S. L. Pira, O. El Mahdi, L. Raibaut, H. Drobecq, J. Dheur, E. Boll and O. Melnyk
Org. Biomol. Chem., 2016,14, 7211-7216
DOI:
10.1039/C6OB01079B,
Paper
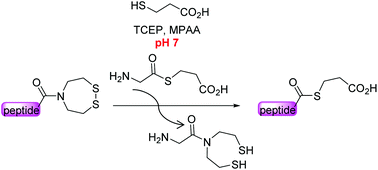
The bis(2-sulfanylethyl)amide (SEA) N,S-acyl shift thioester surrogate has found a variety of useful applications in the field of protein total synthesis. Here we present novel insights into the SEA amide/thioester equilibrium in water which is an essential step in any reaction involving the thioester surrogate properties of the SEA group. We also show that the SEA amide thioester equilibrium can be efficiently displaced at neutral pH for accessing peptide alkylthioesters, i.e. the key components of the native chemical ligation (NCL) reaction.