Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
14C-labelled nanoplastics reveal size-dependent bioaccumulation in juvenile rainbow trout (Oncorhynchus mykiss)
Maya Al-Sid-Cheikh†
 *a,
Joyce W. L. Ang
*a,
Joyce W. L. Ang b,
Gareth T. W. Law
b,
Gareth T. W. Law b,
Ana I. Catarino‡
b,
Ana I. Catarino‡
 c,
Theodore B. Henry
c,
Theodore B. Henry c,
Steve J. Rowland
c,
Steve J. Rowland d,
Marc-Andre Cormier§
d,
Marc-Andre Cormier§
 e and
Richard C. Thompson
e and
Richard C. Thompson a
a
aSchool of Biological and Marine Sciences, University of Plymouth, Drake Circus, Plymouth, PL4 8AA, UK. E-mail: malsid@ed.ac.uk
bRadiochemistry Unit, Department of Chemistry, Faculty of Science, University of Helsinki, A.I. Virtasen Aukio 1, P.O. Box 55, Helsinki, FI-00014, Finland
cSchool of Geography, Earth and Environmental Sciences, University of Plymouth, Drake Circus, Plymouth, PL4 8AA, UK
dInstitute of Life and Earth Sciences Heriot-Watt University, John Muir Building, Edinburgh, EH14 4AS, UK
eDepartment of Earth Sciences, University of Oxford, South Parks Road, Oxford, OX1 3AN, UK
First published on 11th February 2026
Abstract
Concerns have been raised about the occurrence of nanoplastics (NP) in the environment. Herein we investigated size-dependent uptake, biodistribution, and egestion of 20 and 250 nm [14C]nanopolystyrene in juvenile rainbow trout (Oncorhynchus mykiss) at environmentally relevant concentrations: a single-day “high-dose” (250 ng gw.w.−1 (∼ppb) fed once) and a 5 day “low-dose” (8 ng gw.w.−1). Following single-day ‘high’ dose exposure, 20 nm particles – but not 250 nm – were detected in internal tissues, indicating translocation across gut endothelia. Rapid depuration returned NP concentrations to control levels within 10 days, with no evidence of accumulation in organs. The presence of 20 nm NPs in circulation suggests temporary vascular transport without parenchymal retention. Translocation of 20 nm NPs across the gut endothelia and association with blood cells should be investigated to determine the cell types involved and the outcome of these associations. These results indicate that uptake-depuration kinetics for [14C]nanopolystyrene in rainbow trout differ from those of other chemical substances (e.g., metals, persistent organic pollutants) and likely have different implications on organism health. The results of this study and the methods developed provide a means of studying and comparing the fate of NPs in organisms such as bivalves and fish.
Environmental significanceDetection of nanoplastics at environmentally realistic concentrations remains analytically challenging. By combining intrinsic 14C-labelling with quantitative whole-body autoradiography (QWBA), we resolve size-dependent uptake, transient systemic distribution, and rapid depuration of 20 nm and 250 nm polystyrene nanoplastics in juvenile trout under environmentally relevant low-dose dietary exposure. These data clarify how particle size and exposure design govern biodistribution at relevant concentrations and provide benchmarks to inform ecological risk assessment. |
Introduction
Nanoplastics (NPs; defined as plastic particles smaller than 1 μm (ref. 1–4) are a growing environmental concern due to their ability to cross biological barriers,5 raising concerns about potential bioaccumulation and transfer through food webs, which could make them one of the most hazardous forms of plastic litter.6 Global plastic production continues to rise, with 9–14 million tonnes of plastic litter entering the environment annually, potentially increasing to 23–37 million tonnes by 2040.7–10 Despite international efforts, including the ongoing negotiations of the United Nations Plastic Pollution Treaty (INC-4, 2024), understanding of NP behaviour in organisms and ecosystems remains limited, largely due to analytical challenges associated with detecting small particles at environmentally relevant concentrations. Predicted environmental concentrations of NPs—for example, 1 pg L−1 to 15 μg L−1 for ∼50 nm particles and below 0.5 μg L−1 for 5 μm particles—are orders of magnitude lower than those often used in laboratory studies.11 Despite these low concentrations, studies have shown that NPs can translocate within organisms.5Laboratory-based experiments complement environmental monitoring by allowing precise control over NP parameters such as size, morphology, concentration, water chemistry, and exposure regimes. Although these experiments may not fully replicate ecological conditions, they allow investigation of NP behaviour and mechanisms of action, particularly under chronic or complex exposure scenarios. However, experimental studies have been criticized for using particle concentrations in single-day high-dose exposures that exceed environmentally realistic levels.11–13 For example, studies using extrinsic labelling of metal-doped nanopolystyrene (nPS) required excessively high feed concentrations (10 mg Pd as PS–Pd NPs per kg−1, ∼2 g NPs per kg) to detect particles in tissues,14,15 emphasizing the difficulty of measuring NPs at environmentally realistic concentrations. While such high doses provide mechanistic insight, they risk misinterpreting the potential impact of NPs on organisms and ecosystems,16 underscoring the need for studies at environmentally relevant concentrations. Moreover, differences in polymer type and particle morphology may also influence NP behaviour, as polyethylene and polypropylene, or fibres versus spheres, display distinct aggregation and uptake dynamics that remain underexplored in toxicokinetic studies.
Intrinsic radiolabelling of nPS with 14C enables sensitive quantification of NPs at predicted concentrations (<10 μg L−1) and small particle sizes (down to 20 nm,5,17), addressing previous analytical limitations. By incorporating 14C into the polymer backbone, quantitative whole-body autoradiography (QWBA) enables spatially resolved tracking of NP accumulation in tissues, eliminating cross-contamination risks inherent to dissection-based methods.18–20 This method significantly enhances sensitivity and spatial resolution, enabling detection and quantification of NPs in organ substructures and biological fluids, providing a detailed, contamination-free profile of NP distribution.19,20 QWBA has been validated over 20 years as the ‘gold standard21 for producing reliable and precise tissue concentration data, offering accuracy and reproducibility.20 While liquid scintillation counting (LSC) remains a complementary and widely used method for quantifying radiotracers, its reliance on bulk dissection makes it more prone to contamination artefacts, which QWBA can overcome. Together, these approaches allow comparison of NP behaviour based on size: smaller particles (<100 nm) may penetrate biological barriers more readily, while larger particles (>100 nm) may accumulate differently or be cleared via distinct pathways.5,22–24 Such spatially resolved toxicokinetic data are essential to understand NP behaviour, uptake, depuration, and potential toxicity in vertebrates.
We selected nPS as a model system due to its widespread production, accounting for approximately 7% of global polymer output (US Department of Energy, 2024), and its prevalence in environmental samples.25 Its well-characterized chemical structure and predictable behaviour make it an ideal standard material. Incorporation of 14C enables QWBA of [14C]nPS in biological specimens. We investigate the toxicokinetics (i.e. uptake and depuration) of small (20 nm, nPS20) and large (250 nm, nPS250) nPS in rainbow trout (Oncorhynchus mykiss), under two exposure scenarios: a single-day high-dose exposure (250 ng gw.w.−1) and a short-term, environmentally relevant exposure (8 ng gw.w.−1 for 5 days). The smaller size (20 nm) was selected to represent particles capable of crossing biological barriers and entering systemic circulation, as reported for nanoparticles below 100 nm.26–30 The inclusion of 250 nm particles reflects the upper nanoscale range, relevant to environmental NPs and regulatory definitions, and enables assessment of size thresholds for biological barrier penetration. These sizes were chosen not only for comparison but also to evaluate potential limitations in crossing biological barriers such as gut epithelia and lymphoid tissues, which are known to restrict particles above ∼200 nm.23,24,31 This approach enables assessment of size-dependent uptake mechanisms, clearance dynamics, and tissue distribution under controlled conditions in the vertebrate model—critical for accurate environmental risk assessment. By integrating intrinsic radiolabelling with high-resolution autoradiography, this study provides the first spatially resolved quantification of NP biodistribution and clearance in fish exposed to environmentally relevant concentrations.
Materials and methods
Radiosynthesis of [14C]-polystyrene nanoparticles
Radiolabelled polystyrene nanoparticles ([14C]nPS) originated from the same batch previously described and characterized in Al-Sid-Cheikh et al.17 Full synthesis details and physicochemical characterization, including particle size distribution, morphology, and radiolabel incorporation, are provided in that publication and summarised in Text S1. A [14C]-styrene tracer (specific activity 2.22 GBq mmol−1, ARC Inc.) was incorporated into the polymerisation mixture, and the unreacted monomer was removed by repeated ultrafiltration (30 kDa cut-off). Two size fractions were produced—nominal diameters of 20 nm ([14C]nPS20) and 250 nm ([14C]nPS250)—representing the nanoscale and submicrometre size ranges relevant to environmental exposure.Hydrodynamic size and surface charge were determined by dynamic light scattering (DLS) and surface charge (zeta potential) was measured in ultrapure water (18.2 MΩ cm), pH 6 in a sodium chloride solution (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mM), 25 °C. Polydispersity index (PDI) values were 0.07 for [14C]nPS20 and 0.08 for [14C]nPS250, consistent with narrow size distributions. Particle size and morphology were confirmed by transmission electron microscopy (TEM). Representative mean diameters were 25 ± 13 nm and 248 ± 21 nm with zeta potentials of −129 ± 10 mV (20 nm) and −84 ± 12 mV (250 nm), respectively. FTIR and Raman spectra were consistent with the polystyrene backbone (Fig. S1). The detailed synthesis conditions, purification steps, and full physicochemical characterisation are provided in Text S1 and Al-Sid-Cheikh et al. (2020).
mM), 25 °C. Polydispersity index (PDI) values were 0.07 for [14C]nPS20 and 0.08 for [14C]nPS250, consistent with narrow size distributions. Particle size and morphology were confirmed by transmission electron microscopy (TEM). Representative mean diameters were 25 ± 13 nm and 248 ± 21 nm with zeta potentials of −129 ± 10 mV (20 nm) and −84 ± 12 mV (250 nm), respectively. FTIR and Raman spectra were consistent with the polystyrene backbone (Fig. S1). The detailed synthesis conditions, purification steps, and full physicochemical characterisation are provided in Text S1 and Al-Sid-Cheikh et al. (2020).
Ethical approval and animal husbandry
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light
12 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dark cycle. Fish were fed commercial pellets (Nutrafin Basix A-7176) at 2% body weight per day. Exposure tanks were stocked at ∼7–11 g L−1 (28–32 fish of ∼17 ± 2 gw.w. per 60 L tank), a loading that, under continuous flow and oxygenation, maintains stable dissolved oxygen and nitrogenous wastes while supporting normal feeding behaviour. The exposure design followed UK Home Office husbandry standards.
dark cycle. Fish were fed commercial pellets (Nutrafin Basix A-7176) at 2% body weight per day. Exposure tanks were stocked at ∼7–11 g L−1 (28–32 fish of ∼17 ± 2 gw.w. per 60 L tank), a loading that, under continuous flow and oxygenation, maintains stable dissolved oxygen and nitrogenous wastes while supporting normal feeding behaviour. The exposure design followed UK Home Office husbandry standards.[14C]-labelled feed preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) food ratio ≈ 0.5
food ratio ≈ 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (50 g water per 100 g dry feed) to form a homogeneous paste. The paste was spread on a coated, non-stick bench to a thickness of a few millimetres and passed through a metallic mesh to produce uniform pellets. Pellets were dried at 50 °C overnight and stored at room temperature in the dark until use.
1 (50 g water per 100 g dry feed) to form a homogeneous paste. The paste was spread on a coated, non-stick bench to a thickness of a few millimetres and passed through a metallic mesh to produce uniform pellets. Pellets were dried at 50 °C overnight and stored at room temperature in the dark until use.– Single-day high-dose: 250 ± 21.2 ng 14C per gw.w. (14.8 kBq gw.w.−1)
– Low-dose: 8 ± 0.8 ng 14C per gw.w. (4.1 kBq gw.w.−1)
For a batch of 100 g dry feed + 50 g water (wet weight 150 g), the total [14C]nPS required was 37.5 μg (high-dose) and 1.2 μg (low-dose). Batch homogeneity was assessed by LSC (see Solid-sample method) on subsamples taken across the batches.
Exposure design
– Single-day high-dose exposure: one meal formulated to deliver 250 ng gw.w.−1.
– Five day low-dose exposure: one meal per day for 5 consecutive days at 8 ng gw.w.−1 d−1.
After the exposure period, fish were returned to the standard commercial diet (unspiked Nutrafin Basix A-7176) at the acclimation ration (∼2% body mass per d) for the duration of depuration.
– Five day low-dose exposure followed by 10 days of depuration with sampling at 1, 3, 6, 7, 9, 12, and 15 days (where day 1 corresponds to the first exposure day).
T0 corresponds to the start of the exposure. Control fish (particle-free feed and unlabelled nPS) were sampled at the same timepoints.
Total radiocarbon (C) quantification
Liquid scintillation counting (LSC, Hidex 300 SL)
Quantitative whole-body autoradiography (QWBA)
Whole-body autoradiography was conducted on fish sampled from both single-day high-dose and five-day low-dose exposure experiments to visualise tissue distribution of [14C]nPS. Fish were embedded in 2.5% carboxymethyl cellulose, flash-frozen in a liquid N2 bath, and cryosectioned (20 μm) every 0.75 mm. Sections were mounted on tape and exposed for 7 days to phosphor imaging plates, then scanned at 25 μm resolution using a Typhoon FLA 9000. Quantification was performed using AIDA v5.0 SP3 software with calibration against 14C polymer standards.Autoradiograms were validated using internal (serum-embedded or PMMA-embedded) standards and cross-checked on an alternative digital autoradiography platform (BeaQuant). Comparison of the two systems confirmed linearity of response (R2 > 0.99) and no detectable bias (Full details in Text S2; Fig. S2 and S3). The limit of detection (LOD) and limit of quantification (LOQ) for both analytical techniques are reported in Table S2 of the SI.
Toxicokinetic modelling
Time-resolved tissue concentrations were fitted to first-order one-compartment models using the MOSAICbioacc web platform.32–34 Uptake (kuf) and elimination (kee) rate constants, biological half-lives (t1/2), and biomagnification factors (BMFk, BMFss) were derived by Bayesian inference. Model performance was evaluated through posterior predictive checks and 95% credibility intervals. Full model specifications and parameter tables are provided in Text S3 and Fig. S5 and S6.Statistical analyses
All data were processed in R (v4.3.1). Two-way ANOVA followed by Tukey's post hoc tests was used to test the effects of particle size and dose. Results are presented as mean ± SD, and significance was accepted at p < 0.05.Results
Toxicokinetics (TK): uptake and depuration
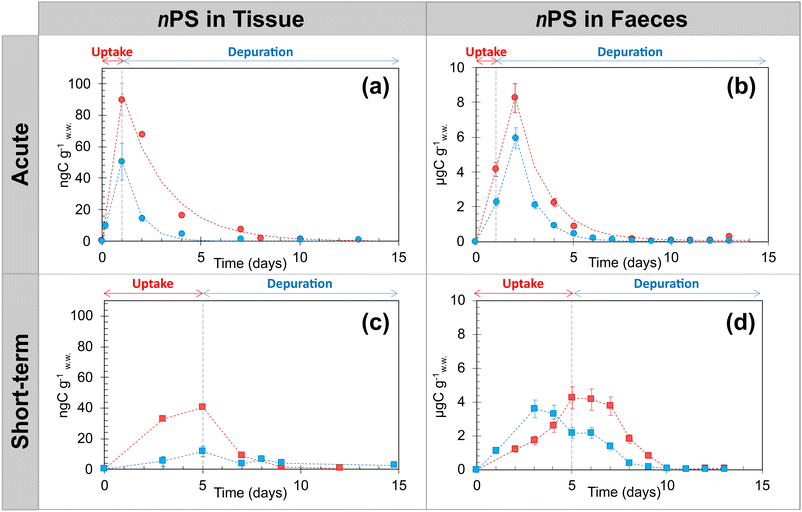
Daily 14C measurements were conducted on tissues (Fig. 1a and c) and faeces (Fig. 1b and d) of O. mykiss exposed to [14C]nPS20 (pink circles) and [14C]nPS250 (blue circles) under two exposure scenarios: a single-day high-dose (single feed at 250 ng gw.w.−1) and short-term low-dose (5 day feed at 8 ng gw.w.−1, Fig. 1). Although all feed was consumed within minutes, uptake is shown over the first day to reflect the time required for gastrointestinal transit and systemic absorption of NPs. The limits of detection (LOD) and quantification (LOQ) for 14C using liquid scintillation counting were 3.14 × 10−3 ng gw.w.−1 and 8.62 × 10−3 ng gw.w.−1, respectively (Table S2).The single-day high-dose (250 ng gw.w.−1) exposure resulted in the highest tissue concentrations, reaching 90.0 ± 6.8 ngC gw.w.−1 for [14C]nPS20 and 51.0 ± 11.9 ngC gw.w.−1. for [14C]nPS250 after a single day (Fig. 1a). Tissue concentration of [14C]nPS250 was consistently lower than that of [14C]nPS20 (∼0.3 and 9.1 times lower, p-value < 0.001, t test, n = 4). In faeces, peak concentrations occurred after 2 days (8.3 ± 0.9 μgC gw.w.−1 for [14C]nPS20; 6.0 ± 0.6 μgC gw.w.−1 for [14C]nPS250; Fig. 1b). Both tissues and faeces exhibited exponential depuration, with near-complete elimination within six days.
During the 5 day low-dose exposure, tissue accumulation reached 40.1 ± 3.1 ngC gw.w.−1 ([14C]nPS20) and 11.8 ± 1.0 ngC gw.w.−1 ([14C]nPS250) after three days (Fig. 1c). The concentration of [14C]nPS20 was consistently higher than that of [14C]nPS250, by approximatively 0.2- to 6.3-fold (Fig. 1c). The concentrations of [14C]nPS in the tissues decreased rapidly between 3 and 6 days. A notable difference was also observed between [14C]nPS20 and [14C]nPS250 concentrations in faeces (Fig. 1d). The peak faecal concentration of [14C]nPS250 (3.6 ± 0.4 μgC gw.w.−1) occurred after 3 days of exposure, whereas the peak for [14C]nPS20 (4.2 ± 0.5 μgC gw.w.−1) was observed after 5 days, coinciding with the end of the exposure period (Fig. 1d). The concentration patterns in faeces during the depuration phase (from day 6 onwards) were similar for both particle sizes, although the larger particles exhibited a faster depuration rate than the smaller ones (Fig. 1d). These differences suggest size-dependent elimination rates or clearance mechanism between the two particle sizes. By day 10, depuration from the tissues was complete for both NP sizes, as confirmed by the low [14C] levels measured in both tissues and faeces.
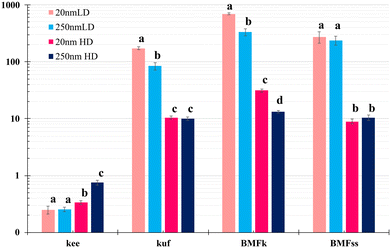
MOSAIC modelling
MOSAICbioacc provided posterior distribution for the uptake rate via food (kuf, d−1), elimination rate via excretion (kee, d−1), and BMF, reported as kinetic BMF (BMFk) and the steady-state BMF (BMFss, Fig. 2). In toxicokinetics, BMF involves at least two trophic levels: the prey (lower trophic level) and the predator (higher trophic level). BMF is defined as the ratio of contaminant concentration in the predator to that in the prey. In this TK model, the term ‘bioaccumulation metrics’ is used generically to denote BMF when exposure occurs via the food,33 and does not necessarily refer to a classical prey–predator relationship. In our study, fish were fed homemade pellets distributed uniformly throughout the tanks, which they consumed as their primary food source.Spatially-resolved analysis: organ-specific patterns
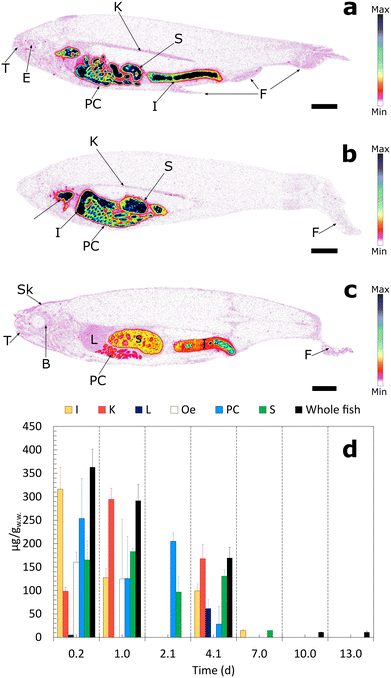
By 1 day (Fig. 3b), activity was concentrated in the pyloric caeca, intestine and kidney, demonstrating the progressive passage of particles through the digestive system. Quantitative analysis (Fig. 3d) showed the highest [14C]nPS20 concentration in the kidney (294.6 μgC gw.w.−1), suggesting early renal accumulation or filtration. [14C]nPS20 was also detected in the liver, with concentrations of 5.4 μgC gw.w.−1 at 1 day and 61.7 μgC gw.w.−1 at 4 days, and in the pyloric caeca, where concentrations remained relatively high (125.5–253.5 μgC gw.w.−1) before decreasing to 28.2 μgC gw.w.−1 by day 4.
At 4 days post-exposure (Fig. 3c), autoradiograms revealed faint but distinct activity near the hepatic and renal regions, as well as in other organs including the eyes, bones, fins, and skin, suggesting particle redistribution from the gastrointestinal tract over time. The measured activities were above the LOD but close to the LOQ (2.89 × 10−3 μg gw.w.−1), indicating that a limited degree of systemic translocation occurred, although the signal intensity prevents precise quantification. The delayed activity observed in the kidney and liver supports the hypothesis that small [14C]nPS20 particles can transiently cross biological barriers.
Discussion
Integrated summary of size-dependent uptake and clearance
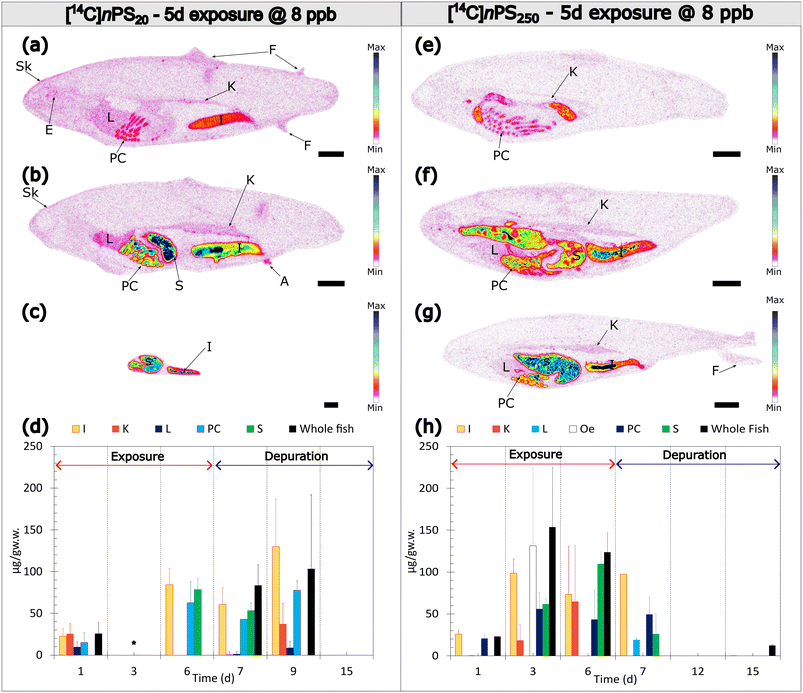
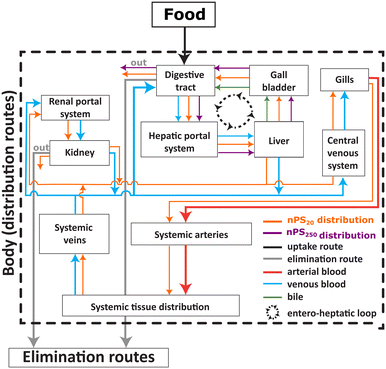
Our combined TK and QWBA data demonstrate a clear size dependence in the dietary uptake and fate of intrinsically 14C-labelled nPS in juvenile rainbow trout. Following ingestion, both sizes (20 and 250 nm) exhibit early localisation within the gastrointestinal (GI) tract, but only the 20 nm fraction shows short-lived, low-level signals beyond the GI tract—most consistently in hepatic and renal regions—before elimination. In contrast, 250 nm particles remain GI-confined across the time course. TK time series in whole-body tissues and faeces (Fig. 1a–d) reveal rapid, first-order depuration for both sizes once feeding ceases, with tissue burdens returning close to background within ∼10 days under the regimes tested. Together, these findings support transient systemic access for 20 nm particles at environmentally relevant doses and minimal parenchymal retention for either size. Fig. 5 provides a conceptual overview of routes and compartments pertinent to dietary uptake and potential enterohepatic recirculation.In this conceptual view, dietary ingestion would first load the stomach and pyloric caeca; sub-100–200 nm particles then cross local epithelia (gut-associated lymphoid tissue: GALT) and enter either the portal venous circulation to the liver or the lymphatic route to systemic blood.23,24,31,35 In the liver, opsonisation and Kupffer cell uptake drive rapid handling, with a proportion returning to the intestinal lumen via bile (enterohepatic recirculation), consistent with the faecal peaks observed in Fig. 1b and d. Larger particles (≈250 nm) are predominantly retained luminally and cleared as faeces because their size exceeds efficient endocytosis thresholds.23,31 Once in blood, particles may associate with circulating cells, facilitating short-range transport; systemically, splenic filtration (∼150 nm) and renal size selectivity (<10 nm filtration; mesangial/peritubular interactions for larger) constrain residence and promote clearance.36–41 Transient ocular/skeletal signals should be interpreted cautiously in light of the skeleton detector-efficiency caveat (Table S5). The arrow weights in Fig. 5 reflect the size-dependence seen in QWBA: robust GI → liver → lumen loops for 20 nm, weak/absent systemic arrows for 250 nm (Fig. 3 and 4).
Why tissue concentrations can exceed faecal concentrations during exposure
Although spiked feed was consumed within minutes, apparent tissue concentrations can temporarily exceed faecal loads during the exposure phase. This arises from two concurrent processes: (i) epithelial absorption of a fraction of ingested particles across the gut wall, followed by a brief presence in vascularised tissues (e.g. liver, kidney) until clearance; and (ii) luminal egestion of the non-absorbed fraction over hours to days. Once particles enter circulation—even briefly—they contribute to measurable tissue burdens, whereas faecal concentrations primarily reflect the non-absorbed fraction. This mechanistic dichotomy—absorption with transient retention vs. luminal egestion—explains the early maxima and the 1–2 day lag to faecal peaks (Fig. 1b and d). Fig. 5 highlights the gut as the primary absorption site and the liver as a secondary processing compartment.35Mechanistic interpretation of epithelial transport and transient systemic access
The spatial patterns resolved by QWBA (Fig. 3 and 4) are consistent with known size constraints on epithelial transport. Nanoparticles in the sub-100–200 nm range can traverse mucosal barriers through endocytic pathways (e.g., clathrin/caveolae-mediated endocytosis, macropinocytosis) and thereby reach portal venous or lymphatic routes. Particles of ∼250 nm are more likely retained luminally and eliminated in faeces.In fish, gut-associated lymphoid tissue (GALT)—a network of immune structures embedded within the intestinal mucosa—plays a central role in antigen sampling and immune surveillance and offers plausible sampling of particulate matter directly from the lumen. GALT contains lymphoid aggregates and specialized epithelial cells capable of internalizing particulate matter from the gut lumen. Sub-100–200 nm particles can cross intestinal epithelia via transcellular endocytic pathways (e.g., clathrin-mediated endocytosis, macropinocytosis), enabling access to GALT and, transiently, systemic circulation.23,24,31,35
Once internalised, particle surfaces will acquire a protein corona that promotes opsonisation and recognition by the mononuclear phagocyte system (MPS)—notably Kupffer cells in the liver and macrophages in the spleen—facilitating fast handling and redistribution.42 This fast handling is well documented for uncoated engineered gold nanoparticles (ENP-Au).36,43,44 We note that the reported size thresholds for splenic slits (around 100 nm)37 and renal filtration (effective mainly for particles <10 nm)38 come from mammalian studies. Since fish differ anatomically and physiologically, these values cannot be directly transferred, and the thresholds for O. mykiss should be viewed as approximate. Within this framework, the renal signal observed for 20 nm particles is most reasonably interpreted as non-glomerular handling (e.g., mesangial/peritubular associations, vascular trapping, immune sequestration) rather than efficient filtration.
The 20 nm kidney signal most plausibly reflects non-glomerular handling (e.g., peritubular uptake, vascular trapping, immune sequestration) rather than filtration. For context, mammalian nanoparticle literature typically places efficient renal filtration in the very small domain (≤∼10 nm). Accordingly, our data do not allow estimation of a fish glomerular size cut-off.
Influence of the dosing regime on apparent accumulation
The two dietary regimes—single-day high-dose and five-day low-dose—map complementary regions of the exposure–response space. The single-day high-dose design, while mechanistically useful to accentuate size differences, can over-represent short-lived systemic signals relative to chronic environmental exposure. The five-day low-dose design better mimics chronic dietary intake: under sustained feeding, apparent accumulation metrics (e.g., BMF) rise during the uptake phase even as elimination proceeds; once exposure stops, rapid depuration collapses these metrics. The qualitative differences evident in Fig. 1 (tissues and faeces) and Fig. 4 (organ traces) highlight that regime choice (dose, duration, feeding schedule) strongly shapes apparent bioaccumulation without implying long retention.Interpreting particulate bioaccumulation metrics (kuf, kee; BMFk, BMFss)
TK fitting (MOSAIC) provides exposure-regime-dependent uptake via food (kuf) and elimination via excretion (kee), along with bioaccumulation metrics (BMFk and BMFss). Two considerations are central for particulates:1) BMF > 1 during multi-day exposure reflects sustained dietary input and short-lived internalisation, not the long biological half-lives characteristic of hydrophobic organic chemicals. Under the five-day low-dose regime, BMF values are higher than under single-day dosing because prolonged feeding raises the numerator (predator burden during uptake), while elimination continues; after exposure ceases, first-order depuration reduces BMF rapidly.
2) Size and regime dependence in kuf and kee track the threshold-like nature of epithelial transport and immune/biliary handling. In the low-dose context, 20 nm particles typically exhibit a higher kuf than 250 nm, consistent with more effective barrier crossing, whereas elimination constants may be similar or slightly slower for 20 nm due to brief residence in hepatic or reticulo-endothelial compartments prior to clearance. In single-day dosing, the 250 nm fraction can show a faster apparent kee consistent with dominant luminal egestion and minimal systemic access.
Practically, BMFk/BMFss should not be read as evidence for trophic magnification in the classical sense for NPs; rather, they quantify exposure-phase internalisation under the tested diet regimes.
QWBA robustness
We established QWBA's robustness through cross-platform comparison (phosphor screens vs. real-time autoradiography), internal embedded standards, and Monte Carlo simulations for detector efficiency across tissues (see SI: Text S2, Fig. S2 and S3, Table S5). Background 14C contributions were minimal, and limits of detection/quantification were consistent between platforms. One caveat is a modest efficiency bias in skeletal regions; because dense mineralised matrices can modestly alter β detection efficiency, skeletal signals were interpreted conservatively. Bone detection were reproducible across sections/animals, anatomically plausible, and—where available—confirmed across platforms. Skeletal signals are reported qualitatively. These checks support confidence in spatial trends while acknowledging known analytical constraints.Post-epithelial handling: MPS, biliary clearance, immune-mediated redistribution
Once across the epithelium, protein-corona formation and opsonisation favour Kupffer cell uptake in the liver and sequestration by splenic macrophages; together these processes constitute rapid MPS handling. Biliary excretion provides a route back to the intestinal lumen (enterohepatic cycling), consistent with faecal peaks following or overlapping tissue maxima (Fig. 1b and d). The immune system (macrophages, neutrophils) can drive redistribution and local inflammatory signalling, potentially contributing to organ-to-organ movement at low levels.45–47 This is captured by QWBA as faint extra-GI signals for 20 nm early in depuration. The absence of persistent muscle burdens and the short whole-body half-lives argue against meaningful long-term storage under the conditions studied.Organ-specific kinetics and spatial–temporal patterns
QWBA images and quantification (Fig. 3 and 4; SI Fig. S6–S9) allow time-resolved insight into organ kinetics. For 20 nm particles under high-dose single-feeding, signals localise rapidly to the stomach, pyloric caeca, intestine, with transient presence in kidney and hepatic regions at early time points (Fig. 3a–d). Under five-day low-dose exposure, GI compartments (stomach, pyloric caeca, intestine) dominate, with lower-intensity but detectable signals in liver, kidney, skin, fins, and eyes (Fig. 4a–d). For 250 nm, signals remain GI-confined and below LOQ in systemic organs at matched time points (Fig. 4e–h; Fig. S6). These patterns align with the size-limited transport and rapid clearance mechanisms described above.Dissolved-chemical paradigm mispredicts NP behaviour
The uptake and depuration kinetics of nPS observed in this study differ fundamentally from those of metals and persistent organic pollutants (POPs). Metals such as methylmercury and cadmium exhibit biological half-lives of weeks to months due to strong binding to proteins and slow elimination,48 whereas POPs (e.g., PCBs, DDT) persist for months to years in fish tissues, driven by high lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow > 6) and limited biotransformation;49,50 these properties result in high biomagnification potential (BMF commonly 5–20, occasionally > 100 in top predators).49–51 In contrast, nPS particles behave as discrete particulates: translocation and clearance are size-dependent, with near-complete depuration under the exposure regimes tested (see Fig. 1; Table S4). NP behaviour therefore does not follow classical bioaccumulation paradigms based on solubility and lipophilicity but is governed by particle size, morphology, and surface properties.5 Comparable size-dependent biodistribution has been reported for ENP-Au and ENP-Ag, including preferential accumulation in liver and spleen.39,52–54 Consequently, risk assessment frameworks designed for dissolved chemicals may not adequately capture the behaviour of NPs characterised by short-lived internalisation and limited potential for long-term retention under environmentally relevant conditions.
Kow > 6) and limited biotransformation;49,50 these properties result in high biomagnification potential (BMF commonly 5–20, occasionally > 100 in top predators).49–51 In contrast, nPS particles behave as discrete particulates: translocation and clearance are size-dependent, with near-complete depuration under the exposure regimes tested (see Fig. 1; Table S4). NP behaviour therefore does not follow classical bioaccumulation paradigms based on solubility and lipophilicity but is governed by particle size, morphology, and surface properties.5 Comparable size-dependent biodistribution has been reported for ENP-Au and ENP-Ag, including preferential accumulation in liver and spleen.39,52–54 Consequently, risk assessment frameworks designed for dissolved chemicals may not adequately capture the behaviour of NPs characterised by short-lived internalisation and limited potential for long-term retention under environmentally relevant conditions.
Environmental realism, exposure design, and limitations
We examined sub-100 nm particles (nanomaterial-like behaviour) alongside 250 nm particles (upper nanoscale), to bracket thresholds for uptake and clearance in relation to definitions of NPs (<1000 nm).6 The single-day high-dose scenario is informative mechanistically (clear size contrast, convenient kinetics) but can over-represent transient systemic signals relative to chronic environmental exposure; conversely, the five-day low-dose regime better mimics chronic dietary intake and yielded distinct dynamics (higher BMF during exposure, similar rapid clearance post-exposure). Together, these regimes map the exposure–response space and emphasise that regime choice (dose and duration) strongly shapes apparent accumulation metrics—even when long-term retention is limited. Several caveats warrant emphasis:• Particle identity: we used pristine, spherical polystyrene as a benchmark to isolate particle size effects at controlled dose, supported by intrinsic 14C labelling and QWBA for sensitive, spatially resolved quantification.5,17,20 Environmental particles, however, are typically aged/heterogeneous: oxidation introduces oxygen-containing groups (e.g., hydroxyl, carbonyl, carboxyl),55–57 roughness increases, and surfaces host biofilms;58 these changes alter hydrophilicity, charge and aggregation, and can enhance contaminant sorption,58–60 thereby modulating membrane interactions, kinetics and toxicity.61 Future work should therefore incorporate aged/weathered polymers and chronic low-level dietary regimes to resolve how surface chemistry, roughness, sorbed contaminants and biofilms impact translocation, tissue distribution and trophic transfer.
• Species physiology: Species-specific physiology (e.g., spleen architecture, renal thresholds) may shift size gates relative to mammalian benchmarks; we therefore avoid hard cut-offs for fish and treat extra-mammalian inferences cautiously.
Despite these limitations, the integrated TK–QWBA approach quantitatively resolves size-dependent biodistribution and clearance at realistic concentrations, providing a contamination-resistant alternative to dissection-based quantification and a strong basis for mechanistic inference.
Ecological and trophic transfer implications
From a consumer perspective, rapid depuration and negligible muscle retention argue for limited long-term body burdens, reducing direct human exposure via fillets under conditions comparable to this study. Ecologically, however, transient internalisation during the diet phase implies the possibility of trophic transfer, especially under chronic, low-level exposures or in species with slower gut transit and different immune handling. Beyond mass balance, functional endpoints (digestion efficiency, mucus production, immune tone, epithelial integrity) deserve attention because the short-lived presence can still modulate physiology in ways not captured by bulk concentration metrics alone.Policy and assessment implications
The data advocate a shift from dissolved-chemical paradigms toward particle-kinetic modelling for NPs. Practically, this means:(i) prioritising chronic, low-dose dietary studies to quantify exposure-phase internalisation without conflating it with long retention;
(ii) incorporating aged/weathered particles (oxidised, roughened, biofilm-bearing; with sorbed co-contaminants) to capture environmental realism; and
(iii) embedding fish-specific physiology (e.g., spleen/renal size selectivity) in bioaccumulation models to avoid mammal-centric biases. These steps will improve ecological realism and regulatory relevance.
Conclusions
Intrinsic 14C labelling combined with QWBA provides a sensitive, contamination-resistant route to quantify and image NP biodistribution at environmentally relevant concentrations. By embedding 14C in the polymer backbone, we achieve accurate spatial quantification without dissection.Our data reveal size-dependent toxicokinetics: 20 nm nPS transiently appears in hepatic/renal regions while 250 nm remains GI-confined; both sizes depurate rapidly (t1/2 ≈ 0.9–2.7 d; near-complete clearance ≤ ∼10 d). Exposure regime matters: multi-day low-dose feeding elevates apparent accumulation during uptake relative to single-day dosing, yet post-exposure depuration is rapid. MOSAIC modelling confirms this with kuf/kee estimates and BMF metrics, where higher BMFk/BMFss under low-dose reflects sustained dietary input, not long retention.
Spatially resolved QWBA shows organ-specific patterns (pyloric caeca, intestine, liver) for 20 nm consistent with GALT-mediated transport and endocytic pathways. A modest detector-efficiency bias in skeletal regions was noted; accordingly, bone signals were interpreted conservatively.
Collectively, NPs in fish behave as particulates, not as dissolved lipophilic chemicals: size, morphology and surface chemistry govern brief internalisation and rapid elimination. These insights argue for particle-kinetic models and chronic, realistic dietary studies—including aged/biofilm-bearing particles—to refine ecological risk, trophic-transfer assessments and policy guidance.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. Conceptualization, MASC, JWLA, GTL, AIC, TBH, SJR, MAC, RCT; data curation MASC, JWLA, GTL; formal analysis, MASC, JWLA; funding acquisition TBH, SJR, RCT; investigation, MASC, JWLA, GTL, AIC, TBH, SJR, MAC, RCT; methodology, MASC, JWLA, GTL; project administration, TBH, SJR, RCT; resources, TBH, SJR, RCT; software, MASC, JWLA; supervision TBH, SJR, RCT; validation, MASC, JWLA, GTL; visualization, MASC, JWLA; roles/writing – original draft, MASC; and writing – review & editing, MASC, JWLA, GTWL, AIC, TBH, SJR, MAC, RCT.Conflicts of interest
There are no conflicts to declare.Abbreviations
| [14C]nPS | 14C-labelled nanopolystyrene particles |
| ATR | Attenuated total reflection |
| BMF | Biomagnification factors |
| BMFk | Biomagnification factors given as probability distributions: kinetic |
| BMFss | BMF at steady-state |
| CMC | Carboxymethylcellulose |
| DLU | Digital light units |
| Dpm | Disintegration per minutes |
| ENPs | Engineered nanoparticles |
| FE | Fraction of electrons emitted |
| FLA | Fluorescent image analyzer |
| FTIR | Fourier-transform infrared spectroscopy |
| kee | Elimination rates of excretion |
| KPS | Potassium persulfate |
| kuf | Uptake rate of food |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MDA | Minimum detectable activity |
| MOSAICbioacc | MOdeling and StAtistical tools for ecotoxicology |
| NaOH | Sodium hydroxide |
| NPs | Nanoplastics |
| nPS | nanopolystyrene |
| pH | potential hydrogen |
| PMMA | Poly(methyl methacrylate) |
| PSL | Photostimulated luminescence |
| Toxicokinetics | TK |
| QWBA | Quantitative-whole body autoradiography |
| S.D. | Standard deviation |
| SDS | Sodium dodecyl sulfate |
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Supplementary information (SI): includes details on the radiosynthesis and characterisation of [14C]nPS (Text S1), calibration and validation of the quantitative whole-body autoradiography method (Text S2), and toxicokinetic modelling (Text S3). Additional data are provided in the form of FTIR and Raman spectroscopy analyses of particles (Fig. S1), calibration curves for QWBA (Fig. S2), comparison of autoradiographs from BeaQuant and phosphor screen autoradiography (Fig. S3), and MOSAIC Bioacc modelling for tissue and faeces analyses (Fig. S4 and S5). Autoradiography images and corresponding physical tissue section photographs from rainbow trout exposed to [14C]nPS under single-day high-dose and chronic conditions are presented in Fig. S6–S9. Experimental design and sampling timelines, limits of detection and quantification for the analytical techniques used, elemental composition of Monte Carlo simulated organs, MOSAIC Bioacc parameter estimates, and emission fractions of 14C from different organs are provided in Tables S1–S5. See DOI: https://doi.org/10.1039/d5en00985e
Acknowledgements
We thank the Heriot Watt University technical staff and Radioprotection supervisor A. Bolton for support throughout the exposure experiment. We thank the University of Plymouth Consolidated Radio-isotope Facility (W. Blake, A. Taylor, G. Millward and N. Crocker) for support for the radioactivity work. This research was part of the RealRiskNano project funded by the Natural Environment Research Council, UK (grant number: NE/N006526/1) to whom we are grateful. All analysis and data were only possible with the Applied RadioIsotope laboratory (grant number: NE/V017616/1), the mobility fellowship from the Royal society (grant number: RM1802-0440), and The CAMS funding (grant number: 600310/10) to whom we are grateful. This research was supported by the scholarship funding from the National Research Foundation, Singapore, and a grant from the Jenny and Antti Wihuri Foundation (grant number: 00220019). We would also like to thank Prof Émilien Pelletier (emeritus) and Ariane Plourde (Director, ISMER-UQAR) for providing access to the CM3600 Leica. This work has received approval for research ethics from Home Office and a proof of approval is available upon request.Notes and references
- J. Gigault, A. Halle, M. Baudrimont, P.-Y. Pascal, F. Gauffre, T.-L. Phi, H. El Hadri, B. Grassl and S. Reynaud, Current Opinion: What Is a Nanoplastic?, Environ. Pollut., 2018, 235, 1030–1034, DOI:10.1016/j.envpol.2018.01.024.
- J. Alexander, L. Barregård, M. Bignami, S. Ceccatelli, B. Cottrill, M. Dinovi, L. Edler, B. Grasl-Kraupp, C. Hogstrand, L. (Ron) Hoogenboom, H. K. Knutsen, C. S. Nebbia, I. Oswald, A. Petersen, V. M. Rogiers, M. Rose, A.-C. Roudot, T. Schwerdtle, C. Vleminckx, G. Vollmer and H. Wallace, EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of microplastics and nanoplastics in food, with particular focus on seafood, EFSA J., 2016, 14(6) DOI:10.2903/j.efsa.2016.4501.
- N. B. Hartmann, T. Hüffer, R. C. Thompson, M. Hassellöv, A. Verschoor, A. E. Daugaard, S. Rist, T. Karlsson, N. Brennholt, M. Cole, M. P. Herrling, M. C. Hess, N. P. Ivleva, A. L. Lusher and M. Wagner, Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris, Environ. Sci. Technol., 2019, 53(3), 1039–1047, DOI:10.1021/acs.est.8b05297.
- J. P. G. L. Frias and R. Nash, Microplastics: Finding a Consensus on the Definition, Mar. Pollut. Bull., 2019, 138, 145–147, DOI:10.1016/j.marpolbul.2018.11.022.
- M. Al-Sid-Cheikh, S. J. Rowland, K. Stevenson, C. Rouleau, T. B. Henry and R. C. Thompson, Uptake, Whole-Body Distribution, and Depuration of Nanoplastics by the Scallop Pecten Maximus at Environmentally Realistic Concentrations, Environ. Sci. Technol., 2018, 52(24), 14480–14486, DOI:10.1021/acs.est.8b05266.
- SAPEA, Scientific Perspective on Microplastics in Nature and Society | SAPEA, Evidence Review Report, 2019, DOI:10.26356/microplastics.
- R. Geyer, J. R. Jambeck and K. L. Law, Production, Use, and Fate of All Plastics Ever Made, Sci. Adv., 2017, 3(7), e1700782, DOI:10.1126/sciadv.1700782.
- L. Lebreton and A. Andrady, Future Scenarios of Global Plastic Waste Generation and Disposal, Palgrave Commun., 2019, 5(1), 6, DOI:10.1057/s41599-018-0212-7.
- I. Tiseo, Plastic Waste Worldwide - Statistics & Facts, 2023, https://www.statista.com/topics/5401/global-plastic-waste/#topicOverview (accessed 2023-07-17) Search PubMed.
- UNEP, C. Llorenç Milà i, C. Alison, L. Peggy, M. Allan, R. Andrew David, S. Aphrodite, S. Steven, T. Elisa, S. Yoni, F. de la José, K. Julia, T. B. Anne, B. Eline, F. Steve, M. Antaya, R. Keiron and B. Andrea, Turning off the Tap: How the World Can End Plastic Pollution and Create a Circular Economy, Geneva, 2023, https://wedocs.unep.org/bitstream/handle/20.500.11822/42277/Plastic_pollution.pdf?sequence=1&isAllowed=y (accessed 2023-07-17) Search PubMed.
- R. Lenz, K. Enders and T. G. Nielsen, Microplastic Exposure Studies Should Be Environmentally Realistic, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(29), E4121–E4122, DOI:10.1073/pnas.1606615113.
- E. M. Cunningham and J. D. Sigwart, Environmentally Accurate Microplastic Levels and Their Absence from Exposure Studies, Integr. Comp. Biol., 2019, 59(6), 1485–1496, DOI:10.1093/icb/icz068.
- D. Materić, H. A. Kjær, P. Vallelonga, J.-L. Tison, T. Röckmann and R. Holzinger, Nanoplastics Measurements in Northern and Southern Polar Ice, Environ. Res., 2022, 208, 112741, DOI:10.1016/j.envres.2022.112741.
- N. J. Clark, F. R. Khan, C. Crowther, D. M. Mitrano and R. C. Thompson, Uptake, Distribution and Elimination of Palladium-Doped Polystyrene Nanoplastics in Rainbow Trout (Oncorhynchus Mykiss) Following Dietary Exposure, Sci. Total Environ., 2023, 854, 158765, DOI:10.1016/j.scitotenv.2022.158765.
- D. M. Mitrano, A. Beltzung, S. Frehland, M. Schmiedgruber, A. Cingolani and F. Schmidt, Synthesis of Metal-Doped Nanoplastics and Their Utility to Investigate Fate and Behaviour in Complex Environmental Systems, Nat. Nanotechnol., 2019, 14(4), 362–368, DOI:10.1038/s41565-018-0360-3.
- M. B. Paul, V. Stock, J. Cara-Carmona, E. Lisicki, S. Shopova, V. Fessard, A. Braeuning, H. Sieg and L. Böhmert, Micro- And Nanoplastics-Current State of Knowledge with the Focus on Oral Uptake and Toxicity, Nanoscale Adv., 2020, 4350–4367, 10.1039/d0na00539h.
- M. Al-Sid-Cheikh, S. J. Rowland, R. Kaegi, T. B. Henry, M.-A. Cormier and R. C. C. Thompson, Synthesis of 14C-Labelled Polystyrene Nanoplastics for Environmental Studies, Commun. Mater., 2020, 1(1), 97, DOI:10.1038/s43246-020-00097-9.
- E. G. Solon, S. K. Balani and F. W. Lee, Whole-Body Autoradiography In Drug Discovery, Curr. Drug Metab., 2002, 3(5), 451–462, DOI:10.2174/1389200023337207.
- E. G. Solon, Use of Radioactive Compounds and Autoradiography to Determine Drug Tissue Distribution, Chem. Res. Toxicol., 2012, 25(3), 543–555, DOI:10.1021/tx200509f.
- E. G. Solon, Autoradiography Techniques and Quantification of Drug Distribution, Cell Tissue Res., 2015, 360(1), 87–107, DOI:10.1007/s00441-014-2093-4.
- E. G. Solon, A. Schweitzer, M. Stoeckli and B. Prideaux, Autoradiography, MALDI-MS, and SIMS-MS Imaging in Pharmaceutical Discovery and Development, AAPS J., 2010, 12(1), 11–26, DOI:10.1208/s12248-009-9158-4.
- M. Al-Sid-Cheikh, C. Rouleau and E. Pelletier, Tissue Distribution and Kinetics of Dissolved and Nanoparticulate Silver in Iceland Scallop (Chlamys Islandica), Mar. Environ. Res., 2013, 86, 21–28, DOI:10.1016/j.marenvres.2013.02.003.
- S. Barua and S. Mitragotri, Challenges Associated with Penetration of Nanoparticles across Cell and Tissue Barriers: A Review of Current Status and Future Prospects, Nano Today, 2014, 9(2), 223–243, DOI:10.1016/j.nantod.2014.04.008.
- A. P. Walczak, E. Kramer, P. J. M. Hendriksen, P. Tromp, J. P. F. G. Helsper, M. van der Zande, I. M. C. M. Rietjens and H. Bouwmeester, Translocation of Differently Sized and Charged Polystyrene Nanoparticles in in Vitro Intestinal Cell Models of Increasing Complexity, Nanotoxicology, 2015, 9(4), 453–461, DOI:10.3109/17435390.2014.944599.
- K. Ziani, C.-B. Ioniţă-Mîndrican, M. Mititelu, S. M. Neacşu, C. Negrei, E. Moroşan, D. Drăgănescu and O.-T. Preda, Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review, Nutrients, 2023, 15(3), 617, DOI:10.3390/nu15030617.
- G. Oberdörster, E. Oberdörster and J. Oberdörster, Nanotoxicology: An Emerging Discipline Evolving from Studies of Ultrafine Particles, Environ. Health Perspect., 2005, 113(7), 823–839, DOI:10.1289/ehp.7339.
- W. G. Kreyling, M. Semmler-Behnke and W. Möller, Ultrafine Particle–Lung Interactions: Does Size Matter?, J. Aerosol Med., 2006, 19(1), 74–83, DOI:10.1089/jam.2006.19.74.
- W. G. Kreyling, M. Semmler, F. Erbe, P. Mayer, S. Takenaka, H. Schulz, G. Oberdörster and A. Ziesenis, Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low, J. Toxicol. Environ. Health, Part A, 2002, 65(20), 1513–1530, DOI:10.1080/00984100290071649.
- M. Semmler-Behnke, J. Lipka, A. Wenk, S. Hirn, M. Schäffler, F. Tian, G. Schmid, G. Oberdörster and W. G. Kreyling, Size Dependent Translocation and Fetal Accumulation of Gold Nanoparticles from Maternal Blood in the Rat, Part. Fibre Toxicol., 2014, 11(1), 33, DOI:10.1186/s12989-014-0033-9.
- W. H. De Jong, W. I. Hagens, P. Krystek, M. C. Burger, A. J. A. M. Sips and R. E. Geertsma, Particle Size-Dependent Organ Distribution of Gold Nanoparticles after Intravenous Administration, Biomaterials, 2008, 29(12), 1912–1919, DOI:10.1016/j.biomaterials.2007.12.037.
- H. Sinnecker, T. Krause, S. Koelling, I. Lautenschläger and A. Frey, The Gut Wall Provides an Effective Barrier against Nanoparticle Uptake, Beilstein J. Nanotechnol., 2014, 5, 2092–2101, DOI:10.3762/bjnano.5.218.
- S. Charles, P. Veber and M. L. Delignette-Muller, MOSAIC: A Web-Interface for Statistical Analyses in Ecotoxicology, Environ. Sci. Pollut. Res., 2018, 25(12), 11295–11302, DOI:10.1007/s11356-017-9809-4.
- A. Ratier and S. Charles, Accumulation-Depuration Data Collection in Support of Toxicokinetic Modelling, Sci. Data, 2022, 9(1), 130, DOI:10.1038/s41597-022-01248-y.
- A. Ratier, C. Lopes, G. Multari, V. Mazerolles, P. Carpentier and S. Charles, New Perspectives on the Calculation of Bioaccumulation Metrics for Active Substances in Living Organisms, Integr. Environ. Assess. Manage., 2022, 18(1), 10–18, DOI:10.1002/ieam.4439.
- X. Qiao, L. Bao, G. Liu and X. Cui, Nanomaterial Journey in the Gut: From Intestinal Mucosal Interaction to Systemic Transport, Nanoscale, 2024, 16(41), 19207–19220, 10.1039/D4NR02480J.
- C. H. J. Choi, J. E. Zuckerman, P. Webster and M. E. Davis, Targeting Kidney Mesangium by Nanoparticles of Defined Size, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(16), 6656–6661, DOI:10.1073/pnas.1103573108.
- E. C. Dreaden, L. A. Austin, M. A. Mackey and M. A. El-Sayed, Size Matters: Gold Nanoparticles in Targeted Cancer Drug Delivery, Ther. Delivery, 2012, 3(4), 457–478, DOI:10.4155/tde.12.21.
- J. E. Zuckerman, C. H. J. Choi, H. Han and M. E. Davis, Polycation-SiRNA Nanoparticles Can Disassemble at the Kidney Glomerular Basement Membrane, Proc. Natl. Acad. Sci. U. S. A., 2012, 109(8), 3137–3142, DOI:10.1073/pnas.1200718109.
- H. Soo Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Renal Clearance of Quantum Dots, Nat. Biotechnol., 2007, 25(10), 1165–1170, DOI:10.1038/nbt1340.
- D. W. Bartlett and M. E. Davis, Physicochemical and Biological Characterization of Targeted, Nucleic Acid-Containing Nanoparticles, Bioconjugate Chem., 2007, 18(2), 456–468, DOI:10.1021/bc0603539.
- A. de Barros, A. Tsourkas, B. Saboury, V. Cardoso and A. Alavi, Emerging Role of Radiolabeled Nanoparticles as an Effective Diagnostic Technique, EJNMMI Res., 2012, 2(1), 39, DOI:10.1186/2191-219X-2-39.
- D. OwensIII and N. Peppas, Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles, Int. J. Pharm., 2006, 307(1), 93–102, DOI:10.1016/j.ijpharm.2005.10.010.
- S. A. Kulkarni and S.-S. Feng, Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery, Pharm. Res., 2013, 30(10), 2512–2522, DOI:10.1007/s11095-012-0958-3.
- X. Liu, N. Huang, H. Li, Q. Jin and J. Ji, Surface and Size Effects on Cell Interaction of Gold Nanoparticles with Both Phagocytic and Nonphagocytic Cells, Langmuir, 2013, 29(29), 9138–9148, DOI:10.1021/la401556k.
- C. Petrarca, E. Clemente, V. Amato, P. Pedata, E. Sabbioni, G. Bernardini, I. Iavicoli, S. Cortese, Q. Niu, T. Otsuki, R. Paganelli and M. Di Gioacchino, Engineered Metal Based Nanoparticles and Innate Immunity, Clin. Mol. Allergy, 2015, 13(1), 13, DOI:10.1186/s12948-015-0020-1.
- E. Vivier and B. Malissen, Innate and Adaptive Immunity: Specificities and Signaling Hierarchies Revisited, Nat. Immunol., 2005, 6(1), 17–21, DOI:10.1038/ni1153.
- A.-C. Greven, T. Merk, F. Karagöz, K. Mohr, M. Klapper, B. Jovanović and D. Palić, Polycarbonate and Polystyrene Nanoplastic Particles Act as Stressors to the Innate Immune System of Fathead Minnow ( Pimephales Promelas ), Environ. Toxicol. Chem., 2016, 35(12), 3093–3100, DOI:10.1002/etc.3501.
- T. W. Clarkson and L. Magos, The Toxicology of Mercury and Its Chemical Compounds, Crit. Rev. Toxicol., 2006, 36(8), 609–662, DOI:10.1080/10408440600845619.
- F. A. P. C. Gobas, A Model for Predicting the Bioaccumulation of Hydrophobic Organic Chemicals in Aquatic Food-Webs: Application to Lake Ontario, Ecol. Modell., 1993, 69(1–2), 1–17, DOI:10.1016/0304-3800(93)90045-T.
- D. Mackay and A. Fraser, Bioaccumulation of Persistent Organic Chemicals: Mechanisms and Models, Environ. Pollut., 2000, 110(3), 375–391, DOI:10.1016/S0269-7491(00)00162-7.
- B. C. Kelly, M. G. Ikonomou, J. D. Blair, A. E. Morin and F. A. P. C. Gobas, Food Web–Specific Biomagnification of Persistent Organic Pollutants, Science, 2007, 317(5835), 236–239, DOI:10.1126/science.1138275.
- M. Al-Sid-Cheikh, C. Rouleau, D. Bussolaro, C. A. Oliveira Ribeiro and E. Pelletier, Tissue Distribution of Radiolabeled 110m Ag Nanoparticles in Fish: Arctic Charr ( Salvelinus Alpinus ), Environ. Sci. Technol., 2019, 53(20), 12043–12053, DOI:10.1021/acs.est.9b04010.
- S. Hirn, M. Semmler-Behnke, C. Schleh, A. Wenk, J. Lipka, M. Schäffler, S. Takenaka, W. Möller, G. Schmid, U. Simon and W. G. Kreyling, Particle Size-Dependent and Surface Charge-Dependent Biodistribution of Gold Nanoparticles after Intravenous Administration, Eur. J. Pharm. Biopharm., 2011, 77(3), 407–416, DOI:10.1016/j.ejpb.2010.12.029.
- G. Sonavane, K. Tomoda and K. Makino, Biodistribution of Colloidal Gold Nanoparticles after Intravenous Administration: Effect of Particle Size, Colloids Surf., B, 2008, 66(2), 274–280, DOI:10.1016/j.colsurfb.2008.07.004.
- D. Feldman, Weathering of Polymers, J. Polym. Sci., Polym. Lett. Ed., 1984, 22(7), 423, DOI:10.1002/pol.1984.130220709.
- D. Feldman, Polymer Weathering: Photo-Oxidation, J. Polym. Environ., 2002, 10(4), 163–173, DOI:10.1023/A:1021148205366.
- B. Gewert, M. M. Plassmann and M. MacLeod, Pathways for Degradation of Plastic Polymers Floating in the Marine Environment, Environ. Sci.: Processes Impacts, 2015, 17(9), 1513–1521, 10.1039/C5EM00207A.
- I. Goßmann, H. Mitsutake, J. Degenhardt, M. E. Simonsen and F. Liu, Biofilms on Plastics Slow Photo-Oxidation While Promoting Surface Degradation, Environ. Sci. Technol., 2025, 59(42), 22866–22873, DOI:10.1021/acs.est.5c08345.
- A. Al Harraq, P. J. Brahana, O. Arcemont, D. Zhang, K. T. Valsaraj and B. Bharti, Effects of Weathering on Microplastic Dispersibility and Pollutant Uptake Capacity, ACS Environ. Au, 2022, 2(6), 549–555, DOI:10.1021/acsenvironau.2c00036.
- F. Yu, Q. Qin, X. Zhang and J. Ma, Characteristics and Adsorption Behavior of Typical Microplastics in Long-Term Accelerated Weathering Simulation, Environ. Sci.: Processes Impacts, 2024, 26(5), 882–890, 10.1039/D4EM00062E.
- S. Oh and E. E. Stache, Recent Advances in Oxidative Degradation of Plastics, Chem. Soc. Rev., 2024, 53(14), 7309–7327, 10.1039/D4CS00407H.
Footnotes |
| † Present address: EaStCHEM School of Chemistry, University of Edinburgh, Joseph Black Building, Edinburgh, EH9 3FJ, United Kingdom. |
| ‡ Present address: Flanders Marine Institute (VLIZ), Research Division, Ocean and Human Health, InnovOcean Campus, Jacobsenstraat 1, 8400 Oostende, Belgium. |
| § Present address: School of Geographical & Earth Sciences, University of Glasgow, Main Building, Glasgow, G12 8QQ, United Kingdom. |
| This journal is © The Royal Society of Chemistry 2026 |