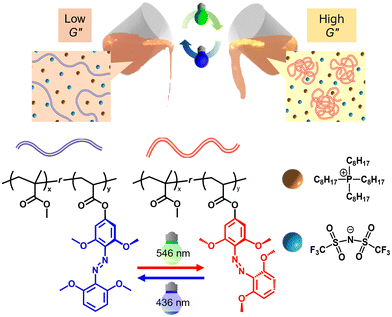
Stimuli-responsive liquid cell scaffold: reversible viscoelasticity switching of a polymer in an ionic liquid by visible-light
Aya
Saruwatari
 ab,
Yuji
Kamiyama
ab,
Yuji
Kamiyama
 a,
Jun
Nakanishi
a,
Jun
Nakanishi
 acd,
Ryota
Tamate
acd,
Ryota
Tamate
 a and
Takeshi
Ueki
a and
Takeshi
Ueki
 *ab
*ab
aResearch Center for Macromolecules and Biomaterials, National Institute for Materials Science, 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan. E-mail: UEKI.Takeshi@nims.go.jp
bGraduate School of Life Science, Hokkaido University, Kita 10, Nishi 8, Kita-ku, Sapporo, Hokkaido 060-0810, Japan
cGraduate School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku-ku, Tokyo 169-8555, Japan
dGraduate School of Advanced Engineering, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan
First published on 6th November 2025
Abstract
Cell culture at liquid|liquid interfaces has gained attention from the perspective of the mechanobiology. We recently reported cell culture at water-immiscible ionic liquids (ILs) interface, which have an advantage of polymer compatibility over the conventional non-polar liquid scaffold capable of interfacial cell culturing. Herein, we report a light-responsive polymer solution in a noncytotoxic IL (NCIL) that reversibly changes its viscosity. Methoxyazobenzene (mAzoA) was selected as the chromophore for reversible photoisomerization under non-phototoxic visible light. For a main polymer, poly(methyl methacrylate) (PMMA) was chosen because of exhibiting upper critical solution temperature (UCST) type phase transition in an NCIL ([P8,8,8,8][TFSI]) near cell culturing temperature. We successfully demonstrated that the loss modulus of the polymer solution was switched reversibly triggered by visible light photochromism of mAzoA. We also revealed that the polymer solution showed no cytotoxicity, confirming that they can be applied to liquid cell scaffold materials. Furthermore, the thermal isomerization of cis-mAzoA to trans-mAzoA was extremely slowed down in [P8,8,8,8][TFSI] (τ1/2 = 2911 h at 37 °C), suggesting the avoidance of undesired aging degradation during cell culturing operation. The system ability to switch between higher and lower viscoelasticity offers a powerful tool for real-time control of the cellular environment.
Introduction
Stimuli-responsive materials are pivotal in modern materials science, offering dynamic control over mechanical properties in response to external stimuli. Viscosity switchable materials are increasingly important in fields like dampers to reduce shocks, control valves, smooth-moving actuators and robotics, drug delivery and microfluids devices such as electro-1–4 and magnetorheological fluids,5–9 where real-time adaptation of material properties is required to match complex environmental or biomedical demands. Polymer solutions offer various approaches to stimuli-responsiveness, allowing various choices depending on the applications. Among external stimuli, light is particularly advantageous due to its non-contact nature, tunable intensity, and precise spatial and temporal control. When combined with polymers, light-responsive solutions offer reversible modulation of viscoelastic properties in a user-defined manner. These features make them promising candidates for dynamic material systems, especially where localized and programmable mechanical environments are required. If we realize photorheologically responsible10,11 and hydrophobic polymer solution, it represents a promising strategy for advancing applications in dynamic cell culture systems and tissue engineering.Within this context, liquid interfaces provide unique environments for investigating cellular responses to mechanical stimuli. In mechanobiology, extremely soft liquid interfaces offer a distinct platform to study how cells perceive and react to their surroundings.12–24 Research at fluorocarbon interfaces has already demonstrated important phenomena, such as selective neural differentiation, the maintenance of undifferentiated states in human mesenchymal stem cells (hMSCs)19–21 as well as significant increases in the strain energy exerted by epithelial cells upon transforming growth factor-β1 treatment, which induces an epithelial–mesenchymal transition (EMT).22 Recent studies have revealed that cell spreading at liquid interfaces involves intricate mechanotransduction pathways, including myosin-based contractility, Rho-associated protein kinase (ROCK) signalling, and actin polymerisation.19,20,24 In particular, the maintenance of hMSC stemness has been linked to focal adhesion kinase (FAK) phosphorylation, ROCK activation, actomyosin contractility, and intracellular metabolic activity.21 These findings highlight the potential of dynamically tunable liquid interfaces as a platform for mechanobiological studies. Liquid cell culture is also essential from a practical standpoint, since liquid|liquid emulsion based 3D culture can significantly increase the culturing area and reduce the cost of regenerative medicine.13,14,17,23 Beyond these advantages, ionic liquids (ILs) present a versatile, non-volatile, non-flammable, and recyclable25–28 alternative to conventional liquid scaffolds such as fluorinated carbons.12–24 We recently discovered hydrophobic and non-cytotoxic ILs (NCILs) and demonstrated that hMSCs could adhere to and spread on NCIL-based liquid scaffolds.29 Owing to their designable chemical structures and broad solubility range, ILs provide a powerful platform to explore how specific liquid properties affect cellular behaviour. Furthermore, their negligible volatility and thermal stability enable their reuse in emulsion-based 3D culture systems, supporting sustainable and scalable approaches to regenerative medicine.28 This aspect opens the door to new possibilities in “bulk manipulation” of scaffold properties, moving beyond “interfacial engineering” to create more sophisticated, tunable platforms for cell culture.
ILs have also been recognized as unique platforms for stimuli-responsive polymers. The phenomenon in which phase-separated components become miscible upon heating is referred to as an upper critical solution temperature (UCST)-type phase transition, whereas the opposite behaviour, where components become miscible upon cooling, is referred to as a lower critical solution temperature (LCST)-type phase transition.30–33 For instance, poly(N-isopropylacrylamide) (PNIPAm) has been widely studied for its LCST-type phase behaviour in water, undergoes an opposite UCST-type phase transition in various hydrophobic ILs, such as a series of 1-alkyl-3-methylimidazolium trifluoromethylsulfonylimide ([Cnmim][TFSI]).31,34–37 When NIPAm was randomly copolymerized with methacrylate monomers bearing azobenzene side chains (P(AzoMA-r-NIPAm)), the UCST-type phase transition temperature in 1-ethyl-3-methylimidazolium trifluoromethylsulfonylimide ([C2mim][TFSI]) changed depending on the photoisomerization state of AzoMA, and solubility switching was observed by light illumination.38 This concept has led to the preparation of processable ion gels that can contribute to the development of next-generation electrochemical devices.39,40
In this study, we demonstrate the concept of a liquid scaffold material based on a light-responsive polymer in an NCIL (Fig. 1). The viscoelasticity of the [P8,8,8,8][TFSI] polymer solution was reversibly switched in response to light illumination. Studies on cell phenotypes such as spreading, proliferation, and differentiation toward direct viscoelastic changes from scaffolds are one of the current aspects in the field of mechanobiology.41–45 If viscoelastically changeable liquid scaffold materials induced by photo stimulation can be established, a sophisticated liquid cell culture platform can be realized. Compared to our previous study using P(AzoMA-r-NIPAm) in [C2mim][TFSI], the polymer solution in this study was specifically designed to be both non-cytotoxic and sufficiently hydrophobic to retain phase separation from the overlaid aqueous medium. For the photoisomerizable unit, we selected methoxyazobenzene (mAzo), which undergoes reversible photoisomerization under non-phototoxic visible light.46–48 We discovered that poly(methyl methacrylate) (PMMA) exhibited UCST type phase transition in [P8,8,8,8][TFSI] approximately 37 °C, the mammalian cell culture temperature. In contrast to the previous system based on PNIPAm and [C2mim][TFSI], we employed hydrophobic PMMA, which exhibits a UCST-type phase transition in [P8,8,8,8][TFSI], an NCIL. This selection ensured both biocompatibility and tunable thermal responsiveness under cell culture conditions.29 Random copolymers of acrylate monomers bearing mAzo (mAzoA: 6-(4-((2,6-dimethoxyphenyl)diazinyl)-3,5-dimethoxyphenoxy)acrylate) and MMA (P(mAzoA-r-MMA)) changed the UCST-type phase transition in response to nonharmful green (546 nm) and blue (436 nm) light. We successfully demonstrated that loss modulus (G″) can be switched reversibly for at least three cycles depending on the photoisomerization state of mAzoA accompanying with the reversible solubility changes of the polymer solution depending on the irradiation light wavelength. In addition, the thermal isomerization of cis-mAzoA to trans-mAzoA was extremely slowed down in [P8,8,8,8][TFSI] (τ1/2 = 2911 h at 37 °C). The results provide an important insight into azobenzene-containing materials, where thermal isomerization has hindered highly flexible material design. Because our scaffold operates at a liquid|liquid boundary, a hydrophobic, water-immiscible IL is essential: it maintains a stable liquid|liquid interface with culture medium and prevents the photoresponsive polymer from leaking into the aqueous phase. Guided by this design requirement, we selected [P8,8,8,8][TFSI] and tuned the UCST of hydrophobic PMMA-based copolymers to operate near 37 °C. In this study, we developed a novel photoresponsive polymer solution whose viscoelasticity can be reversibly modulated under visible light irradiation. Due to its hydrophobic nature and the non-cytotoxicity of both the polymer solution and the light stimulus, this system holds promise as a dynamic liquid scaffold. The interface formed between the polymer–NCIL solution and the culture medium may provide tunable mechanical cues to cells, offering a new strategy for controlling cell behaviour through light-mediated stimuli. The focus of this study is on establishing a visible-light-reponsive, NCIL polymer solution as a materials platform for tunable viscoelasticity, rather than on direct cell-culture experiments, which will be pursued in future work.
Experimental section
Materials
All reagents were purchased from Fujifilm Wako Pure Chemical Corporation (Japan) and Tokyo Chemical Industry (Japan), unless otherwise stated. 4-[2-(2,6-Dimethoxyphenyl)-diazenyl]-3,5-dimethoxyphenol was purchased from NARD institute, Ltd (Japan) and used as received. Methyl methacrylate was purchased from Tokyo Chemical Industry (Japan) and purified by passing them through aluminum oxide (90 active basic 0.063–0.200 mm, Merck, Germany). Lithium trifluoromethylsulfonylimide (Li[TFSI]) and 2,2′-azobis(isobutyronitrile) (AIBN) were obtained from Kanto Chemical (Japan) and used as received.All of the following experiments were performed at room temperature (25 °C) and atmospheric pressure (0.1 MPa), unless otherwise noted.
Synthesis of [P8,8,8,8][TFSI]
Briefly, [P8,8,8,8][TFSI] obtained by ion exchange reaction of cation precursor and corresponding lithium salt as an anion precursor. 10.0 g (17.7 mmol) of tetra-n-octylphosphonium bromide ([P8,8,8,8]Br) and slightly excess (×1.1 by mol) amount of lithium trifluoromethylsulfonylimide (Li[TFSI]) 5.60 g (19.5 mmol) were mixed in 30 mL of ethanol. The reaction was carried out at 80 °C overnight. After evaporation of ethanol at 40 °C using BioChromato's Smart Evaporator™ (BioChromato, Japan), the reaction mixture was washed with water 5 times to remove unreacted water miscible impurity until no precipitate was observed upon addition of AgNO3 to the aqueous phase, indicating the complete removal of residual bromide anions, as previously described.29,49 Clear and colourless liquid was obtained after vacuum-dried at 120 °C overnight and returned to atmospheric pressure with Ar flow.The obtained [P8,8,8,8][TFSI] was confirmed by 1H NMR (400 MHz, JEOL, Japan) in chloroform-d (Fujifilm Wako Pure Chemical Corporation, Japan) (Fig. S1).
Synthesis of mAzoA
First, 4-[2-(2,6-dimethoxyphenyl)-diazenyl]-3,5-dimethoxyacrylate (mAzoA) was prepared from 4-[2-(2,6-dimethoxyphenyl)-diazenyl]-3,5-dimethoxyphenol (Scheme S1). 4-[2-(2,6-Dimethoxyphenyl)-diazenyl]-3,5-dimethoxyphenol (9.55 g, 30 mmol) was dissolved in 90 mL of dichloromethane in a three-neck flask. Triethylamine (8.36 mL, 60 mmol) was added into the solution and the mixture was bubbled with Ar and cooled in an ice bath. Acryloyl chloride (3.65 mL, 45 mmol) was dissolved in 60 mL of dichloromethane bubbled with Ar gas in a two-neck flask. Under a stream of Ar gas, the mixture of acryloyl chloride was added to the solution in three-neck flask dropwise using a syringe. Under an Ar atmosphere, the reaction proceeded at 0 °C and subsequently at room temperature overnight. During the above processes, the mixture was vigorously stirred to facilitate homogeneous mixing. Dichloromethane and unreacted triethylamine was evaporated from the reacted solution using a rotary evaporator, and then ethyl acetate as a poor solvent for triethylammonium chloride was used to dissolve the product. The triethylammonium chloride produced during the reaction was extracted using vacuum filtering. Ethyl acetate was evaporated using a rotary evaporator, and then chloroform was used to dissolve the product. The product was purified by column chromatography using silica gel (neutral, 0.020–0.040 mm, Fujifilm Wako Pure Chemical Corporation, Japan) as a stationary phase and a mixture of hexane and ethyl acetate (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 vol%) as a mobile phase. The thin layer chromatography (TLC) showed three spots. Unreacted substance was adsorbed at origin. The other two spots that correspond to trans-mAzoA and cis-mAzoA were collected. The solution was concentrated by evaporation and subsequently dried under vacuum at 80 °C overnight to obtain a red powder (2.5 g, 22.4% yield). The percent yield was obtained by dividing the actual yield by the theoretical yield (11.2 g) calculated from the amount of 4-[2-(2,6-dimethoxyphenyl)-diazenyl]-3,5-dimethoxyphenol.
50 vol%) as a mobile phase. The thin layer chromatography (TLC) showed three spots. Unreacted substance was adsorbed at origin. The other two spots that correspond to trans-mAzoA and cis-mAzoA were collected. The solution was concentrated by evaporation and subsequently dried under vacuum at 80 °C overnight to obtain a red powder (2.5 g, 22.4% yield). The percent yield was obtained by dividing the actual yield by the theoretical yield (11.2 g) calculated from the amount of 4-[2-(2,6-dimethoxyphenyl)-diazenyl]-3,5-dimethoxyphenol.
The obtained mAzoA was confirmed by 1H NMR (400 MHz, JEOL, Japan) in dimethyl sulfoxide-d6 (Kanto Chemical, Japan) (Fig. S2).
Polymerisation of P(mAzoA-r-MMA)
Copolymers of mAzoA and methyl methacrylate (P(mAzoA-r-MMA)) were synthesized via free-radical polymerisation with different mAzoA/MMA feed ratios (mAzoA/MMA: 2.6/97.4, 4.3/95.7, 8.7/91.3 mol%). Representative procedures for P(mAzoA2.6-r-MMA) (mAzoA/MMA = 2.6/97.4) are described as follows. mAzoA (40.5 mg, 0.109 mmol), MMA (0.437 mL, 4.10 mmol), and 2,2′-azobis (isobutyronitrile) (AIBN, 2.7 mg, 16.4 μmol) were dissolved in 4 mL N,N-dimethylformamide (DMF). The solution was purged with argon gas for 20 min to remove oxygen dissolved in the solution. The reaction was carried out at 65 °C for 24 h. During the above processes, the mixture was vigorously stirred to facilitate homogeneous mixing. The obtained polymer solution was purified by reprecipitation with methanol as a poor solvent. The orange powder was again reprecipitated two times with tetrahydrofuran (THF) as a good solvent and methanol as a poor solvent. Reprecipitation was performed in a poor solvent at least 20 times the volume against the good solvent. The resulting orange powder was collected by filtration and dried under vacuum at 80 °C overnight.Monomer compositions of the P(mAzoA-r-MMA) were determined from the 1H NMR analyses, by calculating the integrated intensity ratio between the peaks for mAzoA and MMA (trans-mAzoA: 7.2–7.4 ppm (Fig. S8(a)), 1H, Ar H; cis-mAzoA: 7.0–7.2 ppm (Fig. S8(a′)), 1H, Ar H; MMA: 0.6–2.2 ppm (Fig. S8(f) and (i)), 5H, CH2CCH3) (Table S3). The molecular weight and polydispersity index were determined by gel permeation chromatography (GPC) with DMF containing 10 mmol L−1 lithium bromide as the eluent (Table S4). The GPC system was equipped with a PU-2080 Plus HPLC pump, a DG-2080-53 3-Line degasser, an RI-4030 refractive index detector, a UV-2075 Plus UV/VIS detector, an LC-NetII/ADC interface box, and a CO-4060 column oven (JASCO, Japan). Two columns (SB-806M HQ, Showa Denko, Japan), which were kept in a column heater at 40 °C, were used for separation. The columns were calibrated using poly(methyl methacrylate) as molecular weight standards.
Preparation of P(mAzoA-r-MMA) solutions in [P8,8,8,8][TFSI]
20 mg of P(mAzoA-r-MMA) was first dissolved in 1 mL dichloromethane (DCM). Then, 1 mL [P8,8,8,8][TFSI] was added to the homogeneous polymer solution. After vigorously mixing well, volatile DCM was evaporated by heating the solution at 85 °C under reduced pressure overnight.Photo- and thermal-isomerization of mAzoA monomer in [P8,8,8,8][TFSI]
To investigate the photoisomerization of mAzoA monomer solution in [P8,8,8,8][TFSI], 0.01 wt% mAzoA monomer in [P8,8,8,8][TFSI] was prepared, and the absorbance spectra were measured using an ultraviolet-visible spectrophotometer (UV-Vis; UV-2600, Shimadzu, Kyoto, Japan). The photoisomerization efficiency was determined from the 1H NMR analyses, by calculating the integrated intensity ratio between the peaks for trans- and cis-mAzoA (Fig. S3). To avoid the influence of deuterated solvent addition on the photoisomerization reaction, measurements were conducted using NMR double tubes to separate acetonitrile-d3 and 10 mM mAzoA monomer solution in [P8,8,8,8][TFSI]. Before measurements, blue light (436 nm, 8.9 mW cm−2) or green light (546 nm, 6.6 mW cm−2) was irradiated by a mercury lamp (SX-UI 251HQ, USHIO, Tokyo, Japan) connected to power supply unit (BA-H250, USHIO, Tokyo, Japan) for 20 min. The wavelength of the irradiated light was switched using bandpass filters (65–199 for blue light and 65–219 for green light, Edmund Optics, US).The thermal isomerization of mAzoA from cis to trans isomer at 37 °C was evaluated by first irradiating the same sample over 2 h with green light (546 nm) to bring mAzoA to a photo-stationary state, and then tracking the cis ratio determined from the 1H NMR analyses for 7 days (Fig. 4(c)). To estimate half-life and activation energy, the kinetics of thermal relaxation were measured at 50, 60, and 70 °C. First order rate constant of cis to trans isomerization, k, is determined by fitting experimental data (Fig. 4(d)) to following the integrated rate law for first order reaction.
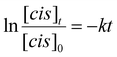 | (1) |
Arrhenius plots (Fig. 4(e)) from the reaction rate constants at each temperature yielded activation energies, Ea, and frequency factors, A. The half-life of cis isomer, t1/2, was determined from the rate constant at 37 °C obtained by Arrhenius equation.
Turbidity measurements
The temperature-dependent phase separation behaviours of PMMA (Table S2), P(trans-mAzoA-r-MMA) and P(cis-mAzoA-r-MMA) (Table S4) were evaluated by measuring the transmittance of the polymer solution (2 w/v%) in [P8,8,8,8][TFSI] using an ultraviolet-visible spectrophotometer (UV-Vis; V-770, JASCO, Japan) with a temperature controllable unit (ETCS-761, JASCO, Japan). After irradiation with blue light (436 nm, 147 mW cm−2) and green light (546 nm, 109 mW cm−2) on the P(trans-mAzoA-r-MMA) and P(cis-mAzoA-r-MMA) solutions, respectively, over 1 h at 85 °C, the polymer solutions were transferred into quartz cells. Photoirradiation was carried out using a spotlight source (LC8, HAMAMATSU Photonics, Japan). The wavelength of the irradiated light was switched using bandpass filters (65–199 for blue light and 65–219 for green light, Edmund Optics, US). The transmittance at 700 nm was measured while the polymer solution was cooled at 0.1 °C min−1. A slow scan rate was chosen to minimize hysteresis and kinetic artifacts known in UCST systems. Temperature control precision was ±0.1 °C min−1 by using thermostatic circulator (PCC-7000, EYELA, Japan). Throughout the measurements, the polymer solution was stirred at 300 rpm and constantly exposed blue or green light from above. The cloud point was determined as the temperature at 50% of the initial transmittance.To ensure that the photoisomerization reaction proceeds as quick as possible after switching the irradiation light, the photoinduced phase transition was measured as follows. A drop of the polymer solution was placed on a glass plate with a shallow concave depression and covered by a cover glass. The optical path length for the turbidity measurements was 800 μm. The glass plate was placed on a hot stage (Imoto, Japan), which enabled temperature control. The transmittance of the polymer solution was monitored at 700 nm, using an Ocean Optics USB-2000 fiber-optic spectrometer at 46 °C ± 0.1 °C min−1.
Rheological measurements
Rheology of P(mAzoA-r-MMA) solution (2 w/v%) in [P8,8,8,8][TFSI] was investigated by an Anton Paar Physica MCR 102 (Anton Paar, Austria) with a standard cell for UV irradiation (P-PTD200/GL). Parallel plate geometry with a diameter of 12 mm and a gap spacing of approximately 0.5 mm was used for the measurement. Blue or green light illuminated the sample from the bottom side for 1 h at 80 °C to reach a photostationary state prior to measurement. Photoirradiation was carried out using a spotlight source (LC8, HAMAMATSU Photonics, Japan). The wavelength of the irradiated light was switched using bandpass filters (65–199 for blue light and 65–219 for green light, Edmund Optics, US). Temperature scanning measurements were performed with a strain of γ = 10%, a frequency of ω = 10 rad s−1 and a cooling rate of 0.1 °C min−1 in the range from 80 to 10 °C were used under continuous each light irradiation. The temperature was controlled using a Peltier temperature controller with ±0.01 °C min−1.The photo switching measurements were also performed with the same strain and frequency. The sample was irradiated with blue light at 60 °C for 1 h and cooled to 25 °C at 1 °C min−1, then the loss modulus was recorded after 1 h, followed by green light irradiation and the loss modulus was also recorded after 1 h. After 2nd cycle, the sample was once heated to 60 °C and cooled to 25 °C at 1 °C min−1, and as in 1st cycle measurements were conducted.
Phototoxicity test
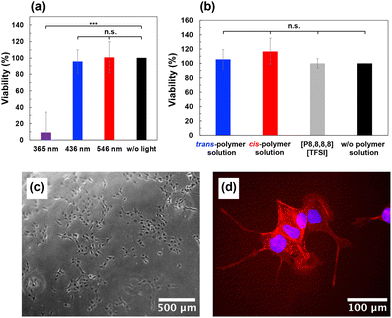
The viability of MDCK cells after irradiation of 365 nm, 436 nm and 546 nm light was evaluated using WST-8 assay (Dojindo Laboratories, Japan), following the manufacturer's protocols. A cell suspension in growth medium (100 µL) was placed into each well of a 96 well cell culture dish at a density of 5 × 103 cells per well. In the experiments of this study, cells were seeded at a density to be almost 100% confluent at the time of measurement according to the growth rate of MDCK cells.50 The plate was then incubated in 5% CO2 at 37 °C for 24 h. The culture medium in each well of 96 well dish was replaced with colourless medium. The culture medium was 10% fetal bovine serum (FBS) (EU origin, BioWest, French) and 1% Penicillin-Streptomycin Solution (Fujifilm Wako Pure Chemical Corporation, Japan) in minimum essential medium (MEM with Earle's Salts and L-Gln, liquid, NACALAI TESQUE, INC, Japan). The same composition of culture medium was used in the subsequent Experimental section. The colourless medium was 10% FBS in Hanks’ balanced salt solution (HBSS, calcium, magnesium, no phenol red, Thermo Fisher Scientific, US). Each well was covered with quartz glass plate, and exposed to UV light (365 nm, 70–85 mW cm−2), blue light (436 nm, 62–75 mW cm−2) and green light (546 nm, 47–56 mW cm−2) for 2 h at 37 °C. Photoirradiation was carried out using a spotlight source (LC8, HAMAMATSU Photonics, Japan). The wavelength of the irradiated light was switched using bandpass filters (65–191 for UV light, 65–199 for blue light and 65–219 for green light, Edmund Optics, US). After replacing the colourless medium with culture medium, the plate was incubated in 5% CO2 at 37 °C for 20 h. WST-8 (10 µL) was added to each well, and the colour reaction proceeded for 1 h. The absorbance at 450 nm was measured using a microplate reader (Model 680, BIO RAD, US), and the viability was calculated based on the absorbance of the unirradiated well set as 100%.Cytotoxicity test for polymer–IL solution and IL
Briefly, cell viability was evaluated by measuring cell metabolism after culturing in a medium pre-saturated with each sample, following ISO 10993-5, “Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity”. The viability of MDCK cells after culturing in the culture medium saturated with P(mAzoA2.4-r-MMA) solution in [P8,8,8,8][TFSI] or [P8,8,8,8][TFSI] was evaluated using WST-8 assay (Dojindo Laboratories, Japan), following the manufacturer's protocols. A cell suspension in a culture medium (10 µL) was placed into each well of a 96 well cell culture dish containing 200 µL of a culture medium at a density of 5 × 103, 2.5 × 103 and 1.3 × 103 cells per well for the 24, 48 and 72 h toxicity test, respectively, followed by incubation in 5% CO2 at 37 °C overnight. 30 µL each of 2 w/v% P(trans-mAzoA2.4-r-MMA) solution in [P8,8,8,8][TFSI], 2 w/v% P(cis-mAzoA2.4-r-MMA) solution in [P8,8,8,8][TFSI] and [C2mim][TFSI] were added to a culture medium and mixed by a vortex mixer, and then the culture medium in each well of 96 well dish was replaced with the supernatant solution collected by centrifugation. As a positive control, the medium was replaced with fresh, unmodified culture medium. The plate was incubated in 5% CO2 at 37 °C for 24, 48 or 72 h followed by the replacement of the medium with fresh culture medium. After incubation for 19 h, the culture medium in each well was removed. Fresh culture medium (100 µL) and WST-8 (10 µL) was added to each well, and the colour reaction proceeded for 1 h. The absorbance at 450 nm was measured using a microplate reader (Multiskan FC, Thermo Fisher Scientific, US), and the viability was quantified by the absorbance ratio of the wells in the presence and the absence of polymer solution or IL samples.Culturing cells on an ion gel
The ion gel was prepared through conventional free radical polymerisation of n-butyl methacrylate (nBuMA) with ethyleneglycol dimethacrylate (EGDMA) as a cross-linker in [P8,8,8,8][TFSI]. nBuMA (0.85 g, 6.0 mmol), EGDMA (59 mg, 0.3 mmol), 2,2′-azobis(isobutyronitrile) (AIBN) (9.9 mg, 0.06 mmol), and [P8,8,8,8][TFSI] (0.2 g) were charged in a glass vial and sealed with a rubber septum, through which argon was bubbled for 15 min at room temperature. The gelation reaction was conducted at 80 °C for 22 h. After the reaction was finished, the ion gel was heated up to 80 °C overnight under vacuum to remove unreacted monomer. The ion gel was then immersed in a solution of fibronectin (2 mL, 6 µg mL−1) in pH 7.4 phosphate buffered saline (PBS) and incubated in 5% CO2 at 37 °C for 2 h. The upper fibronectin solution was washed with 1 mL of culture medium for six times. MDCKs were seeded on the gel at a density of 2.6 × 105 cells per well. After incubation in 5% CO2 at 37 °C for 20 h, cells on the ion gel were stained with phalloidin Alexa Fluor 568 (Life Technologies, A12380, dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) and Hoechst 33342 (Life Technologies, 2548w, dilution of 1
200) and Hoechst 33342 (Life Technologies, 2548w, dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) for 10 min to stain F-actin and nucleus, respectively. Finally, MDCKs on the ion gel were soaked in PBS and observed using Olympus BX51 microscope.
1000) for 10 min to stain F-actin and nucleus, respectively. Finally, MDCKs on the ion gel were soaked in PBS and observed using Olympus BX51 microscope.
Results and discussion
Photoisomerization reaction of mAzoA in [P8,8,8,8][TFSI]
Among various hydrophobic ILs, [P8,8,8,8][TFSI] was selected as the solvent in this study due to its unique combination of properties: it is non-cytotoxic, strongly hydrophobic, and capable of supporting UCST-type phase transitions of PMMA near physiological temperature (37 °C). These features render [P8,8,8,8][TFSI] particularly suitable for use in stimuli-responsive liquid scaffold systems designed for dynamic cell culture environments. First, the photoisomerization of mAzoA in [P8,8,8,8][TFSI] under visible light was investigated. mAzoA was synthesized from mAzo with a hydroxy group at a para-position and acryloyl chloride (Scheme S1 and Fig. S2). Fig. S3 shows the changes in 1H NMR spectra of mAzoA (10 mM) in [P8,8,8,8][TFSI] in response to visible light irradiation. trans- and cis-mAzoA were produced by visible light illumination with blue light at 436 nm (8.9 mW cm−2) and green light at 546 nm (6.6 mW cm−2), respectively. The 1H NMR spectra also confirmed that the ratios of trans- and cis-isomer reached over 80 and 85%, respectively. Fig. S4(a) and (b) show the time course of the absorbance spectra of mAzoA in [P8,8,8,8][TFSI]. Irradiation of trans-type mAzoA monomer at 546 nm decreased the absorption based on the π–π* transition of the trans-type near 300 nm, and blue-shifted the absorption based on the n–π* transition that appeared at 441 nm by 10 nm (Fig. S4(a)). Azobenzene compound generally does not show such blue-shift of the n–π* absorption wavelengths. However, mAzoA is distinguished by its unique n–π* absorption band, which differs between its trans- and cis-forms. It is believed that the introduction of methoxy groups into ortho-position of azobenzene alters geometry of trans-form to nonplanar and increases the highest occupied molecular orbital (HOMO) level of the N atom lone pairs on the trans-form, leading to an increase and a red shift in the n–π* absorption of the trans-form compared to the cis-form.46,47 This unique visible light responsive photoisomerization of mAzo has already been reported in conventional molecular solvents,46–48 whereas, as far as we understand, this is the first report to demonstrate it in an IL. Upon irradiation of the cis-type of mAzoA at 436 nm, a red shift in the n–π* transition absorption peak was observed, with the π–π* transition absorption of the trans-type returning to its original intensity (Fig. S4(b)). The reversible photoisomerization between the cis and trans-forms under visible light illumination was also confirmed (Fig. S4(c)). mAzoA photoisomerization occurred with high isomer yields (over 80%) when alternately irradiated with blue and green light at least three times. The photochromic reaction of mAzoA was completed as quick as within ∼10 min from trans- to cis-type and within ∼0.5 min from cis- to trans-type even in high viscous [P8,8,8,8][TFSI] (390 mPa s at 25 °C, Fig. S5).Thermo-sensitive polymer in [P8,8,8,8][TFSI]
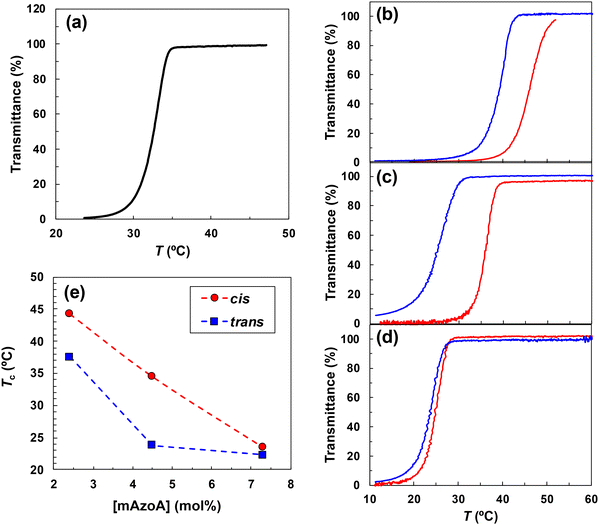
To control the viscosity of liquids by light illumination, we utilised the change of polymer solubility so that UCST- or LCST-type phase transition temperature depends on the wavelength of light. In earlier reports, phototriggered solubility switching in ILs was principally achieved by the photoinduced phase transition of random copolymers composed of photochromic pendant monomers and thermosensitive monomers such as P(AzoMA-r-NIPAm).38,51,52 However, PNIPAm is not able to be utilised for present system, because PNIPAm is eventually water-soluble. There is requisite condition specific to the cell scaffold application that the thermosensitive polymer must be hydrophobic to avoid accidental diffusion into the aqueous culture medium during cell culturing. Therefore, to explore thermosensitive hydrophobic polymer in NCILs, solubility tests were performed for general hydrophobic polymers, such as poly(meth)acrylates and polystyrenes. Table S1 summarizes the solubility tests of the polymers for [P8,8,8,8][TFSI] and previously reported NCILs, triethylpentylphosphonium trifluoromethylsulfonylimide ([P2,2,2,5][TFSI]), tributylmethylphosphonium trifluoromethylsulfonylimide ([P4,4,4,1][TFSI]), and trihexyltetradecylphosphonium trifluoromethylsulfonylimide ([P6,6,6,14][TFSI]).29 Polymers were mixed with each NCIL (2 w/v%) via cosolvent evaporation method, and then the solubility of polymer was judged from the transparency of the solution from 4 to 100 °C. Briefly, poly(meth)acrylates bearing short-alkyl-side-chains less than six are compatible with [P2,2,2,5][TFSI], [P4,4,4,1][TFSI], and [P6,6,6,14][TFSI]. However, poly(meth)acrylates with the alkyl-chain length of six or more and polystyrenes were incompatible with these NCILs. Despite the fact that [P8,8,8,8][TFSI] is a structural isomer of [P6,6,6,14][TFSI], its polymer solubilities was different. P(c-Hex)MA, P(n-Hex)MA, and P(n-Hex)A, which have an alkyl carbon of six and did not dissolve in [P6,6,6,14][TFSI], are soluble in [P8,8,8,8][TFSI]. In contrast, PMA with an alkyl carbon of 1, which was soluble in [P6,6,6,14][TFSI], did not dissolve in [P8,8,8,8][TFSI]. Although [P8,8,8,8][TFSI] and [P6,6,6,14][TFSI] contain the same alkyl chain within the cation structure, [P8,8,8,8][TFSI] appeared to be less polar and exhibited higher solubility against lower polar polymers such as moderately longer alkyl methacrylate. This observation was certainly supported by the lower ET(30) of [P8,8,8,8][TFSI] (44.4 kcal mol−1) than that of [P6,6,614][TFSI] (45.5 kcal mol−1), estimated by well-established solvatochromism measurements using Reichardt's dye.We have found that PMMA showed a clear UCST-type phase transition in [P8,8,8,8][TFSI]. Fig. 2(a) shows the transmittance curve of PMMA (Mw = 1.1 × 105 g mol−1, 2 w/v%) in [P8,8,8,8][TFSI] with a phase transition temperature of Tc = 32.6 °C. In [P8,8,8,8][TFSI], poly(n-decyl acrylate) (P(n-Decy)A) also exhibits thermo-sensitivity (LCST-type transition), however; the phase transition occurs over 80 °C, which is far from mammalian cell culturing temperature. Therefore, we decided to use UCST-type thermo-sensitivity of PMMA showing phase transition temperature near the culturing temperature of 37 °C.
The UCST-type phase separation in polymer/IL solutions is most clearly understood in terms of the Flory–Huggins free energy, ΔGmix = ΔHmix − TΔSmix. UCST arises when mixing is enthalpically unfavourable (ΔHmix > 0) yet accompanied by a positive entropy of mixing (ΔSmix > 0): at low temperatures the enthalpic penalty dominates and the system demixes, whereas heating both reduces the effective interaction penalty and increases the TΔSmix term, so that ΔGmix becomes negative and a homogeneous solution forms. Because the combinatorial entropy of mixing is small for polymers (∝1/N), the miscibility balance is particularly sensitive to the temperature dependence of the interaction parameter χ.36,53 To identify which interactions set χ in our PMMA/[P8,8,8,8][TFSI] system, we examined the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching region by FT-IR in both compatible (soluble) and incompatible (phase-separated) states. No appreciable shift was detected (Fig. S6), indicating that temperature-dependent hydrogen bonding, which can dominate in other systems (e.g., PNIPAm/[C2mim][TFSI])54 is not a major contributor here; likewise, there is no evidence for strong specific electrostatic complexation. We therefore attribute the enthalpic cost primarily to disrupting cohesive IL–IL and polymer–polymer contacts (non-specific, van der Waals interactions) in this low polar IL. In this picture, heating alleviates the relative enthalpic penalty and, together with the (small but positive) polymer mixing entropy, drives dissolution at higher temperatures. Building on this framework, we next quantify the molecular-weight dependence of the critical temperature.
O) stretching region by FT-IR in both compatible (soluble) and incompatible (phase-separated) states. No appreciable shift was detected (Fig. S6), indicating that temperature-dependent hydrogen bonding, which can dominate in other systems (e.g., PNIPAm/[C2mim][TFSI])54 is not a major contributor here; likewise, there is no evidence for strong specific electrostatic complexation. We therefore attribute the enthalpic cost primarily to disrupting cohesive IL–IL and polymer–polymer contacts (non-specific, van der Waals interactions) in this low polar IL. In this picture, heating alleviates the relative enthalpic penalty and, together with the (small but positive) polymer mixing entropy, drives dissolution at higher temperatures. Building on this framework, we next quantify the molecular-weight dependence of the critical temperature.
To design the polymer solution precisely, we evaluated the molecular weight dependence of the phase transition temperature within the Flory–Huggins theory. The relationship between the degree of polymerisation N and the interaction parameter χc at the critical solution temperature (Tcs), where the binodal and spinodal curves overlap, is expressed as follows:30
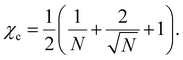 | (2) |
This expression explicitly reflects that the combinatorial entropy of mixing decreases with increasing N, thereby increasing the susceptibility of miscibility to the interaction parameter. Next, we describe the temperature dependence of χ using the contact-energy as follows:
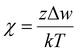 | (3) |
Note that in UCST systems the binodal is bell-shaped: Tc increases with concentration on the dilute side (φ < φc, where φ and φc are volume fraction of polymer and critical volume fraction of polymer giving UCST, respectively), reaches a maximum at φc, and decreases at higher polymer fractions. Our 2 w/v% solutions are on the dilute side, so Tc rises with concentration within this window. In addition, the apparent Tc is known to depend on the scan rate owing to kinetic hysteresis;53,55 all cloud points here were therefore measured on slow cooling at 0.1 °C min−1 to ensure consistency.
Photo- and thermo-sensitive polymer in [P8,8,8,8][TFSI]
We prepared a visible light-responsive polymer, P(mAzoA-r-MMA), by copolymerizing MMA with mAzoA via free-radical polymerisation (Tables S3 and S4). P(mAzoA-r-MMA) (2 w/v%) was mixed with [P8,8,8,8][TFSI] using a cosolvent evaporation method. Fig. 2(b)–(d) show transmittance curve of P(mAzoA-r-MMA)s. Although, Mw/Mns of polymers were slightly dispersed, the Mw of the random copolymers used in the measurements were comparable at 1.1–1.7 × 105 g mol−1, and the amount of mAzoA loaded in the P(mAzoA-r-MMA)s ([mAzoA]) were systematically varied from 2.4–7.3 mol%. Alternatively, the difference in Tcs of P(mAzoA-r-MMA) reflects [mAzoA] and the photoisomerization state of mAzoA. In a previous study, as the amount of azobenzene pendant monomer during the polymerisation in the feed increased, the Mw/Mn of the copolymer and the molecular weight tended to decrease, suggesting that the azobenzene monomers acted as chain transfer agents.38,52,56,57 A similar trend was reported for mAzoA as a comonomer.48 It was difficult to obtain about 1 × 105 g mol−1 of P(mAzoA-r-MMA) at which the polymer was expected to exhibit UCST type phase transition near 37 °C, when the copolymerisation proceeded through reversible addition fragmentation chain transfer (RAFT) polymerisation (chain transfer agent (CTA): 2-phenylpropan-2-yl benzodithioate, monomer concentration: 2 mol L−1, [MMA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [mAzoA] = 97
[mAzoA] = 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mol% in the feed). Thus, P(mAzoA-r-MMA) was synthesized via free-radical polymerisation at a faster reaction rate. Transmittance measurements were performed under visible light irradiation at 546 nm for P(cis-mAzoA-r-MMA) and 436 nm for P(trans-mAzoA-r-MMA) during the cooling process (Fig. 2(b)–(d)). All P(mAzoA-r-MMA) solutions exhibited a clear UCST-type phase transition, but Tcs changes depending on both [mAzoA] and photoisomerization states. Fig. 2(e) shows the relationship between Tcs and [mAzoA]. The width of Tcs sandwiched between trans- and cis-type polymers changed depending on [mAzoA]. This nonlinear tendency has been observed in traditional aqueous azobenzene-containing thermosensitive polymer solutions.58–61 When the azobenzene content was low, it was difficult to generate a polarity contrast according to the photoisomerization state of azobenzene itself, and the Tc difference was not broadened. Conversely, when the azobenzene content was too high, the isomerization efficiency was reduced owing to the neighbouring effect of mAzoA.62,63 For these reasons, the range of the bistable temperature gave a maximum against [mAzoA]. We note that the Tc of PMMA homopolymer (Mw = 1.1 × 105 g mol−1) lies between those of P(mAzoA2.4-r-MMA) and P(mAzoA4.5-r-MMA) in Fig. 2(e). This is readily rationalized when the molecular-weight effect (χc(N)) is considered together with enthalpic tuning by mAzoA. The copolymers used in Fig. 2(b–d) are slightly larger (1.5 × 105 g mol−1 and 1.7 × 10−5 g mol−1; Table S4) than the PMMA in Fig. 2(a). Because Tc increases with Mw in PMMA/[P8,8,8,8][TFSI] (e.g., 32.6 °C (Mw = 1.1 × 105 g mol−1) → 54.7 °C (Mw = 5.0 × 105 g mol−1) → 71.2 °C (Mw = 8.9 × 105 g mol−1); Table S2 and Fig. S7), this N-dependence shifts Tc upward for the copolymers. At [mAzoA] = 2.4 mol%, the N-driven upshift slightly overcompensates the thermodynamic improvement from introducing mAzoA, giving Tc(trans) = 37 °C marginally above Tc(PMMA) = 32.6 °C. At [mAzoA] = 4.5 mol%, the thermodynamic stabilization dominates and Tc(trans) = 23 °C falls below that of PMMA despite the larger Mw. The cis-rich states follow the same trend.
3 mol% in the feed). Thus, P(mAzoA-r-MMA) was synthesized via free-radical polymerisation at a faster reaction rate. Transmittance measurements were performed under visible light irradiation at 546 nm for P(cis-mAzoA-r-MMA) and 436 nm for P(trans-mAzoA-r-MMA) during the cooling process (Fig. 2(b)–(d)). All P(mAzoA-r-MMA) solutions exhibited a clear UCST-type phase transition, but Tcs changes depending on both [mAzoA] and photoisomerization states. Fig. 2(e) shows the relationship between Tcs and [mAzoA]. The width of Tcs sandwiched between trans- and cis-type polymers changed depending on [mAzoA]. This nonlinear tendency has been observed in traditional aqueous azobenzene-containing thermosensitive polymer solutions.58–61 When the azobenzene content was low, it was difficult to generate a polarity contrast according to the photoisomerization state of azobenzene itself, and the Tc difference was not broadened. Conversely, when the azobenzene content was too high, the isomerization efficiency was reduced owing to the neighbouring effect of mAzoA.62,63 For these reasons, the range of the bistable temperature gave a maximum against [mAzoA]. We note that the Tc of PMMA homopolymer (Mw = 1.1 × 105 g mol−1) lies between those of P(mAzoA2.4-r-MMA) and P(mAzoA4.5-r-MMA) in Fig. 2(e). This is readily rationalized when the molecular-weight effect (χc(N)) is considered together with enthalpic tuning by mAzoA. The copolymers used in Fig. 2(b–d) are slightly larger (1.5 × 105 g mol−1 and 1.7 × 10−5 g mol−1; Table S4) than the PMMA in Fig. 2(a). Because Tc increases with Mw in PMMA/[P8,8,8,8][TFSI] (e.g., 32.6 °C (Mw = 1.1 × 105 g mol−1) → 54.7 °C (Mw = 5.0 × 105 g mol−1) → 71.2 °C (Mw = 8.9 × 105 g mol−1); Table S2 and Fig. S7), this N-dependence shifts Tc upward for the copolymers. At [mAzoA] = 2.4 mol%, the N-driven upshift slightly overcompensates the thermodynamic improvement from introducing mAzoA, giving Tc(trans) = 37 °C marginally above Tc(PMMA) = 32.6 °C. At [mAzoA] = 4.5 mol%, the thermodynamic stabilization dominates and Tc(trans) = 23 °C falls below that of PMMA despite the larger Mw. The cis-rich states follow the same trend.
In previous studies, UCST-type phase transitions of P(AzoMA-r-NIPAm) in [C2mim][TFSI] and [C4mim]PF6 were investigated.38,52 The Tcs of P(trans-AzoMA-r-NIPAm) were higher than that of P(cis-AzoMA-r-NIPAm), indicating that the cis-type polymer is more soluble in [C2mim][TFSI] or [C4mim]PF6. It is widely recognized that the polarity of azobenzene changes depending on its photoisomerization state. The dipole moment of trans-azobenzene is 0.5 D, whereas cis-azobenzene exhibits a significantly higher polarity of 3.1 D.64 Consequently, the Tcs of P(trans-AzoMA-r-NIPAm), which is less soluble, are greater than those of P(cis-AzoMA-r-NIPAm), which is more soluble. The increased polarity of the cis-polymer led to stabilised solvation in [C2mim][TFSI] or [C4mim]PF6 solvents with a comparatively higher polarity. However, the present P(mAzoA-r-MMA) system shows that the UCST-type Tcs of trans-type polymer are lower than that of cis-type polymer, indicating that the trans-type polymer is more soluble in [P8,8,8,8][TFSI]. This would come from lower polarity of [P8,8,8,8][TFSI] compared to previously used [C2mim][TFSI] and [C4mim]PF6 (ET(30) = [P8,8,8,8][TFSI]: 44.4 kcal mol−1, [C2mim][TFSI]: 52.2 kcal mol−1,65 [C4mim]PF6: 52.4 kcal mol−1 (ref. 65)). Castner et al., reported that the [P6,6,6,14][TFSI] which is the structural isomer of [P8,8,8,8][TFSI], could absorb non-polar n-hexane as high as 80 mol% due to extremely low polar nature of [P6,6,6,14][TFSI].66 In the discussion of polymer solubility shown in Table S1, it was qualitatively suggested that [P8,8,8,8][TFSI] is less polar than [P6,6,6,14][TFSI]. This collaborative evidence implies that [P8,8,8,8][TFSI] is less polar than [P6,6,6,14][TFSI], which can solubilize nonpolar n-hexane. Related to the order of phase transition temperature depending on photoisomerization state, Irie et al. reported polystyrene-base random copolymer possessing azobenzene67,68 or spirobenzopyran69 pendant exhibited more soluble in their trans-azobenzene or lower polar neutral-spirobenzopyran against non-polar cyclohexane. Consequently, the less polar P(trans-mAzoA-r-MMA) is expected to be more soluble (lower Tc) in a relatively low-polarity solvent, [P8,8,8,8][TFSI].
We further illustrated the photoinduced phase transition of P(mAzoA-r-MMA) in [P8,8,8,8][TFSI] by exploiting the difference in Tc values between the trans- and cis-forms of the polymer. P(mAzoA2.4-r-MMA) was selected for the present study because it exhibited both a greater difference in Tc between trans- and cis-types, enabling more pronounced light-induced solubility switching, than P(mAzoA7.3-r-MMA), and a Tc range better aligned with the physiological cell culture temperature. Specifically, P(mAzoA2.4-r-MMA) showed Tc values of 37 °C (trans-type) and 44 °C (cis-type), whereas P(mAzoA4.5-r-MMA) exhibited Tc values of 23 °C (trans-type) and 34 °C (cis-type), which are less suitable for applications around 37 °C (Fig. 2(b)–(e)). Prior to the measurements, the sample was heated to 80 °C and irradiated with blue light (436 nm) for about 1 h and was confirmed to be transparent. At 46 °C, upon exposure to green light at 546 nm, the polymer solution became turbid, indicating the formation of P(cis-mAzoA2.4-r-MMA) aggregates (Fig. 3). Following the green-light-induced phase transition, the transmittance of the turbid P(cis-mAzoA-r-MMA) solution can be recovered by switching to blue-light irradiation, which converts it back to P(trans-mAzoA-r-MMA). This confirms the reversibility of the visible-light-induced phase transition of the polymer in an NCIL. The transmittance reached a plateau within ∼1 h, indicating that a steady photostationary aggregation state was established. The apparent turbidity change at 46 °C (Fig. 3) is consistent with the cloud-point data shown in Fig. 2. In the turbidity measurements (0.1 °C min−1 cooling), the cloud-point temperature Tc was defined as the temperature giving 50% transmittance. For P(mAzoA2.4-r-MMA), Tc(trans) = 37 °C and Tc(cis) = 44 °C (Fig. 2). At 46 °C, the trans-rich solution is completely transparent, whereas the cis-rich one shows about 70% transmittance. Thus, the light-induced change in transmittance (∼70–75%) observed at 46 °C naturally reflects the difference in optical clarity between the trans- and cis-rich states under isothermal conditions.
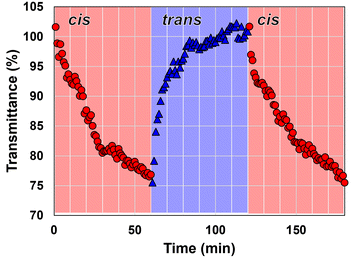 | ||
| Fig. 3 Photoinduced phase transition of 2 w/v% P(mAzoA2.4-r-MMA) in [P8,8,8,8][TFSI] solution at 46 °C under 546 nm light (red circle) or 436 nm light (blue triangle). | ||
Thermal isomerization kinetics of P(cis-mAzoA-r-MMA)
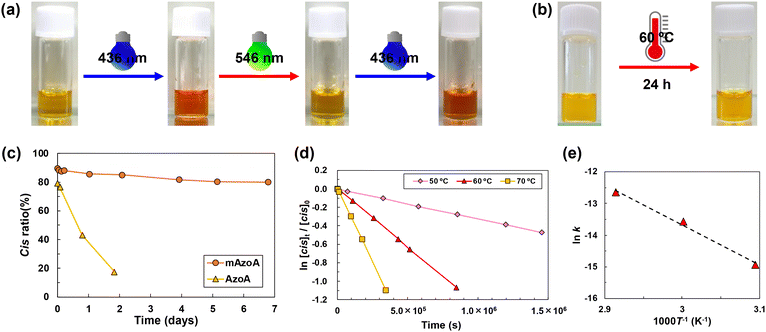
P(mAzoA-r-MMA) in the [P8,8,8,8][TFSI] solution clearly altered its colour depending on the photoisomerization state of mAzoA due to changes in the absorption band corresponding to the n–π* transition (Fig. S4(a) and (b)). P(trans-mAzoA-r-MMA) and P(cis-mAzoA-r-MMA) were orange and yellow, respectively (Fig. 4(a)). For azobenzene compounds, the thermally metastable cis-form gradually returns to the most thermally stable trans-form, without the application of light stimuli. Some previous studies have reported that more than half of the conventional cis-form azobenzene reverted to the trans-form within one day, even under dark conditions.70–75 For all azobenzene-containing cell scaffold materials, this thermal relaxation from cis- to trans-form causes undesired mechanical property degradation over time. However, we found that the P(cis-mAzoA-r-MMA) solution maintained its yellow colour even if it was annealed after 24 h at 60 °C (Fig. 4(b)). Annealing test at 37 °C for cis-mAzoA further confirmed that the ratio of cis-form within the polymer decreased by only 10% after one week, indicating remarkably slow thermal relaxation (Fig. 4(c)). This is clearly different from the result of rapid thermal relaxation from cis- to trans-AzoA (azobenzene acrylate monomer without methoxy groups), which decreased by almost half within 20 h. Fig. 4(d) shows the time course of first-order reaction for thermal cis-to-trans isomerization of mAzoA in [P8,8,8,8][TFSI] at 50, 60, and 70 °C to determine the first-order-rate constant from cis- to trans-mAzoA at each temperature. The slope and intercept of the Arrhenius plot shown in Fig. 4(e) give the activation energy (106 kJ mol−1) and frequency factor (4.0 × 1010 s−1) of the thermal relaxation of mAzoA in [P8,8,8,8][TFSI], respectively. From these values, we could obtain the half-life (τ1/2) of cis-mAzoA in [P8,8,8,8][TFSI] at 37 °C as τ1/2 = 2911 h (121 d), which is over 100 times longer than conventional azobenzene molecules in organic solvents (Table S5).70–75 This means that undesired mechanical property degradation over time can be suppressed compared to conventional azobenzenes, and the controllability of the material can be significantly improved when a combination of P(mAzoA-r-MMA) and [P8,8,8,8][TFSI] is utilised. It has already been reported that the introduction of methoxy groups at the ortho-position of azobenzene not only imparts visible light responsivity but also slows the thermal relaxation rate from the cis- to trans-form.46 We found that the thermal relaxation rate was further reduced in [P8,8,8,8][TFSI], which is low-polarity but can solubilize mAzo.In the earlier report, the thermal relaxation kinetics of 2,2′,6,6′-tetramethoxy-4,4′-diacetamidoazobenzene (mAzoDA) with two acetamide groups at the para-position of mAzo were investigated in detail (Fig. S9 and Table S6).46 The Ea and A for the mAzoDA in dimethyl sulfoxide (DMSO) was calculated from the half-lives of cis-isomer at 4, 25, and 40 °C as 80.2 kJ mol−1 and 6.5 × 107 s−1, respectively. The τ1/2 of mAzoDA is estimated to be 97 h at 37 °C. Although the two τ1/2s are very different, it is unclear whether the difference is due to the functional groups attached to the mAzo moiety or to the solvent environment in which thermal relaxation occurs. Herein, we compared Ea, A, and τ1/2 of mAzoA in [P8,8,8,8][TFSI] with those in DMSO to clarify which factors affect thermal relaxation kinetics, dominantly. Table S6 summarizes the kinetic parameters of mAzoDA in dimethylsulfoxide (DMSO), mAzoA in DMSO (Fig. S10) and mAzoA in [P8,8,8,8][TFSI]. This shows that the effect of the functional groups attached to mAzo is dominant. However, by comparing the results in DMSO and [P8,8,8,8][TFSI], the IL solvent also slowed the thermal relaxation. Specifically, the τ1/2 of mAzoA in [P8,8,8,8][TFSI] was almost one month longer than that in DMSO. The thermal isomerization rate of azobenzene also tends to be slower in ILs than in most organic solvents (Table S5). The Ea and A could not explain the reason for the retardation because of no systematic trend in the Ea and A with τ1/2. It has been reported that a low dielectric constant tends to delay thermal recovery.74,76,77 As mentioned above, [P8,8,8,8][TFSI] is lower polarity than [P6,6,6,14][TFSI] which can solubilize nonpolar n-hexane, so it is obvious that it has a lower dielectric constant than the typical high polar solvent, DMSO. This may explain why the thermal relaxation kinetics are even slower in [P8,8,8,8][TFSI] than in DMSO. However, Asano et al. suggested that the thermal relaxation rate of azobenzene compounds cannot be explained by polarity alone.77 Therefore, a detailed molecular interpretation of the relaxation kinetics of mAzo, particularly in ILs, will be the subject of future studies.
Viscoelasticity switching upon visible-light illumination of P(mAzoA-r-MMA) in [P8,8,8,8][TFSI]
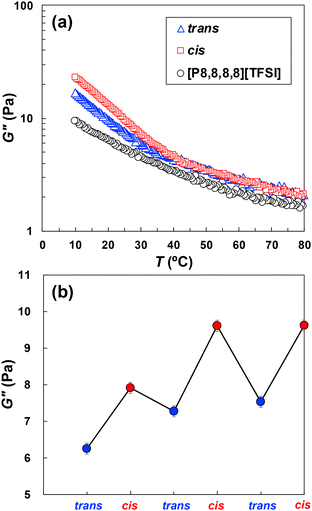
To achieve viscoelasticity switching by alternating visible-light illumination, we first evaluated the temperature dependence of the viscosity of P(mAzoA-r-MMA) in [P8,8,8,8][TFSI] solutions with different photoisomerization states of mAzoA. The loss modulus (G″) of the P(mAzoA-r-MMA) in [P8,8,8,8][TFSI] solution was measured by using rheometer with a parallel plate under 546 nm for cis-type polymer and under 436 nm for trans-type polymer. The measurements were conducted from 80 °C to 10 °C with a cooling rate of 0.1 °C min−1. Fig. 5(a) shows the temperature dependency of the G″ of P(cis-mAzoA-r-MMA), P(trans-mAzoA-r-MMA), and [P8,8,8,8][TFSI] solvent itself. Above Tc of the polymer solution (T > Tc, ∼38 °C), the G″ of the P(mAzoA-r-MMA) in both photoisomerization states are slightly higher than that of [P8,8,8,8][TFSI]. This is understandable because G″ corresponds to the viscosity of the polymer solution which must be higher than that of [P8,8,8,8][TFSI] solvent. Temperature dependency of the G″ against temperature (slope of the G″) is almost comparable among trans-type, cis-type, and [P8,8,8,8][TFSI] solvent. Contrast to this, below Tc of polymer solution (T < Tc), the G″ slope of the more insoluble cis-type polymer becomes significantly upturn compared to more soluble trans-type polymer. This indicates that there is a different G″ dependency on temperature. Therefore, on the condition of T < Tc, straightforward G″ switching upon alternating visible light illumination is possible. Fig. 5(b) shows the G″ switching of the P(mAzoA-r-MMA) at least three times by alternating illumination of 436 nm and 546 nm at 25 °C. The loss modulus changes were highly reproducible, and light-triggered switching was successfully repeated over three cycles with negligible variation (error ∼ 0.15 Pa), indicating sufficient reversibility of the system. Because the polymer concentration, composition, and IL are identical to those in Fig. 3, the timescale for G″ equilibration (∼1 h) reflects the same aggregation-dissolution process; the slightly higher viscosity of [P8,8,8,8][TFSI] at 25 °C may make the equilibration marginally slower. The light-induced change in G″ (∼1–2 Pa) exceeds the experimental repeatability (±0.15 Pa; n = 3) and represents a ∼20–40% relative modulation of the [P8,8,8,8][TFSI] baseline value (G″ ∼ 5.3 Pa at 25 °C, ω = 10 rad s−1). Moreover, temperature-sweep measurements (Fig. S11) show that G″ varies reproducibly within only 0.1–0.2 Pa, confirming that the observed modulation greatly exceeds the experimental scatter. Furthermore, interfacial rheology on the same polymer/IL system (Fig. S13) exhibits a reversible light-dependent change in apparent surface viscosity, supporting that the viscoelastic switching is intrinsic rather than an instrumental artifact.Given the well-known photochemical stability of azobenzene moieties and previous reports of similar IL-based systems maintaining reversible rheological behaviour over multiple cycles,39 the present three-cycle demonstration was adequate. While the photoisomerization of mAzoA occurs within 10 min as shown in Fig. S4, the corresponding change in the G″ under light irradiation proceeds more gradually over approximately 60 min (Fig. S11). This discrepancy suggests that the rate-determining step for the rheological response is not the isomerization itself, but rather the subsequent rearrangement of polymer chain, such as diffusion, aggregation, or changes in entanglement, within the IL environment. Notably, the polymer concentration and molecular structure used in the rheological measurements were identical to those used in the optical transmittance experiments (Fig. 3), where the light-induced phase transition occurred with a comparable timescale. This consistency supports the interpretation that the viscoelastic change observed in rheology reflects the same underlying phase transition dynamics detected optically. The storage modulus (G′) of the polymer solution was also measured under continuous illumination with 436 nm or 546 nm light during temperature sweep, and under alternating light irradiation at constant temperature. The results are shown in Fig. S12(a) and (b), respectively. Although the phase transition process in this study required approximately 1 hour, similar timescales have been reported to influence transcriptional regulators and phenotypic outcomes in stem cells, suggesting relevance to mechanobiological applications. We also confirmed a photoswitchability of the apparent surface viscosity from surface rheological measurement by using a double wall ring geometry. The surface viscosity of P(mAzoA-r-MMA) below Tc could be reversibly switched by alternating visible light illumination (Fig. S13(a) and (b)).
Temperature at which the P(mAzoA-r-MMA) in both photoisomerization states were soluble in the [P8,8,8,8][TFSI] (T > Tc), it is assumed that the microscopic dissolution state of polymer, i.e., conformation, entanglement, aggregation of polymer in the [P8,8,8,8][TFSI] that can affect viscoelasticity of the solution equivalently contribute to G″. Thus G″ values as well as temperature dependency of trans and cis-type polymer were comparable. In contrast, in the temperature range T < Tc, where the aggregation of the polymer occurs, such insoluble state of polymer against G″ does not equally contribute. The G″ in this system is strongly influenced by the IL(solvent)–polymer interactions. When the interaction between the IL and the polymer becomes less favourable, the polymer chains aggregate, leading to increased inter-chain friction and higher G″. In contrast, stronger solvation suppresses aggregation and lowers G″. This trend is consistent with the higher G″ observed in the cis-isomer-rich state, where polymer solubility is reduced. Although FT-IR analysis did not suggest strong specific interactions such as hydrogen bonding, nonspecific van der Waals interactions likely play a dominant role in modulating viscoelastic behaviour. A similar relationship between polymer aggregation and viscosity has been previously reported in a PNIPAm-based thermoresponsive system using azobenzene-functionalized ILs. In that study, photoisomerization of azobenzene units modulated the solubility of PNIPAm, leading to a significant increase in viscosity when the polymer aggregated in the trans-type and a decrease when it was molecularly dissolved in the cis-type.74,75 Although the polymer and IL systems differ, these results support the interpretation that polymer aggregation enhances inter-chain friction and thereby increases the loss modulus in our system as well. Therefore, the less compatible P(cis-mAzoA-r-MMA), at which polymer aggregation proceeds greater, yields a higher viscosity than the more compatible P(trans-mAzoA-r-MMA). Because the inter- and intramolecular frictions causing increased viscosity, such as the friction of the polymer-polymer and/or that of polymer-solvent became more obvious in P(cis-mAzoA-r-MMA). In other words, NCIL species and polymer concentration, which affect the aggregation state of polymers, would modulate G″. Although the absolute change in G″ (1–2 Pa) represents viscous dissipation rather than elasticity (G′), the relative modulation (∼20–40% from baseline G″ ≈ 5.3 Pa at 25 °C, ω = 10 rad s−1) in this study is comparable in magnitude to the relative changes in mechanical modulus that are typical used in hydrogel-based mechanobiology to control cell behaviour.43,78 The monotonic increase of G″ observed in Fig. S11 indicates a slow relaxation process on an hour scale,79 comparable to cellular mechanotransduction dynamics, suggesting that the present light-programmable viscoelastic window is quantitatively relevant to cell-level mechanical environments. This study thus provides a materials platform, and translating the effect to biological responses will be the focus of future work. Electro- or magnetorheological fluids change their shear stress by several orders of magnitude within tens of seconds in response to a voltage of several kV mm−1 or a magnetic field of several hundred mT.1–9 The G″ of the polymer solution in this study changes with visible light irradiation, causing less damage to cells, and the spatial resolution of photostimuli allows the G″ of any arbitrary area to be controlled.
Phototoxicity and cytotoxicity to the NCIL–polymer solution
To explore the biocompatibility of the present system, we investigated the phototoxicity of visible light and the cytotoxicity of P(mAzoA-r-MMA) in a [P8,8,8,8][TFSI] solution. Conventional azobenzene isomerizes from trans- to cis-form via UV light (∼365 nm) irradiation; however, an overdose of such UVA energy poses a serious risk of cytoplasmic organelle damage, cell lysis, cell detachment from other cells and substrates, surface blebbing, apoptosis, and reactive oxygen species (ROS) production.80–83 Phototoxicity tests were performed using blue light at 436 nm and green light at 546 nm, through which photoisomerization of mAzo proceeded. Cell viability was estimated using the WST assay after 2 h of irradiation at 365, 436, and 546 nm. Among epithelial cells, which play key roles in pathological processes such as fibrosis and cancer.84,85 MDCK cells were selected due to their well-defined junctions and rapid proliferation, making them a widely used model for studies on collective cell migration.85–87Fig. 6(a) clearly confirms that visible light at 436 nm and 546 nm showed 100% cell viability, whereas those irradiated with UV light at 365 nm, which is a typical wavelength to induce azobenzene photoisomerization, showed as low as ∼10% viability.We further focused on the non-cytotoxicity of P(mAzoA-r-MMA) in the [P8,8,8,8][TFSI] solution. We recently established a method for the cytotoxicity testing of hydrophobic liquids, including hydrophobic IL and their polymer solutions. The basic idea is inspired from the ISO 10993-5; “Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity”. Briefly, MDCK cells were cultured in a medium saturated with the polymer solution for 24 h and cell viability was determined using the WST-8 assay. Fig. 6(b) shows 100% viability for P(trans-mAzoA2.4-r-MMA) and P(cis-mAzoA2.4-r-MMA) solutions and their solvent [P8,8,8,8][TFSI], indicating non-cytotoxicity. In contrast, [C2mim][TFSI], one of the commonly used ILs, showed serious cytotoxicity (the viability of hMSCs < 10%).28 Furthermore, cell viabilities remained close to 100% even after 48 and 72 h of incubation in medium saturated with the polymer solutions (Fig. S14). We also confirmed that excellent cell adhesion and spreading were observed on the [P8,8,8,8][TFSI] ion gel, suggesting that the present material is non-cytotoxic and does not interfere with cell survival (Fig. 6(c) and (d)). Studies on cell culture at P(mAzoA-r-MMA) in [P8,8,8,8][TFSI] solution and cellular behaviour with respect to G″ switching in response to light stimuli are currently under investigation and are reported elsewhere. According to previous studies,88,89 the phenotype of cell also depends on the interface fluidity. Since the interfacial viscosity at the IL interface not covered by the PNL changes depending on the photoisomerization state (Fig. S13), it could be possible that not only the bulk viscosity but also the interfacial mobility derived from the PNL itself change cell responses.
Conclusions
In this paper, we proposed a visible light-responsive polymer–IL solution that can potentially be used as a liquid cell culture scaffold. Solubility tests of the polymers in four different NCILs revealed that PMMA underwent a UCST-type phase transition covering the cell culture temperature in [P8,8,8,8][TFSI]. Non-harmful visible light responsive azobenzene, mAzoA, possessing four methoxy groups at ortho-position was found to undergo a reversible photochromic reaction in [P8,8,8,8][TFSI]. Furthermore, mAzoA was randomly copolymerized with MMA to form polymer P(mAzoA-r-MMA), which changed its solubility in [P8,8,8,8][TFSI] depending on the temperature. The UCST-type phase-transition temperature varied depending on the photoisomerization state of mAzoA. Because [P8,8,8,8][TFSI] is a relatively low-polar IL, the trans-type polymer exhibits higher solubility (lower UCST-type phase transition temperature). At the appropriate temperature, P(mAzoA-r-MMA) reversibly changed its solubility in [P8,8,8,8][TFSI] in response to visible-light irradiation. By using this feature, it was confirmed that the G″ of the [P8,8,8,8][TFSI] solution can be reversibly changed by illumination with visible-light. The thermal relaxation rate of the thermally metastable cis-type to the equilibrium trans-type polymer in [P8,8,8,8][TFSI] was found to be much slower than that in conventional azobenzene. This kinetically stabilizing effect of the cis-isomer in the IL implies the avoidance of undesired aging degradation of cell scaffold material. The visible light illumination required for the reversible change of G″ was not toxic to MDCK cell. Moreover, the polymer solution and the ion gel made of the [P8,8,8,8][TFSI] polymer solution showed no cytotoxicity, and adhesion and spreading of MDCK cells on the interface of the ion gel was observed. In this study, we realized a new IL scaffold platform with stimuli-responsive properties by exploiting the excellent solubility of polymers in water-immiscible NCILs. Although liquid cell scaffolds such as fluorocarbons and silicones have been explored,12–22 their poor polymer solubility and challenges in incorporating stimuli-responsive functionalities have limited their utility for dynamic mechanical regulation. In contrast, the present system enables external control of scaffold mechanics via light-induced polymer phase transitions. Although the present study concentrates on the material and thermodynamic aspects, the obtained dynamic window (∼25–40% change in G″, relaxation time ∼1 h) is quantitively relevant to cellular mechanotransduction timescales,79 suggesting potential for future cell-level investigations. While the magnitude of G″ changes observed in this system is modest, such variations could still influence cellular responses. For instance, Chaudhuri et al. reported that when the timescale for adhesion and dissociation between substrate and cell is equivalent to the stress relaxation time of the substrate which is associated with G″, a small change in the stress relaxation time results in a large difference in cell spreading area.90–94 Therefore, although further biological validation is required, the spatiotemporal modulation of viscoelasticity achieved in our system may provide a valuable platform for probing mechanobiological phenomena under well-controlled stimuli. In addition, if larger viscoelastic changes are needed, molecular design strategies such as tuning the molecular weight or polymer may further expand the switching range. As demonstrated in our recent work,48 modifying the molecular weight can effectively alter the difference in phase transition temperatures between photoisomerized states, thereby enhancing the magnitude of viscosity modulation. This material could lead to breakthroughs in understanding cellular responses to changing mechanical environments, with potential applications in tissue engineering and stem cell research. Additionally, unlike traditional hydrogel-based scaffolds, our liquid scaffold allows simple cell retrieval process without trypsin treatment due to its fluidity,17 and recyclability after cell culture owing to the thermal stability and negligible volatility of NCILs.28 Cellular mechanobiology by using the present liquid cell scaffold materials inducing the dynamic viscoelasticity changes is currently under investigation.Author contributions
Investigation: A. S.; supervision: T. U.; writing – original draft: A. S. and T. U.; writing – review and editing: Y. K., J. N., R. T. and T. U.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: procedure and results of solubility tests; procedure and results of surface rheological measurement; discussion of the thermal isomerization kinetics of mAzo; 1H-NMR and UV-vis spectra of mAzoA; 1H-NMR and GPC data of P(mAzoA-r-MMA); viscosity of [P8,8,8,8][TFSI]; the results of turbidity test for PMMA solution; comparison of τ1/2 depending on solvent and functional group. A video showing flowability of [P8,8,8,8][TFSI]. See DOI: https://doi.org/10.1039/d5py00876j.Acknowledgements
This work was financially supported by a Grant-in-Aid for JSPS Fellows (22KJ010202 to Aya Saruwatari) and JSPS KAKENHI grants (23H02030 to Takeshi Ueki; and 23K17481 to Jun Nakanishi).References
- Y. Z. Dong, Y. Seo and H. J. Choi, Soft Matter, 2019, 15, 3473–3486 RSC.
- L. Hines, K. Petersen, G. Z. Lum and M. Sitti, Adv. Mater., 2017, 29, 1603483 CrossRef.
- L. Munteanu, A. Munteanu and M. Sedlacik, Prog. Mater. Sci., 2025, 151, 101421 CrossRef CAS.
- Y. Liang, D. Huang, X. Zhou, Z. Wang, Q. Shi, Y. Hong, H. Pu, M. Zhang, J. Wu and W. Wen, Engineering, 2023, 24, 151–171 CrossRef.
- J. de Vicente, D. J. Klingenberg and R. Hidalgo-Alvarez, Soft Matter, 2011, 7, 3701–3710 RSC.
- C. Guerrero-Sanchez, T. Lara-Ceniceros, E. Jimenez-Regalado, M. Raşa and U. S. Schubert, Adv. Mater., 2007, 19, 1740–1747 CrossRef CAS.
- M. Ashtiani, S. H. Hashemabadi and A. Ghaffari, J. Magn. Magn. Mater., 2015, 374, 716–730 CrossRef CAS.
- V. Chauhan, A. Kumar and R. Sham, Manuf. Rev., 2024, 11, 6 Search PubMed.
- H. J. Kim and Y. Seo, Small, 2025, 21, e2410011 CrossRef PubMed.
- A. M. Ketner, R. Kumar, T. S. Davies, P. W. Elder and S. R. Raghavan, J. Am. Chem. Soc., 2007, 129, 1553–1559 CrossRef CAS PubMed.
- H. Y. Lee, K. K. Diehn, K. Sun, T. Chen and S. R. Raghavan, J. Am. Chem. Soc., 2011, 133, 8461–8463 CrossRef PubMed.
- I. Giaever and C. R. Keese, Proc. Natl. Acad. Sci. U. S. A., 1983, 80, 219–222 CrossRef CAS.
- C. R. Keese and I. Giaever, Proc. Natl. Acad. Sci. U. S. A., 1983, 80, 5622–5626 CrossRef CAS.
- C. R. Keese and I. Giaever, Science, 1983, 219, 1448–1449 CrossRef CAS.
- D. Kong, K. D. Q. Nguyen, W. Megone, L. Peng and J. E. Gautrot, Faraday Discuss., 2017, 204, 367–381 RSC.
- D. Kong, W. Megone, K. D. Q. Nguyen, S. Di Cio, M. Ramstedt and J. E. Gautrot, Nano Lett., 2018, 18, 1946–1951 CrossRef CAS PubMed.
- D. Kong, L. Peng, S. Di Cio, P. Novak and J. E. Gautrot, ACS Nano, 2018, 12, 9206–9213 CrossRef CAS PubMed.
- D. Kong, L. Peng, M. Bosch-Fortea, A. Chrysanthou, C. V. J. Alexis, C. Matellan, A. Zarbakhsh, G. Mastroianni, A. Del Rio Hernandez and J. E. Gautrot, Biomaterials, 2022, 284, 121494 CrossRef CAS PubMed.
- X. Jia, K. Minami, K. Uto, A. C. Chang, J. P. Hill, J. Nakanishi and K. Ariga, Adv. Mater., 2020, 32, e1905942 CrossRef CAS.
- X. Jia, J. Song, W. Lv, J. P. Hill, J. Nakanishi and K. Ariga, Nat. Commun., 2022, 13, 3110 CrossRef CAS PubMed.
- W. Lyu, W. Hu, J. Shi, J. Chen, J. Song, Q. Zhang, X. Yuan, D. Li, J. Nakanishi and X. Jia, Adv. Healthcare Mater., 2023, 12, e2300666 CrossRef.
- Z. Lu, M. Tenjimbayashi, J. Zhou and J. Nakanishi, Adv. Mater., 2024, 36, 2403396 CrossRef CAS PubMed.
- E. Mojares, C. Nadal, D. Hayler, H. Kanso, A. Chrysanthou, C. E. Neri Cruz and J. E. Gautrot, Adv. Mater., 2024, 36, e2406333 CrossRef.
- X. Jia, K. Minami, K. Uto, A. C. Chang, J. P. Hill, T. Ueki, J. Nakanishi and K. Ariga, Small, 2019, 15, e1804640 CrossRef.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- J. P. Hallett and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef CAS PubMed.
- J. Nakanishi, T. Ueki, S. Dieb, H. Noguchi, S. Yamamoto and K. Sodeyama, Sci. Technol. Adv. Mater., 2024, 25, 2418287 CrossRef.
- T. Ueki, K. Uto, S. Yamamoto, R. Tamate, Y. Kamiyama, X. Jia, H. Noguchi, K. Minami, K. Ariga, H. Wang and J. Nakanishi, Adv. Mater., 2024, 36, e2310105 CrossRef.
- T. P. Lodge and P. C. Hiemenz, Polymer Chemistry, CRC Press, 2020 Search PubMed.
- R. Tamate and T. Ueki, Chem. Rec., 2023, 23, e202300043 CrossRef CAS.
- X. Q. Yuan, Y. Q. Zhang, Z. Y. Li, F. Huo, Y. H. Dong and H. Y. He, Chin. J. Chem., 2021, 39, 729–744 CrossRef CAS.
- X. Yuan, J. Liu, J. Qin, W. Ma, G. Liu, Y. Wang and H. He, Nano Res., 2023, 16, 4152–4159 CrossRef CAS.
- T. Ueki and M. Watanabe, Chem. Lett., 2006, 35, 964–965 CrossRef CAS.
- H. Asai, K. Fujii, T. Ueki, S. Sawamura, Y. Nakamura, Y. Kitazawa, M. Watanabe, Y.-S. Han, T.-H. Kim and M. Shibayama, Macromolecules, 2013, 46, 1101–1106 CrossRef CAS.
- T. Ueki and M. Watanabe, Bull. Chem. Soc. Jpn., 2012, 85, 33–50 CrossRef CAS.
- T. Ueki, Polym. J., 2014, 46, 646–655 CrossRef CAS.
- T. Ueki, Y. Nakamura, A. Yamaguchi, K. Niitsuma, T. P. Lodge and M. Watanabe, Macromolecules, 2011, 44, 6908–6914 CrossRef CAS.
- T. Ueki, Y. Nakamura, R. Usui, Y. Kitazawa, S. So, T. P. Lodge and M. Watanabe, Angew. Chem., Int. Ed., 2015, 54, 3018–3022 CrossRef CAS PubMed.
- T. Ueki, R. Usui, Y. Kitazawa, T. P. Lodge and M. Watanabe, Macromolecules, 2015, 48, 5928–5933 CrossRef CAS.
- A. M. Kloxin, A. M. Kasko, C. N. Salinas and K. S. Anseth, Science, 2009, 324, 59–63 CrossRef CAS.
- M. Guvendiren and J. A. Burdick, Nat. Commun., 2012, 3, 792 CrossRef.
- C. Yang, M. W. Tibbitt, L. Basta and K. S. Anseth, Nat. Mater., 2014, 13, 645–652 CrossRef CAS PubMed.
- V. Frank, S. Kaufmann, R. Wright, P. Horn, H. Y. Yoshikawa, P. Wuchter, J. Madsen, A. L. Lewis, S. P. Armes, A. D. Ho and M. Tanaka, Sci. Rep., 2016, 6, 24264 CrossRef CAS PubMed.
- K. Hayashi, M. Matsuda, N. Mitake, M. Nakahata, N. Munding, A. Harada, S. Kaufmann, Y. Takashima and M. Tanaka, ACS Appl. Polym. Mater., 2022, 4, 2595–2603 CrossRef CAS.
- A. A. Beharry, O. Sadovski and G. A. Woolley, J. Am. Chem. Soc., 2011, 133, 19684–19687 CrossRef CAS PubMed.
- M. Dong, A. Babalhavaeji, S. Samanta, A. A. Beharry and G. A. Woolley, Acc. Chem. Res., 2015, 48, 2662–2670 CrossRef CAS.
- T. Ueki, Y. Osaka, K. Homma, S. Yamamoto, A. Saruwatari, H. Wang, M. Kamimura and J. Nakanishi, Macromol. Rapid Commun., 2024, 45, e2400419 CrossRef PubMed.
- H. Tokuda, K. Hayamizu, K. Ishii, M. A. B. H. Susan and M. Watanabe, J. Phys. Chem. B, 2004, 108, 16593–16600 CrossRef CAS.
- C. R. Gaush, W. L. Hard and T. F. Smith, Proc. Soc. Exp. Biol. Med., 1966, 122, 931–935 CrossRef CAS PubMed.
- T. Ueki, A. Yamaguchi, N. Ito, K. Kodama, J. Sakamoto, K. Ueno, H. Kokubo and M. Watanabe, Langmuir, 2009, 25, 8845–8848 CrossRef CAS PubMed.
- T. Ueki, Y. Nakamura, T. P. Lodge and M. Watanabe, Macromolecules, 2012, 45, 7566–7573 CrossRef CAS.
- J. Niskanen and H. Tenhu, Polym. Chem., 2017, 8, 220–232 RSC.
- Z. Wang and P. Wu, J. Phys. Chem. B, 2011, 115, 10604–10614 CrossRef CAS.
- R. Kröger, H. Menzel and M. L. Hallensleben, Macromol. Chem. Phys., 1994, 195, 2291–2298 CrossRef.
- A. Altomare, F. Ciardelli, R. Solaro and N. Tirelli, Macromol. Chem. Phys., 1995, 196, 3229–3242 CrossRef CAS.
- D. Kungwatchakun and M. Irie, Makromol. Chem., Rapid Commun., 1988, 9, 243–246 CrossRef CAS.
- M. Irie and D. Kungwatchakun, Proc. Jpn. Acad., Ser. B, 1992, 68, 127–132 CrossRef CAS.
- F. D. Jochum and P. Theato, Polymer, 2009, 50, 3079–3085 CrossRef CAS.
- X. Tao, Z. Gao, T. Satoh, Y. Cui, T. Kakuchi and Q. Duan, Polym. Chem., 2011, 2, 2068–2073 RSC.
- F. D. Jochum and P. Theato, Macromolecules, 2009, 42, 5941–5945 CrossRef CAS.
- C. Barrett, A. Natansohn and P. Rochon, Macromolecules, 1994, 27, 4781–4786 CrossRef CAS.
- M. Concilio, V. P. Beyer and C. R. Becer, Polym. Chem., 2022, 13, 6423–6474 RSC.
- G. S. Kumar and D. C. Neckers, Chem. Rev., 1989, 89, 1915–1925 CrossRef CAS.
- H. Tokuda, S. Tsuzuki, M. A. Susan, K. Hayamizu and M. Watanabe, J. Phys. Chem. B, 2006, 110, 19593–19600 CrossRef CAS PubMed.
- M. Liang, S. Khatun and E. W. Castner Jr., J. Chem. Phys., 2015, 142, 121101 CrossRef PubMed.
- M. Irie and H. Tanaka, Macromolecules, 1983, 16, 210–214 CrossRef CAS.
- M. Irie and W. Schnabel, Macromolecules, 1985, 18, 394–398 CrossRef CAS.
- M. Irie, T. Iwayanagi and Y. Taniguchi, Macromolecules, 1985, 18, 2418–2422 CrossRef CAS.
- J. M. Nerbonne and R. G. Weiss, J. Am. Chem. Soc., 2002, 100, 5953–5954 CrossRef.
- T. Asano, T. Okada, S. Shinkai, K. Shigematsu, Y. Kusano and O. Manabe, J. Am. Chem. Soc., 1981, 103, 5161–5165 CrossRef CAS.
- M. A. Strauss and H. A. Wegner, Angew. Chem., Int. Ed., 2021, 60, 779–786 CrossRef CAS.
- R. J. W. Le Fèvre and J. Northcott, J. Chem. Soc., 1953, 867–870 RSC.
- N. Nishimura, T. Sueyoshi, H. Yamanaka, E. Imai, S. Yamamoto and S. Hasegawa, Bull. Chem. Soc. Jpn., 1976, 49, 1381–1387 CrossRef CAS.
- K. Matczyszyn, W. Bartkowiak and J. Leszczynski, J. Mol. Struct., 2001, 565–566, 53–57 CrossRef CAS.
- J. Dokic, M. Gothe, J. Wirth, M. V. Peters, J. Schwarz, S. Hecht and P. Saalfrank, J. Phys. Chem. A, 2009, 113, 6763–6773 CrossRef CAS PubMed.
- K. Baba, H. Ono, E. Itoh, S. Itoh, K. Noda, T. Usui, K. Ishihara, M. Inamo, H. D. Takagi and T. Asano, Chem. – Eur. J., 2006, 12, 5328–5333 CrossRef CAS PubMed.
- Material-based Mechanobiology, ed. J. Nakanishi and K. Uto, The Royal Society of Chemistry, Cambridge, UK, 2022 Search PubMed.
- Z. Lu, M. Tenjimbayashi, J. Zhou and J. Nakanishi, Adv. Mater., 2024, 36, 2403396 CrossRef CAS PubMed.
- J. Z. Beer, K. M. Olvey, S. A. Miller, D. P. Thomas and D. E. Godar, Photochem. Photobiol., 1993, 58, 676–681 CrossRef CAS.
- E. Straface, P. U. Giacomoni and W. Malorni, J. Photochem. Photobiol., B, 2001, 63, 52–60 CrossRef CAS PubMed.
- W. Malorni, G. Donelli, E. Straface, M. T. Santini, S. Paradisi and P. U. Giacomoni, J. Photochem. Photobiol., B, 1994, 26, 265–270 CrossRef CAS PubMed.
- S. Yamamoto, H. Ikegami, K. Yamaguchi and J. Nakanishi, ChemPhotoChem, 2018, 2, 786–790 CrossRef CAS.
- E. P. Mihalko and A. C. Brown, ACS Biomater. Sci. Eng., 2018, 4, 1149–1161 CrossRef CAS.
- J. Nakanishi, Chem. Rec., 2017, 17, 611–621 CrossRef CAS PubMed.
- J. D. Dukes, P. Whitley and A. D. Chalmers, BMC Cell Biol., 2011, 12, 43 CrossRef CAS PubMed.
- M. Poujade, E. Grasland-Mongrain, A. Hertzog, J. Jouanneau, P. Chavrier, B. Ladoux, A. Buguin and P. Silberzan, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 15988–15993 CrossRef CAS PubMed.
- J. H. Seo and N. Yui, Biomaterials, 2013, 34, 55–63 CrossRef CAS PubMed.
- J. H. Seo, S. Kakinoki, Y. Inoue, T. Yamaoka, K. Ishihara and N. Yui, J. Am. Chem. Soc., 2013, 135, 5513–5516 CrossRef CAS PubMed.
- O. Chaudhuri, L. Gu, D. Klumpers, M. Darnell, S. A. Bencherif, J. C. Weaver, N. Huebsch, H. P. Lee, E. Lippens, G. N. Duda and D. J. Mooney, Nat. Mater., 2016, 15, 326–334 CrossRef CAS.
- H. P. Lee, L. Gu, D. J. Mooney, M. E. Levenston and O. Chaudhuri, Nat. Mater., 2017, 16, 1243–1251 CrossRef CAS.
- Z. Gong, S. E. Szczesny, S. R. Caliari, E. E. Charrier, O. Chaudhuri, X. Cao, Y. Lin, R. L. Mauck, P. A. Janmey, J. A. Burdick and V. B. Shenoy, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, E2686–E2695 CAS.
- S. Nam, R. Stowers, J. Lou, Y. Xia and O. Chaudhuri, Biomaterials, 2019, 200, 15–24 CrossRef CAS PubMed.
- O. Chaudhuri, J. Cooper-White, P. A. Janmey, D. J. Mooney and V. B. Shenoy, Nature, 2020, 584, 535–546 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2026 |