porosity, etc.), and also in the chemistry that is involved upon PS elimination or transformation.
Limits of the review
We will not consider the following issues, for which the reader is directed to possible references (among others) and that are closely connected to the topic of this review: micro- and nano-casting of wood31–37 or textiles38 by metal oxide powders, impregnation of wood as precursors of SiC or SiOC,39 PS-to-carbon synthesis,40–45 PS-to-biofuel and chemical synthesis,46 preparation of catalysts,47 PS in electro-conductive composites,48 MO@PS as a material for biotechnology for enzyme support,49 bacteria encapsulation,50–53 coating of MO nanoparticles by PS or modified-PS (for diagnosis, therapy, catalysis and remediation).54–56 Interested readers are also referred to the literature on magnetic nanoparticles,57,58 or metal and metal hydroxides.27,59–61To be comprehensive as much as possible, we limited this minireview to a few natural polysaccharides like cellulose, chitosan, chitin, amylose (starch), alginate and carrageenan. Related publications with a broader scope are also recommended.24,62–68
Part 1: the different ways to associate metal oxides and polysaccharides
In nature, microorganisms can form deposits of nanoparticles of different inorganic compounds, and in some cases of metal oxides or hydroxides of metals such as iron, manganese or uranium, these processes are important for biogeochemical cycles of metals throughout the environment.4 Mineralisation processes generally involve proteins, but some of them are glycosylated.69,70In laboratories, among the earliest work dealing with associations of cellulose and inorganic materials, one can mention, back in the 1940s, the infiltration of cellulose with inorganic salts to produce a “metallic shadow casting” to improve the contrast of the cellulose in the early age of microscopy.71,72 The formation of inorganic phases was not pursued at that time. Later, different researchers, focusing on assessing the fossilisation process of wood and algae, performed infiltration and impregnation of these materials to produce their silification.73 Amongst early work on the formation of metal oxides with polysaccharides one can mention the patent of Union Carbide74,75 by Elliot and Huggins on NaAlO2–Al2O3.76
Otherwise, progress in the sol–gel “Chimie douce” and periodic mesoporous silica fields77,78 has also probably generated ideas. Before the use of glucose as a template in the synthesis of mesoporous silica,79,80 polysaccharides were first considered as templates that can control the growth of functional MO particles at the nanoscale, as an alternative to the Pechini method: photocatalyst TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81,82 and magnetic Fe2O3 nanoparticles prepared with cellulose or alginate,83 superconductor YBaCuO prepared with cellulose84,85 or luminescent LaMnO3 prepared with starch.60 Sol–gel remains the most suited process to take advantage of PS, but alternative approaches (PVD, ALD, mineralisation) recently paved the way for alternative formation of MO@PS hybrids and templated metal oxides (see Part 3).
81,82 and magnetic Fe2O3 nanoparticles prepared with cellulose or alginate,83 superconductor YBaCuO prepared with cellulose84,85 or luminescent LaMnO3 prepared with starch.60 Sol–gel remains the most suited process to take advantage of PS, but alternative approaches (PVD, ALD, mineralisation) recently paved the way for alternative formation of MO@PS hybrids and templated metal oxides (see Part 3).
Sol–gel is indeed well suited for coating complex shapes, but the use of PS in sol–gel processes goes far beyond this application. It quickly became evident that PS have several advantages during the sol–gel process: (a) they are compatible with many green chemistry principles, (b) PS provides flexible scaffolds/porogens for monolithic nanocomposites and can be easily removed, (c) their hydroxyl groups are reactive in promoting/accelerating polycondensation, or in sequestering cations [Mn+] or hydroxylated cations [M(OH)m+] that can undergo nucleation/growth processes, somewhat similarly to citric acid in Pechini's process, (d) entanglement of PS fibers defines a macro- to nano-cavity that can stabilize nascent MO and control their growth, (e) many simple PS characteristics (MW, solubility, functionality, concentration, etc.) can induce different MO structures and properties.
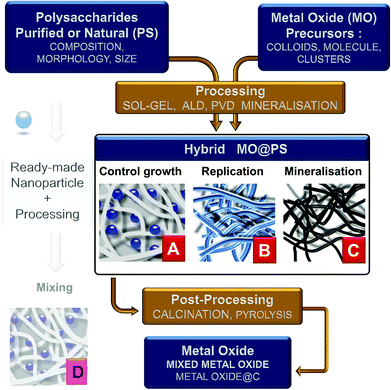
Four main different approaches can be tentatively classified as follows:
(1) the use of dissolved PS in homogeneous solution or as hydrogels into which a metal oxide precursor is introduced. Therefore, the polysaccharide is expected to control the chemistry and growth of the nanoparticles (situation A in Fig. 3);
(2) PS is used as an insoluble template onto which a more or less dense layer of metal oxides is formed/deposited. Here the PS fibers are considered as a mold for the preparation of hollow or porous oxide structures (situation B in Fig. 3);
(3) the reaction and therefore the chemical transformation of the fibers with a metal-containing reagent. In contrast to the two previous approaches, here the polysaccharide is a template and an O-source for oxide formation (situation C in Fig. 3);
(4) the impregnation of polysaccharide fibers by ready-made MO nanoparticles as a filler (situation D in Fig. 3). This is frequently carried out for the treatment of textiles, packaging, papers, etc. This is a whole world in itself and is beyond the scope of this review; see, for example, pre-formed silicalite nanoparticles mixed in starch gel,86,87 or preformed luminescent particles LaVO4:Ln3+ nanorods in cellulose gels,88 or preformed silica nanoparticles for the preparation of chitosan-silica for separation membranes,89 or chitin/TiO2 needles for tissue engineering scaffolds.90
This classification does not account for all situations, thus there is no clear limit between a deposit of some nanoparticles distributed along the fibers and complete coverage of these fibers by these nanoparticles. In addition, it is sometimes difficult to distinguish between PS “truly dissolved”, fibers that form a gel (more or less solvated and concentrated) and “totally insoluble” fibers. This question is becoming even more complicated because of two advances: new nano-sized polysaccharides (nanocrystals, nanofibers, aerogels) and new media in which fibers are “dissolved”, e.g. NaOH/urea,91 DMSO/TBAF,92 or ionic liquids.93,94 Regarding this, there is growing interest in the preparation of hybrid PS/MO by electro-spinning of blends, e.g. ZnO–SnO2@Cellulose acetate95 and SiO2@Chitin.96
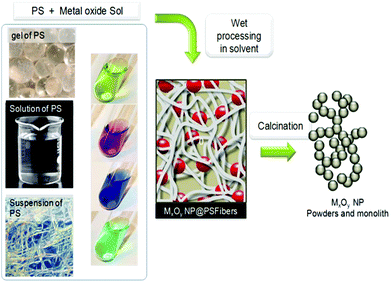
The last step of the process is calcination to remove the PS template at generally 500–600 °C. Combustion of their organic scaffold likely forms dispersed oxides in conditions under which sintering is not favored. To remove the PS, other means have been explored: dissolution in complex media, or enzyme treatment like in the case of dextran removed by dextranase.97 Ionic liquids could be alternatives that remain to be explored with this purpose.93 This makes possible the recycling of the PS, but so far, this has not been reported yet, probably due to no economical interest with regard to the cheapness of PS and the cost of such recycling.
Thermal oxidation of PS is considered as globally benign or with low impact by itself on MO. However, all PS do not behave equally, for example thermal decomposition of alginate is sensitive to cations with the formation of metal oxalate,98 and differs from chitosan, chitin,99,100 or cellulose.101 This situation could be affected by at least two factors, i.e. MO (as a crust, nanoparticles or free ions) and PS-induced nano-sizing. Much attention should be paid to this step and could lead to “element-doping” with PS.
Consequently, the next section (Part 2) is devoted to a range of combinations dealing with soluble and jellified polysaccharides. Data clearly identifying replication of insoluble natural or refined polysaccharide fibers are presented in Part 3, while Part 4 concerns mineralisation through chemical reactions of PS.
Part 2: controlled growth of metal oxide nanoparticles throughout polysaccharide fibers
This approach, schematically depicted in Fig. 4, takes advantage of the functionality of polysaccharides to control hydrolysis/polycondensation and nucleation growth processes – it is sometimes called “in situ precipitation”. It benefits from the fact that polysaccharides are able to absorb metal cations.102–105 This absorption may occur through different modes: complexation, solvation, M–OH⋯HO–PS H-bonding and also depends on the PS functionality, and the experimental procedure (pH and concentration) (illustration in Fig. 5).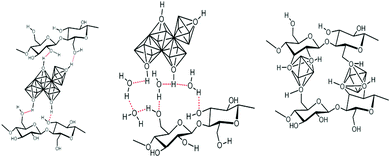 | ||
| Fig. 5 Schematic representation of the possible interaction between functions of polysaccharide units and metal-containing species. | ||
It has long been recognized that “a distinguishing feature of polysaccharides is that they sharply accelerate the kinetics of sol–gel processes.… Such acceleration is attributed to a catalytic effect of polysaccharides on the silica synthesis.”106 Various studies have shown the formation of metal–cellulose complexes, e.g. Fe(III)–cellulose,107 Cu(II)–cellulose,108 Zn(II)–cellulose,109 Pb(II) and Cd(II),110 or Cr(III) and Al(III).111 This could improve or introduce properties of the cellulose (mechanical, absorbent, anti-microbial and -fungal, UV self-cleaning, dissolution), but could also be detrimental to its stability.112 These authors highlighted the important role of both the pH and the residual carboxylic group of the cellulose.
In this context, the effect of the type of PS on MO nanoparticles is an important question. For example, in pioneering work on the preparation of maghemite with polysaccharides, it was observed that “starch and guluronic-rich alginate retains large amounts of iron … compared to chitosan”.113
More recently, the structure and functionality of the polysaccharide were found to not only modify the microstructure and morphology of TiO2, but they also induced a different phase, with rutile being obtained using β-cyclodextrin and chitosan, anatase being obtained using starch.114 Comparisons of this type are unfortunately still rare. The possibility of combining two PS is also limited to very few examples (see the case of improved chemical and mechanical stability of Fe3O4 particles55). Other processes like ALD are less commonly used. In that case, the PS reactivity seems to be less involved since the metal oxide nanoparticles are formed before their contact with PS, but their anchoring depends on the functionality of the surface.115,116
The resulting MO@Cellulose composite is sometimes the targeted material, but generally the metal oxide is targeted and recovered after calcination, unfortunately more seldomly a MO@C is sought by a pyrolytic treatment such as LiFePO4/C,117 or TiO2@C (shown in Fig. 6).118
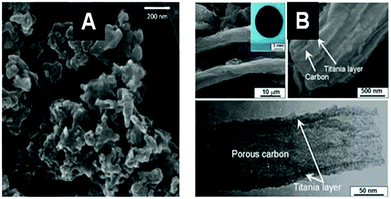 | ||
| Fig. 6 SEM (A) and TEM (B) images of an example of a MO@C composite obtained by pyrolytic treatment of the corresponding hybrid MO@PS. (A) LiFePO4@C from filter paper after impregnation with (LiNO3/FeSO4) and pyrolysis under Ar + H2 (Reprinted from ref. 117, Copyright (2013), with permission from Elsevier). (B) TiO2@C from ashless filter paper impregnated with TTB and pyrolysis under N2 (Reprinted from ref. 118, Copyright (2010), with permission from Wiley and Sons). | ||
Otherwise, a diverse range of metal oxides and supports have been explored for application or fundamental purposes. In the best cases, metal oxide nanoparticles with a ∅ < 50 nm are homogeneously dispersed along the fibers inside the micro- or nanofiber entanglement. Although the metal oxide structure has an effect, the most common morphology is round-shape, which is not the most attractive, but in some rare cases PS like cellulose allows the formation of unusual morphologies such as TiO2 cubes119 or flowers.120 Recently, cellulose in an ionic liquid was used as a new medium for metal oxide formation, showing a pronounced effect on the morphology and size when compared to aqueous processes (e.g. Na0.5K0.5NbO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121). The solvent effect on sol–gel chemistry is well-known; this is the first example of a PS-IL medium that combines the properties of PS and ILs.
121). The solvent effect on sol–gel chemistry is well-known; this is the first example of a PS-IL medium that combines the properties of PS and ILs.
MONP@Cellulose . (See Table 1: MO@Cellulose devoted to metal oxides and Table 2: Mixed-MO@Cellulose devoted to mixed metal oxides in ESI.†) Cellulose is the most popular polysaccharide. It is found in organisms ranging from bacteria to animals, forming a polymeric chain made of β(1→4) linked D-glucose units.
The preparation of Fe2O3/Fe3O4,125,126 or YBaCuO84,85 with cellulose is among the first studies on this topic and examples of MONP@Cellulose before calcination are presented in Fig. 7. No benchmark has been established in this area concerning the type of cellulose, but cellulose pulp (or purified) is frequently used and bacterial cellulose is now increasingly popular as a nano-sized PS.
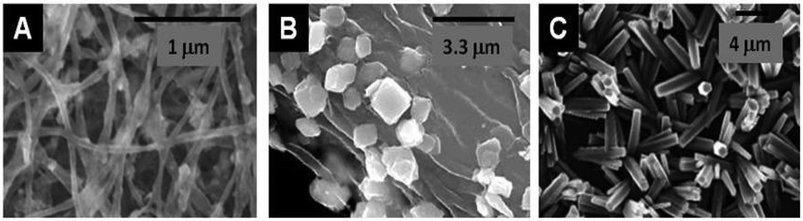 | ||
| Fig. 7 SEM images of examples of different metal oxide nanoparticles assembled with or produced from cellulose-type PS (A) Fe3O4@BNC (from Acetobacter xylinum) (Reprinted from ref. 122, Copyright (2011), with permission from Elsevier); (B) BiVO4@Cellulose (from Eucalyptus globulus wood cellulose fibers) (Reprinted from ref. 123, Copyright (2005), with permission from Elsevier); and (C) ZnO@Cellulose layer (from filter paper).124 | ||
The use of cellulose avoids having to rely on a catalyst in many cases. Besides this common observation and the effectiveness of using cellulose in conjunction with sol–gel, there are two other issues that have been less discussed and need more studies. The first concerns the effect of cellulose degradation on calcination, which is an important point for some applications. For example, in the case of phosphor, some residual carbon can be present depending on the type of cellulose and the temperature, with an impact on the luminescence.127,128
The second point is the potentially reducing behavior of cellulose, depending on the temperature and atmosphere. Indeed, cellulose is clearly recognized as being a reducing agent in the formation of metal nanoparticles like Pt,129 Au,61 or Ag130 and when metal cations with high oxidation potential are involved in the effective reduction of hydroxyl groups, e.g. see the case of Mn(VII),131,132 leading possibly to aldehyde and carboxylic functions, or C–C bond cleavage.
MONP@Alginate . (See Table 3: MO@Alginate in ESI.†) Alginate is extracted from brown seaweed and consists of mannuronic and guluronic acid residues randomly sequenced along the polysaccharide chain; it is also used in many ways for different materials.133,134 Examples of MO prepared with alginates are shown in Fig. 8. It is generally recognized that the buckled polyguluronate segments of alginate strongly bind [Mn+] multivalent metal cations, preferentially to Na+, to form microcrystalline arrays through a mechanism known as the ‘egg-box’ model.135
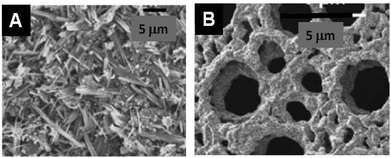 | ||
| Fig. 8 SEM images of examples of different metal oxide nanoparticles assembled with or produced from alginate-type PS. (A) La0.67Sr0.33MnO3 with sodium alginate140 and (B) CuO after calcination of Cu-alginate aerogel from sodium alginate (Reprinted from ref. 138, Copyright (2007), with permission from Elsevier). | ||
Prior works targeted the formation of paramagnetic beads of alginate by reaction with iron salt for the potential preparation of biotechnology materials,113,136,137 extended now to many other oxides. Basically alginates like chitosan are frequently processed as true solutions or after jellification by the addition of multivalent cations. An important novelty in the process concerns the use of supercritical CO2 drying of the gel after metal oxide formation. These conditions preserve the shape and high specific surface area of the material before calcination.
In some cases, “no memory of the fibrillar structure of the polysaccharide can be observed in the morphology and organization of oxide crystals”,138 but in the preparation of YBa2Cu4O8 “there is more evidence of the effect of either the polyguluronate/polymannuronate ratio or the counter cation (ammonium/sodium) on metal oxide formation.”139,140 Again, the nature of the oxide is critical in the ability to control its morphology from the PS. Interestingly, the chemical reactivity of alginate seems to be involved at two levels: first the carboxylate would act as a reducing agent, as proposed in the well-known citrate method (Pechini's method), and secondly the decomposition polymer products help prevent significant mass transport and sintering to leave sponge-like material.27,141
MONP@Chitosan and chitin . (See Table 4: MO@Chitosan or chitin in ESI.†) Chitosan is an abundant polysaccharide obtained by deacetylation of chitin, a naturally occurring polysaccharide found in fungi and the exoskeleton of insects and crustaceans, already used for many applications (food, cosmetics, biomedical and pharmaceutical applications142).
In early work, chitosan was mixed with ready-made Fe3O4 nanoparticles to produce a polyelectrolytic support for cell separation processes.143 Earlier, SiO2@Chitosan was targeted as artificial skin due to the high oxygen permeability of such a “membrane”,144,145 but recent work focuses on TiO2 as MO (example shown in Fig. 9A). Alternatively, and more frequently, chitosan is engaged as alcogel beads in which a precursor of the metal oxide diffuses from an alcoholic solution to give rise to MO coated chitosan hybrid microspheres. Similar to alginate, before calcination and biotemplate elimination, a key step of the process is the supercritical drying with CO2, which can prevent the collapse of the opened structure of the alcogel.146 Alternatively, chitosan can be associated with a solvothermal process.147
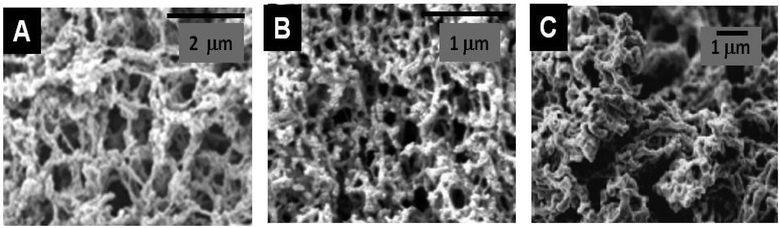 | ||
| Fig. 9 SEM images of different metal oxide nanoparticles assembled with different PS (A) TiO2@Chitosan (20% acetylation) (Reprinted from ref. 157, Copyright (2010), with permission from Elsevier); (B) TiO2 after calcination of the TiO2@Agarose composite (Reprinted with permission from ref. 158, Copyright (2006) American Chemical Society); and (C) YBa2Cu3O7@Dextran (Reprinted with permission from ref. 159, Copyright (2007) American Chemical Society). | ||
The NH2 group of the chitosan structure, a distinctive feature of this polysaccharide, is important for understanding its reactivity and chemistry. This group has been identified as one of the key components in the formation of the fibrous structure of oxides.146,148 Based on IR-data of SiO2@Chitosan, the Si–O−⋯+H2N– interaction is evidenced,149 along with high hydrogen bonding.150 The presence of the –NH2 group suggests that it could be possible to N-dope some oxides prepared with chitosan and after calcination – a situation that has not yet been reported, suggesting the loss of this element upon calcination.147
In a limited number of cases, the presence of chitosan was postulated to orient the morphology of the recovered metal oxide, e.g. in the case of YBa2Cu4O8 nanowires,151 in contrast to round-shaped nanoparticles obtained in the absence of chitosan, a key result showing the effect of PS and promoting their uses.
Chitin has been less used for MONP formation than for replication (see the next section). However, colloidal solution of chitin crystals can be combined with the sol–gel process, thus giving rise to nanocomposites and porous oxides like SiO2 and TiO2.96,152
MONP@Starch . (See Table 5: MO@Starch, MO@Dextran, MO@Carrageenan, MO@Agarose in ESI.†) Starch is produced by green plants, D-glucose sequences of D-glucose units lead to a spiral structure in linear amylose and in a highly branched structure in amylopectin. Easy to dissolve in water it is routinely combined with cellulose in the preparation of paper and nanocomposites.153
Due to its solubility, starch was tested as a “structure directing agent” or a “porogen”, offering the advantages to be easily removed with water, see for example for the preparation of Ni–BaCe0.9Y0.1O2.95 cermet fuel cell electrodes.154 Early studies used starch for the preparation of oxide Sr-doped LaMnO3 and Sr-doped La(Fe,Co)O3. The purpose is clearly to use starch as an alternative to citric acids in the Pechini method.60 It was found that starch plays an important role in the formation of homogeneously dispersed nanoparticles, while also having a marked impact on the crystal phase.114 Indeed, aqueous Ti3+ solutions lead to anatase with starch, while rutile is obtained with cyclodextrin or chitosan. Although very attractive, the interpretation of such data needs investigations to promote the use of PS.
In the case of ZnO, starch layers were found to be efficient for growing uniformly oriented nanorods, which is evidence of the strong interaction of the initial cluster oxide with the surface of such PS.124 Other studies support the assumption that nucleation and initial crystal growth occur through starch/[Metal] species, but show that it is possible to control the morphology and avoid nanorod formation.155
Starch was also combined with readymade nanoparticles (silicalite for macroporous sponge-like monoliths with meso-/macroporosity86), especially as coating to ensure their biocompatibility (SPION superparamagnetic iron oxide nanoparticles156).
MONP@Carrageenan . Carrageenans are linear sulphated polysaccharides that are extracted from red edible seaweeds, and this family has at least 10 different chemical structures depending on the number and position of the –OSO3− group (α, β, γ, ι, κ, λ, μ, δ, θ, etc.). The number of studies is limited but, interestingly, it was reported that the presence of carrageenan limits the growth of metal oxide particles in the case of Fe2O3 and that the type of carrageenan plays an important role, “with ι-carrageenan nanoparticles showing higher average sizes than κ- and λ-carrageenan.”160 These different behaviours are probably related to the number of charges (–OSO3−) per monomer unit, a key parameter for understanding the [Fe3+]/carrageenan interaction by charge neutralization between sulphate groups and hydrolyzed oxyhydroxide iron species.161 Overall, carrageenan chains crosslink via metal cations, and in turn create a protective layer around iron oxide particles, thus producing a self-assembled nanoreactor for hydrolysis and condensation of oxyhydroxide iron species.
MONP@Agarose. Agarose is generally extracted from cell walls of certain algae species with essentially galactose subunits. The alcoholic gel is used as a reaction medium for the formation of metal oxides by hydrolysis/polycondensation of metal alkoxides absorbed in the gel, and also as a soluble porogen (see an example in Fig. 9B). As in the case of other gels, the impregnation process parameters (mineral/organic ratio, number of cycles, temperature, etc.) are crucial in the diffusion of titanium precursors into templates and the maintenance of a porous structure in the resulting titania beads. This could explain the differences observed with respect to previously published findings. Interestingly, the reducing ability of agarose towards Au salt was combined with its porogen properties to generate the Au@TiO2 catalyst,162 an approach that should be generalized to other PS for the preparation of the new composite MONP@C@M'O.
MONP@Dextran is a branched polymer of dextrose (glucose) of very high molecular weight excreted by lactic-acid bacteria, with the best-known being Leuconostoc mesenteroides and Streptococcus mutans. The use of this polymer for metal oxide preparation is limited but, interestingly, in the case of YBa2Cu3O7, the use of dextran is reported to lead to a uniform crystal size and morphology and a macroporous structure (Fig. 9C). With the same PS, calcination of a freeze-dried metal oxide–dextran aqueous mixture leads to foamy porous open structures that are easy to calcine with less sintering.
Part 3: templating of polysaccharide fibers
Bio-replication, which differs from biomorphism,163 is the direct replication of a structure found in an organism. This is a very general approach to the synthesis of materials that is implemented to take advantage of hierarchical micro- and nanostructures produced by living organisms (schematically depicted in Fig. 10).12,39,64,164–169 The initial aim was to demonstrate the possibility of precisely molding “raw” natural structures with MO. This is still relevant when taking advantage of cheap raw materials via the shortest possible loop, and when preparing metal oxides with complex morphologies and hierarchical architectures (see different examples in Fig. 11). Several examples have been reported from the coarse- to the nano-scale: eggshell membranes,170 human hairs,171,172 silk fibers,173 pollen,174,175 diatoms,176 bacteria,87,177,178 viruses,179 protein cages,180,181 and collagen.182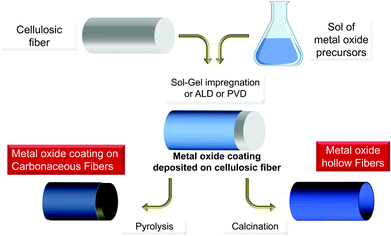 | ||
| Fig. 10 General pathway for metal oxide preparation by impregnation/deposition with PS, leading to hybrids MxOy@PS and then MxOy or MxOy@C materials upon thermal treatment. | ||
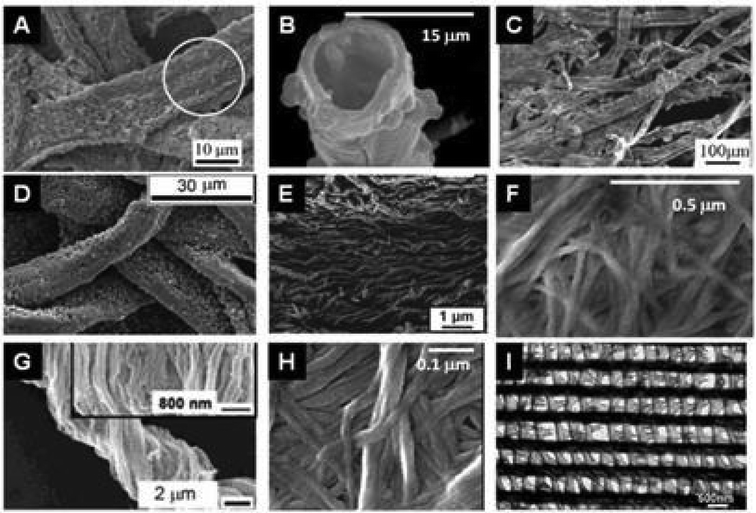 | ||
| Fig. 11 SEM images of the replication of polysaccharide fibers with metal oxides deposited by sol–gel or ALD techniques. (A) MoO3@Cellulose (commercial paper) after calcination at 500 °C (Reprinted from ref. 198, Copyright (2008), with permission from Wiley and Sons); (B) TiO2 hollow fibers after calcination of TiO2@Cellulose (woven cotton);189 (C) MnO2@Cellulose from lens paper before calcination (Reprinted with permission from ref. 199, Copyright (2011) American Chemical Society); (D) ZnO@Cellulose from filter paper before calcination (Reprinted from ref. 200, Copyright (2011), with permission from Wiley); (E) SiO2 nanotubes after calcination of SiO2@Cellulose from filter paper (Reprinted with permission from ref. 201, Copyright (2011) American Chemical Society); (F) Fe3O4@Cellulose from bacterial cellulose of G. xylinum (Reprinted from ref. 202, Copyright (2011), with permission from Elsevier); (G) TiO2 after calcination of TiO2@Cellulose from natural cotton;1 (H) V2O5@Cellulose from bacterial G. xylinum before calcination (Reprinted from ref. 203, Copyright (2013), with permission from Springer); and (I) Al2O3 replicas after calcination of Al2O3@butterfly wing by ALD (Reprinted with permission from ref. 201, Copyright (2006) American Chemical Society). | ||
In such replication processes, only the outermost part of the surface of the PS is supposed to be involved in the chemical processes. This can be assumed to occur in the same manner as the process involved in the controlled growth of MONP above: by complexation/solvation of cation or hydroxycation species through the organic function of the biotemplate (carboxylate, hydroxyl, amine and amide groups, etc.). In the case of ZnO, for example, it was found that “cellulosic fibers act as hydrophilic substrates for the heterogeneous nucleation of ZnO”;183 and this surface can be pre-seeded with metal salt.184,185
The inner part of the fibers is probably not involved in these nucleation and seeding processes. Calcination is generally used to isolate the inorganic replica but pyrolysis could be of interest in the preparation of MO@C composites. “Biotemplating” or “bioreplicas” are therefore appropriate, in contrast to “mineralisation”, “petrifaction” or “fossilization”, which should be limited to effective chemical transformations of the template in its massive part.
PS fibers are replicated through a sol–gel process, but a few exceptions are reported with other techniques, leading to a thick MO deposit of 20 to several nm, covering the template, and made of round shaped and aggregated nanoparticles of ∅ < 50 nm. Besides the many different supports highlighted in the table, different “modified cellulose” supports have also been used, e.g. cellulose acetate.186–188
TiO 2 -Cellulose. (See Table 6 Biotemplating of cellulosic fibers for TiO2@Cellulose for TiO2 formation in ESI.†) Cellulose fibers are the most popular biotemplates here because of their insolubility, which render them more appropriate for bio-replication than for nanoparticle growth control. Otherwise, the most frequently targeted oxide is TiO2 due to the numerous potential applications of this oxide: sensors for hydrogen, oxygen, humidity, glucose and hydrogen peroxide, anodes for electrochemical devices, and photocatalysts for oxidation and reduction. In pioneering studies, TiO2 micro- or nanotubes were already targeted with cotton fibers as the template.189,190
MO replication is achieved mainly by the impregnation or suction/filtration technique using a sol of TiO2-precursors, the experimental details being one of the knowhow of the authors. An alternative to this process is hydrolysis/condensation of the MO precursor by exposure to air or moisture once it has been absorbed by the cellulose fibers.191,192 The third possibility involves the ALD process, which can be combined with multi-oxide formation and for which the presence of the hydroxyl group at the cellulose surface seems to “simplify nucleation of water-based ALD processes”.193
Calcination (generally at 500 °C) is used to remove the template but the possibility of removing the cellulose by urea/NaOH treatment has also been reported.194 Generally, anatase is the common phase but rutile has been reported in one case, using flame calcination after impregnation, although it was recognized that “the cellulose template plays key role not only in obtaining the nanotubular structures, but also in achieving smaller particle sizes”.195 Exceptionally, cellulose is also considered as a source of carbon that could lead to a C@TiO2 nanocomposite (anatase + rutile),196 this could be further developed if considering the new materials required for energy and catalysis.
Besides relatively refined cellulosic materials, raw materials directly collected from nature, essentially plant parts, were biotemplated. In these cases, besides the cellulose-rich part of the body (skin, leave seeds, pith, etc.), other biopolymers or salts can be present and interfere in the process. Despite or because of this, some interesting and effective properties have been reported, giving rise to a direct and greener process, mainly for the potential preparation of photocatalysts (see Table 7 Biotemplating of plants for TiO2@Plant and TiO2 formation in ESI†).
M x O y -Cellulose. (See Table 8 Biotemplating of Cellulose for MO@Cellulose and MO formation in ESI.†) Sol–gel processing is the most commonly used, note that chemical modification of cellulose can be performed to enhance the formation of, for example, SiO2 around fibers.197 The ALD technique is attractive, it provides a possibility to adjust the thickness of the metal oxide layer by increasing or decreasing the number of cycles, more precise than sol–gel processing.
M x O y @PS and M x O y @modified-PS or M x O y @raw material . Cellulose is a kind of benchmark to test bio-replication, therefore other polysaccharides are less reported also because some are soluble in water.
Chitin, more than chitosan, is often used due to its abundance in chitinized exoskeletons. Butterfly wings have been templated by different oxides: SiO2 (CVD),205 Al2O3 (ALD),204 TiO2 (impregnation),206 TiO2 (ALD),207 Sn–TiO2 (sol–gel),208 SnO2 (sol–gel),209 ZnO (sol–gel),210,211 BaTiO3 (LbL),212 TiO2 and SiO2 (sol–gel).213 Other chitin biotemplates have also been tested like beetle cuticles in weevil and longhorn families, and used for the production of inorganic photonic structures by a sol–gel biotemplating method.214 Besides arthropods, chitin is also found in marine organisms and biotemplating of a macroporous β-chitin sponge-like monolith of cuttlebone was performed by acidic demineralisation after impregnation with sodium silicate, which provided good replication control.215 The objective in all cases is to take advantage of the unique hierarchical structure of the biotemplate, especially for photonic materials, since hierarchical porosity is beneficial for catalysis or electrodes. The ALD process was used to deposit Al2O3 on spines of sea mouse (Aphrodita aculeata) made of chitin and proteins.216
Chitosan flakes have recently been reported as insoluble biotemplates for the preparation of bulk and mesoporous silica. Here again, the role of the amino group is a key for understanding the role of the biotemplate.217
At that point, the wood structure replication can be mentioned, but because the template is a raw mixture of non-purified biopolymers, it will not be detailed. Since the early work of Drum et al. on the silicification of betula wood by an aqueous solution of silicate,218 many other wood/MO couples have been investigated, leading to the preparation of MO with complex and hierarchical structures: pine wood (templating Al2O3, TiO2 and ZrO2;219 NiO;220 SrAl2O4:Eu2+;221 Fe2O3;222 SiO2;223 Y–ZrO2;224 to SiOC225); fir wood (templating ZnO;226 TiO2;227 Mn3O4 and Mn2O3;228 Fe2O3;222,229 and Cr2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230); cedar, cypress and judas wood (to TiO2
230); cedar, cypress and judas wood (to TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 227); paulownia (templating Fe2O3;222,229 Cr2O3;230 Mn3O4 and Mn2O3228) and lauan wood (templating Fe2O3
227); paulownia (templating Fe2O3;222,229 Cr2O3;230 Mn3O4 and Mn2O3228) and lauan wood (templating Fe2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222), poplar wood (templating SiO2
222), poplar wood (templating SiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223); cedar wood (templating TiO2
223); cedar wood (templating TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 and zeolite232); spruce wood (templating SiO2;233,234 to Ce0.5Zr0.5O2
231 and zeolite232); spruce wood (templating SiO2;233,234 to Ce0.5Zr0.5O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31); and bamboo (templating zeolite232).
31); and bamboo (templating zeolite232).
M x O y -PS through hard templates. Another approach related to the MO@PS topic is the use of micrometer size granules of PS for nano-casting. Microspheres of starch infiltrated with TiO2 sol in supercritical CO2 lead to the formation of mesoporous–macroporous anatase (2 nm to 5 μm).235 In a different approach, starch granules were first converted into carbonaceous material by a solvothermal process,236 and then used as sacrificial templates for the preparation of ZnO microspheres after impregnation with zinc(II) acetate.237,238 This approach was initially pioneered with glucose as a precursor for carbonaceous dots used, for example, for the production of metal and mixed metal oxides.239–245 With the development of carbon dots, this approach could be fruitfully further enhanced to associate these dots with MO.
Part 4: chemical reaction of biopolymers with metal oxide precursors
In the studies mentioned above, cellulose was exclusively considered as a template and its reactivity was generally not considered, except for the outermost layer. But in other domains, biotemplates have often been considered as a C-provider for the production of carbides, such as SiC246–249 or250 B4C,251,252 TiC,253,254 C-doped TiO2,147,255 C-doped SiO2,256 or as a reducing agent for the preparation of metal particles,129 and recently metal fibers.257 However, biotemplates are very seldom considered as an O-source functioning as both a template and reagent, e.g. gas/solid metathesis between TiF4 and SiO2-based diatoms.258Polysaccharides are indeed highly reactive materials with a high oxygen content, thus making them promising O-providers. In the past, alcohols and ethers have been used in the preparation of metal oxides by the so-called non-hydrolytic sol–gel process.259,260Fig. 12 schematically illustrates such a general route where polysaccharides are considered as both templates and reagents.
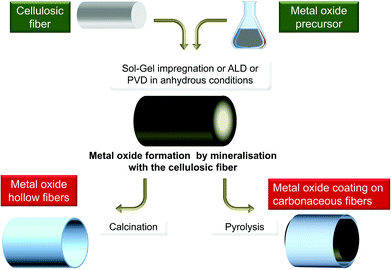 | ||
| Fig. 12 General scheme of the polysaccharide mineralisation process leading to hybrid MxOy@PS and then to either MxOy or MxOy@C materials. | ||
An ALD-type process has been developed and is based, in the case of the formation of TiO2, on the reaction for TTIP with-and-on dried nanofibers of nanocellulose aerogels (Fig. 13). Considering the temperature used in the process, i.e. 190 °C at pressures of 1–5 kPa for 2 h, different reactions can occur: with cellulose hydroxyl groups to produce the alcohol of the MO precursor, thermal decomposition of titanium isopropoxide, and reactions with traces of water.261–263
 | ||
| Fig. 13 SEM images of ZnO@NCA (A, B); TiO2@NCA (C) and Al2O3@NCA (D) obtained reactive ALD with nanocellulose aerogel (Reprinted with permission from ref. 262, Copyright (2011) American Chemical Society). | ||
Recently, we have reported a mineralisation, under strictly anhydrous conditions similar to non-hydrolytic sol–gel processes. TiO2 can be obtained by the reaction between TiCl4 as the titanium-source and a cellulosic material like Ferula communis piths or cotton wool acting as the oxygen source.264 This is a one-pot metathetic liquid/solid reaction.
The most important difference from other approaches is that, under mild conditions (<80 °C), mineralisation leads to the formation of nanoparticles with a non-round shape, i.e. nanorod-like structures assembled in needle3 (Fig. 14) or lacework formation,265 a situation that has never been reported in sol–gel biotemplating processes. In addition, this approach can be combined with pre-impregnation of the cellulose and is efficient on aerogel of nanocellulose.
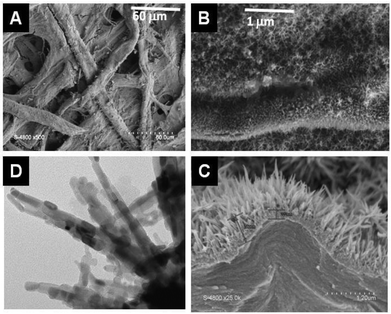 | ||
| Fig. 14 SEM (A, B, C) and TEM (D) picture of TiO2@ filter paper obtained by mineralization of filter paper by TiCl4 under anhydrous conditions at 80 °C.3 | ||
Perspectives and concluding remarks
Green, dark green, pale green… How is this Green Chemistry? Answers to this question can be given in relation to the basic principles of “green chemistry”, and by comparison with other methods that aim to control the synthesis of metal oxides.In terms of maximizing the amount of raw material with no (or limited) environmental impact and toxicity, PS and water are certainly appropriate chemicals, the use of MO precursors cannot be avoided by any process. PS is an ideal sustainable substance, although some of them require purification or extraction like for nanocrystals and nanofibers. Anyway, the use of PS is certainly “greener” than that of surfactants and additives issued from petrochemical industry, and generally used for the same purpose.
In terms of by products, the chemicals produced by the process are usually alcohols (produced by the hydrolysis of the MO precursor) and CO2 during calcination (plus traces of S and N containing chemicals in some cases), the latter two compounds exhibiting no particular toxicity and being easily mastered by the chemist. However, to limit contamination by metals and maximize the incorporation of all materials used in the process, special attention must be paid to the presence of clusters and metal oxide nanoparticles in the final solution after the filtration step.
In terms of energy, one may argue of the necessity to remove the PS by thermal treatment (although elimination could be done with enzyme or IL extraction in some cases). But firstly, the syntheses of MO require generally a thermal treatment to improve the crystallinity of MO, the temperatures are generally equivalent or even higher than the one required for the elimination of the PS. Secondly, when the thermal treatment is performed under an inert atmosphere, it leads to the carbon phase provided by the PS, a situation that cannot be attained when using surfactants or additives, and that allows, in one step, the formation of MO@C composites. Finally, most of the oxides thus synthesized are required by green chemistry, especially in the catalytic and photocatalysis processes. Therefore, it is among the greenest approaches to the synthesis of materials expected in Green Chemistry.
Over the last two decades, there has been a significant increase in the development of approaches and in research on the use of PS for MO preparation. This is certainly only the beginning of the story considering the importance of polysaccharides as potential materials and reagents, and of metal oxides as functional materials for chemical applications and devices. Of course, the development of chemistry around the nano-sizing and chemical modification of PS is a major issue for the development of this domain, as mentioned above. At the end of this review, we propose to keep in mind five important issues.
First, a precise rationalisation of the effect of the polysaccharide on the formation of the oxide is not possible at present because of the tremendous diversity of processing parameters and the complexity of PS structures. This is further hampered by the lack of a precise description and analyses of what happens during nanoparticle growth or replication. The descriptions of the experimental procedures should always be accurate and detailed. Comparison of different PS engaged under the same conditions would greatly help to understand and rationalise their specificity and interest in these processes.
Secondly, the decomposition/elimination of biopolymers leading to the oxide is an important step requiring greater consideration: for instance, nano-sizing of PS and MO coatings may modify the thermal behaviour of PS, which is an important point with respect to the formation of C-doped MO or C@MO composites.
Thirdly, the reactivity of PS, beyond its role as a template, opens new avenues in terms of the transfer of elements to the targeted MO and of redox reaction before or during thermal treatment for the formation of other composites (M@C, M@MO@C, etc.). Examples of the synthesis of metal nanoparticles,130,266–269 or metal oxides (magnetite,270 hausmannite131) are useful tools in such perspective.
The use of PS is usually to control MO properties including porosity. With the exception of some water-soluble PS (alginates), the results in this area are not very remarkable, especially compared to the results obtained with surfactants. However, the availability of new forms of PS (nanocrystals, nanofibers) offers hope for progress in this area.
Finally, in addition to other advances in PS processing, the latter could be modified by chemical reaction or simply by adsorption of chemicals inside fibers. This offers numerous combinations for the potential preparation of complex MO@PS multifunctional composites.
From a general point of view, the sum of these results shows the diversity and richness of this synthetic pathway to MO, using abundant natural polymers, and which can also lead to the formation of composite and nanocomposite MO@C. Although today we lack a fine and precise understanding of the specific character of each PS, it is already possible today to take advantage of them to access materials of high demand in new technologies and devices.
Abbreviation
| PS | Polysaccharide |
| MO | Metal oxide |
| MMO | Mixed metal oxide |
| NP | Nanoparticle |
| TTIP | Ti(OiPr)4 |
| TTB | Ti(OBu)4 |
| iMM&SC | Mastering morphology and size control |
| SSA | Specific surface area |
| PVD | Plasma vapour deposition |
| ALD | Atomic layer deposition |
| A@B | For mixed material like composite or core shell materials, the A@B is used which means that A is quantitatively the minor phase and B the major phase, for example TiO2@C is mostly carbon covered or mixed with TiO2. |
Acknowledgements
The authors thank the Institut Charles Gerhardt, Pôle Balard and LabEx cheMISyst for financial support.Notes and references
- J. Huang and T. Kunitake, J. Am. Chem. Soc., 2003, 125, 11834–11835 CrossRef CAS PubMed
.
- S. Chen, B. Zhou, W. Hu, W. Zhang, N. Yin and H. Wang, Carbohydr. Polym., 2013, 92, 1953–1959 CrossRef CAS PubMed
.
- R. G. Nair, S. K. Samdarshi and B. Boury, Eur. J. Inorg. Chem., 2013, 5303–5310 CrossRef
.
- M. Fomina, J. M. Charnock, S. Hillier, R. Alvarez and G. M. Gadd, Environ. Microbiol., 2007, 9, 1696–1710 CrossRef CAS PubMed
.
- M. Sprynskyy, I. Kovalchuk and B. a. Buszewski, J. Hazard. Mater., 2010, 181, 700–707 CrossRef CAS PubMed
.
- R. E. Dunin-Borkowski, M. R. McCartney, R. B. Frankel, D. A. Bazylinski, M. Posfai and P. R. Buseck, Science, 1998, 282, 1868–1870 CrossRef CAS PubMed
.
- G. M. Gadd, Microbiology, 2010, 156, 609–643 CrossRef CAS PubMed
.
- L. Addadi, D. Joester, F. Nudelman and S. Weiner, Chem. – Eur. J., 2006, 12, 980–987 CrossRef CAS PubMed
.
- C. E. Killian and F. H. Wilt, Chem. Rev., 2008, 108, 4463–4474 CrossRef CAS PubMed
.
- D. Faivre and D. Schüler, Chem. Rev., 2008, 108, 4875–4898 CrossRef CAS PubMed
.
- A. M. Belcher, X. H. Wu, R. J. Christensen, P. K. Hansma, G. D. Stucky and D. E. Morse, Nature, 1996, 381, 56–58 CrossRef CAS
.
-
H. Cölfen, in Biomineralization II Mineralization Using Synthetic Polymers and Templates, Springer-Verlag, Berlin, 2007, pp. 1–77 Search PubMed
.
-
E. Bäeuerlein, Handbook of Biomineralization: Biomimetic and bioinspired chemistry, Wiley-VCH, 2007 Search PubMed
.
- P. Calvert and P. Rieke, Chem. Mater., 1996, 8, 1715–1727 CrossRef CAS
.
- J. Aizenberg, L. Addadi, S. Weiner and G. Lambert, Adv. Mater., 1996, 8, 222–226 CrossRef CAS
.
- R. L. Brutchey and D. E. Morse, Chem. Rev., 2008, 108, 4915–4934 CrossRef CAS PubMed
.
- M. B. Dickerson, K. H. Sandhage and R. R. Naik, Chem. Rev., 2008, 108, 4935–4978 CrossRef CAS PubMed
.
- S. V. Patwardhan, Chem. Commun., 2011, 47, 7567–7582 RSC
.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed
.
- A. Kubacka, M. Fernandez-Garcia and G. Colon, Chem. Rev., 2011, 112, 1555–1614 CrossRef PubMed
.
- X. Zhao, B. M. Sanchez, P. J. Dobson and P. S. Grant, Nanoscale, 2011, 3, 839–855 RSC
.
- Y.-F. Sun, S.-B. Liu, F.-L. Meng, J.-Y. Liu, Z. Jin, L.-T. Kong and J.-H. Liu, Sensors, 2012, 12, 2610–2631 CrossRef CAS PubMed
.
- P. Tingaut, T. Zimmermann and G. Sebe, J. Mater. Chem., 2012, 22, 20105–20111 RSC
.
- N. Lin, J. Huang and A. Dufresne, Nanoscale, 2012, 4, 3274–3294 RSC
.
- S. Elazzouzi-Hafraoui, J.-L. Putaux and L. Heux, J. Chem. Phys., 2009, 113, 11069–11075 CrossRef CAS PubMed
.
- S. Elazzouzi-Hafraoui, Y. Nishiyama, J.-L. Putaux, L. Heux, F. Dubreuil and C. Rochas, Biomacromolecules, 2008, 9, 57–65 CrossRef CAS PubMed
.
- S. Sarkar, E. Guibal, F. Quignard and A. K. SenGupta, J. Nanopart. Res., 2012, 14, 1–24 CrossRef
.
- D. Klemm, F. Kramer, S. Moritz, T. Lindström, M. Ankerfors, D. Gray and A. Dorris, Angew. Chem., Int. Ed., 2011, 50, 5438–5466 CrossRef CAS PubMed
.
- S. Geresh and S. Arad, Bioresour. Technol., 1991, 38, 195–201 CrossRef CAS
.
-
C. Zollfrank, P. Cromme, M. Rauch, H. Scheel, M. H. Kostova, K. Gutbrod, S. Gruber and D. V. Opdenbosch, in Bioinspired, Biomimetic and Nanobiomaterials, 2012, pp. 13–25 Search PubMed
.
- A. S. Deshpande, I. Burgert and O. Paris, Small, 2006, 2, 994–998 CrossRef CAS PubMed
.
- F. Graziola, F. Girardi, R. Di Maggio, E. Callone, E. Miorin, M. Negri, K. Müller and S. Gross, Prog. Org. Coat., 2012, 74, 479–490 CrossRef CAS
.
- H. Budunoglu, A. Yildirim, M. O. Guler and M. Bayindir, ACS Appl. Mater. Interfaces, 2011, 3, 539–545 CAS
.
- S. Wang, R. Mahlberg, S. Jaemsae, J. Nikkola, J. Mannila, A.-C. Ritschkoff and J. Peltonen, Prog. Org. Coat., 2011, 71, 274–282 CrossRef CAS
.
- S. Saha, D. Kocaefe, D. Sarkar, Y. Boluk and A. Pichette, J. Coat. Technol. Res., 2011, 8, 183–190 CrossRef CAS
.
- M. Petrič, Rev. Adhes. Adhes., 2013, 1, 216–247 CrossRef
.
- T. Valdés-Soli and A. B. Fuertes, Mater. Res. Bull., 2006, 41, 2187–2197 CrossRef
.
- T. Nypelö and O. J. Rojas, Am. Ceram. Soc. Bull., 2012, 91, 28–31 Search PubMed
.
- M. Singh, J. Martínez-Fernández and A. R. de Arellano-López, Curr. Opin. Solid State Mater. Sci., 2003, 7, 247–254 CrossRef CAS
.
- Z.-G. Zhao, Z.-F. Liu and M. Miyauchi, Chem. Commun., 2010, 46, 3321–3323 RSC
.
- L. Zhao, X. Chen, X. Wang, Y. Zhang, W. Wei, Y. Sun, M. Antonietti and M.-M. Titirici, Adv. Mater., 2010, 22, 3317–3321 CrossRef CAS PubMed
.
- M.-M. Titirici, M. Antonietti and N. Baccile, Green Chem., 2008, 10, 1204–1212 RSC
.
- A. Kruse, A. Funke and M.-M. Titirici, Curr. Opin. Chem. Biol., 2013, 17, 515–521 CrossRef CAS PubMed
.
- B. Hu, K. Wang, L. Wu, S.-H. Yu, M. Antonietti and M.-M. Titirici, Adv. Mater., 2010, 22, 813–828 CrossRef CAS PubMed
.
- N. Baccile, G. Laurent, C. Coelho, F. Babonneau, L. Zhao and M.-M. Titirici, J. Phys. Chem. C, 2011, 115, 8976–8982 CAS
.
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed
.
-
A. l. P. Rauter, N. M. Xavier, S. D. Lucas, M. Santos and H. Derek, in Advances in Carbohydrate Chemistry and Biochemistry, Academic Press, 2010, pp. 29–99 Search PubMed
.
- Z. Shi, G. O. Phillips and G. Yang, Nanoscale, 2013, 5, 3194–3201 RSC
.
- Y. Ohmori and Y. Kurokawa, J. Biotechnol., 1994, 33, 205–209 CrossRef CAS PubMed
.
- T. Coradin, N. Nassif and J. Livage, Appl. Microbiol. Biotechnol., 2003, 61, 429–434 CrossRef CAS PubMed
.
- M. Perullini, M. Amoura, M. Jobbagy, C. Roux, J. Livage, T. Coradin and S. A. Bilmes, J. Mater. Chem., 2011, 21, 8026–8031 RSC
.
- E. Callone, R. Campostrini, G. Carturan, A. Cavazza and R. Guzzon, J. Mater. Chem., 2008, 18, 4839–4848 RSC
.
- T. Coradin and J. Livage, J. Sol-Gel Sci. Technol., 2003, 26, 1165–1168 CrossRef CAS
.
- A. M. G. C. Dias, A. Hussain, A. S. Marcos and A. C. A. Roque, Biotechnol. Adv., 2011, 29, 142–155 CrossRef CAS PubMed
.
- Z. Liu, H. Wang, C. Liu, Y. Jiang, G. Yu, X. Mu and X. Wang, Chem. Commun., 2012, 48, 7350–7352 RSC
.
- J. Wu and M. J. Sailor, Adv. Funct. Mater., 2009, 19, 733–741 CrossRef CAS
.
- S. Liu, D. Tao and L. Zhang, Powder Technol., 2012, 217, 502–509 CrossRef CAS
.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. Vander Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS PubMed
.
- S. Padalkar, J. R. Capadona, S. J. Rowan, C. Weder, Y.-H. Won, L. A. Stanciu and R. J. Moon, Langmuir, 2010, 26, 8497–8502 CrossRef CAS PubMed
.
- L.-W. Tai, H. U. Anderson and P. A. Lessing, J. Am. Ceram. Soc., 1992, 75, 3490–3494 CrossRef CAS
.
- Z. Li, A. Friedrich and A. Taubert, J. Mater. Chem., 2008, 18, 1008–1014 RSC
.
-
J. Li, Y. Gu and J. Huang, in Nanostructured Biomaterials, Springer, Berlin, Heidelberg, 2010, pp. 133–164 Search PubMed
.
- Z. Schnepp, Angew. Chem., Int. Ed., 2013, 52, 1096–1108 CrossRef CAS PubMed
.
- R. A. Caruso and M. Antonietti, Chem. Mater., 2001, 13, 3272–3282 CrossRef CAS
.
- I. Ichinose and T. Kunitake, Chem. Rec., 2002, 2, 339–351 CrossRef CAS PubMed
.
- S. Lou, X. Guo, T. Fan and D. Zhang, Energy Environ. Sci., 2012, 5, 9195–9216 CAS
.
- C. Aimé and T. Coradin, J. Polym. Sci., Part B: Polym. Phys., 2012, 50, 669–680 CrossRef
.
- R. J. Martin-Palma and A. Lakhtakia, Int. J. Smart Nano Mater., 2012, 4, 83–90 CrossRef
.
-
F. Marin, P. Narayanappa and S. Motreuil, in Molecular Biomineralization, Springer, Berlin, Heidelberg, 2011, pp. 353–395 Search PubMed
.
- C. Pavat, I. Zanella-Cléon, M. Becchi, D. Medakovic, G. Luquet, N. Guichard, G. Alcaraz, J.-L. Dommergues, A. Serpentini, J.-M. Lebel and F. Marin, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2012, 161, 303–314 CrossRef CAS PubMed
.
- W. G. Kinsinger and C. W. Hock, Ind. Eng. Chem. Res., 1948, 40, 1711–1716 CrossRef CAS
.
-
C. W. Hock and H. Mark, in Cellulose, ed. E. Ott, Interscience Publishers Inc., New York, 1946, p. 346 Search PubMed
.
- C. C. Walters, L. Margulis and E. S. Barghoorn, Precambrian Res., 1977, 5, 241–248 CrossRef CAS
.
-
B. H. Hamling, U.S. Pat., 1973, 3,736,160 Search PubMed
.
-
B. H. Hamling, U.S. Pat., 1968, 3,385,915 Search PubMed
.
- A. G. Elliot and R. A. Huggins, J. Am. Ceram. Soc., 1975, 58, 497–500 CrossRef CAS
.
- T. Yanagisawa, T. Shimizu, K. Kuroda and C. Kato, Bull. Chem. Soc. Jpn., 1990, 63, 988–992 CrossRef CAS
.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson and E. W. Sheppard, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS
.
- Y. Wei, D. Jin, T. Ding, W.-H. Shih, X. Liu, S. Z. D. Cheng and Q. Fu, Adv. Mater., 1998, 10, 313–316 CrossRef CAS
.
- Y. Wei, J. Xu, H. Dong, J. H. Dong, K. Qiu and S. A. Jansen-Varnum, Chem. Mater., 1999, 11, 2023–2029 CrossRef CAS
.
- J. H. Jean and T. A. Ring, Colloids Surf., 1988, 29, 273–291 CrossRef CAS
.
- J. H. Jean and T. A. Ring, Am. Ceram. Soc. Bull., 1986, 65, 1574–1577 CAS
.
- E. Kroll, F. M. Winnik and R. F. Ziolo, Chem. Mater., 1996, 8, 1594–1596 CrossRef CAS
.
- T. Itoh, M. Uzawa and H. Uchikawa, J. Mater. Sci. Lett., 1988, 7, 130–132 CrossRef CAS
.
- T. Itoh and H. Uchikawa, J. Mater. Sci. Lett., 1988, 7, 693–694 CrossRef CAS
.
- B. Zhang, S. A. Davis and S. Mann, Chem. Mater., 2002, 14, 1369–1375 CrossRef CAS
.
- B. Zhang, S. A. Davis, N. H. Mendelson and S. Mann, Chem. Commun., 2000, 781–782 RSC
.
- Q. Wang, Z. Zhang, Y. Zheng, W. Cai and Y. Yu, CrystEngComm, 2012, 14, 4786–4793 RSC
.
- Y.-L. Liu, C.-Y. Hsu, Y.-H. Su and J.-Y. Lai, Biomacromolecules, 2004, 6, 368–373 CrossRef PubMed
.
- R. Jayakumar, R. Ramachandran, V. V. Divyarani, K. P. Chennazhi, H. Tamura and S. V. Nair, Int. J. Biol. Macromol., 2011, 48, 336–344 CrossRef CAS PubMed
.
- T. Bechtold, A. P. Manian, H. B. Oztürk, U. Paul, B. Siroka, J. Siroky, H. Soliman, L. T. T. Vo and H. Vu-Manh, Carbohydr. Polym., 2013, 93, 316–323 CrossRef CAS PubMed
.
- A. Ostlund, D. Lundberg, L. Nordstierna, K. Holmberg and M. Nydén, Biomacromolecules, 2009, 10, 2401–2407 CrossRef PubMed
.
- A. Brandt, J. Grasvik, J. P. Hallett and T. Welton, Green Chem., 2013, 15, 550–583 RSC
.
- S. Sen, J. D. Martin and D. S. Argyropoulos, ACS Sustainable Chem. Eng., 2013, 1, 858–870 CrossRef CAS
.
- R. Liu, Y. Huang, A. Xiao and H. Liu, J. Alloys Compd., 2010, 503, 103–110 CrossRef CAS
.
- B. Alonso and E. Belamie, Angew. Chem., Int. Ed., 2010, 49, 8201–8204 CrossRef CAS PubMed
.
- Y.-Y. Kim, K. Hore, S. R. Hall and D. Walsh, Small, 2009, 5, 913–918 CrossRef CAS PubMed
.
- K. S. Khairou, J. Therm. Anal. Calorim., 2002, 69, 583–588 CrossRef CAS
.
- Y. S. Nam, W. H. Park, D. Ihm and S. M. Hudson, Carbohydr. Polym., 2010, 80, 291–295 CrossRef CAS
.
- M. J. Zohuriaan and F. Shokrolahi, Polym. Test., 2004, 23, 575–579 CrossRef CAS
.
- A. L. Sullivan and R. Ball, Atmos. Environ., 2012, 47, 133–141 CrossRef CAS
.
- P. Sipos, T. G. S. Pierre, E. Tombacz and J. Webb, J. Inorg. Biochem., 1995, 58, 129–138 CrossRef CAS
.
- Y. Ogiwara and H. Kubota, J. Polym. Sci., Part A: Polym. Chem., 1969, 7, 2087–2095 CrossRef CAS
.
- P. Chan, W. Chuaanusorn, M. Nesterova, P. Sipos, T. Stpierre, J. Ward and J. Webb, Aust. J. Chem., 1995, 48, 783–792 CrossRef CAS
.
- E. M. Coe, R. D. Bereman and W. T. Monte, J. Inorg. Biochem., 1995, 60, 149–153 CrossRef CAS
.
- Y. A. Shchipunov and T. y. Y. Karpenko, Langmuir, 2004, 20, 3882–3887 CrossRef CAS PubMed
.
- A. Kongdee and T. Bechtold, Carbohydr. Polym., 2004, 56, 47–53 CrossRef CAS
.
- H. E. Emam, A. P. Manian, B. Siroka and T. Bechtold, Carbohydr. Polym., 2012, 90, 1345–1352 CrossRef CAS PubMed
.
- Q. Xu and L.-F. Chen, J. Appl. Polym. Sci., 1999, 71, 1441–1446 CrossRef CAS
.
- L. V. A. Gurgel, K. O. Junior, R. Gil Pereira de Freitas and L. F. Gil, Bioresour. Technol., 2008, 99, 3077–3083 CrossRef CAS PubMed
.
- N. K. Yurkshtovich, N. V. Golub, F. N. Kaputskii, T. L. Yurkshtovich and R. I. Kosterova, Russ. J. Appl. Chem., 2004, 77, 471–475 CrossRef CAS
.
- A. Potthast, U. Henniges and G. Banik, Cellulose, 2008, 15, 849–859 CrossRef CAS
.
- F. Llanes, C. Diaz, H. Ryan and R. H. Marchessault, Int. J. Polym. Mater. Polym. Biomater., 2002, 51, 537–545 CrossRef CAS
.
- S.-J. Bao, C. Lei, M.-W. Xu, C.-J. Cai, C.-J. Cheng and C. M. Li, CrystEngComm, 2013, 15, 4694–4699 RSC
.
- E. Rauwel, G. Clavel, M.-G. Willinger, P. Rauwel and N. Pinna, Angew. Chem., Int. Ed., 2008, 47, 3592–3595 CrossRef CAS PubMed
.
- E. Rauwel, M.-G. Willinger, F. Ducroquet, P. Rauwel, I. Matko, D. Kiselev and N. Pinna, J. Phys. Chem. C, 2008, 112, 12754–12759 CAS
.
- D. Jugovic, M. Mitric, M. Milovic, B. Jokic, M. Vukomanovic, D. Suvorov and D. Uskokovic, Powder Technol., 2013, 246, 539–544 CrossRef CAS
.
- X. Liu, Y. Gu and J. Huang, Chem. – Eur. J., 2010, 16, 7730–7740 CrossRef CAS PubMed
.
- Y. Zhou, E.-Y. Ding and W.-D. Li, Mater. Lett., 2007, 61, 5050–5052 CrossRef CAS
.
- X. Peng and E. Ding, Micro Nano Lett., 2011, 998–1001 CAS
.
- D. C. Green, S. Glatzel, A. M. Collins, A. J. Patil and S. R. Hall, Adv. Mater., 2012, 24, 5767–5772 CrossRef CAS PubMed
.
- W. Zhang, S. Chen, W. Hu, B. Zhou, Z. Yang, N. Yin and H. Wang, Carbohydr. Polym., 2011, 86, 1760–1767 CrossRef CAS
.
- M. A. B. Barata, M. C. Neves, C. Pascoal Neto and T. Trindade, Dyes Pigm., 2005, 65, 125–127 CrossRef CAS
.
- Z. Ibupoto, K. Khun, M. Eriksson, M. AlSalhi, M. Atif, A. Ansari and M. Willander, Materials, 2013, 6, 3584–3597 CrossRef CAS
.
- R. H. Marchessault, S. Ricard and P. Rioux, Carbohydr. Res., 1992, 224, 133–139 CrossRef CAS
.
- L. Raymond, J. F. Revol, R. H. Marchessault and D. H. Ryan, Polymer, 1995, 36, 5035–5043 CrossRef CAS
.
- Y. Young-Hoon, K. Kab-Young and P. Un-Kyu, Ceram. Int., 2012, 38, 1599–1603 CrossRef
.
- Y. H. Song, M. O. Kim, M. K. Jung, K. Senthil, T. Masaki, K. Toda and D. H. Yoon, Mater. Lett., 2012, 77, 121–124 CrossRef CAS
.
- L. Johnson, W. Thielemans and D. A. Walsh, Green Chem., 2011, 13, 1686–1693 RSC
.
- T. Mochochoko, O. S. Oluwafemi, D. N. Jumbam and S. P. Songca, Carbohydr. Polym., 2013, 98, 290–294 CrossRef CAS PubMed
.
- J. Yin, F. Gao, Y. Wu, J. Wang and Q. Lu, CrystEngComm, 2010, 12, 3401–3403 RSC
.
- W. Jiang, K. Zhang, L. Wei, D. Yu, J. Wei and Y. Chen, Nanoscale, 2013, 5, 11108–11117 RSC
.
- M. Robitzer, L. David, C. Rochas, F. D. Renzo and F. Quignard, Langmuir, 2008, 24, 12547–12552 CrossRef CAS PubMed
.
- F. Quignard, F. Renzo and E. Guibal, Carbohydr. Sustainable Dev. I, 2010, 294, 165–197 CAS
.
- G. T. Grant, E. R. Morris, D. A. Rees, P. J. C. Smith and D. Thom, FEBS Lett., 1973, 32, 195–198 CrossRef CAS
.
- N. M. Pope, R. C. Alsop, Y.-A. Chang and A. K. Smith, J. Biomed. Mater. Res., 1994, 28, 449–457 CrossRef CAS PubMed
.
- T. Hamaya, T. Takizawa, H. Hidaka and K. Horikoshi, J. Chem. Eng. Jpn., 1993, 26, 223–224 CrossRef CAS
.
- R. Horga, F. Di Renzo and F. Quignard, Appl. Catal., A, 2007, 325, 251–255 CrossRef CAS
.
- Z. A. C. Schnepp, S. C. Wimbush, S. Mann and S. R. Hall, Adv. Mater., 2008, 20, 1782–1786 CrossRef CAS
.
- Z. Schnepp, S. C. Wimbush, S. Mann and S. R. Hall, CrystEngComm, 2010, 12, 1410–1415 RSC
.
- Z. Schnepp, S. R. Hall, M. J. Hollamby and S. Mann, Green Chem., 2011, 13, 272–275 RSC
.
- M. Rinaudo, Prog. Polym. Sci., 2006, 31, 603–632 CrossRef CAS
.
- H. Honda, A. Kawabe, M. Shinkai and T. Kobayashi, J. Ferment. Bioeng., 1998, 86, 191–196 CrossRef CAS
.
- T. Suzuki and Y. Mizushima, J. Ferment. Bioeng., 1997, 84, 128–132 CrossRef CAS
.
- Y. Mizushima, J. Non-Cryst. Solids, 1992, 144, 305–307 CrossRef CAS
.
- A. El Kadib and M. Bousmina, Chem. – Eur. J., 2012, 18, 8264–8277 CrossRef PubMed
.
- J. Matos, P. Atienzar, H. Garcia and J. C. Hernandez-Garrido, Carbon, 2013, 53, 169–181 CrossRef CAS
.
- X. Guan, J. Du, X. Meng, Y. Sun, B. Sun and Q. Hu, J. Hazard. Mater., 2012, 215–216, 1–16 CrossRef CAS PubMed
.
- S. S. Rashidova, D. S. Shakarova, O. N. Ruzimuradov, D. T. Satubaldieva, S. V. Zalyalieva, O. A. Shpigun, V. P. Varlamov and B. D. Kabulov, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 800, 49–53 CrossRef CAS
.
- J.-T. Yeh, C.-L. Chen and K.-S. Huang, Mater. Lett., 2007, 61, 1292–1295 CrossRef CAS
.
- S. R. Hall, Adv. Mater., 2006, 18, 487–490 CrossRef CAS
.
- A. Sachse, V. Hulea, K. L. Kostov, N. Marcotte, M. Y. Boltoeva, E. Belamie and B. Alonso, Chem. Commun., 2012, 48, 10648–10650 RSC
.
- F. Xie, E. Pollet, P. J. Halley and L. Averous, Prog. Polym. Sci., 2013, 38, 1590–1628 CrossRef CAS
.
- G. Taillades, P. Batocchi, A. Essoumhi, M. Taillades, D. J. Jones and J. Rozière, Microporous Mesoporous Mater., 2011, 145, 26–31 CrossRef CAS
.
- A. Taubert and G. Wegner, J. Mater. Chem., 2002, 12, 805–807 RSC
.
- D. K. Kim, M. Mikhaylova, F. H. Wang, J. Kehr, B. Bjelke, Y. Zhang, T. Tsakalakos and M. Muhammed, Chem. Mater., 2003, 15, 4343–4351 CrossRef CAS
.
- A. El Kadib, K. Molvinger, M. Bousmina and D. Brunel, J. Catal., 2010, 273, 147–155 CrossRef CAS
.
- J. F. Zhou, M. F. Zhou and R. A. Caruso, Langmuir, 2006, 22, 3332–3336 CrossRef CAS PubMed
.
- D. Walsh, S. C. Wimbush and S. R. Hall, Chem. Mater., 2007, 19, 647–649 CrossRef CAS
.
- A. L. Daniel-da-Silva, T. Trindade, B. J. Goodfellow, B. F. O. Costa, R. N. Correia and A. M. Gil, Biomacromolecules, 2007, 8, 2350–2357 CrossRef CAS PubMed
.
- F. Jones, H. Cölfen and M. Antonietti, Colloid Polym. Sci., 2000, 278, 491–501 CAS
.
- X. Wang, D. R. G. Mitchell, K. Prince, A. J. Atanacio and R. A. Caruso, Chem. Mater., 2008, 20, 3917–3926 CrossRef CAS
.
- M. Kellermeier, H. Cölfen and J. M. García-Ruiz, Eur. J. Inorg. Chem., 2012, 2012, 5123–5144 CrossRef CAS
.
- E. Dujardin and S. Mann, Adv. Mater., 2004, 16, 1125–1129 CrossRef CAS
.
- W. Shenton, T. Douglas, M. Young, G. Stubbs and S. Mann, Adv. Mater., 1999, 11, 253–256 CrossRef CAS
.
- S. A. Davis, M. Breulmann, K. H. Rhodes, B. Zhang and S. Mann, Chem. Mater., 2001, 13, 3218–3226 CrossRef CAS
.
-
Y. Shin and G. J. Exarhos, in The Nanoscience and Technology of Renewable Biomaterials, John Wiley & Sons, Ltd, 2009, pp. 315–335 Search PubMed
.
- J. Huang and Y. Gu, Curr. Opin. Colloid Interface Sci., 2011, 16, 470–481 CrossRef
.
- Y. Kurokawa and K. Hanaya, Carbohydr. Polym., 1995, 27, 313–320 CrossRef CAS
.
- D. Yang, L. Qi and J. Ma, Adv. Mater., 2002, 14, 1543–1546 CrossRef CAS
.
- S. Liu and J. He, J. Am. Chem. Soc., 2005, 88, 3513–3514 CAS
.
- K. Kim, Biomacromolecules, 2003, 4, 908 CrossRef PubMed
.
- E. L. Mayes, F. Vollrath and S. Mann, Adv. Mater., 1998, 10, 801–805 CrossRef CAS
.
- S. R. Hall, V. M. Swinerd, F. N. Newby, A. M. Collins and S. Mann, Chem. Mater., 2006, 18, 598–600 CrossRef CAS
.
- W. Brandon Goodwin, I. J. Gomez, Y. Fang, J. C. Meredith and K. H. Sandhage, Chem. Mater., 2013, 25, 4529–4536 CrossRef CAS
.
- M. W. Anderson, S. M. Holmes, N. Hanif and C. S. Cundy, Angew. Chem., Int. Ed., 2000, 39, 2707–2710 CrossRef CAS
.
- S. A. Davis, S. L. Burkett, N. H. Mendelson and S. Mann, Nature, 1997, 385, 420–423 CrossRef CAS
.
- S. Chia, J. Urano, F. Tamanoi, B. Dunn and J. I. Zink, J. Am. Chem. Soc., 2000, 122, 6488–6489 CrossRef CAS
.
- D. J. Evans, J. Mater. Chem., 2008, 18, 3746–3754 RSC
.
- T. Douglas, E. Strable, D. Willits, A. Aitouchen, M. Libera and M. Young, Adv. Mater., 2002, 14, 415–418 CrossRef CAS
.
- M. Uchida, M. T. Klem, M. Allen, P. Suci, M. Flenniken, E. Gillitzer, Z. Varpness, L. O. Liepold, M. Young and T. Douglas, Adv. Mater., 2007, 19, 1025–1042 CrossRef CAS
.
- Y. Ono, Y. Kanekiyo, K. Inoue, J. Hojo, M. Nango and S. Shinkai, Chem. Lett., 1999, 28, 475–476 CrossRef
.
- G. Gonçalves, P. A. A. P. Marques, C. P. Neto, T. Trindade, M. Peres and T. Monteiro, Cryst. Growth Des., 2009, 9, 386–390 Search PubMed
.
- H. Wang, A. Zakirov, S. U. Yuldashev, J. Lee, D. Fu and T. Kang, Mater. Lett., 2011, 65, 1316–1318 CrossRef CAS
.
- S. V. Costa, A. S. Gonçalves, M. A. Zaguete, T. Mazon and A. F. Nogueira, Chem. Commun., 2013, 49, 8096–8098 RSC
.
- R. A. Caruso and J. H. Schattka, Adv. Mater., 2000, 12, 1921–1923 CrossRef CAS
.
- J. Zeng, R. Li, S. Liu and L. Zhang, ACS Appl. Mater. Interfaces, 2011, 3, 2074–2079 CAS
.
- T. Kimura, Phys. Chem. Chem. Phys., 2013, 15, 15056–15061 RSC
.
- H. Imai, M. Matsuta, K. Shimizu, H. Hirashima and N. Negishi, J. Mater. Chem., 2000, 10, 2005–2006 RSC
.
- K. Shimizu, H. Imai, H. Hirashima and K. Tsukuma, Thin Solid Films, 1999, 351, 220–224 CrossRef CAS
.
- P. Rodriguez, L. Reinert, M. Comet, J. Kighelman and H. Fuzellier, J. Chem. Phys., 2007, 106, 102–108 CAS
.
- A. B. Shishmakov, Y. V. Mikushina, O. V. Koryakova, M. S. Valova and L. A. Petrov, Russ. J. Appl. Chem., 2012, 85, 1514–1518 CrossRef CAS
.
- J. S. Jur, W. J. Sweet, C. J. Oldham and G. N. Parsons, Adv. Funct. Mater., 2011, 21, 1993–2002 CrossRef CAS
.
- Y. Gu and J. Huang, J. Mater. Chem., 2009, 19, 3764–3770 RSC
.
- J. Zhao, Y. Gu and J. Huang, Chem. Commun., 2011, 47, 10551–10553 RSC
.
- Y. Luo, X. Liu and J. Huang, J. Nanosci. Nanotechnol., 2013, 13, 582–588 CrossRef PubMed
.
- C. Zollfrank, H. Scheel and P. Greil, Adv. Mater., 2007, 19, 984–987 CrossRef CAS
.
- L. Jia, K. Fung-luen and D. H. L. Ng, J. Am. Ceram. Soc., 2008, 91, 1350–1353 CrossRef
.
- L. Zhou, J. He, J. Zhang, Z. He, Y. Hu, C. Zhang and H. He, J. Phys. Chem. C, 2011, 115, 16873–16878 CAS
.
- M. Estruga, I. Gonzalez-Valls, C. Domingo, M. Lira-Cantu and J. A. Ayllón, Eur. J. Inorg. Chem., 2011, 2011, 821–825 CrossRef
.
- Y. Zhang, X. Liu and J. Huang, ACS Appl. Mater. Interfaces, 2011, 3, 3272–3275 CAS
.
- H. Zhu, S. Jia, T. Wan, Y. Jia, H. Yang, J. Li, L. Yan and C. Zhong, Carbohydr. Polym., 2011, 86, 1558–1564 CrossRef CAS
.
- J. Gutierrez, S. M. Fernandes, I. Mondragon and A. Tercjak, Cellulose, 2013, 20, 1301–1311 CrossRef CAS
.
- J. Huang, W. Wang and Z. L. Wang, Nano Lett., 2006, 6, 2325–2331 CrossRef CAS PubMed
.
- G. Cook, P. L. Timms and C. Göltner-Spickermann, Angew. Chem., Int. Ed., 2003, 42, 557–559 CrossRef CAS PubMed
.
- W. Zhang, D. Zhang, T. Fan, J. Gu, J. Ding, H. Wang, Q. Guo and H. Ogawa, Chem. Mater., 2008, 21, 33–40 CrossRef
.
- D. P. Gaillot, O. Deparis, V. Welch, B. K. Wagner, J. P. Vigneron and C. J. Summers, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 2008, 78, 031922 CrossRef
.
- M. R. Weatherspoon, Y. Cai, M. Crne, M. Srinivasarao and K. H. Sandhage, Angew. Chem., Int. Ed., 2008, 47, 7921–7923 CrossRef CAS PubMed
.
- F. Song, H. Su, J. Han, D. Zhang and Z. Chen, Nanotechnology, 2009, 20, 495502 CrossRef PubMed
.
- W. Zhang, D. Zhang, T. Fan, J. Ding, Q. Guo and H. Ogawa, Microporous Mesoporous Mater., 2006, 92, 227–233 CrossRef
.
- W. Zhang, D. Zhang, T. Fan, J. Ding, J. Gu, Q. Guo and H. Ogawa, Mater. Sci. Eng., C, 2009, 29, 92–96 CrossRef
.
- J. P. Vernon, Y. Fang, Y. Cai and K. H. Sandhage, Angew. Chem., Int. Ed., 2010, 49, 7765–7768 CrossRef CAS PubMed
.
- C. Mille, E. C. Tyrode and R. W. Corkery, RSC Adv., 2013, 3, 3109–3117 RSC
.
- J. W. Galusha, L. R. Richey, M. R. Jorgensen, J. S. Gardner and M. H. Bartl, J. Mater. Chem., 2010, 20, 1277–1284 RSC
.
- W. Ogasawara, W. Shenton, S. A. Davis and S. Mann, Chem. Mater., 2000, 12, 2835–2837 CrossRef CAS
.
- F. Mumm, M. Kemell, M. Leskelä and P. Sikorski, Bioinspir. Biomim., 2010, 5, 026005 CrossRef CAS PubMed
.
- V. Puchol, J. El Haskouri, J. Latorre, C. Guillem, A. Beltran, D. Beltran and P. Amoros, Chem. Commun., 2009, 2694–2696 RSC
.
- R. W. Drum, Science, 1968, 161, 175–176 CAS
.
- J. Cao, C. R. Rambo and H. Sieber, Ceram. Int., 2004, 30, 1967–1970 CrossRef CAS
.
- Z. Liu, T. Fan and D. Zhang, J. Am. Ceram. Soc., 2006, 89, 662–665 CrossRef CAS
.
- M. H. Kostova, C. Zollfrank, M. Batentschuk, F. Goetz-Neunhoeffer, A. Winnacker and P. Greil, Adv. Funct. Mater., 2009, 19, 599–603 CrossRef CAS
.
- Z. Liu, T. Fan, W. Zhang and D. Zhang, Microporous Mesoporous Mater., 2005, 85, 82–88 CrossRef CAS
.
- Y. Shin, J. Liu, J. H. Chang, Z. Nie and G. J. Exarhos, Adv. Mater., 2001, 13, 728–732 CrossRef CAS
.
- C. R. Rambo, J. Cao and H. Sieber, Mater. Chem. Phys., 2004, 87, 345–352 CrossRef CAS
.
- C. Zollfrank, R. Kladny, H. Sieber and P. Greil, J. Eur. Ceram. Soc., 2004, 24, 479–487 CrossRef CAS
.
- Z. Liu, T. Fan, J. Ding, D. Zhang, Q. Guo and H. Ogawa, Ceram. Int., 2008, 34, 69–74 CrossRef CAS
.
- T. Ota, M. Imaeda, H. Takase, M. Kobayashi, N. Kinoshita, T. Hirashita, H. Miyazaki and Y. Hikichi, J. Am. Ceram. Soc., 2000, 83, 1521–1523 CrossRef CAS
.
- X. Li, T. Fan, Z. Liu, J. Ding, Q. Guo and D. Zhang, J. Eur. Ceram. Soc., 2006, 26, 3657–3664 CrossRef CAS
.
- Z. Liu, T. Fan, J. Gu, D. Zhang, X. Gong, Q. Guo and J. Xu, Mater. Trans., 2007, 48, 878 CrossRef CAS
.
- T. Fan, X. Li, Z. Liu, J. Gu, D. Zhang and Q. Guo, J. Am. Ceram. Soc., 2006, 89, 3511–3515 CrossRef CAS
.
- S. Zhu, D. Zhang, Z. Chen, G. Zhou, H. Jiang and J. Li, J. Nanopart. Res., 2010, 12, 2445–2456 CrossRef CAS
.
- A. Dong, Y. Wang, Y. Tang, N. Ren, Y. Zhang, Y. Yue and Z. Gao, Adv. Mater., 2002, 14, 926–929 CrossRef CAS
.
- G. Fritz-Popovski, D. Van Opdenbosch, C. Zollfrank, B. Aichmayer and O. Paris, Adv. Funct. Mater., 2013, 23, 1265–1272 CrossRef CAS
.
- D. Van Opdenbosch, G. Fritz-Popovski, O. Paris and C. Zollfrank, J. Mater. Res., 2011, 26, 1193–1202 CrossRef CAS
.
- T. Lin-Qi, N. Wei, Z. Hong-Ying, X. Qun and J. Jian-Xia, BioResources, 2009, 4, 38–48 Search PubMed
.
- M. Zheng, Y. Liu, Y. Xiao, Y. Zhu, Q. Guan, D. Yuan and J. Zhang, J. Phys. Chem. C, 2009, 113, 8455–8459 CAS
.
- G. Patrinoiu, J. M. Caldera-Moreno, D. C. Culita, R. Birjega, R. Ene and O. Carp, J. Solid State Chem., 2013, 202, 291–299 CrossRef CAS
.
- G. Patrinoiu, M. Tudose, J. M. Caldera-Moreno, R. Birjega, P. Budrugeac, R. Ene and O. Carp, J. Solid State Chem., 2012, 186, 17–22 CrossRef CAS
.
- J. Zhang, S. Wang, Y. Wang, M. Xu, H. Xia, S. Zhang, W. Huang, X. Guo and S. Wu, Sens. Actuators, B, 2009, 139, 411–417 CrossRef CAS
.
- X. Sun and Y. Li, Angew. Chem., Int. Ed., 2004, 43, 597–601 CrossRef PubMed
.
- J. Yu and X. Yu, Environ. Sci. Technol., 2008, 42, 4902–4907 CrossRef CAS PubMed
.
- J. Yu, X. Yu, B. Huang, X. Zhang and Y. Dai, Cryst. Growth Des., 2009, 9, 1474–1480 CAS
.
- Q. Wang, H. Li, L. Chen and X. Huang, Carbon, 2001, 39, 2211–2214 CrossRef CAS
.
- X. Lai, J. Li, B. A. Korgel, Z. Dong, Z. Li, F. Su, J. Du and D. Wang, Angew. Chem., Int. Ed., 2011, 50, 2738–2741 CrossRef CAS PubMed
.
- X. Sun, J. Liu and Y. Li, Chem. – Eur. J., 2006, 12, 2039–2047 CrossRef CAS PubMed
.
- L. Wen, Y. Ma, B. Dai, Y. Zhou, J. Liu and C. Pei, Mater. Res. Bull., 2013, 48, 687–690 CrossRef CAS
.
- Y. Shin and G. Exarhos, Cellulose, 2007, 14, 269–279 CrossRef CAS
.
- A. T. Harris and A. R. Maddocks, Mater. Sci. Technol., 2010, 26, 375–378 CrossRef CAS
.
- T. L. Church, S. Fallani, J. Liu, M. Zhao and A. T. Harris, Catal. Today, 2012, 190, 98–106 CrossRef CAS
.
- Y. Shin, C. Wang, W. D. Samuels and G. J. Exarhos, Mater. Lett., 2007, 61, 2814–2817 CrossRef CAS
.
- X. Tao, L. Dong, X. Wang, W. Zhang, B. J. Nelson and X. Li, Adv. Mater., 2010, 22, 2055–2059 CrossRef CAS PubMed
.
- J. Du, Q. Li, Y. Xia, X. Cheng, Y. Gan, H. Huang, W. Zhang and X. Tao, J. Alloys Compd., 2013, 581, 128–132 CrossRef CAS
.
- Y. Shin, X. S. Li, C. Wang, J. R. Coleman and G. J. Exarhos, Adv. Mater., 2004, 16, 1212–1215 CrossRef CAS
.
- Y. Gotoh, K. Fujimura, M. Koike, Y. Ohkoshi, M. Nagura, K. Akamatsu and S. Deki, Mater. Res. Bull., 2001, 36, 2263–2275 CrossRef CAS
.
- H. Haiping, W. Yuxia and T. Honggao, J. Phys.: Condens. Matter, 2002, 14, 11867 CrossRef
.
- W. Ren, Z. Ai, F. Jia, L. Zhang, X. Fan and Z. Zou, Appl. Catal., B, 2007, 69, 138–144 CrossRef CAS
.
- Y. Gu, D. Jia and J. Huang, CrystEngComm, 2013, 15, 8924–8928 RSC
.
- R. R. Unocic, F. M. Zalar, P. M. Sarosi, Y. Cai and K. H. Sandhage, Chem. Commun., 2004, 796–797 RSC
.
- A. Vioux, Chem. Mater., 1997, 9, 2292–2299 CrossRef
.
-
M. Niederberger and N. Pinna, Metal Oxide Nanoparticles in organic solvent, Spinger-Verlag, London, 2007 Search PubMed
.
- M. Kettunen, R. J. Silvennoinen, N. Houbenov, A. Nykänen, J. Ruokolainen, J. Sainio, V. Pore, M. Kemell, M. Ankerfors, T. Lindström, M. Ritala, R. H. A. Ras and O. Ikkala, Adv. Funct. Mater., 2011, 21, 510–517 CrossRef CAS
.
- J. T. Korhonen, P. Hiekkataipale, J. Malm, M. Karppinen, O. Ikkala and R. H. A. Ras, ACS Nano, 2011, 5, 1967–1974 CrossRef CAS PubMed
.
- J. T. Korhonen, M. Kettunen, R. H. A. Ras and O. Ikkala, ACS Appl. Mater. Interfaces, 2011, 3, 1813–1816 CAS
.
- B. Boury, R. G. Nair, S. K. Samdarshi, T. Makiabadi and P. H. Mutin, New J. Chem., 2012, 36, 2196–2200 RSC
.
- B. Boury and S. Plumejeau, un-published results, 2014.
- K. Benaissi, L. Johnson, D. A. Walsh and W. Thielemans, Green Chem., 2010, 12, 220–222 RSC
.
- M. Wu, S. Kuga and Y. Huang, Langmuir, 2008, 24, 10494–10497 CrossRef CAS PubMed
.
- X. Wu, C. Lu, W. Zhang, G. Yuan, R. Xiong and X. Zhang, J. Mater. Chem. A, 2013, 1, 8645–8652 CAS
.
- R. Xiong, C. Lu, W. Zhang, Z. Zhou and X. Zhang, Carbohydr. Polym., 2013, 95, 214–219 CrossRef CAS PubMed
.
- J. L. T. Hage, R. D. Schuiling and S. P. Vriend, Can. Metall. Q., 1999, 38, 267–276 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4gc00957f |
| This journal is © The Royal Society of Chemistry 2015 |